Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries
Abstract
1. Introduction
- (1)
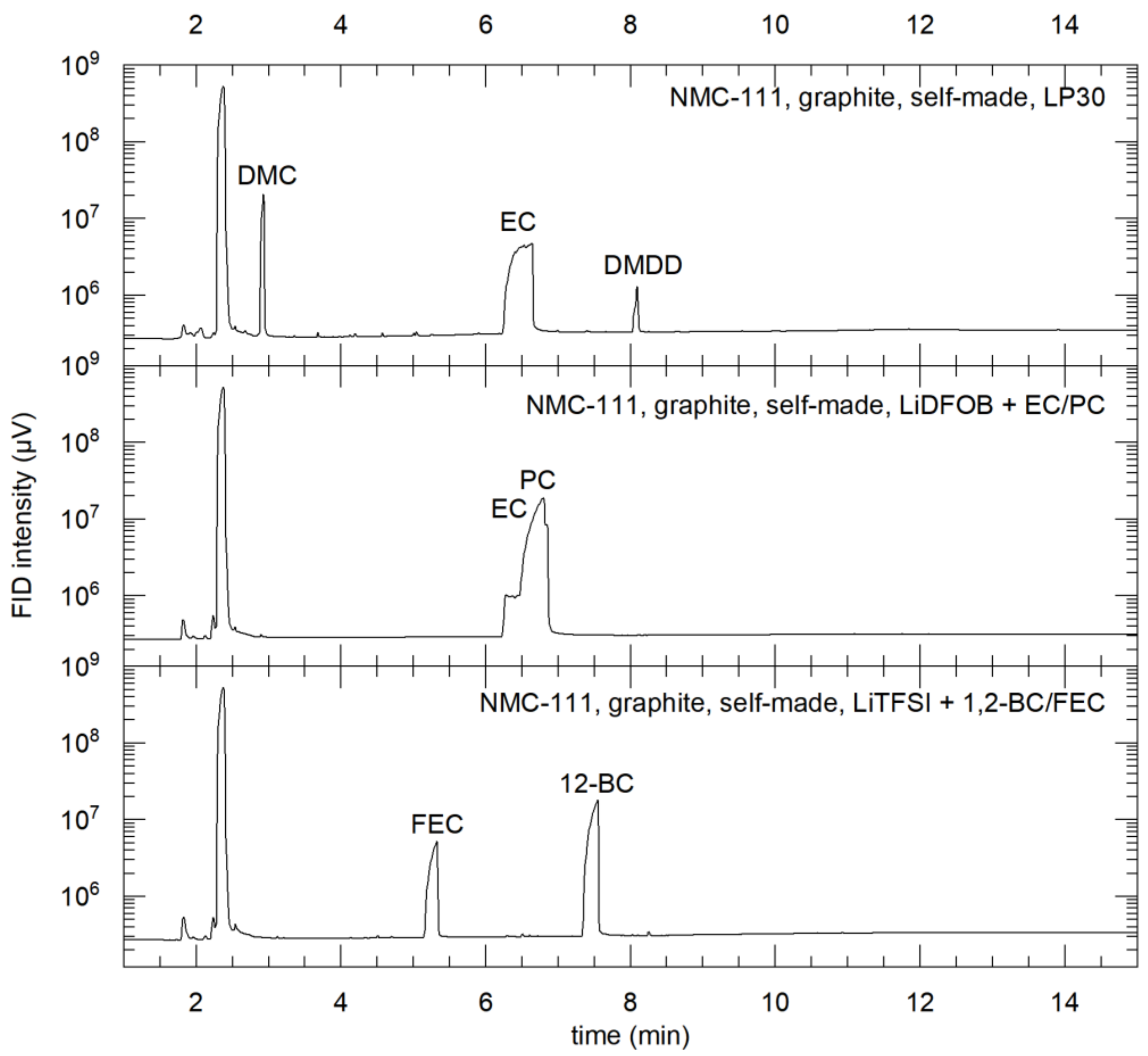
- An LP30-standard Li-ion battery electrolyte containing ethylene carbonate (EC) and dimethyl carbonate (DMC) in equivalent volumetric parts as solvents and 1 M LiPF6 as a conductive salt;
- (2)
- Ethylene carbonate (EC) and propylene carbonate (PC) + 1 M LiDFOB;
- (3)
- 1,2-butylene carbonate (1,2-BC) and fluoroethylene carbonate (FEC) + 1 M LiTFSI.
2. Materials and Methods
2.1. Chemicals
2.2. Electrolytes
2.3. Electrode and Cell Preparation
2.4. Li-Ion Battery Cells
2.5. Methods
2.5.1. Gas Chromatography Coupled to Mass Spectrometry (GC-MS, Gas)
2.5.2. Gas Chromatography Coupled to Mass Spectrometry (GC-MS, Liquid)
2.5.3. Light Microscopy
2.5.4. Mandrel Bend Test
2.5.5. Resistance
2.5.6. Rheology
2.5.7. Scanning Electron Microscope (SEM)
2.5.8. Solubility
2.5.9. Thermogravimetric Analysis (TGA-IR)
2.5.10. Overcharging Abuse Test
3. Results
3.1. Electrolyte Formulations and Electrolyte Characteristics
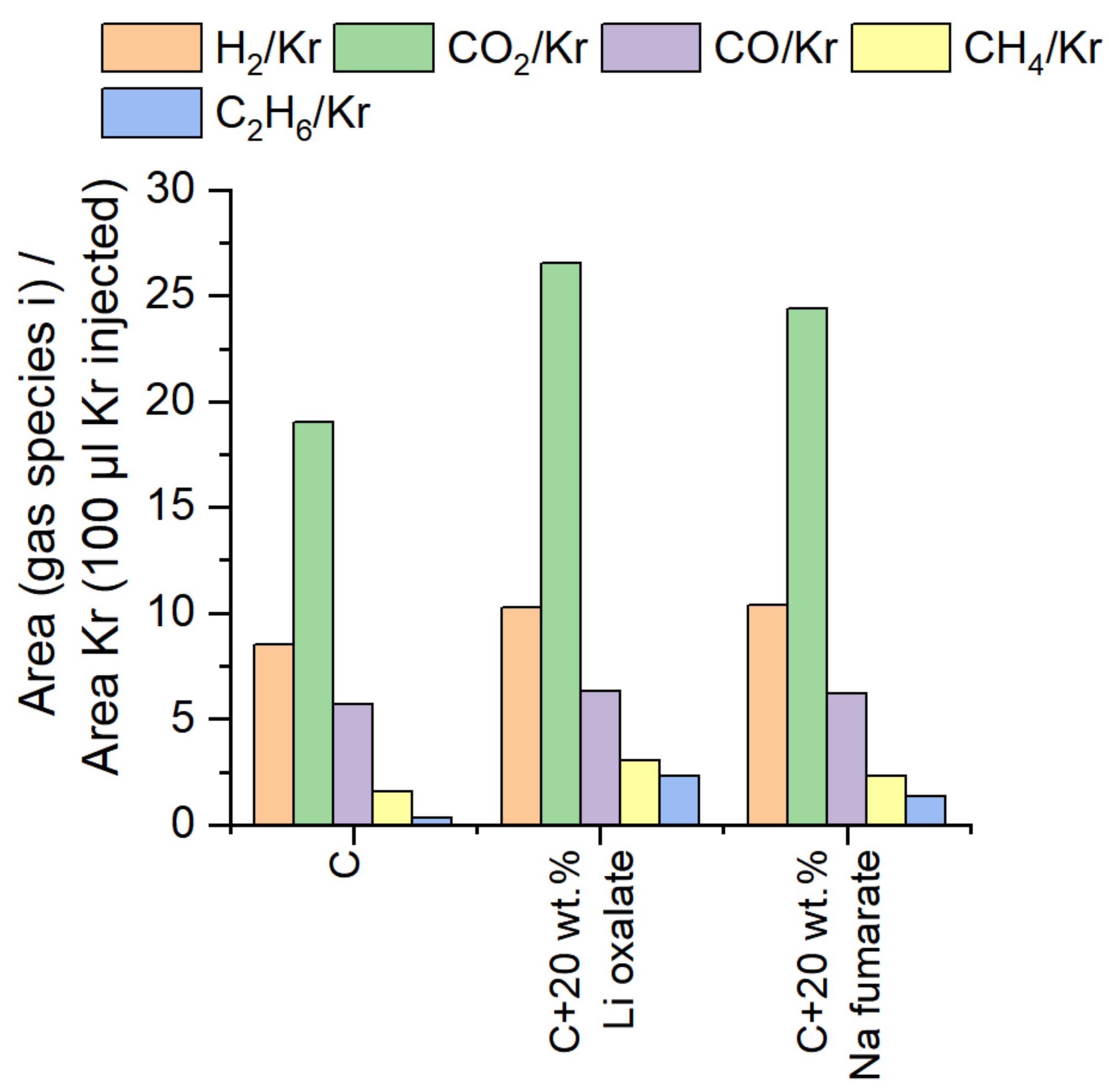
3.2. Flame Retardants and CO2 Release
3.3. Electrode Slurry Preparation and Anode Characterization
3.4. Assembly and Testing of Lithium-Ion Cells
3.5. Post-Mortem Analysis of the Li-Ion Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, E.P.; Orendorff, C.J. How electrolytes influence battery safety. Electrochem. Soc. Interface 2012, 21, 45–49. [Google Scholar] [CrossRef]
- Doughty, D.H.; Roth, E.P. A general discussion of Li ion battery safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar]
- Hou, J.; Wang, L.; Feng, X.; Terada, J.; Lu, L.; Yamazaki, S.; Su, A.; Kuwajima, Y.; Chen, Y.; Hidaka, T.; et al. Thermal Runaway of Lithium-Ion Batteries Employing Flame-Retardant Fluorinated Electrolytes. Energy Environ. Mater. 2022, 0, 1–7. [Google Scholar] [CrossRef]
- Orendorff, C.J. The role of separators in lithium-ion cell safety. Electrochem. Soc. Interface 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Ravdel, B.; Abraham, K.M.; Gitzendanner, R.; DiCarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119–121, 805–810. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Mu, X.; Zhou, M.; Cai, W.; Song, L.; Xing, W.; Hu, Y. Polyphosphazenes-based flame retardants: A review. Compos. B Eng. 2020, 202, 108397–108414. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Metal-organic frameworks for flame retardant polymers application: A critical review. Compos. A Appl. Sci. Manuf. 2020, 139, 106113–106123. [Google Scholar] [CrossRef]
- Gao, F.; Liu, H.; Yang, K.; Zeng, C.; Wang, S.; Fan, M.; Wang, H. A review on materials for flame retarding and improving the thermal stability of lithium ion batteries. Int. J. Electrochem. Sci. 2020, 15, 1391–1411. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Indris, S.; Heinzmann, R.; Schulz, M.; Hofmann, A. Ionic liquid based electrolytes: Correlating Li diffusion coefficients and battery performance. J. Electrochem. Soc. 2014, 161, A2036–A2041. [Google Scholar] [CrossRef]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Lee, D.J.; Im, D.; Ryu, Y.-G.; Lee, S.; Yoon, J.; Lee, J.; Choi, W.; Jung, I.; Lee, S.; Doo, S.-G. Phosphorus derivatives as electrolyte additives for lithium-ion battery: The removal of O2 generated from lithium-rich layered oxide cathode. J. Power Sources 2013, 243, 831–835. [Google Scholar] [CrossRef]
- Dagger, T.; Grützke, M.; Reichert, M.; Haetge, J.; Nowak, S.; Winter, M.; Schappacher, F.M. Investigation of lithium ion battery electrolytes containing flame retardants in combination with the film forming electrolyte additives vinylene carbonate, vinyl ethylene carbonate and fluoroethylene carbonate. J. Power Sources 2017, 372, 276–285. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-retardant additives for lithium-ion batteries. J. Power Sources 2003, 119–121, 383–387. [Google Scholar] [CrossRef]
- Matsumoto, K.; Martinez, M.; Gutel, T.; Mailley, S.; De vito, E.; Patoux, S.; Inoue, K.; Utsugi, K. Stability of trimethyl phosphate non-flammable based electrolyte on the high voltage cathode (LiNi0.5Mn1.5O4). J. Power Sources 2015, 273, 1084–1088. [Google Scholar] [CrossRef]
- Milien, M.S.; Beyer, H.; Beichel, W.; Klose, P.; Gasteiger, H.A.; Lucht, B.L.; Krossing, I. Lithium bis(2,2,2-trifluoroethyl)phosphate Li[O2P(OCH2CF3)2]: A high voltage additive for LNMO/Graphite cells. J. Electrochem. Soc. 2018, 165, A2569–A2576. [Google Scholar] [CrossRef]
- Pires, J.; Castets, A.; Timperman, L.; Santos-Peña, J.; Dumont, E.; Levasseur, S.; Tessier, C.; Dedryvère, R.; Anouti, M. Tris(2,2,2-trifluoroethyl) phosphite as an electrolyte additive for high-voltage lithium-ion batteries using lithium-rich layered oxide cathode. J. Power Sources 2015, 296, 413–425. [Google Scholar] [CrossRef]
- Wu, B.; Pei, F.; Wu, Y.; Mao, R.; Ai, X.; Yang, H.; Cao, Y. An electrochemically compatible and flame-retardant electrolyte additive for safe lithium ion batteries. J. Power Sources 2013, 227, 106–110. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Li, B.; Li, W.; Li, X.; Hu, S. Tris (pentafluorophenyl) phosphine: An electrolyte additive for high voltage Li-ion batteries. Electrochem. Commun. 2012, 18, 123–126. [Google Scholar] [CrossRef]
- Todorov, Y.M.; Aoki, M.; Mimura, H.; Fujii, K.; Yoshimoto, N.; Morita, M. Thermal and electrochemical properties of nonflammable electrolyte solutions containing fluorinated alkylphosphates for lithium-ion batteries. J. Power Sources 2016, 332, 322–329. [Google Scholar] [CrossRef]
- Sogawa, M.; Kawanoue, H.; Todorov, Y.M.; Hirayama, D.; Mimura, H.; Yoshimoto, N.; Morita, M.; Fujii, K. Solvation-controlled lithium-ion complexes in a nonflammable solvent containing ethylene carbonate: Structural and electrochemical aspects. Phys. Chem. Chem. Phys. 2018, 20, 6480–6486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hofmann, A.; Hanemann, T. Low-flammable electrolytes with fluoroethylene carbonate based solvent mixtures and lithium bis(trifluoromethanesulfonyl)imide for lithium-ion batteries. Electrochim. Acta 2019, 298, 960–972. [Google Scholar] [CrossRef]
- Zu, C.; Yu, H.; Li, H. Enabling the thermal stability of solid electrolyte interphase in Li-ion battery. Infomat 2021, 3, 648–661. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z.; et al. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235–4245. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Q.; Li, K.; Ping, P.; Jiang, L.; Sun, J. A self-cooling and flame-retardant electrolyte for safer lithium ion batteries. Sustain. Energy Fuels 2018, 2, 1323–1331. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Demirocak, D.; Srinivasan, S.; Stefanakos, E. A review on nanocomposite materials for rechargeable Li-ion batteries. Appl. Sci. 2017, 7, 731–757. [Google Scholar] [CrossRef]
- Gogia, A.; Wang, Y.; Rai, A.K.; Bhattacharya, R.; Subramanyam, G.; Kumar, J. Binder-free, thin-film ceramic-coated separators for improved safety of lithium-ion batteries. ACS Omega 2021, 6, 4204–4211. [Google Scholar] [CrossRef]
- Zeng, G.; Zhao, J.; Feng, C.; Chen, D.; Meng, Y.; Boateng, B.; Lu, N.; He, W. Flame-retardant bilayer separator with multifaceted van der Waals interaction for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 26402–26411. [Google Scholar] [CrossRef]
- Luo, X.; Lu, X.; Chen, X.; Chen, Y.; Song, C.; Yu, C.; Wang, N.; Su, D.; Wang, C.; Gao, X.; et al. A robust flame retardant fluorinated polyimide nanofiber separator for high-temperature lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 14788–14798. [Google Scholar] [CrossRef]
- Liu, H.; Yang, F.; Xiang, M.; Cao, Y.; Wu, T. Development of multilayer polypropylene separators for lithium-ion batteries via an industrial process. Ind. Eng. Chem. Res. 2021, 60, 11611–11620. [Google Scholar] [CrossRef]
- Yan, N.; Ding, L.; Wu, T.; Zhang, S.; Yang, F.; Cao, Y.; Xiang, M. Shutdown-functionalized poly ethylene-vinyl alcohol sulfonate lithium and poly (vinyl alcohol) composite lithium-ion battery separator. J. Electrochem. Soc. 2021, 168, 110510–110519. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 3935–3943. [Google Scholar] [CrossRef]
- Liao, C.; Mu, X.; Han, L.; Li, Z.; Zhu, Y.; Lu, J.; Wang, H.; Song, L.; Kan, Y.; Hu, Y. A flame-retardant, high ionic-conductivity and eco-friendly separator prepared by papermaking method for high-performance and superior safety lithium-ion batteries. Energy Storage Mater. 2022, 48, 123–132. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shin, S.H.; Moon, S.H. Flame retardant coated polyolefin separators for the safety of lithium ion batteries. Korean J. Chem. Eng. 2016, 33, 285–289. [Google Scholar] [CrossRef]
- Peng, L.; Shen, X.; Dai, J.; Wang, X.; Zeng, J.; Huang, B.; Li, H.; Zhang, P.; Zhao, J. Three-dimensional coating layer modified polyolefin ceramic-coated separators to enhance the safety performance of lithium-ion batteries. J. Electrochem. Soc. 2019, 166, A2111–A2120. [Google Scholar] [CrossRef]
- Arise, I.; Miyahara, Y.; Miyazaki, K.; Abe, T. Functional role of aramid coated separator for dendrite suppression in lithium-ion batteries. J. Electrochem. Soc. 2022, 169, 010536–010545. [Google Scholar] [CrossRef]
- Sharma, G.; Jin, Y.; Lin, Y.S. Lithium ion batteries with alumina separator for improved safety. J. Electrochem. Soc. 2017, 164, A1184–A1191. [Google Scholar] [CrossRef]
- Ye, Y.; Chou, L.-Y.; Liu, Y.; Wang, H.; Lee, H.K.; Huang, W.; Wan, J.; Liu, K.; Zhou, G.; Yang, Y.; et al. Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 2020, 5, 786–793. [Google Scholar] [CrossRef]
- Boldyrev, V.V.; Nev’yantsev, I.S.; Mikhailov, Y.I.; Khairetdinov, E.F. The mechanism of the thermal decomposition of oxalates. Kinet. Katal. 1970, 11, 306–311. [Google Scholar]
- Girgis, M.M.; El-Awad, A.M. Kinetics and mechanism of thermal decomposition of lithium oxalate catalysed by CdI_,Co,Fe204 (X = 0.0, 0.5 and 1.0) ferrospinel additives. Thermochim. Acta 1993, 214, 291–303. [Google Scholar] [CrossRef]
- Dollimore, D.; Tinsley, D. The thermal decomposition of oxalates. Part XlP: The thermal decomposition of Lithium oxalate. J. Chem. Soc. A 1971, 304. [Google Scholar]
- Papazian, H.A.; Pizzolato, P.J.; Patrick, J.A. Thermal decomposition of oxalates of ammonium and potassium. J. Am. Ceram. Soc. 1971, 54, 250–254. [Google Scholar] [CrossRef]
- Hofmann, A.; Wang, Z.; Bautista, S.P.; Weil, M.; Müller, F.; Löwe, R.; Schneider, L.; Mohsin, I.U.; Hanemann, T. Comprehensive characterization of propylene carbonate based liquid electrolyte mixtures for sodium-ion cells. Electrochim. Acta 2022, 403, 139670–139687. [Google Scholar] [CrossRef]
- Shanmukaraj, D.; Grugeon, S.; Laruelle, S.; Douglade, G.; Tarascon, J.-M.; Armand, M. Sacrificial salts: Compensating the initial charge irreversibility in lithium batteries. Electrochem. Commun. 2010, 12, 1344–1347. [Google Scholar] [CrossRef]
- Solchenbach, S.; Wetjen, M.; Pritzl, D.; Schwenke, K.U.; Gasteiger, H.A. Lithium oxalate as capacity and cycle-life enhancer in LNMO/Graphite and LNMO/SiG full cells. J. Electrochem. Soc. 2018, 165, A512–A524. [Google Scholar] [CrossRef]
- Muraishi, K. Thermal decomposition of alkali metal malonate anhydrides in various atmospheres. Thermochim. Acta 1990, 164, 401–409. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J. Electrode design optimization of lithium secondary batteries to enhance adhesion and deformation capabilities. Energy 2014, 75, 525–533. [Google Scholar] [CrossRef]
- Larkin, P. Environmental Dependence of Vibrational Spectra; Elsevier: Amsterdam, The Netherlands, 2011; pp. 55–62. [Google Scholar]
- Ionashiro, E.Y.; Caires, F.J.; Siqueira, A.B.; Lima, L.S.; Carvalho, C.T. Thermal behaviour of fumaric acid, sodium fumarate and its compounds with light trivalent lanthanides in air atmosphere. J. Therm. Anal. Calorim. 2011, 108, 1183–1188. [Google Scholar] [CrossRef]
- Caires, F.J.; Lima, L.S.; Carvalho, C.T.; Giagio, R.J.; Ionashiro, M. Thermal behaviour of malonic acid, sodium malonate and its compounds with some bivalent transition metal ions. Thermochim. Acta 2010, 497, 35–40. [Google Scholar] [CrossRef]
- Bauer, W.; Nötzel, D. Rheological properties and stability of NMP based cathode slurries for lithium ion batteries. Cer. Internat. 2014, 40, 4591–4598. [Google Scholar] [CrossRef]
- Bitsch, B.; Dittmann, J.; Schmitt, M.; Scharfer, P.; Schabel, W.; Willenbacher, N. A novel slurry concept for the fabrication of lithium-ion battery electrodes with beneficial properties. J. Power Sources 2014, 265, 81–90. [Google Scholar] [CrossRef]
- Cushing, A.; Zheng, T.; Higa, K.; Liu, G. Viscosity analysis of battery electrode slurry. Polymers 2021, 13, 4033–4041. [Google Scholar] [CrossRef]
- Diehm, R.; Kumberg, J.; Dörrer, C.; Müller, M.; Bauer, W.; Scharfer, P.; Schabel, W. In situ investigations of simultaneous two-layer slot die coating of component-graded anodes for improved high-energy Li-ion batteries. Energy Technol. 2020, 8, 1901251. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.-C.; Liu, T.-J.; Fan, T.; Tsou, E.-Y.; Tiu, C. An effective mixing for lithium ion battery slurries. Adv. Chem. Eng. Sci. 2014, 04, 515–528. [Google Scholar] [CrossRef]
- Ma, F.; Fu, Y.; Battaglia, V.; Prasher, R. Microrheological modeling of lithium ion battery anode slurry. J. Power Sources 2019, 438, 226994. [Google Scholar] [CrossRef]
- Ouyang, L.; Wu, Z.; Wang, J.; Qi, X.; Li, Q.; Wang, J.; Lu, S. The effect of solid content on the rheological properties and microstructures of a Li-ion battery cathode slurry. RSC Adv. 2020, 10, 19360–19370. [Google Scholar] [CrossRef]
- Jeschull, F.; Brandell, D.; Wohlfahrt-Mehrens, M.; Memm, M. Water-soluble binders for lithium-ion battery graphite electrodes: Slurry rheology, coating adhesion, and electrochemical performance. Energy Technol. 2017, 5, 2108–2118. [Google Scholar] [CrossRef]
- Gordon, R.; Orias, R.; Willenbacher, N. Effect of carboxymethyl cellulose on the flow behavior of lithium-ion battery anode slurries and the electrical as well as mechanical properties of corresponding dry layers. J. Mater. Sci. 2020, 55, 15867–15881. [Google Scholar] [CrossRef]
- Lim, S.; Kim, S.; Ahn, K.H.; Lee, S.J. The effect of binders on the rheological properties and the microstructure formation of lithium-ion battery anode slurries. J. Power Sources 2015, 299, 221–230. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J. A large deformation and fracture model of lithium-ion battery cells treated as a homogenized medium. J. Electrochem. Soc. 2020, 167, 120503–120504. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Huttner, F.; Haselrieder, W.; Kwade, A. The influence of different post-drying procedures on remaining water content and physical and electrochemical properties of lithium-ion batteries. Energy Technol. 2019, 8, 1900245. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of lithium-ion battery anodes for use in high-energy cells: Influence of electrode thickness on drying time, adhesion, and crack formation. Energy Technol. 2019, 7, 1900722. [Google Scholar] [CrossRef]
- Schilling, A.; Schmitt, J.; Dietrich, F.; Dröder, K. Analyzing bending stresses on lithium-ion battery cathodes induced by the assembly process. Energy Technol. 2016, 4, 1502–1508. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kobayashi, Y.; Ohtsuka, T.; Tsuruta, T.; Nakamura, K.; Tsukagoshi, Y. Computational evaluation of bending fatigue test on electrode of lithium-ion battery. In Proceedings of the 6th European Conference on Computational Mechanics; 7th European Conference on Computational Fluid Dynamics, Glasgow, Scotland, 11-15 June 2018. [Google Scholar]
- Pierson, H.O. Handbook of Carbon Graphite Diamond and Fullerenes. Properties, Processing and Applications; Noyes Publications: Norwich, NY, USA, 1994. [Google Scholar]
- Matsumoto, K.; Inoue, K.; Nakahara, K.; Yuge, R.; Noguchi, T.; Utsugi, K. Suppression of aluminum corrosion by using high concentration LiTFSI electrolyte. J. Power Sources 2013, 231, 234–238. [Google Scholar] [CrossRef]
- Quilty, C.D.; Bock, D.C.; Yan, S.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C. Probing sources of capacity fade in LiNi0.6Mn0.2Co0.2O2 (NMC622): An operando XRD study of Li/NMC622 batteries during extended cycling. J. Phys. Chem. C 2020, 124, 8119–8128. [Google Scholar] [CrossRef]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2(NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Kawamura, T.; Okada, S.; Yamaki, J.-i. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, F.; Wang, W.; Zhao, Y.; Liang, Z.; Yan, D. Overcharge failure investigation of lithium-ion batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Chen, S.; Zhu, J.; Han, G.; Dai, H. Revealing the impact of slight electrical abuse on the thermal safety characteristics for lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 12858–12870. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Hofmann, A.; Uhlmann, N.; Ziebert, C.; Wiegand, O.; Schmidt, A.; Hanemann, T. Preventing Li-ion cell explosion during thermal runaway with reduced pressure. Appl. Therm. Eng. 2017, 124, 539–544. [Google Scholar] [CrossRef]
- Meisel, T.; Halmos, Z.; Seybold, K.; Pungor, E. The thermal decomposition of alkali metal formates. J. Therm. Anal. 1975, 7, 73–80. [Google Scholar] [CrossRef]
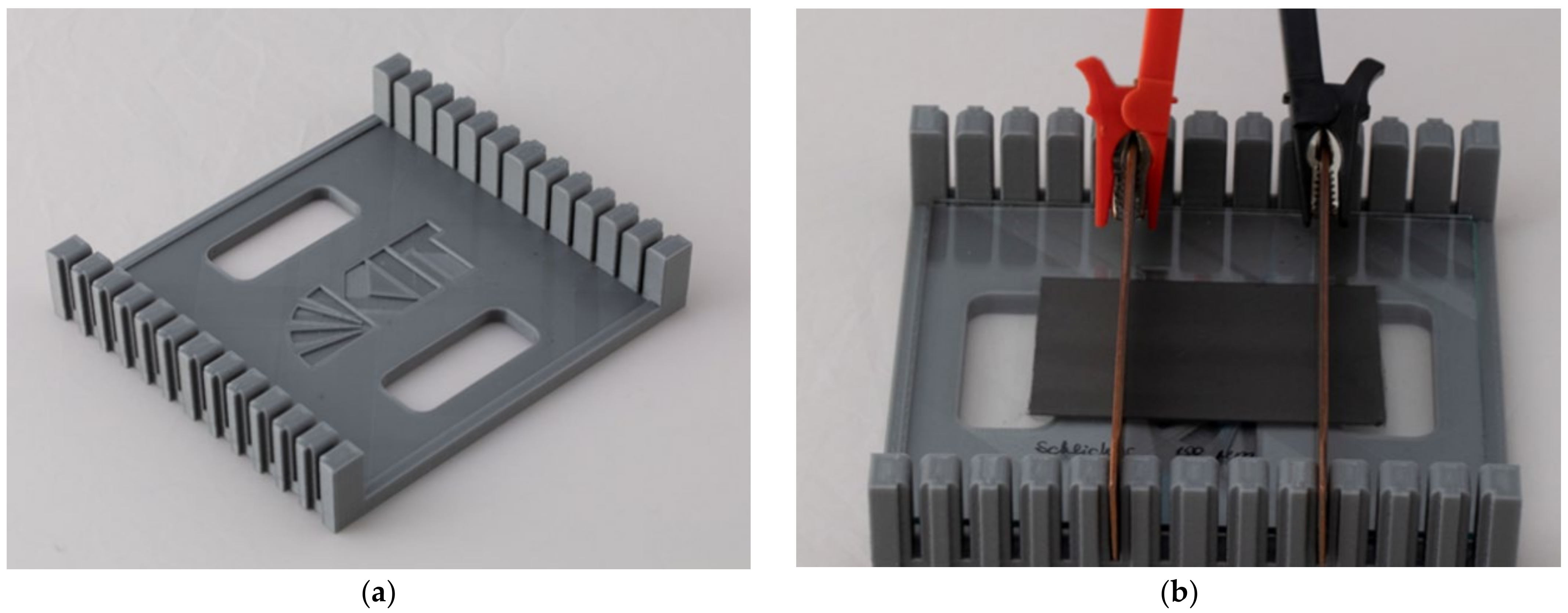
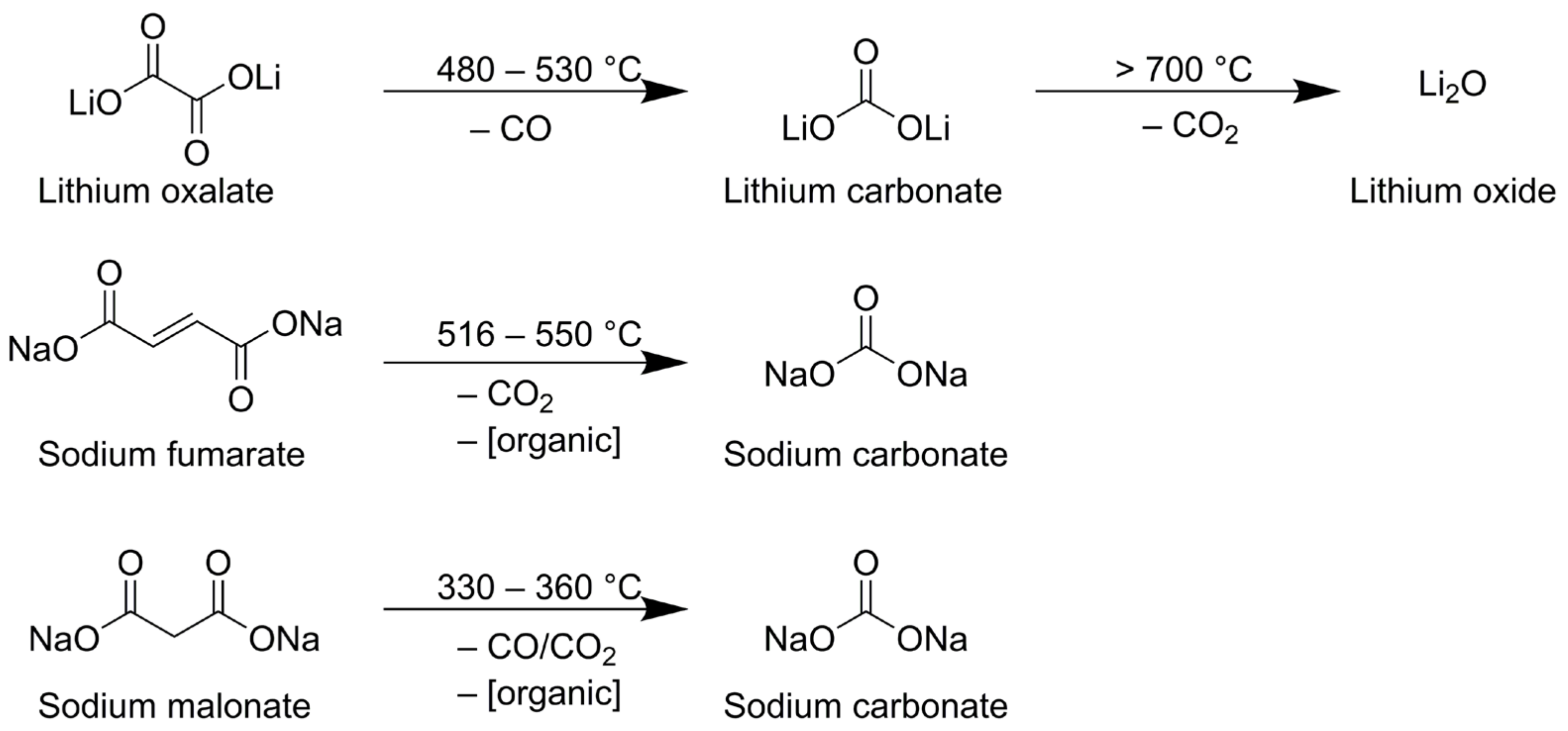

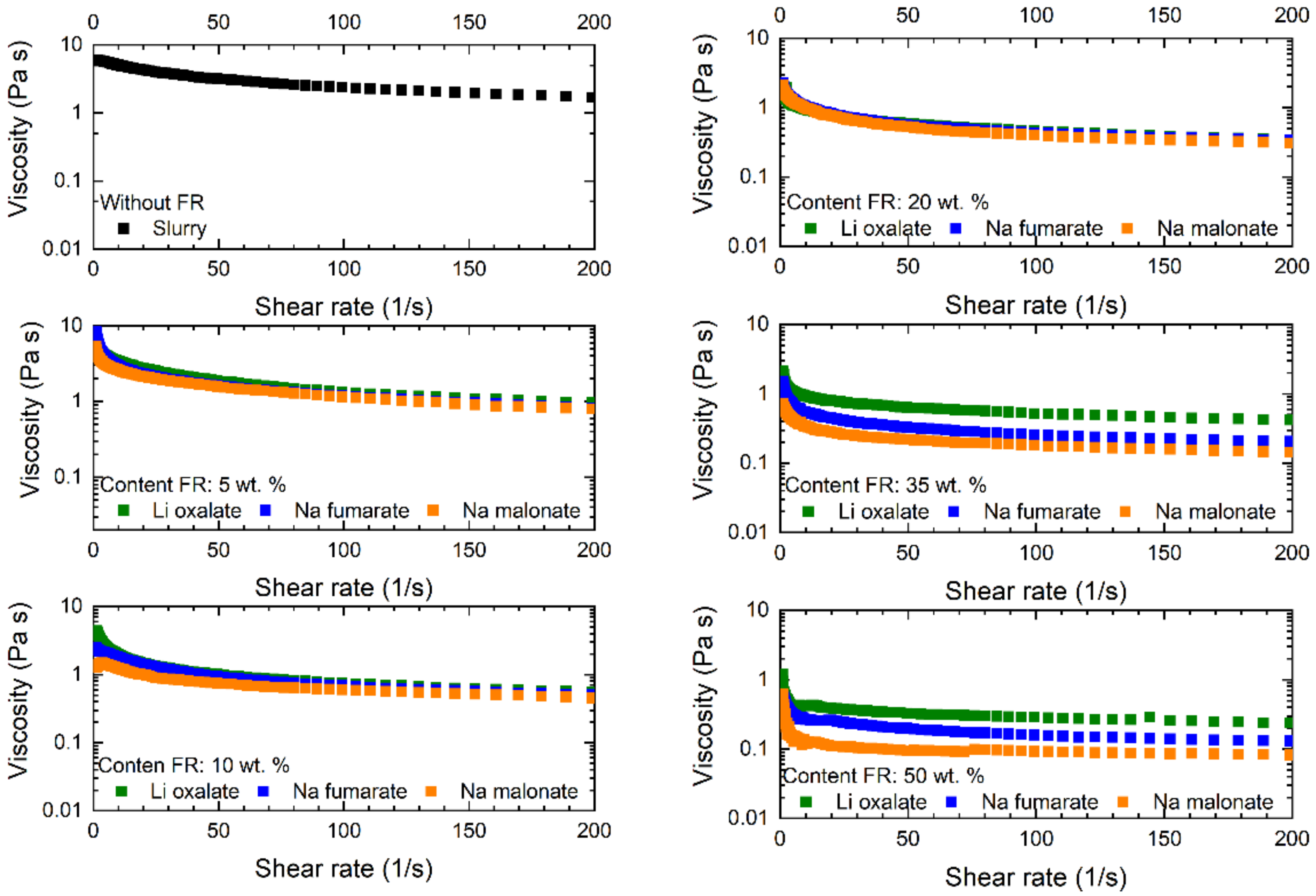
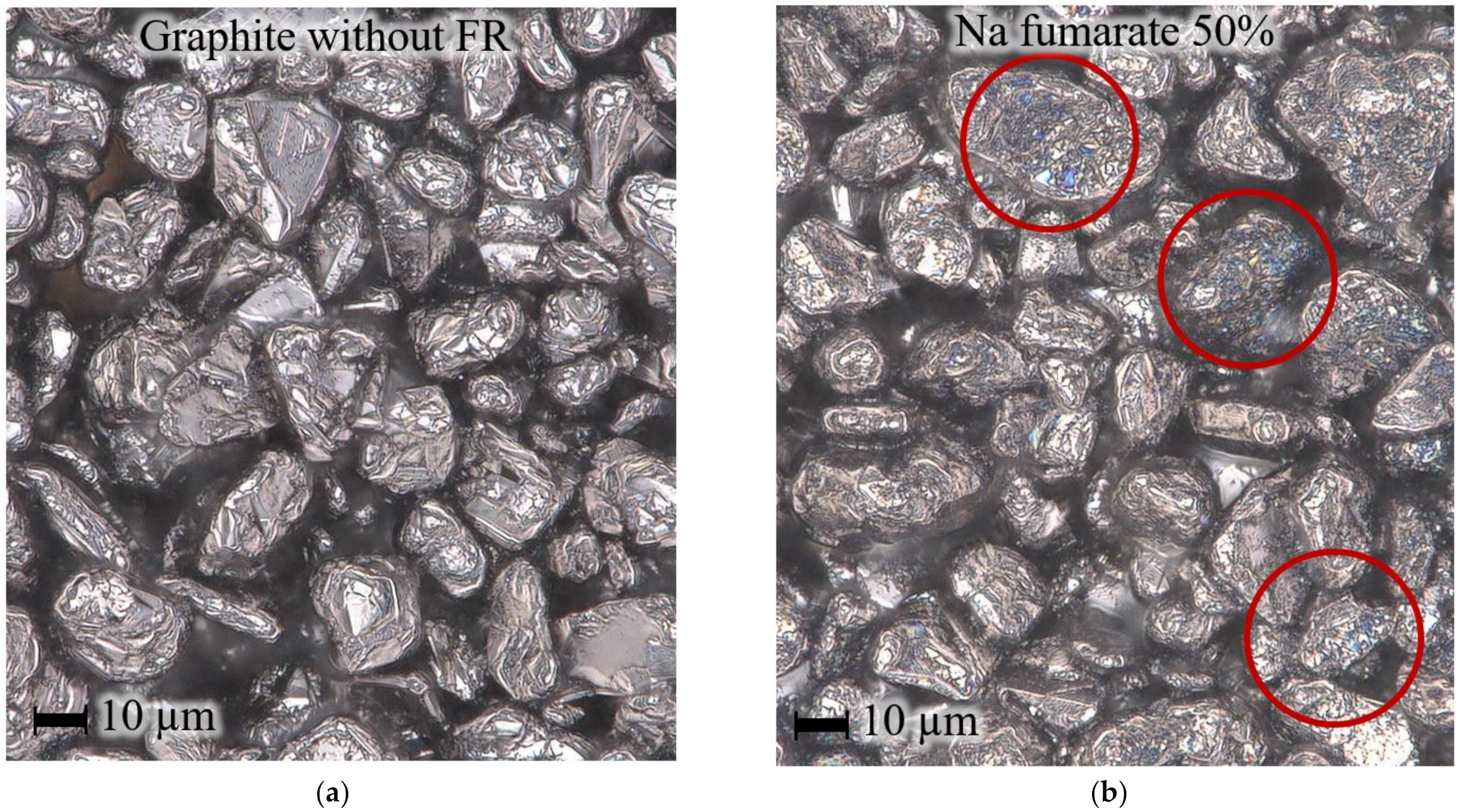
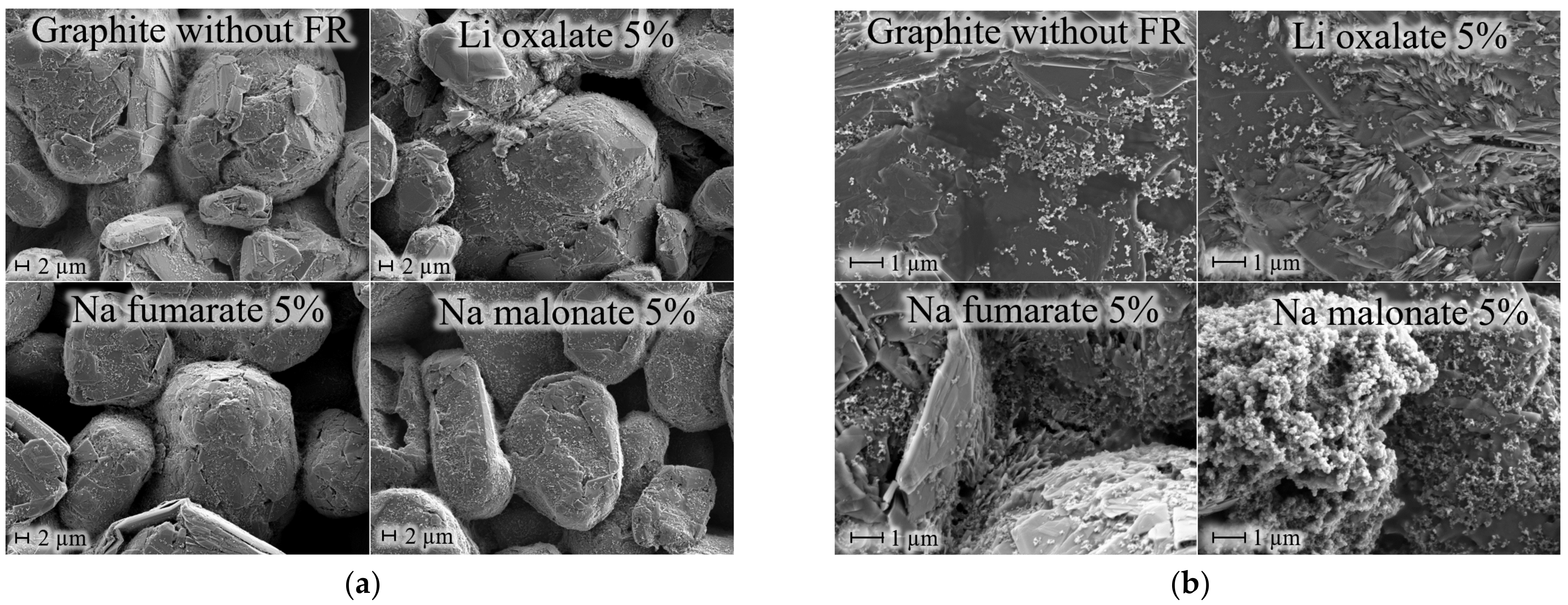



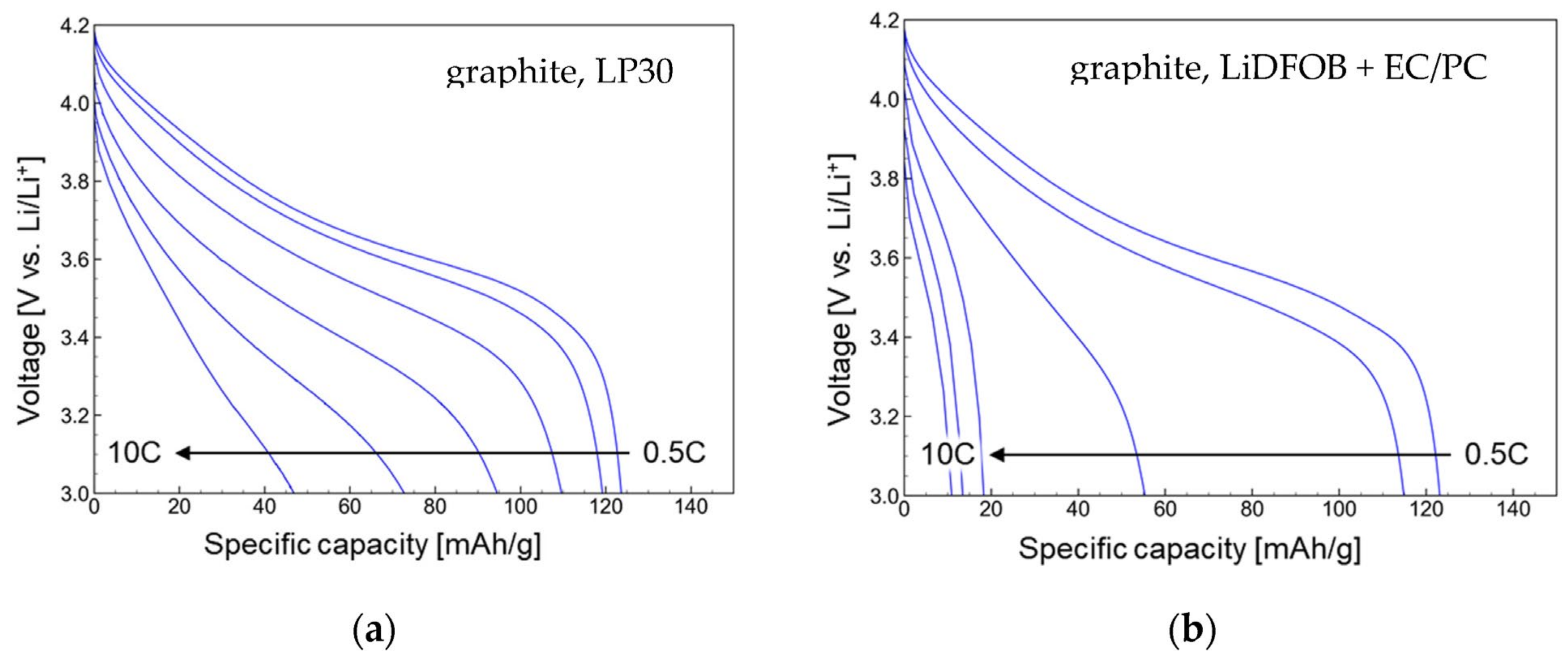
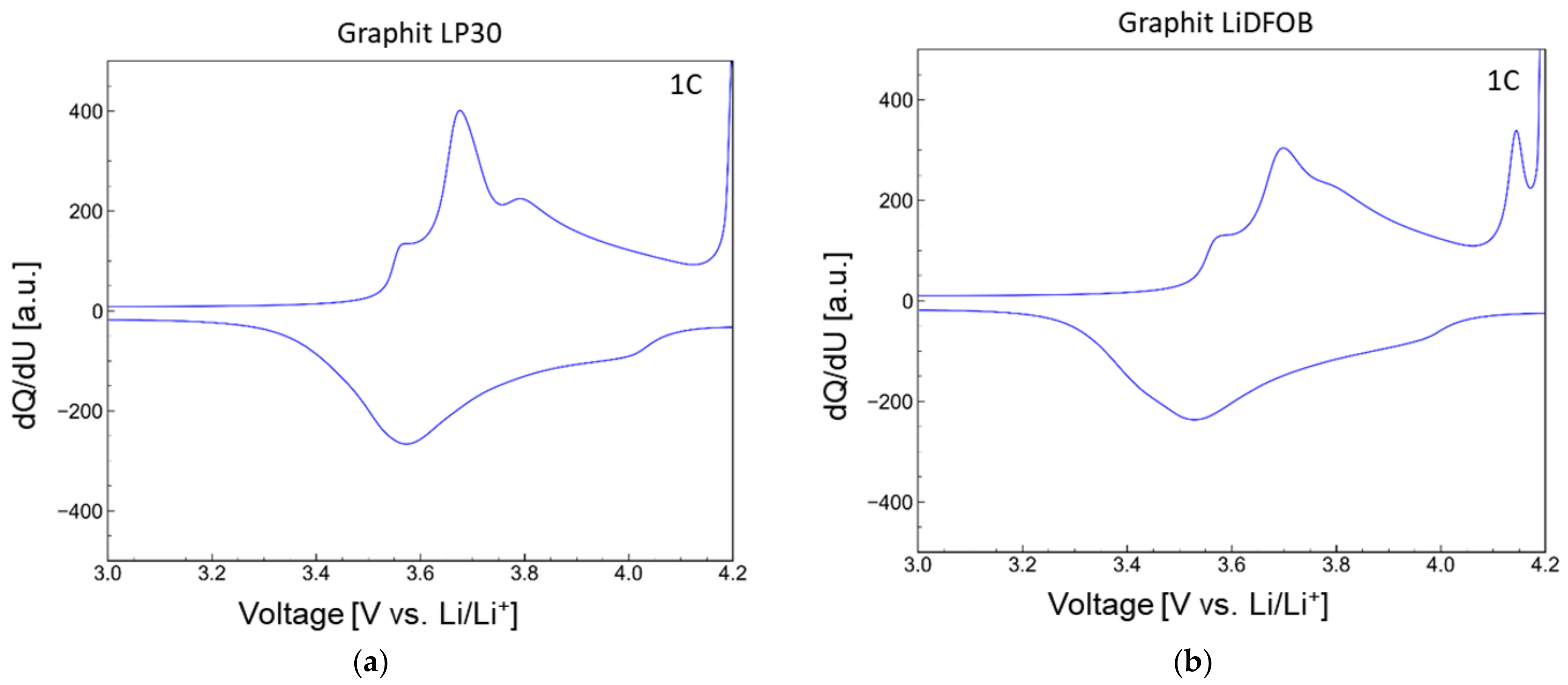
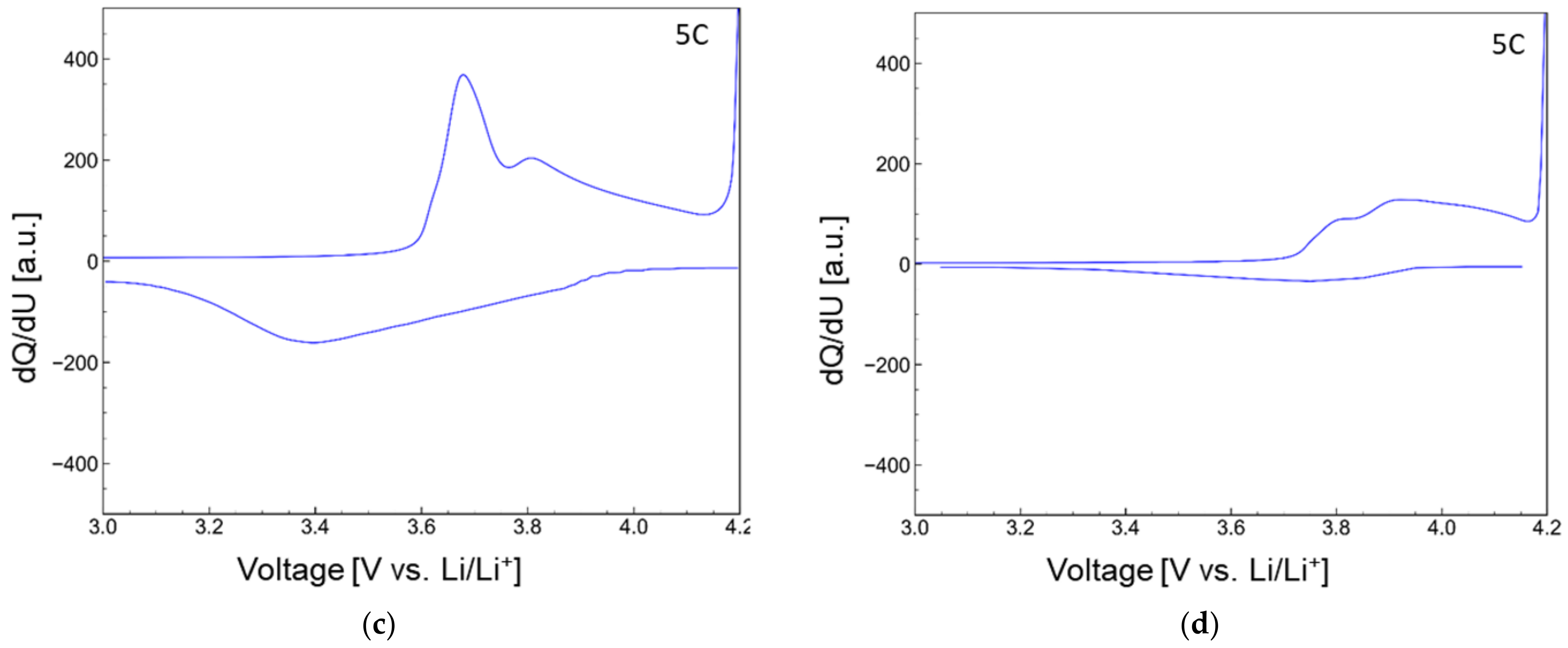
| wt.% of FR in anode layer | 5 | 10 | 20 | 35 | 50 |
|---|---|---|---|---|---|
| m (slurry), (g) | 10 | 10 | 10 | 10 | 10 |
| m (FR), (g) | 0.2758 | 0.5822 | 1.3100 | 2.8215 | 5.2400 |
| m (FR)/ml water in slurry, (mg/mL) | 49 | 98 | 196 | 343 | 490 |
| m (added water), (g) | 0.2505 | 0.5289 | 1.1190 | 2.5631 | 4.7600 |
| m (SBR binder), (g) | 0.2105 | 0.2222 | 0.2500 | 0.3077 | 0.4000 |
| Film thickness, (µm) | 145 | 160 | 210 | 345 | 470 |
| Electrolyte Solvent Composition | EC/DMC | EC/PC | 1,2-BC/FEC |
|---|---|---|---|
| Conducting salt | LiPF6 | LiDFOB | LiTFSI |
| c (conducting salt), mol∙kg−1 | 0.77 | 0.75 | 0.75 |
| Density ρ, 298.15 K, g∙cm−3 | 1.3 | 1.32 | 1.39 |
| Glass point (Tg, taken from DSC at 10 K∙min−1), (°C) | −72.8 | −93.4 | −95.0 |
| Flash point (fp), (°C) | 25.0 | 162 | 149.0 |
| Melting point (mp., taken from DSC at 10 K∙min−1), (°C) | −4.6 | − | − |
| Eox, Li|Pt, 298.15 K, (V) | 4.7 | 4.5 | 4.8 |
| Viscosity η at 298.15 K (mPa∙s) | 3.9 | 6.6 | 12.0 |
| Conductivity κ at 293.15 K (mS∙cm−1) | 10.7 | 7.4 | 2.8 |
| FR wt.% | Li Oxalate | Na Fumarate | Na Malonate | |||
|---|---|---|---|---|---|---|
| Split | Detachment | Split | Detachment | Split | Detachment | |
| 5 | NA | NA | NA | NA | NA | NA |
| 10 | 2 mm | NA | NA | NA | 2 mm | NA |
| 20 | 2 mm | NA | 8 mm | NA | 20 mm | NA |
| 35 | 3 mm | NA | 25 mm | NA | 25 mm | NA |
| 50 | 3 mm | NA | 32 mm | 10 mm | 32 mm | 10 mm |
| Layer Thickness [µm] | Specific Electric Resistivity [10−3 Ohm·m] |
|---|---|
| 80 | 1.16 |
| 240 | 0.93 |
| 750 | 1.35 |
| 1040 | 1.16 |
| Specific Electrical Resistivity ρ [10−3 Ohm·m]. | |||||
|---|---|---|---|---|---|
| 5 wt.% | 10 wt.% | 20 wt.% | 35 wt.% | 50 wt.% | |
| Li-oxalate | 4 | 4 | 5 | 14 | 118 |
| Na-fumarate | 5 | 7 | 12 | 18 | 80 |
| Na-malonate | 3 | 3 | 11 | 123 | 417 |
| Lithium Oxalate | Sodium Fumarate | Sodium Malonate | |
|---|---|---|---|
| Molecular mass [g∙mol−1] | 101.90 | 160.04 | 148.03 |
| Temperature range [°C] | 300–600 | 300–600 | 300–600 |
| Evolved gas | CO | CO2 | CO/CO2 (70:30) |
| Mass loss [%] | 27.9 | 27.5 | 22.6 |
| mol-eq. CO2 [mol] per 1 mol FR | 0.996 | 0.626 | 0.471 + 0.202 = 0.673 |
| mL CO2 per 1 g FR | 218.9 | 87.5 | 101.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulik, N.; Hofmann, A.; Nötzel, D.; Müller, M.; Reuter, I.; Müller, F.; Smith, A.; Hanemann, T. Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries. Batteries 2023, 9, 82. https://doi.org/10.3390/batteries9020082
Fulik N, Hofmann A, Nötzel D, Müller M, Reuter I, Müller F, Smith A, Hanemann T. Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries. Batteries. 2023; 9(2):82. https://doi.org/10.3390/batteries9020082
Chicago/Turabian StyleFulik, Natalia, Andreas Hofmann, Dorit Nötzel, Marcus Müller, Ingo Reuter, Freya Müller, Anna Smith, and Thomas Hanemann. 2023. "Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries" Batteries 9, no. 2: 82. https://doi.org/10.3390/batteries9020082
APA StyleFulik, N., Hofmann, A., Nötzel, D., Müller, M., Reuter, I., Müller, F., Smith, A., & Hanemann, T. (2023). Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries. Batteries, 9(2), 82. https://doi.org/10.3390/batteries9020082






