Abstract
Aqueous zinc batteries (AZBs) are one of the most promising large-scale energy storage devices by virtue of their high specific capacity, high degree of safety, non-toxicity, and significant economic benefits. However, Zn anodes in aqueous electrolyte suffer from zinc dendrites and side reactions, which lead to a low coulombic efficiency and short life cycle of the cell. Since electrolytes play a key role in the Zn plating/stripping process, versatile strategies have been developed for designing an electrolyte to handle these issues. Among these strategies, electrolyte additives are considered to be promising for practical application because of the advantages of low cost and simplicity. Moreover, the resulting electrolyte can maximally preserve the merits of the aqueous electrolyte. The availability and effectiveness of additives have been demonstrated by tens of research works. Up to now, it has been essential and timely to systematically overview the progress of electrolyte additives in mild acidic/neutral electrolytes. These additives are classified as metal ion additives, surfactant additives, SEI film-forming additives, and complexing additives, according to their functions and mechanisms. For each category of additives, their functional mechanisms, as well as the latest developments, are comprehensively elaborated. Finally, some perspectives into the future development of additives for advanced AZBs are presented.
1. Introduction
The growing concerns about the environment and sustainability in world energy markets have promoted the development and utilization of renewable energy sources [1]. In order to cope with the intermittent features of renewable energy, efficient energy storage systems (ESSs) are greatly desired. Lithium-ion batteries (LIBs) are deemed as one of most important ESSs by virtue of their high energy and power densities. Nevertheless, safety issues and high costs limit their more widespread applications [2,3,4,5].
Recently, aqueous rechargeable multivalent metal-ion batteries have been considered as promising alternatives to conventional LIBs due to their high degree of safety, desirable performance, and low cost [6,7,8]. In particular, Zn anodes deliver the merits of a high theoretical capacity (820 mAh g−1), low redox potential (−0.763 V vs. SHE), non-toxicity, and abundant reserves (0.02 wt% of the enclosure content). Therefore, aqueous zinc batteries (AZBs) demonstrate promising potential in large-scale energy storage systems and have received significant attention [9,10]. However, their practical applications have been hindered by some problems residing in Zn anodes, such as side reactions and zinc dendrites. These deteriorate the AZB’s performance and, thus, are desired to be resolved [11,12,13].
Intensive research has focused on developing and optimizing Zn metal anodes in terms of their compositions, architectures, etc. [14,15]. Indeed, these strategies can alleviate the aforementioned issues to some extent. On the other hand, further investigation in aqueous electrolyte is urgently needed, since electrolytes plays a central role in the Zn plating/stripping process and provide channels for ion transportation [16,17]. To this end, versatile manipulating strategies have been reported for designing electrolytes for highly reversible and dendrite-free Zn anodes, including a zinc salt design, the water-in-salt strategy, co-solvent strategies, hydrogel electrolytes, and additives [18]. Among these strategies, adjusting electrolyte composition by adding a small quantity of additives is regarded as promising because of the advantages of low cost and simplicity. Moreover, the resulting electrolyte can maximally preserve the merits of the aqueous electrolyte. The availability and effectiveness of additives in solving the Zn anode problems have been demonstrated by a number of reported studies.
To date, several excellent reviews have given a good overview of the development of electrolytes from the perspectives of co-solvent strategies, hydrogel electrolytes, etc. [19,20,21], but systematic reviews focusing on electrolyte additives are scarce. Since mild acidic/neutral electrolytes are considered to be promising due to their relatively high stability with zinc metal and their use by most of the recently reported works, we are, thus, motivated to summarize the state-of-the-art development in electrolyte additives for highly reversible and dendrite-free Zn anodes in mildly acidic/neutral electrolytes. Herein, challenges with Zn anodes are briefly introduced at first, including zinc dendrites and side reactions. After that, recent developments in electrolyte additives are summarized and thoroughly elaborated. According to their functions and mechanisms, additives are classified into four categories: metal ion additives, surfactant additives, solid electrolyte interphase (SEI) film-forming additives, and complexing additives. Lastly, some perspectives into the future development of additives for advanced AZBs are presented.
2. Challenges with Zn Anode
With the advantages of high security, low price, abundant reserves, and environmental friendliness, zinc metal, thereby, extensively serves as the anode in AZBs. However, as known to us, Zn anodes in aqueous electrolyte suffer from issues including zinc dendrites and side reactions, which are detrimental to the performance of AZBs [22]. The formation of zinc dendrites is usually induced by the uneven distribution of the electric field and the uncontrolled 2D diffusion of Zn2+ on the anode surface [23]. Specifically, the initiation of dendrites is usually formed at the preferential nucleation sites of the Zn anode surface. Next, zinc ions are inclined to deposit on the sites exhibiting minimal surface energy, which are usually the bulges of initial dendrites, causing the so-called “tip effect” [24,25]. As a result, the electric field on the zinc anode surface is distributed unevenly, accelerating the dendritic growth [24]. The brittle zinc dendrites produced during charging tend to fracture and form dead zinc when discharging, seriously affecting the performance of AZBs. Terrifically, dendrites will penetrate the separators, which eventually induces short circuits in the AZBs.
In terms of the side-reaction issues of Zn anodes, hydrogen evolution (HER) is the most concerning one among corrosion, HERs, and passivation [26]. Hydrogen evolution side reactions will continuously reduce the coulombic efficiency of the cell [27]. Moreover, a portion of the produced H2 will adsorb on the Zn surface. Zn deposition is prevented on the site adsorbed by H2, which further accelerates the uneven Zn deposition and dendritic growth [28]. In a word, dendrite formation and HER side reactions are the root causes of unsatisfactory electrochemical performance of a Zn anode [29].
3. Electrolyte Additives
Although aqueous electrolytes possess superiority in safety, cost, and ionic conductivity, issues with zinc anodes in aqueous electrolytes, including dendritic growth and side reactions, as mentioned in Section 2, need to be resolved. In this regard, researchers have made continuous efforts and adopted various strategies, such as solvent regulation [30], water-confined separators [31], and hydrogel electrolytes [32]. Among these strategies, additives can not only address the above-mentioned problems well, but also save materials and avoid the complex fabrication procedures. Trace amounts of additives in aqueous electrolyte can greatly improve the electrochemical performance of the zinc anode [33].
Various additives have been adopted to improve the properties of aqueous zinc batteries. According to their functions and mechanisms, we classify the additives into four categories, as follows:
- (1)
- Metal ion additives: metal ions can be reduced before zinc to provide additional deposition sites, or they cannot be reduced during zinc deposition and form an electrostatic shielding layer to suppress the tip effect.
- (2)
- Surfactant additives: the molecules/ions that adsorb on the electrode surface, thereby, modulate the anode/electrolyte interfacial environment and homogenize the Zn2+ flux.
- (3)
- SEI film-forming additives: the additives will be consumed by reactions to form a protective interfacial layer on the anode surface, which can prevent the hydrogen evolution reaction and dendrite formation by suppressing the 2D diffusion of Zn2+ on the substrate surface.
- (4)
- Complexing additives: the interaction between these additives and zinc ions is stronger than that between zinc ions and water, and they can enter the solvation sheath of Zn2+, weaken the activity of water molecules, and change the zinc deposition kinetics.
In this section, we will review the recent developments and detailed mechanisms for each category of these additives.
3.1. Metal Ion Additives
Metal ion additives with different reduction potentials inhibit the formation of zinc dendrites through different mechanisms. To be specific, metal ions with high reduction potentials (Bi3+, Pd2+, Co2+, Ni2+) will be reduced before Zn2+ and form uniformly distributed metal sites in situ that can be used as heterogeneous nucleation sites. Therefore, metal ion additives effectively inhibit the undesirable 2D diffusion of Zn2+ and dendritic growth [34,35,36,37]. Chang et al. adopted 1.67 mmol L−1 of Pd2+ and Ni2+ as additives to investigate their inhibitory effects on Zn dendrites. From the scanning electron microscopy (SEM) images (Figure 1a,c), it can be seen that the morphology of the zinc deposition after adding Pd2+ displayed smaller spherical depositions compared to that without any additives. However, tiny Zn dendrites were still observed when adding Ni2+ and Cu2+ to the electrolyte (Figure 1b,d). Although the preferential deposition of both Pd2+ and Ni2+ additives increased nucleation sites and effectively inhibited the formation of Zn dendrites, Zn2+ tended to form zinc alloys with Pd2+ rather than with Ni2+; thus, Pd2+ led to a more homogeneous zinc deposition [37].
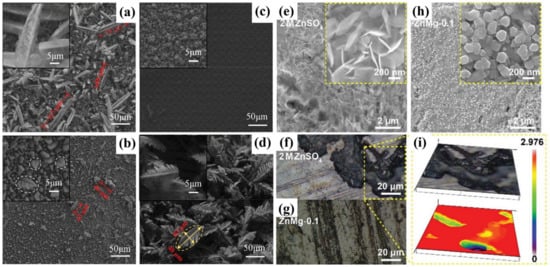
Figure 1.
SEM images of Zn deposits obtained at −1.5 V vs. Zn/Zn2+ for 400 s with different additives of (a) none, (b) Ni2+, (c) Pb2+, and (d) Cu2+ [37]; SEM images of Zn deposition in (e) 2 M ZnSO4 and (h) ZnMg-0.1 electrolytes after 20 cycles at the fully charged state. Optical images of Zn deposition in (f) 2 M ZnSO4 and (g) ZnMg-0.1 electrolytes, and (i) zoom in view of (f) [38].
Similarly, Ma et al. found that the addition of 0.2 mol L−1 CoSO4 to aqueous electrolyte can also exhibit an inhibitory effect on Zn dendrites. Furthermore and interestingly, the electrochemical window of the electrolyte was widened to 2.2 V. As a result, the Zn/Co3O4 battery delivered great durability, which maintained 92% capacity retention even after 5000 cycles [36].
On the other hand, metal ions with lower reduction potentials (Na+, Mg2+, Mn2+, Ce3+) can homogenize the flux of Zn2+ through a cationic electrostatic shielding mechanism, thereby inhibiting the tip effect [37,38,39,40]. Xu et al. demonstrated the successful use of a Zn/NaMnO2 battery by adding 0.25 mol L−1 Na2SO4 to the ZnSO4 electrolyte. They observed that the added Na+ ions would form an electrostatic shield around the tips of Zn. This would drive Zn2+ ions to deposit in non-tip regions, preventing the formation of Zn dendrites. As expected, a homogeneous Zn deposition morphology was observed in the modified electrolyte, and the Zn/Zn symmetric cells exhibited a low overpotential (48.8 mV) and excellent cycling stability (300 h) [41].
Moreover, the addition of metal ions can also mitigate the occurrence of side reactions. Taking the Mg2+ additive as an example, 0.1 M MgSO4 was introduced into the ZnSO4 electrolyte. It was found that Mg2+ showed strong interactions with water molecules. Such strong interactions were demonstrated to decrease the water activity and, thus, mitigate the hydrogen evolution. At the same time, flatter zinc deposition morphology was also obtained (Figure 1e–i). Additionally, Mg2+ and Zn2+ share the solvated H2O form (Zn2+Mg2+(H2O)6), which reduces water molecule activity. Furthermore, the Mg2+ ions can form an electrostatic shield layer around the Zn surface due to electrostatic attraction. This layer isolates water from the zinc metal to prevent HERs, thereby reducing side reactions. Meanwhile, this layer can also drive Zn2+ ions to deposit in non-tip regions because of electrostatic repulsion, leading to a flatter deposition layer (Figure 2). As a result, the assembled Zn/Zn symmetric batteries with a MgSO4 addition showed smaller overpotential (85.9 mV) and achieved a great durability that maintained 98.7% capacity retention after 10,000 cycles [38].
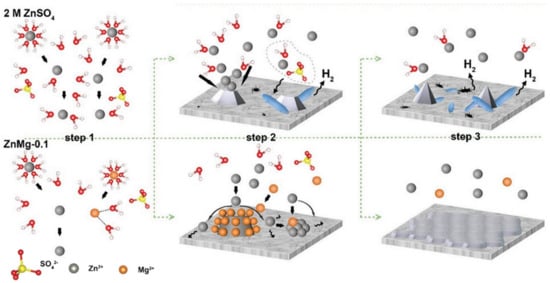
Figure 2.
The scheme illustration of the Zn deposition process in the electrolyte without (top row) and with 0.1 M MgSO4 additive (bottom row) [38].
3.2. Surfactant Additives
Unlike metal ion additives, surfactant additives can spontaneously distribute at the interface between the electrode and electrolyte due to reduced surface tension, preventing direct contact between active water and the zinc anode, thereby inhibiting the hydrogen evolution side reactions. Furthermore, the re-establishment of the interfacial environment changes the Zn2+ deposition kinetics and inhibits the growth of zinc dendrites. Considering these merits, the surfactant additives will be elaborated on in this section. There are four types, according to the dissociation properties of their polar groups: cationic surfactants, anionic surfactants, amphoteric surfactants, and non-ionic surfactants.
Cationic surfactants, especially ammonium surfactants such as tetrabutylammonium sulphate (TBA2SO4) and triethylmethylammonium chloride (TMA), have been used as additives in electrolytes to flatten the surface energy distribution [42,43,44,45,46,47,48]. For example, Zhu et al. added 29 mg L−1 cationic surfactant TBA2SO4 to the electrolyte and found that TBA+ with hydrophobic chains was adsorbed on the zinc surface to avoid hydrogen evolution. At the same time, the formed protective layer prevented the transport of solvated Zn2+, which slowed down the Zn plating and ensured uniform deposition (Figure 3a,b) [42]. However, this inhibition effect of Zn deposition needs to be carefully controlled, so as to prevent HERs and achieve an acceptable rate capability. For this purpose, Guan et al. investigated three quaternary ammonium surfactants, benzyl dimethyl dodecyl ammonium chloride (DDBAC), dodecyl trimethyl ammonium chloride (DTAC), and benzyl trimethyl ammonium chloride (TMBAC), and analyzed the effects of their different hydrophobic groups on the modulation of Zn deposition behavior [43]. Experimental and theoretically calculated results revealed that the hydrophobic groups on surfactants were the decisive factor in affecting the kinetic process of zinc-ion deposition (Figure 4a). Among them, TMBAC obtained the brighter zinc deposition than did DTAC or DDBAC (Figure 3c–f) because the more hydrophobic groups on and higher surface activity of DTAC and DDBAC increased the polarization overpotential and destabilized the adsorption layer. Therefore, the Zn/Zn symmetric cells with addition of 0.5 g L−1 TMBA+ exhibited the best performance, which achieved the compact deposition morphology without producing byproducts, and were able to cycle stably for more than 500 h at 10 mA cm−2 and 5 mAh cm−2.
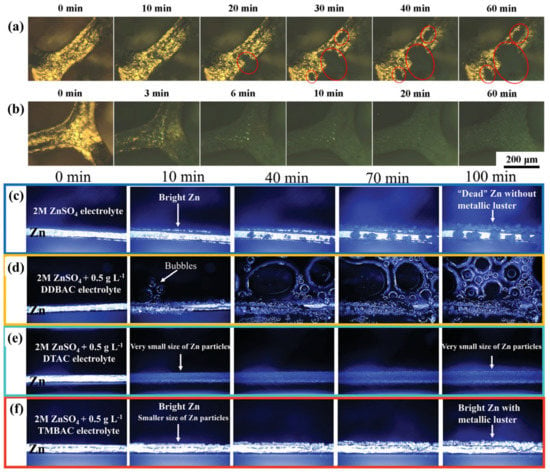
Figure 3.
In situ optical microscopy observations of the Zn deposition process at 10 mA cm−2 in (a) blank and (b) 0.05 mM TBA2SO4 additive electrolytes [42]. In situ optical microscopy observations of Zn deposition at 5 mA cm−2 in electrolytes with different additives: (c) Blank, (d) 0.5 g L−1 DDBAC, (e) 0.5 g L−1 DTAC, and (f) 0.5 g L−1 TMBAC [43].
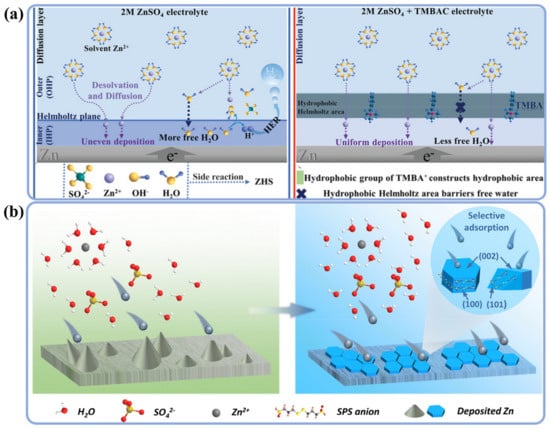
Figure 4.
The scheme illustration of the Zn deposition process in 2M ZnSO4 electrolyte (a) with or without TMBAC [43] and (b) with or without SPS additive [49].
Additionally, anionic surfactants such as sodium dodecyl benzene sulfonate (SDBS) and sodium 3,3′-dithiodipropane sulfonate (SPS) have been reported and proved to be effective in solving Zn dendritic growth and anode corrosion issues [49,50,51]. Wang et al. used 10 mmol L−1 SPS as an electrolyte additive in 1M Zn(OTf)2, and first-principles calculations revealed that SPS− tended to adsorb on other crystalline planes ((100) and (101)) rather than on the (002). As expected, deposition of Zn in the direction of (002) was effectively controlled (Figure 4b). Thus, dendritic growth and HERs were significantly inhibited at the same time. Benefitting from SPS−, the Zn/Zn symmetric cell cycled stably for more than 4000 h at 1 mA cm−2 and 1 mAh cm−2 [49].
In addition, natural amphoteric surfactants such as arginine (Arg) and cysteine (Cys) have also been extensively studied [52,53,54,55]. For example, Lu et al. added 0.1 mol L−1 of the hydrophilic amino acids, including Arg, serine (Ser), and glutamic acid (Glu), to aqueous electrolyte to investigate the factors that influence the stability of the adsorption layer. DFT calculations revealed that Arg+ delivered the highest adsorption energy and a better stability of the adsorption layer. Because the adsorbed Arg layer can isolate water and guide Zn2+ flux during the plating process, the electrolyte with Arg+ showed a better ability to inhibit HERs and dendritic growth. Consequently, the assembled cell exhibited ultra-long stable cycling, even up to 2200 h at 5 mA cm−2 and 4 mAh cm−2 [52].
Furthermore, non-ionic surfactants can also be adsorbed on the Zn surface to regulate the Zn deposition process, such as polyethylene oxide (PEO), poly(4-styrenesulfonic acid sodium) (PSS), and poly(acrylamide-co-methylacrylate) copolymer (APA) [56,57,58,59,60]. It is worth noting that the hydrophilicity and hydrophobicity of the surfactants are of great significance in rebuilding the interfacial environment. Different from other kinds of surfactants, the hydrophilicity of polymer surfactants can be facially adjusted in many ways so as to optimize the AZB performance. For instance, a hydrophilicity-tunable nonionic copolymer additive, 1 wt.% APA, was introduced to traditional Zn(OTf)2 electrolyte. The Zn/Zn symmetric cells achieved a superior cycle life (Figure 5a–c) and a high CE with an average of 99.3% (Figure 5e). Surprisingly, the operating temperature of the Zn/Zn cell was significantly widened, ranging from 50 °C down to −30 °C (Figure 5d), because the hydrophilicity-tunable APA tuned the coordination environment of Zn2+ and, thus, lowered the freezing point of the electrolyte (Figure 5f,g) [56]. Finally, Table 1 summarizes the surfactant additives and corresponding Zn anode cycling performance.
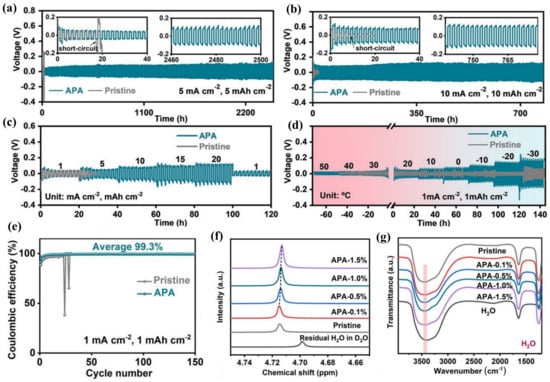
Figure 5.
Cycling performance of Zn/Zn cells cycled at (a) 5 mA cm−2, (b) 10 mA cm−2, and (c) at varied current densities; (d) cycling performance of Zn/Zn cells cycled under varied temperatures at 1 mA cm−2; (e) coulombic efficiency of Zn/Cu cells at 1 mA cm−2 and 1 mAh cm−2; (f) 1H NMR spectra in D2O and (g) infrared spectra in H2O for pristine- and APA-containing electrolytes [56].

Table 1.
Summary of surfactant additives.
3.3. SEI Film-Forming Additives
The adsorption layers at the electrode/electrolyte interface can be constructed by adding surfactants; in a like manner, a protective SEI film can be formed in situ on the anode surface by adding “SEI film-forming” additives. The SEI formed in situ can homogenize the Zn deposition and avoid HER occurrence by facilitating the 3D diffusion of Zn2+ and preventing the direct contact between the active water and the zinc, respectively. Zeng et al. revealed that Zn2+-SEI composed of Zn phosphates could be generated by introducing 25 mmol L−1 Zn(H2PO4) 2 into the electrolyte. The Zn3(PO4) 2 4H2O layer (~140 nm thick) formed in situ presented features of uniformity and denseness and was endowed with rapid transport kinetics, which inhibited electrolyte erosion and facilitated even Zn deposition. Therefore, the Zn/Zn cell presented stable cycling for over 1200 h at 1 mA cm−2 and 1 mAh cm−2 [61].
As known to us, fluorinated SEI has been extensively investigated in LIBs. Inspired by this, Wang et al. introduced 0.5 mol L−1 of alkylammonium salt (Me3EtNOTF) to aqueous electrolyte. Through the depth profiles of XPS F1s spectra (Figure 6a–c), it can be seen that a fluorinated and hydrophobic interphase was formed in situ, as schematically illustrated in Figure 6d. The as-formed SEI was able to guard the Zn from water (but allow Zn2+ to diffuse through) and promote lateral rather than vertical Zn2+ migration and deposition, thereby preventing parasitic reactions and suppressing dendritic growth (Figure 7a–f). Therefore, the Zn/VOPO4 coin cell presented stable cycling for over 6000 h at 2.0 A g−1 in sharp contrast to that without additives (Figure 6e) [62]. Moreover, inspired by the bioadhesive of marine mussels, Zeng et al. formed a polydopamine SEI by an in situ reaction with 50 mmol L−1 of dopamine (DA) additive. Consequently, the Zn/V2O5 full battery gave ultra-long stable cycling up to 1000 cycles at a current density of 1 A g−1, with a limited electrolyte (9 μL mAh−1) and a low anode/cathode capacity ratio (~2) [63].
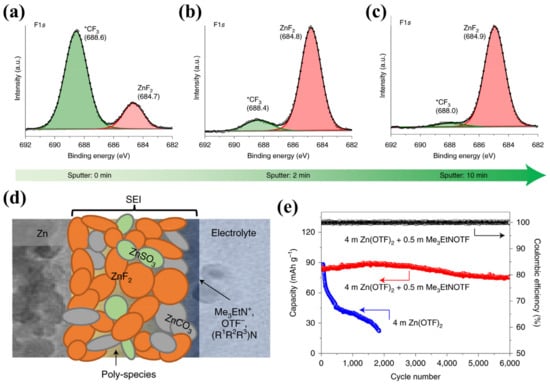
Figure 6.
XPS F1s spectra generated after Ar+ sputtering for (a) 0 min, (b) 2 min, and (c) 10 min; (d) the scheme illustration of proposed SEI, consisting of small nodular particles embedded within a polymeric framework. (e) Cycling performance of the Zn/VOPO4 coin cell in both electrolytes at 2.0 A g−1 [62].
Additionally, some 2D materials, such as graphene oxide (GO) and MXene, have been used as “SEI forming” additives in electrolytes in order to construct a self-assembled SEI [64,65,66]. Abdulla et al. added 0.16 wt% of GO to ZnSO4 electrolyte. The GO added to the electrolyte formed an interface layer by closely adsorbing on the zinc surface. The GO layer could promote a homogeneous distribution of surface energy and provide more nucleation sites by virtue of its abundant polar functional groups (Figure 7g–l). As a result, the Zn/Zn symmetric cells were able to cycle stably for 200 h at 10 mA cm−2, 5 mAh cm−2 [66].
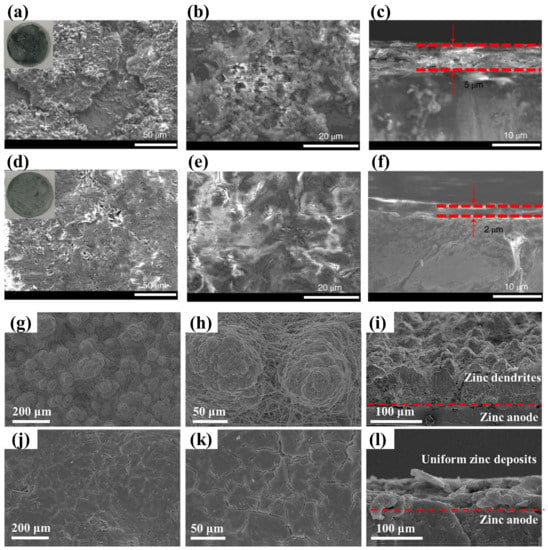
Figure 7.
SEM images of the zinc anode at different scales: (a) 50 um, (b) 20 um, and (c) cross-section image without Me3EtNOTF additive after 50 cycles. SEM images of the zinc anode at different scales: (d) 50 um, (e) 20 um, and (f) cross-section image with 0.5 m Me3EtNOTF additive after 50 cycles [62]. SEM images after 200 plating/stripping cycles in the blank electrolyte at different scales: (g) 200 um, (h) 50 um, and (i) cross-section image; SEM images after 200 plating/stripping cycles in the electrolyte with GO electrolyte additive at different scales: (j) 200 um, (k) 50 um, and (l) cross-section image [66].
3.4. Complexing Additives
The complexing additives added to aqueous electrolyte play a crucial role in reconstituting the solvated sheath layer of Zn2+, which directly influences the zinc deposition kinetics and side reactions. Hitherto, according to the reports in the literature, halogen and amine molecules are the most commonly used complexing additives. Table 2 summarizes the complexing additives and corresponding Zn anode cycling performance.

Table 2.
Summary of complexing additives.
By investigating a series of halogenated Zn salts, Jin et al. found that, unlike [Zn(H2O)6]2+ in the ZnSO4 electrolyte, in the ZnCl2 electrolyte, [ZnCl2(H2O)6] was the dominant ionic state; in the ZnI2 electrolyte, [ZnI]42− was the dominant ionic state, and in the ZnBr2 electrolyte, it was [ZnBr2(H2O)6] and [ZnBr]42− that coexisted in the ionic state. They further demonstrated that ionic complexes containing anions could reduce the water within the Zn2+ solvation sheath to form solvation structures, such as [ZnBr]42− and [ZnI]42−, because the interaction force between ionic complexes containing anions and Zn2+ was stronger than that between H2O and Zn2+. This prevents HERs, endowing halogenated Zn salt electrolytes with excellent electrochemical performance. Consequently, the Zn/carbon cloth asymmetric battery was capable of maintaining long-term cycling of over 5000 cycles at 160 mA cm−2, 2.6 mAh cm−2 in 1 mol L−1 ZnI2 electrolyte [67].
As for amine molecules containing lone pairs of electrons, numerous studies have studied their potential as complexing additives [68]. For example, Han et al. added 1 mol L−1 of the ammonium acetate (NH4OAc) as a pH buffer to aqueous electrolyte to investigate the factors that influence the stability of the interface, as illustrated in Figure 8a. According to the density functional theory calculation, NH4+ induced an electrostatic shielding effect at the interface (Figure 8b). At the same time, OAc− as a pH buffer could effectively inhibit the occurrence of pH-sensitive side reactions. Therefore, it showed a better ability in inhibiting the dendritic growth and hydrogen evolution, leading to greatly improved cycling stability (Figure 8c). Consequently, the assembled Zn/Zn cell exhibited ultra-long stable cycling, even up to 3500 h at 1 mA cm−2 and 1 mAh cm−2 [68]. Furthermore, Chen et al. introduced 4 mol L−1 of NH4I into the Zn(OAc)2 electrolyte and found that the NH4+ and I− could participate in the solvation structure and reduce the water activity, thus inhibiting the occurrence of side reactions. Additionally, NH4+ was able to construct an electrostatic shield by adsorption to inhibit Zn dendrites. The assembled Zn/Cu half-battery achieved a cycle life of 200 h with a coulombic efficiency of 99.8% at 1 mA cm−2 [69]. Furthermore, substituted amines or other complexing agents containing amines have also demonstrated their roles in modulating the solvation sheath, such as ethylene diamine tetra acetic acid tetrasodium salt (Na4EDTA) and triethylamine hydrochloride (TEHC) [70,71,74,75]. As demonstrated by Qian et al., the coordination effect of the amine ligand could be further enhanced by the induction effect of the substituent. As such, the ethyl substituent was chosen to modify the amine group, and the effects of 0.5 mol L−1 triethylamine hydrochloride, diethylamine hydrochloride, ethylamine hydrochloride, and ammonium chloride were investigated. As compared to NH4Cl, TEHC possessed a stable electron-donating ability, which enhanced its binding to Zn2+ and optimized the solvation shell of Zn2+. Benefitting from TEHC, the Zn/Zn symmetric cell showed stable cycling for more than 1100 h at 1 mA cm−2 and 1 mAh cm−2 [72].
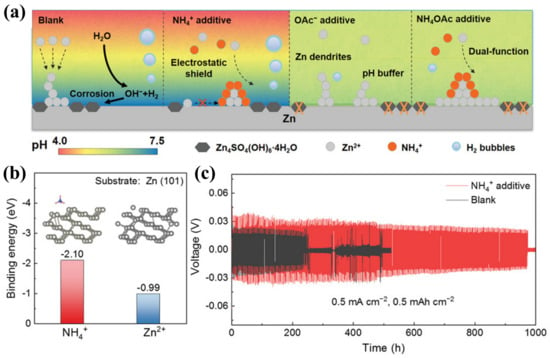
Figure 8.
(a) The scheme illustration of the Zn deposition and evolution process in blank electrolytes or with different additives; (b) binding energy for Zn2+ and NH4+ on Zn (101); (c) lifespan of Zn/Zn cells in the blank electrolytes and with the NH4+ additive [68].
Zhang et al. introduced 75 mmol L−1 of Na4EDTA as an additive into the ZnSO4 electrolyte and found that the EDTA anion could be adsorbed on the Zn surface to suppress side reactions. Remarkably, the EDTA anion was allowed to enter the Zn2+ solvation shell thanks to the strong bonding between Zn2+ and the EDTA anion. Thus, the desolvation process of hydrated Zn2+ could be accelerated to achieve uniform Zn deposition. Hence, the addition of Na4EDTA to the electrolyte enabled the assembled cell to deliver a high coulombic efficiency of 99.5% and ultra-long cycle life of 2500 h [71]. Furthermore, it is increasingly urgent to establish effective selection criteria by which complexing additives can be selected reasonably. Yang’s group pioneered the use of the equilibrium constant of the complexation reaction (K) as a general criterion to accurately select a suitable complexing agent. By comparing the K values of Zn acetate, Zn oxalate, Zn glycinate, Zn nitrite triacetate, and Zn EDTA (Figure 9a), EDTA was found to exhibit the highest K value and chelated energies in the selected matrix (Figure 9b). Through a calculation of corrosion reaction pathways and energy intervals, it was proved that EDTA exhibited better inhibition of anode corrosion (Figure 9c–e). Meanwhile, it exhibited smoother galvanization, and ultimately, the Zn/Zn symmetric cells enabled long-term stable cycling for over 3000 h at 5 mA cm−2 and 1 mAh cm−2 [73].
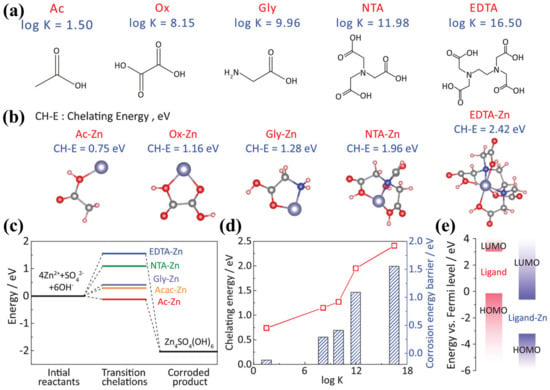
Figure 9.
(a) Molecular models of Ac, Ox, Gly, NTA, and EDTA; (b) the optimized molecular structures and corresponding chelation energies of Ac-Zn, Ox-Zn, Gly-Zn, NTA-Zn, and EDTA-Zn; (c) corrosion reaction pathways with different Zn–ligand complexes in various electrolytes; (d) relationship between log K value and the chelation energy and (e) energy intervals between the LUMO and HOMO of Ligand and Ligand-Zn [73].
4. Conclusions and Perspectives
Aqueous zinc batteries are deemed as one of the most promising large-scale energy storage systems due to their excellent sustainability and economics. Nevertheless, some issues, including zinc dendrites and side reactions, are the central obstacles restricting their large-scale applications. Among various strategies to cope with these issues, additives have received significant attention, owing to their facile and material-saving features. In this review, recent developments in electrolyte additives were comprehensively overviewed according to their functions and mechanisms, and the additives were classified into metal ion additives, surfactant additives, SEI film-forming additives, and complexing additives.
In summary, the four types of additives can largely suppress the formation of zinc dendrites due to distinct mechanisms. However, their abilities to inhibit HERs as well as corrosion are quite different. Metal ion additives are less satisfactory for the inhibition of the HER. Only the metal ions with reduction potentials lower than that of Zn2+ can separate water molecules from Zn metal, to some extent, by forming an acceptably tight electrostatic shield layer. Surfactant additives can spontaneously form a tight protective layer via surface tension to block water molecules, but the low ionic conductivity of this protective layer significantly reduces the rate performance of the cell. Screen and optimization of the molecular structure of the surfactant is needed to achieve a high-rate capability. The SEI film-forming additives can form a dense and highly ionically conductive SEI, which effectively blocks water molecules to suppress HERs without sacrificing cell performance. However, the adhesion of the SEI film to zinc is troublesome, and it may easily peel off after repeated cycles. The complexing additive reduces the activity of water molecules through complexation with Zn2+ to inhibit the HER. However, compared to the former three additives with effective concentrations of mmol/L, complexing additives often work in the mol/L range, which increases the cost and diminishes the economic advantage of aqueous electrolytes.
In spite of these achievements, continuous efforts are needed to promote the development of additives from the following viewpoints:
- (1)
- Developing multifunctional additives that can solve both anode and cathode issues simultaneously is of great importance. For example, some metal ion additives can suppress the dissolution of metal ions from cathodes and, simultaneously, inhibit the dendritic growth of anodes. To this end, more advanced additives are urgently needed to modify both the anode and cathode at the same time, aiming at achieving a better overall performance of the battery.
- (2)
- Exploring the working principles of additives plays a key role in guiding the future development and design of electrolyte additives. It is essential to systematically investigate the influence of additive structure on the electrochemical performance, so as to establish the relationship of structure–mechanism performance on additives.
- (3)
- Studying the interactions between different additives in electrolyte is highly desired as well, which can help realize the synergistic effects that arise from various additives added to the electrolyte, achieving further improved cell performance.
Author Contributions
Conceptualization, T.C.; formal analysis, Y.G.; investigation, Q.S. and T.Y.; resources, Y.W. and G.H.; data summary, H.S.; writing—original draft preparation, Q.S.; writing—review and editing, X.L. and T.C.; funding acquisition, T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China (No. 51902301), Natural Science Foundation of Zhejiang Province (LY21E020006), and Shanghai Pujiang Program (No. 21PJ1411100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the support of the National Science Foundation of China (No. 51902301). We also acknowledge the support of the Natural Science Foundation of Zhejiang Province (LY21E020006) and Shanghai Pujiang Program (No. 21PJ1411100).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, D.; Yang, H.Y.; Bai, Y. Tactics to Optimize Conversion-Type Metal Fluoride/Sulfide/Oxide Cathodes toward Advanced Lithium Metal Batteries. Nano Res. 2022. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.-F.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Z.; Lv, Y.; Tang, A.; Dai, L.; Wang, L.; He, Z. Perovskite enables high performance vanadium redox flow battery. Chem. Eng. J. 2022, 443, 136341. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Kidanu, W.G.; Yang, H.; Park, S.; Hur, J.; Kim, I.T. Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries. Batteries 2022, 8, 208. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Zhong, C.; Wu, T.; Chen, Z.; Hu, W.; Amine, K.; Lu, J. Recent Advances in Flexible Zinc-Based Rechargeable Batteries. Adv. Energy Mater. 2018, 9, 1802605. [Google Scholar] [CrossRef]
- Wu, X.; Markir, A.; Xu, Y.; Zhang, C.; Leonard, D.P.; Shin, W.; Ji, X. A Rechargeable Battery with an Iron Metal Anode. Adv. Funct. Mater. 2019, 29, 1900911. [Google Scholar] [CrossRef]
- Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries 2021, 7, 40. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2019, 2, 237–260. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, N.; Liu, Z.; Tang, Z.; Zapien, J.A.; Chen, S.; Fan, J.; Zhi, C. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32, 1908121. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Islam, S.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.-k.; Kim, J. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Thieu, N.A.; Li, W.; Chen, X.; Hu, S.; Tian, H.; Tran, H.N.N.; Li, W.; Reed, D.M.; Li, X.; Liu, X. An Overview of Challenges and Strategies for Stabilizing Zinc Anodes in Aqueous Rechargeable Zn-Ion Batteries. Batteries 2023, 9, 41. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef]
- Nagy, T.; Nagy, L.; Erdélyi, Z.; Baradács, E.; Deák, G.; Zsuga, M.; Kéki, S. “In Situ” Formation of Zn Anode from Bimetallic Cu-Zn Alloy (Brass) for Dendrite-Free Operation of Zn-Air Rechargeable Battery. Batteries 2022, 8, 212. [Google Scholar] [CrossRef]
- Liu, J.; Nie, N.; Wang, J.; Hu, M.; Zhang, J.; Li, M.; Huang, Y. Initiating a wide-temperature-window yarn zinc ion battery by a highly conductive iongel. Mater. Today Energy 2020, 16, 100372. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Zeng, X.; Li, D.; Mao, J.; Guo, Z. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 2020, 13, 3917–3949. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.; Lai, F.; Liu, T.; Shearing, P.R.; Parkin, I.P.; He, G.; Brett, D.J.L. Rechargeable aqueous Zn-based energy storage devices. Joule 2021, 5, 2845–2903. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2022, 452, 214297. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Zhao, Y.; Hong, H.; Li, H.; Huang, Z.; Liang, G.; Yang, Q.; Zhi, C. Insight on Organic Molecules in Aqueous Zn-Ion Batteries with an Emphasis on the Zn Anode Regulation. Adv. Energy Mater. 2022, 12, 2102707. [Google Scholar] [CrossRef]
- Guo, S.; Qin, L.; Zhang, T.; Zhou, M.; Zhou, J.; Fang, G.; Liang, S. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries. Energy Storage Mater. 2021, 34, 545–562. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of Zinc Dendrite Growth in Zinc-Based Batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, G.; Guo, Y.; Liu, Z.; Yan, B.; Wang, D.; Huang, Z.; Li, X.; Fan, J.; Zhi, C. Do Zinc Dendrites Exist in Neutral Zinc Batteries: A Developed Electrohealing Strategy to In Situ Rescue In-Service Batteries. Adv. Mater. 2019, 31, e1903778. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Bayaguud, A.; Fu, Y.; Zhu, C. Interfacial parasitic reactions of zinc anodes in zinc ion batteries: Underestimated corrosion and hydrogen evolution reactions and their suppression strategies. J. Energy Chem. 2022, 64, 246–262. [Google Scholar] [CrossRef]
- Banik, S.J.; Akolkar, R. Suppressing Dendritic Growth during Alkaline Zinc Electrodeposition using Polyethylenimine Additive. Electrochim. Acta 2015, 179, 475–481. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Zeng, Z.; Qin, J.; Huang, Y. Strategies of regulating Zn2+solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 2022, 15, 499–528. [Google Scholar] [CrossRef]
- Qin, H.; Chen, W.; Kuang, W.; Hu, N.; Zhang, X.; Weng, H.; Tang, H.; Huang, D.; Xu, J.; He, H. A Nature-Inspired Separator with Water-Confined and Kinetics-Boosted Effects for Sustainable and High-Utilization Zn Metal Batteries. Small 2023, 19, e2300130. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Huang, F.; Cai, Y.; Li, Y.; Ke, H.; Lv, P.; Wei, Q. “Water-in-Salt” Nonalkaline Gel Polymer Electrolytes Enable Flexible Zinc-Air Batteries with Ultra-Long Operating Time. Adv. Funct. Mater. 2022, 32, 2203204. [Google Scholar] [CrossRef]
- Geng, Y.; Pan, L.; Peng, Z.; Sun, Z.; Lin, H.; Mao, C.; Wang, L.; Dai, L.; Liu, H.; Pan, K.; et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022, 51, 733–755. [Google Scholar] [CrossRef]
- Gilman, F.; Mansfeld, S. The effect of lead ions on the dissolution and deposition characteristics of a zinc single crystal in 6M KOH. J. Electrochem. Soc. 1970, 171, 588–597. [Google Scholar]
- Wang, J.M.; Zhang, L.; Zhang, C.; Zhang, J.Q. Effects of bismuth ion and tetrabutylammonium bromide on the dendritic growth of zinc in alkaline zincate solutions. J. Power Sources 2001, 102, 139–143. [Google Scholar]
- Ma, L.; Chen, S.; Li, H.; Ruan, Z.; Tang, Z.; Liu, Z.; Wang, Z.; Huang, Y.; Pei, Z.; Zapien, J.A.; et al. Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co(iii) rich-electrode. Energy Environ. Sci. 2018, 11, 2521–2530. [Google Scholar] [CrossRef]
- Chang, G.; Liu, S.; Fu, Y.; Hao, X.; Jin, W.; Ji, X.; Hu, J. Inhibition Role of Trace Metal Ion Additives on Zinc Dendrites during Plating and Striping Processes. Adv. Mater. Interfaces 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Wang, P.; Xie, X.; Xing, Z.; Chen, X.; Fang, G.; Lu, B.; Zhou, J.; Liang, S.; Fan, H.J. Mechanistic Insights of Mg2+-Electrolyte Additive for High-Energy and Long-Life Zinc-Ion Hybrid Capacitors. Adv. Energy Mater. 2021, 11, 2101158. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, F.; Zhao, Y.; Wang, H.; Huang, Y.; Wu, F.; Chen, R.; Li, L. A Self-Regulated Electrostatic Shielding Layer toward Dendrite-Free Zn Batteries. Adv. Mater. 2022, 34, e2203104. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, J.; Wang, Y.; Wu, X.; Ma, P.; Zhang, X.; Wang, G.; Yan, X. A rechargeable aqueous zinc/sodium manganese oxides battery with robust performance enabled by Na2SO4 electrolyte additive. Energy Storage Mater. 2021, 38, 299–308. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic Surfactant-Type Electrolyte Additive Enables Three-Dimensional Dendrite-Free Zinc Anode for Stable Zinc-Ion Batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Guan, K.; Tao, L.; Yang, R.; Zhang, H.; Wang, N.; Wan, H.; Cui, J.; Zhang, J.; Wang, H.; Wang, H. Anti-Corrosion for Reversible Zinc Anode via a Hydrophobic Interface in Aqueous Zinc Batteries. Adv. Energy Mater. 2022, 12, 2103557. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Che, Y.; Cheng, L.; Zhang, H.; Chen, J.; Xie, F.; Wang, N.; Jin, Y.; Meng, H. Arginine Cations Inhibiting Charge Accumulation of Dendrites and Boosting Zn Metal Reversibility in Aqueous Rechargeable Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6855–6863. [Google Scholar] [CrossRef]
- Yao, R.; Qian, L.; Sui, Y.; Zhao, G.; Guo, R.; Hu, S.; Liu, P.; Zhu, H.; Wang, F.; Zhi, C.; et al. A Versatile Cation Additive Enabled Highly Reversible Zinc Metal Anode. Adv. Energy Mater. 2021, 12, 2102780. [Google Scholar] [CrossRef]
- Qiu, Q.; Chi, X.; Huang, J.; Du, Y.; Liu, Y. Highly Stable Plating/Stripping Behavior of Zinc Metal Anodes in Aqueous Zinc Batteries Regulated by Quaternary Ammonium Cationic Salts. ChemElectroChem 2021, 8, 858–865. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, J.; Li, Y.; Zhou, X.; Ding, S.; Zheng, Q.; Lin, D.; Zhao, J.; Xu, B. Insights into Zn anode surface chemistry for dendrite-free Zn ion batteries. J. Mater. Chem. A 2022, 10, 11288–11297. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, X.; Sun, J.; Wang, W.; Wang, M.; Yuan, Y.; Chuai, M.; Chen, N.; Hu, H.; Chen, W. Nucleophilic Interfacial Layer Enables Stable Zn Anodes for Aqueous Zn Batteries. Nano Lett. 2022, 22, 3298–3306. [Google Scholar] [CrossRef]
- Lin, Y.; Mai, Z.; Liang, H.; Li, Y.; Yang, G.; Wang, C. Dendrite-free Zn anode enabled by anionic surfactant-induced horizontal growth for highly-stable aqueous Zn-ion pouch cells. Energy Environ. Sci. 2023, 16, 687–697. [Google Scholar] [CrossRef]
- Hao, J.; Long, J.; Li, B.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Yang, Z.; Pang, W.K.; Guo, Z. Toward High-Performance Hybrid Zn-Based Batteries via Deeply Understanding Their Mechanism and Using Electrolyte Additive. Adv. Funct. Mater. 2019, 29, 1903605. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Tian, Q.; Chen, J.; Xu, X.; Han, X.; Xu, J. Stabilizing zinc deposition with sodium lignosulfonate as an electrolyte additive to improve the life span of aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 601, 486–494. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino Acid-Induced Interface Charge Engineering Enables Highly Reversible Zn Anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Meng, Q.; Zhao, R.; Cao, P.; Bai, Q.; Tang, J.; Liu, G.; Zhou, X.; Yang, J. Stabilization of Zn anode via a multifunctional cysteine additive. Chem. Eng. J. 2022, 447, 137471. [Google Scholar] [CrossRef]
- Zhou, T.; Mu, Y.; Chen, L.; Li, D.; Liu, W.; Yang, C.; Zhang, S.; Wang, Q.; Jiang, P.; Ge, G.; et al. Toward stable zinc aqueous rechargeable batteries by anode morphology modulation via polyaspartic acid additive. Energy Storage Mater. 2022, 45, 777–785. [Google Scholar] [CrossRef]
- Miao, Z.; Liu, Q.; Wei, W.; Zhao, X.; Du, M.; Li, H.; Zhang, F.; Hao, M.; Cui, Z.; Sang, Y.; et al. Unveiling unique steric effect of threonine additive for highly reversible Zn anode. Nano Energy 2022, 97, 107145. [Google Scholar] [CrossRef]
- Niu, B.; Li, Z.; Luo, D.; Ma, X.; Yang, Q.; Liu, Y.-E.; Yu, X.; He, X.-r.; Qiao, Y.; Wang, X. Nano-scaled hydrophobic confinement of aqueous electrolyte by nonionic amphiphilic polymer for long-lasting and wide-temperature zn-based energy storage. Energy Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Jin, Y.; Han, K.S.; Shao, Y.; Sushko, M.L.; Xiao, J.; Pan, H.; Liu, J. Stabilizing Zinc Anode Reactions by Polyethylene Oxide Polymer in Mild Aqueous Electrolytes. Adv. Funct. Mater. 2020, 30, 2003932. [Google Scholar] [CrossRef]
- Bani Hashemi, A.; Kasiri, G.; La Mantia, F. The effect of polyethyleneimine as an electrolyte additive on zinc electrodeposition mechanism in aqueous zinc-ion batteries. Electrochim. Acta 2017, 258, 703–708. [Google Scholar] [CrossRef]
- Yan, M.; Xu, C.; Sun, Y.; Pan, H.; Li, H. Manipulating Zn anode reactions through salt anion involving hydrogen bonding network in aqueous electrolytes with PEO additive. Nano Energy 2021, 82, 105739. [Google Scholar] [CrossRef]
- Yan, M.; Dong, N.; Zhao, X.; Sun, Y.; Pan, H. Tailoring the Stability and Kinetics of Zn Anodes through Trace Organic Polymer Additives in Dilute Aqueous Electrolyte. ACS Energy Lett. 2021, 6, 3236–3243. [Google Scholar] [CrossRef]
- Zeng, X.; Mao, J.; Hao, J.; Liu, J.; Liu, S.; Wang, Z.; Wang, Y.; Zhang, S.; Zheng, T.; Liu, J.; et al. Electrolyte Design for In Situ Construction of Highly Zn2+-Conductive Solid Electrolyte Interphase to Enable High-Performance Aqueous Zn-Ion Batteries under Practical Conditions. Adv. Mater. 2021, 33, e2007416. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C.; Chen, L.; Vatamanu, J.; Hu, E.; Hourwitz, M.J.; et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat. Nanotechnol. 2021, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xie, K.; Liu, S.; Zhang, S.; Hao, J.; Liu, J.; Pang, W.K.; Liu, J.; Rao, P.; Wang, Q.; et al. Bio-inspired design of anin situmultifunctional polymeric solid–electrolyte interphase for Zn metal anode cycling at 30 mA cm−2 and 30 mAh cm−2. Energy Environ. Sci. 2021, 14, 5947–5957. [Google Scholar] [CrossRef]
- Sun, C.; Wu, C.; Gu, X.; Wang, C.; Wang, Q. Interface Engineering via Ti(3)C(2)T(x) MXene Electrolyte Additive toward Dendrite-Free Zinc Deposition. Nano-Micro Lett. 2021, 13, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, S.; Yuan, Z.; Zhu, J.; Zhao, Z.; Niu, Z. Direct Self-Assembly of MXene on Zn Anodes for Dendrite-Free Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 2861–2865. [Google Scholar] [CrossRef]
- Abdulla, J.; Cao, J.; Zhang, D.; Zhang, X.; Sriprachuabwong, C.; Kheawhom, S.; Wangyao, P.; Qin, J. Elimination of Zinc Dendrites by Graphene Oxide Electrolyte Additive for Zinc-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4602–4609. [Google Scholar] [CrossRef]
- Jin, S.; Yin, J.; Gao, X.; Sharma, A.; Chen, P.; Hong, S.; Zhao, Q.; Zheng, J.; Deng, Y.; Joo, Y.L.; et al. Production of fast-charge Zn-based aqueous batteries via interfacial adsorption of ion-oligomer complexes. Nat. Commun. 2022, 13, 2283. [Google Scholar] [CrossRef]
- Han, D.; Wang, Z.; Lu, H.; Li, H.; Cui, C.; Zhang, Z.; Sun, R.; Geng, C.; Liang, Q.; Guo, X.; et al. A Self-Regulated Interface toward Highly Reversible Aqueous Zinc Batteries. Adv. Energy Mater. 2022, 12, 2102982. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Lu, Y.; Ni, Y.; Lin, L.; Hao, Z.; Yan, Z.; Zhao, Q.; Chen, J. Halogenated Zn(2+) Solvation Structure for Reversible Zn Metal Batteries. J. Am. Chem. Soc. 2022, 144, 18435–18443. [Google Scholar] [CrossRef]
- Luo, M.; Wang, C.; Lu, H.; Lu, Y.; Xu, B.B.; Sun, W.; Pan, H.; Yan, M.; Jiang, Y. Dendrite-free zinc anode enabled by zinc-chelating chemistry. Energy Storage Mater. 2021, 41, 515–521. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hao, J.; Luo, D.; Zhang, P.F.; Zhang, B.; Davey, K.; Lin, Z.; Qiao, S.Z. Dual-Function Electrolyte Additive for Highly Reversible Zn Anode. Adv. Energy Mater. 2021, 11, 2102010. [Google Scholar] [CrossRef]
- Qian, L.; Yao, W.; Yao, R.; Sui, Y.; Zhu, H.; Wang, F.; Zhao, J.; Zhi, C.; Yang, C. Cations Coordination-Regulated Reversibility Enhancement for Aqueous Zn-Ion Battery. Adv. Funct. Mater. 2021, 31, 2105736. [Google Scholar] [CrossRef]
- Meng, R.; Li, H.; Lu, Z.; Zhang, C.; Wang, Z.; Liu, Y.; Wang, W.; Ling, G.; Kang, F.; Yang, Q.H. Tuning Zn-Ion Solvation Chemistry with Chelating Ligands toward Stable Aqueous Zn Anodes. Adv. Mater. 2022, 34, e2200677. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Li, T.; Liu, X.; Yuan, Z.; Li, X. Functional complexed zincate ions enable dendrite-free long cycle alkaline zinc-based flow batteries. Nano Energy 2022, 102, 107697. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, P.; Qin, A.; Cui, G.; Mai, W. Metal-coordination chemistry guiding preferred crystallographic orientation for reversible zinc anode. Energy Storage Mater. 2022, 49, 463–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).