A Facile Two-Step Thermal Process for Producing a Dense, Phase-Pure, Cubic Ta-Doped Lithium Lanthanum Zirconium Oxide Electrolyte for Upscaling
Abstract
:1. Introduction
2. Materials and Methods
3. Results
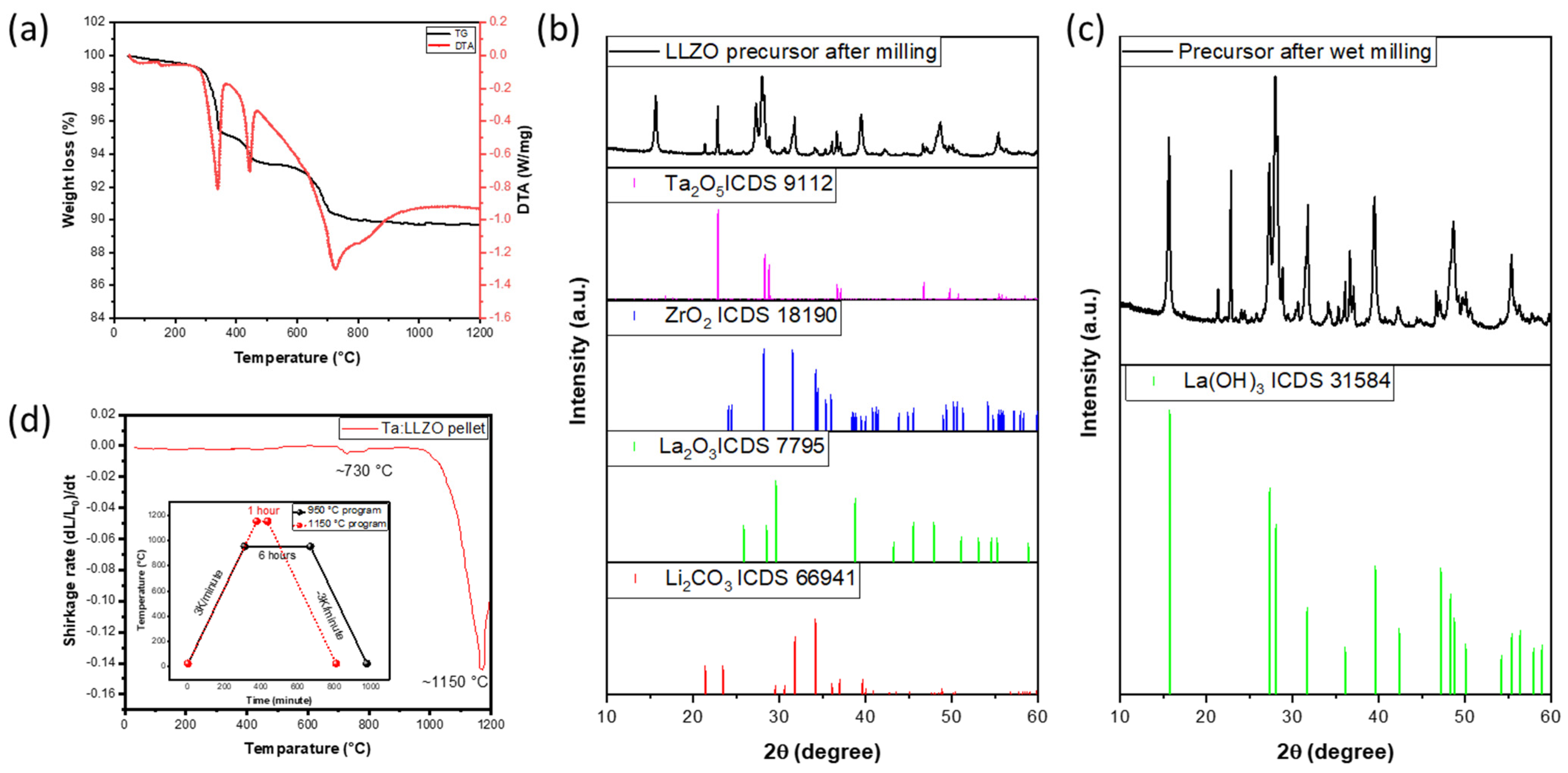
3.1. Thermal Analysis
3.2. Phase Analysis
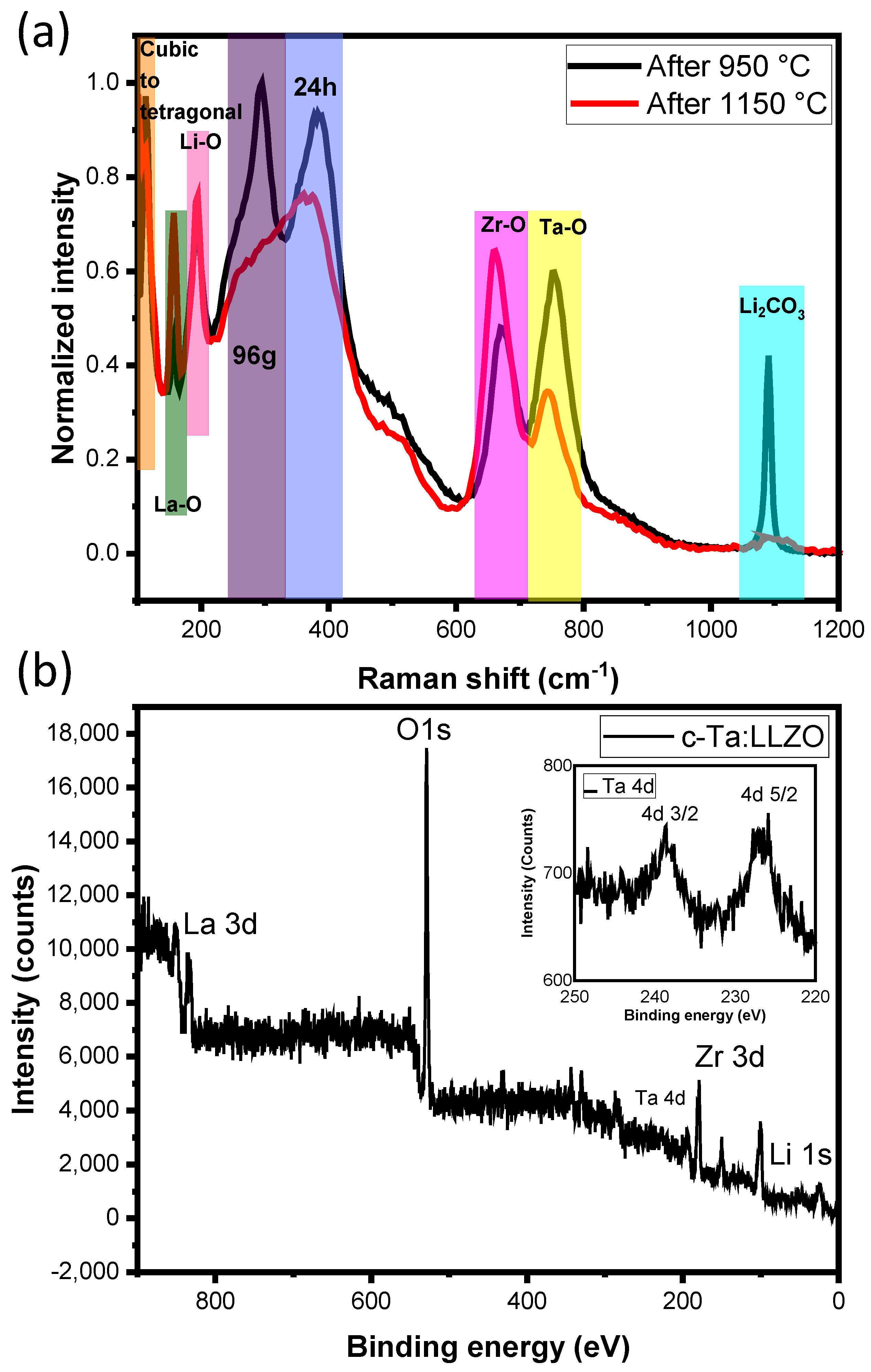
3.3. Vibration and Elemental Analysis
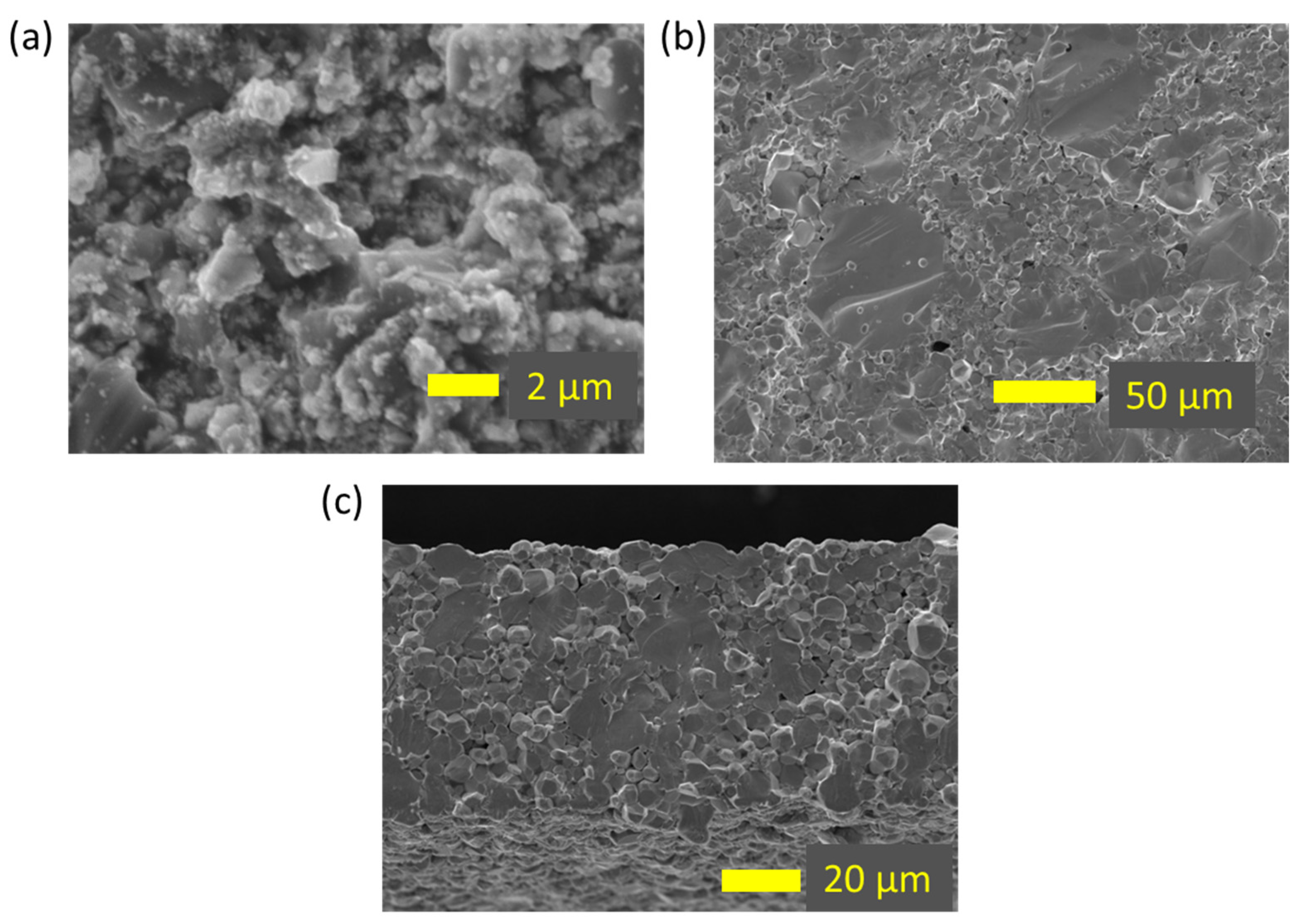
3.4. Microstructural Analysis
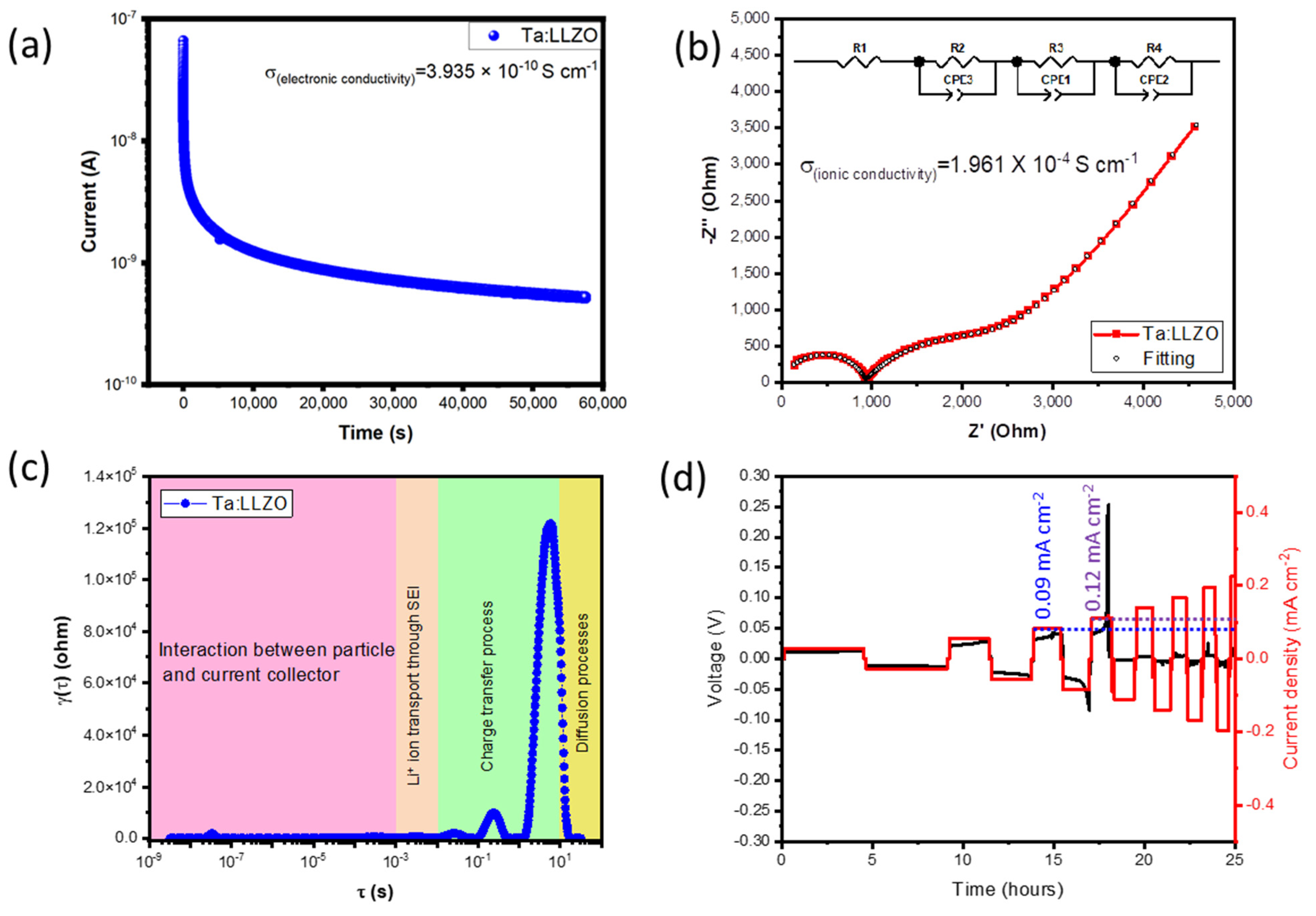
3.5. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, F.; Guo, W.; Zeng, D.; Sun, Z.; Gao, J.; Li, J.; Zhao, B.; He, B.; Han, X. A Simple and Highly Efficient Method toward High-Density Garnet-Type LLZTO Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 30313–30319. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ion. 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Buannic, L.; Orayech, B.; López Del Amo, J.-M.; Carrasco, J.; Katcho, N.A.; Aguesse, F.; Manalastas, W.; Zhang, W.; Kilner, J.; Llordés, A. Dual Substitution Strategy to Enhance Li+ Ionic Conductivity in Li7La3Zr2O12 Solid Electrolyte. Chem. Mater. 2017, 29, 1769–1778. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, K.; Chen, D.; Zeng, J.; Luo, B. Spark plasma sintering plus heat-treatment of Ta-doped Li7La3Zr2O12 solid electrolyte and its ionic conductivity. Mater. Res. Express 2020, 7, 025518. [Google Scholar] [CrossRef]
- Clemenceau, T.; Andriamady, N.; Kumar, M.K.; Badran, A.P.; Avila, V.; Dahl, K.; Hopkins, M.; Vendrell, X.; Marshall, D.; Raj, R. Flash sintering of Li-ion conducting ceramic in a few seconds at 850 °C. Scr. Mater. 2019, 172, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Tu, R.; Shen, Q.; Zhang, L. Field assisted sintering of dense Al-substituted cubic phase Li7La3Zr2O12 solid electrolytes. J. Power Sources 2014, 268, 960–964. [Google Scholar] [CrossRef]
- Li, H.-Y.; Huang, B.; Huang, Z.; Wang, C.-A. Enhanced mechanical strength and ionic conductivity of LLZO solid electrolytes by oscillatory pressure sintering. Ceram. Int. 2019, 45, 18115–18118. [Google Scholar] [CrossRef]
- Yang, L.; Dai, Q.; Liu, L.; Shao, D.; Luo, K.; Jamil, S.; Liu, H.; Luo, Z.; Chang, B.; Wang, X. Rapid sintering method for highly conductive Li7La3Zr2O12 ceramic electrolyte. Ceram. Int. 2020, 46, 10917–10924. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Karabay, D.T.; Kovalenko, M.V. On the feasibility of all-solid-state batteries with LLZO as a single electrolyte. Sci. Rep. 2022, 12, 1177. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, F.; Shen, Q.; Zhang, L.; Chen, C. Effect of the lithium ion concentration on the lithium ion conductivity of Ga-doped LLZO. Mater. Res. Express 2019, 6, 085546. [Google Scholar] [CrossRef]
- Yi, E.; Wang, W.; Kieffer, J.; Laine, R.M. Flame made nanoparticles permit processing of dense, flexible, Li+ conducting ceramic electrolyte thin films of cubic-Li7La3Zr2O12 (c-LLZO). J. Mater. Chem. A 2016, 4, 12947–12954. [Google Scholar] [CrossRef]
- Badami, P.; Smetaczek, S.; Limbeck, A.; Rettenwander, D.; Chan, C.K.; Kannan, A.N.M. Facile synthesis of Al-stabilized lithium garnets by a solution-combustion technique for all solid-state batteries. Mater. Adv. 2021, 2, 5181–5188. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Hu, Z.; Huang, Y.; Zhu, X. MgO or Al2O3 crucible: Which is better for the preparation of LLZ-based solid electrolytes? Ceram. Int. 2020, 46, 3367–3373. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.-Q.; Guo, X.-X. Densification and lithium ion conductivity of garnet-type Li7La3Zr2−xTaxO12 (x = 0.25) solid electrolytes. Chin. Phys. B 2013, 22, 078201. [Google Scholar] [CrossRef]
- Weller, J.M.; Whetten, J.A.; Chan, C.K. Synthesis of fine cubic Li7La3Zr2O12 powders in molten LiCl–KCl eutectic and facile densification by reversal of Li+/H+ exchange. ACS Appl. Energy Mater. 2018, 1, 552–560. [Google Scholar] [CrossRef]
- Xia, W.; Xu, B.; Duan, H.; Guo, Y.; Kang, H.; Li, H.; Liu, H. Ionic conductivity and air stability of Al-doped Li7La3Zr2O12 sintered in alumina and Pt crucibles. ACS Appl. Mater. Interfaces 2016, 8, 5335–5342. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, Y.; Guo, H.; Song, Z.; Xiu, T.; Badding, M.E.; Wen, Z. None-mother-powder method to prepare dense Li-garnet solid electrolytes with high critical current density. ACS Appl. Energy Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef]
- Sato, M.; Garcia-Mendez, R.; Sakamoto, J. Mapping hot-pressed Li6.25Al0.25La3Zr2O12 (LLZO) grains and grain boundaries through a simple thermal grooving technique. J. Asian Ceram. Soc. 2020, 8, 793–803. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, C.H.; Jarry, A.; Chen, W.; Ye, Y.; Zhu, J.; Kostecki, R.; Persson, K.; Guo, J.; Salmeron, M.; et al. Interrelationships among grain size, surface composition, air stability, and interfacial resistance of Al-substituted Li7La3Zr2O12 solid electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 17649–17655. [Google Scholar] [CrossRef]
- Heo, T.W.; Grieder, A.; Wang, B.; Wood, M.; Hsu, T.; Akhade, S.A.; Wan, L.F.; Chen, L.-Q.; Adelstein, N.; Wood, B.C. Microstructural impacts on ionic conductivity of oxide solid electrolytes from a combined atomistic-mesoscale approach. npj Comput. Mater. 2021, 7, 214. [Google Scholar] [CrossRef]
- Inada, R.; Takeda, A.; Yamazaki, Y.; Miyake, S.; Sakurai, Y.; Thangadurai, V. Effect of postannealing on the properties of a Ta-doped Li7La3Zr2O12 solid electrolyte degraded by Li dendrite penetration. ACS Appl. Energy Mater. 2020, 3, 12517–12524. [Google Scholar] [CrossRef]
- Fritsch, C.; Zinkevich, T.; Indris, S.; Etter, M.; Baran, V.; Bergfeldt, T.; Knapp, M.; Ehrenberg, H.; Hansen, A.-L. Garnet to hydrogarnet: Effect of post synthesis treatment on cation substituted LLZO solid electrolyte and its effect on Li ion conductivity. RSC Adv. 2021, 11, 30283–30294. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, K.; Chen, L.; Lu, Y.; Li, Y.; Wang, C.-A. Sintering behavior of garnet-type Li6.4La3Zr1.4Ta0.6O12 in Li2CO3 atmosphere and its electrochemical property. Int. J. Appl. Ceram. Technol. 2017, 14, 921–927. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Hu, D.; Chen, F.; Shen, Q.; Zhang, L.; Dong, S. Synergistic regulation of garnet-type Ta-doped Li7La3Zr2O12 solid electrolyte by Li+ concentration and Li+ transport channel size. Electrochim. Acta 2019, 296, 823–829. [Google Scholar] [CrossRef]
- Yi, M.; Liu, T.; Wang, X.; Li, J.; Wang, C.; Mo, Y. High densification and Li-ion conductivity of Al-free Li7-xLa3Zr2−xTaxO12 garnet solid electrolyte prepared by using ultrafine powders. Ceram. Int. 2019, 45, 786–792. [Google Scholar] [CrossRef]
- Ramakumar, S.; Deviannapoorani, C.; Dhivya, L.; Shankar, L.S.; Murugan, R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog. Mater. Sci. 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Zeier, W.G.; Zhou, S.; Lopez-Bermudez, B.; Page, K.; Melot, B.C. Dependence of the Li-Ion Conductivity and Activation Energies on the Crystal Structure and Ionic Radii in Li6MLa2Ta2O12. ACS Appl. Mater. Interfaces 2014, 6, 10900–10907. [Google Scholar] [CrossRef]
- Miara, L.J.; Ong, S.P.; Mo, Y.; Richards, W.D.; Park, Y.; Lee, J.-M.; Lee, H.S.; Ceder, G. Effect of Rb and Ta Doping on the Ionic Conductivity and Stability of the Garnet Li7+2x–y(La3–xRbx)(Zr2–yTay)O12 (0 ≤ x ≤ 0.375, 0 ≤ y ≤ 1) Superionic Conductor: A First Principles Investigation. Chem. Mater. 2013, 25, 3048–3055. [Google Scholar] [CrossRef]
- Gajraj, V.; Kumar, A.; Indris, S.; Ehrenberg, H.; Kumar, N.; Mariappan, C.R. Influence of Al on the structure and ion transport in garnet-type Li7La3−xAlxZr2O12 solid electrolytes for Li-ion batteries. Ceram. Int. 2022, 48, 29238–29246. [Google Scholar] [CrossRef]
- Gonzalez Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Larraz, G.; Orera, A.; Sanjuán, M.L. Cubic phases of garnet-type Li7La3Zr2O12: The role of hydration. J. Mater. Chem. A 2013, 1, 11419–11428. [Google Scholar] [CrossRef]
- Hirose, E.; Niwa, K.; Kataoka, K.; Akimoto, J.; Hasegawa, M. Structural stability of the Li-ion conductor Li7La3Zr2O12 investigated by high-pressure in-situ X-ray diffraction and Raman spectroscopy. Mater. Res. Bull. 2018, 107, 361–365. [Google Scholar] [CrossRef]
- Rettenwander, D.; Welzl, A.; Cheng, L.; Fleig, J.; Musso, M.; Suard, E.; Doeff, M.M.; Redhammer, G.J.; Amthauer, G. Synthesis, crystal chemistry, and electrochemical properties of Li7–2xLa3Zr2–xMoxO12 (x = 0.1–0.4): Stabilization of the cubic garnet polymorph via substitution of Zr4+ by Mo6+. Inorg. Chem. 2015, 54, 10440–10449. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Rustad, J.R. The effect of tetrahedral versus octahedral network-blocking atom substitutions on lithium ion conduction in LLZO garnet. arXiv 2016, arXiv:1605.08598. [Google Scholar]
- Zhu, Y.; Connell, J.G.; Tepavcevic, S.; Zapol, P.; Garcia-Mendez, R.; Taylor, N.J.; Sakamoto, J.; Ingram, B.J.; Curtiss, L.A.; Freeland, J.W.; et al. Dopant-dependent stability of garnet solid electrolyte interfaces with lithium metal. Adv. Energy Mater. 2019, 9, 1803440. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Song, Z.; Rui, K.; Wang, Q.; Xiu, T.; Badding, M.E.; Wen, Z. Manipulating Li2O atmosphere for sintering dense Li7La3Zr2O12 solid electrolyte. Energy Storage Mater. 2019, 22, 207–217. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Zhao, S.-X.; Xu, Y.-H.; Nan, C.-W. Effect of the morphology of Li–La–Zr–O solid electrolyte coating on the electrochemical performance of spinel LiMn1.95Ni0.05O3.98F0.02 cathode materials. J. Mater. Chem. A 2014, 2, 18889–18897. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Li, W.; Xue, Y.; Yang, J.; Li, S. Realization of superior ionic conductivity by manipulating the atomic rearrangement in Al-doped Li7La3Zr2O12. Ceram. Int. 2023, 49, 10462–10470. [Google Scholar] [CrossRef]
- Hahn, M.; Rosenbach, D.; Krimalowski, A.; Nazarenus, T.; Moos, R.; Thelakkat, M.; Danzer, M.A. Investigating solid polymer and ceramic electrolytes for lithium-ion batteries by means of an extended Distribution of Relaxation Times analysis. Electrochim. Acta 2020, 344, 136060. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, M.; Huang, T.; Yu, A. Detection of lithium plating in lithium-ion batteries by distribution of relaxation times. J. Power Sources 2021, 496, 229867. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Carrillo, R.E.; Wood, V. EIS2MOD: A DRT-based modeling framework for li-ion cells. IEEE Trans. Ind. Appl. 2021, 58, 1429–1439. [Google Scholar] [CrossRef]
- Flatscher, F.; Philipp, M.; Ganschow, S.; Wilkening, H.M.R.; Rettenwander, D. The natural critical current density limit for Li7La3Zr2O12 garnets. J. Mater. Chem. A 2020, 8, 15782–15788. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Ai, B.; Zhao, S.; Zhang, W.; Pan, K.; Zhang, L. Improvement of the interface between the lithium anode and a garnet-type solid electrolyte of lithium batteries using an aluminum-nitride layer. Nanomaterials 2022, 12, 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppiah, D.; Komissarenko, D.; Yüzbasi, N.S.; Liu, Y.; Warriam Sasikumar, P.V.; Hadian, A.; Graule, T.; Clemens, F.; Blugan, G. A Facile Two-Step Thermal Process for Producing a Dense, Phase-Pure, Cubic Ta-Doped Lithium Lanthanum Zirconium Oxide Electrolyte for Upscaling. Batteries 2023, 9, 554. https://doi.org/10.3390/batteries9110554
Karuppiah D, Komissarenko D, Yüzbasi NS, Liu Y, Warriam Sasikumar PV, Hadian A, Graule T, Clemens F, Blugan G. A Facile Two-Step Thermal Process for Producing a Dense, Phase-Pure, Cubic Ta-Doped Lithium Lanthanum Zirconium Oxide Electrolyte for Upscaling. Batteries. 2023; 9(11):554. https://doi.org/10.3390/batteries9110554
Chicago/Turabian StyleKaruppiah, Diwakar, Dmitrii Komissarenko, Nur Sena Yüzbasi, Yang Liu, Pradeep Vallachira Warriam Sasikumar, Amir Hadian, Thomas Graule, Frank Clemens, and Gurdial Blugan. 2023. "A Facile Two-Step Thermal Process for Producing a Dense, Phase-Pure, Cubic Ta-Doped Lithium Lanthanum Zirconium Oxide Electrolyte for Upscaling" Batteries 9, no. 11: 554. https://doi.org/10.3390/batteries9110554
APA StyleKaruppiah, D., Komissarenko, D., Yüzbasi, N. S., Liu, Y., Warriam Sasikumar, P. V., Hadian, A., Graule, T., Clemens, F., & Blugan, G. (2023). A Facile Two-Step Thermal Process for Producing a Dense, Phase-Pure, Cubic Ta-Doped Lithium Lanthanum Zirconium Oxide Electrolyte for Upscaling. Batteries, 9(11), 554. https://doi.org/10.3390/batteries9110554







