Integration of Electrode Markings into the Manufacturing Process of Lithium-Ion Battery Cells for Tracking and Tracing Applications
Abstract
1. Introduction
2. State of the Art
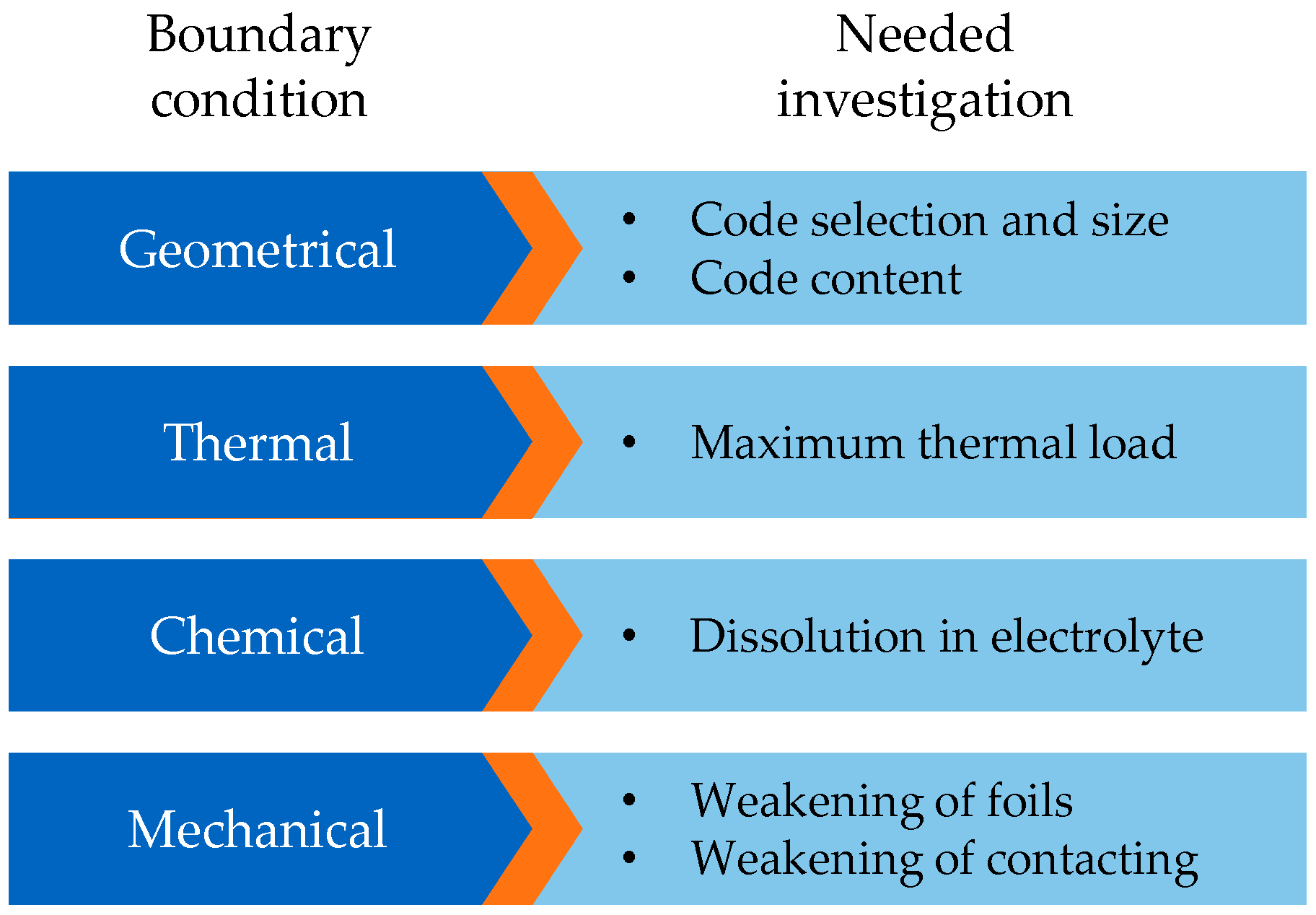
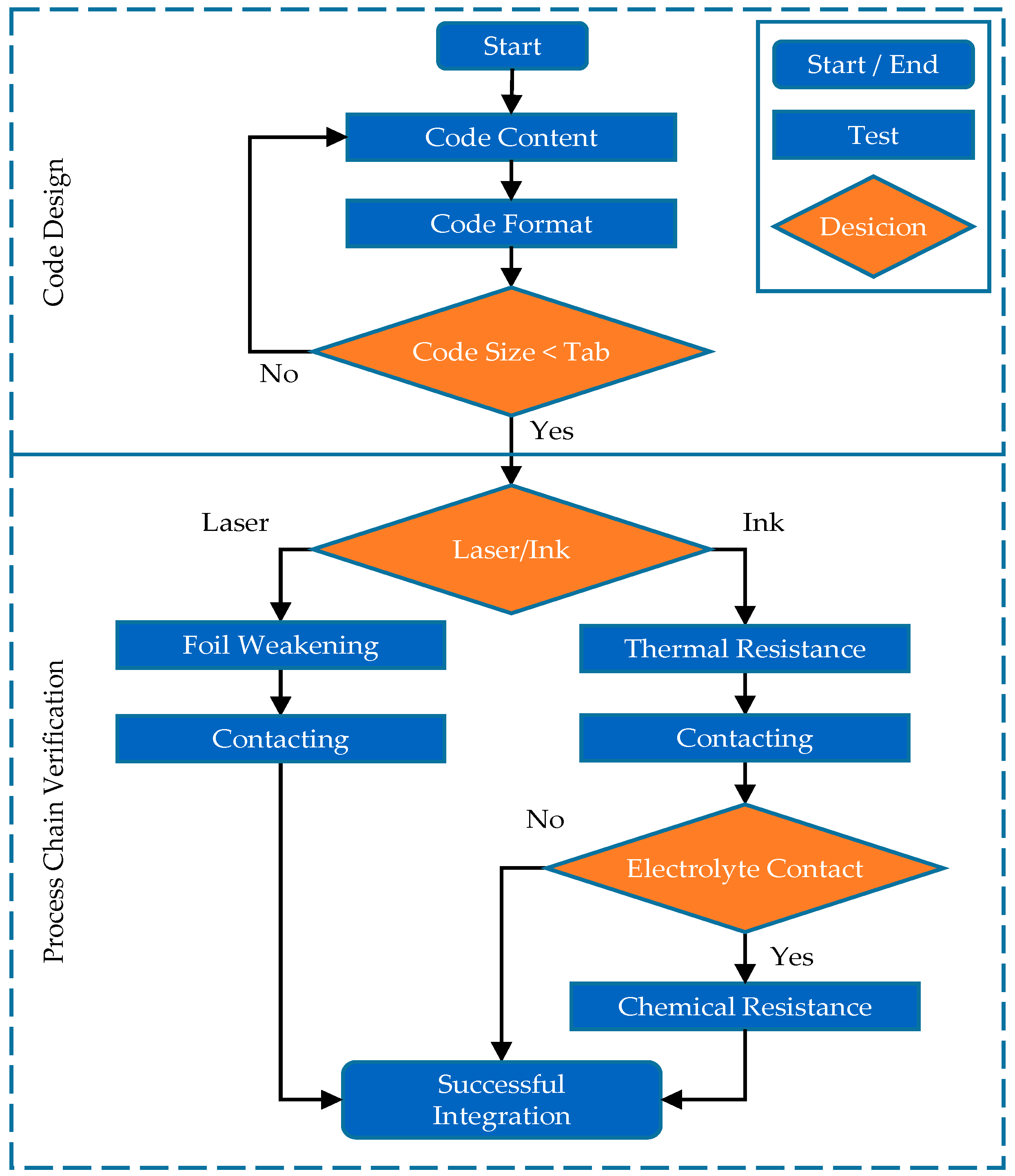
3. Approach and Experimental Scope for the Integration of Marking into Lithium-Ion Battery Cell Production
4. Materials and Methods
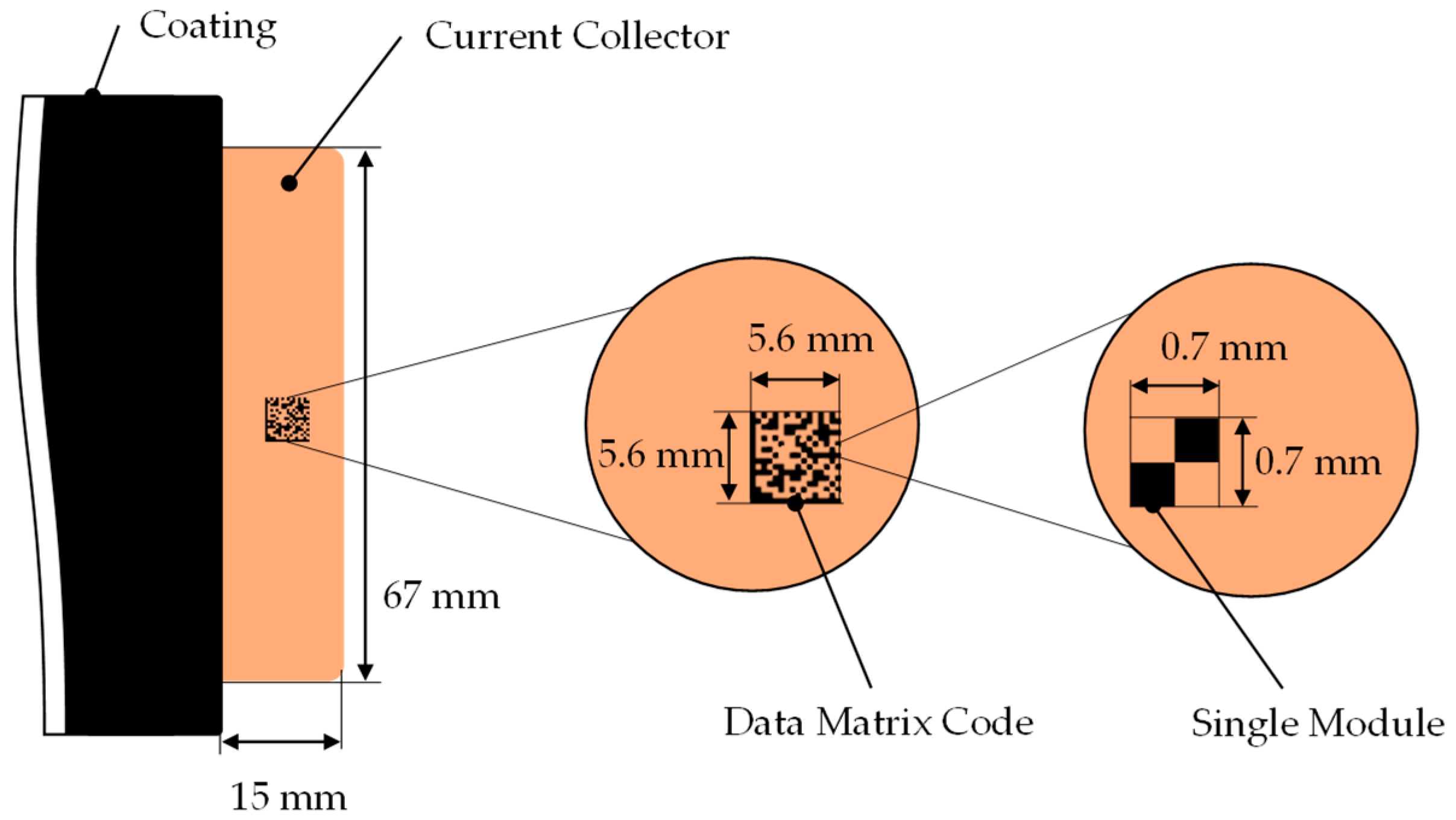
4.1. Geometrical Constraints/Code Design and Application
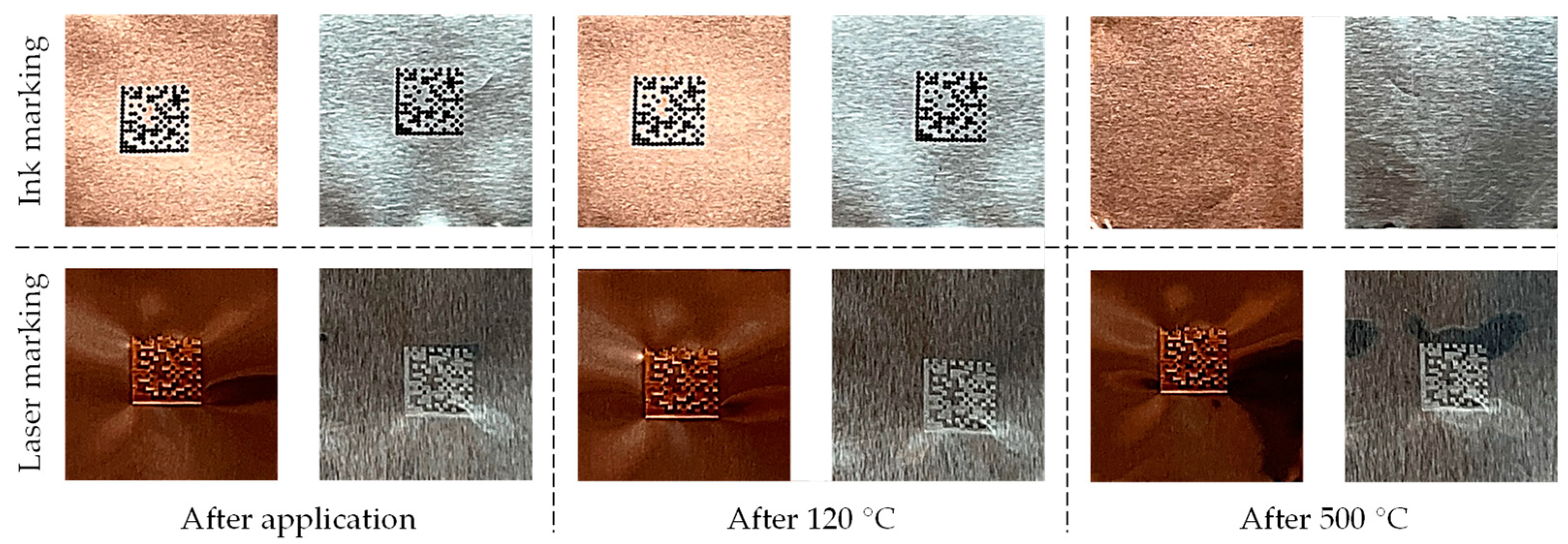
4.2. Thermal Constraints/Thermal Resistance
4.3. Chemical Constraints/Chemical Resistance
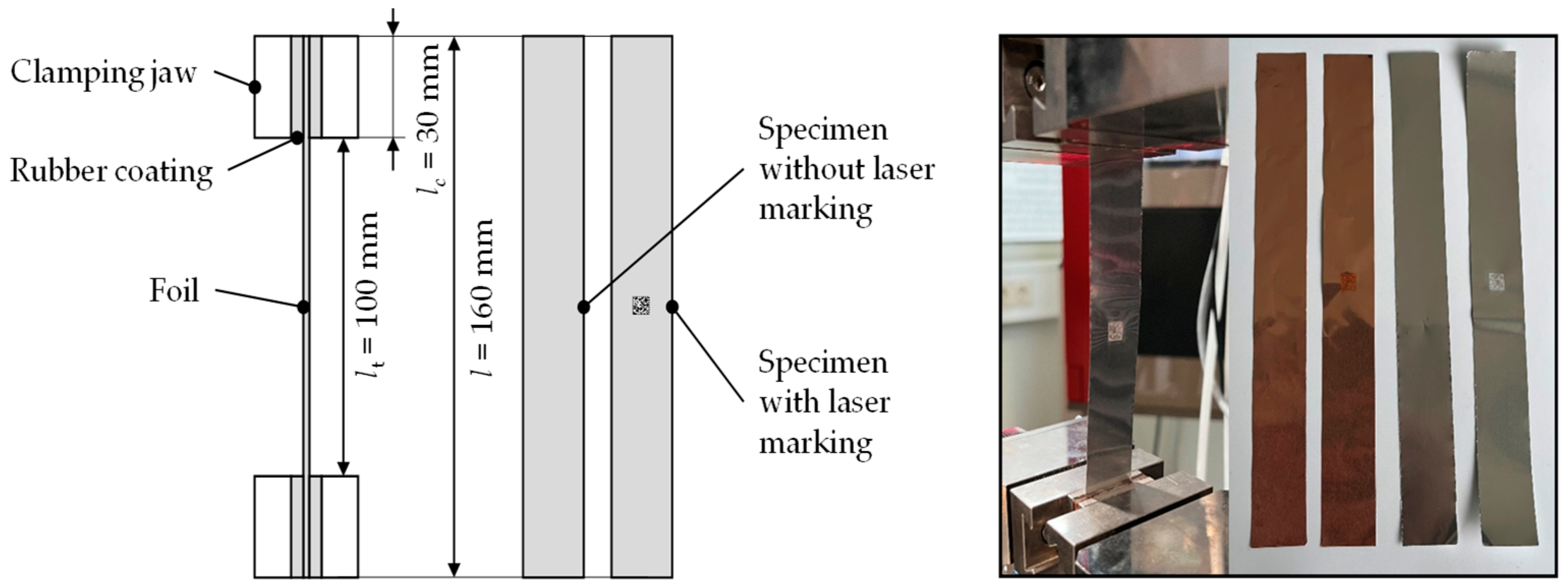
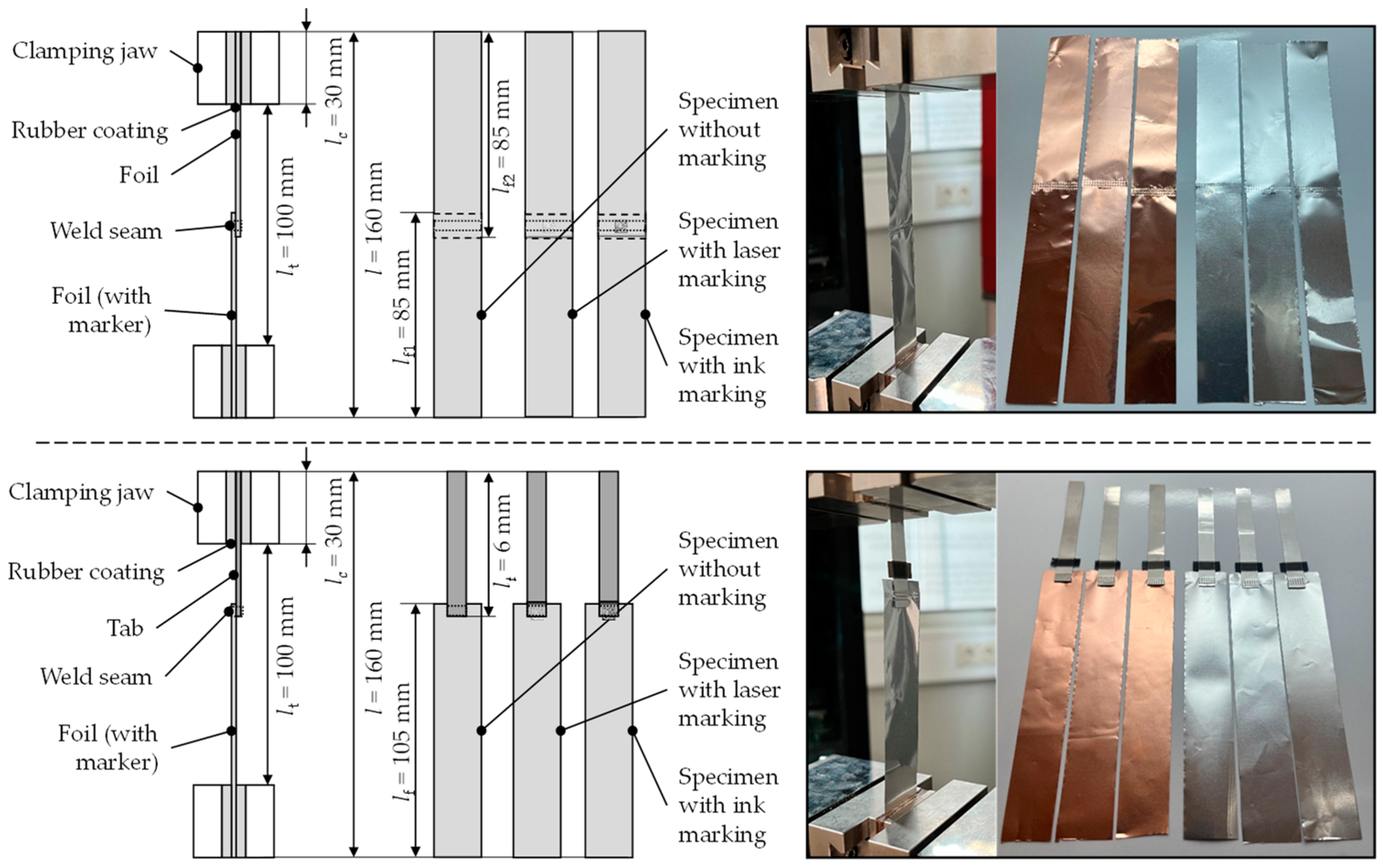
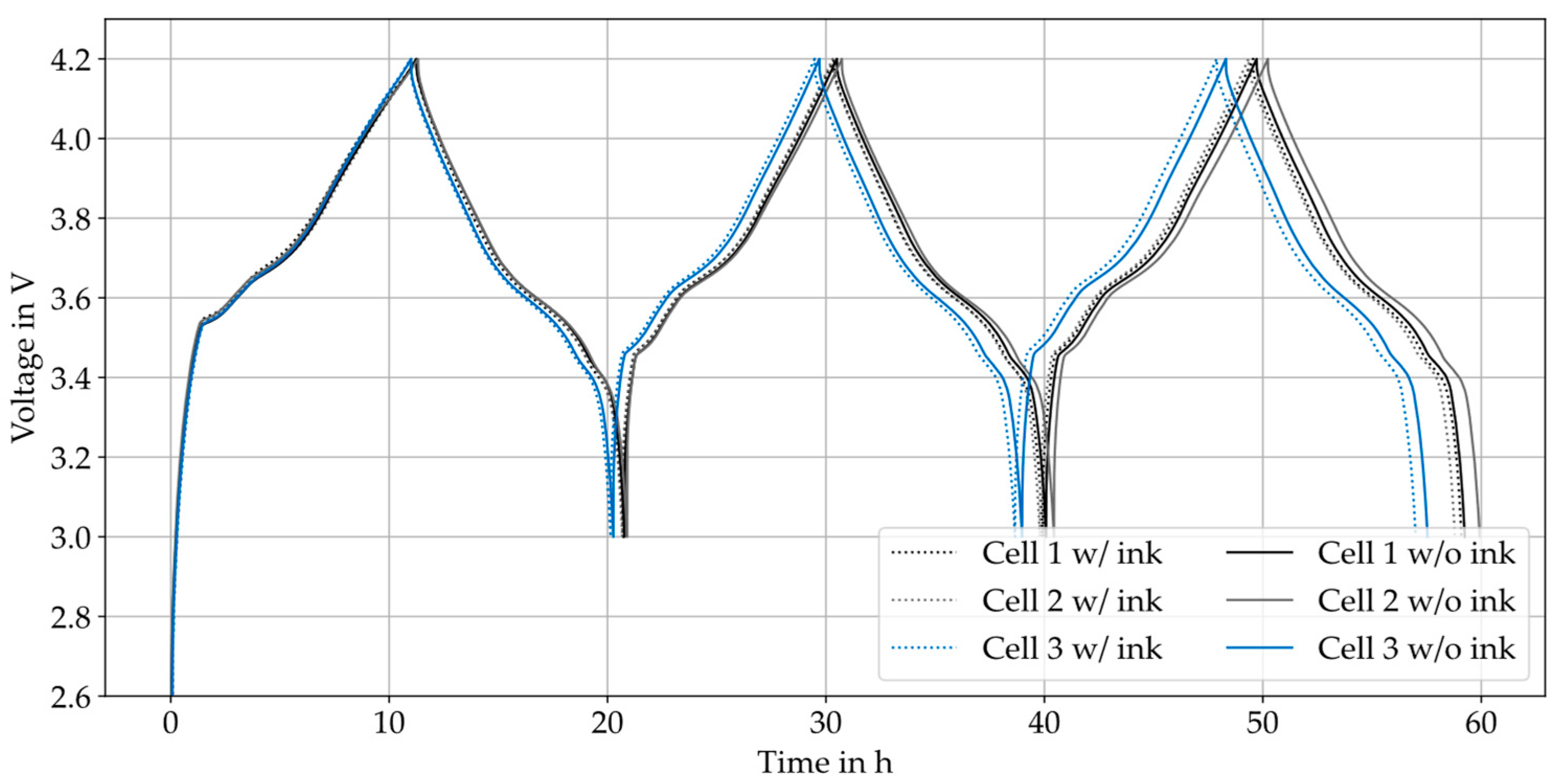
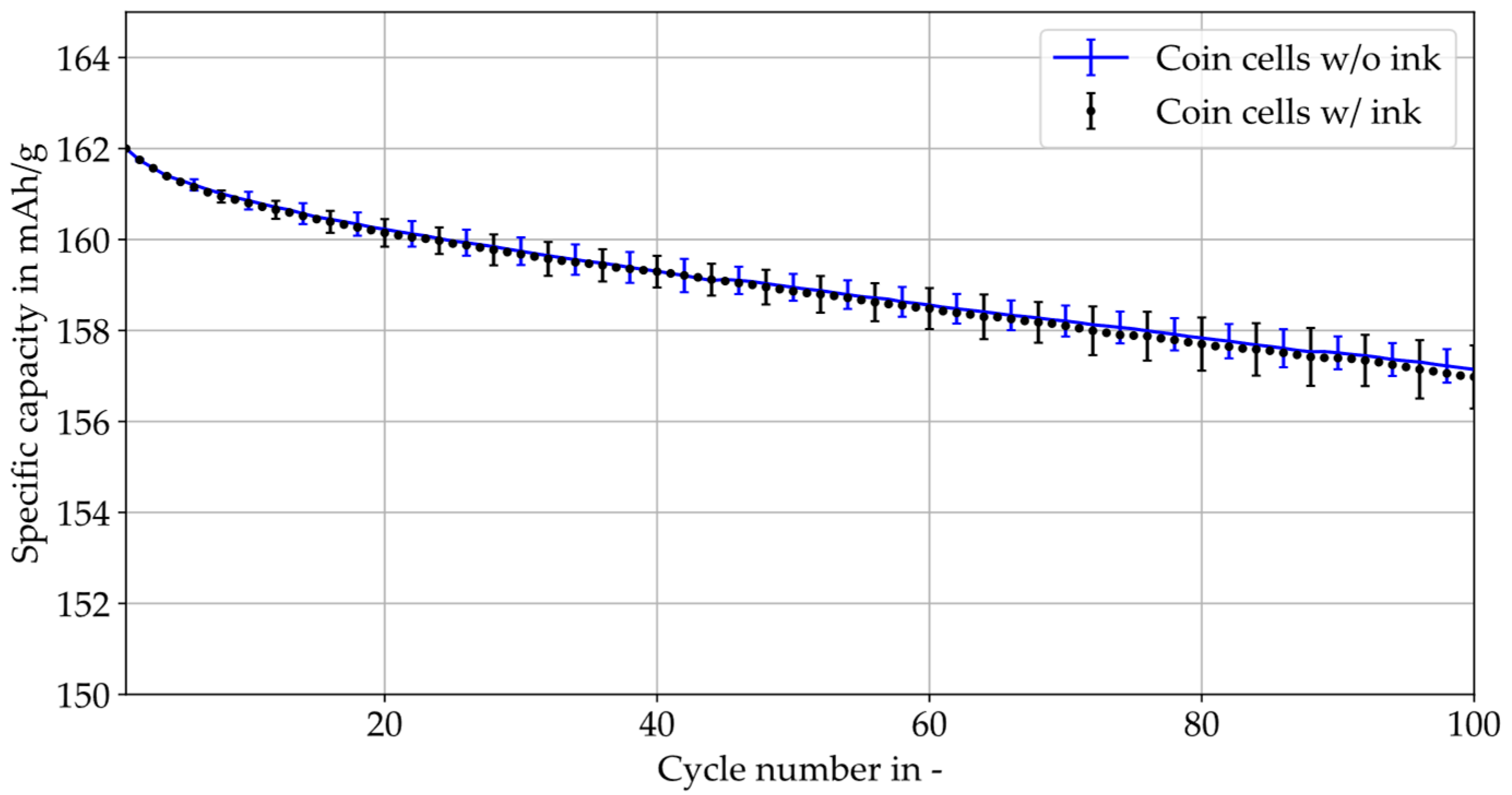
4.4. Mechanical Constraints/Foil Weakening and Contacting
5. Results and Discussion
5.1. Geometrical Constraints/Code Design and Application
5.2. Thermal Constraints/Thermal Resistance
5.3. Chemical Constraints/Chemical Resistance
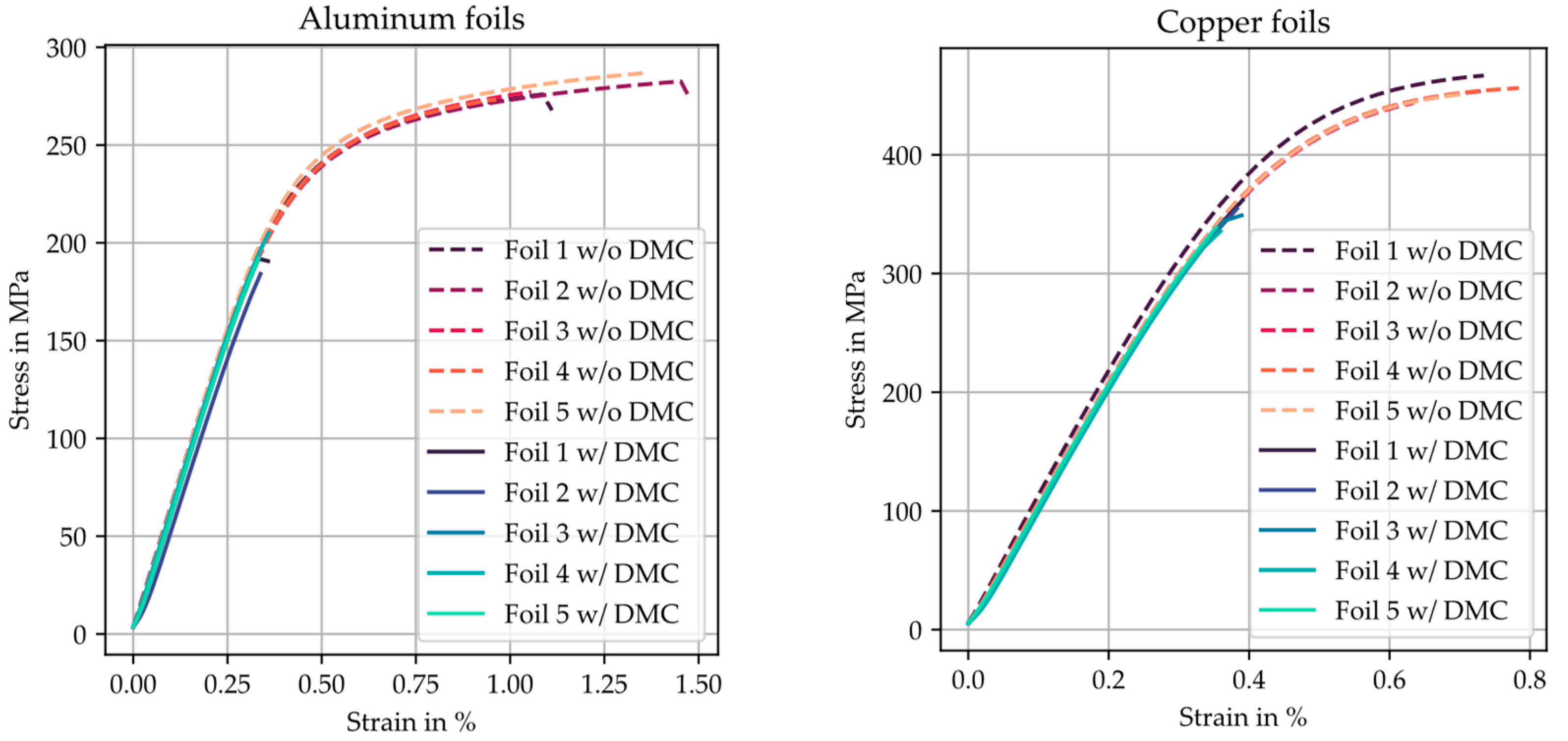
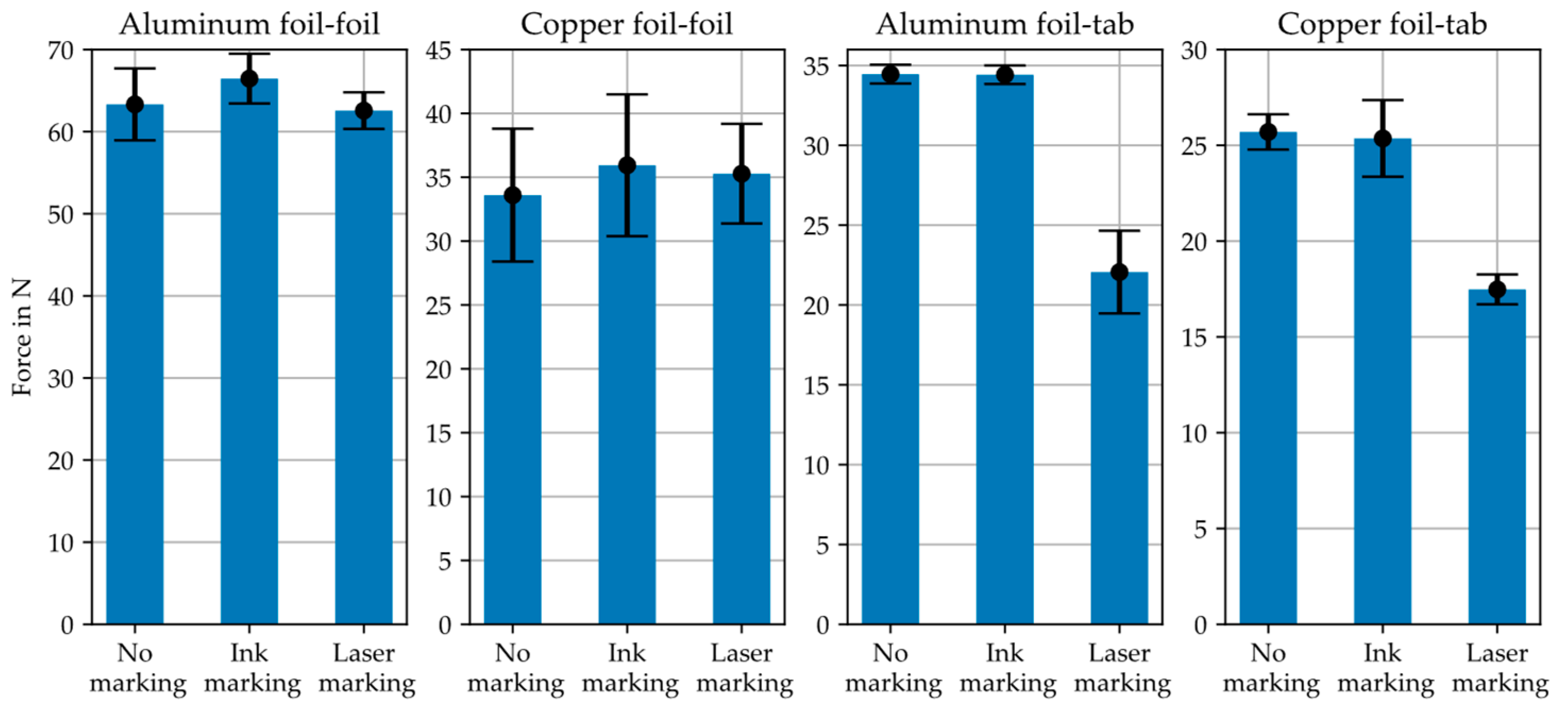
5.4. Mechanical Constraints/Foil Weakening and Contacting
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okafor, C.L.; Ahove, M.A.; Odewumi, S.G. Intergovernmental panel on climate change. climate change 2021: The physical science basis. In Intergovernmental Panel on Climate Change; Springer International Publishing: Cham, Switzerland, 2021; pp. 13–18. [Google Scholar]
- Trenberth, K.E.; Fasullo, J.T. Tracking Earth’s Energy: From El Niño to Global Warming. Surv. Geophys. 2012, 33, 413–426. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hoerpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Pillot, C. The Rechargeable Battery Market and Main Trends 2018-2030. In Proceedings of the International Congress for Battery Recycling, Avicenne Energy Information for Growth, Paris, France, 28 May 2019. [Google Scholar]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Schnell, J.; Nentwich, C.; Endres, F.; Kollenda, A.; Distel, F.; Knoche, T.; Reinhart, G. Data mining in lithium-ion battery cell production. J. Power Sources 2019, 413, 360–366. [Google Scholar] [CrossRef]
- Schnell, J.; Reinhart, G. Quality Management for Battery Production: A Quality Gate Concept. Procedia CIRP 2016, 57, 568–573. [Google Scholar] [CrossRef]
- Stock, S.; Ceruti, A.; Günter, F.J.; Reinhart, G. Introducing Inline Process and Product Analysis for the Lean Cell Finalization in Lithium-Ion Battery Production. Procedia CIRP 2021, 104, 1052–1058. [Google Scholar] [CrossRef]
- Turetskyy, A.; Thiede, S.; Thomitzek, M.; Drachenfels, N.; von Pape, T.; Herrmann, C. Toward Data-Driven Applications in Lithium-Ion Battery Cell Manufacturing. Energy Technol. 2020, 8, 1900136. [Google Scholar] [CrossRef]
- Thiede, S.; Turetskyy, A.; Kwade, A.; Kara, S.; Herrmann, C. Data mining in battery production chains towards multi-criterial quality prediction. CIRP Ann. 2019, 68, 463–466. [Google Scholar] [CrossRef]
- Haug, A.; Zachariassen, F.; van Liempd, D. The costs of poor data quality. JIEM 2011, 4, 168–193. [Google Scholar] [CrossRef]
- Guenther, T.; Billot, N.; Schuster, J.; Schnell, J.; Spingler, F.B.; Gasteiger, H.A. The Manufacturing of Electrodes: Key Process for the Future Success of Lithium-Ion Batteries. AMR 2016, 1140, 304–311. [Google Scholar] [CrossRef]
- Riexinger, G.; Doppler, J.P.; Haar, C.; Trierweiler, M.; Buss, A.; Schoebel, K.; Ensling, D.; Bauernhansl, T. Integration of Traceability Systems in Battery Production. Procedia CIRP 2020, 93, 125–130. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 9000:2015-11(en) Quality Management Systems—Fundamentals and Vocabulary; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- Pizzuti, T.; Mirabelli, G.; Sanz-Bobi, M.A.; Goméz-Gonzaléz, F. Food Track & Trace ontology for helping the food traceability control. J. Food Eng. 2014, 120, 17–30. [Google Scholar] [CrossRef]
- Heyder, M.; Theuvsen, L.; Hollmann-Hespos, T. Investments in tracking and tracing systems in the food industry: A PLS analysis. Food Policy 2012, 37, 102–113. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Steinberger, G.; Rothmund, M. A model and prototype implementation for tracking and tracing agricultural batch products along the food chain. Food Control 2010, 21, 112–121. [Google Scholar] [CrossRef]
- Rotunno, R.; Cesarotti, V.; Bellman, A.; Introna, V.; Benedetti, M. Impact of Track and Trace Integration on Pharmaceutical Production Systems. Int. J. Eng. Bus. Manag. 2014, 6, 25. [Google Scholar] [CrossRef]
- Wessel, J.; Turetskyy, A.; Wojahn, O.; Herrmann, C.; Thiede, S. Tracking and Tracing for Data Mining Application in the Lithium-ion Battery Production. Procedia CIRP 2020, 93, 162–167. [Google Scholar] [CrossRef]
- Wittine, N.; Wenzel, S.; Kern, C.; Refflinghaus, R.; Trostmann, T. Introduction of Traceability into the Continuous Improvement Process of SMEs; Publish-Ing: Hannover, Germany, 2020. [Google Scholar]
- Sommer, A.; Leeb, M.; Haghi, S.; Guenter, F.J.; Reinhart, G. Marking of Electrode Sheets in the Production of Lithium-Ion Cells as an Enabler for Tracking and Tracing. Procedia CIRP 2021, 104, 1011–1016. [Google Scholar] [CrossRef]
- GS1 Germany GmbH. GS1 DataMatrix Guideline: Overview and Technical Introduction to the Use of GS1 DataMatrix, 2.5.1; GS1 AISBL: Cologne, Germany, 2018; pp. 1–60. [Google Scholar]
- National Aeronautics and Space Administration. Applying Data Matrix Identification Symbols on Aerospace Parts: NASA-STD-6002D; National Aeronautics and Space Administration: Washington, DC, USA, 2008; pp. 29–32.
- Lazov, L.; Deneva, H.; Narica, P. Laser Marking Methods. ETR 2015, 1, 108. [Google Scholar] [CrossRef]
- Shawn Lee, S.; Hyung Kim, T.; Jack Hu, S.; Cai, W.W.; Abell, J.A.; Li, J. Characterization of Joint Quality in Ultrasonic Welding of Battery Tabs. J. Manuf. Sci. Eng. 2013, 135, 541. [Google Scholar] [CrossRef]
- Liukkonen, M.; Tsai, T.-N. Toward decentralized intelligence in manufacturing: Recent trends in automatic identification of things. Int. J. Adv. Manuf. Technol. 2016, 87, 2509–2531. [Google Scholar] [CrossRef]
- Kern, C.; Refflinghaus, R.; Trostmann, T.; Wenzel, S.; Wittine, N.; Herrlich, F. Concept for selecting and integrating traceability systems in the continuous improvement process of SMEs, Excellence in Services. In Proceedings of the 22nd International Conference, Thessaloniki, Greece, 28–31 October 2019; pp. 350–363. [Google Scholar]
- International Organization for Standardization; International Electrotechnical Commission. ISO/IEC 16022:2006 (E): Information Technology—Automatic Identification and Data Capture Techniques—Data Matrix Bar Code Symbology Specification; Beuth Verlag GmbH: Berlin, Germany, 2006. [Google Scholar]
- International Organization for Standardization; International Electrotechnical Commission. ISO/IEC 18004:2015 (E): Information Technology—Automatic Identification and Data Capture Techniques—QR Code Bar Code Symbology Specification; Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- International Organization for Standardization; International Electrotechnical Commission. ISO/IEC 24724 (E): Information Technology—Automatic Identification and Data Capture Techniques—GS1 DataBar Bar Code Symbology Specification; Beuth Verlag GmbH: Berlin, Germany, 2011. [Google Scholar]
- Kriegler, J.; Hille, L.; Stock, S.; Kraft, L.; Hagemeister, J.; Habedank, J.B.; Jossen, A.; Zaeh, M.F. Enhanced performance and lifetime of lithium-ion batteries by laser structuring of graphite anodes. Appl. Energy 2021, 303, 117693. [Google Scholar] [CrossRef]
- Schreiner, D.; Zuend, T.; Guenter, F.J.; Kraft, L.; Stumper, B.; Linsenmann, F.; Schueßler, M.; Wilhelm, R.; Jossen, A.; Reinhart, G.; et al. Comparative Evaluation of LMR-NCM and NCA Cathode Active Materials in Multilayer Lithium-Ion Pouch Cells: Part I Production, Electrode Characterization, and Formation. J. Electrochem. Soc. 2021, 168, 30507. [Google Scholar] [CrossRef]
- Guenter, F.J.; Roessler, S.; Schulz, M.; Braunwarth, W.; Gilles, R.; Reinhart, G. Influence of the Cell Format on the Electrolyte Filling Process of Lithium-Ion Cells. Energy Technol. 2020, 8, 1801108. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, T.H.; Hu, S.J.; Cai, W.W.; Abell, J.A. Joining Technologies for Automotive Lithium-Ion Battery Manufacturing: A Review. In Proceedings of the ASME 2010 International Manufacturing Science and Engineering Conference, Erie, PA, USA, 12–15 October 2010; pp. 541–549. [Google Scholar]
- Grabmann, S.; Tomcic, L.; Zaeh, M.F. Laser beam welding of copper foil stacks using a green high power disk laser. Procedia CIRP 2020, 94, 582–586. [Google Scholar] [CrossRef]
- Grabmann, S.; Kriegler, J.; Harst, F.; Guenter, F.J.; Zaeh, M.F. Laser welding of current collector foil stacks in battery production–mechanical properties of joints welded with a green high-power disk laser. Int. J. Adv. Manuf. Technol. 2022, 118, 2571–2586. [Google Scholar] [CrossRef]
- Guenter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the Electrolyte Quantity on Lithium-Ion Cells. J. Electrochem. Soc. 2019, 166, A1709–A1714. [Google Scholar] [CrossRef]


| Cells | 1st Cycle | 2nd Cycle | 3rd Cycle | Mean Capacity in mAh | SD | |||
|---|---|---|---|---|---|---|---|---|
| Mean CE in % | SD | Mean CE in % | SD | Mean CE in % | SD | |||
| w/o ink | 84.4 | 0.2 | 98.6 | 0.2 | 99.0 | 0.1 | 4.270 | 0.1 |
| w/ink | 83.6 | 0.2 | 98.4 | 0.2 | 98.8 | 0.1 | 4.213 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, A.; Leeb, M.; Weishaeupl, L.; Daub, R. Integration of Electrode Markings into the Manufacturing Process of Lithium-Ion Battery Cells for Tracking and Tracing Applications. Batteries 2023, 9, 89. https://doi.org/10.3390/batteries9020089
Sommer A, Leeb M, Weishaeupl L, Daub R. Integration of Electrode Markings into the Manufacturing Process of Lithium-Ion Battery Cells for Tracking and Tracing Applications. Batteries. 2023; 9(2):89. https://doi.org/10.3390/batteries9020089
Chicago/Turabian StyleSommer, Alessandro, Matthias Leeb, Lukas Weishaeupl, and Ruediger Daub. 2023. "Integration of Electrode Markings into the Manufacturing Process of Lithium-Ion Battery Cells for Tracking and Tracing Applications" Batteries 9, no. 2: 89. https://doi.org/10.3390/batteries9020089
APA StyleSommer, A., Leeb, M., Weishaeupl, L., & Daub, R. (2023). Integration of Electrode Markings into the Manufacturing Process of Lithium-Ion Battery Cells for Tracking and Tracing Applications. Batteries, 9(2), 89. https://doi.org/10.3390/batteries9020089






