All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs
Abstract
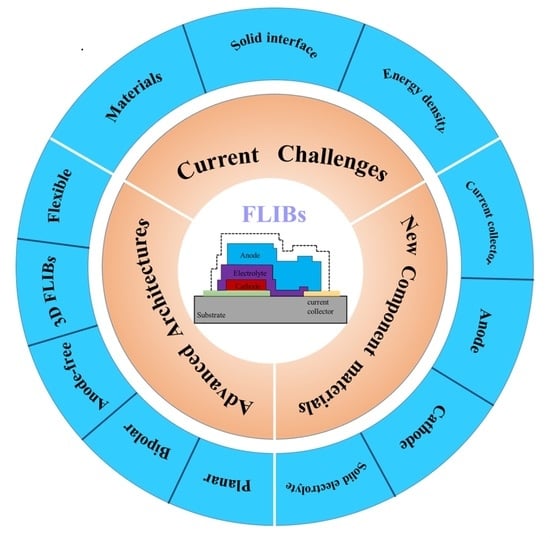
1. Introduction
2. History and Challenges
2.1. Brief History of TFLIBs
2.2. Current Challenges
2.2.1. Materials and Deposition Technologies
2.2.2. Solid-Solid Interfaces
2.2.3. Energy Density
2.2.4. Charging Rate
2.2.5. Manufacturing Cost
3. Batteries Materials
3.1. Current Collectors
3.1.1. Surface Coatings
3.1.2. Alloyed Current Collectors
3.1.3. 3D-Current Collectors
3.2. Anode Materials
3.2.1. Li-Based Anodes
Surface Modification of Li-Anodes
Li-Alloyed Anodes
3.2.2. Si-Based Anodes
Pure Si Anodes
3.2.3. Metal Oxides Anodes
3.3. Cathode Materials
3.3.1. High Voltage Cathodes
3.3.2. High-Capacity Cathodes
3.4. Electrolytes
3.4.1. Perovskites
3.4.2. NASICON
3.4.3. LISICON and Thio-LISICON
3.4.4. Garnets
3.4.5. Sulfides
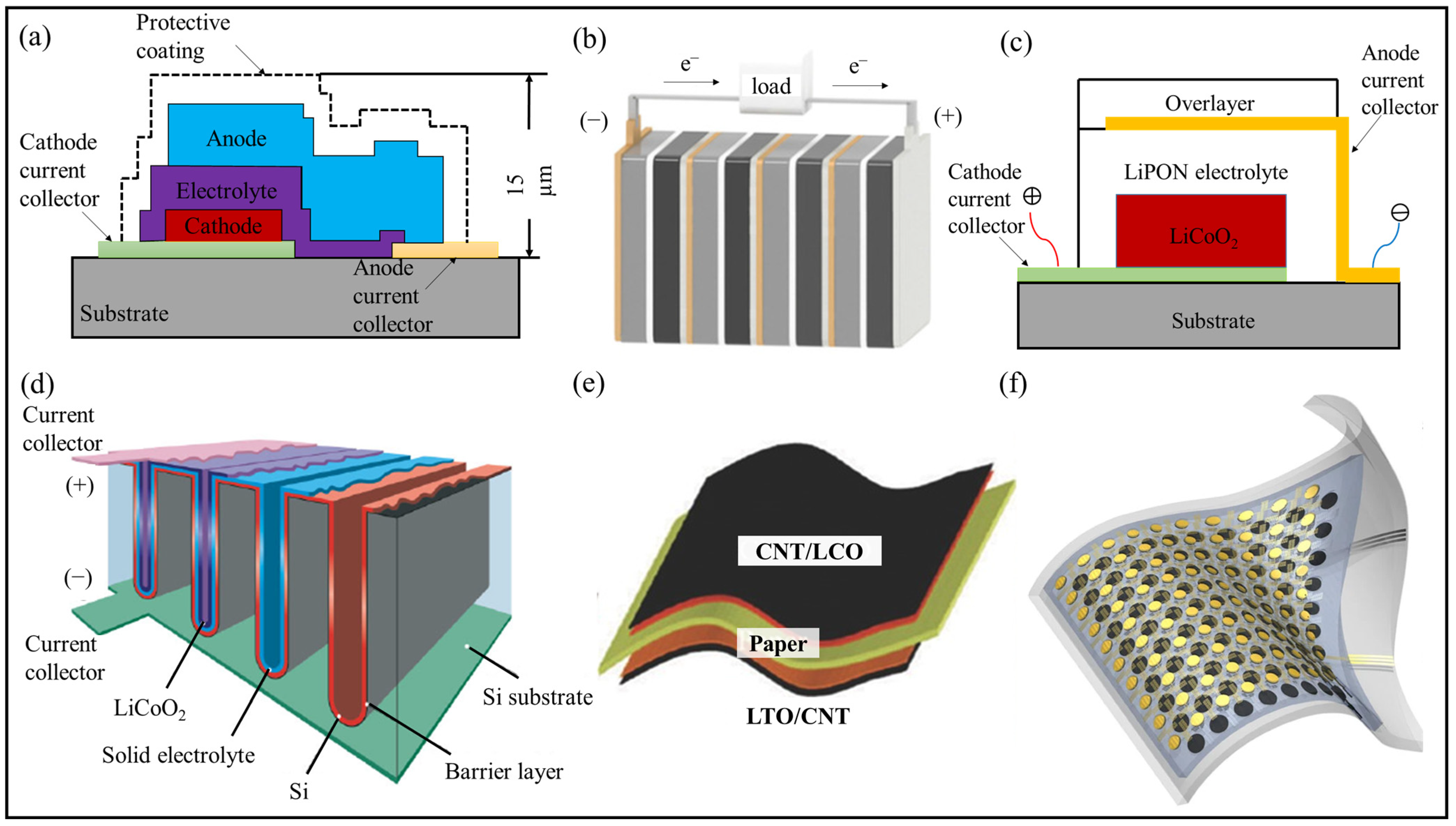
4. Architecture Designs
4.1. Planar TFLIBs
4.2. Bipolar TFLIBs
4.3. Anode-Free TFLIBs
4.4. 3D TFLIBs
4.4.1. 3D Cathodes
4.4.2. 3D Anodes
4.4.3. 3D Electrolytes
4.4.4. 3D Thin-Film Batteries
4.5. Other Novel TFLIBs
5. Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Notation | Definition |
| ASSBs | All-solid-state batteries |
| TFLIBs | All-solid-state thin-film Li-ion batteries |
| LIBs | Li-ion batteries |
| SSEs | Solid-state electrolytes |
| PVD | Physical vapor deposition |
| CVD | Chemical vapor deposition |
| PLD | Pulsed Laser Deposition |
| ALD | Atomic Layer Deposition |
| PECVD | Plasma-enhanced chemical vapor deposition |
| LPCVD | Low-pressure chemical vapor deposition |
| MOCVD | Metal-Organic Chemical Vapour Deposition |
| CCs | Current collector |
| 3D | Three-dimensional |
| SEI | Solid electrolyte interphase |
| RT | Room temperature |
| EC | Ethylene carbonate |
| PC | Propylene carbonate |
| DEC | Diethyl carbonate |
| DMC | Dimethyl carbonate |
| LLT | Lithium lanthanum titanates |
| LLZO | Li7La3Zr2O12 |
| LLCZN | Li7La2.75Ca0.25Zr1.75Nb0.25O12 |
| LMNO | LiNi0.5Mn1.5O4 |
| LiPON | Lithium phosphorus oxynitride |
| LCP | LiCoPO4 |
| NCM532 | LiNi0.5Mn0.3Co0.2O2 |
| NCM111 | LiNi0.3Mn0.3Co0.3O2 |
| NCM811 | LiNi0.8Mn0.1Co0.1O2 |
| NCM622 | LiNi0.6Mn0.2Co0.2O2 |
| LATP | Li1+xAlxTi2−x (PO4)3 |
| LAGP | Li1+xAlxGe2−x (PO4)3 |
| SSE | Solid-state electrolytes |
| LLZTO | Li6.75La3Zr1.75Ta0.25O12 |
| MLG | multilayer-graphene |
| LTO | Li4Ti5O12 |
| LCO | LiCoO2 |
| TNO | TiNb2O7 |
| GF | Graphite film |
References
- Palacín, M.R. Recent advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Y.-F.; Ma, J.; Ling, H.; Kang, F.; He, Y.-B. Progress and Perspective of All-Solid-State Lithium Batteries with High Performance at Room Temperature. Energy Fuels 2020, 34, 13456–13472. [Google Scholar] [CrossRef]
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO–LUMO misconception. Energy Environ. Sci. 2018, 11, 2306–2309. [Google Scholar] [CrossRef]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Takehara, Z.-i. Future prospects of the lithium metal anode. J. Power Sources 1997, 68, 82–86. [Google Scholar] [CrossRef]
- Takeda, Y.; Yamamoto, O.; Imanishi, N. Lithium Dendrite Formation on a Lithium Metal Anode from Liquid, Polymer and Solid Electrolytes. Electrochemistry 2016, 84, 210–218. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, G.; Ma, A.; Chen, W.; Shao, L.; Shen, C.; Xie, K. High-Performance Solid Composite Polymer Electrolyte for all Solid-State Lithium Battery Through Facile Microstructure Regulation. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose Nanofiber Supported 3D Interconnected BN Nanosheets for Epoxy Nanocomposites with Ultrahigh Thermal Management Capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Hou, H.; Xu, Q.; Pang, Y.; Li, L.; Wang, J.; Zhang, C.; Sun, C. Efficient Storing Energy Harvested by Triboelectric Nanogenerators Using a Safe and Durable All-Solid-State Sodium-Ion Battery. Adv. Sci. 2017, 4, 1700072. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Gittleson, F.S.; El Gabaly, F. Non-Faradaic Li+ Migration and Chemical Coordination across Solid-State Battery Interfaces. Nano Lett. 2017, 17, 6974–6982. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.Z.; Xin, H.L.; Han, L.; Grillon, N.; Guy-Bouyssou, D.; Bouyssou, E.; Proust, M.; Meng, Y.S. Effects of cathode electrolyte interfacial (CEI) layer on long term cycling of all-solid-state thin-film batteries. J. Power Sources 2016, 324, 342–348. [Google Scholar] [CrossRef]
- Lobe, S.; Dellen, C.; Finsterbusch, M.; Gehrke, H.G.; Sebold, D.; Tsai, C.L.; Uhlenbruck, S.; Guillon, O. Radio frequency magnetron sputtering of Li7La3Zr2O12 thin films for solid-state batteries. J. Power Sources 2016, 307, 684–689. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.-i.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem. Eng. J. 2022, 430, 132876. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Wook Shin, D.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Lin, J.; Lin, L.; Qu, S.; Deng, D.; Wu, Y.; Yan, X.; Xie, Q.; Wang, L.; Peng, D. Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries. Energy Environ. Mater. 2022, 5, 133–156. [Google Scholar] [CrossRef]
- Fenech, M.; Sharma, N. Pulsed Laser Deposition-based Thin Film Microbatteries. Chem. Asian J. 2020, 15, 1829–1847. [Google Scholar] [CrossRef]
- Liang, X.; Tan, F.; Wei, F.; Du, J. Research progress of all solid-state thin film lithium Battery. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012138. [Google Scholar] [CrossRef]
- Yang, G.; Abraham, C.; Ma, Y.; Lee, M.; Helfrick, E.; Oh, D.; Lee, D. Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films. Appl. Sci. 2020, 10, 4727. [Google Scholar] [CrossRef]
- Clement, B.; Lyu, M.; Sandeep Kulkarni, E.; Lin, T.; Hu, Y.; Lockett, V.; Greig, C.; Wang, L. Recent Advances in Printed Thin-Film Batteries. Engineering 2022, 13, 238–261. [Google Scholar] [CrossRef]
- Xia, Q.; Zan, F.; Zhang, Q.; Liu, W.; Li, Q.; He, Y.; Hua, J.; Liu, J.; Xu, J.; Wang, J.; et al. All-Solid-State Thin Film Lithium/Lithium-Ion Microbatteries for Powering the Internet of Things. Adv. Mater. 2023, 35, 2200538. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Armstrong, D.; Brown, Z.L.; Bu, J.; Castell, M.R.; Chen, P.; Cocks, A.; Corr, S.A.; Cussen, E.J.; Darnbrough, E.; et al. 2020 roadmap on solid-state batteries. J. Phys. Energy 2020, 2, 032008. [Google Scholar] [CrossRef]
- Hull, M.N. Recent progress in the development of solid electrolyte batteries. Energy Convers. 1970, 10, 215–226. [Google Scholar] [CrossRef]
- Liang, C.C.; Bro, P. A High-Voltage, Solid-State Battery System: I. Design Considerations. J. Electrochem. Soc. 1969, 116, 1322. [Google Scholar] [CrossRef]
- Megahed, S.; Scrosati, B. Lithium-ion rechargeable batteries. J. Power Sources 1994, 51, 79–104. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Gruzalski, G.R.; Zuhr, R.A.; Choudhury, A.; Luck, C.F.; Robertson, J.D. Electrical properties of amorphous lithium electrolyte thin films. Solid State Ion. 1992, 53–56, 647–654. [Google Scholar] [CrossRef]
- West, W.C.; Whitacre, J.F.; White, V.; Ratnakumar, B.V. Fabrication and testing of all solid-state microscale lithium batteries for microspacecraft applications. J. Micromech. Microeng. 2002, 12, 58. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D Integrated All-Solid-State Rechargeable Batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Cras, F.L.; Pecquenard, B.; Dubois, V.; Phan, V.-P.; Guy-Bouyssou, D. All-Solid-State Lithium-Ion Microbatteries Using Silicon Nanofilm Anodes: High Performance and Memory Effect. Adv. Energy Mater. 2015, 5, 1501061. [Google Scholar] [CrossRef]
- Garbayo, I.; Struzik, M.; Bowman, W.J.; Pfenninger, R.; Stilp, E.; Rupp, J.L.M. Glass-Type Polyamorphism in Li-Garnet Thin Film Solid State Battery Conductors. Adv. Energy Mater. 2018, 8, 1702265. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; He, Y.-B.; Kang, F.; Chen, L.; Li, H.; Yang, Q.-H. Solid-state lithium batteries: Safety and prospects. eScience 2022, 2, 138–163. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Tong, L.; Wang, P.; Fang, W.; Guo, X.; Bao, W.; Yang, Y.; Shen, S.; Qiu, F. Interface Engineering of Silicon/Carbon Thin-Film Anodes for High-Rate Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 29242–29252. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Ali, N. 7.04—PVD Technology in Fabrication of Micro- and Nanostructured Coatings. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 49–84. [Google Scholar] [CrossRef]
- Putkonen, M.; Aaltonen, T.; Alnes, M.; Sajavaara, T.; Nilsen, O.; Fjellvåg, H. Atomic layer deposition of lithium containing thin films. J. Mater. Chem. 2009, 19, 8767–8771. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Pei, A.; Chen, K.; Vailionis, A.; Li, Y.; Zheng, G.; Sun, J.; Cui, Y. Robust Pinhole-free Li3N Solid Electrolyte Grown from Molten Lithium. ACS Cent. Sci. 2018, 4, 97–104. [Google Scholar] [CrossRef]
- Tian, C.; Lin, F.; Doeff, M.M. Electrochemical Characteristics of Layered Transition Metal Oxide Cathode Materials for Lithium Ion Batteries: Surface, Bulk Behavior, and Thermal Properties. Acc. Chem. Res. 2018, 51, 89–96. [Google Scholar] [CrossRef]
- Pan, Q.; Barbash, D.; Smith, D.M.; Qi, H.; Gleeson, S.E.; Li, C.Y. Correlating Electrode–Electrolyte Interface and Battery Performance in Hybrid Solid Polymer Electrolyte-Based Lithium Metal Batteries. Adv. Energy Mater. 2017, 7, 1701231. [Google Scholar] [CrossRef]
- Zhang, W.; Schröder, D.; Arlt, T.; Manke, I.; Koerver, R.; Pinedo, R.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. (Electro)chemical expansion during cycling: Monitoring the pressure changes in operating solid-state lithium batteries. J. Mater. Chem. A 2017, 5, 9929–9936. [Google Scholar] [CrossRef]
- Koerver, R.; Zhang, W.; de Biasi, L.; Schweidler, S.; Kondrakov, A.O.; Kolling, S.; Brezesinski, T.; Hartmann, P.; Zeier, W.G.; Janek, J. Chemo-mechanical expansion of lithium electrode materials—On the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 2018, 11, 2142–2158. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity Fade in Solid-State Batteries: Interphase Formation and Chemomechanical Processes in Nickel-Rich Layered Oxide Cathodes and Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Bucci, G.; Talamini, B.; Renuka Balakrishna, A.; Chiang, Y.-M.; Carter, W.C. Mechanical instability of electrode-electrolyte interfaces in solid-state batteries. Phys. Rev. Mater. 2018, 2, 105407. [Google Scholar] [CrossRef]
- Zhang, W.; Richter, F.H.; Culver, S.P.; Leichtweiss, T.; Lozano, J.G.; Dietrich, C.; Bruce, P.G.; Zeier, W.G.; Janek, J. Degradation Mechanisms at the Li10GeP2S12/LiCoO2 Cathode Interface in an All-Solid-State Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 22226–22236. [Google Scholar] [CrossRef]
- Morimoto, H.; Awano, H.; Terashima, J.; Shindo, Y.; Nakanishi, S.; Ito, N.; Ishikawa, K.; Tobishima, S.-i. Preparation of lithium ion conducting solid electrolyte of NASICON-type Li1+xAlxTi2−x(PO4)3 (x = 0.3) obtained by using the mechanochemical method and its application as surface modification materials of LiCoO2 cathode for lithium cell. J. Power Sources 2013, 240, 636–643. [Google Scholar] [CrossRef]
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface Stability in Solid-State Batteries. Chem. Mater. 2016, 28, 266–273. [Google Scholar] [CrossRef]
- Okumura, T.; Nakatsutsumi, T.; Ina, T.; Orikasa, Y.; Arai, H.; Fukutsuka, T.; Iriyama, Y.; Uruga, T.; Tanida, H.; Uchimoto, Y.; et al. Depth-resolved X-ray absorption spectroscopic study on nanoscale observation of the electrode–solid electrolyte interface for all solid state lithium ion batteries. J. Mater. Chem. 2011, 21, 10051–10060. [Google Scholar] [CrossRef]
- Bron, P.; Roling, B.; Dehnen, S. Impedance characterization reveals mixed conducting interphases between sulfidic superionic conductors and lithium metal electrodes. J. Power Sources 2017, 352, 127–134. [Google Scholar] [CrossRef]
- Löbberding, H.; Wessel, S.; Offermanns, C.; Kehrer, M.; Rother, J.; Heimes, H.; Kampker, A. From Cell to Battery System in BEVs: Analysis of System Packing Efficiency and Cell Types. World Electr. Veh. J. 2020, 11, 77. [Google Scholar] [CrossRef]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent Insights into Rate Performance Limitations of Li-ion Batteries. Batter. Supercaps 2021, 4, 268–285. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Zhu, G.-L.; Zhao, C.-Z.; Huang, J.-Q.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast Charging Lithium Batteries: Recent Progress and Future Prospects. Samll 2019, 15, 1805389. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, W.; Zhang, X.; Ke, Y.; Qiu, Z.; Luo, J.; Tang, Y.; Wang, C.; Yuan, Y.; Huang, Y. A review on structuralized current collectors for high-performance lithium-ion battery anodes. Appl. Energy 2020, 276, 115464. [Google Scholar] [CrossRef]
- Rehnlund, D.; Lindgren, F.; Böhme, S.; Nordh, T.; Zou, Y.; Pettersson, J.; Bexell, U.; Boman, M.; Edström, K.; Nyholm, L. Lithium trapping in alloy forming electrodes and current collectors for lithium based batteries. Energy Environ. Sci. 2017, 10, 1350–1357. [Google Scholar] [CrossRef]
- Deng, W.; Zhu, W.; Zhou, X.; Liu, Z. Graphene nested porous carbon current collector for lithium metal anode with ultrahigh areal capacity. Energy Stor. Mater. 2018, 15, 266–273. [Google Scholar] [CrossRef]
- Li, S.; Church, B.C. Electrochemical stability of aluminum current collector in aqueous rechargeable lithium-ion battery electrolytes. J. Appl. Electrochem. 2017, 47, 839–853. [Google Scholar] [CrossRef]
- Ma, T.; Xu, G.-L.; Li, Y.; Wang, L.; He, X.; Zheng, J.; Liu, J.; Engelhard, M.H.; Zapol, P.; Curtiss, L.A.; et al. Revisiting the Corrosion of the Aluminum Current Collector in Lithium-Ion Batteries. J. Phys. Chem. Lett. 2017, 8, 1072–1077. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Yang, Y.; Lv, Y.; Ren, Y.; Li, W.; Li, C. Corrosion Protection of Copper Current Collector Of Lithium- Ion Batteries by Doped Polypyrrole Coatings. Int. J. Electrochem. Sci. 2020, 15, 2667–2676. [Google Scholar] [CrossRef]
- Du, J.; Liu, Y.; Mo, X.; Li, Y.; Li, J.; Wu, X.; Ouyang, M. Impact of high-power charging on the durability and safety of lithium batteries used in long-range battery electric vehicles. Appl. Energy 2019, 255, 113793. [Google Scholar] [CrossRef]
- Killer, M.; Farrokhseresht, M.; Paterakis, N.G. Implementation of large-scale Li-ion battery energy storage systems within the EMEA region. Appl. Energy 2020, 260, 114166. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Schreiber, M. Current Collectors for Positive Electrodes of Lithium-Based Batteries. J. Electrochem. Soc. 2005, 152, A2105. [Google Scholar] [CrossRef]
- Kanamura, K.; Toriyama, S.; Shiraishi, S.; Takehara, Z.i. Studies on Electrochemical Oxidation of Nonaqueous Electrolytes Using In Situ FTIR Spectroscopy: I. The Effect of Type of Electrode on On-Set Potential for Electrochemical Oxidation of Propylene Carbonate Containing 1.0 mol dm−3. J. Electrochem. Soc. 1995, 142, 1383. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Y.; Liu, Q. Electrochemical behavior and application of a silver electrode in a 1 M LiPF6 solution. J. Alloys Compd. 2018, 758, 1–4. [Google Scholar] [CrossRef]
- Gu, Y.; Federici, J.F. Fabrication of a Flexible Current Collector for Lithium Ion Batteries by Inkjet Printing. Batteries 2018, 4, 42. [Google Scholar] [CrossRef]
- Fredriksson, W.; Edström, K. XPS study of duplex stainless steel as a possible current collector in a Li-ion battery. Electrochim. Acta 2012, 79, 82–94. [Google Scholar] [CrossRef]
- Shironita, S.; Ihsan, N.; Konakawa, K.; Souma, K.; Umeda, M. Investigation of nitriding treated Ni-free stainless steel as current collector for 5 V-class Li-ion secondary cell. Electrochim. Acta 2019, 295, 1052–1056. [Google Scholar] [CrossRef]
- Myung, S.-T.; Sasaki, Y.; Sakurada, S.; Sun, Y.-K.; Yashiro, H. Electrochemical behavior of current collectors for lithium batteries in non-aqueous alkyl carbonate solution and surface analysis by ToF-SIMS. Electrochim. Acta 2009, 55, 288–297. [Google Scholar] [CrossRef]
- Gao, T.; Qu, Q.; Zhu, G.; Shi, Q.; Qian, F.; Shao, J.; Zheng, H. A self-supported carbon nanofiber paper/sulfur anode with high-capacity and high-power for application in Li-ion batteries. Carbon 2016, 110, 249–256. [Google Scholar] [CrossRef]
- Myung, S.-T.; Sasaki, Y.; Saito, T.; Sun, Y.-K.; Yashiro, H. Passivation behavior of Type 304 stainless steel in a non-aqueous alkyl carbonate solution containing LiPF6 salt. Electrochim. Acta 2009, 54, 5804–5812. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Doberdò, I.; Löffler, N.; Laszczynski, N.; Cericola, D.; Penazzi, N.; Bodoardo, S.; Kim, G.-T.; Passerini, S. Enabling aqueous binders for lithium battery cathodes-Carbon coating of aluminum current collector. J. Power Sources 2014, 248, 1000–1006. [Google Scholar] [CrossRef]
- Lu, L.-L.; Zhang, Y.; Pan, Z.; Yao, H.-B.; Zhou, F.; Yu, S.-H. Lithiophilic Cu–Ni core–shell nanowire network as a stable host for improving lithium anode performance. Energy Stor. Mater. 2017, 9, 31–38. [Google Scholar] [CrossRef]
- Hou, Z.; Yu, Y.; Wang, W.; Zhao, X.; Di, Q.; Chen, Q.; Chen, W.; Liu, Y.; Quan, Z. Lithiophilic Ag Nanoparticle Layer on Cu Current Collector toward Stable Li Metal Anode. ACS Appl. Mater. Interfaces 2019, 11, 8148–8154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, W.; Zhou, G.; Huang, Z.; Zhang, Y.; Lyu, R.; Wu, H.; Yun, Q.; Kang, F.; Yang, Q.-H. Vertically Aligned Lithiophilic CuO Nanosheets on a Cu Collector to Stabilize Lithium Deposition for Lithium Metal Batteries. Adv. Energy Mater. 2018, 8, 1703404. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.I.; Wee, J.-H.; Kim, C.H.; Ahn, B.W.; Lee, J.W.; Shu, S.J.; Terrones, M.; Kim, Y.A.; Yang, C.-M. Few-layer graphene coated current collectors for safe and powerful lithium ion batteries. Carbon 2019, 153, 495–503. [Google Scholar] [CrossRef]
- Jiang, J.; Nie, P.; Ding, B.; Wu, W.; Chang, Z.; Wu, Y.; Dou, H.; Zhang, X. Effect of Graphene Modified Cu Current Collector on the Performance of Li4Ti5O12 Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 30926–30932. [Google Scholar] [CrossRef]
- Baggetto, L.; Verhaegh, N.A.M.; Niessen, R.A.H.; Roozeboom, F.; Jumas, J.-C.; Notten, P.H.L. Tin Nitride Thin Films as Negative Electrode Material for Lithium-Ion Solid-State Batteries. J. Electrochem. Soc. 2010, 157, A340. [Google Scholar] [CrossRef]
- Cho, G.-b.; Kim, B.-m.; Noh, J.-p.; Im, Y.-m.; Lee, S.-h.; Kim, K.-w.; Nam, T.-h. Electrochemical properties of Si film electrodes with TiNi shape memory alloy as a current collector. J. Alloys Compd. 2013, 577, S190–S194. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, K.; Zhu, S.; Luo, W.; Wang, Y.; Li, Y.; Hitz, E.; Yao, Y.; Dai, J.; Wan, J.; et al. Reduced Graphene Oxide Films with Ultrahigh Conductivity as Li-Ion Battery Current Collectors. Nano Lett. 2016, 16, 3616–3623. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, J.; Zhao, Y.; Song, R.; Wang, Z.; Huang, Z.; Shi, M.; Ye, Y.; He, D.; Mu, S. Lifting the energy density of lithium ion batteries using graphite film current collectors. J. Power Sources 2020, 455, 227991. [Google Scholar] [CrossRef]
- Choi, B.N.; Seo, J.Y.; Kim, B.; Kim, Y.S.; Chung, C.-H. Electro-deposition of the lithium metal anode on dendritic copper current collectors for lithium battery application. Appl. Surf. Sci. 2020, 506, 144884. [Google Scholar] [CrossRef]
- Cao, L.; Li, L.; Xue, Z.; Yang, W.; Zou, H.; Chen, S.; Liu, Z. The aluminum current collector with honeycomb-like surface and thick Al2O3 film increased durability and enhanced safety for lithium-ion batteries. J. Porous Mater. 2020, 27, 1677–1683. [Google Scholar] [CrossRef]
- Yuan, W.; Qiu, Z.; Huang, Y.; Wang, C.; Tang, Y. Honeycomb-Inspired Surface-Patterned Cu@CuO Composite Current Collector for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1900445. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Stor. Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Zhang, X.; Zhu, Y. Strategies to Improve the Performance of Li Metal Anode for Rechargeable Batteries. Front. Chem. 2020, 8, 409. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Shiga, T.; Kato, Y.; Kondo, H.; Okuda, C.-a. Self-extinguishing electrolytes using fluorinated alkyl phosphates for lithium batteries. J. Mater. Chem. A 2017, 5, 5156–5162. [Google Scholar] [CrossRef]
- Ates, T.; Keller, M.; Kulisch, J.; Adermann, T.; Passerini, S. Development of an all-solid-state lithium battery by slurry-coating procedures using a sulfidic electrolyte. Energy Stor. Mater. 2019, 17, 204–210. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-Generation Lithium Metal Anode Engineering via Atomic Layer Deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef]
- Matsuda, S.; Kubo, Y.; Uosaki, K.; Nakanishi, S. Insulative Microfiber 3D Matrix as a Host Material Minimizing Volume Change of the Anode of Li Metal Batteries. ACS Energy Lett. 2017, 2, 924–929. [Google Scholar] [CrossRef]
- Bordes, A.; De Vito, E.; Haon, C.; Secouard, C.; Montani, A.; Marcus, P. Investigation of Lithium Insertion Mechanisms of a Thin-Film Si Electrode by Coupling Time-of-Flight Secondary-Ion Mass Spectrometry, X-ray Photoelectron Spectroscopy, and Focused-Ion-Beam/SEM. ACS Appl. Mater. Interfaces 2015, 7, 27853–27862. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.L.; Morgan, B.J.; Passerini, S.; Teobaldi, G. Density functional theory screening of gas-treatment strategies for stabilization of high energy-density lithium metal anodes. J. Power Sources 2015, 296, 150–161. [Google Scholar] [CrossRef]
- Li, N.-W.; Yin, Y.-X.; Yang, C.-P.; Guo, Y.-G. An Artificial Solid Electrolyte Interphase Layer for Stable Lithium Metal Anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef]
- Zhang, Y.-j.; Bai, W.-q.; Wang, X.-l.; Xia, X.-h.; Gu, C.-d.; Tu, J.-p. In situ confocal microscopic observation on inhibiting the dendrite formation of a-CNx/Li electrode. J. Mater. Chem. A 2016, 4, 15597–15604. [Google Scholar] [CrossRef]
- Yan, K.; Lee, H.-W.; Gao, T.; Zheng, G.; Yao, H.; Wang, H.; Lu, Z.; Zhou, Y.; Liang, Z.; Liu, Z.; et al. Ultrathin Two-Dimensional Atomic Crystals as Stable Interfacial Layer for Improvement of Lithium Metal Anode. Nano Lett. 2014, 14, 6016–6022. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961–969. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Cheng, X.-B.; Zhang, Q. Advances in Interfaces between Li Metal Anode and Electrolyte. Adv. Mater. Interfaces 2018, 5, 1701097. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Gong, Y.; Fu, Z.; Xie, H.; Yao, Y.; Liu, B.; Carter, M.; Wachsman, E.; Hu, L. Transient Behavior of the Metal Interface in Lithium Metal–Garnet Batteries. Angew. Chem. Int. Ed. 2017, 56, 14942–14947. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Gong, Y.; Liu, B.; Zhu, Y.; Xu, S.; Yao, Y.; Luo, W.; Wang, C.; Lacey, S.D.; Dai, J.; et al. Toward garnet electrolyte-based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Li, B.; Song, H.; Cheng, Z.; Chen, M.; He, P.; Zhou, H. Germanium Thin Film Protected Lithium Aluminum Germanium Phosphate for Solid-State Li Batteries. Adv. Energy Mater. 2018, 8, 1702374. [Google Scholar] [CrossRef]
- He, M.; Cui, Z.; Chen, C.; Li, Y.; Guo, X. Formation of self-limited, stable and conductive interfaces between garnet electrolytes and lithium anodes for reversible lithium cycling in solid-state batteries. J. Mater. Chem. A 2018, 6, 11463–11470. [Google Scholar] [CrossRef]
- Feng, W.; Dong, X.; Li, P.; Wang, Y.; Xia, Y. Interfacial modification of Li/Garnet electrolyte by a lithiophilic and breathing interlayer. J. Power Sources 2019, 419, 91–98. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Roddatis, V.; Chandran, C.V.; Ma, Q.; Uhlenbruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Moon, T.; Kim, C.; Park, B. Electrochemical performance of amorphous-silicon thin films for lithium rechargeable batteries. J. Power Sources 2006, 155, 391–394. [Google Scholar] [CrossRef]
- Omampuliyur, R.S.; Bhuiyan, M.; Han, Z.; Jing, Z.; Li, L.; Fitzgerald, E.A.; Thompson, C.V.; Choi, W.K. Nanostructured Thin Film Silicon Anodes for Li-Ion Microbatteries. J. Nanosci. Nanotechnol. 2015, 15, 4926–4933. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Baggetto, L.; Niessen, R.A.H.; Knoops, H.C.M.; Donders, M.E.; van Dongen, T.; de Croon, M.H.J.M.; Kessels, W.M.M.; Notten, P.H.L. Towards High Energy Density 3D-integrated Lithium-ion Micro-batteries. MRS Online Proc. Libr. 2011, 1168, 1. [Google Scholar] [CrossRef]
- Ohara, S.; Suzuki, J.; Sekine, K.; Takamura, T. A thin film silicon anode for Li-ion batteries having a very large specific capacity and long cycle life. J. Power Sources 2004, 136, 303–306. [Google Scholar] [CrossRef]
- Deng, H.; Park, M.; Chung, C.Y.; Park, C.-H.; Joo, S.-K. Effect of Substrate Surface Conditions on Electrochemical Performance of Si Thin Film Anode. ECS Trans. 2007, 2, 105. [Google Scholar] [CrossRef]
- Takamura, T.; Ohara, S.; Uehara, M.; Suzuki, J.; Sekine, K. A vacuum deposited Si film having a Li extraction capacity over 2000 mAh/g with a long cycle life. J. Power Sources 2004, 129, 96–100. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.P.; Yang, L.C.; Wang, B.; Wu, Y.P.; Takamura, T. The structural evolution and lithiation behavior of vacuum-deposited Si film with high reversible capacity. Electrochim. Acta 2008, 53, 5660–5664. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Cho, J. Challenges in Accommodating Volume Change of Si Anodes for Li-Ion Batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar] [CrossRef]
- Cho, G.-b.; Noh, J.-p.; Sung, H.-j.; Lee, S.-h.; Im, Y.-m.; Ahn, H.-j.; Kim, K.-w. Patterned Si thin film electrodes for enhancing structural stability. Nanoscale Res. Lett. 2012, 7, 20. [Google Scholar] [CrossRef]
- Baggetto, L.; Danilov, D.; Notten, P.H.L. Honeycomb-Structured Silicon: Remarkable Morphological Changes Induced by Electrochemical (De)Lithiation. Adv. Mater. 2011, 23, 1563–1566. [Google Scholar] [CrossRef]
- Polat, B.D.; Eryilmaz, O.L.; Keleş, O.; Erdemir, A.; Amine, K. Compositionally graded SiCu thin film anode by magnetron sputtering for lithium ion battery. Thin Solid Film. 2015, 596, 190–197. [Google Scholar] [CrossRef]
- Hwang, C.-M.; Lim, C.-H.; Yang, J.-H.; Park, J.-W. Electrochemical properties of negative SiMox electrodes deposited on a roughened substrate for rechargeable lithium batteries. J. Power Sources 2009, 194, 1061–1067. [Google Scholar] [CrossRef]
- Kim, J.-B.; Jun, B.-S.; Lee, S.-M. Improvement of capacity and cyclability of Fe/Si multilayer thin film anodes for lithium rechargeable batteries. Electrochim. Acta 2005, 50, 3390–3394. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Wu, Z.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. 3D nanostructured multilayer Si/Al film with excellent cycle performance as anode material for lithium-ion battery. J. Alloys Compd. 2016, 657, 559–564. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, M.; Du, Z.; Obrovac, M.N. The Electrochemistry of Amorphous Si-B Thin Film Electrodes in Li Cells. J. Electrochem. Soc. 2016, 163, A192. [Google Scholar] [CrossRef]
- Li, H.; Bai, H.; Tao, Z.; Chen, J. Si–Y multi-layer thin films as anode materials of high-capacity lithium-ion batteries. J. Power Sources 2012, 217, 102–107. [Google Scholar] [CrossRef]
- Wang, Y.H.; He, Y.; Xiao, R.J.; Li, H.; Aifantis, K.E.; Huang, X.J. Investigation of crack patterns and cyclic performance of Ti–Si nanocomposite thin film anodes for lithium ion batteries. J. Power Sources 2012, 202, 236–245. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Chang, C.-C.; Duh, J.-G. Silicon nitride coated silicon thin film on three dimensions current collector for lithium ion battery anode. J. Power Sources 2016, 325, 64–70. [Google Scholar] [CrossRef]
- Lin, J.; Guo, J.; Liu, C.; Guo, H. Artificial solid electrolyte interphase with in-situ formed porosity for enhancing lithiation of silicon wafer. J. Power Sources 2016, 336, 401–407. [Google Scholar] [CrossRef]
- Jiao, M.; Wang, Y.; Ye, C.; Wang, C.; Zhang, W.; Liang, C. High-capacity SiOx (0≤x≤2) as promising anode materials for next-generation lithium-ion batteries. J. Alloys Compd. 2020, 842, 155774. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.; Han, S.A.; Kim, J.; Malgras, V.; Heo, Y.-U.; Kim, H.; Lee, S.-M.; Liu, H.K.; Dou, S.X.; et al. Everlasting Living and Breathing Gyroid 3D Network in Si@SiOx/C Nanoarchitecture for Lithium Ion Battery. ACS Nano 2019, 13, 9607–9619. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Xu, C.; Zhu, J.; Wei, X.; Zhou, L.; He, L.; Yang, W.; Mai, L. Ultrafine Nickel-Nanoparticle-Enabled SiO2 Hierarchical Hollow Spheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2018, 28, 1704561. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Li, N.; Patrissi, C.J.; Che, G.; Martin, C.R. Rate Capabilities of Nanostructured LiMn2O4 Electrodes in Aqueous Electrolyte. J. Electrochem. Soc. 2000, 147, 2044. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on Advanced Materials for Li-ion Batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Cunha, D.M.; Hendriks, T.A.; Vasileiadis, A.; Vos, C.M.; Verhallen, T.; Singh, D.P.; Wagemaker, M.; Huijben, M. Doubling Reversible Capacities in Epitaxial Li4Ti5O12 Thin Film Anodes for Microbatteries. ACS Appl. Energy Mater. 2019, 2, 3410–3418. [Google Scholar] [CrossRef]
- Daramalla, V.; Penki, T.R.; Munichandraiah, N.; Krupanidhi, S.B. Fabrication of TiNb2O7 thin film electrodes for Li-ion micro-batteries by pulsed laser deposition. Mater. Sci. Eng. B 2016, 213, 90–97. [Google Scholar] [CrossRef]
- Daramalla, V.; Venkatesh, G.; Kishore, B.; Munichandraiah, N.; Krupanidhi, S.B. Superior Electrochemical Performance of Amorphous Titanium Niobium Oxide Thin Films for Li-Ion Thin Film Batteries. J. Electrochem. Soc. 2018, 165, A764. [Google Scholar] [CrossRef]
- Rambabu, A.; Senthilkumar, B.; Dayamani, A.; Krupanidhi, S.B.; Barpanda, P. Preferentially oriented SrLi2Ti6O14 thin film anode for Li-ion micro-batteries fabricated by pulsed laser deposition. Electrochim. Acta 2018, 269, 212–216. [Google Scholar] [CrossRef]
- Maximov, M.Y.; Novikov, P.A.; Nazarov, D.V.; Rymyantsev, A.M.; Silin, A.O.; Zhang, Y.; Popovich, A.A. Characterization and Electrochemical Performance at High Discharge Rates of Tin Dioxide Thin Films Synthesized by Atomic Layer Deposition. J. Electron. Mater. 2017, 46, 6571–6577. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, H.-Y.; Ha, T.-S.; Baik, H.-K.; Lee, S.-M. Amorphous Lithium Nickel Vanadate Thin-Film Anodes for Rechargeable Lithium Microbatteries. Electrochem. Solid-State Lett. 2002, 5, A138. [Google Scholar] [CrossRef]
- Wei, K.; Zhao, Y.; Cui, Y.; Wang, J.; Cui, Y.; Zhu, R.; Zhuang, Q.; Xue, M. Lithium phosphorous oxynitride (LiPON) coated NiFe2O4 anode material with enhanced electrochemical performance for lithium ion batteries. J. Alloys Compd. 2018, 769, 110–119. [Google Scholar] [CrossRef]
- Khamlich, S.; Nuru, Z.Y.; Bello, A.; Fabiane, M.; Dangbegnon, J.K.; Manyala, N.; Maaza, M. Pulsed laser deposited Cr2O3 nanostructured thin film on graphene as anode material for lithium-ion batteries. J. Alloys Compd. 2015, 637, 219–225. [Google Scholar] [CrossRef]
- Teng, X.; Qin, Y.; Wang, X.; Li, H.; Shang, X.; Fan, S.; Li, Q.; Xu, J.; Cao, D.; Li, S. A Nanocrystalline Fe2O3 Film Anode Prepared by Pulsed Laser Deposition for Lithium-Ion Batteries. Nanoscale Res. Lett. 2018, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Lim, S.; Cheung, J.; Sharma, N. Mechanistic insights into the phenomena of increasing capacity with cycle number: Using pulsed-laser deposited MoO2 thin film electrodes. Phys. Chem. Chem. Phys. 2019, 21, 25779–25787. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhao, Y.; Guo, Q.; Yang, S.; Ding, G.; Mei, Y.; Huang, G. Cycling-Induced Capacity Increase of Graphene Aerogel/ZnO Nanomembrane Composite Anode Fabricated by Atomic Layer Deposition. Nanoscale Res. Lett. 2019, 14, 69. [Google Scholar] [CrossRef]
- Dhara, A.; Sarkar, S.K.; Mitra, S. Controlled 3D Carbon Nanotube Architecture Coated with MoOx Material by ALD Technique: A High Energy Density Lithium-Ion Battery Electrode. Adv. Mater. Interfaces 2017, 4, 1700332. [Google Scholar] [CrossRef]
- Gregorczyk, K.E.; Kozen, A.C.; Chen, X.; Schroeder, M.A.; Noked, M.; Cao, A.; Hu, L.; Rubloff, G.W. Fabrication of 3D Core–Shell Multiwalled Carbon Nanotube@RuO2 Lithium-Ion Battery Electrodes through a RuO2 Atomic Layer Deposition Process. ACS Nano 2015, 9, 464–473. [Google Scholar] [CrossRef]
- Sinha, S.; Ramasamy, H.V.; Nandi, D.K.; Didwal, P.N.; Cho, J.Y.; Park, C.-J.; Lee, Y.-S.; Kim, S.-H.; Heo, J. Atomic layer deposited zinc oxysulfide anodes in Li-ion batteries: An efficient solution for electrochemical instability and low conductivity. J. Mater. Chem. A 2018, 6, 16515–16528. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Xue, M.; Wu, C.; Wu, X.; Fu, Z. The electrochemistry of nanostructured In2O3 with lithium. J. Power Sources 2006, 162, 1373–1378. [Google Scholar] [CrossRef]
- Takehara, Z.i.; Ogumi, Z.; Uchimoto, Y.; Endo, E.; Kanamori, Y. Thin Film Solid-State Lithium Batteries Prepared by Consecutive Vapor-Phase Processes. J. Electrochem. Soc. 1991, 138, 1574. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Sakurai, Y. Characteristics of Li/MoO3−x thin film batteries. Solid State Ion. 2001, 144, 59–64. [Google Scholar] [CrossRef]
- Baba, M.; Kumagai, N.; Fujita, N.; Ohta, K.; Nishidate, K.; Komaba, S.; Groult, H.; Devilliers, D.; Kaplan, B. Fabrication and electrochemical characteristics of all-solid-state lithium-ion rechargeable batteries composed of LiMn2O4 positive and V2O5 negative electrodes. J. Power Sources 2001, 97–98, 798–800. [Google Scholar] [CrossRef]
- Chiu, K.F. Lithium cobalt oxide thin films deposited at low temperature by ionized magnetron sputtering. Thin Solid Films 2007, 515, 4614–4618. [Google Scholar] [CrossRef]
- Iriyama, Y.; Nishimoto, K.; Yada, C.; Abe, T.; Ogumi, Z.; Kikuchi, K. Charge-Transfer Reaction at the Lithium Phosphorus Oxynitride Glass Electrolyte/Lithium Manganese Oxide Thin-Film Interface and Its Stability on Cycling. J. Electrochem. Soc. 2006, 153, A821. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Dudney, N.J.; Lance, M.J. Characterization and Performance of LiFePO4 Thin-Film Cathodes Prepared with Radio-Frequency Magnetron-Sputter Deposition. J. Electrochem. Soc. 2007, 154, A805. [Google Scholar] [CrossRef]
- Shobana, M.K. Metal oxide coated cathode materials for Li ion batteries—A review. J. Alloys Compd. 2019, 802, 477–487. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Review of 5-V electrodes for Li-ion batteries: Status and trends. Ionics 2013, 19, 951–988. [Google Scholar] [CrossRef]
- Yang, X.; Yang, T.; Liang, S.; Wu, X.; Zhang, H. Modification of LiNi0.5Mn1.5O4 high potential cathode from the inner lattice to the outer surface with Cr3+-doping and Li+-conductor coating. J. Mater. Chem. A 2014, 2, 10359–10364. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kang, K. Surface-Modified Spinel LiNi0.5Mn1.5O4 for Li-Ion Batteries. J. Korean Ceram. Soc 2018, 55, 21–35. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Q.; Yang, G.; Yang, Z.; Zhang, L.; Long, H.; Huang, Y.; Lu, P. High-stability 5V spinel LiNi0.5Mn1.5O4 sputtered thin film electrodes by modifying with aluminium oxide. Electrochim. Acta 2014, 136, 450–456. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, B.; Wang, Z.; Lu, C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sources 2014, 256, 20–27. [Google Scholar] [CrossRef]
- Sun, Y.K.; Lee, Y.S.; Yoshio, M.; Amine, K. Synthesis and Electrochemical Properties of ZnO-Coated LiNi0.5Mn1.5O4 Spinel as 5 V Cathode Material for Lithium Secondary Batteries. Electrochem. Solid-State Lett. 2002, 5, A99. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Sun, S.; Wan, N.; Pan, D.; Bai, Y.; Zhu, H.; Hu, Y.-S.; Dai, S. Improved electrochemical performance of spinel LiMn1.5Ni0.5O4 through MgF2 nano-coating. Nanoscale 2015, 7, 15609–15617. [Google Scholar] [CrossRef]
- Park, B.C.; Kim, H.B.; Myung, S.T.; Amine, K.; Belharouak, I.; Lee, S.M.; Sun, Y.K. Improvement of structural and electrochemical properties of AlF3-coated Li[Ni1/3Co1/3Mn1/3]O2 cathode materials on high voltage region. J. Power Sources 2008, 178, 826–831. [Google Scholar] [CrossRef]
- Lu, C.; Wu, H.; Zhang, Y.; Liu, H.; Chen, B.; Wu, N.; Wang, S. Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J. Power Sources 2014, 267, 682–691. [Google Scholar] [CrossRef]
- Konishi, H.; Suzuki, K.; Taminato, S.; Kim, K.; Zheng, Y.; Kim, S.; Lim, J.; Hirayama, M.; Son, J.-Y.; Cui, Y.; et al. Effect of surface Li3PO4 coating on LiNi0.5Mn1.5O4 epitaxial thin film electrodes synthesized by pulsed laser deposition. J. Power Sources 2014, 269, 293–298. [Google Scholar] [CrossRef]
- Jang, W.H.; Kim, M.C.; Lee, S.N.; Ahn, J.Y.; Aravindan, V.; Lee, Y.S. Enhanced elevated temperature performance of LiFePO4 modified spinel LiNi0.5Mn1.5O4 cathode. J. Alloys Compd. 2014, 612, 51–55. [Google Scholar] [CrossRef]
- Deng, Y.; Mou, J.; Wu, H.; Jiang, N.; Zheng, Q.; Lam, K.H.; Xu, C.; Lin, D. A superior Li2SiO3-Composited LiNi0.5Mn1.5O4 Cathode for High-Voltage and High-Performance Lithium-ion Batteries. Electrochim. Acta 2017, 235, 19–31. [Google Scholar] [CrossRef]
- Lv, S.; Li, M.; Luo, X.; Cheng, J.; Li, Z. High-voltage LiNi0.5Mn1.5O4 thin film cathodes stabilized by LiPON solid electrolyte coating to enhance cyclic stability and rate capability. J. Alloys Compd. 2020, 815, 151636. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, N.; Lang, Y.; Sun, K. Enhanced rate performance of carbon-coated LiNi0.5Mn1.5O4 cathode material for lithium ion batteries. Electrochim. Acta 2011, 56, 4058–4064. [Google Scholar] [CrossRef]
- Fang, X.; Ge, M.; Rong, J.; Zhou, C. Graphene-oxide-coated LiNi0.5Mn1.5O4 as high voltage cathode for lithium ion batteries with high energy density and long cycle life. J. Mater. Chem. A 2013, 1, 4083–4088. [Google Scholar] [CrossRef]
- Yi, T.-F.; Xie, Y.; Ye, M.-F.; Jiang, L.-J.; Zhu, R.-S.; Zhu, Y.-R. Recent developments in the doping of LiNi0.5Mn1.5O4 cathode material for 5 V lithium-ion batteries. Ionics 2011, 17, 383–389. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, T.; Zhang, Y.; Yan, L.; Mao, S.S.; Xie, J. Surface-segregated, high-voltage spinel lithium-ion battery cathode material LiNi0.5Mn1.5O4 cathodes by aluminium doping with improved high-rate cyclability. J. Alloys Compd. 2017, 703, 289–297. [Google Scholar] [CrossRef]
- Aklalouch, M.; Amarilla, J.M.; Rojas, R.M.; Saadoune, I.; Rojo, J.M. Chromium doping as a new approach to improve the cycling performance at high temperature of 5V LiNi0.5Mn1.5O4-based positive electrode. J. Power Sources 2008, 185, 501–511. [Google Scholar] [CrossRef]
- Liu, J.; Manthiram, A. Understanding the Improvement in the Electrochemical Properties of Surface Modified 5 V LiMn1.42Ni0.42Co0.16O4 Spinel Cathodes in Lithium-ion Cells. Chem. Mater. 2009, 21, 1695–1707. [Google Scholar] [CrossRef]
- Wang, H.; Xia, H.; Lai, M.O.; Lu, L. Enhancements of rate capability and cyclic performance of spinel LiNi0.5Mn1.5O4 by trace Ru-doping. Electrochem. Commun. 2009, 11, 1539–1542. [Google Scholar] [CrossRef]
- Xu, X.X.; Yang, J.; Wang, Y.Q.; NuLi, Y.N.; Wang, J.L. LiNi0.5Mn1.5O3.975F0.05 as novel 5V cathode material. J. Power Sources 2007, 174, 1113–1116. [Google Scholar] [CrossRef]
- Amine, K.; Yasuda, H.; Yamachi, M. Olivine LiCoPO4 as 4.8 V Electrode Material for Lithium Batteries. Electrochem. Solid-State Lett. 2000, 3, 178. [Google Scholar] [CrossRef]
- Zhang, M.; Garcia-Araez, N.; Hector, A.L. Understanding and development of olivine LiCoPO4 cathode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14483–14517. [Google Scholar] [CrossRef]
- Ruzgas, T.; Larpant, N.; Shafaat, A.; Sotres, J. Cover Feature: Wireless, Battery-Less Biosensors Based on Direct Electron Transfer Reactions. ChemElectroChem 2019, 6, 5165. [Google Scholar] [CrossRef]
- Wu, B.; Xu, H.; Mu, D.; Shi, L.; Jiang, B.; Gai, L.; Wang, L.; Liu, Q.; Ben, L.; Wu, F. Controlled solvothermal synthesis and electrochemical performance of LiCoPO4 submicron single crystals as a cathode material for lithium ion batteries. J. Power Sources 2016, 304, 181–188. [Google Scholar] [CrossRef]
- Laszczynski, N.; Birrozzi, A.; Maranski, K.; Copley, M.; Schuster, M.E.; Passerini, S. Effect of coatings on the green electrode processing and cycling behaviour of LiCoPO4. J. Mater. Chem. A 2016, 4, 17121–17128. [Google Scholar] [CrossRef]
- Konishi, S.; Murayama, D.; Itadani, A.; Uematsu, K.; Toda, K.; Sato, M.; Arimitsu, N.; Aoki, T.; Yamaguchi, T. Improvement in Electrochemical Performance of LiCoPO4 Using Furnace Blacks with High Surface Areas as a Carbon-based Composite Material. Electrochemistry 2017, 85, 643–646. [Google Scholar] [CrossRef]
- Örnek, A. An impressive approach to solving the ongoing stability problems of LiCoPO4 cathode: Nickel oxide surface modification with excellent core–shell principle. J. Power Sources 2017, 356, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Yu, Z.; Ming, H.; Li, M.; Zhang, S.; Yang, Y. AlF3-modified LiCoPO4 for an advanced cathode towards high energy lithium-ion battery. Ceram. Int. 2018, 44, 1312–1320. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Sergey, C.; Lee, H.; Yoon, J.; Kang, Y.-M.; Yoon, W.-S. Advances in the Cathode Materials for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2020, 59, 2578–2605. [Google Scholar] [CrossRef]
- Molenda, J. Electronic limitations of lithium diffusibility. From layered and spinel toward novel olivine type cathode materials. Solid State Ion. 2005, 176, 1687–1694. [Google Scholar] [CrossRef]
- Yu, F.D.; Que, L.F.; Wang, Z.B.; Zhang, Y.; Xue, Y.; Liu, B.S.; Gu, D.M. Layered-spinel capped nanotube assembled 3D Li-rich hierarchitectures for high performance Li-ion battery cathodes. J. Mater. Chem. A 2016, 4, 18416–18425. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Sun, Y.; Feng, X.; Chen, C. Three-dimensional porous V2O5 cathode with ultra high rate capability. Energy Environ. Sci. 2011, 4, 2854–2857. [Google Scholar] [CrossRef]
- Pearse, A.; Schmitt, T.; Sahadeo, E.; Stewart, D.M.; Kozen, A.; Gerasopoulos, K.; Talin, A.A.; Lee, S.B.; Rubloff, G.W.; Gregorczyk, K.E. Three-Dimensional Solid-State Lithium-Ion Batteries Fabricated by Conformal Vapor-Phase Chemistry. ACS Nano 2018, 12, 4286–4294. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wan, Y.; Assenmacher, W.; Mader, W.; Yuan, G.; Lu, L. Facile synthesis of chain-like LiCoO2 nanowire arrays as three-dimensional cathode for microbatteries. NPG Asia Mater. 2014, 6, e126. [Google Scholar] [CrossRef]
- Maximov, M.; Nazarov, D.; Rumyantsev, A.; Koshtyal, Y.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Medvedev, O.; Popovich, A. Atomic Layer Deposition of Lithium–Nickel–Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries. Energies 2020, 13, 2345. [Google Scholar] [CrossRef]
- Wang, G.X.; Lindsay, M.J.; Ionescu, M.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. Physical and electrochemical characterization of LiNi0.8Co0.2O2 thin-film electrodes deposited by laser ablation. J. Power Sources 2001, 97–98, 298–302. [Google Scholar] [CrossRef]
- Qi, Z.; Tang, J.; Huang, J.; Zemlyanov, D.; Pol, V.G.; Wang, H. Li2MnO3 Thin Films with Tilted Domain Structure as Cathode for Li-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3461–3468. [Google Scholar] [CrossRef]
- Ramana, C.V.; Zaghib, K.; Julien, C.M. Pulsed-laser deposited LiNi0.8Co0.15Al0.05O2 thin films for application in microbatteries. Appl. Phys. Lett. 2007, 90, 021916. [Google Scholar] [CrossRef]
- Deng, J.; Xi, L.; Wang, L.; Wang, Z.; Chung, C.Y.; Han, X.; Zhou, H. Electrochemical performance of LiNi1/3Co1/3Mn1/3O2 thin film electrodes prepared by pulsed laser deposition. J. Power Sources 2012, 217, 491–497. [Google Scholar] [CrossRef]
- Jacob, C.; Lynch, T.; Chen, A.; Jian, J.; Wang, H. Highly textured Li(Ni0.5Mn0.3Co0.2)O2 thin films on stainless steel as cathode for lithium-ion battery. J. Power Sources 2013, 241, 410–414. [Google Scholar] [CrossRef]
- Phillip, N.D.; Westover, A.S.; Daniel, C.; Veith, G.M. Structural Degradation of High Voltage Lithium Nickel Manganese Cobalt Oxide (NMC) Cathodes in Solid-State Batteries and Implications for Next Generation Energy Storage. ACS Appl. Energy Mater. 2020, 3, 1768–1774. [Google Scholar] [CrossRef]
- Yan, B.; Liu, J.; Song, B.; Xiao, P.; Lu, L. Li-rich Thin Film Cathode Prepared by Pulsed Laser Deposition. Sci. Rep. 2013, 3, 3332. [Google Scholar] [CrossRef]
- Fehse, M.; Trócoli, R.; Ventosa, E.; Hernández, E.; Sepúlveda, A.; Morata, A.; Tarancón, A. Ultrafast Dischargeable LiMn2O4 Thin-Film Electrodes with Pseudocapacitive Properties for Microbatteries. ACS Appl. Mater. Interfaces 2017, 9, 5295–5301. [Google Scholar] [CrossRef]
- Lim, J.; Lee, S.; Suzuki, K.; Kim, K.; Kim, S.; Taminato, S.; Hirayama, M.; Oshima, Y.; Takayanagi, K.; Kanno, R. Synthesis, structure and electrochemical properties of novel Li–Co–Mn–O epitaxial thin-film electrode using layer-by-layer deposition process. J. Power Sources 2015, 279, 502–509. [Google Scholar] [CrossRef]
- Kuwata, N.; Kudo, S.; Matsuda, Y.; Kawamura, J. Fabrication of thin-film lithium batteries with 5-V-class LiCoMnO4 cathodes. Solid State Ion. 2014, 262, 165–169. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Yang, G.; Peng, Q.; Lu, P.X. Excellent Electrochemical Performance and Thermal Stability of LiNi0.5Mn1.5O4 Thin-Film Cathode Prepared by Pulsed Laser Deposition. Adv. Mater. Res. 2014, 853, 83–89. [Google Scholar] [CrossRef]
- Fujimoto, D.; Kuwata, N.; Matsuda, Y.; Kawamura, J.; Kang, F. Fabrication of solid-state thin-film batteries using LiMnPO4 thin films deposited by pulsed laser deposition. Thin Solid Films 2015, 579, 81–88. [Google Scholar] [CrossRef][Green Version]
- Mosa, J.; Aparicio, M.; Durán, A.; Laberty-Robert, C.; Sanchez, C. Nanocrystalline mesoporous LiFePO4 thin-films as cathodes for Li-ion microbatteries. J. Mater. Chem. A 2014, 2, 3038–3046. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Rana, J.; Stan, M.; Kloepsch, R.; Li, J.; Schumacher, G.; Welter, E.; Zizak, I.; Banhart, J.; Winter, M. Structural Changes in Li2MnO3 Cathode Material for Li-Ion Batteries. Adv. Energy Mater. 2014, 4, 1300998. [Google Scholar] [CrossRef]
- Li, N.; Hwang, S.; Sun, M.; Fu, Y.; Battaglia, V.S.; Su, D.; Tong, W. Unraveling the Voltage Decay Phenomenon in Li-Rich Layered Oxide Cathode of No Oxygen Activity. Adv. Energy Mater. 2019, 9, 1902258. [Google Scholar] [CrossRef]
- Sathiya, M.; Abakumov, A.M.; Foix, D.; Rousse, G.; Ramesha, K.; Saubanère, M.; Doublet, M.L.; Vezin, H.; Laisa, C.P.; Prakash, A.S.; et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 2015, 14, 230–238. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, S.H.; Nahm, K.S.; Chung, H.T.; Lee, Y.S.; Lee, J.H.; Boo, S.; Kim, J. Structural and electrochemical study of Li[CrxLi(1−x)/3Mn2(1−x)/3]O2 (0≤x≤0.328) cathode materials. J. Alloys Compd. 2008, 449, 343–348. [Google Scholar] [CrossRef]
- Chen, R.; Ren, S.; Knapp, M.; Wang, D.; Witter, R.; Fichtner, M.; Hahn, H. Disordered Lithium-Rich Oxyfluoride as a Stable Host for Enhanced Li+ Intercalation Storage. Adv. Energy Mater. 2015, 5, 1401814. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yan, K.; Wan, S.; He, F.; Sun, B.; Wang, G. Towards high-energy-density lithium-ion batteries: Strategies for developing high-capacity lithium-rich cathode materials. Energy Stor. Mater. 2021, 34, 716–734. [Google Scholar] [CrossRef]
- Jin, X.; Xu, Q.; Liu, H.; Yuan, X.; Xia, Y. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim. Acta 2014, 136, 19–26. [Google Scholar] [CrossRef]
- Qing, R.-P.; Shi, J.-L.; Xiao, D.-D.; Zhang, X.-D.; Yin, Y.-X.; Zhai, Y.-B.; Gu, L.; Guo, Y.-G. Enhancing the Kinetics of Li-Rich Cathode Materials through the Pinning Effects of Gradient Surface Na+ Doping. Adv. Energy Mater. 2016, 6, 1501914. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Guo, H.; Li, X.; He, Z.; Li, T. Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. Ceram. Int. 2015, 41, 11396–11401. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Chen, H.; Huang, Z.; Li, X.; Guo, H.; Wang, R. Electrochemical performance of zirconium doped lithium rich layered Li1.2Mn0.54Ni0.13Co0.13O2 oxide with porous hollow structure. J. Power Sources 2015, 299, 334–341. [Google Scholar] [CrossRef]
- Laisa, C.P.; Ramesha, R.N.; Ramesha, K. Enhanced electrochemical performance of lithium rich layered cathode materials by Ca2+ substitution. Electrochim. Acta 2017, 256, 10–18. [Google Scholar] [CrossRef]
- Yu, R.; Wang, G.; Liu, M.; Zhang, X.; Wang, X.; Shu, H.; Yang, X.; Huang, W. Mitigating voltage and capacity fading of lithium-rich layered cathodes by lanthanum doping. J. Power Sources 2016, 335, 65–75. [Google Scholar] [CrossRef]
- Pan, L.; Xia, Y.; Qiu, B.; Zhao, H.; Guo, H.; Jia, K.; Gu, Q.; Liu, Z. Structure and electrochemistry of B doped Li(Li0.2Ni0.13Co0.13Mn0.54)1-xBxO2 as cathode materials for lithium-ion batteries. J. Power Sources 2016, 327, 273–280. [Google Scholar] [CrossRef]
- Kapylou, A.; Song, J.H.; Missiul, A.; Ham, D.J.; Kim, D.H.; Moon, S.; Park, J.H. Improved Thermal Stability of Lithium-Rich Layered Oxide by Fluorine Doping. ChemPhysChem 2018, 19, 116–122. [Google Scholar] [CrossRef]
- Li, B.; Yan, H.; Ma, J.; Yu, P.; Xia, D.; Huang, W.; Chu, W.; Wu, Z. Manipulating the Electronic Structure of Li-Rich Manganese-Based Oxide Using Polyanions: Towards Better Electrochemical Performance. Adv. Funct. Mater. 2014, 24, 5112–5118. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, B.; Yan, K.; Zhang, J.; Wang, C.; Wang, G. Aegis of Lithium-Rich Cathode Materials via Heterostructured LiAlF4 Coating for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 33260–33268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, X.; Yu, R.; Zhou, J.; Huang, Y.; Cao, S.; Wang, Y.; Tang, K.; Wu, C.; Wang, X. Improvement of the Cycling Stability of Li-Rich Layered Mn-Based Oxide Cathodes Modified by Nanoscale LaPO4 Coating. ACS Appl. Energy Mater. 2019, 2, 3532–3541. [Google Scholar] [CrossRef]
- Shi, S.J.; Tu, J.P.; Mai, Y.J.; Zhang, Y.Q.; Gu, C.D.; Wang, X.L. Effect of carbon coating on electrochemical performance of Li1.048Mn0.381Ni0.286Co0.286O2 cathode material for lithium-ion batteries. Electrochim. Acta 2012, 63, 112–117. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Sakurai, Y.; Yamaki, J.-i. Reversibility of LiNiO2 cathode. Solid State Ion. 1997, 95, 275–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, J.; Zhang, J.; Cheng, F.; Chen, J. LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries. J. Mater. Chem. A 2019, 7, 20958–20964. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.-L.; Deng, Y.-P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, C.; Wang, F.; Pan, F. Ni/Li Disordering in Layered Transition Metal Oxide: Electrochemical Impact, Origin, and Control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Hu, Z.; Cui, G.; Chen, L. Identifying and Addressing Critical Challenges of High-Voltage Layered Ternary Oxide Cathode Materials. Chem. Mater. 2019, 31, 6033–6065. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhang, Q.; Cheng, F.; Chen, J. Recent advances in Ni-rich layered oxide particle materials for lithium-ion batteries. Particuology 2020, 53, 1–11. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Guohui, Y. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Cao, X.; He, Y.; Wu, K.; Yang, J.; Zhou, H.; Liu, W.; Sun, X. Thiol-Branched Solid Polymer Electrolyte Featuring High Strength, Toughness, and Lithium Ionic Conductivity for Lithium-Metal Batteries. Adv. Mater. 2020, 32, 2001259. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Inseok, S.; Steve, W.M. New Developments in Solid Electrolytes for Thin-Film Lithium Batteries. In Lithium Ion Batteries; Ilias, B., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 5. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, X.; Zheng, C.; Liu, X.; Chen, Z.; Yang, W.; Lin, J.; Huang, F. Chemistry Design Towards a Stable Sulfide-Based Superionic Conductor Li4Cu8Ge3S12. Angew. Chem. Int. Ed. 2019, 58, 7673–7677. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwahara, H. Ionic conduction in perovskite-type oxide solid solution and its application to the solid electrolyte fuel cell. Energy Convers. 1971, 11, 105–111. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Huggins, R.A. Fast ionic conductivity in lithium nitride. Mater. Res. Bull. 1978, 13, 23–32. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium Lanthanum Titanates: A Review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Morales, M.; Laffez, P.; Chateigner, D.; Vickridge, I. Characterisation of lanthanum lithium titanate thin films deposited by radio frequency sputtering on [100]-oriented MgO substrates. Thin Solid Films 2002, 418, 119–128. [Google Scholar] [CrossRef]
- Ohta, H.; Mizoguchi, T.; Aoki, N.; Yamamoto, T.; Sabarudin, A.; Umemura, T. Lithium-ion conducting La2/3−xLi3xTiO3 solid electrolyte thin films with stepped and terraced surfaces. Appl. Phys. Lett. 2012, 100, 173107. [Google Scholar] [CrossRef]
- Furusawa, S.-i.; Tabuchi, H.; Sugiyama, T.; Tao, S.; Irvine, J.T.S. Ionic conductivity of amorphous lithium lanthanum titanate thin film. Solid State Ion. 2005, 176, 553–558. [Google Scholar] [CrossRef]
- Ahn, J.-K.; Yoon, S.-G. Characteristics of perovskite (Li0.5La0.5)TiO3 solid electrolyte thin films grown by pulsed laser deposition for rechargeable lithium microbattery. Electrochim. Acta 2004, 50, 371–374. [Google Scholar] [CrossRef]
- West, W.C.; Hood, Z.D.; Adhikari, S.P.; Liang, C.; Lachgar, A.; Motoyama, M.; Iriyama, Y. Reduction of charge-transfer resistance at the solid electrolyte—Electrode interface by pulsed laser deposition of films from a crystalline Li2PO2N source. J. Power Sources 2016, 312, 116–122. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Y.; Banis, M.N.; Sun, Q.; Adair, K.R.; Li, R.; Sham, T.-K.; Sun, X. Atomic Layer Deposition of Lithium Niobium Oxides as Potential Solid-State Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1654–1661. [Google Scholar] [CrossRef]
- Ohnishi, T.; Takada, K. Synthesis and orientation control of Li-ion conducting epitaxial Li0.33La0.56TiO3 solid electrolyte thin films by pulsed laser deposition. Solid State Ion. 2012, 228, 80–82. [Google Scholar] [CrossRef]
- Wei, J.; Ogawa, D.; Fukumura, T.; Hirose, Y.; Hasegawa, T. Epitaxial Strain-Controlled Ionic Conductivity in Li-Ion Solid Electrolyte Li0.33La0.56TiO3 Thin Films. Cryst. Growth Des. 2015, 15, 2187–2191. [Google Scholar] [CrossRef]
- Kim, S.; Hirayama, M.; Cho, W.; Kim, K.; Kobayashi, T.; Kaneko, R.; Suzuki, K.; Kanno, R. Low temperature synthesis and ionic conductivity of the epitaxial Li0.17La0.61TiO3 film electrolyte. CrystEngComm 2014, 16, 1044–1049. [Google Scholar] [CrossRef]
- Lü, X.; Wu, G.; Howard, J.W.; Chen, A.; Zhao, Y.; Daemen, L.L.; Jia, Q. Li-rich anti-perovskite Li3OCl films with enhanced ionic conductivity. Chem. Comm. 2014, 50, 11520–11522. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, T.; Chen, X.; Doerrer, C.; Jagger, B.; Speller, S.C.; Grovenor, C.R.M. Fabrication of Li1+xAlxGe2-x(PO4)3 thin films by sputtering for solid electrolytes. Solid State Ion. 2020, 354, 115397. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Yang, B.; Li, Q.; Wu, B.; Zhao, J.; Ma, L.; Liu, Y.; An, H. Preparation and ion conduction of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte films using radio frequency sputtering. Solid State Ion. 2020, 346, 115224. [Google Scholar] [CrossRef]
- Ling, Q.; Yu, Z.; Xu, H.; Zhu, G.; Zhang, X.; Zhao, Y.; Yu, A. Preparation and electrical properties of amorphous Li-Al-Ti-P-O thin film electrolyte. Mater. Lett. 2016, 169, 42–45. [Google Scholar] [CrossRef]
- Nakagawa, A.; Kuwata, N.; Matsuda, Y.; Kawamura, J. Characterization of Stable Solid Electrolyte Lithium Silicate for Thin Film Lithium Battery. J. Phys. Soc. Japan. 2010, 79, 98–101. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Osada, M.; Zhang, L.; Sasaki, T.; Watanabe, M. Solid electrolyte, thio-LISICON, thin film prepared by pulsed laser deposition. J. Power Sources 2005, 146, 707–710. [Google Scholar] [CrossRef]
- Loho, C.; Djenadic, R.; Bruns, M.; Clemens, O.; Hahn, H. Garnet-Type Li7La3Zr2O12 Solid Electrolyte Thin Films Grown by CO2-Laser Assisted CVD for All-Solid-State Batteries. J. Electrochem. Soc. 2017, 164, A6131. [Google Scholar] [CrossRef]
- Kazyak, E.; Chen, K.-H.; Wood, K.N.; Davis, A.L.; Thompson, T.; Bielinski, A.R.; Sanchez, A.J.; Wang, X.; Wang, C.; Sakamoto, J.; et al. Atomic Layer Deposition of the Solid Electrolyte Garnet Li7La3Zr2O12. Chem. Mater. 2017, 29, 3785–3792. [Google Scholar] [CrossRef]
- Reinacher, J.; Berendts, S.; Janek, J. Preparation and electrical properties of garnet-type Li6BaLa2Ta2O12 lithium solid electrolyte thin films prepared by pulsed laser deposition. Solid State Ion. 2014, 258, 1–7. [Google Scholar] [CrossRef]
- Kuwata, N.; Kawamura, J.; Toribami, K.; Hattori, T.; Sata, N. Thin-film lithium-ion battery with amorphous solid electrolyte fabricated by pulsed laser deposition. Electrochem. Commun. 2004, 6, 417–421. [Google Scholar] [CrossRef]
- Xie, J.; Sendek, A.D.; Cubuk, E.D.; Zhang, X.; Lu, Z.; Gong, Y.; Wu, T.; Shi, F.; Liu, W.; Reed, E.J.; et al. Atomic Layer Deposition of Stable LiAlF4 Lithium Ion Conductive Interfacial Layer for Stable Cathode Cycling. ACS Nano 2017, 11, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Meng, X.; Elam, J.W. Atomic Layer Deposition of LixAlyS Solid-State Electrolytes for Stabilizing Lithium-Metal Anodes. ChemElectroChem 2016, 3, 858–863. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Hama, S.; Tatsumisago, M. Preparation of Highly Lithium-Ion Conductive 80Li2S20P2S5 Thin-Film Electrolytes Using Pulsed Laser Deposition. J. Am. Ceram. Soc. 2010, 93, 765–768. [Google Scholar] [CrossRef]
- Quan, Z.; Hirayama, M.; Sato, D.; Zheng, Y.; Yano, T.-a.; Hara, K.; Suzuki, K.; Hara, M.; Kanno, R. Effect of excess Li2S on electrochemical properties of amorphous Li3PS4 films synthesized by pulsed laser deposition. J. Am. Ceram. Soc. 2017, 100, 746–753. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Yada, C.; Rosciano, F.; Roling, B. Correlation between micro-structural properties and ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 ceramics. J. Power Sources 2011, 196, 6456–6464. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Chen, R.; Chen, S.; Wang, G. Preparation and performance of novel Li–Ti–Si–P–O–N thin-film electrolyte for thin-film lithium batteries. J. Power Sources 2009, 189, 467–470. [Google Scholar] [CrossRef]
- Kim, H.-S.; Choi, K.S.; Kim, E.J.; Kang, K.; Kim, J.H.; Kim, J.; Yoon, C.S. Characterization of Sputter-Deposited LiZr2(PO4)3 Thin Film Solid Electrolyte. J. Electrochem. Soc. 2015, 162, A2080. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-Type Materials in Contact with Lithium Metal: Formation of Mixed Conducting Interphases (MCI) on Solid Electrolytes. J. Phys. Chem. C 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Chen, X.; Lü, X.; Cui, Z.; Xin, S.; Xue, L.; Jia, Q.; Goodenough, J.B. Mastering the interface for advanced all-solid-state lithium rechargeable batteries. PNAS 2016, 113, 13313–13317. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; West, A.R. The A-C Conductivity of Polycrystalline LISICON, Li2 + 2x Zn1-x GeO4, and a Model for Intergranular Constriction Resistances. J. Electrochem. Soc. 1983, 130, 662–669. [Google Scholar] [CrossRef]
- Robertson, A.D.; West, A.R.; Ritchie, A.G. Review of crystalline lithium-ion conductors suitable for high temperature battery applications. Solid State Ion. 1997, 104, 1–11. [Google Scholar] [CrossRef]
- Kanno, R.; Hata, T.; Kawamoto, Y.; Irie, M. Synthesis of a new lithium ionic conductor, thio-LISICON–lithium germanium sulfide system. Solid State Ion. 2000, 130, 97–104. [Google Scholar] [CrossRef]
- Murayama, M.; Kanno, R.; Irie, M.; Ito, S.; Hata, T.; Sonoyama, N.; Kawamoto, Y. Synthesis of New Lithium Ionic Conductor Thio-LISICON—Lithium Silicon Sulfides System. J. Solid State Chem. 2002, 168, 140–148. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Meesala, Y.; Liao, Y.-K.; Jena, A.; Yang, N.-H.; Pang, W.K.; Hu, S.-F.; Chang, H.; Liu, C.-E.; Liao, S.-C.; Chen, J.-M.; et al. An efficient multi-doping strategy to enhance Li-ion conductivity in the garnet-type solid electrolyte Li7La3Zr2O12. J. Mater. Chem. A 2019, 7, 8589–8601. [Google Scholar] [CrossRef]
- Rawlence, M.; Garbayo, I.; Buecheler, S.; Rupp, J.L.M. On the chemical stability of post-lithiated garnet Al-stabilized Li7La3Zr2O12 solid state electrolyte thin films. Nanoscale 2016, 8, 14746–14753. [Google Scholar] [CrossRef] [PubMed]
- Saccoccio, M.; Yu, J.; Lu, Z.; Kwok, S.C.T.; Wang, J.; Yeung, K.K.; Yuen, M.M.F.; Ciucci, F. Low temperature pulsed laser deposition of garnet Li6.4La3Zr1.4Ta0.6O12 films as all solid-state lithium battery electrolytes. J. Power Sources 2017, 365, 43–52. [Google Scholar] [CrossRef]
- Kalita, D.J.; Lee, S.H.; Lee, K.S.; Ko, D.H.; Yoon, Y.S. Ionic conductivity properties of amorphous Li–La–Zr–O solid electrolyte for thin film batteries. Solid State Ion. 2012, 229, 14–19. [Google Scholar] [CrossRef]
- Lobe, S.; Dellen, C.; Windmüller, A.; Tsai, C.L.; Vondahlen, F.; Uhlenbruck, S.; Guillon, O. Challenges regarding thin film deposition of garnet electrolytes for all-solid-state lithium batteries with high energy density. Ionics 2018, 24, 2199–2208. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nature Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Solid-State Batteries: Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1970311. [Google Scholar] [CrossRef]
- Lepley, N.D.; Holzwarth, N.A.W.; Du, Y.A. Structures, Li+ mobilities, and interfacial properties of solid electrolytes Li3PS4 and Li3PO4 from first principles. Phys. Rev. B 2013, 88, 104103. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium-ion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Dudney, N.J.; Neudecker, B.J. Solid state thin-film lithium battery systems. Curr. Opin. Solid State Mater. Sci. 1999, 4, 479–482. [Google Scholar] [CrossRef]
- Neudecker, B.J.; Dudney, N.J.; Bates, J.B. “Lithium-Free” Thin-Film Battery with In Situ Plated Li Anode. J. Electrochem. Soc. 2000, 147, 517–523. [Google Scholar] [CrossRef]
- Jung, K.-N.; Shin, H.-S.; Park, M.-S.; Lee, J.-W. Solid-State Lithium Batteries: Bipolar Design, Fabrication, and Electrochemistry. ChemElectroChem 2019, 6, 3842–3859. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.-S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Garche, J. On the historical development of the lead/acid battery, especially in Europe. J. Power Sources 1990, 31, 401–406. [Google Scholar] [CrossRef]
- Bullock, K.R. Progress and Challenges in Bipolar Lead-Acid Battery Development. J. Electrochem. Soc. 1995, 142, 1726. [Google Scholar] [CrossRef]
- Shen, D.H.; Halpert, G. Design concepts of high power bipolar rechargeable lithium battery. J. Power Sources 1993, 43, 327–338. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Gambe, Y.; Sun, Y.; Honma, I. Development of Bipolar All-solid-state Lithium Battery Based on Quasi-solid-state Electrolyte Containing Tetraglyme-LiTFSA Equimolar Complex. Sci. Rep. 2015, 5, 8869. [Google Scholar] [CrossRef]
- Yoshima, K.; Harada, Y.; Takami, N. Thin hybrid electrolyte based on garnet-type lithium-ion conductor Li7La3Zr2O12 for 12 V-class bipolar batteries. J. Power Sources 2016, 302, 283–290. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Jiang, H.; Xiong, S.; Feng, J.; Qian, Y. Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 2020, 78, 105344. [Google Scholar] [CrossRef]
- Weber, R.; Genovese, M.; Louli, A.J.; Hames, S.; Martin, C.; Hill, I.G.; Dahn, J.R. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 2019, 4, 683–689. [Google Scholar] [CrossRef]
- Zhang, J.-G. Anode-less. Nat. Energy 2019, 4, 637–638. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.-G. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Woo, J.-J.; Maroni, V.A.; Liu, G.; Vaughey, J.T.; Gosztola, D.J.; Amine, K.; Zhang, Z. Symmetrical Impedance Study on Inactivation Induced Degradation of Lithium Electrodes for Batteries Beyond Lithium-Ion. J. Electrochem. Soc. 2014, 161, A827. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Pollard, T.P.; Fan, X.; Borodin, O.; Wang, C. Electrolyte design for Li metal-free Li batteries. Mater. Today 2020, 39, 118–126. [Google Scholar] [CrossRef]
- Assegie, A.A.; Chung, C.-C.; Tsai, M.-C.; Su, W.-N.; Chen, C.-W.; Hwang, B.-J. Multilayer-graphene-stabilized lithium deposition for anode-Free lithium-metal batteries. Nanoscale 2019, 11, 2710–2720. [Google Scholar] [CrossRef]
- Kim, S.; Jung, C.; Kim, H.; Thomas-Alyea, K.E.; Yoon, G.; Kim, B.; Badding, M.E.; Song, Z.; Chang, J.; Kim, J.; et al. The Role of Interlayer Chemistry in Li-Metal Growth through a Garnet-Type Solid Electrolyte. Adv. Energy Mater. 2020, 10, 1903993. [Google Scholar] [CrossRef]
- Chen, C.; Eichel, R.A.; Notten, P.H.L. Metal-organic chemical vapor deposition enabling all-solid-state Li-ion microbatteries: A short review. J. Electroceram. 2017, 38, 230–247. [Google Scholar] [CrossRef]
- Horowitz, Y.; Strauss, E.; Peled, E.; Golodnitsky, D. How to Pack a Punch-Why 3D Batteries are Essential. Isr. J. Chem. 2021, 61, 38–50. [Google Scholar] [CrossRef]
- Shaijumon, M.M.; Perre, E.; Daffos, B.; Taberna, P.-L.; Tarascon, J.-M.; Simon, P. Nanoarchitectured 3D Cathodes for Li-Ion Microbatteries. Adv. Mater. 2010, 22, 4978–4981. [Google Scholar] [CrossRef]
- Mattelaer, F.; Geryl, K.; Rampelberg, G.; Dendooven, J.; Detavernier, C. Amorphous and Crystalline Vanadium Oxides as High-Energy and High-Power Cathodes for Three-Dimensional Thin-Film Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 13121–13131. [Google Scholar] [CrossRef] [PubMed]
- Labyedh, N.; Mattelaer, F.; Detavernier, C.; Vereecken, P.M. 3D LiMn2O4 thin-film electrodes for high rate all solid-state lithium and Li-ion microbatteries. J. Mater. Chem. A 2019, 7, 18996–19007. [Google Scholar] [CrossRef]
- Shui, J.L.; Jiang, G.S.; Xie, S.; Chen, C.H. Thin films of lithium manganese oxide spinel as cathode materials for secondary lithium batteries. Electrochim. Acta 2004, 49, 2209–2213. [Google Scholar] [CrossRef]
- Kim, S.-J.; Moon, S.-H.; Kim, M.-C.; So, J.-Y.; Han, S.-B.; Kwak, D.-H.; Bae, W.-G.; Park, K.-W. Micro-patterned 3D Si electrodes fabricated using an imprinting process for high-performance lithium-ion batteries. J. Appl. Electrochem. 2018, 48, 1057–1068. [Google Scholar] [CrossRef]
- Létiche, M.; Eustache, E.; Freixas, J.; Demortière, A.; De Andrade, V.; Morgenroth, L.; Tilmant, P.; Vaurette, F.; Troadec, D.; Roussel, P.; et al. Atomic Layer Deposition of Functional Layers for on Chip 3D Li-Ion All Solid State Microbattery. Adv. Energy Mater. 2017, 7, 1601402. [Google Scholar] [CrossRef]
- Moitzheim, S.; Balder, J.E.; Poodt, P.; Unnikrishnan, S.; De Gendt, S.; Vereecken, P.M. Chlorine Doping of Amorphous TiO2 for Increased Capacity and Faster Li+-Ion Storage. Chem. Mater. 2017, 29, 10007–10018. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Huang, G.; Xu, B.; Wang, B.; Pan, R.; Men, C.; Mei, Y. ZnO Nanomembrane/Expanded Graphite Composite Synthesized by Atomic Layer Deposition as Binder-Free Anode for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 38522–38529. [Google Scholar] [CrossRef]
- Put, B.; Vereecken, P.M.; Meersschaut, J.; Sepúlveda, A.; Stesmans, A. Electrical Characterization of Ultrathin RF-Sputtered LiPON Layers for Nanoscale Batteries. ACS Appl. Mater. Interfaces 2016, 8, 7060–7069. [Google Scholar] [CrossRef]
- Nisula, M.; Shindo, Y.; Koga, H.; Karppinen, M. Atomic Layer Deposition of Lithium Phosphorus Oxynitride. Chem. Mater. 2015, 27, 6987–6993. [Google Scholar] [CrossRef]
- Nasreldin, M.; de Mulatier, S.; Delattre, R.; Ramuz, M.; Djenizian, T. Flexible and Stretchable Microbatteries for Wearable Technologies. Adv. Mater. Technol. 2020, 5, 2000412. [Google Scholar] [CrossRef]
- He, Y.; Matthews, B.; Wang, J.; Song, L.; Wang, X.; Wu, G. Innovation and challenges in materials design for flexible rechargeable batteries: From 1D to 3D. J. Mater. Chem. A 2018, 6, 735–753. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Kou, T.; Song, Y.; Liu, T.; Li, Y. Paper-Based Electrodes for Flexible Energy Storage Devices. Adv. Sci. 2017, 4, 1700107. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat Commun. 2013, 4, 1543. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nature Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Chen, Z.; Liu, K.; Zhou, G.; Sun, Y.; Song, M.-S.; Bao, Z.; Cui, Y. Stretchable Lithium-Ion Batteries Enabled by Device-Scaled Wavy Structure and Elastic-Sticky Separator. Adv. Energy Mater. 2017, 7, 1701076. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; La Mantia, F.; Yang, Y.; Cui, Y. Thin, Flexible Secondary Li-Ion Paper Batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Too, C.O.; Wallace, G.G. Electrochemical Properties of Graphene Paper Electrodes Used in Lithium Batteries. Chem. Mater. 2009, 21, 2604–2606. [Google Scholar] [CrossRef]
- Zhao, X.; Hayner, C.M.; Kung, M.C.; Kung, H.H. In-Plane Vacancy-Enabled High-Power Si–Graphene Composite Electrode for Lithium-Ion Batteries. Adv. Energy Mater. 2011, 1, 1079–1084. [Google Scholar] [CrossRef]
- Rana, K.; Kim, S.D.; Ahn, J.-H. Additive-free thick graphene film as an anode material for flexible lithium-ion batteries. Nanoscale 2015, 7, 7065–7071. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Z.; Zhu, M.; Lu, Y. Polyacrylic Acid Assisted Assembly of Oxide Particles and Carbon Nanotubes for High-Performance Flexible Battery Anodes. Adv. Energy Mater. 2015, 5, 1401207. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Zhu, X.-D.; Duan, Z.-Q.; Xie, X.-M. Flexible and robust MoS2–graphene hybrid paper cross-linked by a polymer ligand: A high-performance anode material for thin film lithium-ion batteries. Chem. Commun. 2013, 49, 10305–10307. [Google Scholar] [CrossRef] [PubMed]
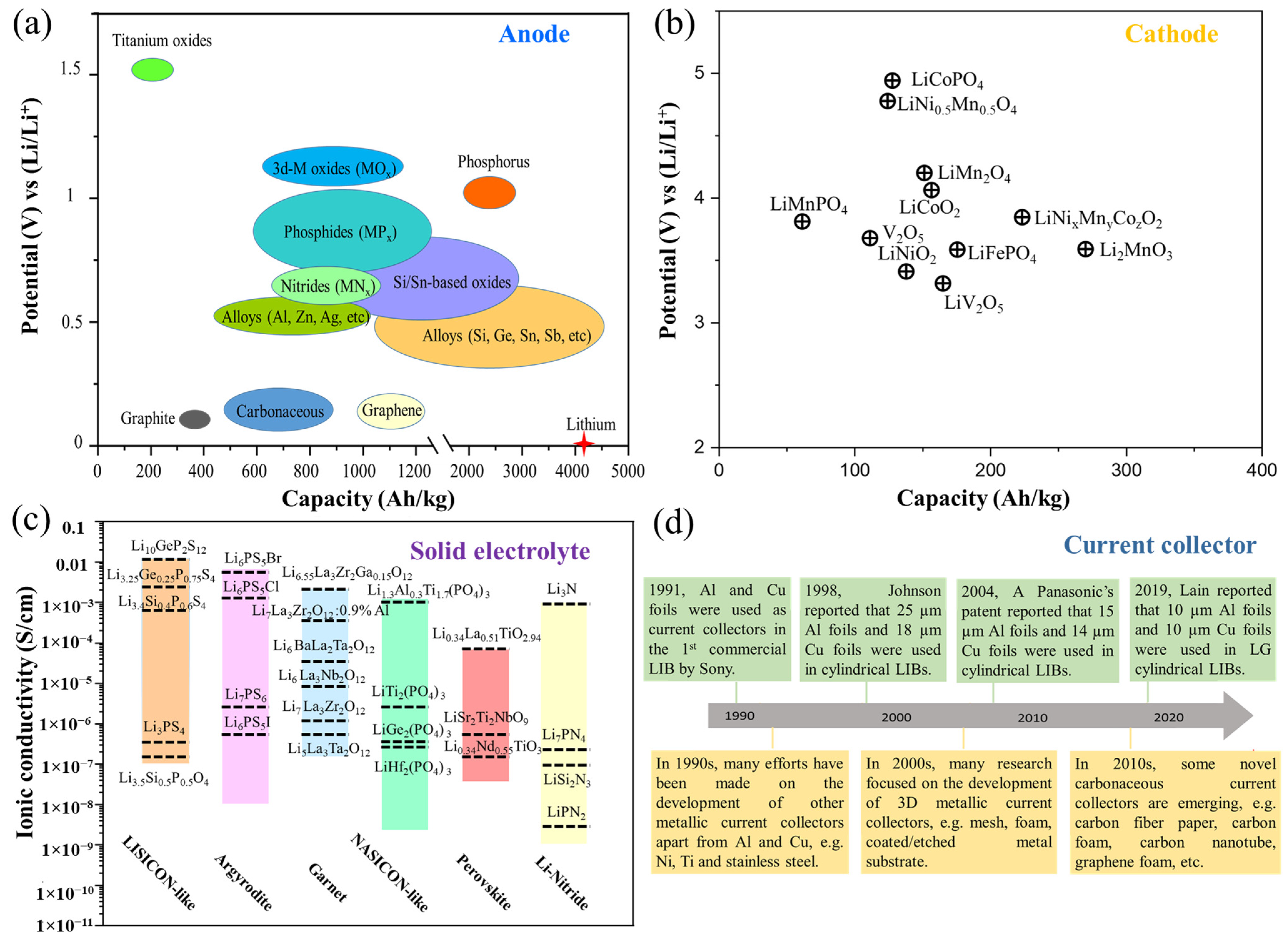
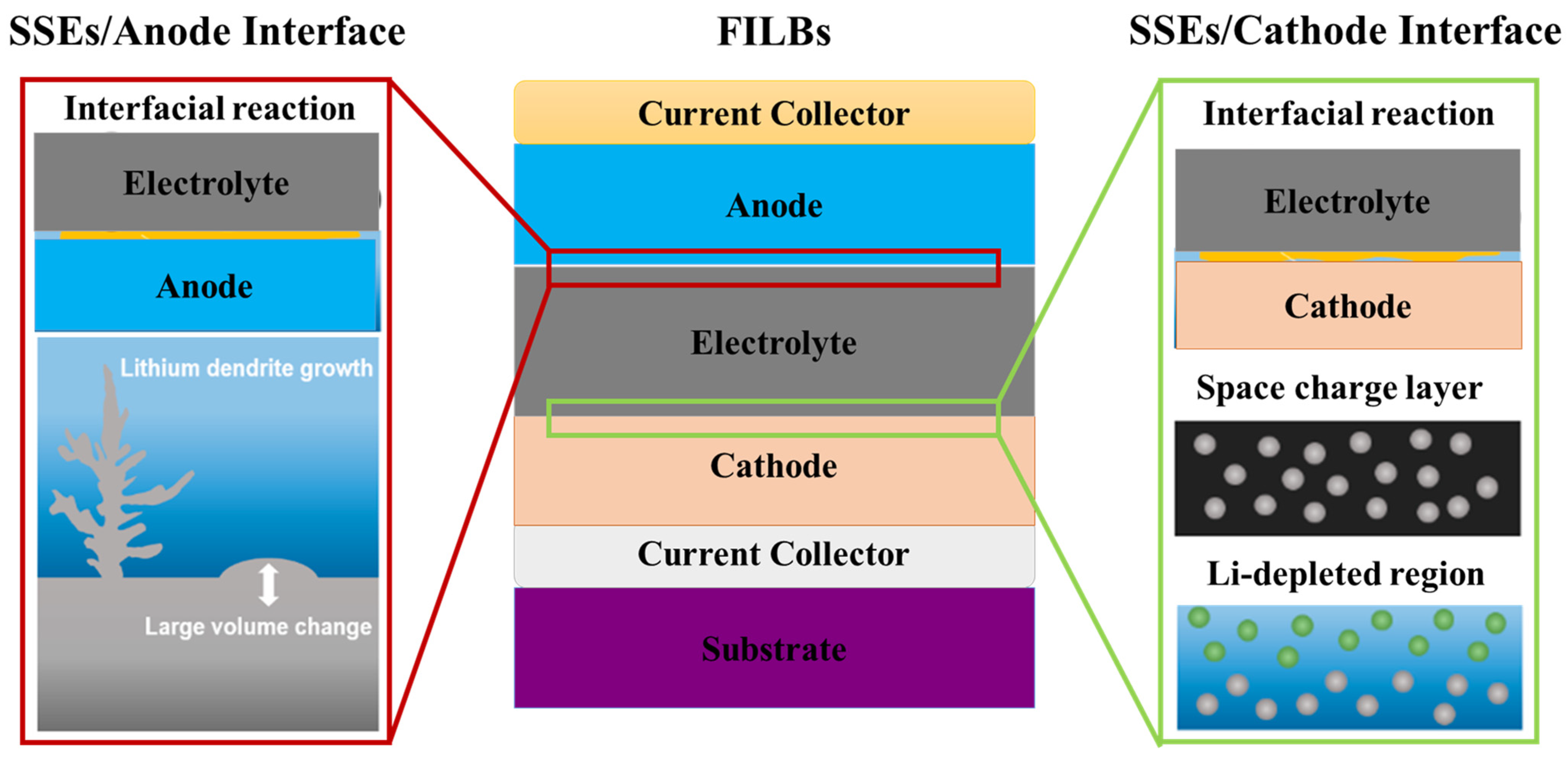
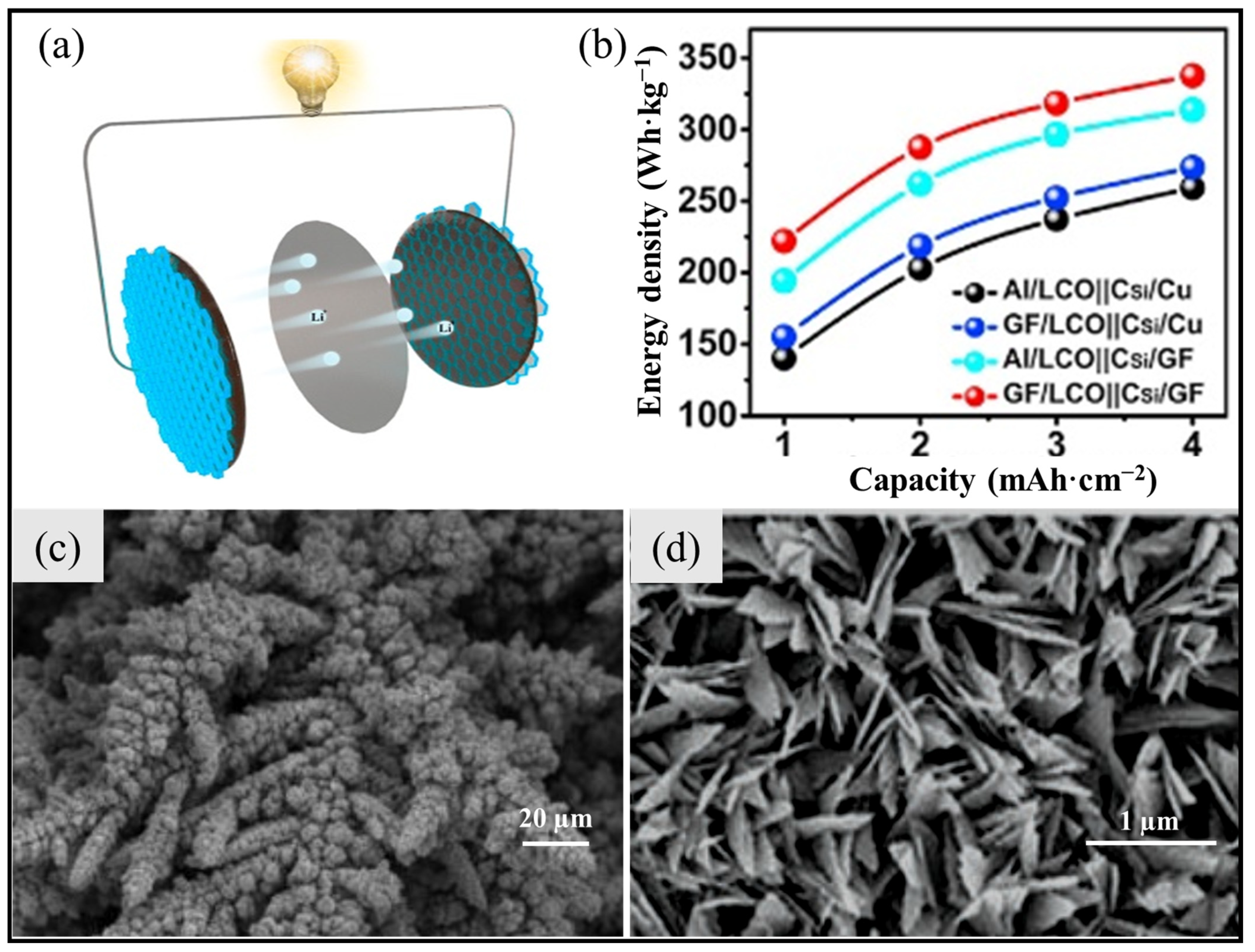
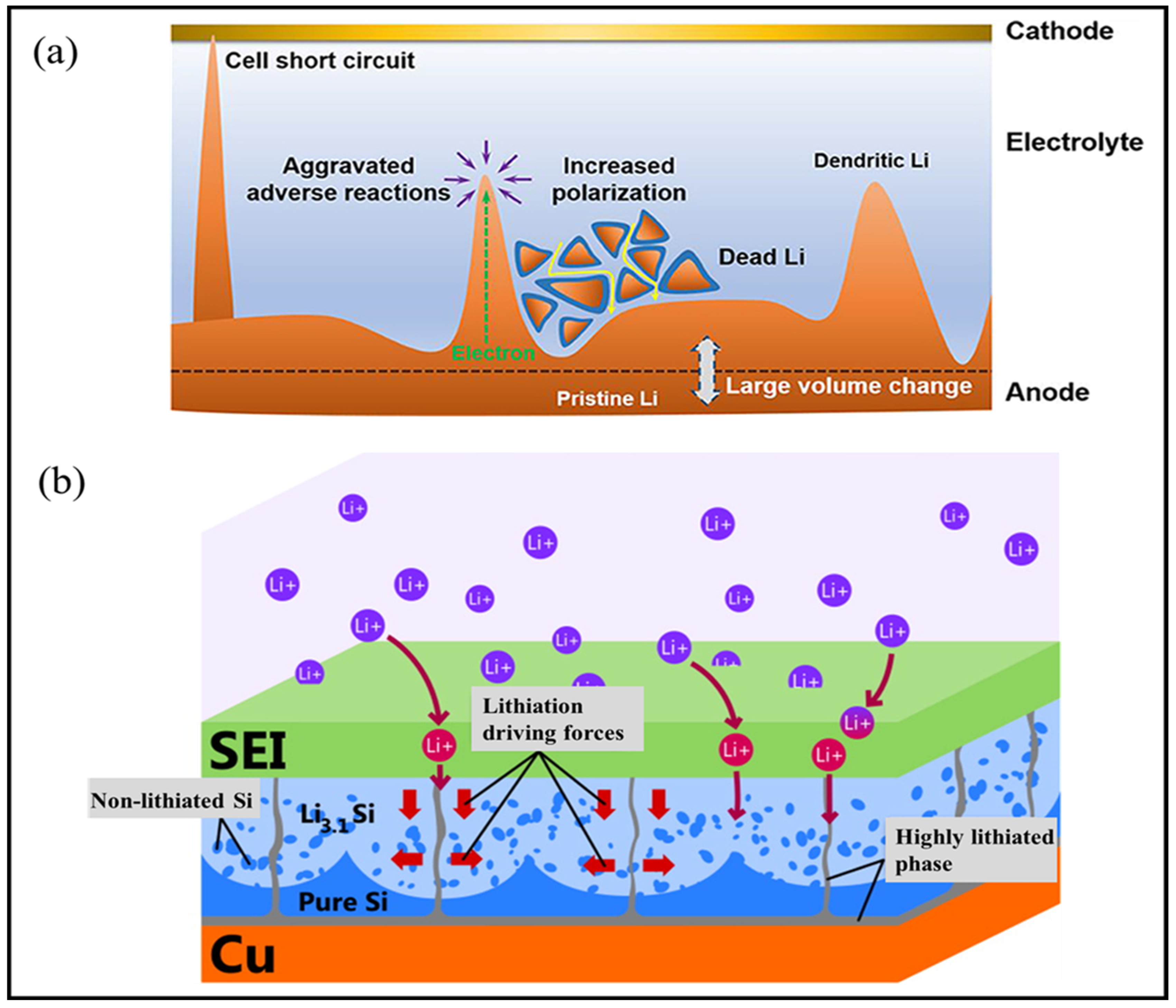

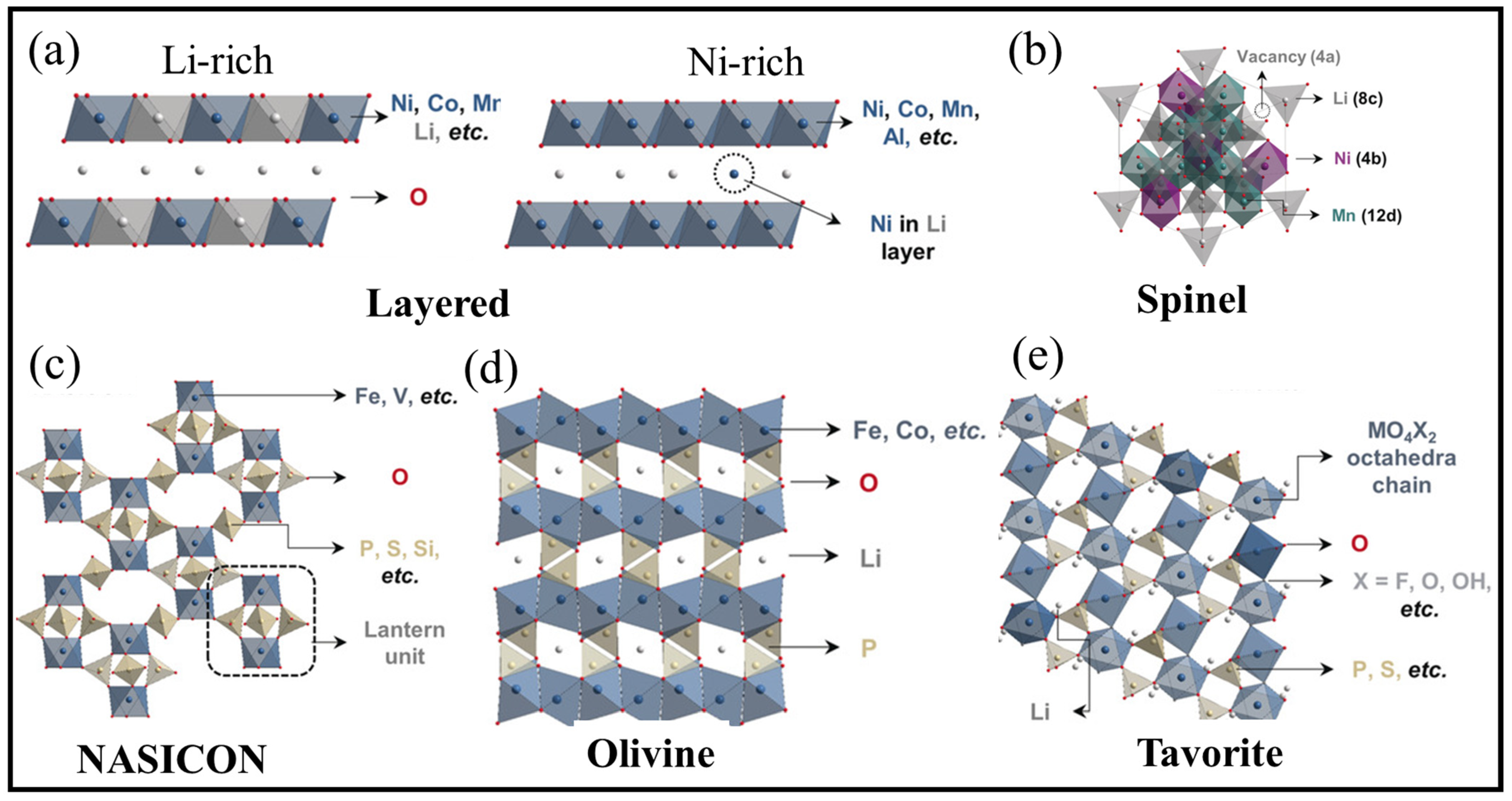
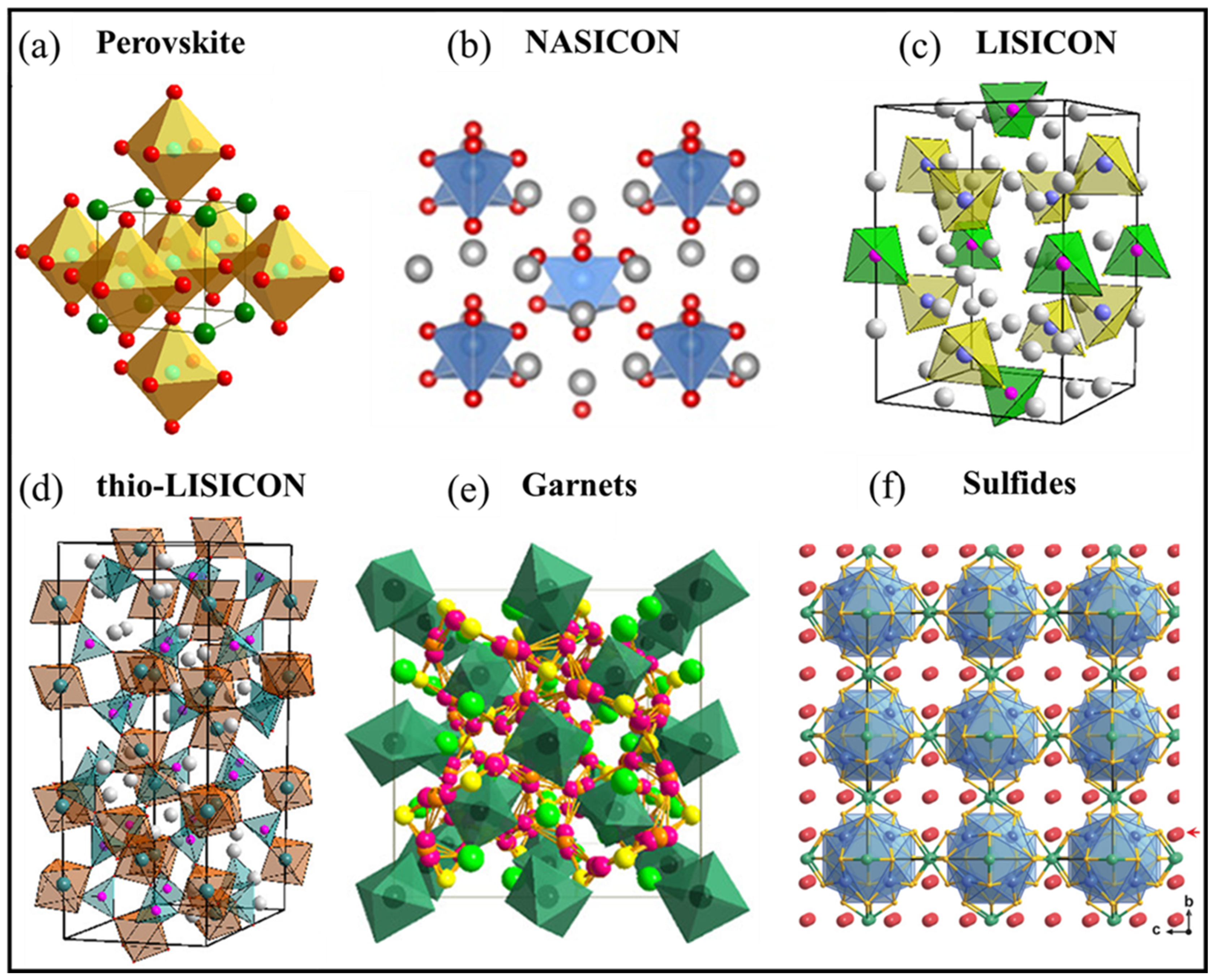


| Current Collectors | Electrode | Electrolyte | Voltage Window | Reference |
|---|---|---|---|---|
| Au | cathode | 1 M LiClO4 in PC 1 M LiPF6 in EC/DMC | 3–5 3–4.4 | [65] |
| Ag | cathode | 1 M LiClO4 in PC 1 M LiPF6 in EC/DMC | 3–3.7 | [66] |
| Al | cathode | 1 M LiClO4 in PC 1 M LiPF6 in EC/DMC | 1.5–5.5 1.5–5 | [65] |
| Ni | cathode | 1 M LiPF6 in EC/DMC | 3–4.5 | [67] |
| Stainless steel | cathode | 1 M LiPF6 in EC/DMC | 3–4.5 | [68] |
| Stainless steel | cathode | 1 M LiPF6 in EC/DMC | 1.5–5.5 | [69] |
| Cr | cathode/anode | 1 M LiPF6 in EC/DMC | 0–4 | [70] |
| Ti | cathode/anode | 1 M LiPF6 in EC/DMC | 0–4 | [70] |
| TiN | cathode/anode | 1 M LiPF6 in EC/DMC | 0–4.12 | [69] |
| Carbon fiber paper | cathode/anode | 1 M LiPF6 in EC/DMC | 1.5–3 | [71] |
| Stainless steel | cathode/anode | 1 M LiPF6 in EC/DMC | 2–3.4 | [72] |
| Fe | anode | 1 M LiPF6 in EC/DMC | 0–3.2 | [70] |
| Cu | anode | 1 M LiPF6 in EC/DMC | 0–3 | [70] |
| Metal Oxides Anode | Reaction Mechanism | Initial Discharge Capacity (mAh·g−1) | Coulomb Efficiency | Reference |
|---|---|---|---|---|
| LTO | intercalation | 313 | 95.0% | [136] |
| TiNb2O7(TNO) | intercalation | 398 | 92.0% | [137] |
| TiNb2O7 | intercalation | 460 | 99.0% | [138] |
| SrLi2Ti6O14 | intercalation | 175 | 91.4% | [139] |
| SnO2 | alloy | 650 | 99.5% | [140] |
| LNVO | conversion | 871 | 98.5% | [141] |
| NiFe2O4 | conversion | 849 | 67.7% | [142] |
| Co2O3 | conversion | 1235 | 56.0% | [143] |
| Fe2O3 | conversion | 951 | 100% | [144] |
| MoO2 | conversion | 1510 | - | [145] |
| ZnO | conversion | 1200 | - | [146] |
| MoOx | conversion | 1118 | 81.8% | [147] |
| RuO2 | conversion | 1200 | 33.0% | [148] |
| ZnOS | conversion | 1271 | 64.1% | [149] |
| In2O3 | conversion | 1083 | 81.5% | [150] |
| Metal Oxides Anode | Crystal Structure | Capacity | Voltage Range (V) | Rate Performance | Reference |
|---|---|---|---|---|---|
| V2O5 | Layer | 142 mAh·g−1 | 2.5–4.0 | 86.7 mAh·g−1 at 56 C | [191] |
| LiV2O5 | 36 μAh·cm−2·μm−1 | 2.4–3.6 | [192] | ||
| HT-LiCoO2 | Layer | 135 mAh·g−1 | 3.0–4.2 | 103 mAh·g−1 at 10 C | [193] |
| LiNiO2 | Layer | 23 μAh·cm−2·μm−1 | 2.8–4.2 | [194] | |
| LiNi0.8Co0.2O2 | Layer | 125 mAh·g−1 | 2.8–4.4 | [195] | |
| Li2MnO3 | Layer | 267 mAh·g−1 | 2.0–4.8 | 147.84 mAh·g−1 at 9.3 C | [196] |
| LiNi0.8Co0.15Al0.05O2 (NCA) | Layer | 197 mAh·g−1 | 2.5–4.2 | [197] | |
| LiNi1/3Co1/3Mn1/3O2 (NMC333) | Layer | 177 mAh·g−1 | 2.8–4.5 | 60 mAh·g−1 at 2 C | [198] |
| LiNi0.5Mn0.3Co0.2O2 (NMC532) | Layer | 167 mAh·g−1 | 3.0–4.5 | [199] | |
| LiNi0.6Mn0.2Co0.2O2 (NMC622) | Layer | 79 mAh·g−1 | 2.5–4.5 | [200] | |
| Li1.2Mn0.55Ni0.15Co0.1O2 | Layer | 250 mAh·g−1 | 2.0–4.8 | [201] | |
| LiMn2O4 (LMO) | Spinel | 82 mAh·g−1 | 67 mAh·g−1 at 348 C | [202] | |
| Li 0.92Co0.65Mn1.35O4 | Spinel | 340 mAh·g−1 | 1.6–4.5 | 268 mAh·g−1 at 10 C | [203] |
| LiCoMnO4 | Spinel | 107 mAh·g−1 | 3.0–5.0 | [204] | |
| LiNi0.5Mn1.5O4 | Spinel | 117 mAh·g−1 | 3.0–5.0 | 108 mAh·g−1 at 10 C | [205] |
| LiMnPO4 | Olivine | 24 mAh·g−1 | 3.5–4.4 | [206] | |
| LiFePO4 | Olivine | 159 mAh·g−1 | 3.0–4.0 | 153 mAh·g−1 at 7.5 C | [207] |
| Electrolyte | Crystal Structure | Deposition Method | Ea (eV) | Ionic Conductivity (S·cm−1) | Reference |
|---|---|---|---|---|---|
| LiPON | PLD | 1.5·10−8 | [249] | ||
| LixNbyO | ALD | 0.62 | 6.4·10−8 | [250] | |
| Li0.33La0.56TiO3 (LLTO) | Perovskite | PLD | 0.35 | 3.5·10−5 | [251] |
| epitaxial Li0.33La0.56TiO3 | Perovskite | PLD | 0.34 | 6.7·10−4 | [252] |
| Li0.17La0.61TiO3 | Perovskite | PLD | 0.25 | 3.8·10−4 | [253] |
| Li0.5La0.5TiO2 | Perovskite | PLD | 1.1·10−5 | [248] | |
| Li3OCl | anti-perovskite | PLD | 0.36 | 8.9·10−6 | [254] |
| Li 1+xAlxGe2−x(PO4)3 (LAGP) | NASICON | sputtering | >10−4 | [255] | |
| Li1.5Al0.5Ge1.5(PO4)3 | NASICON | sputtering | 0.25 | 1.29·10−6 | [256] |
| Li0.5Al0.29Ti1.35P2.9 (LATP) | NASICON | sputtering | 6.5·10−6 | [257] | |
| Li2O-SiO2 | LISICON | PLD | 4.1·10−7 | [258] | |
| Li3.25Ge0.25P0.75S4 | thio-LISICON | PLD | 0.38 | 1.7·10−4 | [259] |
| Li7La3Zr2O12 (LLZO) | Garnet | CVD | 0.50 | 4.2·10−6 | [260] |
| Al-doped LLZO | Garnet | ALD | 0.63 | 1.0·10−8 | [261] |
| Li6BaLa2Ta2O12 | Garnet | PLD | 0.42 | 2.0·10−6 | [262] |
| Li2O-V2O5-SiO2(LVSO) | PLD | 0.54 | 2.5·10−7 | [263] | |
| LiAlF4 | ALD | 3.5·10−8 | [264] | ||
| LiAl0.3S | ALD | 0.48 | 2.5·10−7 | [265] | |
| Li2S-P2O5 | PLD | 0.45 | 7.9·10−5 | [266] | |
| Li3PS4 | PLD | 0.47 | 5.3·10−4 | [267] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Chen, C.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs. Batteries 2023, 9, 186. https://doi.org/10.3390/batteries9030186
Wu B, Chen C, Danilov DL, Eichel R-A, Notten PHL. All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs. Batteries. 2023; 9(3):186. https://doi.org/10.3390/batteries9030186
Chicago/Turabian StyleWu, Baolin, Chunguang Chen, Dmitri L. Danilov, Rüdiger-A. Eichel, and Peter H. L. Notten. 2023. "All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs" Batteries 9, no. 3: 186. https://doi.org/10.3390/batteries9030186
APA StyleWu, B., Chen, C., Danilov, D. L., Eichel, R.-A., & Notten, P. H. L. (2023). All-Solid-State Thin Film Li-Ion Batteries: New Challenges, New Materials, and New Designs. Batteries, 9(3), 186. https://doi.org/10.3390/batteries9030186