In-Situ Plasticized LLZTO-PVDF Composite Electrolytes for High-Performance Solid-State Lithium Metal Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Experiment
2.2. Preparation of Electrolyte Support System
2.3. Preparation of Polymerization Precursors
2.4. Preparation of Batteries
2.5. Electrochemical Test
2.6. Materials Characterization
3. Results
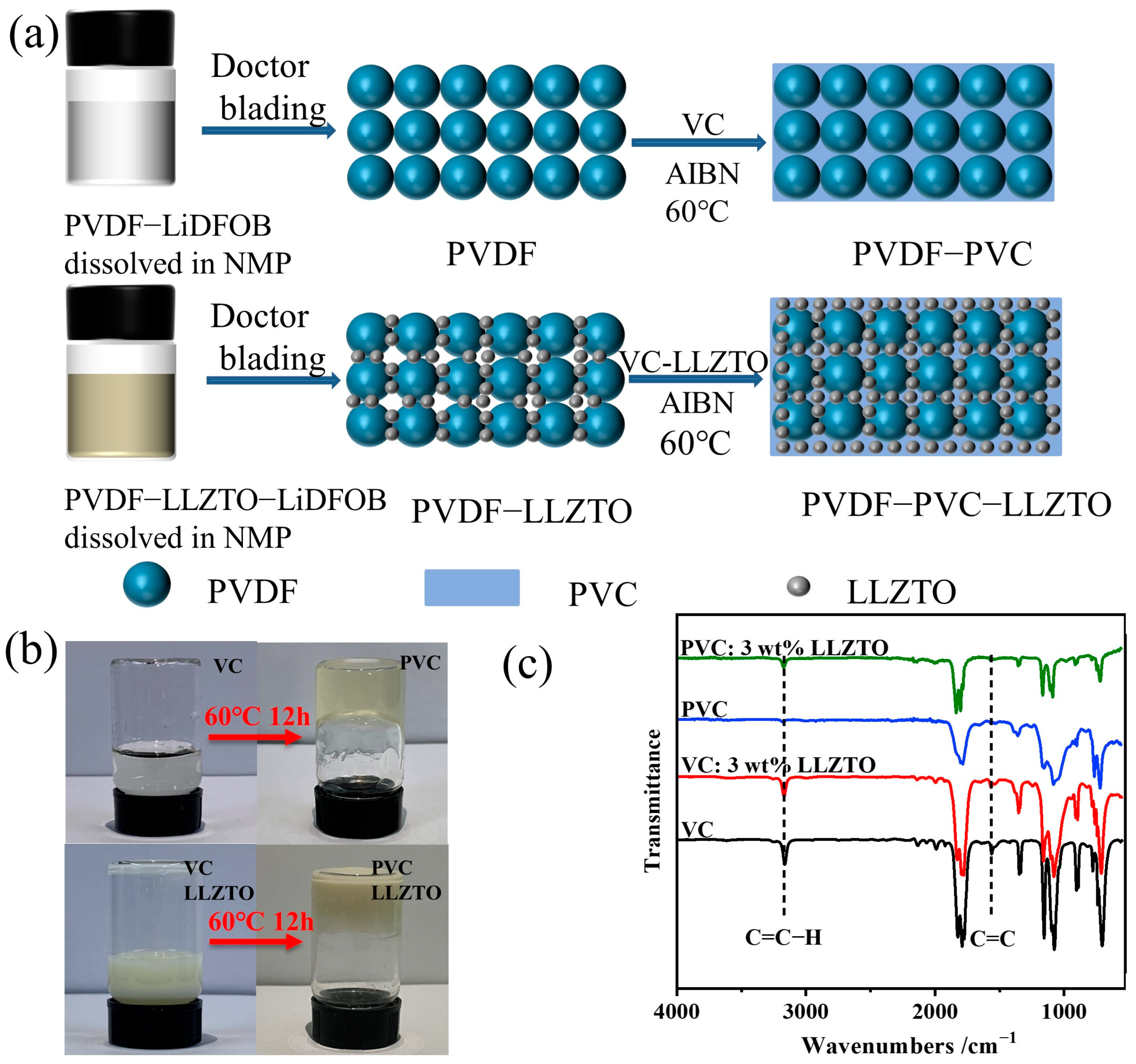
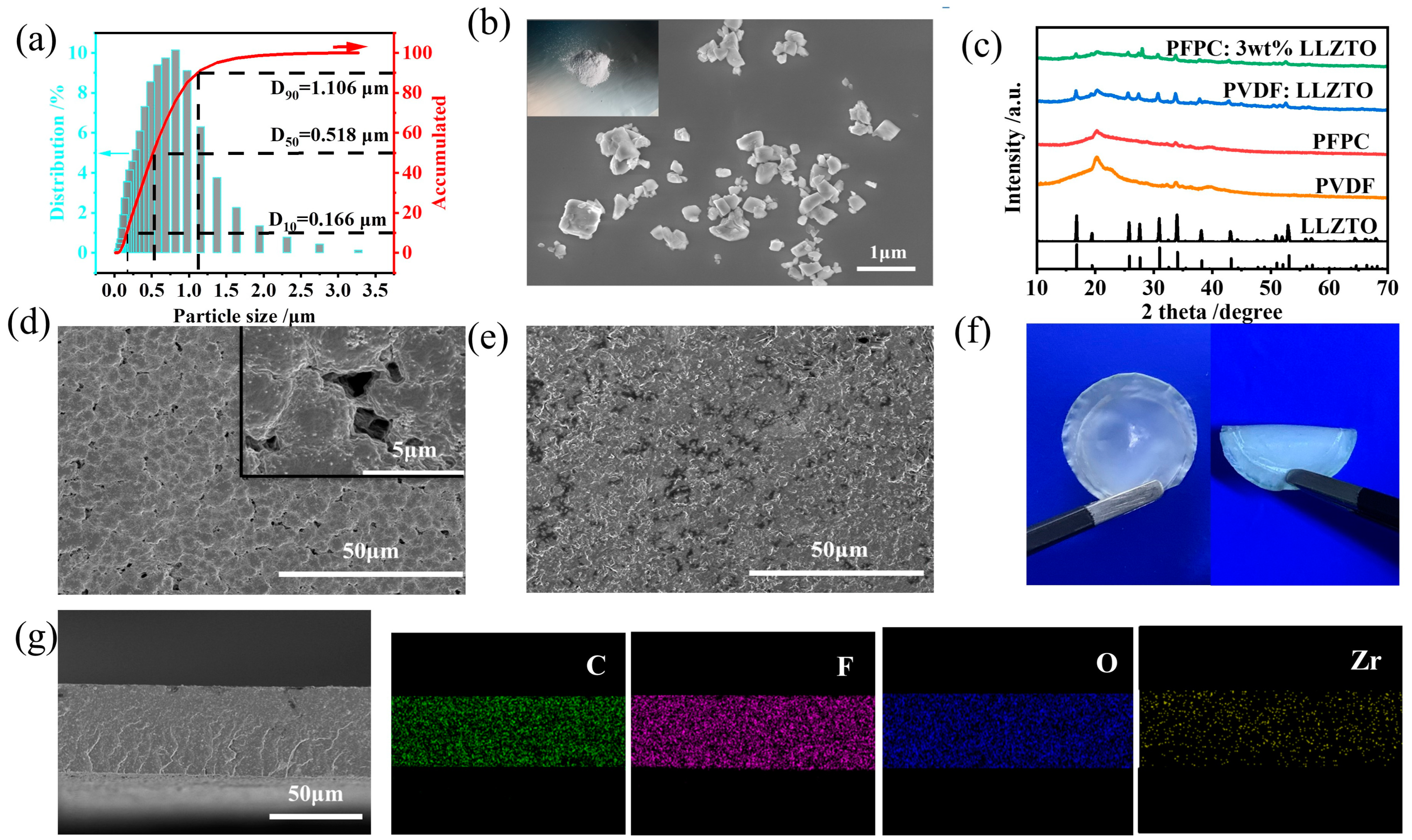
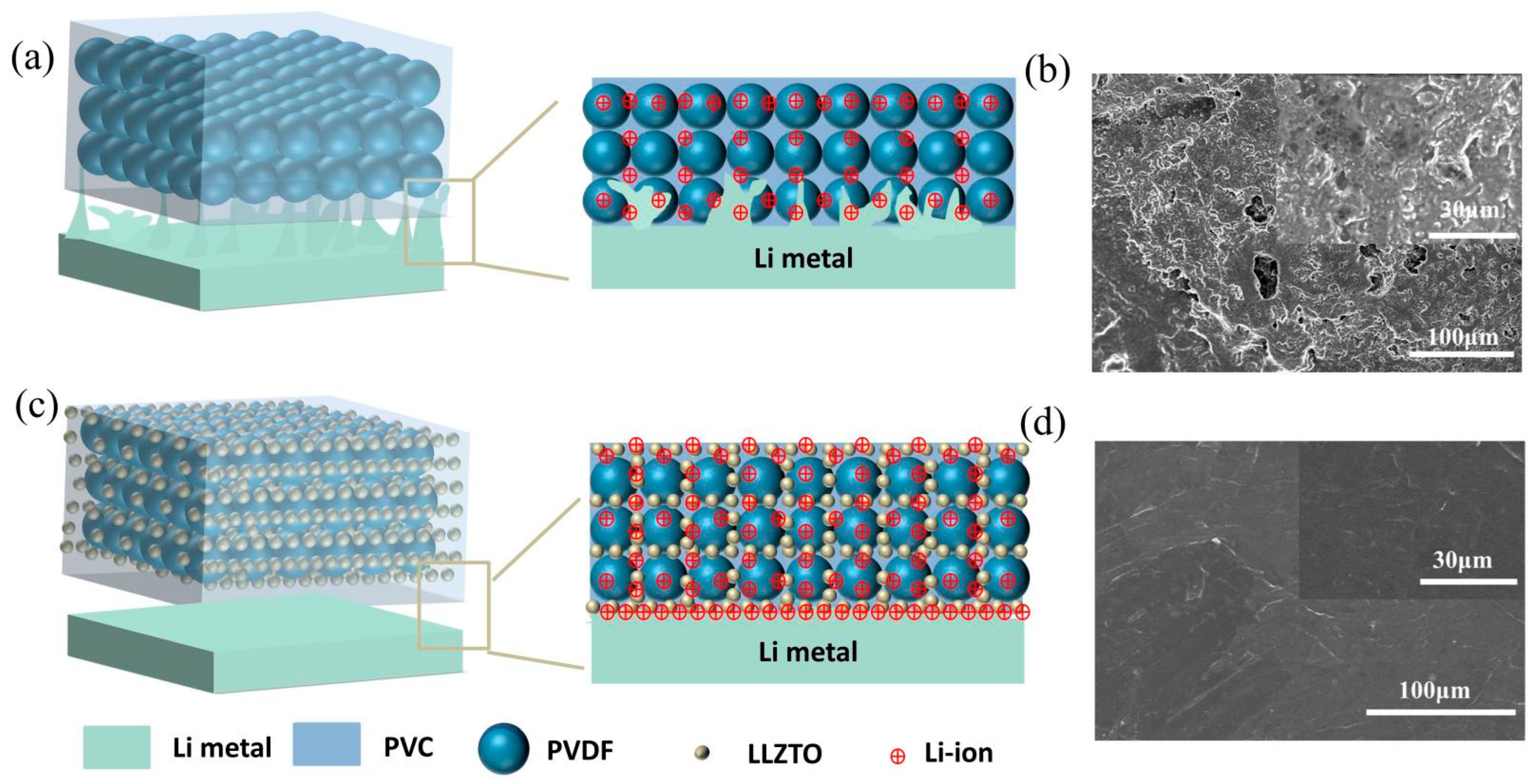
3.1. Preparation and Physical Characterization of Electrolytes
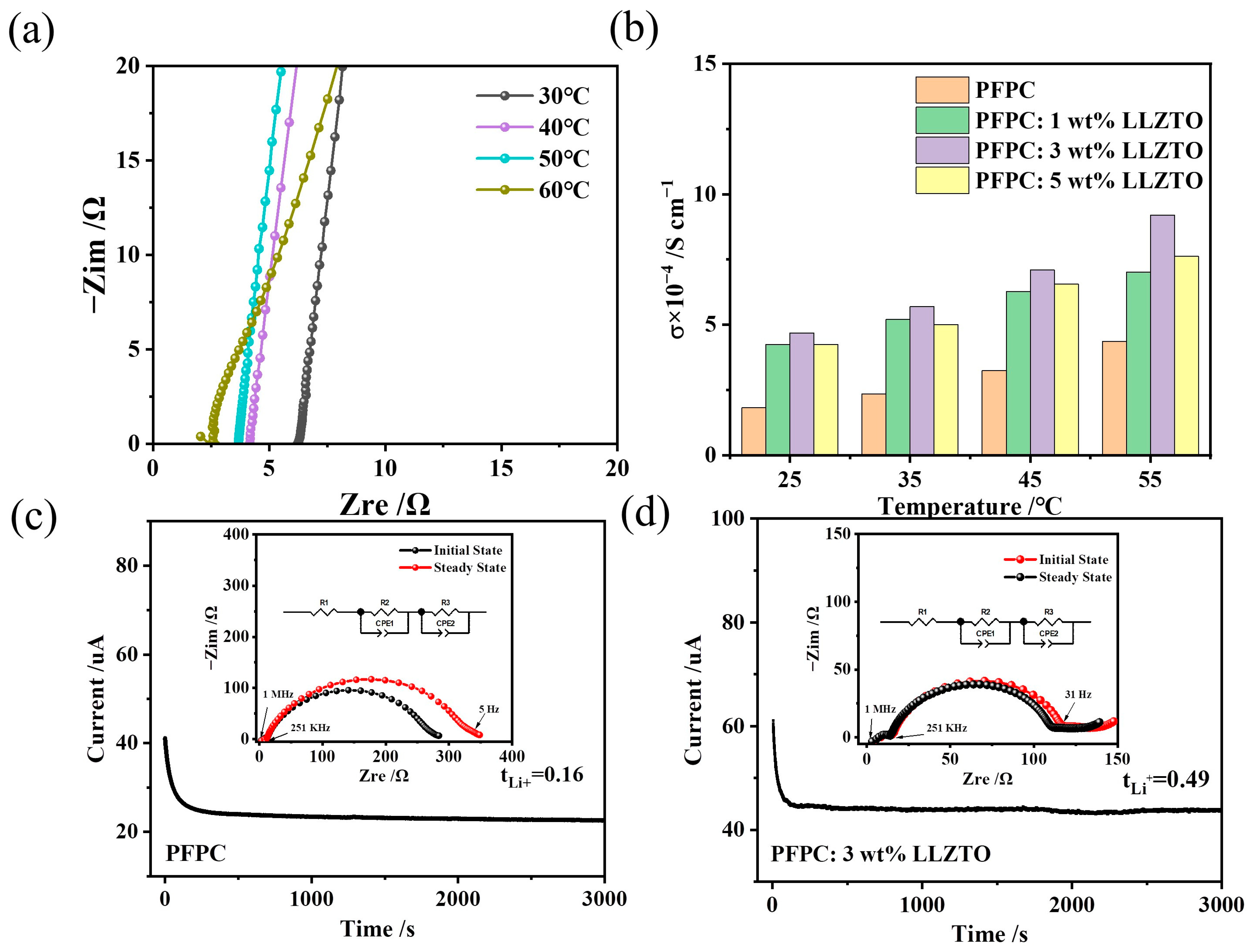
3.2. Electrochemical Performance of Electrolytes
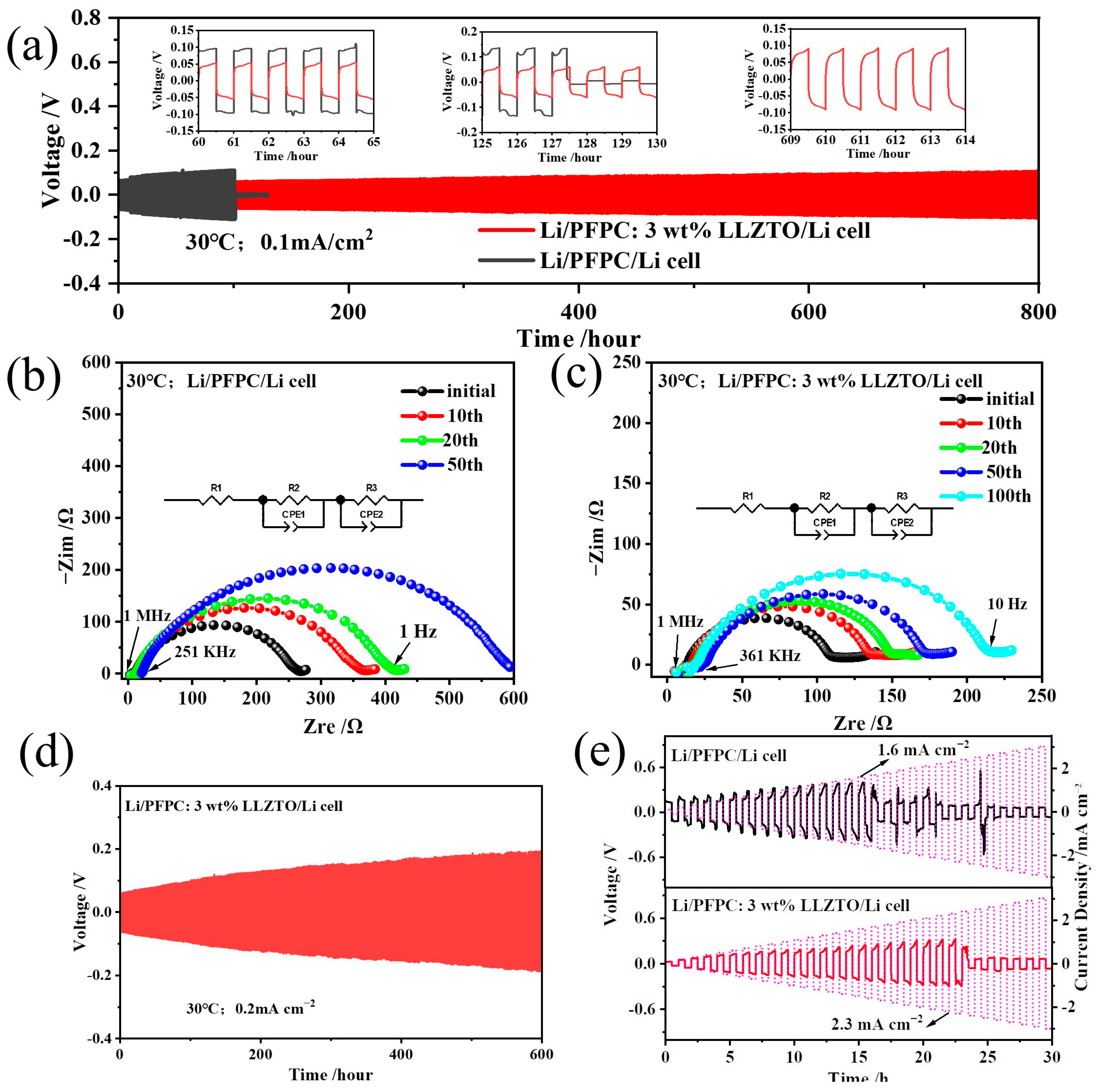
3.3. Stability against Electrolytes for Lithium Metal
3.4. Solid-State Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Li, J.; Yin, S.; Yuan, C.; Hu, Z.; Wang, L.; Li, Y.; Xu, J. Safety issues caused by internal short circuits in lithium-ion batteries. J. Mat. Chem. A 2018, 6, 21475–21484. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mat. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Jia, M.; Zhao, N.; Huo, H.; Guo, X. Comprehensive Investigation into Garnet Electrolytes Toward Application-Oriented Solid Lithium Batteries. Electrochem. Electrochem. Energy Rev. 2020, 3, 656–689. [Google Scholar] [CrossRef]
- Zhao, N.; Khokhar, W.; Bi, Z.; Shi, C.; Guo, X.; Fan, L.-Z.; Nan, C.-W. Solid Garnet Batteries. Joule 2019, 3, 1190–1199. [Google Scholar] [CrossRef]
- Huang, L.; Gao, J.; Bi, Z.; Zhao, N.; Wu, J.; Fang, Q.; Wang, X.; Wan, Y.; Guo, X. Comparative Study of Stability against Moisture for Solid Garnet Electrolytes with Different Dopants. Energies 2022, 15, 3206. [Google Scholar] [CrossRef]
- Miao, X.; Guan, S.; Ma, C.; Li, L.; Nan, C.W. Role of Interfaces in Solid-State Batteries. Adv. Mater. 2022, e2206402. [Google Scholar] [CrossRef]
- Li, J.; Cai, Y.; Wu, H.; Yu, Z.; Yan, X.; Zhang, Q.; Gao, T.Z.; Liu, K.; Jia, X.; Bao, Z. Polymers in Lithium-Ion and Lithium Metal Batteries. Adv. Energy Mater. 2021, 11, 2003239. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Choo, Y.; Halat, D.M.; Villaluenga, I.; Timachova, K.; Balsara, N.P. Diffusion and migration in polymer electrolytes. Prog. Polym. Sci. 2020, 103, 101220. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.; Zhao, Y.; Ye, Y.; Li, Y.; Liu, J. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2022, 35, e2110423. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Jia, M.; Muhammad, K.T.; Guo, X. Insight into the key factors in high Li + transference number composite electrolytes for solid lithium batteries. ChemSusChem 2022, 16, e202201801. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Mu, S.; Huang, W.; Sun, W.; Zhao, N.; Jia, M.; Bi, Z.; Guo, X. Heterogeneous electrolyte membranes enabling double-side stable interfaces for solid lithium batteries. J. Energy Chem. 2021, 60, 162–168. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xue, C.; Xin, C.; Lin, Y.; Shen, Y.; Li, L.; Nan, C.W. Self-Suppression of Lithium Dendrite in All-Solid-State Lithium Metal Batteries with Poly(vinylidene difluoride)-Based Solid Electrolytes. Adv. Mater. 2019, 31, e1806082. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, T.; Cui, J.; Shen, X.; Shi, C.; Cao, S.; Zhao, J. A homogenous solid polymer electrolyte prepared by facile spray drying method is used for room-temperature solid lithium metal batteries. Nano Res. 2021, 16, 5080–5086. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Zhai, P.; Yang, Z.; Wei, Y.; Guo, X.; Gong, Y. Two-Dimensional Fluorinated Graphene Reinforced Solid Polymer Electrolytes for High-Performance Solid-State Lithium Batteries. Adv. Energy Mater. 2022, 12, 2200967. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Niu, X.; Xin, C.; Xue, C.; Wang, S.; Shen, Y.; Zhang, L.; Li, L.; Nan, C.W. High Cycling Stability for Solid-State Li Metal Batteries via Regulating Solvation Effect in Poly(Vinylidene Fluoride)-Based Electrolytes. Batter. Supercaps 2020, 3, 876–883. [Google Scholar] [CrossRef]
- Jie, J.; Liu, Y.; Cong, L.; Zhang, B.; Lu, W.; Zhang, X.; Liu, J.; Xie, H.; Sun, L. High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. J. Energy Chem. 2020, 49, 80–88. [Google Scholar] [CrossRef]
- Ding, P.; Lin, Z.; Guo, X.; Wu, L.; Wang, Y.; Guo, H.; Li, L.; Yu, H. Polymer electrolytes and interfaces in solid-state lithium metal batteries. Mater. Today 2021, 51, 449–474. [Google Scholar] [CrossRef]
- Bi, Z.; Huang, W.; Mu, S.; Sun, W.; Zhao, N.; Guo, X. Dual-interface reinforced flexible solid garnet batteries enabled by in-situ solidified gel polymer electrolytes. Nano Energy 2021, 90, 106498. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.W.; Shen, Y. Synergistic Coupling between Li(6.75)La(3)Zr(1.75)Ta(0.25)O(12) and Poly(vinylidene fluoride) Induces High Ionic Conductivity, Mechanical Strength, and Thermal Stability of Solid Composite Electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, Y.; Wang, J.; Bai, Z.; Cao, R.; Wang, N.; Dou, S.; Huang, F. A Quasi-Double-Layer Solid Electrolyte with Adjustable Interphases Enabling High-Voltage Solid-State Batteries. Adv. Mater. 2022, 34, e2107183. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Deng, S.; Cui, P.; Yao, X. 10 mum-Thick High-Strength Solid Polymer Electrolytes with Excellent Interface Compatibility for Flexible All-Solid-State Lithium-Metal Batteries. Adv. Mater. 2021, 33, e2100353. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.-Y.; Guo, X. High-performance lithium metal batteries with ultraconformal interfacial contacts of quasi-solid electrolyte to electrodes. Energy Storage Mater. 2020, 29, 149–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Lin, Z.; Wang, K.; Chen, M.; Xu, K.; Li, W. Highly safe and cyclable Li-metal batteries with vinylethylene carbonate electrolyte. Nano Energy 2020, 74, 104860. [Google Scholar] [CrossRef]
- Chai, J.; Liu, Z.; Ma, J.; Wang, J.; Liu, X.; Liu, H.; Zhang, J.; Cui, G.; Chen, L. In Situ Generation of Poly (Vinylene Carbonate) Based Solid Electrolyte with Interfacial Stability for LiCoO(2) Lithium Batteries. Adv. Sci. 2017, 4, 1600377. [Google Scholar] [CrossRef]
- Chen, S.; Che, H.; Feng, F.; Liao, J.; Wang, H.; Yin, Y.; Ma, Z.F. Poly(vinylene carbonate)-Based Composite Polymer Electrolyte with Enhanced Interfacial Stability To Realize High-Performance Room-Temperature Solid-State Sodium Batteries. ACS. Appl. Mater. Interfaces 2019, 11, 43056–43065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, R.; Jia, D.; Cui, Y.; Liu, Q.; Liu, S.; Wu, D. Ultrathin Yet Robust Single Lithium-Ion Conducting Quasi-Solid-State Polymer-Brush Electrolytes Enable Ultralong-Life and Dendrite-Free Lithium-Metal Batteries. Adv. Mater. 2021, 33, e2100943. [Google Scholar] [CrossRef]
- Zha, W.; Li, J.; Li, W.; Sun, C.; Wen, Z. Anchoring succinonitrile by solvent-Li+ associations for high-performance solid-state lithium battery. Chem. Eng. J. 2021, 406, 126754. [Google Scholar] [CrossRef]
- Zha, W.; Li, W.; Ruan, Y.; Wang, J.; Wen, Z. In situ fabricated ceramic/polymer hybrid electrolyte with vertically aligned structure for solid-state lithium batteries. Energy Storage Mater. 2021, 36, 171–178. [Google Scholar] [CrossRef]
- Yan, Y.; Ju, J.; Dong, S.; Wang, Y.; Huang, L.; Cui, L.; Jiang, F.; Wang, Q.; Zhang, Y.; Cui, G. In Situ Polymerization Permeated Three-Dimensional Li +-Percolated Porous Oxide Ceramic Framework Boosting All Solid-State Lithium Metal Battery. Adv. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Jiang, T.; He, P.; Wang, G.; Shen, Y.; Nan, C.W.; Fan, L.Z. Solvent-Free Synthesis of Thin, Flexible, Nonflammable Garnet-Based Composite Solid Electrolyte for All-Solid-State Lithium Batteries. Adv. Energy Mater. 2020, 10, 1903376. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, C.; Cao, Y.; Guo, X. Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J. Power Sources 2014, 248, 642–646. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, Y.Y. New Insights into the Compositional Dependence of Li-Ion Transport in Polymer-Ceramic Composite Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Shen, Y.; Shen, M.; Liu, X.; Chen, H.; Liu, C.; Kang, T.; Jin, F.; Li, L.; Li, J.; et al. Superionic Conductors via Bulk Interfacial Conduction. J. Am. Chem. Soc. 2020, 142, 18035–18041. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, G.; Li, X.; Zhang, S.; Chen, R.; Wang, X.; Gao, Y.; Wang, Z.; Chen, L. Polymer electrolytes based on interactions between [solvent-Li+] complex and solvent-modified polymer. Energy Storage Mater. 2022, 51, 443–452. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.M.; Zhu, J.K.; Wu, J.F.; Yang, H.; Wei, L.; Guo, X. Ionic Conduction in Composite Polymer Electrolytes: Case of PEO:Ga-LLZO Composites. ACS Appl. Mater. Interfaces 2019, 11, 784–791. [Google Scholar] [CrossRef]
- Grugeon, S.; Jankowski, P.; Cailleu, D.; Forestier, C.; Sannier, L.; Armand, M.; Johansson, P.; Laruelle, S. Towards a better understanding of vinylene carbonate derived SEI-layers by synthesis of reduction compounds. J. Power Sources 2019, 427, 77–84. [Google Scholar] [CrossRef]
- Lim, K.; Popovic, J.; Maier, J. Ion transport and growth behavior of solid electrolyte interphases on Li and Na with liquid electrolytes based on impedance analysis. J. Mater. Chem. A 2023, 11, 5725–5733. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Cui, P.; Yao, X. High-Rate Solid Polymer Electrolyte Based Flexible All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 34649–34655. [Google Scholar] [CrossRef]
- Barai, P.; Fuchs, T.; Trevisanello, E.; Kim, H.K.; Richter, F.H.; Janek, J.; Srinivasan, V. Reaction Current Heterogeneity at the Interface between a Lithium Electrode and Polymer/Ceramic Composite Electrolytes. ACS Appl. Energy Mater. 2023, 6, 2160–2177. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Chen, P.Y.; Zhang, R.; Chen, X.; Li, B.Q.; Zhang, X.Q.; Cheng, X.B.; Zhang, Q. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 2018, 4, eaat3446. [Google Scholar] [CrossRef]
- Su, D.L.; Cui, J.; Zhai, P.B.; Guo, X.X. Mechanism Study on Garnet-type Li6.4La3Zr1.4Ta0.6O12 Regulating the Solid Electrolyte Interphases of Si/C Anodes. J. Inorg. Mater. 2022, 37, 802–808. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhai, P.; Zhao, N.; Guo, X. In-Situ Plasticized LLZTO-PVDF Composite Electrolytes for High-Performance Solid-State Lithium Metal Batteries. Batteries 2023, 9, 257. https://doi.org/10.3390/batteries9050257
Yu X, Zhai P, Zhao N, Guo X. In-Situ Plasticized LLZTO-PVDF Composite Electrolytes for High-Performance Solid-State Lithium Metal Batteries. Batteries. 2023; 9(5):257. https://doi.org/10.3390/batteries9050257
Chicago/Turabian StyleYu, Xinjie, Pengbo Zhai, Ning Zhao, and Xiangxin Guo. 2023. "In-Situ Plasticized LLZTO-PVDF Composite Electrolytes for High-Performance Solid-State Lithium Metal Batteries" Batteries 9, no. 5: 257. https://doi.org/10.3390/batteries9050257
APA StyleYu, X., Zhai, P., Zhao, N., & Guo, X. (2023). In-Situ Plasticized LLZTO-PVDF Composite Electrolytes for High-Performance Solid-State Lithium Metal Batteries. Batteries, 9(5), 257. https://doi.org/10.3390/batteries9050257







