Abstract
Owing to the unique virtues of specific energy/power densities, lithium-ion capacitors (LICs) have been increasingly attracting research attention. However, the LICs are greatly restrained by the slow Li+-reaction kinetics of battery-type anodes, which is still a challenging task. In this work, we construct a superior LIC using ultrafine MnO/dual N-doped carbon (MnO/DNC) anode and activated N-doped porous carbon (ANC) derived from a homologous polypyrrole precursor. The uniform MnO ultrafine particles (~10 nm size) are well encapsulated into a dual-carbon framework, which provides fast ion/electron transportation and structural cushion for high-rate and long-durable energy storage. Accordingly, the anodic MnO/DNC achieves an impressive rate performance (179 mAh g−1 @10 A g−1) and a stable 500-cycling lifespan. The as-constructed LICs could deliver a large specific energy of 172 Wh kg−1 at 200 W kg−1 and retain at 37 Wh kg−1 even at a high specific power of 15 kW kg−1. It is believed that the design strategy of confining ultrafine conversion-type anode materials into a dual-carbon structure will expedite the development of advanced LICs.
1. Introduction
Over the past few decades, lithium-ion batteries (LIBs) have occupied a dominant place among energy storage devices in both scientific and technological fields on account of their high specific energy [1]. However, their power output was greatly restricted by the inherent disadvantages of sluggish reaction kinetics [2]. As a promising alternative, lithium-ion capacitors (LICs) provide reliable possibilities to synchronously achieve high specific energy as well as specific power by combining a battery-type anode and capacitive-type cathode [3]. Nevertheless, the discrepancy of electrochemical behavior in two electrodes, especially the inferior cycling and rate performance of anode materials induced by slower kinetic, plague the development of LICs [3,4]. Therefore, developing the advanced electrode materials with superior kinetics is crucially important for boosting the LICs toward excellent comprehensive performance [4].
Transition metal oxides (TMOs) have been systematically studied as anode materials, including Fe2O3 [5], MoO2 [6], MnO [7], NiO [8], and CoO [9]. Among these, MnO has raised widespread interest for its low cost, low redox potential (below 0.5 V), high theoretical capacity (756 mAh g−1), and environmental friendliness [10]. Nevertheless, its inherent drawbacks including the huge volume expansion (171%) and poor electrical conductivity greatly hinder its applications [11,12]. Aiming to alleviate the above problems, many kinds of strategies have been developed, including the structure designing [13], oxygen depletion [14] and MnO/C hybridizing [15]. One of the most facile and efficacious strategies is constructing the composite of MnO and carbon, which is beneficial for the improvement of the overall electrical conductivity along with restraining the volume change during the cycling. Wang et al. [16] prepared MnO/carbon nanocages composite by a bio-gel derived process, which shows distinct superiority for lithium storage (805 mAh g−1 @1 A g−1), in sharp contrast to pristine MnO (317 mAh g−1 @1 A g−1). Up to date, various composite strategies for MnO-based nanostructures and carbon frameworks have been designed, such as MnO@C nanocages [16], carbon-anchored MnO nanosheets [17], interconnected MnO/C aerogels [18] and MnO@C nanorods [19]. However, it is still a challenge to achieve the satisfactory tolerance of its volume expansion from multiple directions and maintain the stable interface during cycling [20]. Moreover, it is also necessary to confine the particle size of MnO to reduce structural tension and shorten the diffusion path [21]. Hence, effective efforts are still highly essential to construct novel architectures for the combination of MnO and carbon materials with the purpose of improving the electrochemical performance of LICs.
Herein, we demonstrate a dual-carbon compositing approach toward constructing the novel MnO/C anode material, in which the MnO ultrafine particles (~10 nm in size) are confined in the dual N-doped carbon matrix (MnO/DNC) consisting of the carbon substrate and carbon coating layer. Benefiting from the rapid ion/electron transportation as well as the structural robustness, the MnO/DNC achieves an outstanding rate performance (474 and 179 mAh g−1 at 1 and 10 A g−1, respectively), and a stable 500-cycling lifespan is achieved. Finally, a superior LIC using ultrafine MnO/DNC anode and activated N-doped carbon (ANC) cathode derived from a homologous polypyrrole (PPy) precursor is successfully constructed, which delivers a high specific energy of 172 Wh kg−1, a high specific power of 15 kW kg−1, and good cycling performance (a capacity retention of 85.1% over 1000 cycles).
2. Materials and Methods
2.1. Chemicals
All chemical substances are directly used without any additional treatments. Dopamine, tris-(hydroxymethyl)-aminoethane, cetrimonium bromide (CTAB), and ammonium persulfate ((NH4)2S2O8) were obtained from Macklin. Others were purchased from Aladdin.
2.2. Materials Preparation
PPy preparation: First, 7.3 g of CTAB was added into 120 mL of 1 M HCl, which was followed by adding 13.7 g of (NH4)2S2O8 oxidant. White flocculent reaction templates were formed rapidly in the above solution. Subsequently, the reaction system was cooled down to 0–5 °C in an ice bath, and 8.3 mL of purified pyrrole monomers was added and kept for 24 h. After filtering and purifying by 1 M HCl, DI water, and ethanol, the as-obtained black precipitates were dried at the temperature of 80 °C.
PPy/MnCO3/PDA preparation: The as-prepared PPy nanowebs (150 mg) were dispersed into the DI water (50 mL) with ultrasonication for 30 min (solution A). Afterwards, 130 mg of KMnO4 was added into the DI water (30 mL), which was marked as solution B. The mixture of solution A and B was put into the Teflon-lined stainless steel autoclave (100 mL) and maintained at 80 °C for 12 h. After the reaction, the resulting PPy/MnCO3 powders were obtained. Then, 200 mg of the above precursor was dispersed into the tris-buffer solution (0.01 M, 100 mL). After adding 20 mg of dopamine, the PPy/MnCO3/PDA was obtained.
MnO/DNC preparation: The as-prepared PPy/MnCO3/PDA and PPy were annealed at 700 °C for 1.5 h with a ramping rate of 5 °C min−1 under the protection of Ar gas to form the MnO/dual N-doped carbon (MnO/DNC) and N-doped carbon (NC) for comparison.
ANC preparation: The PPy precursor was mixed with KOH (weight ratio: 3/1) and then annealed at 650 °C for 1 h (5 °C min−1) under Ar flow. The products were subsequently rinsed using 1 M HCl and DI water until the filtrate was neutral. The product was then dried at 80 °C to obtain the activated N-doped carbon (ANC).
2.3. Materials Characterizations
The scanning electron microscopy (SEM, FEI NanoSEM 450) equipped with an energy-dispersive X-ray (EDX) system and transmission electron microscopy (TEM, FEI Talos F200X) were used to observe the morphology and structure of the samples. The structural information was inspected by X-ray diffraction (XRD) and Raman microscope techniques. The thermogravimetric analysis (TGA) was used to determine the composition content under air condition. The surface characteristics of the samples, including the specific surface area and related pore size distribution, were studied by Micromeritics ASAP 2020 at 77 K. The compositional state and examined by X-ray photoelectron spectra (XPS, PHI VersaProbe III).
2.4. Electrochemical Evaluations
A CR2025 coin-type half-cell was fabricated in an Ar-filled glove box to assess the electrochemical properties. In a typical preparation of the working electrode, a slurry was firstly obtained using the active materials, poly-vinylidene fluoride (PVDF) as the binder, and carbon black as the conductive agent, which were dispersed with a mass ratio of 8:1:1 in N-methyl-2-pyrrolidone (NMP) solvent. Then, the electrodes were prepared by coating the sticky slurry on the current collectors of copper (anode) or aluminum (cathode), which was followed by vacuum drying at 120 °C for 12 h. The mass loading of active materials was about 1.5 mg cm−2 in anode. The electrolyte was 1 M LiPF6 in EC/DMC/DEC (1:1:1 by volume) solvent. The separator was the commercial Celgard 2500 film. A galvanostatic intermittent titration technique (GITT) was performed at 0.1 A g−1 under a testing condition of 30 min current pulse and 2 h relaxation. The cyclic voltammetry (CV) curves and electrochemical impedance spectroscopy (EIS, 105~10−1 Hz frequency) were recorded using a CHI 660D electrochemical workstation.
For the assembly of LICs, the MnO/DNC and ANC (3:1 by mass ratio) were used as the anode and cathode, respectively. The MnO/DNC should be prelithiated by contacting with a pure Li plate in electrolyte. The following equations were used to determine the specific energy (E) and specific power (P) of LICs on the basis of both electrode masses (M):
Herein, V presents the average discharging potential of the initial and final states, I stands for the current density (A), and t means the total discharging time (h).
3. Results and Discussion
Figure 1 schematically shows the preparation process. The PPy nanowebs are firstly prepared by a simple soft-template method [22], which then hydrothermally reacts with KMnO4. During this reaction process, the superficial C atoms of PPy are oxidized (C → CO2 + H2O) [23], leading to the creation of defects on the PPy surface [21]. Simultaneously, MnO4− is reduced to Mn2+ (Mn7+ → Mn2+) and forms MnCO3 nanoparticles with the dissolved CO2 in the solvent, which prefers to locate at the surface defects of PPy and thus form the PPy/MnCO3 composite. Afterwards, the dopamine is self-polymerized conformally to form a coating layer on the PPy/MnCO3 (denoted as PPy/MnCO3/PDA) [24]. Further carbonization treatment gives rise to the N-doped carbon substrate from PPy and the outer N-doped carbon coating layer over MnO from PDA, leading to the architecture of superfine MnO encapsulated in the dual N-doped carbon substrate. The N-doping sites in the framework will contribute to additional pseudocapacitance in the lithium storage process and enhance the overall electrical conductivity of MnO/DNC [25]. The N-doped carbon nanowebs (NC) derived from the direct carbonization of PPy are also prepared and work as the control sample.
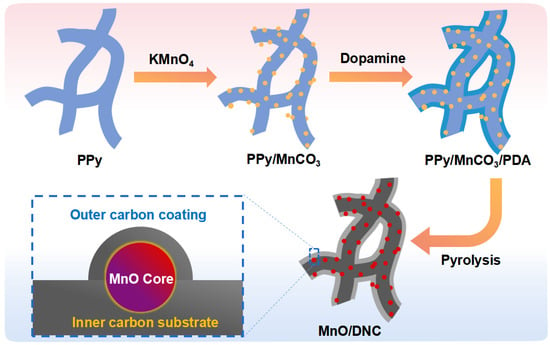
Figure 1.
Schematic synthesis of MnO/DNC.
The as-obtained PPy presents the crosslinked nanoweb structure with a diameter of 60–80 nm (Figure 2a) [23]. After loading the MnCO3 and PDA, the fiber diameter of PPy/MnCO3/PDA increases, and the surface becomes rougher (Figure S2a). The XRD pattern further confirms the presence of crystalline MnCO3 with well-indexed peaks (No. PDF#44-1472, Figure S2b). After carbonization, the PPy and PDA are transformed into N-doped carbon, and the MnCO3 was pyrolyzed into MnO nanoparticles (No. PDF#07-0230, Figure S3). As shown in Figure 2b, the MnO/DNC well inherits the nanonetwork structure. The ultrafine MnO nanoparticles were further characterized by TEM. As displayed in Figure 2c,d, the size of MnO nanoparticles is about 5–10 nm, which distributed uniformly on the fibers without obvious agglomeration. The outer carbon layer conformally covers MnO nanoparticles (Figure 2e), with a d-spacing of 0.22 nm (Figure 2f), corresponding to the (200) plane of cubic phase MnO [26]. Figure 2g shows the EDX elemental mapping of MnO/DNC, indicating that Mn/O are concentrated in the particles area, while C/N disperses both in the carbon fiber and particles, proving the structural characteristics of N doping in both the carbon coating and fiber network.
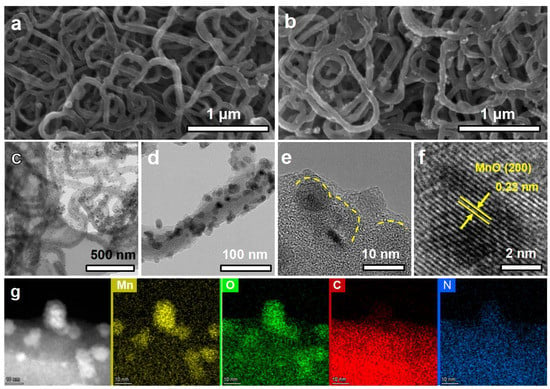
Figure 2.
(a) SEM image of PPy, (b) SEM image, (c–e) TEM images, (f) High-resolution TEM image, and (g) EDS mapping of MnO/DNC.
The sharp peak observed at 643 cm−1 in the Raman spectra of MnO/DNC (Figure 3a) corresponds to the Mn-O bond [26]. The board peak located at 1343 cm−1 originates from the disordered carbon (D band), while another board peak centered at 1591 cm−1 is induced by graphitic carbon (G band) in a dual-carbon framework [27]. The intensity ratio of D/G bands (ID/IG) is relatively high (0.95), indicating its low crystallinity, which is in favor of fast Li+ diffusion [18]. As shown in Figure 3b, the carbon content in MnO/DNC was confirmed using the TGA method. The sharp loss before 450 °C can be ascribed to the burning of carbon content (C + O2 → CO2), and the oxidation of MnO to Mn3O4 follows above 500 °C [26], indicating the proportion of carbon is 52 wt% based on the overall evaluation. The nitrogen adsorption–desorption isotherm of MnO/DNC (Figure S4a) displays a considerably high Brunauer–Emmett–Teller (BET) specific surface area (159 m2 g−1), with a preponderant pore size around 1–1.5 nm (Figure S4b), demonstrating the predominance of micropores/mesopore, which could provide enhanced electrolyte permeability and tolerate the volume change of MnO [28]. The XPS curve of MnO/DNC shows the coexistence of Mn, O, C, and N elements (Figure 3c). The high-resolution Mn 2p spectra is shown in Figure 3d, where two peaks are situated at 652.5 and 640.8 eV, standing for Mn 2p1/2 and Mn 2p3/2, respectively [12]. Three subpeaks centered at 288.9, 286.4, and 284.7 eV in the C 1s spectra (Figure 3e) correspond to the C-O/C=O, C-N and C-C bonds, respectively [29]. The N 1s spectra (Figure 3f) can be divided into graphitic-N (N3), pyrrolic-N (N5), and pyridinic-N (N6), centered at 402.4, 400.1, and 398.0 eV, respectively [30].
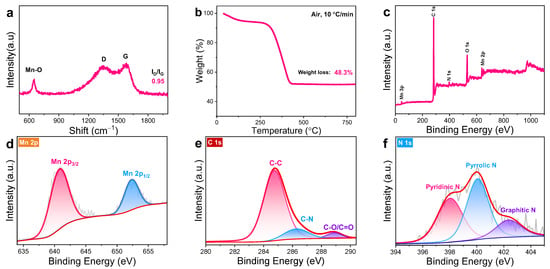
Figure 3.
(a) Raman spectra, (b) TG curve, (c) XPS survey spectra of MnO/DNC and its high-resolution XPS spectra of (d) Mn 2p, (e) C 1s, and (f) N 1s.
The electrochemical performance of MnO/DNC for Li+ storage was then tested. From the first three CV curves of anodic MnO/DNC at a scanning rate of 0.1 mV s−1 (Figure 4a), weak peaks at 0.5–2 V during the first cathodic scan can be observed, which can be attributed to the formation of the solid electrolyte interface (SEI) film and the irreversible decomposition of electrolyte [31], and they vanish in the later cycles. The sharp peak below 0.1 V stands for the reduction of MnO to Mn accompanied by the formation of Li2O [32]. Subsequently, it moves to 0.3 V due to the enhanced reaction kinetics caused by the as-formed Li2O and Mn [11]. Correspondingly, a peak centered at 1.32 V during the anodic scans indicates the reverse oxidization of Mn to MnO and decomposition of Li2O [33]. The overlapping of the later CV curves further manifests the stability of MnO/DNC. Figure 4b,c displays the cycling performance of MnO/DNC at 0.1 A g−1 (0.132 C) and the galvanostatic charge–discharge (GCD) curves. The initial discharge specific capacity of MnO/DNC is 1105 mAh g−1, which drops to 721 mAh g−1 (865 F g−1) due to the decomposition of electrolyte and formation of an SEI layer [18] and shows an initial Coulombic efficiency (ICE) of 65%. On account of the conductive enhancement and structural stability enabled by the dual carbon structures of network and coating layer, the specific discharge capacity of MnO/DNC slightly attenuates to 682 mAh g−1 after 100 charge–discharge cycles. The GDC curves are almost overlapped starting from the 2nd cycle, especially for the discharge curves, which proves the excellent cycling stability of MnO/DNC [33]. The rate performance measurement (Figure 4d) shows that the specific discharge capacities of MnO/DNC electrodes are 752, 645, 547, 474 and 406 mAh g−1 at 0.1, 0.2, 0.5, 1 and 2 A g−1, respectively. Capacities of 294 and 179 mAh g−1 can be still maintained under the high rates of 5 and 10 A g−1, corresponding to the high capacity retention of 39 and 24%, respectively. In comparison, the NC only delivers 491, 393, 326, 281, 246, 202, 174 mAh g−1 at corresponding rates, confirming the high capacity contribution of MnO. Figure 4e shows the long-term cycling stability of MnO/DNC electrode tested at 2 A g−1. The initial sharp increase corresponds to the activation of electrode material caused by the gradual infiltration of electrolyte [34]. Subsequently, the capacity attenuates, which could be due to the decomposition of the electrolyte and the fluctuation of SEI films caused by the volume change of MnO particles. The pulverized MnO particles and Li2O promoted the oxidation process to high-valance manganese oxides, which contributes to the second capacity increase [12,24,35]. In addition, the formation of an active gel-like film also participates in the increase in capacity [17]. Finally, the decay and growth reach an equilibrium, flattening the capacity curve. The discharge capacity at 25 cycles is 404 mAh g−1 and retains 393 mAh g−1 after 500 cycles, which corresponds to a remarkable capacity retention of 97.2%. A comparison of present work with other reported MnO-based anodes in terms of the rate performance and cycling performance has been conducted (Table S1), demonstrating the decisive role of the dual-carbon framework for improving the electrochemical performance of MnO.
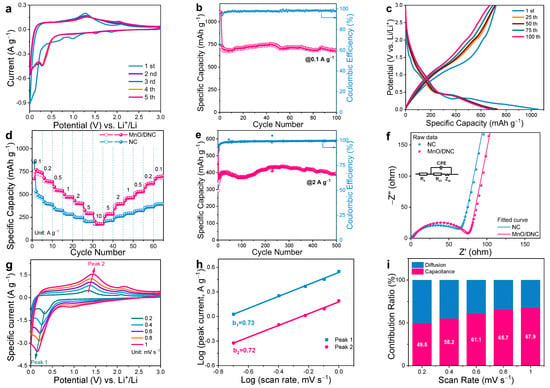
Figure 4.
(a) CV at 0.1 mV s−1; (b) Cycling performance and (c) GCD curves of MnO/DNC at 0.1 A g−1; (d) Rate performance of NC and MnO/DNC; (e) Long-term cycling performance of MnO/DNC at 2 A g−1; (f) EIS curves, equivalent circuit diagram, and fitted EIS curves of electrodes; (g) CV curves at each scan rate; (h) Linear fitting of log(i)–log(v) curves; (i) and Capacitive-controlled proportion of MnO/DNC at various scan rates.
Systematical dynamical evaluations are further conducted to verify the fast reaction kinetics. To analyze the overall conductivity, EIS was firstly performed (Figure 4f). Based on the fitted EIS curves [36], the NC shows a lower electron-transfer resistance (Rct) of 51 Ω due to its pure carbon nature. Benefited by the dual-carbon framework, the Rct of MnO/DNC is only slightly higher (72 Ω) than NC. Detailed SEI layer resistance (Rs) and Rct values of MnO/DNC and NC are provided in Table S2. Moreover, the diffusion coefficient of Li+ () can be further obtained using the following equations [36]:
According to the slopes of linear fitting in Figure S5, the calculated value of MnO/DNC is 2.35 × 10−13 cm2 s−1, which is close to that of NC (3.18 × 10−13 cm2 s−1).
The electrochemical behavior of MnO/DNC was then determined using the peak current (i) obtained from the CV curves (Figure 4g) and corresponding scan rate (v) according to the following formula [15]:
The log(i)–log(v) curves give the b value of the electrodes by a fitting slope. According to Bruce Dunn’s studies, a b value of 0.5 stands for a complete diffusion-controlled behavior, while a b value of 1.0 represents a surface-controlled process [15]. The b values of the cathodic and anodic peaks are found to be 0.73 and 0.72 (Figure 4h), respectively, indicating that the electrochemical behavior of MnO/DNC is mainly controlled by the capacitive process. The specific contribution ratio of capacitive-controlled capacity was quantitatively evaluated by dividing the response current (i) into capacitiance response (k1v) and diffusion response (k2v1/2) parts using the following equation [37]:
According to the calculation results, the capacitive contribution ratios of MnO/DNC increase from 49.5 to 65.7%, when the scan rate rises from 0.2 to 0.8 mV s−1 (Figure 4i). The increasing trend and a dominant ratio of 67.9% at a scan rate of 1 mV s−1 (Figure S6) can be attributed to the porous surface provided by the web-like dual-carbon structure and rapid Li+ absorption on the N-doped sites [37].
The GITT was also conducted to investigate the diffusion coefficients of Li+ at each stage of charge and discharge through the following equation [38]:
As shown in Figure S7, the varies in the range of 10−10~10−12 cm−2 s−1 during the whole cycle, confirming the rapid reaction kinetics of MnO/DNC [38]. The superior kinetics of MnO/DNC can be attributed to the following reasons. Firstly, the conformal carbon coating on the surface of MnO nanoparticles significantly enhances the conductivity of the composite. Secondly, the carbon framework with a generated porous structure provides sufficient ion channels and contributes to the infiltration of the electrolyte [39]. Moreover, the defective N5 and N6 sites contribute to more surface capacitance [40], which enables a higher reaction rate than the diffusion behaviors.
The cathode materials with micropores also play a key role in improving the specific energy of LICs [41]. Therefore, the homologous PPy was chemically activated to prepare the activated N-doped carbon (ANC) with high capacity as a favorable alternative of commercially activated carbon (AC), which suffers from low capacity (40~70 mAh g−1) [7,42,43]. As shown in Figure 5a,b, the edge of ANC presents a two-dimensional nanosheet structure, which is significantly different from that of the precursor (PPy). Therefore, it can be presumed that the drastic change of morphology comes from the structure evolution from the one-dimensional nanofiber of the original network to two-dimensional under the high temperature and KOH chemical etching. The EDS test of ANC (Figure 5c) demonstrated the presence of heteroatom N in ANC. As shown in Figure 5d, the ANC exhibited a much higher volume of nitrogen adsorption and desorption than AC, with a BET specific surface area as high as 3436 m2 g−1, displaying fundamental superiority to commercial AC (1404 m2 g−1). Meanwhile, the density function theory (DFT) pore-size distribution of ANC (Figure S8) shows more dominance of 1–3 nm. Subsequently, the electrochemical performance of ANC was tested within 2–4.5 V. It displays the discharge capacities of 111 mAh g−1 (160 F g−1) at 0.1 A g−1, greatly surpassing that of AC (71 mAh g−1). Capacities of 107, 102, 95, 84, 57 and 30 mAh g−1 (154, 147, 137, 121, 82 and 43 F g−1) are achieved at 0.2, 0.5, 1, 2, 5, and 10 A g−1, respectively, further demonstrating its superiority. Moreover, the long-span cycling performance of the ANC further confirms its availability. The capacity augmentation during the first 400 cycles can be ascribed to the activation process of electrode materials caused by the gradual infiltration of electrolyte [22,44].
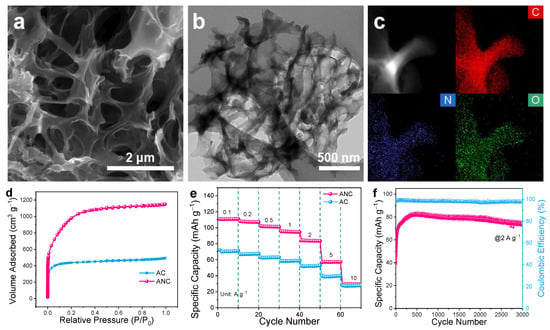
Figure 5.
(a) SEM image, (b) TEM image and (c) EDS mapping of ANC; (d) BET curves and (e) Rate performance of AC and ANC; (f) Long-term cycling test of ANC at 2 A g−1.
To verify the practical application of MnO/DNC and ANC, the prelithiated MnO/DNC and ANC were used as the anode and cathode to construct a full LIC. Figure 6a shows the charge storage mechanism of MnO/DNC//ANC LICs, where the physical adsorption/desorption of PF6− occurs at the ANC cathode, which is accompanied by the insertion/extraction of Li+ in the MnO/DNC anode. The mass ratio of the anode/cathode was tuned for achieving the optimal performance. Therefore, the rate test of the MnO/DNC//ANC LICs assembled based on various ratios (anode/cathode) was conducted (Figure S9). It is found that the LIC with the mass ratio of 1:3 shows the obvious superiority. Therefore, all the electrochemical tests afterward used the LIC assembled with the anode/cathode ratio of 1:3. The rectangular CV curves of LICs at various scan rates (Figure 6b) indicate the significant capacitive behavior, well matching the approximately linear GCD curves (Figure 6c) [45]. Note that the deviation from the original shape at high scan rates is caused by the totally different reaction mechanisms of two electrodes. As shown in the Ragone plot (Figure 6d), benefiting from the high capacity of the cathode and the excellent rate performance of anodic MnO/DNC, the as-constructed LIC can deliver a high specific energy of 172 W h kg−1 at 200 W kg−1 and maintain 37 W h kg−1 even at a high specific power of 15,037 W kg−1, exhibiting obvious superiority compared with the previously reported LICs [7,27,32,42,43,45,46,47,48,49]. Moreover, the present LIC delivers a superior cycle stability at 1 A g−1 with a capacity retention of 85.1% over 1000 cycles (Figure 6e).
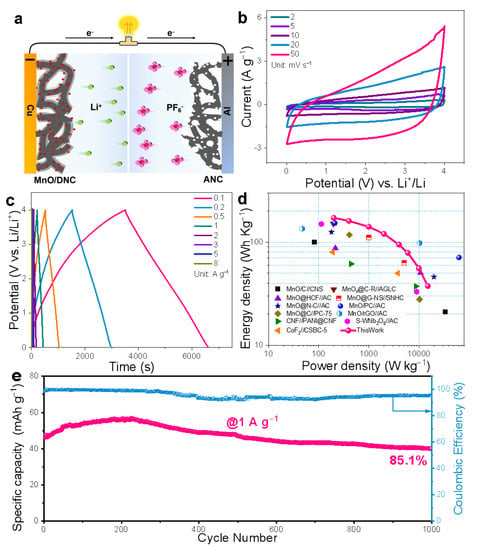
Figure 6.
(a) Schematic diagram of the energy storage mechanism of LIC based on the MnO/DNC//ANC; (b) CV curves and (c) GCD curves of the LIC; (d) Ragone plots of the LIC with comparison of previously reported LICs; (e) Cycling stability at 1 A g−1 of the LIC.
4. Conclusions
In conclusion, we have presented a successful demonstration of a superior anodic MnO/DNC and LIC based on its homologous ANC cathode. For the battery-type anode, both the web-like substrate and extra carbon coating provide fast ion/electron passage and stable interface for ultrafine MnO. This dual-carbon structure enables the MnO/DNC with a high capacity of 179 mAh g−1 at 10 A g−1 along with a superior cycling stability. For the capacitive-type cathode, the homologous ANC exhibits a high capacity of 111 mAh g−1 at 0.1 A g−1 with a surface area of 3436 m2 g−1. Combining the merits of both cathode and anode, the LIC based on the MnO/DNC and ANC presents a high specific energy of 172 Wh kg−1, a high specific power of 15 kW kg−1 as well as promising lifetime. This work offers a facile and effective strategy for the development of advanced electrode materials for high-performance LICs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries9050241/s1, Figure S1: SEM images of (a) PPy and (b) MnO/DNC at different magnification. Figure S2: (a) SEM image and (b) XRD pattern of PPy/MnCO3/PDA. Figure S3: XRD pattern of MnO/DNC. Figure S4: (a) BET curve and (b) the corresponding pore-size distribution of MnO/DNC. Figure S5: The linear relationship between Z′ and the ω−1/2 of NC and MnO/DNC. Figure S6: Capacitive contribution (pink region) of the MnO/DNC electrode at 1 mV s−1. Figure S7: GITT curve and calculated of MnO/DNC. Figure S8: The corresponding pore-size distribution of AC and ANC. Figure S9: Rate capabilities of MnO/DNC//ANC LICs with different mass ratios. Table S1: The comparison of rate performance and cycling performance of present work with other reported MnO-based anodes. Table S2: Fitted impedance parameters and equivalent circuit.
Author Contributions
Conceptualization, J.-G.W., Y.Z. and D.L.; methodology, D.L., Y.G., Z.H., L.R., M.J. and Y.C.; validation, D.L., Y.G., Z.H., L.R., M.J. and Y.C.; investigation, D.L., Y.G., Z.H., L.R., M.J. and Y.C.; resources, J.-G.W. and Y.Z.; data curation, D.L. and Y.G.; writing—original draft preparation, D.L. and Y.G.; writing—review and editing, J.-G.W. and Y.Z.; supervision, J.-G.W. and Y.Z.; funding acquisition, J.-G.W. All authors have read and agreed to the published version of the manuscript.
Funding
The work is supported by the National Natural Science Foundation of China (52272239, 22109044, and 51821091) and Fundamental Research Funds for the Central Universities (D5000210894 and 3102019JC005).
Data Availability Statement
For data, please refer to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khaligh, A.; Zhihao, L. Battery, ultracapacitor, fuel cell, and hybrid energy storage systems for electric, hybrid electric, fuel cell, and plug-in hybrid electric vehicles: State of the art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Pasquier, A.D.; Plitz, I.; Gural, J.; Badway, F.; Amatucci, G.G. Power-ion battery: Bridging the gap between Li-ion and supercapacitor chemistries. J. Power Sources 2004, 136, 160–170. [Google Scholar] [CrossRef]
- Han, P.; Xu, G.; Han, X.; Zhao, J.; Zhou, X.; Cui, G. Lithium ion capacitors in organic electrolyte system: Scientific problems, material development, and key technologies. Adv. Energy Mater. 2018, 8, 1801243. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Kanjanaboos, P.; Lokhande, V.C.; Praserthdam, S.; Lokhande, C.D.; Shaikh, J.S. Engineering of battery type electrodes for high performance lithium ion hybrid supercapacitors. ChemElectroChem 2021, 8, 4686–4724. [Google Scholar] [CrossRef]
- Jiang, T.; Bu, F.; Feng, X.; Shakir, I.; Hao, G.; Xu, Y. Porous Fe2O3 nanoframeworks encapsulated within three-dimensional graphene as high-performance flexible anode for lithium-ion battery. ACS Nano 2017, 11, 5140–5147. [Google Scholar] [CrossRef]
- Hou, C.; Yang, W.; Xie, X.; Sun, X.; Wang, J.; Naik, N.; Pan, D.; Mai, X.; Guo, Z.; Dang, F.; et al. Agaric-like anodes of porous carbon decorated with MoO2 nanoparticles for stable ultralong cycling lifespan and high-rate lithium/sodium storage. J. Colloid Interface Sci. 2021, 596, 396–407. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, Z.; Li, H.; Wang, Y.; Wang, X. Reduced graphene oxide encapsulated MnO microspheres as an anode for high-rate lithium ion capacitors. New Carbon Mater. 2021, 36, 573–584. [Google Scholar] [CrossRef]
- Zou, F.; Chen, Y.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Gao, M.; Zhu, Y. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 2016, 10, 377–386. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Xia, Q.; Zhang, H.; Li, Z.; Wang, H.; Li, X.; Zuo, F.; Zhang, F.; Wang, X.; et al. Operando magnetometry probing the charge storage mechanism of CoO lithium-Ion batteries. Adv. Mater. 2021, 33, 2006629. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Zhao, Y.; Jia, B. A review on the synthesis of manganese oxide nanomaterials and their applications on lithium-ion batteries. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; Wang, J.; Nan, D.; Wei, C. Scale-up production of high-tap-density carbon/MnOx/carbon nanotube microcomposites for Li-ion batteries with ultrahigh volumetric capacity. Chem. Eng. J. 2018, 354, 220–227. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, Z.; Wang, L.; Zhu, Y.; Li, Q.; Liang, J.; Yu, Y.; Huang, T.; Tang, K.; Qian, Y.; et al. Synthesis of MnO@C core–shell nanoplates with controllable shell thickness and their electrochemical performance for lithium-ion batteries. J. Mater. Chem. 2012, 22, 17864. [Google Scholar] [CrossRef]
- Huang, S.; Yang, L.; Gao, M.; Zhang, Q.; Xu, G.; Liu, X.; Cao, J.; Wei, X. Well-dispersed MnO-quantum-dots/N-doped carbon layer anchored on carbon nanotube as free-standing anode for high-performance Li-Ion batteries. Electrochim. Acta 2019, 319, 302–311. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, W.; Chen, N.; Chen, S.; Xu, W.; Cai, R.; Brown, C.L.; Yang, D.; Yao, X. Generating oxygen vacancies in MnO hexagonal sheets for ultralong life lithium storage with high capacity. ACS Nano 2019, 13, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Wei, H.; Yan, X.; Guo, Z. Carbon-coated MnO microparticulate porous nanocomposites serving as anode materials with enhanced electrochemical performances. Nano Energy 2014, 9, 41–49. [Google Scholar] [CrossRef]
- Hou, C.; Tai, Z.; Zhao, L.; Zhai, Y.; Hou, Y.; Fan, Y.; Dang, F.; Wang, J.; Liu, H. High performance MnO@C microcages with a hierarchical structure and tunable carbon shell for efficient and durable lithium storage. J. Mater. Chem. A 2018, 6, 9723–9736. [Google Scholar] [CrossRef]
- Xiao, Y.; Cao, M. Carbon-anchored MnO nanosheets as an anode for high-rate and long-life lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 12840–12849. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.; Zhou, C.; Li, A.; Gong, Y.; Muhammad, N.; Song, H. Controlled synthesis of porous 3D interconnected MnO/C composite aerogel and their excellent lithium-storage properties. Electrochim. Acta 2019, 306, 143–150. [Google Scholar] [CrossRef]
- Lin, J.; Yu, L.; Liu, W.; Dong, H.; Dong, X.; Zhao, M.; Zhai, Y.; Xiang, J.; Chen, M. In situ construction of one-dimensional porous MnO@C nanorods for electrode materials. New J. Chem. 2021, 45, 4422–4426. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; He, T.; Amiinu, I.S.; Kou, Z.; Li, J.; Mu, S. Smart reconstruction of dual-carbon decorated MnO for anode with high-capacity and ultralong-life lithium storage properties. Carbon 2017, 115, 95–104. [Google Scholar] [CrossRef]
- He, Y.; Xu, P.; Zhang, B.; Du, Y.; Song, B.; Han, X.; Peng, H. Ultrasmall MnO nanoparticles supported on nitrogen-doped carbon nanotubes as efficient anode materials for sodium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 38401–38408. [Google Scholar] [CrossRef] [PubMed]
- Qie, L.; Chen, W.; Wang, Z.; Shao, Q.; Li, X.; Yuan, L.; Hu, X.; Zhang, W.; Huang, Y. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Qi, X.; Yu, J.; Zhang, Z.; Wei, J.; Yang, Z. Agaric-assisted synthesis of core-shell MnO@C microcubes as super-high-volumetric-capacity anode for lithium-ion batteries. Carbon 2020, 162, 36–45. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Liu, Z.; Wu, H.; Zhao, H.; Liu, H.; Zhang, Y. Cycling-induced structure refinement of MnO nanorods wrapped by N-doped carbon with internal void space for advanced lithium-ion anodes. Appl. Surf. Sci. 2019, 479, 386–394. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, J.; Nie, P.; Zhu, Q.; Chen, C.; He, W.; Zhang, T.; Dou, H.; Zhang, X. Hierarchical N-doped hollow carbon microspheres as advanced materials for high-performance lithium-ion capacitors. J. Mater. Chem. A 2020, 8, 3956–3966. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhang, C.; Jin, D.; Xie, K.; Wei, B. Synthesis of ultralong MnO/C coaxial nanowires as freestanding anodes for high-performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 13699–13705. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Shi, J.; Liu, W.; Shi, Z.; Chen, S.; Wang, X.; Wang, H. Two-dimensional biomass-derived carbon nanosheets and MnO/carbon electrodes for high-performance Li-ion capacitors. J. Mater. Chem. A 2017, 5, 15243–15252. [Google Scholar] [CrossRef]
- Peng, H.; Hao, G.; Chu, Z.; Lin, J.; Lin, X.; Cai, Y. Mesoporous Mn3O4/C microspheres fabricated from MOF template as advanced lithium-ion battery anode. Cryst. Growth Des. 2017, 17, 5881–5886. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Xia, K.; Han, B.; Zhou, C.; Gao, Q.; Wang, H.; Pu, S.; Wu, J. Facile synthesis of hierarchically porous N/P codoped carbon with simultaneously high-level heteroatom-doping and moderate porosity for high-performance supercapacitor electrodes. ACS Sustainable Chem. Eng. 2019, 7, 5717–5726. [Google Scholar] [CrossRef]
- Pei, C.; Ding, R.; Yu, X.; Feng, L. Electrochemical oxygen reduction reaction performance boosted by N, P doped carbon layer over manganese dioxide nanorod. ChemCatChem 2019, 11, 4617–4623. [Google Scholar] [CrossRef]
- Pei, X.; Mo, D.; Lyu, S.; Zhang, J.; Fu, Y. Preparation of novel two-stage structure MnO micrometer particles as lithium-ion battery anode materials. RSC Adv. 2018, 8, 28518–28524. [Google Scholar] [CrossRef]
- Jiang, S.; Yun, S.; Cao, H.; Zhang, Z.; Feng, H.; Chen, H. Porous carbon matrix-encapsulated MnO in situ derived from metal-organic frameworks as advanced anode materials for Li-ion capacitors. Sci. China Mater. 2021, 65, 59–68. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, Z.; Rong, H.; Guo, L.; Jiang, Z. High performance of yolk-shell structured MnO@nitrogen doped carbon microspheres as lithium ion battery anode materials and their in operando X-ray diffraction study. Electrochim. Acta 2018, 282, 719–727. [Google Scholar] [CrossRef]
- Zheng, F.; Yin, Z.; Xia, H.; Bai, G.; Zhang, Y. Porous MnO@C nanocomposite derived from metal-organic frameworks as anode materials for long-life lithium-ion batteries. Chem. Eng. J. 2017, 327, 474–480. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Q.; Li, M.; Jiang, K.; Hu, Z.; Chu, J. High-capacity and long-life lithium storage boosted by pseudocapacitance in three-dimensional MnO-Cu-CNT/graphene anodes. Nanoscale 2018, 10, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, J.; Zeng, Y.; Zhang, Y.; Guo, H. Morphological and structural evolution of MnO@C anode and its application in lithium-ion capacitors. ACS Appl. Energy Mater. 2019, 2, 8345–8358. [Google Scholar] [CrossRef]
- Le, Z.; Liu, F.; Nie, P.; Li, X.; Liu, X.; Bian, Z.; Chen, G.; Wu, H.B.; Lu, Y. Pseudocapacitive sodium storage in mesoporous single-crystal-like TiO2-graphene nanocomposite enables high-performance sodium-Ion capacitors. ACS Nano 2017, 11, 2952–2960. [Google Scholar] [CrossRef]
- Sun, Q.; Cao, Z.; Zhang, J.; Cheng, H.; Zhang, J.; Li, Q.; Ming, H.; Liu, G.; Ming, J. Metal catalyst to construct carbon nanotubes networks on metal oxide microparticles towards designing high-performance electrode for high-voltage lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2009122. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Dong, K.; AiminHao; Luo, S.; Liu, Y.; Wang, Q.; Zhang, Y.; Wang, Z. Fabrication of porous carbon with controllable nitrogen doping as anode for high-performance potassium-ion batteries. ChemElectroChem 2019, 6, 3699–3707. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, Z.; An, Y.; Zhu, Q.; Xu, Y.; Zang, S.; Dou, H.; Zhang, X. Defect-rich and N-doped hard carbon as a sustainable anode for high-energy lithium-ion capacitors. J. Colloid Interface Sci. 2020, 567, 75–83. [Google Scholar] [CrossRef]
- Zou, K.; Cai, P.; Cao, X.; Zou, G.; Hou, H.; Ji, X. Carbon materials for high-performance lithium-ion capacitor. Curr. Opin. Electrochem. 2020, 21, 31–39. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, Z.; Ayub, M.; Li, S.; Ma, X.; Xu, C. High energy and power lithium-ion capacitor based on MnO-encased graphene spheres anode and hollow carbon nano-rods cathode. Chem. Eng. Sci. 2021, 245, 116968. [Google Scholar] [CrossRef]
- Chen, Z.; He, B.; Yan, D.; Yu, X.; Li, W. Peapod-like MnO@hollow carbon nanofibers film as self-standing electrode for Li-ion capacitors with enhanced rate capacity. J. Power Sources 2020, 472, 228501. [Google Scholar] [CrossRef]
- Chen, M.; Le, T.; Zhou, Y.; Kang, F.; Yang, Y. Enhanced electrode matching assisted by in situ etching and co-doping toward high-rate dual-carbon lithium-ion capacitors. ACS Sustain. Chem. Eng. 2021, 9, 10054–10061. [Google Scholar] [CrossRef]
- Yue, C.; Qiu, D.; Kang, C.; Li, M.; Qiu, C.; Xian, L.; Wang, F.; Yang, R. Multidimensional dual-carbon skeleton network confined MnOx anode boosting high-performance lithium-ion hybrid capacitors. ACS Appl. Energy Mater. 2021, 4, 11268–11278. [Google Scholar] [CrossRef]
- Luan, Y.; Yin, J.; Cheng, K.; Ye, K.; Yan, J.; Zhu, K.; Wang, G.; Cao, D. Facile synthesis of MnO porous sphere with N-doped carbon coated layer for high performance lithium-ion capacitors. J. Electroanal. Chem. 2019, 852, 113515. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.; Guo, L.; Dong, X.; Chen, Z.; Li, W. Hard@soft integrated morning glory like porous carbon as a cathode for a high-energy lithium ion capacitor. ACS Appl. Mater. Interfaces 2018, 10, 43946–43952. [Google Scholar] [CrossRef]
- Han, C.; Tong, J.; Tang, X.; Zhou, D.; Duan, H.; Li, B.; Wang, G. Boost anion storage capacity using conductive polymer as a pseudocapacitive cathode for high-energy and flexible lithium ion capacitors. ACS Appl. Mater. Interfaces 2020, 12, 10479–10489. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, S.; Cheng, C.; Wu, D.; Wang, G.; Hou, L.; Yuan, C. Single-crystal nano-subunits assembled accordion-shape WNb2O8 framework with high Ionic/electronic conductivities towards Li-ion capacitors. Small 2022, 18, 2107987. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).