1. Introduction
Due to their large capacity, long lifespan, and high security, vanadium redox flow batteries (VRFBs) are in widespread use for energy storage [
1,
2,
3,
4,
5,
6]. As the energetic material for energy storage and the heart of energy conversion, the vanadium electrolyte has a crucial impact on the characteristics and efficiency of VRFBs [
7,
8]. Generally, the action of impurity ions is one of the major causes of electrolyte performance degradation [
9,
10]. The presence of impurity ions has been reported to not only influence the run temperature and sustainability of the electrolyte but also impair the energy efficiency of VRFBs [
11].
Fe ions are common and abundant impurity ions in V
2O
5, which is the raw material of vanadium electrolytes. In the vanadium shale wet extraction process, it is extremely easy for Fe ions to enter the acid leach solution from the raw vanadium shale ore and it is difficult to remove them completely, with them ultimately remaining in the vanadium products [
12,
13]. In addition, Fe ions enter the electrolyte from the tubes and frame material during the charge/discharge cycles of VRFBs [
14]. Related studies have shown that not only was the stability of the electrolyte substantially reduced but side reactions and capacity decay occurred as well if the vanadium electrolyte contained more than 1.095 g·L
−1 of Fe ions [
15]. Therefore, to maintain the operating performance of VRFBs, periodic treatment of the electrolyte to remove Fe is required.
The most commonly used methods for removing Fe impurity ions are chemical precipitation, solvent extraction, and ion exchange [
16,
17,
18]. Lzadi [
19] et al. added hydroxide and jarosite to an electrolyte solution for the electrowinning of copper to reduce the concentration of Fe ions. Wang [
20] et al. reported that P507 was used to remove Fe impurity ions from a H
2SO
4 leaching solution of coal fly ash with a removal rate of more than 97.6%. Lv [
21] et al. used S957 resin to remove Fe impurity ions from a mixed solution containing nitric acid and phosphoric acid, and they achieved a significant removal rate under optimum conditions. However, vanadium electrolytes are characterized by high vanadium content, high acid concentration, and low levels of impurity ions [
22,
23]. Chemical precipitation and solvent extraction processes to remove Fe impurity ions require chemical addition, which contaminates the electrolyte, and ion exchange processes can also cause substantial vanadium loss. Hence, there is a necessity to find a process for eliminating Fe ions that is better suited to the characteristics of the vanadium electrolyte.
With the benefits of minimal energy consumption and environmental friendliness, CDI is a water treatment technology of the future [
24,
25,
26,
27]. The basic principle of CDI is that the charge-carrying ions in the solution migrate toward the electrode and accumulate on its surface due to the effect of the electrofield, thereby achieving ion removal [
28,
29]. For the improvement of the adsorption efficiency and separability of CDI electrodes, other scholars added resin to CDI electrodes and prepared resin/carbon composite electrodes. Duan [
30] et al. investigated the properties and adsorption behavior of composite electrodes made of resin and carbon, carbon electrodes, and resin and found that the resin/carbon composite electrodes had lower specific capacitance, higher adsorption capacity, and better selectivity. Zhou [
31,
32] et al. used ZG-A-PX resin/carbon composite electrodes to remove Al and P from a H
2SO
4 leach solution of stone coal with removal rates of 31.8% and 63.3%, respectively. It is evident that CDI allows for the selective adsorption of the target element in a complicated solution.
This study is aimed at developing a new process to remove Fe impurities from spent vanadium electrolytes. LSC-957 resin/carbon composite electrodes were prepared for CDI, and the optimum process parameters for CDI adsorption were established. Through viscosity and conductivity measurements, electrochemical performance characterization, and charge/discharge tests, the Fe ions were successfully separated from the spent electrolyte, and the properties of the regenerated electrolyte were substantially enhanced. This research has provided a number of practical guidelines for the regeneration and recycling of vanadium electrolytes.
2. Materials and Methods
2.1. Chemicals
LSC-957 chelating resin (
Table 1) with high affinity for Fe from China Xi’an Lanxiao Technology Co., Ltd. and activated carbon (average diameter 0.038 mm) from China Hunan Durban Activated Carbon Co., Ltd. were used for the preparation of the composite electrodes. The graphite collector electrodes were used to charge the electrode materials, whose length was 100 mm, whose width was 50 mm, and whose thickness was 2 mm. Analytical pure DMAC as the solvent was supplied by China Shanghai Sinopharm Chemical Reagent Co., Ltd. Analytical pure PVDF as the binder was acquired from China Shanghai Sigma Aldrich Co., Ltd. All the vanadium electrolyte in this study was prepared by dissolving vanadium oxychloride sulfate in a sulfuric acid solution, with vanadium ions as the active substance and sulfuric acid as the supporting electrolyte. The spent vanadium electrolyte used in this study suffered severe performance degradation due to the enrichment of Fe impurity ions, which required the removal of Fe and regeneration to meet the criteria for continued use, and its composition is shown in
Table 2.
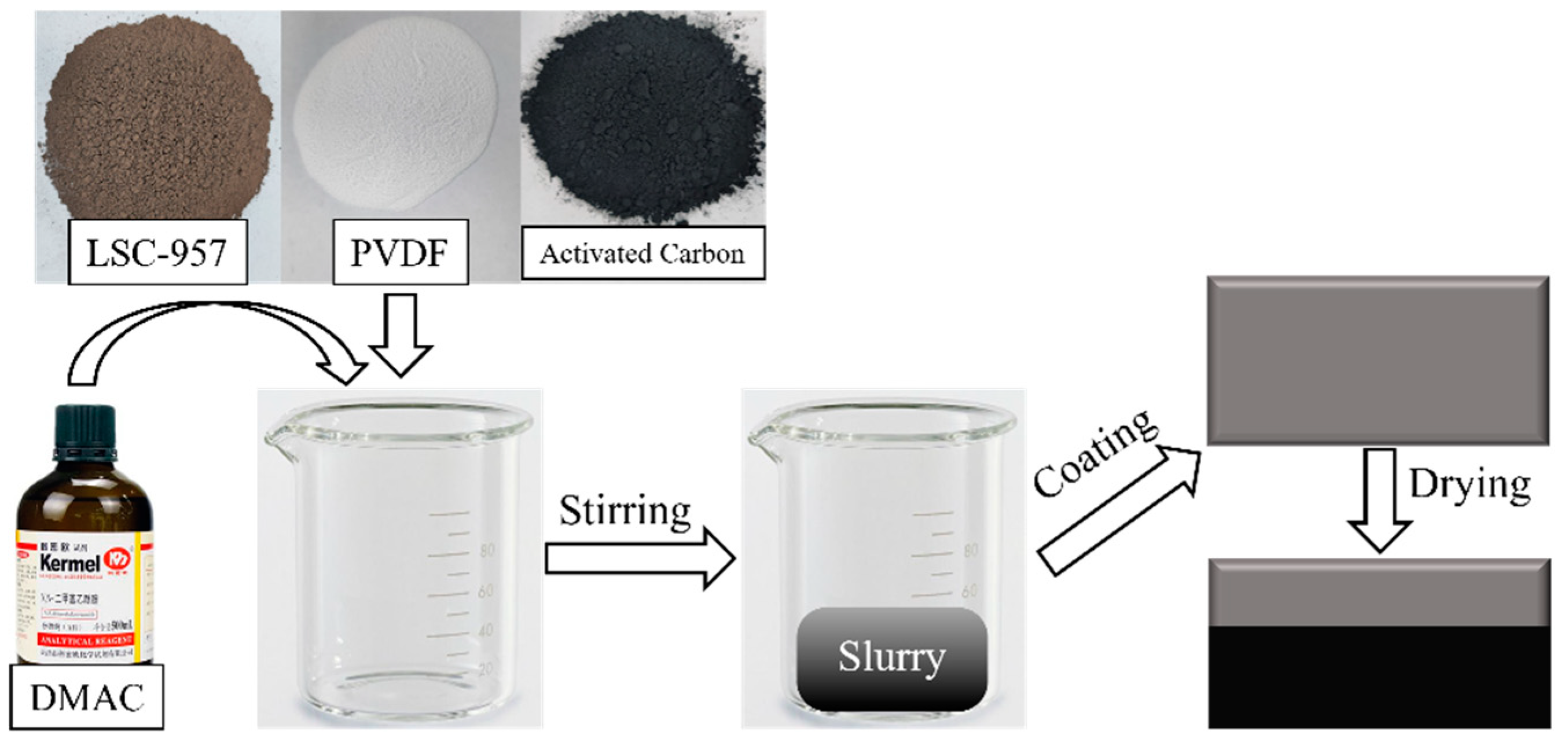
2.2. Preparation of the Composite Electrodes
The resin needs to be pretreated with acid and alkali solutions before use to remove soluble impurities to ensure the purity of the resin. Firstly, the LSC-957 resin was immersed in 5% NaOH solution and rinsed with deionized water to a neutral pH after 12 h and then filtered. Subsequently, the resin was immersed in 5% H
2SO
4 solution and rinsed with deionized water to a neutral pH after 12 h and then filtered and dried at 60 °C to a constant weight. The resin was treated with a vibrating mill for 1 s, sieved, and retained the products of ≤74 μm particle size. Finally, the resin powder, activated carbon, and PVDF were dissolved in DMAC in a certain mass ratio and mixed thoroughly for 4 h. The resulting suspension was applied evenly to the graphite collector electrodes and then dried at a temperature of 60 °C in a drying oven. The composite electrodes were then produced. The manufacturing process of LSC-957 resin/carbon composite electrodes is shown in
Figure 1.
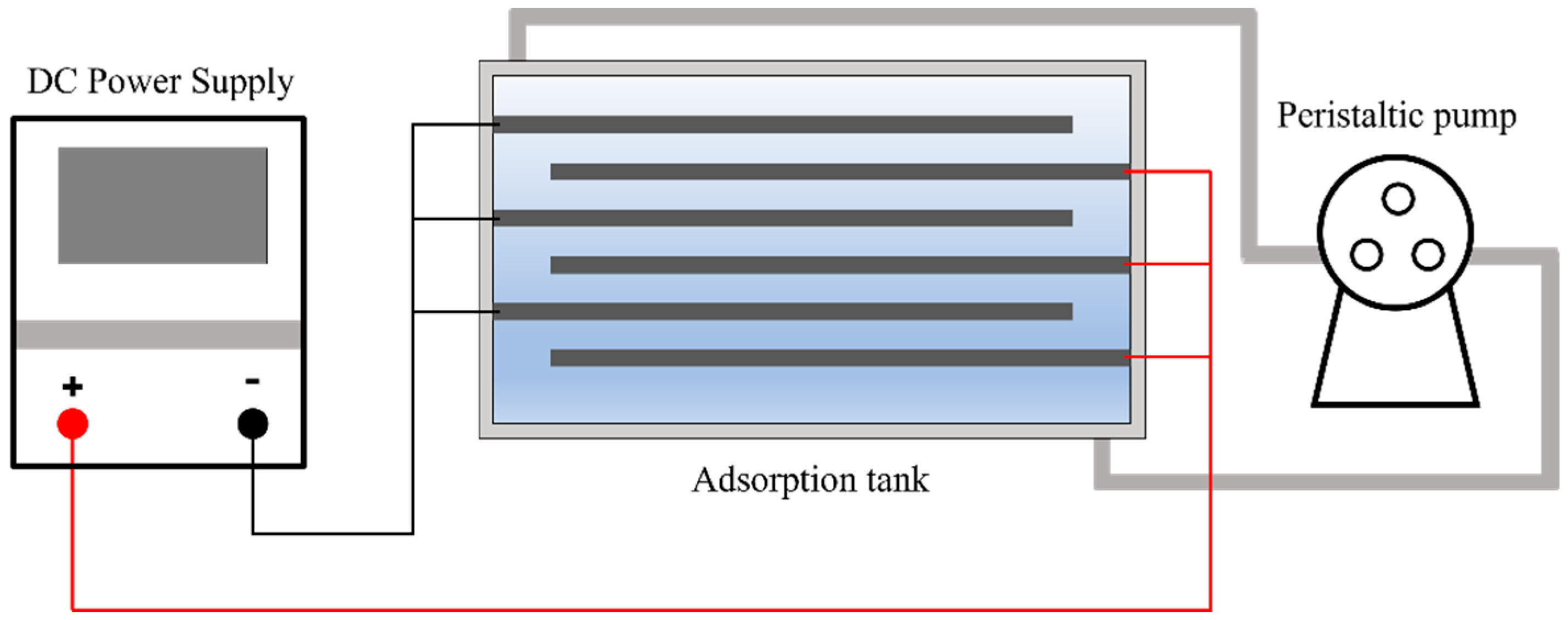
2.3. CDI Treatment
The CDI unit mainly includes an adsorption tank, a peristaltic pump, a parallel splitter, and a DC power supply (IT6861A, ITECH Co., Ltd., Nanjing, China) (
Figure 2). In the CDI adsorption treatment, in the adsorption tank, the graphite collector electrodes connected to the cathode and the composite electrodes connected to the anode were placed in parallel, and the circulation of the spent electrolyte in the adsorption tank was realized by the action of the peristaltic pump. The same constant voltage was applied to each pair of parallel electrodes during the CDI treatment. After a certain period of time of adsorption, the adsorbed electrolyte was discharged.
The following Equations (1) and (2) were used to calculate the ion adsorption rate and separation factor of the composite electrodes [
33]:
where
D is the ion adsorption rate (%);
S(a,b) is the separation factor of a and b ions;
C0 is the initial concentration of ions in the electrolyte (mg·L
−1);
C1 is the concentration of ions in the electrolyte after CDI adsorption treatment (mg·L
−1);
V0 is the initial volume of the electrolyte (L);
V1 is the volume of the electrolyte after CDI adsorption treatment (L);
Ca,0 and
Cb,0 are the initial concentrations of a and b ions in the electrolyte (mg·L
−1), respectively; and
Ca,1 and
Cb,1 are the concentrations of a and b ions in the electrolyte after CDI adsorption treatment (mg·L
−1), respectively.
2.4. Analytical Test Methods
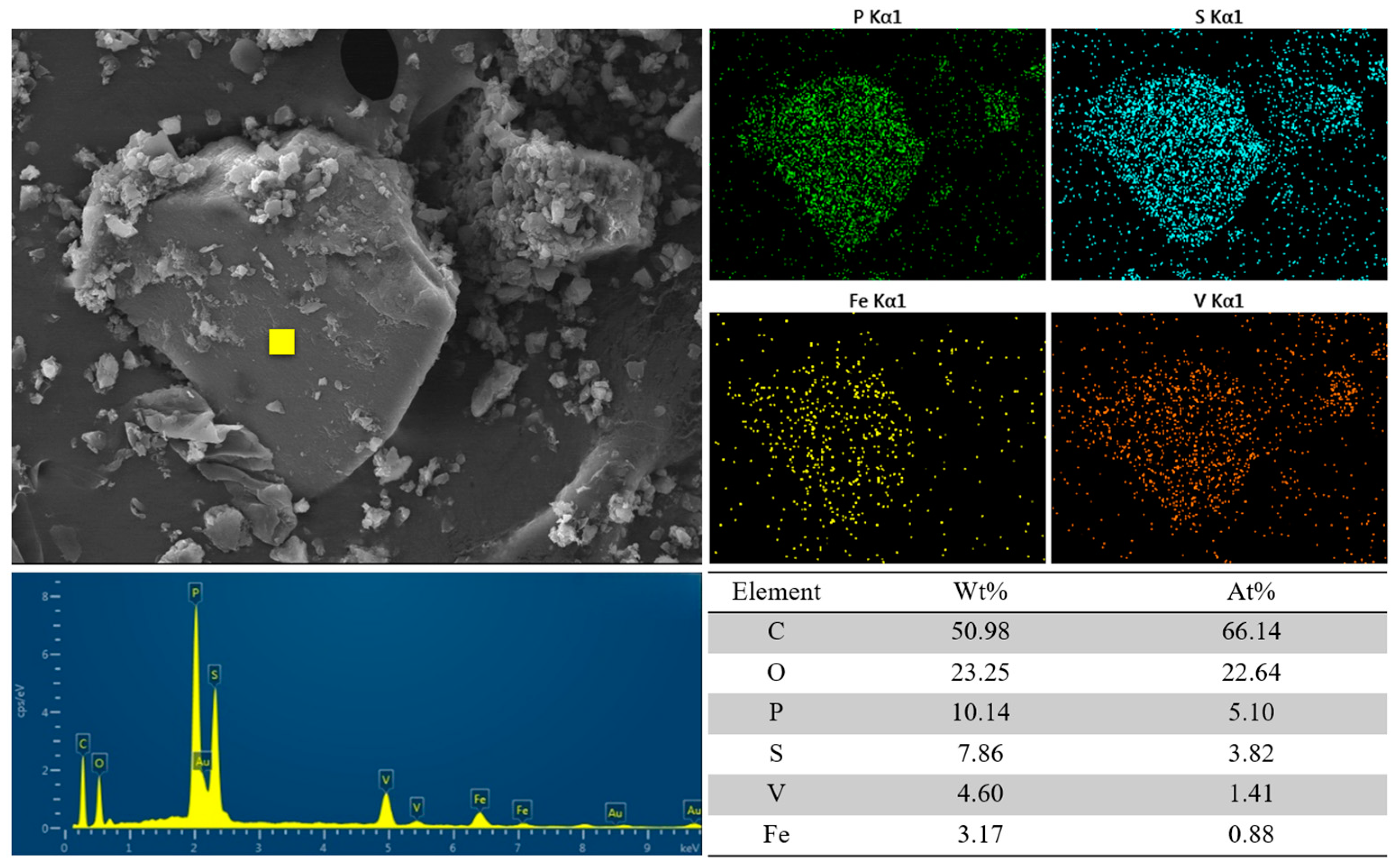
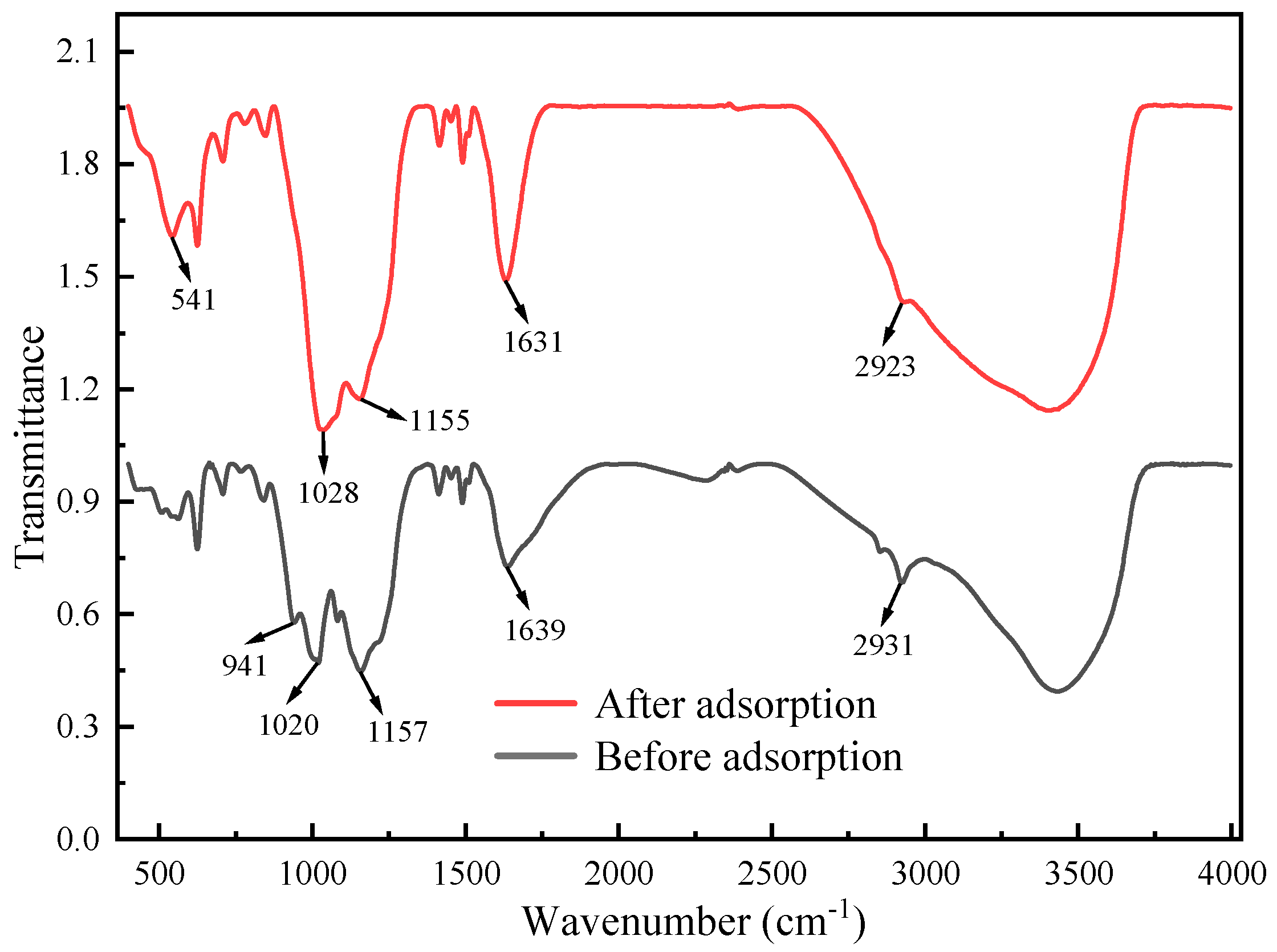
Using N-phenyl-o-aminobenzoic acid as an indicator, the V concentration was measured by means of titration with (NH4)2Fe(SO4)2·6H2O solution. ICP-OES (model 730, Agilent Co., Ltd., Shanghai, China) was used to determine the concentrations of the other ions in the electrolyte. The surface morphology of the electrode materials was analyzed by SEM-EDS (JSM-IT300, JEOL Co., Ltd., Tokyo, Japan). The changes before and after the adsorption of the composite electrodes were analyzed by FTIR (VERTEX70, Bruker, Germany).
The conductivity and viscosity of the electrolyte were measured using a conductivity meter and an Ubbelohde viscometer, respectively, and both were tested three times and averaged.
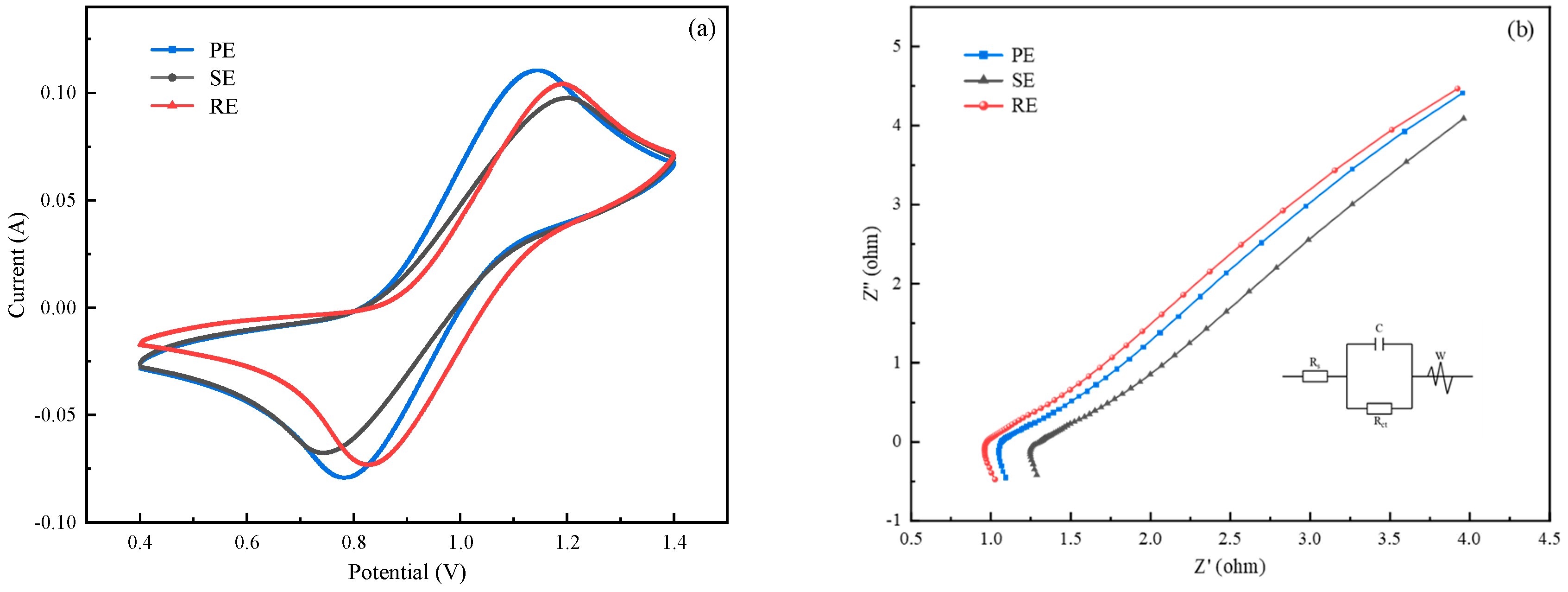
For electrochemical testing, a three-electrode system was used. A graphite electrode (1 cm × 1 cm) was selected as the working electrode, a platinum electrode (1 cm × 1 cm) was selected as the counter electrode, and a saturated calomel electrode filled with potassium chloride solution was selected as the reference electrode. CV and EIS tests of electrolytes were run on a CHI 660E electrochemical workstation using a 3-electrode system. The CV test set the voltage range to 0.4–1.4 V and the scan rate to 10 mV·S−1, and the EIS spectra were collected from 1 Hz to 105 Hz over a frequency range of 5 mV using an equivalent circuit to fit the test results.
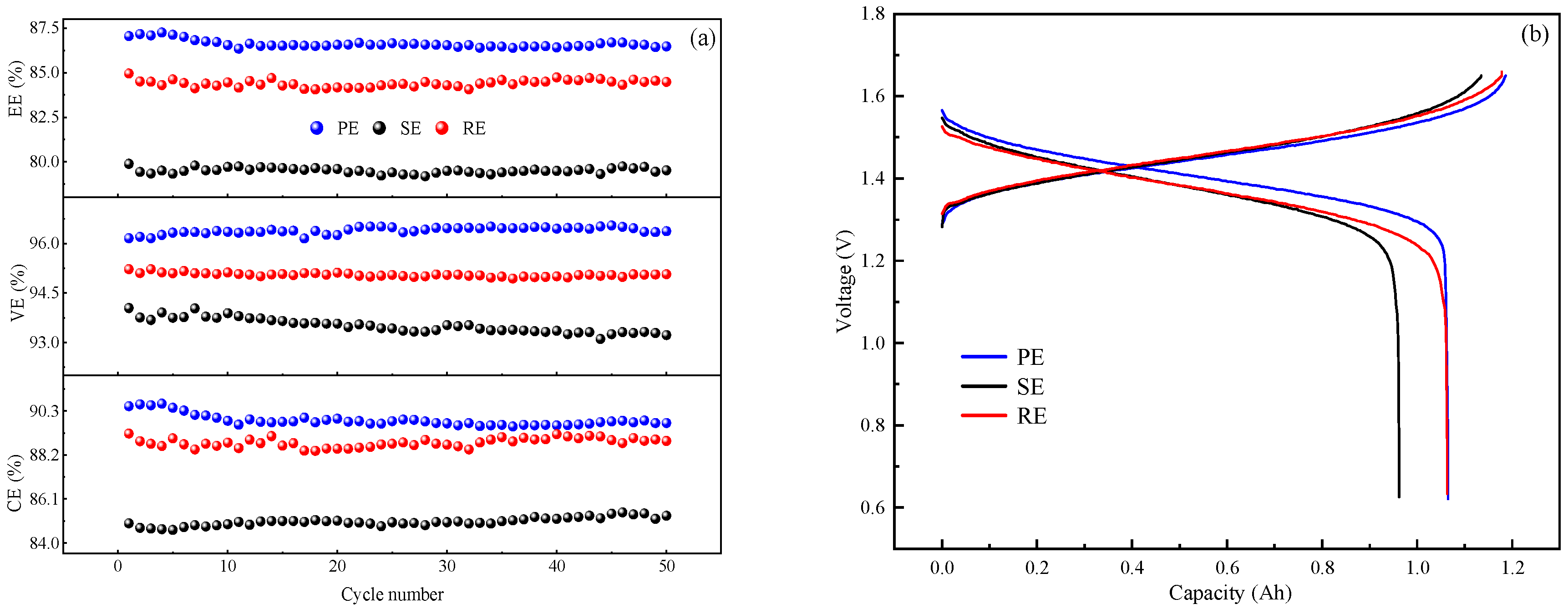
The vanadium battery system used for battery charge/discharge tests consists of a single stack, positive and negative electrode reservoirs, two peristaltic pumps, and a battery test system. The electrolyte was stored in a reservoir and transported to the single stack by a peristaltic pump through a hose, while a graphite felt electrode (8 cm × 8 cm) was placed on the positive and negative side of the stack, and a Nafion 117 membrane was sandwiched between the two electrodes to provide separation. A Land CT2001A (5 V/10 A) battery system was used to test the charge/discharge performance of the electrolytes, the current density was set to 40 mA·cm−2, the voltage range was 0.65–1.65 V, and the flow rate was set to 60 mL·min−1.
4. Conclusions
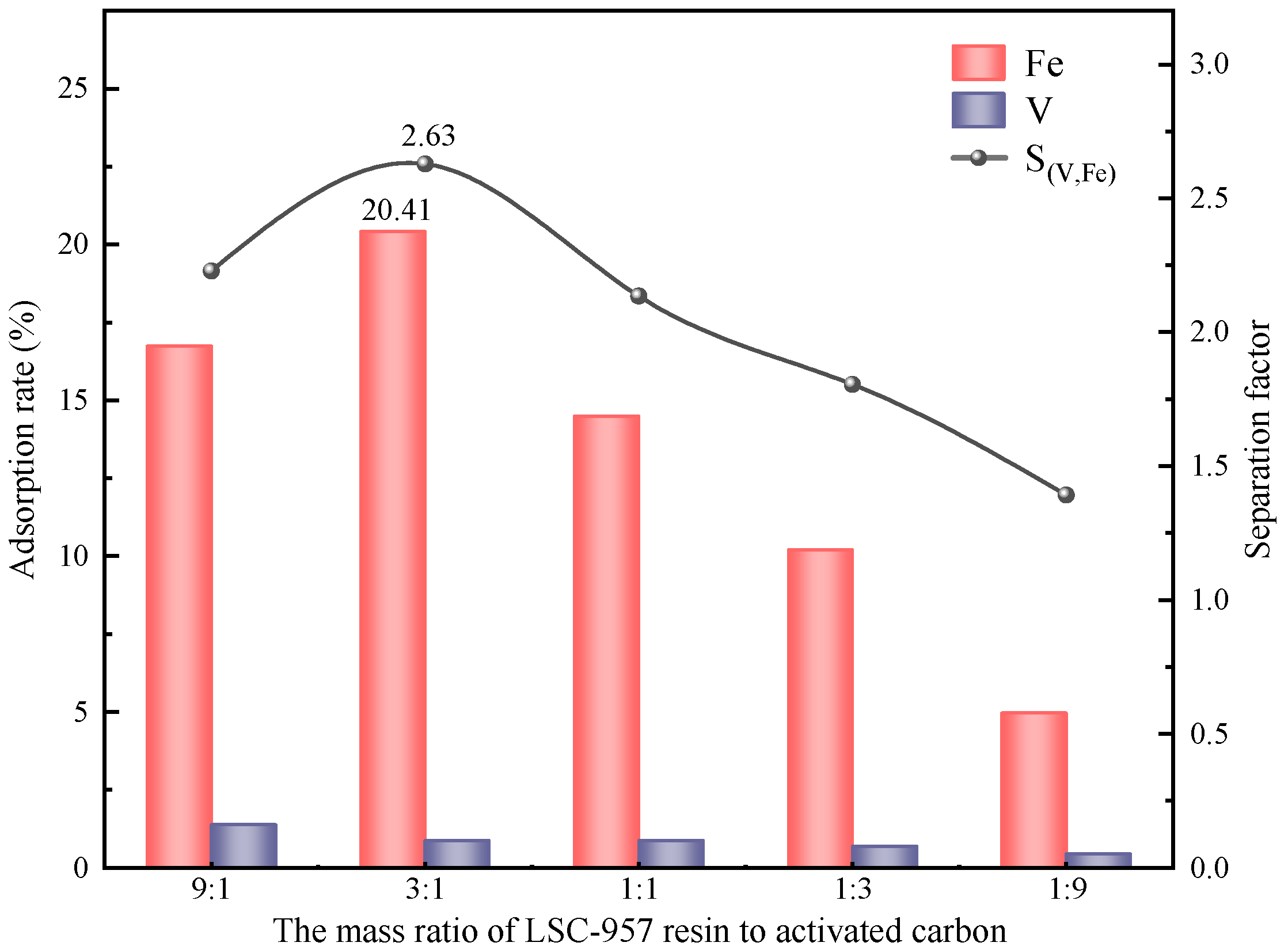
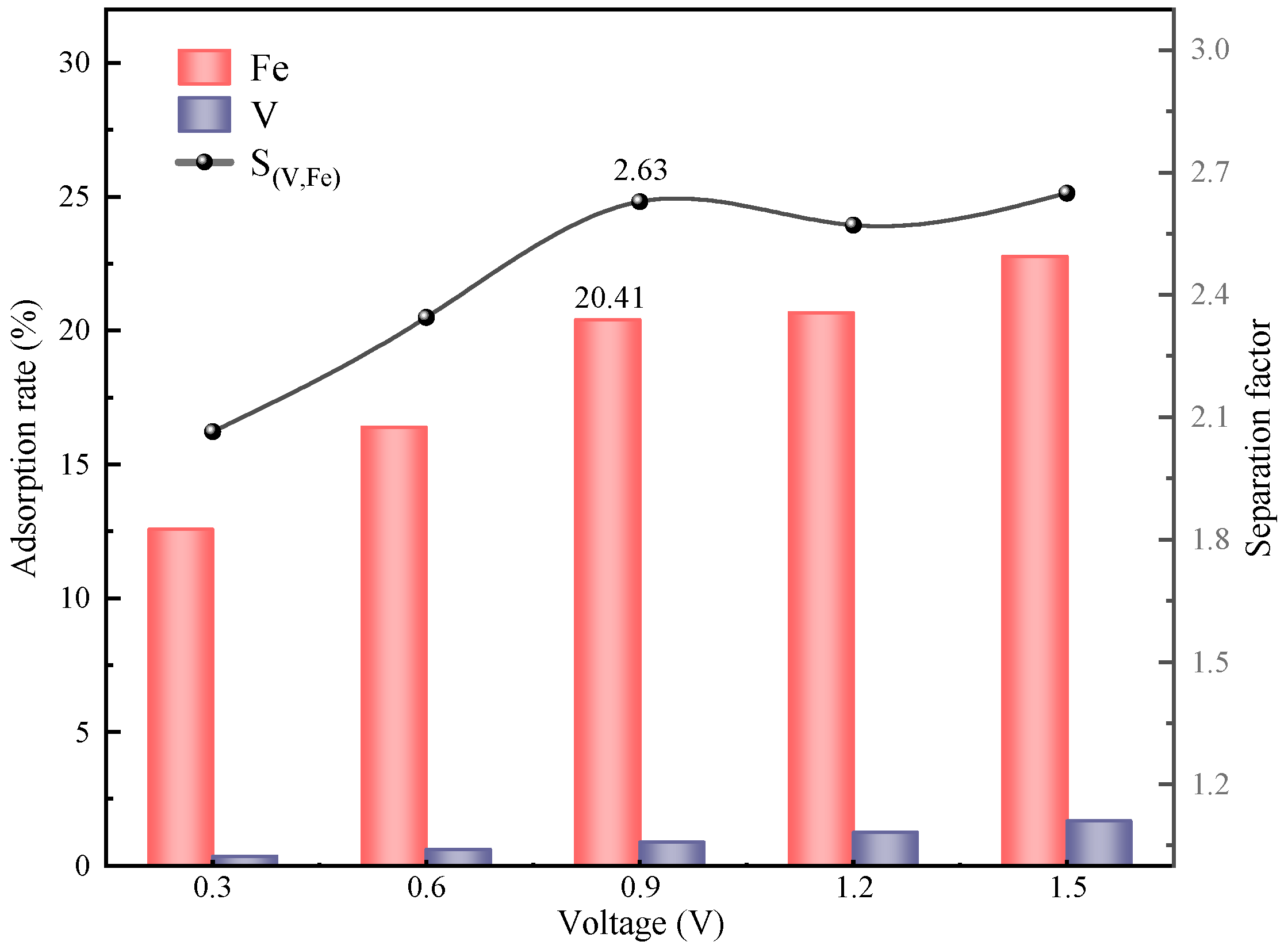
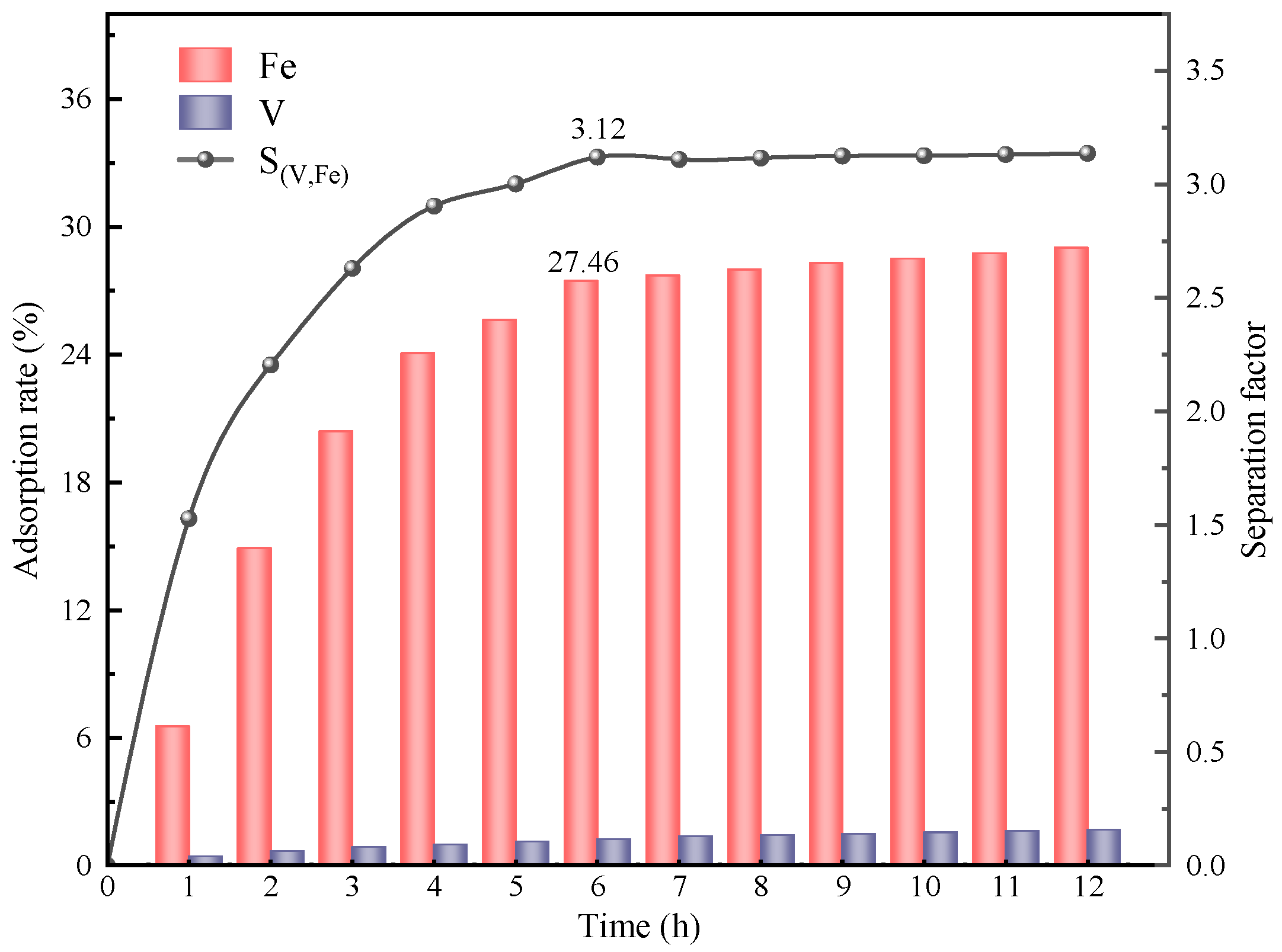
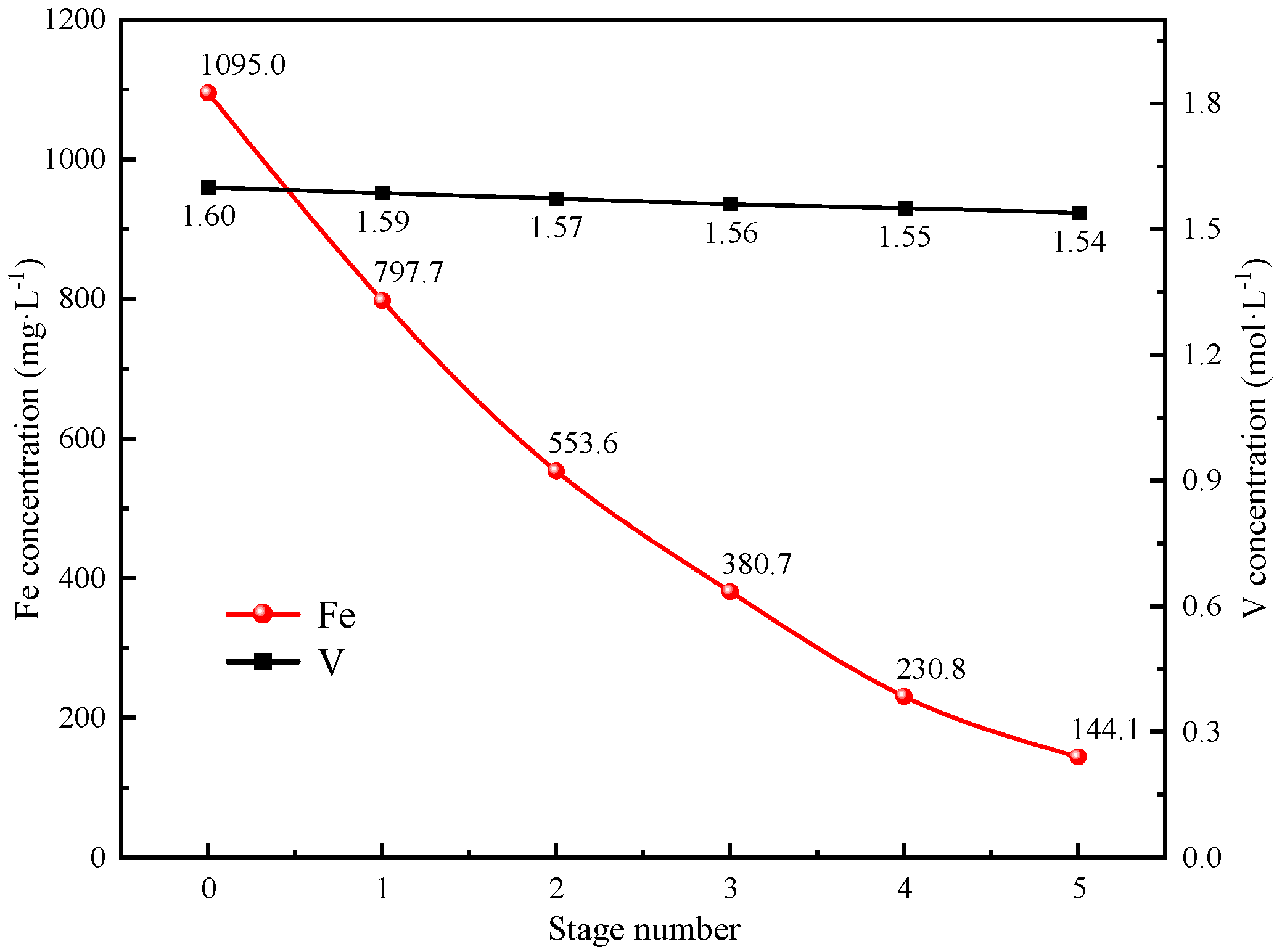
In summary, the removal of Fe impurity ions from spent vanadium electrolytes is well achieved by CDI based on LSC-957 resin/carbon composite electrodes. The composite electrodes were prepared with the mass ratio of LSC-957 resin to activated carbon of 3:1, and the spent electrolyte was treated at a constant voltage of 0.9 V for 6 h. After the five stages of CDI treatment, the adsorption rate of Fe impurity ions was 86.84%, the concentration of Fe impurity ions in the electrolyte was in accordance with the requirements of the national standard, and the loss rate of V was only 3.8%.
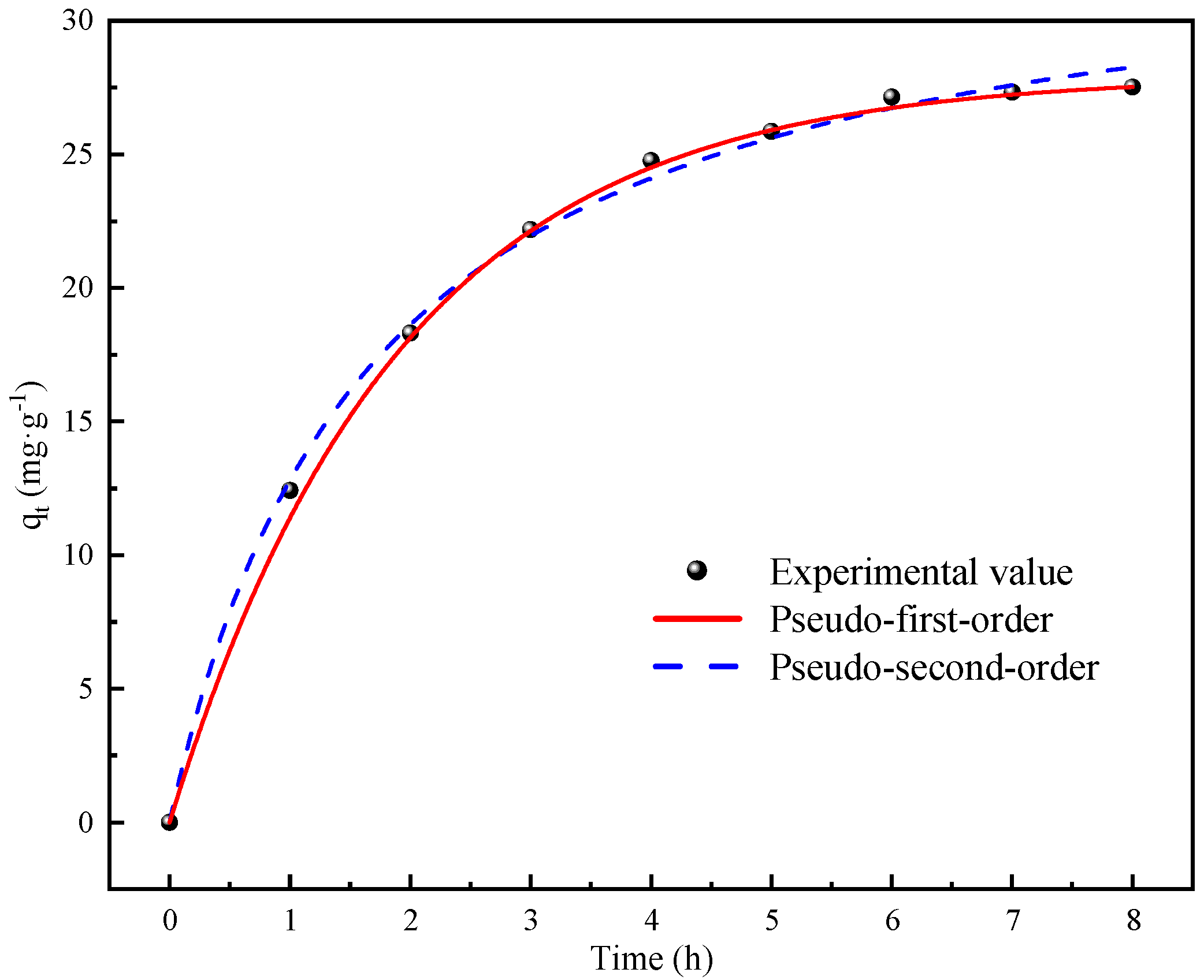
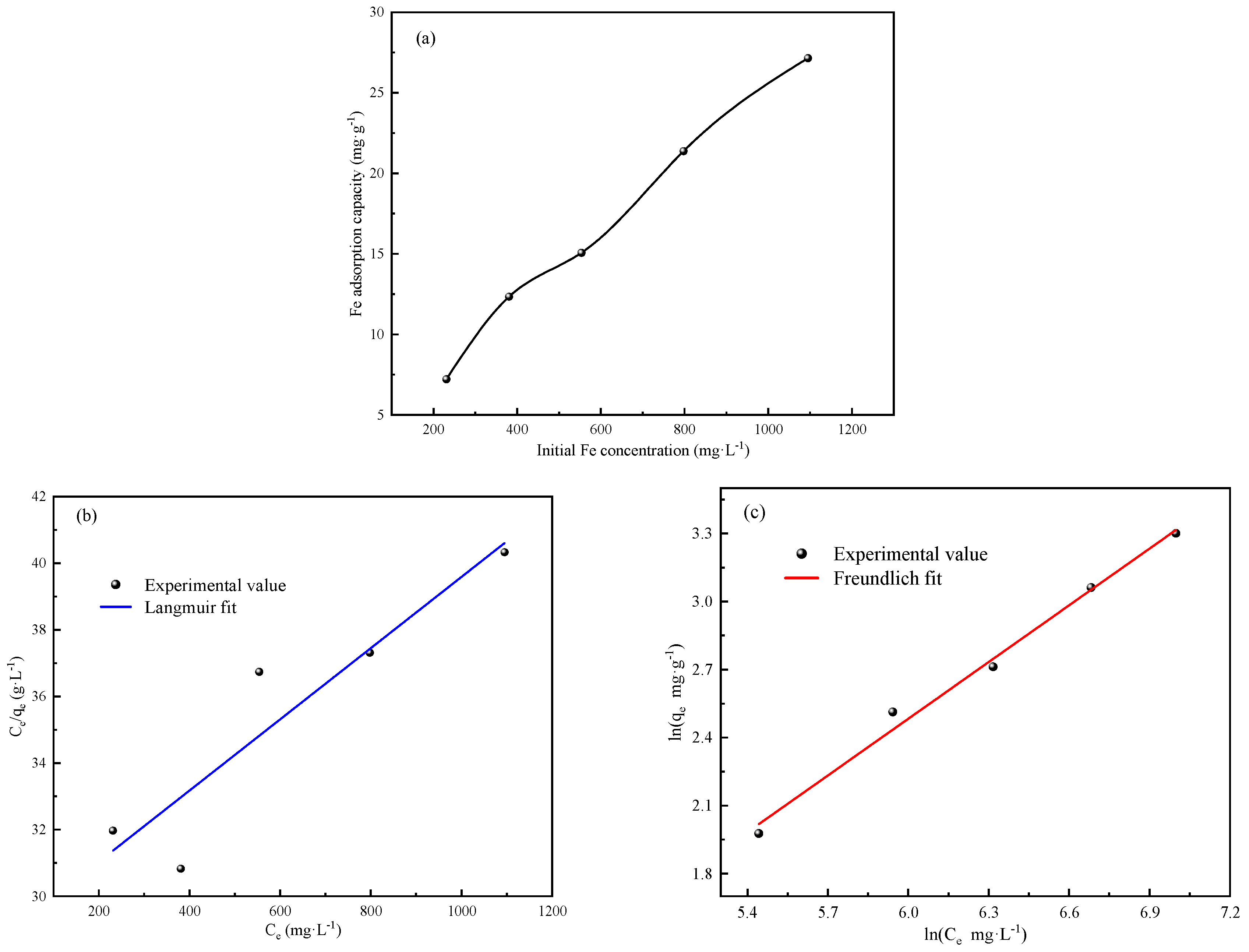
The results of the kinetic fit and isothermal adsorption models’ fit showed that the CDI treatment involved both physical adsorption and chemical reactions. The SEM-EDS images and FTIR analysis showed that the CDI adsorption process includes not only the electrostatic interaction between Fe impurity ions and composite electrodes, but also the chelating coordination reaction between Fe ions and phosphonic acid and sulfonic acid groups on the LSC-957 resin.
Through the characterization process, the performance of the regenerated electrolyte was significantly restored and reached the standard level for further use. This study demonstrates the feasibility of using CDI for the removal of impurity ions from spent vanadium electrolytes and provides an example in the recycling of vanadium electrolytes.