1. Introduction
The battery as an energy storage system is an emerging technology nowadays. The development of battery energy storage systems (BESS) has been increasing significantly. The total BESS energy deployed globally was 11 GWh in 2017 and increased to 100–167 GWh by 2030 [
1]. Within usage, such as grid applications [
2,
3], electric vehicles [
4], and electric aircrafts [
5], utilize BESS to store and deliver energy when the loads require it. Lithium-ion batteries (LIB) have a larger demand in the world market compared to other batteries. The LIB has a longer lifespan, lower maintenance, higher energy density, and is more efficient compared to other batteries [
6].
The main concept being investigated is how the aging of LIBs due to charge and discharge cycles and high current fast charging. This will provide insights into the safety and reliability of BESS systems. To measure the battery’s reliability, the manufacturers assume that if the LIB capacity is reduced by 20% of its initial or rated capacity, the LIB is in end-of-life (EOL). This is due to the fact that the capacity reduces rapidly after EOL [
7]. To increase the battery’s safety, battery management systems (BMS) are utilized to make sure there is no over-charging or over-discharging of each battery. In this case, battery balancing based on the BMS is needed. In addition, the BMS should monitor the internal temperature of the battery to mitigate thermal runaway and the performance of LIB. This internal temperature measurement is required to maintain the LIB’s lifetime and maximize the use of LIB.
Despite the extreme growth of LIB, there are limitations to estimating the LIB internal temperature rapidly and effectively. The main functions of LIB, such as aging [
8,
9], safety [
8], and performance [
8,
9], are extremely affected by temperature. Ref. [
8] found that the LIB’s capacity was reduced by 98.75% at −40 °C. This means the performance decreases significantly, and the LIB does not generate maximum energy to the load. Moreover, since the LIB’s impedance increases, the heat generated by the internal resistance can reduce the LIB’s lifetime. In [
9], the author explained how LIB degradation also accelerates with temperature, which can damage LIB at high temperatures and reduce performance at low temperatures. Ref. [
10] explained that the LIB’s thermal runaway is affected by electrical abuse, such as overcharge, and heat abuse, such as high environmental temperatures. The ability to estimate the LIB internal temperature during the operation as early detection is a necessary function of BMS. This is due to the non-linear behavior of LIB, which can reduce performance at low temperature and trigger thermal runaway at high temperature. Moreover, in electric mobility applications such as electric vehicles, the LIB’s internal temperature estimation is urgently needed since there is extreme fluctuation of temperature during winter and summer. Consequently, the ability to track and measure the LIB’s internal temperature for BMS as early detection is critical to maintain the LIB’s lifetime and maximize the use of LIB.
The existing methods for measuring the LIB internal temperature use a temperature sensor mounted on the surface of the LIB [
11,
12]. Surface-mounted thermistors or thermocouples are common strategies for estimating the surface temperature of LIB. The data show that the temperature sensor can detect the surface or the external temperature of the LIB, but it cannot detect the LIB’s internal temperature. Moreover, in certain scenarios, for high current charging and discharging, the LIB internal temperature is significantly different compared to the surface temperature. Ref. [
13] suggested a pulse resistance approach by calculating the load fluctuations based on the load profile. This method is promising, but the sensing board needs to be sensitive, and the probability of achieving pulse load can depend on the applications. Ref. [
14] proposed an optic cable, Fiber Bragg Grating (FBG), to measure the LIB’s internal temperature. However, it needs to be put inside battery cells. Another temperature measurement approach is to use mathematical model-based estimators [
15]. The mathematical model provides a way to apply the general laws of thermodynamics to an LIB. However, this method has a high computation, and additional information needs to be added before the equation can model the thermodynamic law.
Normally, the models are described using different types of observer-based methods, as they can account for nonlinear dynamic behaviors and the variation of uncertainties [
16]. This method is promising; however, data processing is burdening the LIB internal temperature. In addition, as prediction precision highly depends on the aforementioned parameters, parameterization test data need to be done for all possible scenarios, such as high charging and discharging, at low and high temperatures to be sufficiently representative. To accommodate the LIB internal temperature measurement, electrochemical impedance spectroscopy (EIS) based on an Inductor Capacitor (
LC) resonant tank is proposed in this paper.
EIS [
17] is the method used to inject current or voltage into the LIB and measure the response of voltage or current in the LIB terminal. The voltage and current data can be used to estimate the LIB lifetime since the battery resistance is influenced by the LIB internal temperature. Scientists have proposed some EIS methods to measure LIB’s impedance. The common method [
12] is to use a frequency response analyzer (FRA) to measure LIB impedance. This method gives high accuracy; however, this method is costly and bulky since this method needs a commercial analyzer. In addition, this method cannot be utilized as an online impedance measurement due to the fact that the LIB needs to be unplugged from the systems and connected to the analyzer before testing.
There are many papers describing the impedance characteristics of LIB with an increase in temperature [
18]. Observations of SoC-independence and temperature-dependence of the charge transfer resistance (
Rct) and ohmic resistance (
Rohm) were also reported in [
19,
20]. The charge transfer is an intrinsic property of almost all Li-ion cells that transforms a solvated Li
+ in the electrolyte on both sides of the electrode. The resistance associated with the charge transfer process derives the indicator to measure the LIB internal temperature. However, estimating
Rct and
Rohm requires a wide range of frequency impedance measurements and curve fitting of the collected data to an electrical equivalent circuit model (ECM).
The authors of [
21], as shown in
Figure 1a, used the BMS topology to measure the LIB’s impedance. However, the data processing of windows [
21] and Fast Fourier Transform (FFT) of the impedance measurement burden the BMS. Furthermore, data acquisition (DAQ) is needed to process the data from the voltage and current sensing board. The authors of [
22], as shown in
Figure 1b, proposed embedded impedance spectroscopy into a bidirectional battery charger/converter. This method injected the sinusoidal current into the LIB during charging or released the sinusoidal current into the load during discharging, and measured the voltage and current responses from the LIB. The voltage and current data were processed (voltage and current peak, angle estimation, and frequency) to estimate the LIB impedance. However, this method burdens the data processing and needs an additional control modification in the bidirectional battery charger/converter since the sinusoidal signal is formed by the converter. Moreover, during the discharging mode, the load can be affected by the ripple injected by the battery.
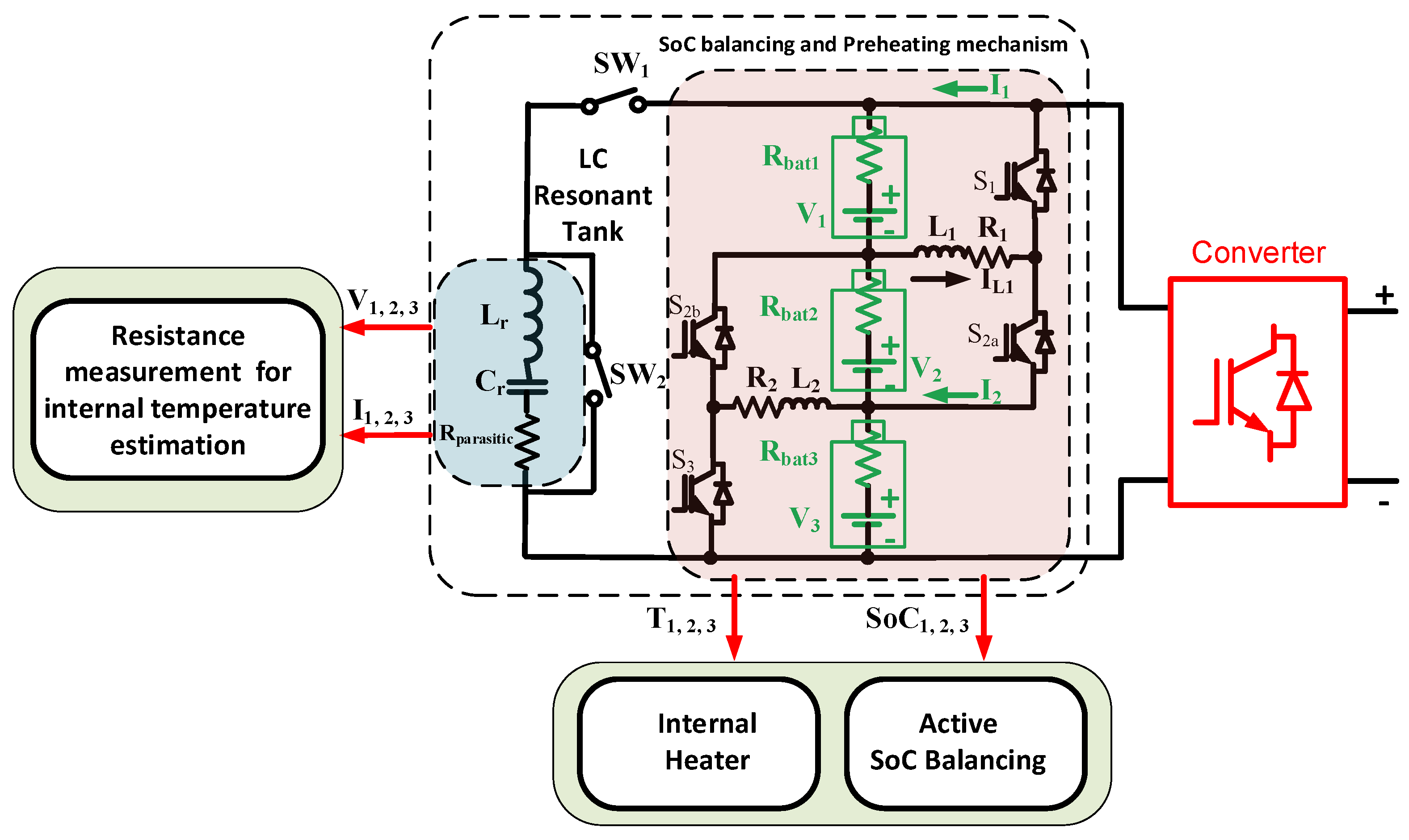
This paper proposes an internal resistance measurement using an
LC resonant tank [
23] to estimate the LIB internal temperature for the BMS, as shown in
Figure 2. The
LC resonant tank will inject an oscillation current into the battery pack due to the resonation of the inductor and capacitor. The voltage in the terminal batteries responds to the current depending on the internal resistance of the batteries. The voltage and current will be measured, and the battery’s internal resistance can be obtained. The LIB internal temperatures can be estimated based on the LIB internal measurements. Since there is no phase angle between voltage and current estimation, Fast Fourier Transform (FFT) is not needed. Data processing needs to detect the first cycle of the voltage and current peak only. In addition, the heating mechanism, and the state-of-charge (SoC) balancing modes can be used with the same topology. The internal preheating mechanism mode exists to warm up the battery at a temperature lower than zero. This is due to the LIB impedance behavior, which can be far higher at low temperatures compared to room temperature. The active SoC balancing mode equalizes the SoC level in each LIB. This strategy is to ensure no over-charging or under-charging of the LIBs. Moreover, SoC balancing helps to simplify the impedance measurement, since the LIB’s impedance data depend on the SoC level.
The advantages of the proposed paper are based on the following:
A new method to measure the LIB’s resistance for LIB internal temperature measurement based on an LC resonant tank.
The proposed LC resonant tank for LIB resistance measurement reduces data processing since windows and FFT are not needed.
To integrate the LC resonant tank into the BMS.
This paper is organized as follows:
Section 2 describes the method of the
LC resonant tank method, the preheating mechanism, and SoC balancing.
Section 3 explains the battery management systems used in this proposed method.
Section 4 gives the simulation and experimental results to validate the proposed LIB internal temperature measurement method. Finally,
Section 5 elaborates the conclusions.
3. Battery Management Systems
The batteries deployed in this proposed method are LIB 18650, which has the same internal and surface temperatures described in [
16]. Thus, the thermodynamical model for the battery’s internal resistance and losses is:
where
m is the LIB’s mass,
T is the cell temperature,
Tamb is the cell ambient/room temperature,
c is the specific heat capacity of the cell,
h is the equivalent heat transfer coefficient,
Rbat(n) is the internal LIB
B(n) resistance,
i(n) is the current in LIB
B(n), and
S is the LIB’s surface area,
Plosses(n) is the power used for the heating mechanism in LIB
B(n),
i(n),rms is the rms current in LIB
B(n), and
EL(n) is the energy in inductor
L(n).
The preheat mechanism condition is activated if the internal resistance based on the
LC resonant tank detects the maximum resistance. The balancing condition is triggered during the battery charging or discharging condition, since the balancing is active balancing. For the preheating mechanism, the duty cycle is 0.5 to ensure that all series LIBs have the same energy of heating mode, as described in
Figure 5.
Figure 5a shows the current flow at the upper loop of the proposed circuit. The current flows from the LIB to the inductor to charge the inductor. When the inductor voltage is higher than the inductor, the current flows back to the LIB. The same current flows occur in the lower circuit, as shown in
Figure 5b. Due to the current flows forward and backward, the internal resistance and the electrochemical reaction create heat, as in (9–10). This heat is utilized to preheat the battery during the sub-zero condition. In this case, the rms current and the internal resistance are the main factors that determine how fast the LIBs can be heated up from −5° to 10° Celsius.
The balancing mode is activated when SoC
B1,
B2, and
B3 are not at the same level. If the SoC level is not the same, the higher SoC level LIB will supply power to the other LIBs, which have a lower SoC level.
Figure 5a shows the current flow at the upper loop of the balancing circuit. The current flows from the LIB to the inductor to charge the inductor. When the inductor voltage is higher than the LIB, the current flows back to the LIB. The same current flows occur in the lower circuit, as shown in
Figure 5b. To make sure the energy is transferred from the higher SoC level to the lower SoC level, the duty cycle is set to 60%. It is different from the preheating mechanism, since the preheating mechanism needs to ensure that the preheating in each LIB is the same. Active balancing ensures that the balancing mode can be operated simultaneously during the charge or discharge load conditions. The duty cycle varies for the SoC balancing condition, and it is based on how much the SoC differs between each LIB. To ensure that the inductor can be protected from high current, the maximum duty cycle is 0.6 to limit the current.
4. Simulation and Experiment Results
The simulation used for this proposed method is PSIM, and the system parameters for the simulation are shown in
Table 1. As in
Figure 2, three series LIBs are used for the simulation, while two inductors are utilized to store the energy from the LIBs.
Figure 6 shows the balancing and preheating modes.
As shown in
Figure 6, the heating mechanism duration is the first 100 s. The slope of the SoC level of each LIB is negative due to the losses during the preheating mechanism. In
Figure 6a, battery
B1 delivers the power to inductor
L1, and the current returns to
B1 to heat up
B1. In the lower circuit,
B2 injects power to
L1 (with the
S1 and
S2a are compliment switches) and the current returns to
B2 to heat up
B2 as shown in
Figure 6b. The losses are the energy needed to heat up the battery due to the LIB’s internal resistance. In addition, there are losses due to the parasitic in the
L1. When the
LC resonant tank detects that the internal resistance is lower than at zero temperature, the preheating mechanism will be stopped.
For the condition after 100 s, the SoC levels are not the same; thus, the balancing is activated. The
S1 is triggered on since the
B1 has a higher SoC level.
L1 will receive energy from the charger and transfer it to the
B2 or
B3 as shown in
Figure 6a for charging conditions. While
B1 will be charged slowly due to the
S1 being on. For the discharging condition, The
S2a is triggered off since the
B2 has a higher SoC level.
L1 will receive energy from the
B2 and transfer it to the
B1. Additionally,
L2 will receive energy from the
B2 and transfer it to the
B3 as shown in
Figure 6b for the discharging condition. When the SoC levels are the same, the switches are triggered off, and the balancing condition is deactivated. The duration of balancing during charging is 450 s, while balancing during discharging is 550 s.
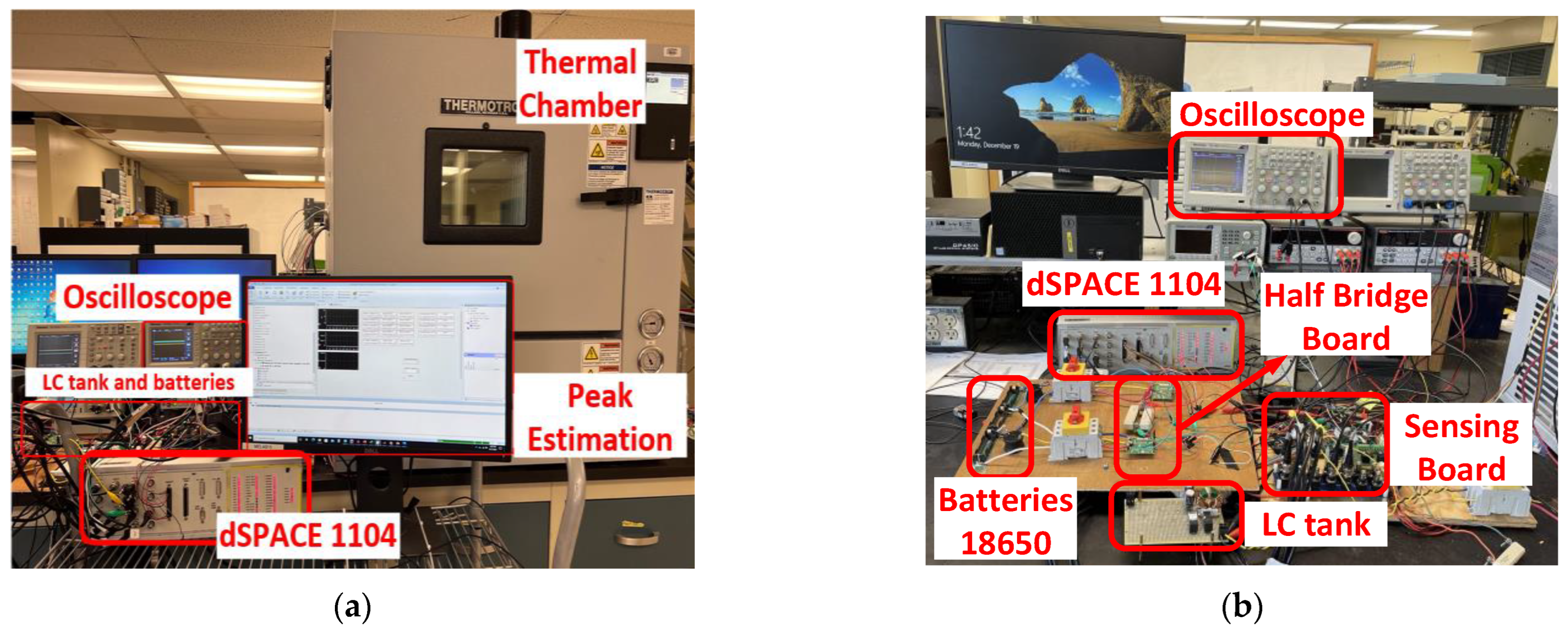
Figure 7 shows the experimental setup of the
LC resonant, preheating mechanism, and SoC balancing.
Figure 7a shows the testbed for the preheating mechanism. Three LIBs (18650) are placed inside the thermal chamber, and dSPACE 1104 is used to control the preheating mechanism. Two half-bridge boards are used for the inductor ladder converter. The thermal camera FLIR ONE is used to measure the batteries’ body temperature. For balancing and
LC resonant tanks, a thermal chamber is not needed. The
LC resonant tank for internal resistance spectroscopy is shown in
Figure 7b. It has a resonant inductor and capacitor (Lr = 1.4 mH, Cr = 5600 uF), a sensing board, an oscilloscope, and dSPACE 1104. The scope records the current and voltage when the resonant occurs. Afterward, dSPACE 1104 measures the peak of the voltage and current and estimates the resistance. Once the resistance is recorded, it can be used to estimate the batteries’ lifetime.
Figure 8 shows the experimental results of SoC balancing LIBs in series.
Figure 8a shows the experimental waveforms of SoC balancing during charging.
B2 SoC is 70%, while the SoC in
B1 and
B3 are 60% and 50%, respectively. The
B2 SoC is higher than
B1 and
B3 SoC, thus the
B2 transfers energy to
B1, and
B3. After
B2 and
B1 reach the same SoC level, both of
B2 and
B1 will transfer the energy to
B3. The total time needed for balancing is 410 s.
Figure 8b shows the experimental waveforms of SoC balancing during discharging.
B1 SoC is 70%, while the SoC in
B2 and
B3 are 60% and 50%, respectively. The
B1 SoC is higher than
B2 and
B3 SoC, thus the
B1 transfers energy to
B2, and
B3. After
B1 and
B2 reach the same SoC level, both of
B1 and
B2 will transfer energy to
B3. The total time needed for balancing is 400 s.
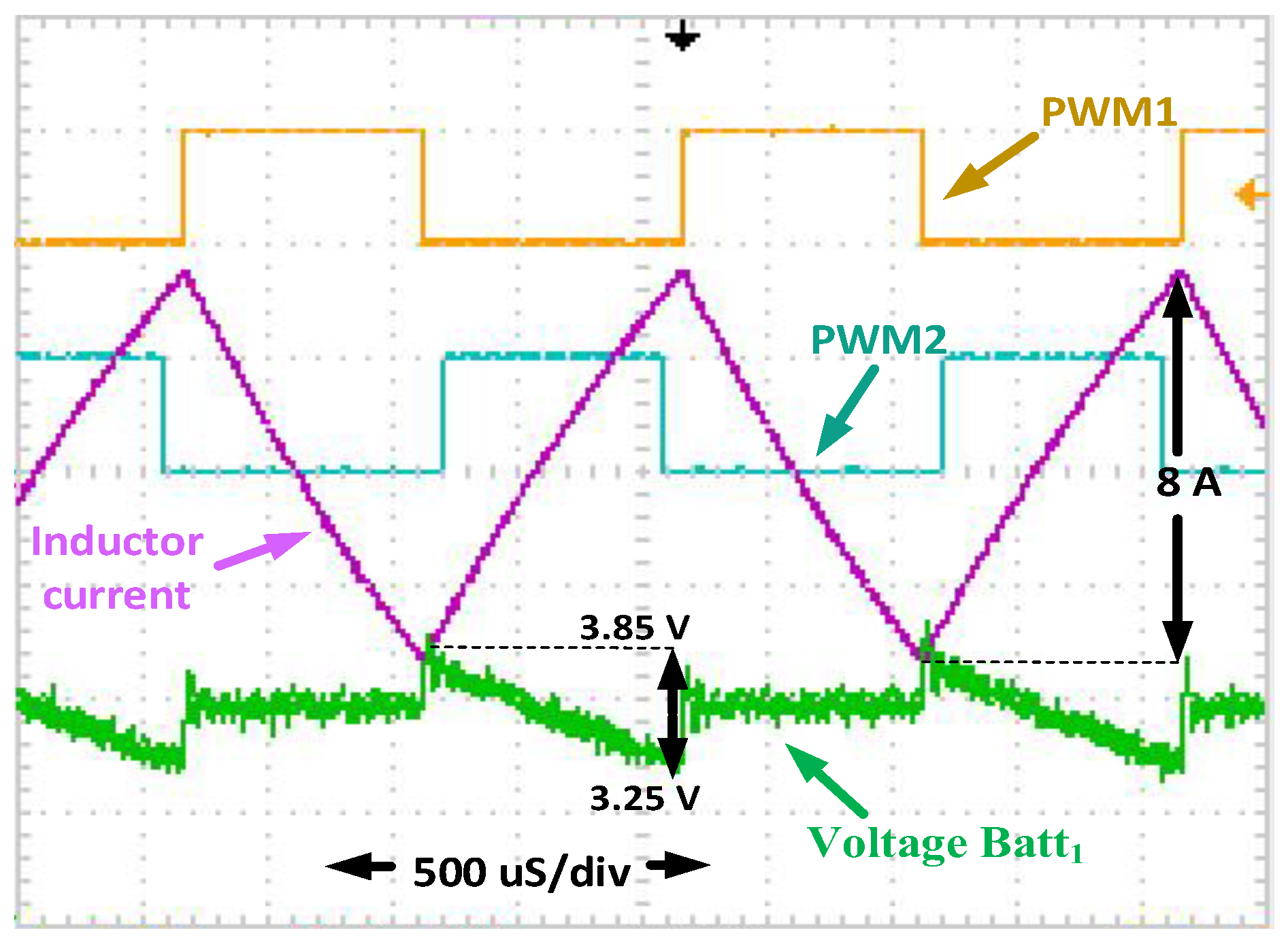
Figure 9 shows the preheating mechanism waveforms. It shows the current through the inductor,
PWM1 and
PWM2, and the voltage of
B1 during preheating. When the
PWM1 is turned on and the
PWM2 is turned off, the current is circulating in
B1. The
B1 is discharging
L1 when the voltage in
B1 is higher than the voltage in
L1. When the voltage of
L1 is higher than the voltage of
B1, the current flows from
L1 to
B1. It is shown that the inductor current increases and decreases while
B1 is charging or discharging. In this condition, the duty cycle is 50% to ensure that the energy is balanced between
B1 and
L1.
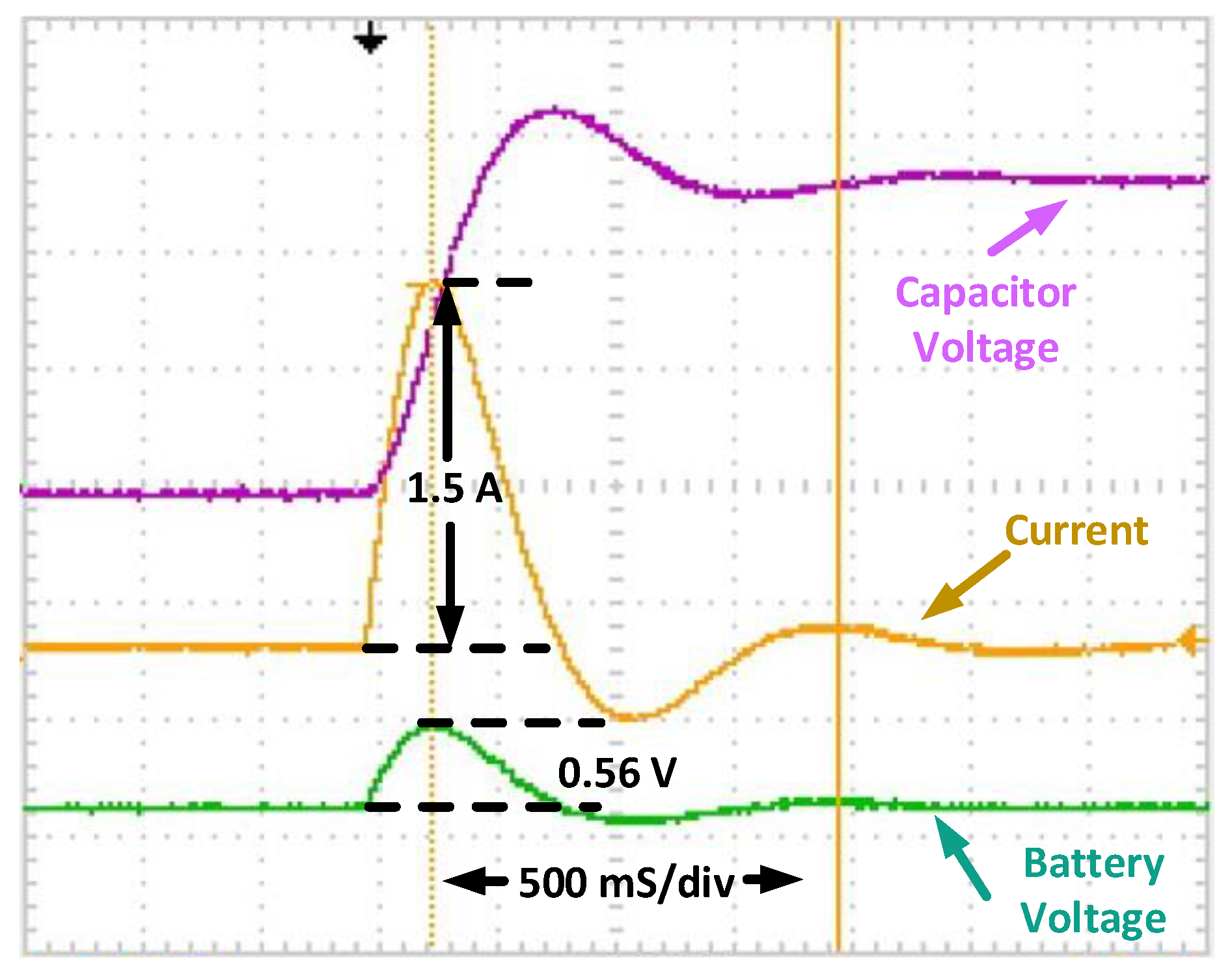
Figure 10 explains the experimental waveforms of
LC resonant tank conditions. In this mode, the battery is injecting current into the
LC resonant tank, and oscillation occurs at the resonant frequency. The peak current and voltage of the oscillation at the resonant frequency are measured, and the resistance can be calculated from (3 and 7). dSPACE DS1104 is utilized to track the peak and the mean of the current and voltage. Through this, FFT and additional data processing are not needed. Moreover, the
LC resonant tank can keep the resonant frequency stable at 56 Hz as a resonant frequency of Lr = 1.4 mH, Cr = 5600. The LIB voltage and current are in phase, with a peak of 3.92 V (or 0.56 V from the rated 2.8 V) and 1.5 A.
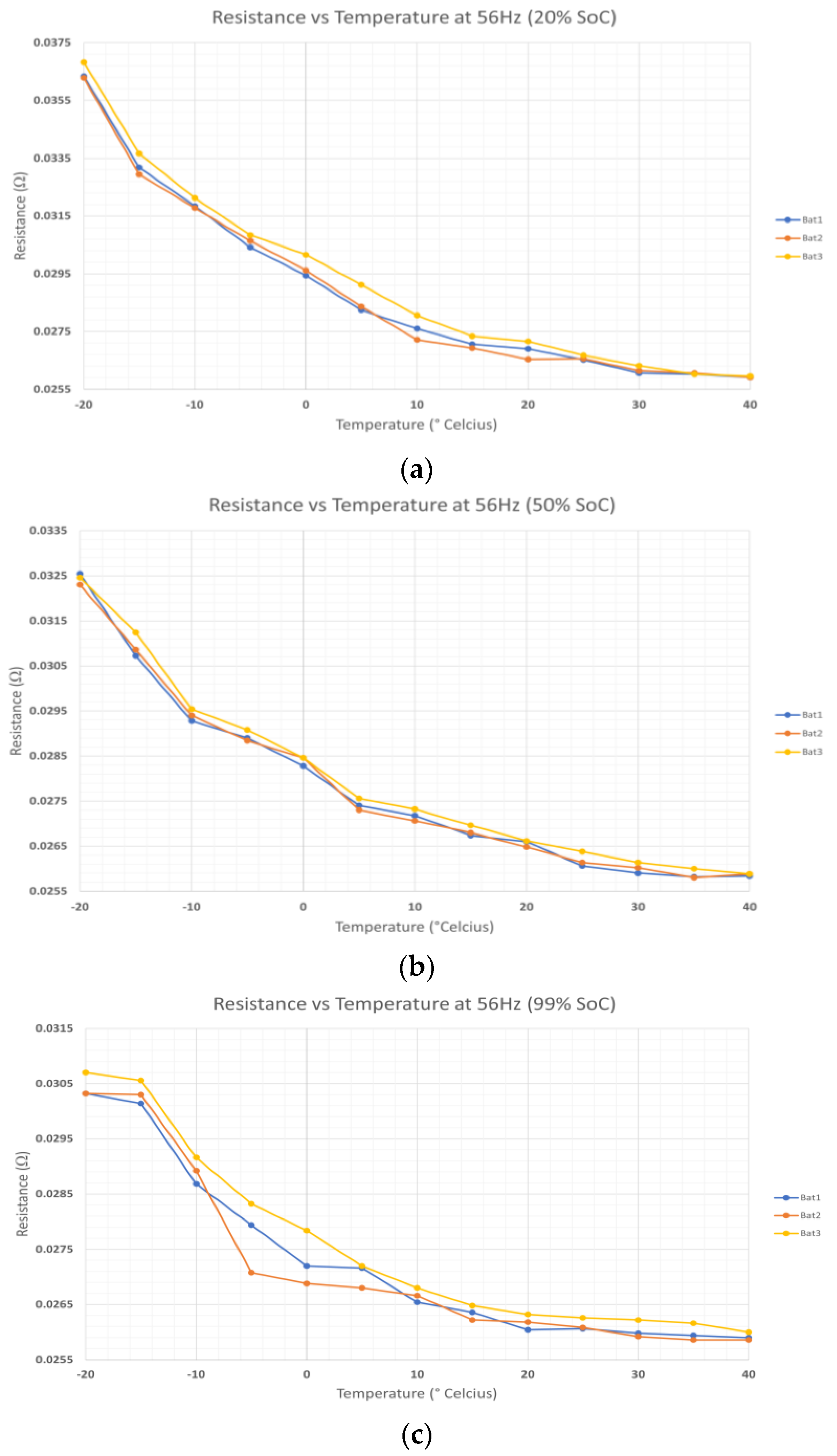
Figure 11 shows the resistance data of
B1,
B2, and
B3. The data are obtained by putting the LIBs inside the thermal chamber to control the temperature. Then, the LIBs are connected to the
LC resonant tank and the BMS. The temperature is set from −20 to 40 °C with an interval of 5 °C. The data show that
Rohm and
Rct are affected by temperature. At low temperatures,
Rohm and
Rct are higher due to the kinetics of the reactions. In addition, the free ions due to the dissociation of molecule scattering during collisions with the solvent molecules increase the
Rohm. While, at high temperatures, R
ohm and R
ct are significantly lower than at high temperatures. In addition,
B3 has the highest resistance compared to the other LIBs at each interval temperature. This can be due to the different characteristics of each battery.
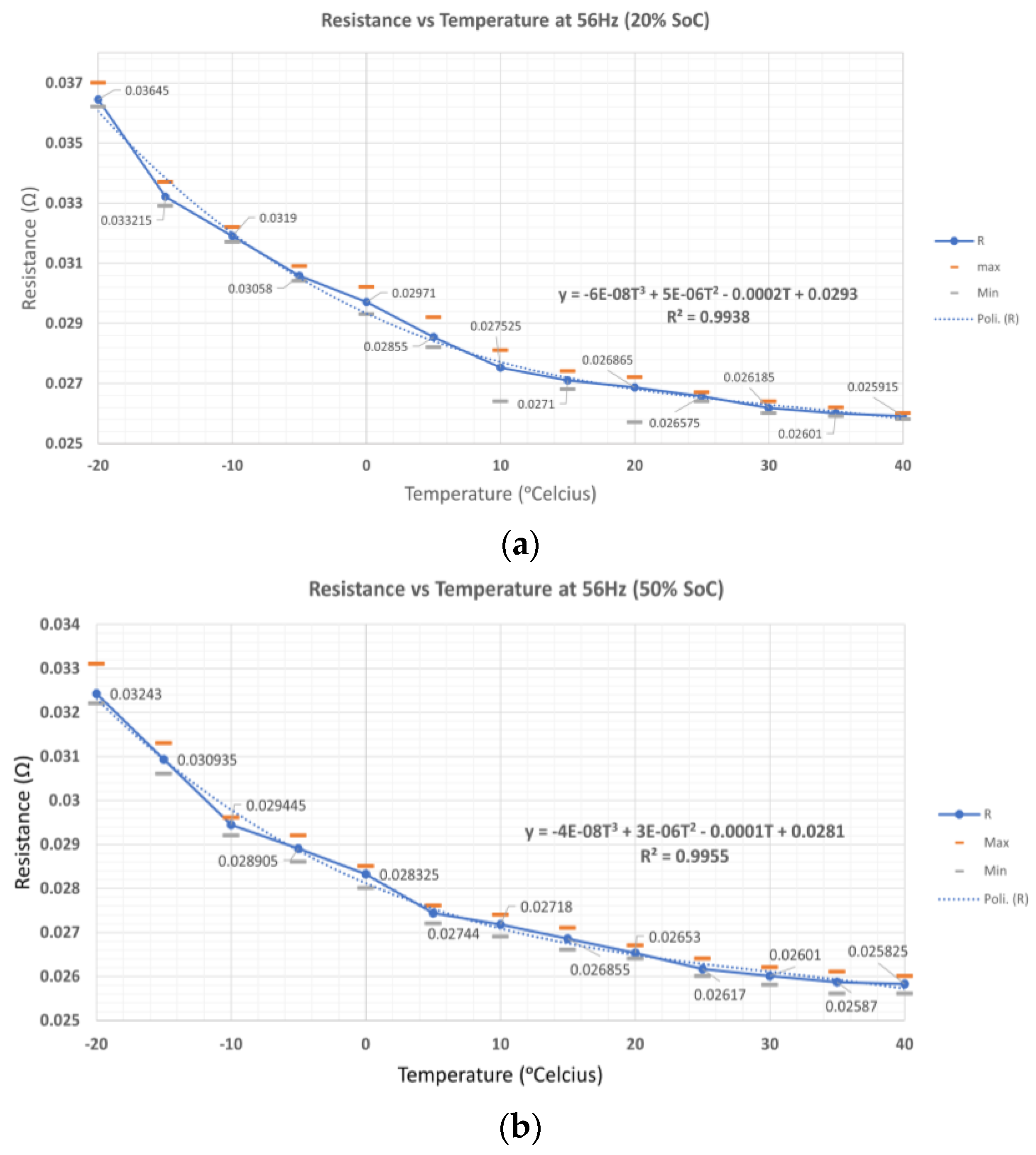
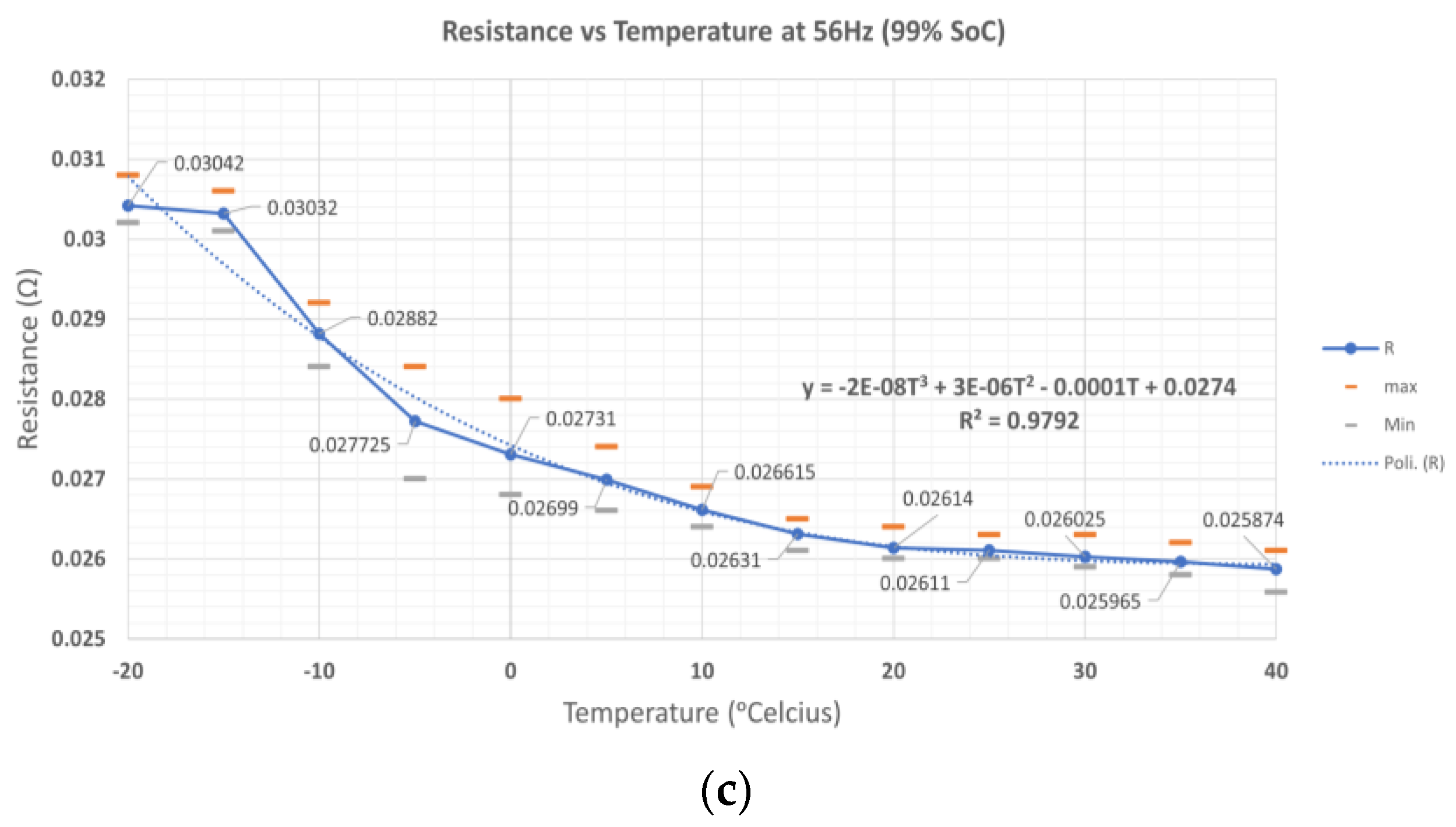
Figure 12 shows the mean values of
B1,
B2, and
B3 and the curve fitting by polynomial fitting. The mean data show that R
ohm and R
ct are affected by temperature. The curve fitting with order three can fit the data with the coefficient of determination of 0.9928, 0.9955, and 0.9792 at 20%, 50%, and 99% SoC levels, as shown in
Table 2. At different SoC levels, resistance is significantly different at low temperatures. At a low SoC level, resistance is higher than at a high SoC level. At high temperatures, the resistance shows a small difference between 20%, 50%, and 99% SoC levels. This condition occurs at a low-frequency point (56 Hz in this case). At low temperatures, the impedance at a higher SoC level will be greater than the impedance at a lower SoC level. Since the coefficient of determination (order three polynomial) is higher at the 50% SoC level, the polynomial curve fitting at this SoC level can be used to measure the LIB internal temperature.
As seen in
Table 3, the proposed method can measure the resistance in the LIBs. The 18650 battery is tested by an FRA to validate the resistance data obtained by this proposed method, as shown in
Figure 12. This shows that the
LC resonant tank can measure
B1 and
B3 with 17.87% and 17.73% errors compared to the FRA. While
B2 has an 18.14% error. This is due to the parasitics in the testbed. The cable resistance and turn-on switch resistance can affect the resistance measurement.
The preheating mechanism is shown in
Figure 13.
Figure 13a shows the batteries before preheating begins. The thermal chamber temperature is set to −5 °C for 24 h to make sure the electrochemical inside the LIBs are the same as the thermal chamber temperature, as shown in
Figure 13a. After 24 h, the preheating mode is activated, and the switches begin to operate with a 50% duty cycle.
Figure 13b shows the results after the preheating mode is completed. The preheating duration is 34 min from −5 °C to 10 °C. During the preheating mechanism, there is energy consumption, which can be translated as a capacity loss in the LIBs. The rate of energy loss due to the preheating mode is 0.214%/°C to preheat the LIBs from −5 °C to 10 °C. The rate of energy loss is measured before the preheating condition and after the preheating condition is completed.
Table 4 describes the
LC resonant tank method compared to the other methods [
21,
22,
29]. It is shown that the proposed method has less data computation compared with other methods, such as the two-pulse load test, integrated EIS into BMS, and battery charger as an EIS measurement.