Integrated Design of a Functional Composite Electrolyte and Cathode for All-Solid-State Li Metal Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Cathodes
2.3. Preparation of ID-FCC and Cells’ Assembly
2.4. Material Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
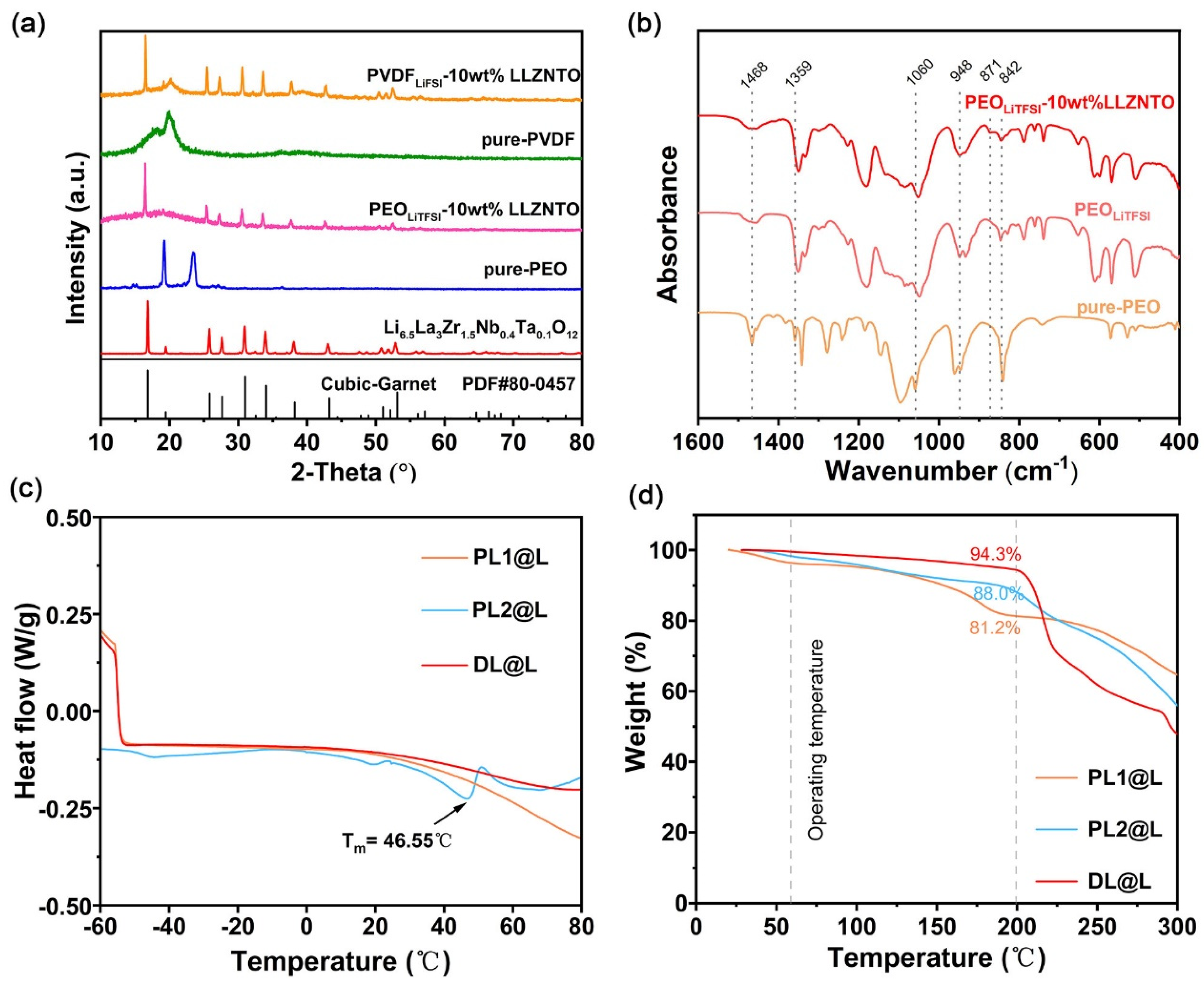
3.1. Characterization of Composite Membranes
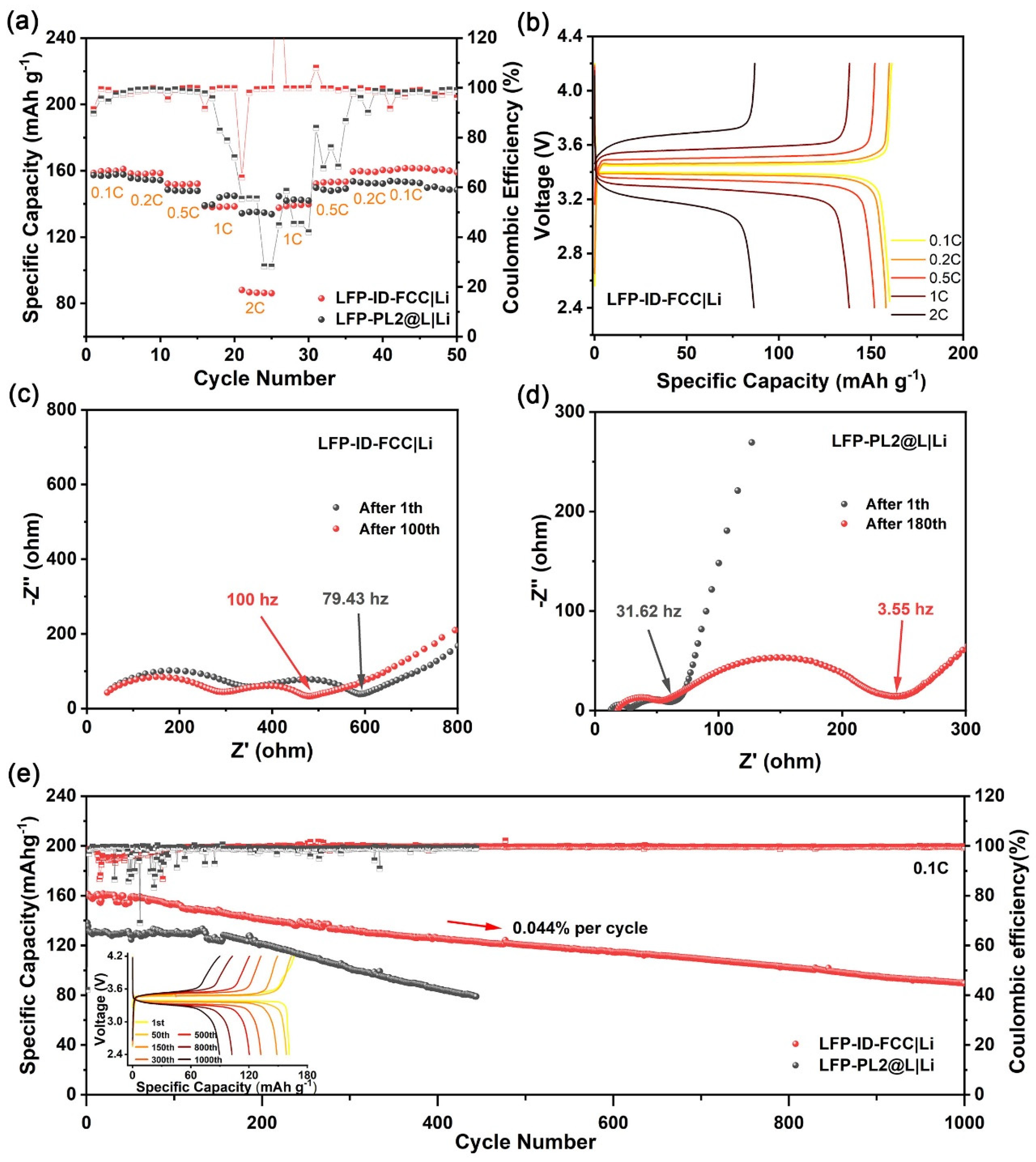
3.2. Electrochemical Characterization of Composite Membranes
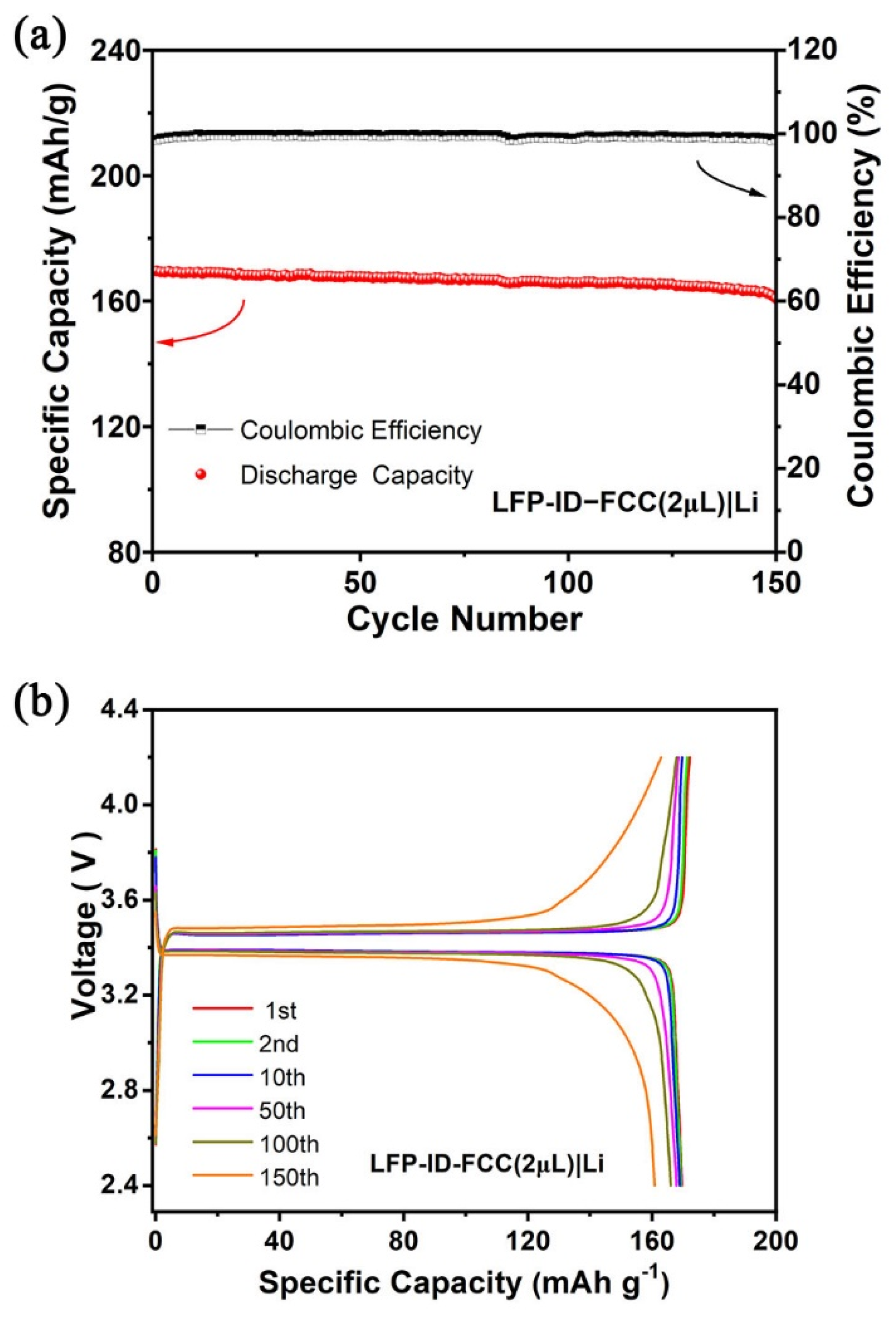
3.3. Performance of Composite Membranes in Full Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Goodenough, J.B. How we made the Li-ion rechargeable battery. Nat. Electron. 2018, 1, 204. [Google Scholar] [CrossRef]
- Hu, Y.-S. Batteries: Getting solid. Nat. Energy 2016, 1, 16042. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Ji, X.; Hou, S.; Wang, P.; He, X.; Piao, N.; Chen, J.; Fan, X.; Wang, C. Solid-state electrolyte design for lithium dendrite suppression. Adv. Mater. 2020, 32, 2002741. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat Mater 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Huang, S.; Cao, P. Solid-state inorganic electrolytes (oxides, sulfides, and halides). In Advanced Ceramics for Energy Storage, Thermoelectrics and Photonics; Cao, P., Chen, Z.-G., Xia, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 4. [Google Scholar]
- Raju, M.M.; Altayran, F.; Johnson, M.; Wang, D.; Zhang, Q. Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview. Electrochem 2021, 2, 390–414. [Google Scholar] [CrossRef]
- Cao, S.; Song, S.; Xiang, X.; Hu, Q.; Zhang, C.; Xia, Z.; Xu, Y.; Zha, W.; Li, J.; Gonzale, P.M.; et al. Modeling, Preparation, and Elemental Doping of Li7La3Zr2O12 Garnet-Type Solid Electrolytes: A Review. J. Korean Ceram. Soc. 2019, 56, 111–129. [Google Scholar] [CrossRef]
- Song, X.; Zhang, T.; Christopher, T.D.; Guo, Y.; Huang, S.; Liu, Y.; Söhnel, T.; Cao, P. Achieving enhanced densification and superior ionic conductivity of garnet electrolytes via a co-doping strategy coupled with pressureless sintering. J. Eur. Ceram. Soc. 2022, 42, 5023–5028. [Google Scholar] [CrossRef]
- Zhang, T.; Christopher, T.D.; Huang, S.; Söhnel, T.; Liu, Y.g.; Cao, P. Electrochemical properties of Li6+yLa3−yBayNbZrO12 lithium garnet oxide solid-state electrolytes with co-doping barium and zirconium. J. Alloys Compd. 2021, 862, 158600. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Z.; Fan, A.; Liu, X.; Wang, H.; Ma, W.; Zhu, L.; Zhang, X. A high energy and power all-solid-state lithium battery enabled by modified sulfide electrolyte film. J. Power Sources 2021, 485, 229325. [Google Scholar] [CrossRef]
- Alexander, G.V.; Indu, M.; Kamakshy, S.; Murugan, R. Development of stable and conductive interface between garnet structured solid electrolyte and lithium metal anode for high performance solid-state battery. Electrochim. Acta 2020, 332, 135511. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Dong, D.; Zhou, B.; Sun, Y.; Zhang, H.; Zhong, G.; Dong, Q.; Fu, F.; Qian, H.; Lin, Z.; Lu, D. Polymer electrolyte glue: A universal interfacial modification strategy for all-solid-state Li batteries. Nano Lett. 2019, 19, 2343–2349. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Feng, Y.; Tan, R.; Zuo, Y.; Gao, R.; Zhao, Y.; Han, L.; Wang, Z.; Pan, F. Flexible composite solid electrolyte facilitating highly stable “soft contacting” Li–electrolyte interface for solid state lithium-ion batteries. Adv. Energy Mater. 2017, 7, 1701437. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, K.-H.; Cho, S.-J.; Choi, S.; Park, S.; Lee, S.-Y. Printable solid-state lithium-ion batteries: A new route toward shape-conformable power sources with aesthetic versatility for flexible electronics. Nano Lett. 2015, 15, 5168–5177. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Bella, F.; Nair, J.R.; Mecerreyes, D.; Gerbaldi, C. Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 2016, 1, 678–682. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Fullerton-Shirey, S.K.; Maranas, J.K. Effect of LiClO4 on the structure and mobility of PEO-based solid polymer electrolytes. Macromolecules 2009, 42, 2142–2156. [Google Scholar] [CrossRef]
- Pożyczka, K.; Marzantowicz, M.; Dygas, J.R.; Krok, F. Ionic conductivity and lithium transference number of poly (ethylene oxide): LiTFSI system. Electrochim. Acta 2017, 227, 127–135. [Google Scholar] [CrossRef]
- Nkosi, F.P.; Valvo, M.; Mindemark, J.; Dzulkurnain, N.A.; Hernández, G.; Mahun, A.; Abbrent, S.; Brus, J.; Kobera, L.; Edström, K. Garnet-Poly(ε-caprolactone-co-trimethylene carbonate) Polymer-in-Ceramic Composite Electrolyte for All-Solid-State Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 2531–2542. [Google Scholar] [CrossRef]
- Xie, H.; Yang, C.; Fu, K.K.; Yao, Y.; Jiang, F.; Hitz, E.; Liu, B.; Wang, S.; Hu, L. Flexible, Scalable, and Highly Conductive Garnet-Polymer Solid Electrolyte Templated by Bacterial Cellulose. Adv. Energy Mater. 2018, 8, 1703474. [Google Scholar] [CrossRef]
- Li, Y.; Wong, K.W.; Ng, K.M. Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J. Mater. Chem. A 2016, 52, 4369–4372. [Google Scholar] [CrossRef]
- Pervez, S.A.; Ganjeh-Anzabi, P.; Farooq, U.; Trifkovic, M.; Roberts, E.P.; Thangadurai, V. Fabrication of a Dendrite-Free all Solid-State Li Metal Battery via Polymer Composite/Garnet/Polymer Composite Layered Electrolyte. Adv. Mater. Interfaces 2019, 6, 1900186. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Pu, Y.; Li, Y.; Xin, S.; Li, X.; Chen, J.; Goodenough, J.B. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 2019, 31, 1805574. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tang, W.; Zhang, X.; Deng, L.; Luo, J. High energy density solid state lithium metal batteries enabled by sub-5 µm solid polymer electrolytes. Adv. Mater. 2021, 33, 2105329. [Google Scholar] [CrossRef]
- Aurbach, D.; Talyosef, Y.; Markovsky, B.; Markevich, E.; Zinigrad, E.; Asraf, L.; Gnanaraj, J.S.; Kim, H.-J. Design of electrolyte solutions for Li and Li-ion batteries: A review. Electrochim. Acta 2004, 50, 247–254. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Chen, B.; Wang, L.; Yue, L.; Liu, H.; Zhang, J.; Liu, Z.; Cui, G. A strategy to make high voltage LiCoO2 compatible with polyethylene oxide electrolyte in all-solid-state lithium ion batteries. J. Electrochem. Soc. 2017, 164, A3454. [Google Scholar] [CrossRef]
- Aurbach, D.; Granot, E. The study of electrolyte solutions based on solvents from the “glyme” family (linear polyethers) for secondary Li battery systems. Electrochim. Acta 1997, 42, 697–718. [Google Scholar] [CrossRef]
- Xia, Y.; Fujieda, T.; Tatsumi, K.; Prosini, P.P.; Sakai, T. Thermal and electrochemical stability of cathode materials in solid polymer electrolyte. J. Power Sources 2001, 92, 234–243. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Song, X.; Zhang, T.; Huang, S.; Mi, J.; Zhang, Y.; Travas-Sejdic, J.; Turner, A.P.; Gao, W.; Cao, P. Constructing a PVDF-based composite solid-state electrolyte with high ionic conductivity Li6.5La3Zr1.5Ta0.1Nb0.4O12 for lithium metal battery. J. Power Sources 2023, 564, 232849. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.-W.; Shen, Y. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly (vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Zhang, X.Q.; Cheng, X.B.; Zhang, R.; Xu, R.; Chen, P.Y.; Peng, H.J.; Huang, J.Q.; Zhang, Q. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl. Acad. Sci. USA 2017, 114, 11069–11074. [Google Scholar] [CrossRef]
- Ling, H.; Shen, L.; Huang, Y.; Ma, J.; Chen, L.; Hao, X.; Zhao, L.; Kang, F.; He, Y.B. Integrated Structure of Cathode and Double-Layer Electrolyte for Highly Stable and Dendrite-Free All-Solid-State Li-Metal Batteries. ACS Appl. Mater. Interfaces 2020, 12, 56995–57002. [Google Scholar] [CrossRef]
- Croce, F.; Focarete, M.L.; Hassoun, J.; Meschini, I.; Scrosati, B. A safe, high-rate and high-energy polymer lithium-ion battery based on gelled membranes prepared by electrospinning. Energy Environ. Sci. 2011, 4, 921–927. [Google Scholar] [CrossRef]
- Homann, G.; Stolz, L.; Nair, J.; Laskovic, I.C.; Winter, M.; Kasnatscheew, J. Poly(ethylene oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: Evaluating the role of electrolyte oxidation in rapid cell failure. Sci. Rep. 2020, 10, 4390. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, R.; Sun, J.; Wu, M.; Zhao, T. Polyoxyethylene (PEO)|PEO-Perovskite|PEO Composite Electrolyte for All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 46930–46937. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, P.; Jia, H.; Zhu, J.; Selvan, R.K.; Li, Y.; Dong, X.; Du, Z.; Angunawela, I.; Wu, N.; et al. High-Performance 3-D Fiber Network Composite Electrolyte Enabled with Li-Ion Conducting Nanofibers and Amorphous PEO-Based Cross-Linked Polymer for Ambient All-Solid-State Lithium-Metal Batteries. Adv. Fiber Mater. 2019, 1, 46–60. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, N.; Sun, J.; Du, F.; Li, Y.; Guo, X. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery. J. Power Sources 2017, 372, 1–7. [Google Scholar] [CrossRef]
- Falco, M.; Castro, L.; Nair, J.R.; Bella, F.; Bardé, F.; Meligrana, G.; Gerbaldi, C. UV-Cross-Linked Composite Polymer Electrolyte for High-Rate, Ambient Temperature Lithium Batteries. ACS Appl. Energy Mater. 2019, 2, 1600–1607. [Google Scholar] [CrossRef]
- Wu, M.; Liu, D.; Qu, D.; Xie, Z.; Li, J.; Lei, J.; Tang, H. 3D Coral-like LLZO/PVDF Composite Electrolytes with Enhanced Ionic Conductivity and Mechanical Flexibility for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 52652–52659. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Lei, D.; Yang, W.; Liu, C.; Shi, K.; Hao, X.; Shen, L.; Lv, W.; Li, B.; Yang, Q.-H.; et al. Low Resistance-Integrated All-Solid-State Battery Achieved by Li7La3Zr2O12 Nanowire Upgrading Polyethylene Oxide (PEO) Composite Electrolyte and PEO Cathode Binder. Adv. Funct. Mater. 2019, 29, 1805301. [Google Scholar] [CrossRef]
- Zhuang, H.; Ma, W.; Xie, J.; Liu, X.; Li, B.; Jiang, Y.; Huang, S.; Chen, Z.; Zhao, B. Solvent-free synthesis of PEO/garnet composite electrolyte for high-safety all-solid-state lithium batteries. J. Alloys Compd. 2021, 860, 157915. [Google Scholar] [CrossRef]


| Materials (IPHE//Cathode) | Processing Method | Discharge Capacity in mA h g−1 (Rate) @Temperature | Capacity Retention Rate | Ref. |
|---|---|---|---|---|
| PEOLiTFSI-LLZO//LFP | Blend | 155 (0.1 C) @60 °C | 87% @100 cycles | [37] |
| PEOLiTFSI-LLZTO//LFP | Blend | 148.6 (0.2 C) @55 °C | 93.6% @100 cycles | [34] |
| PEOLiTFSI-3D LLTO//LFP | Blend | 147.6 (0.5 C); 135.0 (2 C) @60 °C | / 79.0% @300 cycles | [41] |
| PEO-LLZTO//LFP | Blend | 141.5 (0.1 C) @60 °C | ~75% @200 cycles | [42] |
| PEOLiClO4-LLTO//LFP | Crosslinked | 147 (0.1 C) @25 °C | 98% @100 cycles | [43] |
| PEO-LLZTO-[BMIM]TF2N //LFPLiTFSI | Blend | 133.2 (0.1 C) @25 °C | 88% @150 cycles | [44] |
| PEOLiTFSI/G4-LLZO//LFP | Crosslinked | 163 (0.1 C) @60 °C | 66% @200 cycles | [45] |
| PVDFLiClO4-3D LLZAO//LFP | Blend | 168.3 (0.1 C); 151.8 (1 C) @RT | 95.2% @200 cycles / | [46] |
| PEOLiTFSI-3D LLZAO//LFP | Blend | 162.7 (0.1 C); 163 (0.5 C) @60 °C | 91.7% @120 cycles 97.4% @70 cycles | [47] |
| PEOLiTFSI-LLZTO//LFP | Blend | 149.5 (0.2 C) @55 °C | 93.4% @100 cycles | [48] |
| PEOLiTFSILATP/PEO@PVDF-HFPLiTFSI//LFP | Integrated design | 142.6 (0.1 C) @45 °C | 91.7% @100 cycles | [38] |
| PEOLiTFSI/PVDFLiFSI-LLZNTO //LFPLLZNTO | Integrated design | 161.5 (0.1 C) @60 °C | 94.7% @100 cycles 78.7% @400 cycles 56.1% @1000 cycles | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Fan, R.; Huang, S.; Zhao, J.; Zhang, Y.; Dai, W.; Zhao, C.; Song, X.; Cao, P. Integrated Design of a Functional Composite Electrolyte and Cathode for All-Solid-State Li Metal Batteries. Batteries 2023, 9, 320. https://doi.org/10.3390/batteries9060320
Zhang Z, Fan R, Huang S, Zhao J, Zhang Y, Dai W, Zhao C, Song X, Cao P. Integrated Design of a Functional Composite Electrolyte and Cathode for All-Solid-State Li Metal Batteries. Batteries. 2023; 9(6):320. https://doi.org/10.3390/batteries9060320
Chicago/Turabian StyleZhang, Zhenghang, Rongzheng Fan, Saifang Huang, Jie Zhao, Yudong Zhang, Weiji Dai, Cuijiao Zhao, Xin Song, and Peng Cao. 2023. "Integrated Design of a Functional Composite Electrolyte and Cathode for All-Solid-State Li Metal Batteries" Batteries 9, no. 6: 320. https://doi.org/10.3390/batteries9060320
APA StyleZhang, Z., Fan, R., Huang, S., Zhao, J., Zhang, Y., Dai, W., Zhao, C., Song, X., & Cao, P. (2023). Integrated Design of a Functional Composite Electrolyte and Cathode for All-Solid-State Li Metal Batteries. Batteries, 9(6), 320. https://doi.org/10.3390/batteries9060320







