Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery
Abstract
1. Introduction
2. Experiment
2.1. Synthesis Materials
2.2. Preparation of Zn-Air Batteries
2.3. Characterization
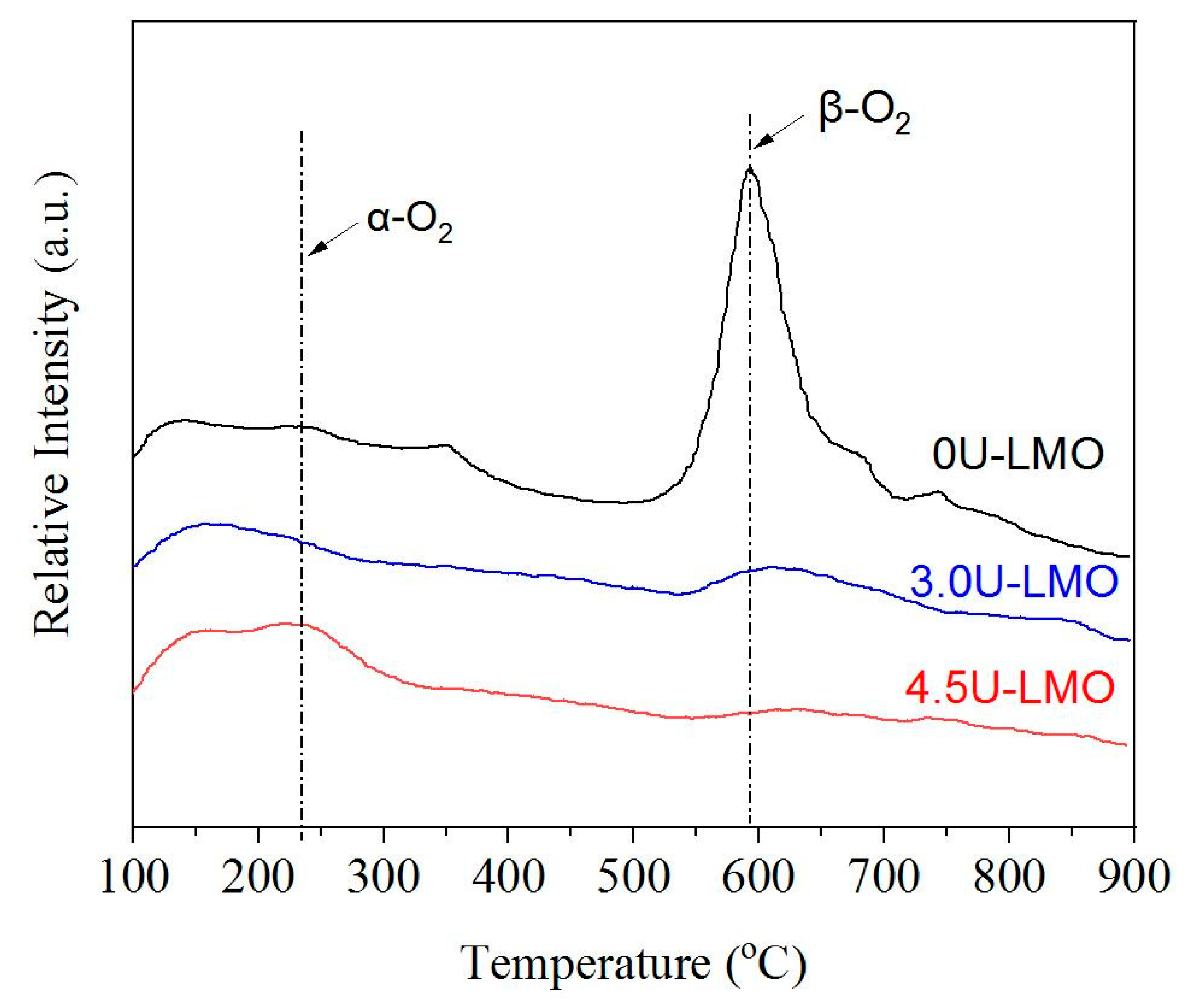
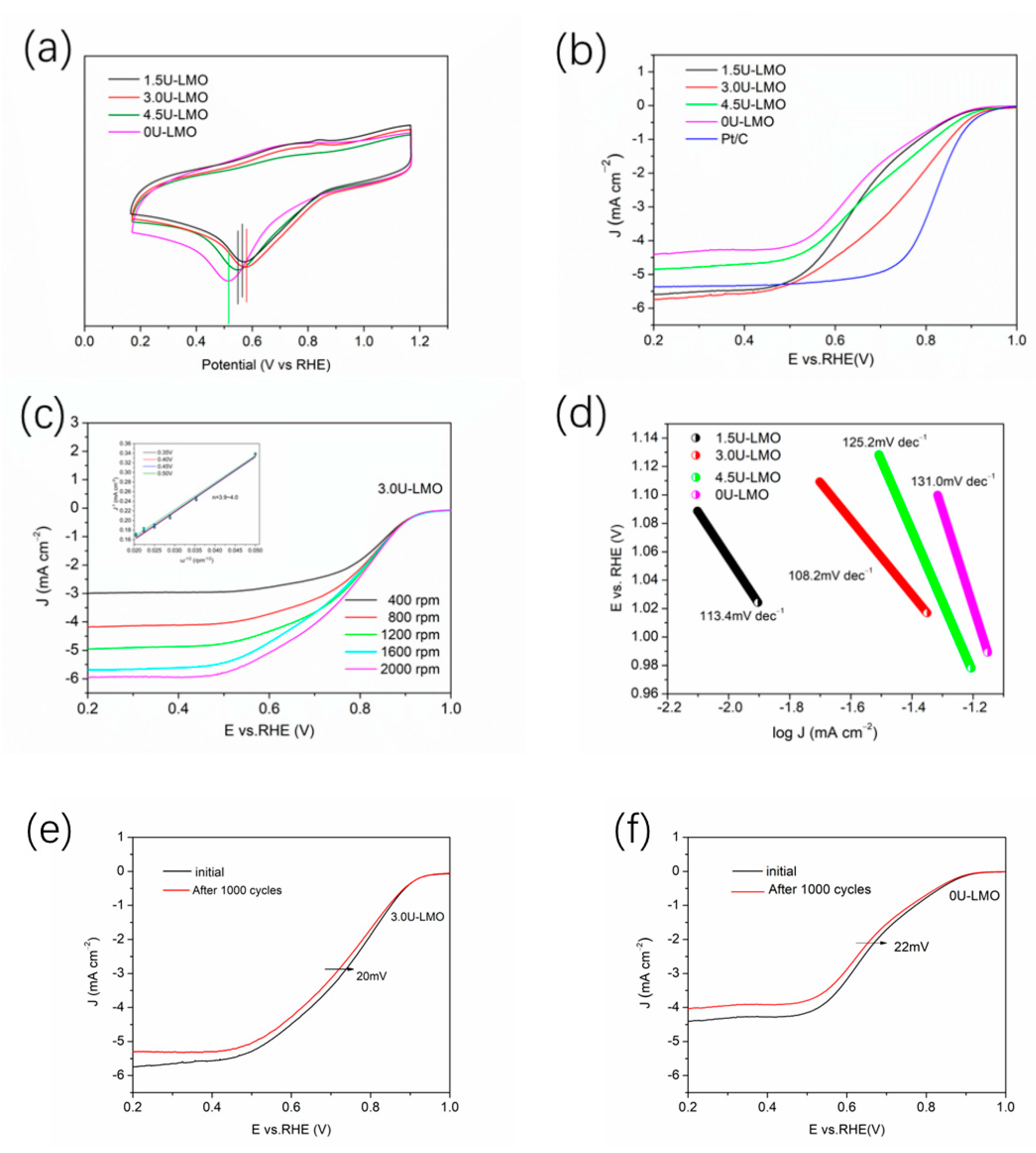
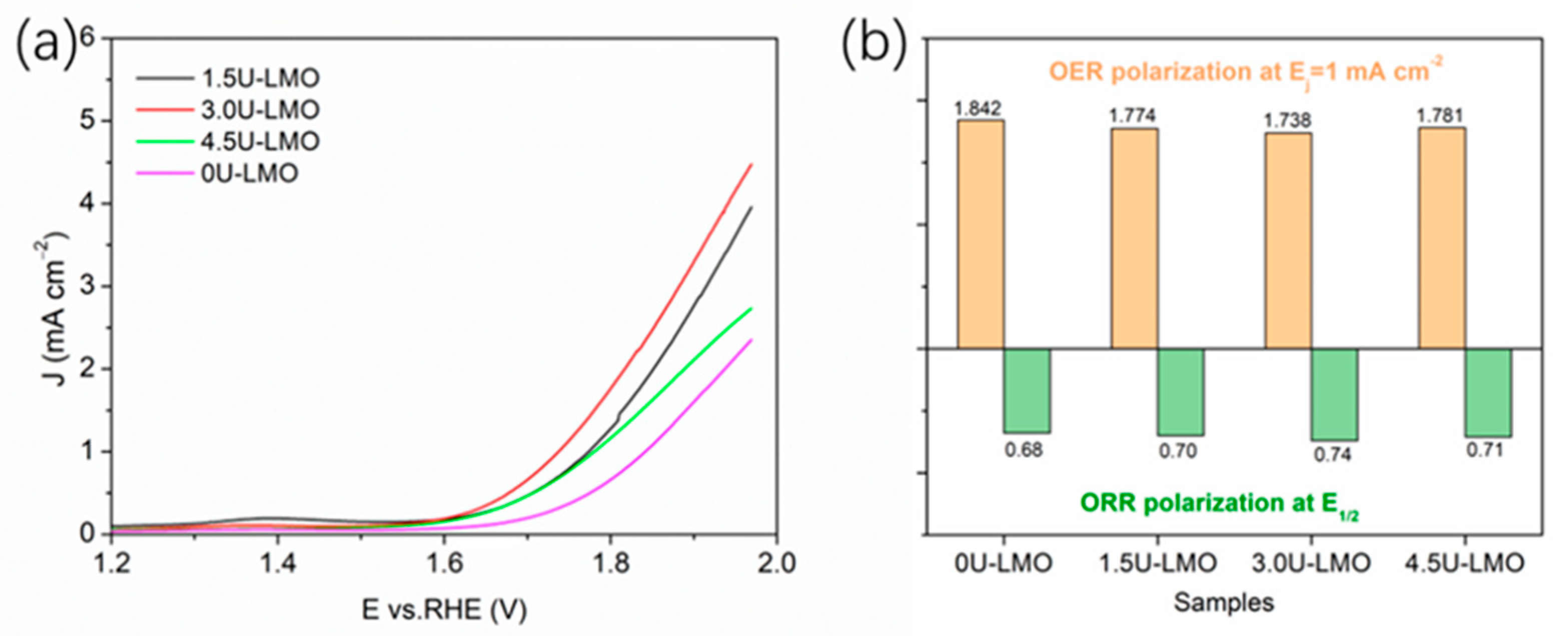
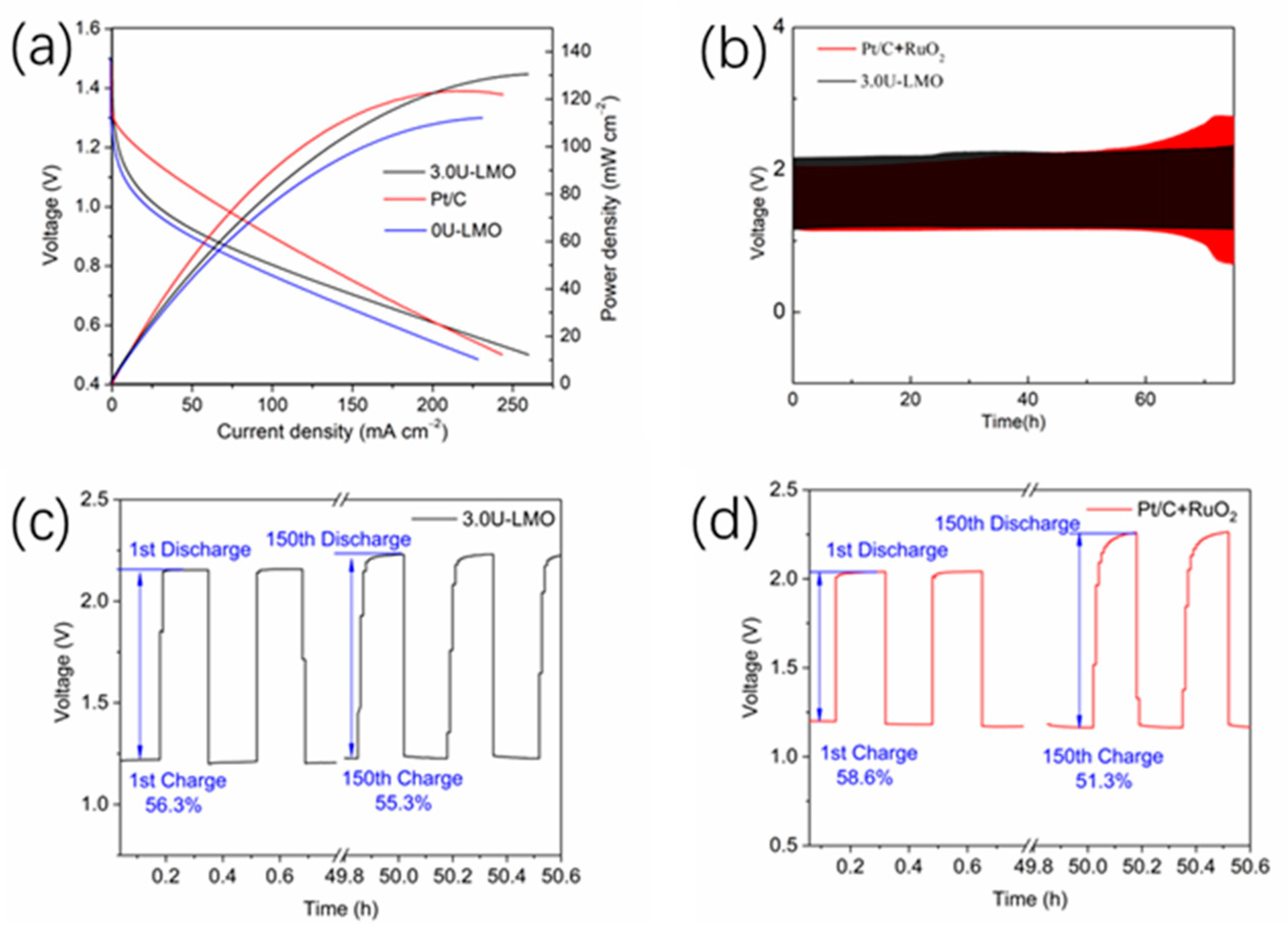
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, P.; Chen, B.; Xu, H.R.; Zhang, H.C.; Cai, W.Z.; Ni, M.; Liu, M.L.; Shao, Z.P. Flexible Zn- and Li-air Batteries: Recent Advances, Challenges, and Future Perspectives. Energy Environ. Sci. 2017, 10, 2056–2080. [Google Scholar] [CrossRef]
- Hao, J.N.; Li, X.L.; Zeng, X.H.; Li, D.; Mao, J.F.; Guo, Z.P. Deeply Understanding the Zn Anode Behaviour and Corresponding Improvement Strategies in Different Aqueous Zn-Based Batteries. Energy Environ. Sci. 2020, 13, 3917–3949. [Google Scholar] [CrossRef]
- Chen, G.B.; Liu, P.; Liao, Z.Q.; Sun, F.F.; He, Y.H.; Zhong, H.X.; Zhang, T.; Zschech, E.; Chen, M.W.; Wu, G.; et al. Zinc-Mediated Template Synthesis of Fe-N-C Electrocatalysts with Densely Accessible Fe-N-x Active Sites for Efficient Oxygen Reduction. Adv. Mater. 2020, 32, 7. [Google Scholar] [CrossRef]
- Li, J.; Xue, H.; Xu, N.; Zhang, X.; Wang, Y.; He, R.; Huang, H.; Qiao, J. Co/Ni Dual-Metal Embedded in Heteroatom Doped Porous Carbon Core-Shell Bifunctional Electrocatalyst for Rechargeable Zn-Air Batteries. Mater. Rep. Energy 2022, 2, 100090. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Xu, X.M.; Liu, P.Y.; Ran, R.; Jiang, S.P.; Wu, H.W.; Shao, Z.P. A Function-Separated Design of Electrode for Realizing High-Performance Hybrid Zinc Battery. Adv. Energy Mater. 2020, 10, 2002992. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Xu, X.M.; Wang, W.; Shao, Z.P. Recent Advances in Metal-Organic Framework Derivatives as Oxygen Catalysts for Zinc-Air Batteries. Batter. Supercaps 2019, 2, 272–289. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, Z.; Chen, S.; Chang, Y.; Hsueh, K. Effects of Cell Design Parameters on Zinc-Air Battery Performance. Batteries 2022, 8, 92. [Google Scholar] [CrossRef]
- Qiu, K.; Trudgeon, D.; Li, X.; Yufit, V.; Chakrabarti, B.; Brandon, N.; Shah, A. Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries. Batteries 2022, 8, 106. [Google Scholar] [CrossRef]
- Dai, Y.W.; Yu, J.; Cheng, C.; Tan, P.; Ni, M. Mini-Review of Perovskite Oxides as Oxygen Electrocatalysts for Rechargeable Zinc-Air Batteries. Chem. Eng. J. 2020, 397, 16. [Google Scholar] [CrossRef]
- Zheng, Q.L.; Zhang, Y.D.; Su., C.; Zhao, L.; Guo, Y.M. Nonnoble Metal Oxides for High-performance Zn-air batteries: Design strategies and Future challenges. Asia-Pac. J. Chem. Eng. 2022, 17, e2776. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.F.; Ni, M.; Pan, W.D.; Luo, S.J.; Leung, D.Y.C. Rechargeable Zn-Air Batteries: Recent Trends and Future Perspectives. Renew. Sustain. Energy Rev. 2022, 154, 19. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal-Air Batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.N.; Shi, L.N.; Huang, H. Oxygen Reduction Reaction Activity of LaMn1-xCoxO3-Graphene Nanocomposite for Zinc-Air Battery. Electrochim. Acta 2015, 161, 115–123. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Feng, F.X.; Zhang, C.H.; Zheng, Q.L.; Wang, C.C.; Hu, H.B.; Wu, M.Z.; Guo, Y.M. Enhanced Catalytic Activity of LaMnO3 by A-Site Substitution as Air Electrode of Zn-Air Batteries with Attractive Durability. Energy Fuels 2020, 34, 10170–10177. [Google Scholar] [CrossRef]
- Kuai, L.; Kan, E.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.-P.; Huotari, S.; Wang, W.; Geng, B. Mesoporous LaMnO3+δ Perovskite from Spray−Pyrolysis with Superior Performance for Oxygen Reduction Reaction and Zn−Air Battery. Nano Energy 2018, 43, 81–90. [Google Scholar] [CrossRef]
- Miao, H.; Wu, X.Y.; Chen, B.; Wang, Q.; Wang, F.; Wang, J.T.; Zhang, C.F.; Zhang, H.C.; Yuan, J.L.; Zhang, Q.J. A-site Deficient/Excessive Effects of LaMnO3 Perovskite as Bifunctional Oxygen Catalyst for Zinc-Air Batteries. Electrochim. Acta 2020, 333, 11. [Google Scholar] [CrossRef]
- You, M.; Gui, L.; Ma, X.; Wang, Z.; Xu, Y.; Zhang, J.; Sun, J.; He, B.; Zhao, L. Electronic Tuning of SrIrO3 Perovskite Nanosheets by Sulfur Incorporation to Induce Highly Efficient and Long-Lasting Oxygen Evolution in Acidic Media. Appl. Catal. B Environ. 2021, 298, 120562. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Y.; Wang, Z.; Xu, Y.; Gui, L.; He, B.; Zhao, L. Probing Dynamic Self-Reconstruction on Perovskite Fluorides toward Ultrafast Oxygen Evolution. Adv. Sci. 2022, 9, 2201916. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, R. Catalytic Activity for Methane Combustion of the Perovskite-Type La1−xSrxCoO3-δ Oxide Prepared by the Urea Decomposition Method. Appl. Catal. B Environ. 2010, 98, 147–153. [Google Scholar] [CrossRef]
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent Advances and Prospective in Ruthenium-Based Materials for Electrochemical Water Splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ahmad, N.; Alam, M.; Adil, S.F.; Ramay, S.M.; Albadri, A.; Ahmad, A.; Al-Enizi, A.M.; Alrayes, B.F.; Assal, M.E.; et al. Physico-Chemical Properties and Catalytic Activity of the Sol-Gel Prepared Ce-Ion Doped LaMnO3 Perovskites. Sci. Rep. 2019, 9, 7747. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, W.; Chu, X.; Cong, Y.; Huang, K.; Feng, S. Unfolding BOB Bonds for an Enhanced ORR Performance in ABO3-Type Perovskites. Small 2019, 15, e1803513. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Duan, X.; Li, J.; Dai, J.; Liu, B.; Wang, S.; Zhou, W.; Shao, Z. Boosting Performance of Lanthanide Magnetism Perovskite for Advanced Oxidation through Lattice Doping with Catalytically Inert Element. Chem. Eng. J. 2019, 355, 721–730. [Google Scholar] [CrossRef]
- Kim, W.S.; Anoop, G.; Lee, H.J.; Lee, S.S.; Kwak, J.H.; Lee, H.J.; Jo, J.Y. Facile Synthesis of Perovskite LaMnO3+δ Nanoparticles for the Oxygen Reduction Reaction. J. Catal. 2016, 344, 578–582. [Google Scholar] [CrossRef]
- Xiang, F.; Chen, X.; Yu, J.; Ma, W.; Li, Y.; Yang, N. Synthesis of Three-Dimensionally Ordered Porous Perovskite Type LaMnO3 for Al-Air Battery. J. Mater. Sci. Technol. 2018, 34, 1532–1537. [Google Scholar] [CrossRef]
- Li, B.; Yang, Q.L.; Peng, Y.; Chen, J.J.; Deng, L.; Wang, D.; Hong, X.W.; Li, J.H. Enhanced Low-Temperature Activity of LaMnO3 for Toluene Oxidation: The Effect of Treatment with an Acidic KMnO4. Chem. Eng. J. 2019, 366, 92–99. [Google Scholar] [CrossRef]
- Zhu, J.J.; Li, H.L.; Zhong, L.Y.; Xiao, P.; Xu, X.L.; Yang, X.G.; Zhao, Z.; Li, J.L. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Liu, X.; Mi, J.; Shi, L.; Liu, H.; Liu, J.; Ding, Y.; Shi, J.; He, M.; Wang, Z.; Xiong, S.; et al. In Situ Modulation of A-Site Vacancies in LaMnO3.15 Perovskite for Surface Lattice Oxygen Activation and Boosted Redox Reactions. Angew. Chem.-Int. Ed. 2021, 60, 26747–26754. [Google Scholar] [CrossRef] [PubMed]
- Shaterian, M.; Enhessari, M.; Rabbani, D.; Asghari, M.; Salavati-Niasari, M. Synthesis, Characterization and Photocatalytic Activity of LaMnO3 Nanoparticles. Appl. Surf. Sci. 2014, 318, 213–217. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Hasan, S.; Osman, N. Role of CA-EDTA on the Synthesizing Process of Cerate-Zirconate Ceramics Electrolyte. J. Chem. 2013, 2013, 908340. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Wang, C.; Zhang, C.; Guo, Y. CoO Enhanced Oxygen Evolution Kinetics of LaMnO3 Perovskite as a Potential Cathode for Rechargeable Zn-Air Batteries. Energy Fuels 2021, 36, 1091–1099. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Liu, T.; Feng, F.; Wang, C.; Hu, H.; Wu, M.; Ni, M.; Shao, Z. The Synergistic Effect Accelerates the Oxygen Reduction/Evolution Reaction in a Zn-Air Battery. Front. Chem. 2019, 7, 524. [Google Scholar] [CrossRef]
- Sakulkhaemaruethai, S.; Sreethawong, T. Synthesis of Mesoporous-Assembled TiO2 Nanocrystals by a Modified Urea-Aided Sol-Gel Process and their Outstanding Photocatalytic H2 Production Activity. Int. J. Hydrogen Energy 2011, 36, 6553–6559. [Google Scholar] [CrossRef]
- Thongchanthep, C.; Thountom, S. The Synthesis of Ba0.7Sr0.3TiO3 Ceramics Prepared by Sol–Gel Combustion Method with Urea as Fuel. Ceram. Int. 2015, 41, S95–S99. [Google Scholar] [CrossRef]
- Xu, W.; Apodaca, N.; Wang, H.; Yan, L.; Chen, G.; Zhou, M.; Ding, D.; Choudhury, P.; Luo, H. A-site Excessive (La0.8Sr0.2)1+xMnO3 Perovskite Oxides for Bifunctional Oxygen Catalyst in Alkaline Media. ACS Catal. 2019, 9, 5074–5083. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zeng, K.; Wang, S.; Zhang, H.; Li, X.; Wang, Z.; Zhang, C. Revealing the Strong Interaction Effect of MnOx Nanoparticles and Nb2O5 Supports with Variable Morphologies on Catalytic Propane Oxidation. Appl. Surf. Sci. 2022, 576, 13. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Wang, M.; Liu, X.; Shang, S.; Wang, Z.; Zhang, C. Micro-meso Hierarchical ZSM-5 Zeolite Supported RuOx Nanoparticles for Activity Enhancement of Catalytic Vinyl Chloride Oxidation. Appl. Surf. Sci. 2022, 606, 154906. [Google Scholar] [CrossRef]
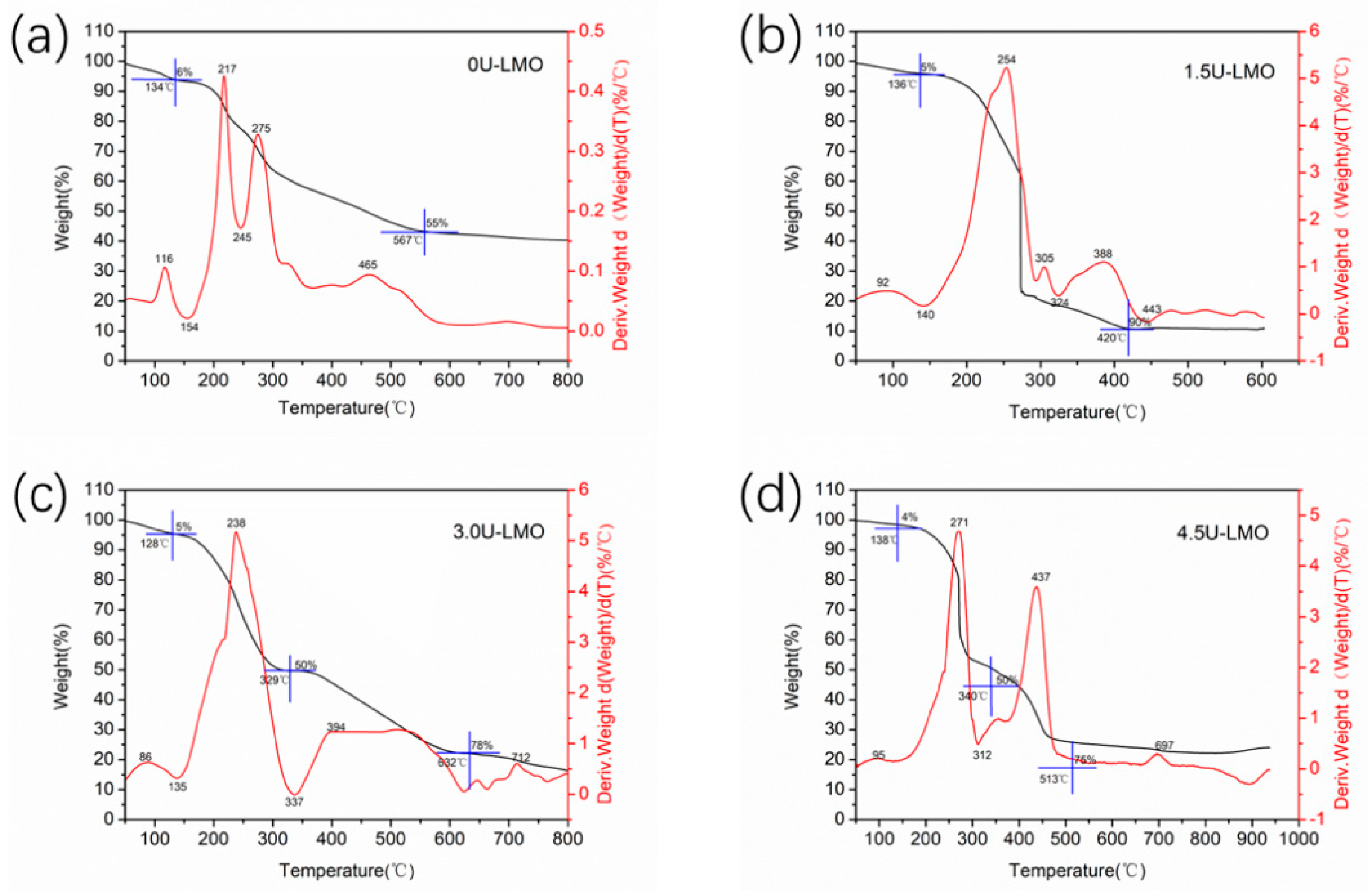
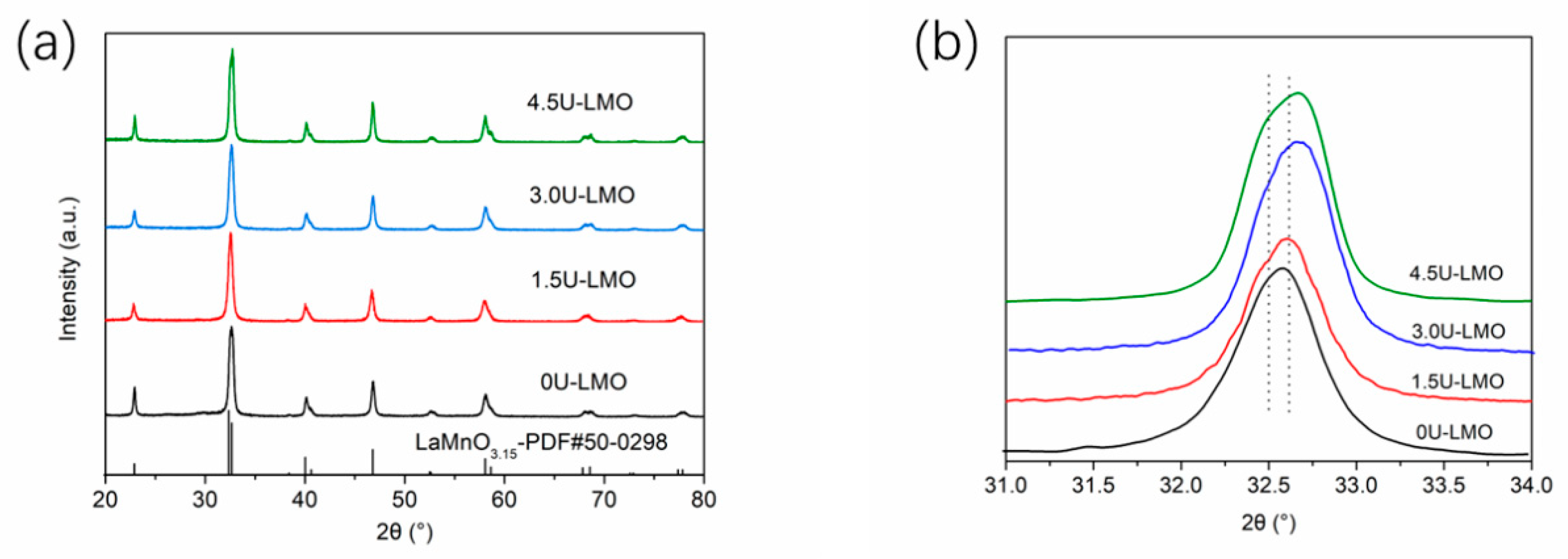
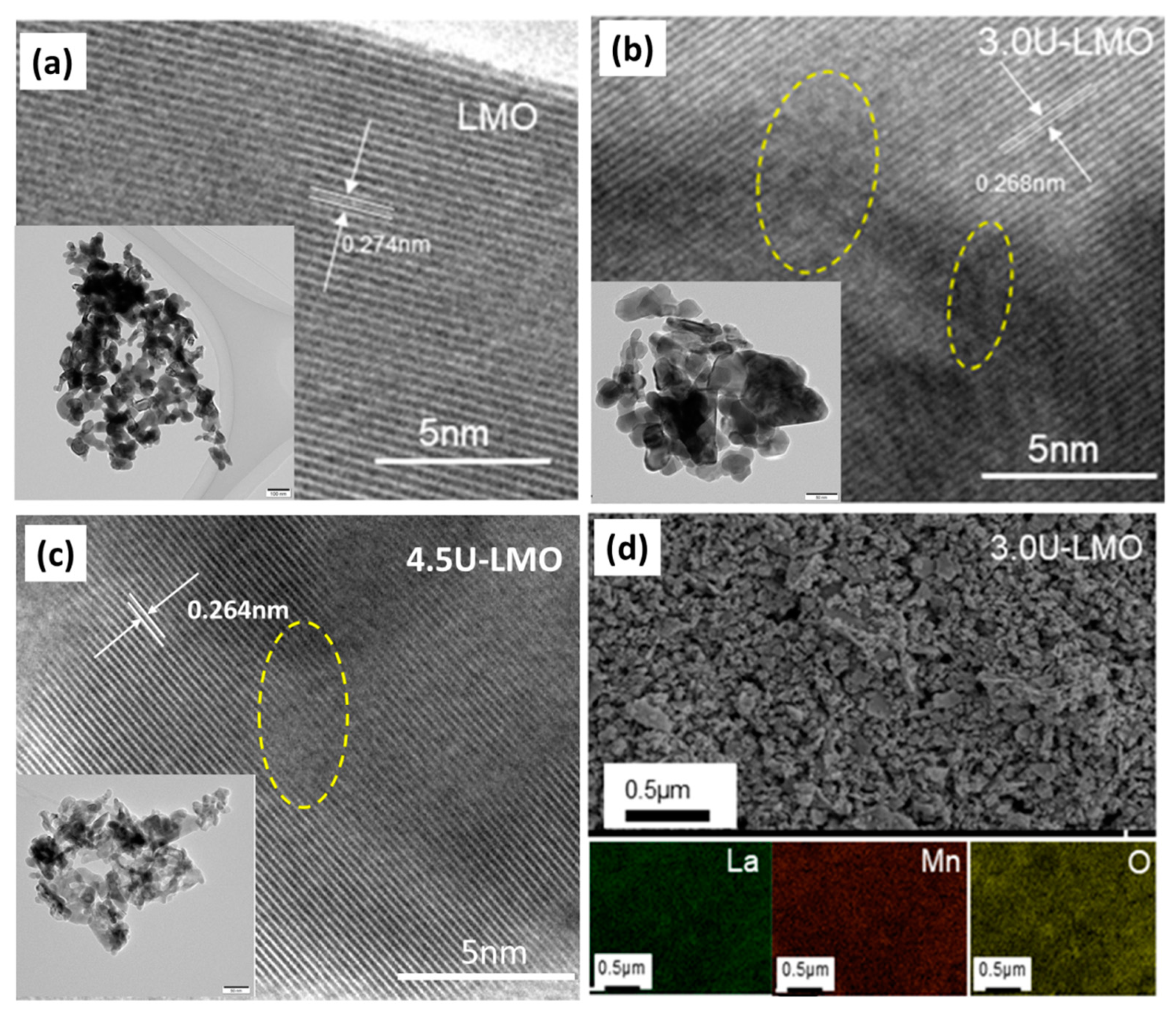
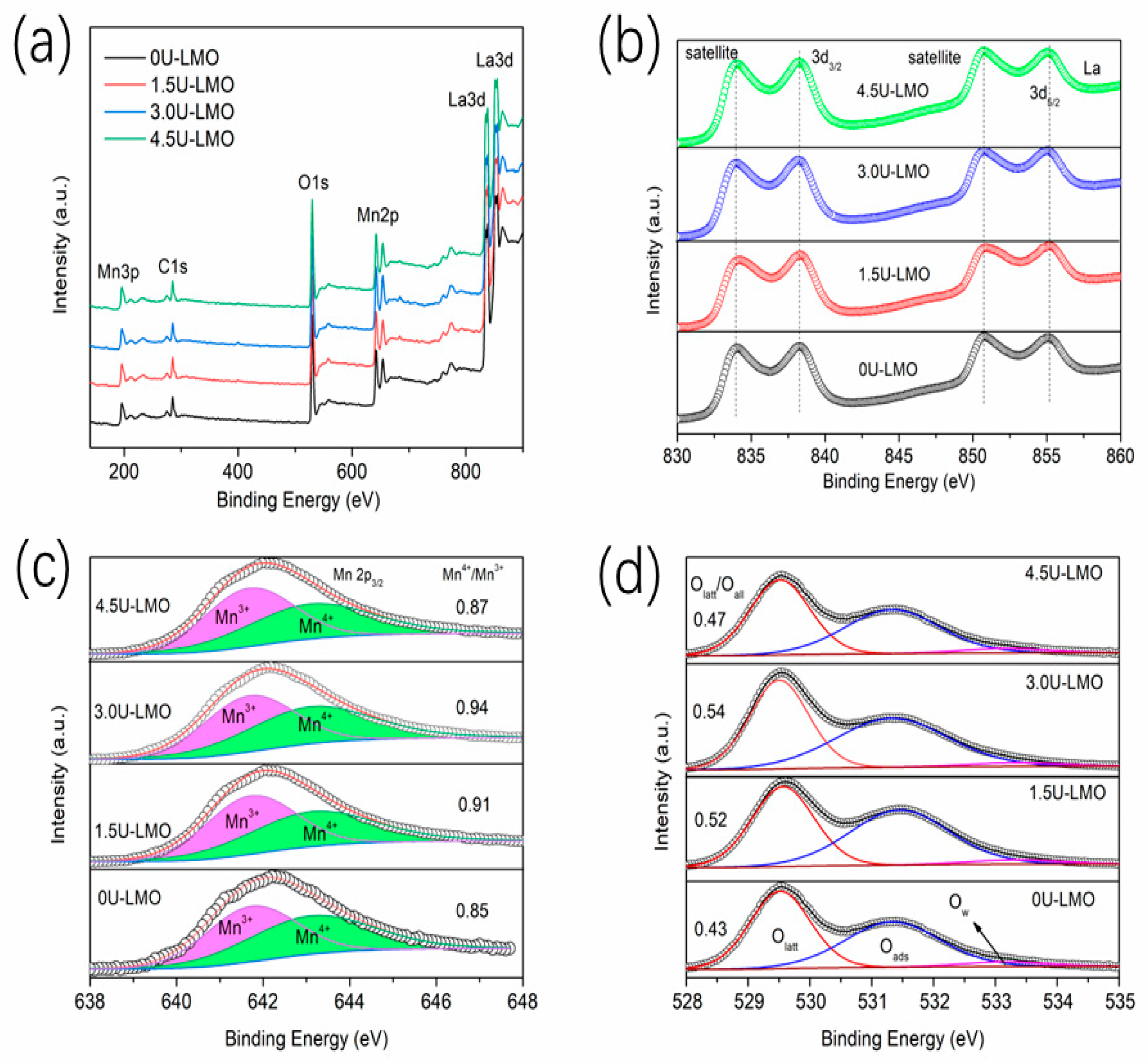
| Samples | Space Group | a | b | c | La-Site Occupy | Rw |
|---|---|---|---|---|---|---|
| 1.5U-LMO | R-3c | 5.5006 (3) | 5.5006 (3) | 13.3471 (4) | 0.90908 | 13.26 |
| 3.0U-LMO | R-3c | 5.5070 (8) | 5.5070 (8) | 13.3757 (8) | 0.86238 | 14.65 |
| 4.5U-LMO | R-3c | 5.5034 (9) | 5.5034 (9) | 13.3248 (3) | 0.93393 | 13.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.; Zheng, Q.; Yu, Y.; Wang, C.; Jiang, S.; Zhang, H.; Liu, Y.; Guo, Y. Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery. Batteries 2023, 9, 90. https://doi.org/10.3390/batteries9020090
Luo K, Zheng Q, Yu Y, Wang C, Jiang S, Zhang H, Liu Y, Guo Y. Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery. Batteries. 2023; 9(2):90. https://doi.org/10.3390/batteries9020090
Chicago/Turabian StyleLuo, Kaikai, Qilong Zheng, Yi Yu, Chunchang Wang, Shanshan Jiang, Haijuan Zhang, Yu Liu, and Youmin Guo. 2023. "Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery" Batteries 9, no. 2: 90. https://doi.org/10.3390/batteries9020090
APA StyleLuo, K., Zheng, Q., Yu, Y., Wang, C., Jiang, S., Zhang, H., Liu, Y., & Guo, Y. (2023). Urea-Assisted Sol-Gel Synthesis of LaMnO3 Perovskite with Accelerated Catalytic Activity for Application in Zn-Air Battery. Batteries, 9(2), 90. https://doi.org/10.3390/batteries9020090







