Garnet-Type Zinc Hexacyanoferrates as Lithium, Sodium, and Potassium Solid Electrolytes
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Characterization
3.2. Ionic Conductivity
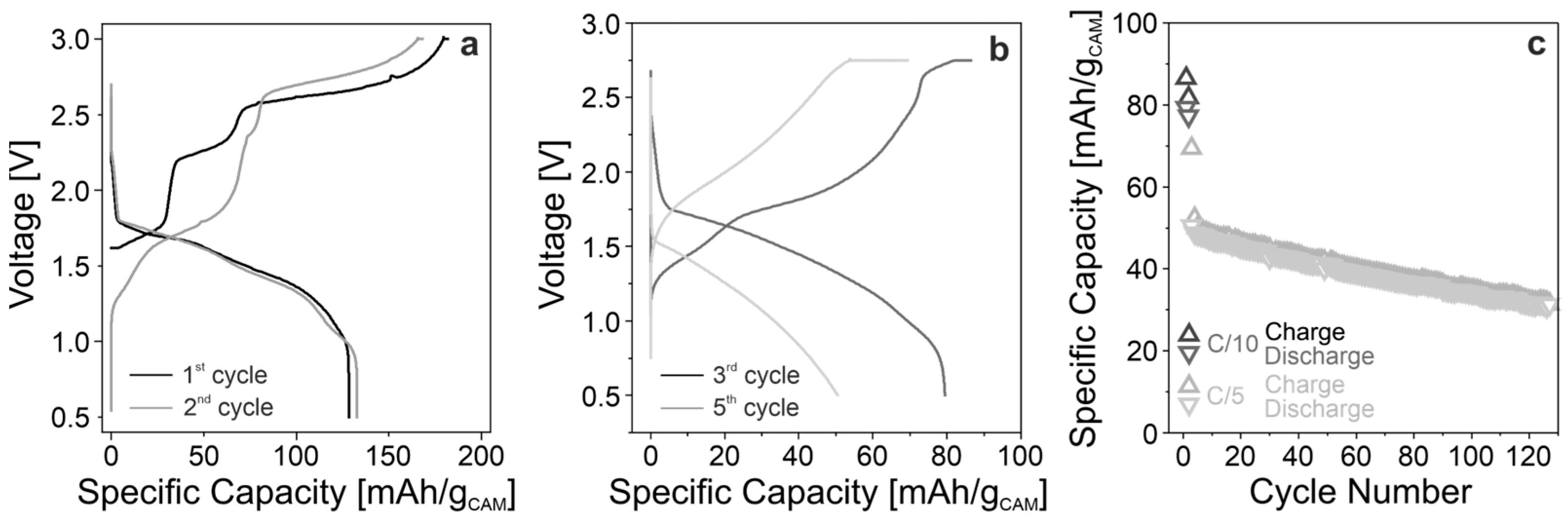
3.3. Electrochemical Testing
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Li, Y.; Xin, S.; Goodenough, J.B. Rechargeable Sodium All-Solid-State Battery. ACS Cent. Sci. 2017, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.-S.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, J.; Qi, X.; Rong, X.; Shao, Y.; Feng, W.; Nie, J.; Hu, Y.-S.; Li, H.; Huang, X.; et al. A new Na[(FSO2)(n-C4F9SO2)N]-based polymerelectrolyte for solid-state sodium batteries. J. Mater. Chem. A 2017, 5, 7738–7743. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, Y.; Li, H.; Chen, L.; Huang, X.; Zhou, Z. An sodium bis(trifluoromethanesulfonyl)imide-based polymer electrolyte for solid-state sodium batteries. Acta Phys. Chim. Sin. 2018, 34, 213–218. [Google Scholar]
- Moreno, J.S.; Armand, M.; Berman, M.B.; Greenbaum, S.G.; Scrosati, B.; Panero, S. Composite PEOn:NaTFSI polymer electrolyte: Preparation, thermal and electrochemical characterization. J. Power Sources 2014, 248, 695–702. [Google Scholar] [CrossRef]
- Song, S.; Kotobuki, M.; Zheng, F.; Xu, C.; Savilov, S.V.; Hu, N.; Lu, L.; Wang, Y.; Li, W.D.Z. A hybrid polymer/oxide/ionic-liquid solid electrolyte for Na-metal batteries. J. Mater. Chem. A 2017, 5, 6424–6431. [Google Scholar] [CrossRef]
- Goodwin, L.E.; Till, P.; Bhardwaj, M.; Nazer, N.; Adelhelm, P.; Tietz, F.; Zeier, W.G.; Richter, F.H.; Janek, J. Protective NaSICON Interlayer between a Sodium–Tin Alloy Anode and Sulfide-Based Solid Electrolytes for All-Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2023, 15, 50457–50468. [Google Scholar] [CrossRef]
- Wu, J.-F.; Wang, Q.; Guo, X. Sodium-ion conduction in Na2Zn2TeO6 solid electrolytes. J. Power Sources 2018, 402, 513–518. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, B.; Xie, Z.; Zhou, Z. NASICON-Type Na3Zr2Si2PO12 Solid-State Electrolytes for Sodium Batteries. ChemElectroChem 2021, 8, 1035–1047. [Google Scholar] [CrossRef]
- Yu Yao, Y.-F.; Kummer, J.T. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 1967, 29, 2453–2475. [Google Scholar] [CrossRef]
- Wu, E.A.; Banerjee, S.; Tang, H.; Richardson, P.M.; Doux, J.M.; Qi, J.; Zhu, Z.; Grenier, A.; Li, Y.; Zhao, E.; et al. A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries. Nat. Commun. 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Schlem, R.; Banik, A.; Eckhardt, M.; Zobel, M.; Zeier, W.G. Na3−xEr1−xZrxCl6—A Halide-Based Fast Sodium-Ion Conductor with Vacancy-Driven Ionic Transport. ACS Appl. Energy Mater. 2020, 3, 10164–10173. [Google Scholar] [CrossRef]
- Kwak, H.; Lyoo, J.; Park, J.; Han, Y.; Asakura, R.; Remhof, A.; Battaglia, C.; Kim, H.; Hong, S.T.; Jung, Y.S. Na2ZrCl6 enabling highly stable 3 V all-solid-state Na-ion batteries. Energy Storage Mater. 2021, 37, 47–54. [Google Scholar] [CrossRef]
- Tsuji, F.; Nasu, A.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Mechanochemical synthesis and characterization of Na3–xP1–xWxS4 solid electrolytes. J. Power Sources 2021, 506, 230100. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 856. [Google Scholar] [CrossRef]
- Banerjee, A.; Park, K.H.; Heo, J.W.; Nam, Y.J.; Moon, C.K.; Oh, S.M.; Hong, S.-T.; Jung, Y.S. Na3SbS4: A Solution Processable Sodium Superionic Conductor for All-Solid-State Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 9634–9638. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, K.H.; Choi, Y.E.; Lee, J.Y.; Jung, Y.S. Aqueous-solution synthesis of Na3SbS4 solid electrolytes for all-solid-state Na-ion batteries. J. Mater. Chem. A 2018, 6, 840–844. [Google Scholar] [CrossRef]
- Kim, T.; Hyeok Ahn, S.; Song, Y.Y.; Jin Park, B.; Lee, C.; Choi, A.; Kim, M.H.; Seo, D.H.; Jung, S.K.; Lee, H.W. Prussian Blue-Type Sodium-ion Conducting Solid Electrolytes for All Solid-State Batteries. Angew. Chem. Int. Ed. 2023, 62, e202309852. [Google Scholar] [CrossRef]
- Pshinko, G.N.; Puzyrnaya, L.N.; Shunkov, V.S.; Kosorukov, A.A.; Demchenko, V.Y. Removal of Cesium and Strontium Radionuclides from Aqueous Media by Sorption onto Magnetic Potassium Zinc Hexacyanoferrate(II). Radiochemistry 2016, 58, 491–497. [Google Scholar] [CrossRef]
- Kawamura, S.; Kuraku, H.; Kurotaki, K. Adsorption characteristics of radionuclides on nickel hexacyanoferrate(II). Anal. Chim. Acta 1970, 81, 91–97. [Google Scholar] [CrossRef]
- Jassal, V.; Shanker, U.; Kaith, B.S.; Shankar, S. Green synthesis of potassium zinc hexacyanoferrate nanocubes and their potential application in photocatalytic degradation of organic dyes. RSC Adv. 2015, 5, 26141–26149. [Google Scholar] [CrossRef]
- Arida, H.A.; Aglan, R.F. A Solid-State Potassium Selective Electrode Based on Potassium Zinc Ferrocyanide Ion Exchanger. Anal. Lett. 2003, 36, 895–907. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.A.; Vazquez-Samperio, J.; Cabrera-Sierra, R.; Reguera, E. Materials for aqueous sodium-ion batteries: Cation mobility in a zinc hexacyanoferrate electrode. RSC Adv. 2016, 6, 108627–108634. [Google Scholar] [CrossRef]
- He, B.; Man, P.; Zhang, Q.; Wang, C.; Zhou, Z.; Li, C.; Wei, L.; Yao, Y. Conversion Synthesis of Self-Standing Potassium Zinc Hexacyanoferrate Arrays as Cathodes for High-Voltage Flexible Aqueous Rechargeable Sodium-Ion Batteries. Small 2019, 15, 1905115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Il Kim, Y.; Park, J.K.; Choi, J.W. Sodium zinc hexacyanoferrate with a well-defined open framework as a positive electrode for sodium ion batteries. Chem. Commun. 2012, 48, 8416–8418. [Google Scholar] [CrossRef]
- Heo, J.W.; Chae, M.S.; Hyoung, J.; Hong, S.T. Rhombohedral Potassium–Zinc Hexacyanoferrate as a Cathode Material for Nonaqueous Potassium-Ion Batteries. Inorg. Chem. 2019, 58, 3065–3072. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Lu, Y.; Liu, J.; Guo, B.; Xiao, P.; Lee, J.J.; Yang, X.Q.; Henkelman, G.; Goodenough, J.B. Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery. J. Am. Chem. Soc. 2015, 137, 2658–2664. [Google Scholar] [CrossRef]
- Xiao, P.; Song, J.; Wang, L.; Goodenough, J.B.; Henkelman, G. Theoretical Study of the Structural Evolution of a Na2FeMn(CN)6 Cathode upon Na Intercalation. Chem. Mater. 2015, 27, 3763–3768. [Google Scholar] [CrossRef]
- Nguyen, H.; Banerjee, A.; Wang, X.; Tan, D.; Wu, E.A.; Doux, J.-M.; Stephens, R.; Verbist, G.; Meng, Y.S. Single-step synthesis of highly conductive Na3PS4 solid electrolyte for sodium all solid-state batteries. J. Power Sources 2019, 435, 126623. [Google Scholar] [CrossRef]
- Siebert, H.; Jentsch, W. Rhomboedrisch kristallisierende Zink-hexacyanometallate(III) Zn3[M(CN)8]2. Z. Naturforsch. 1981, 36b, 123–124. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Reguera, E.; Lima, E.; Balmaseda, J.; Martínez-García, R.; Yee-Madeira, H. An atypical coordination in hexacyanometallates: Structure and properties of hexagonal zinc phases. J. Phys. Chem. Solids 2007, 68, 1630–1642. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.; Ramos-Sánchez, G.; Guzmán, G.; Avila, M.; González, I.; Reguera, E. Water effect on sodium mobility in zinc hexacyanoferrate during charge/discharge processes in sodium ion-based battery. Solid State Ion. 2017, 312, 67–72. [Google Scholar] [CrossRef]
- Avila, M.; Rodríguez-Hernández, J.; Lemus-Santana, A.A.; Reguera, E. Cation mobility and structural changes on the water removal in zeolite-like zinc hexacyanometallates (II). J. Phys. Chem. Solids 2011, 72, 988–993. [Google Scholar] [CrossRef]
- Liu, X.; Gong, H.; Han, C.; Cao, Y.; Li, Y.; Sun, J. Barium ions act as defenders to prevent water from entering prussian blue lattice for sodium-ion battery. Energy Storage Mater. 2023, 57, 118–124. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian Blue Analogs as Battery Materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef]
- Takachi, M.; Fukuzumi, Y.; Moritomo, Y. Na+ diffusion kinetics in nanoporous metal-hexacyanoferrates. Dalt. Trans. 2016, 45, 458–461. [Google Scholar] [CrossRef]
- Nordstrand, J.; Toledo-Carrillo, E.; Kloo, L.; Dutta, J. Sodium to cesium ions: A general ladder mechanism of ion diffusion in prussian blue analogs. Phys. Chem. Chem. Phys. 2022, 24, 12374–12382. [Google Scholar] [CrossRef]
- Moritomo, Y.; Igarashi, K.; Kim, J.; Tanaka, H. Size Dependent Cation Channel in Nanoporous Prussian Blue Lattice. Appl. Phys. Express 2009, 2, 085001. [Google Scholar] [CrossRef]
- Ning, Z.; Li, G.; Melvin, D.L.R.; Chen, Y.; Bu, J.; Spencer-Jolly, D.; Liu, J.; Hu, B.; Gao, X.; Perera, J.; et al. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature 2023, 618, 287–293. [Google Scholar] [CrossRef]
- Ma, Q.; Ortmann, T.; Yang, A.; Sebold, D.; Burkhardt, S.; Rohnke, M.; Tietz, F.; Fattakhova-Rohlfing, D.; Janek, J.; Guillon, O. Enhancing the Dendrite Tolerance of NaSICON Electrolytes by Suppressing Edge Growth of Na Electrode along Ceramic Surface. Adv. Energy Mater. 2022, 12, 2201680. [Google Scholar] [CrossRef]
- Medenbach, L.; Bender, C.L.; Haas, R.; Mogwitz, B.; Pompe, C.; Adelhelm, P.; Schröder, D.; Janek, J. Origins of Dendrite Formation in Sodium–Oxygen Batteries and Possible Countermeasures. Energy Technol. 2017, 5, 2265–2274. [Google Scholar] [CrossRef]
- Singh, D.K.; Henss, A.; Mogwitz, B.; Gautam, A.; Horn, J.; Krauskopf, T.; Burkhardt, S.; Sann, J.; Richter, F.H.; Janek, J. Li6PS5Cl microstructure and influence on dendrite growth in solid-state batteries with lithium metal anode. Cell Rep. Phys. Sci. 2022, 3, 101043. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, L.; Pan, H.; Lu, X.; Gu, L.; Hu, Y.-S.; Li, H.; Armand, M.; Ikuhara, Y.; Chen, L.; et al. Direct atomic-scale confirmation of three-phase storage mechanism in Li4Ti5O12 anodes for room-temperature sodium-ion batteries. Nat. Commun. 2013, 4, 1870. [Google Scholar] [CrossRef] [PubMed]
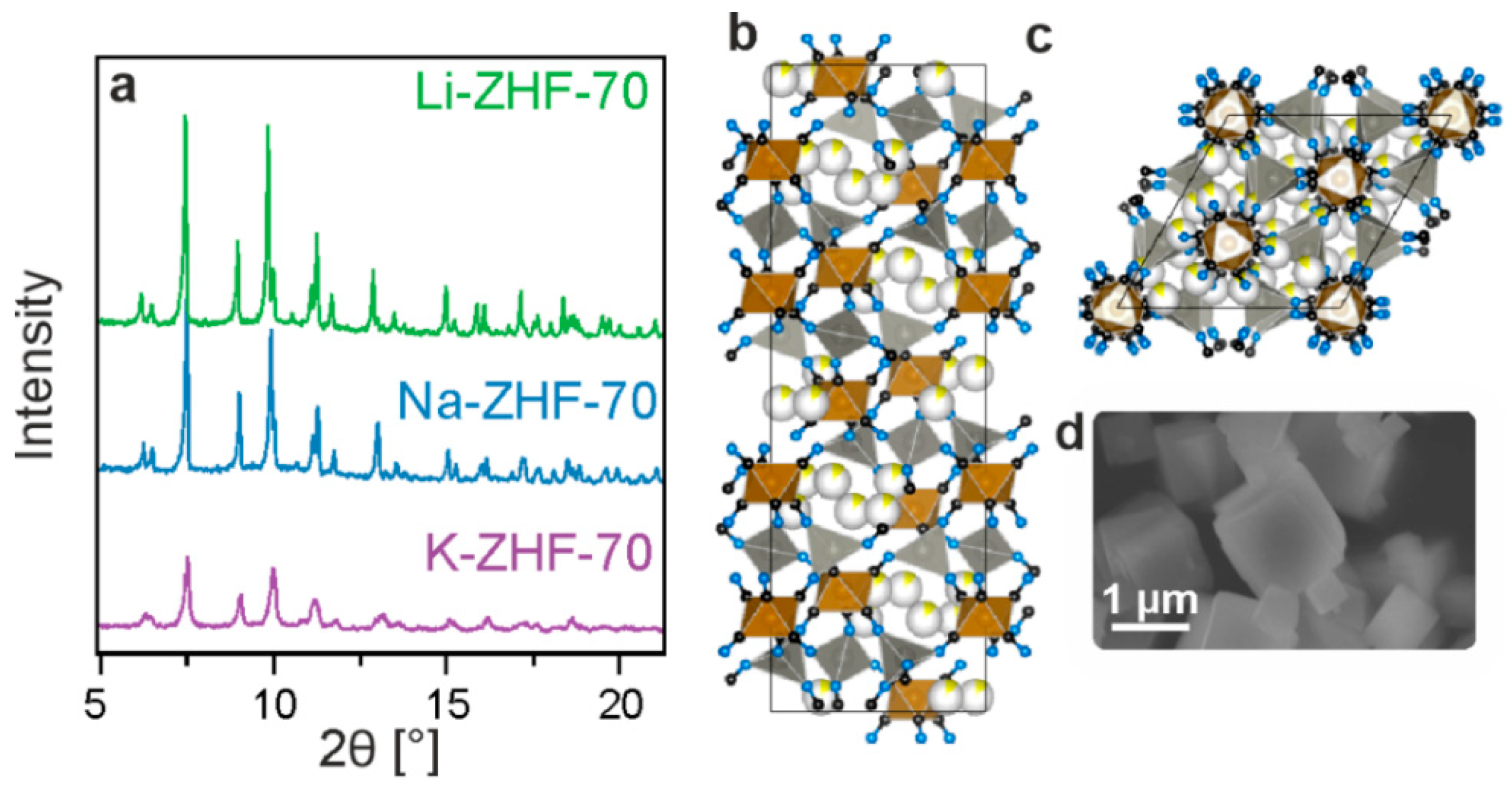
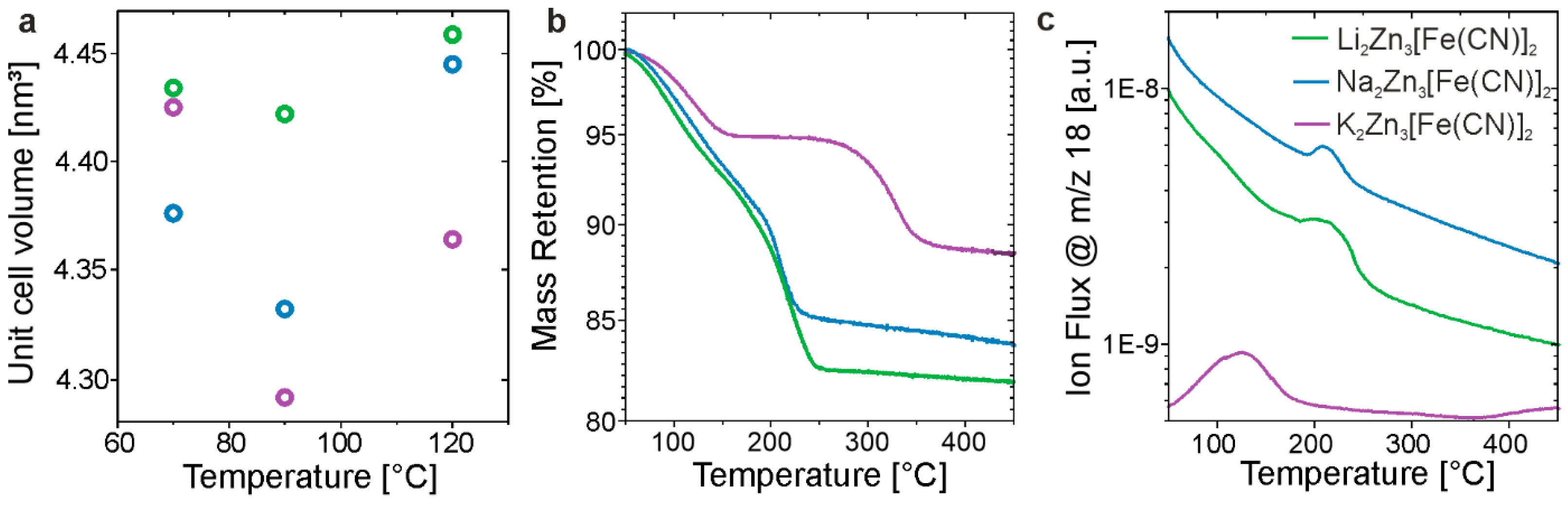
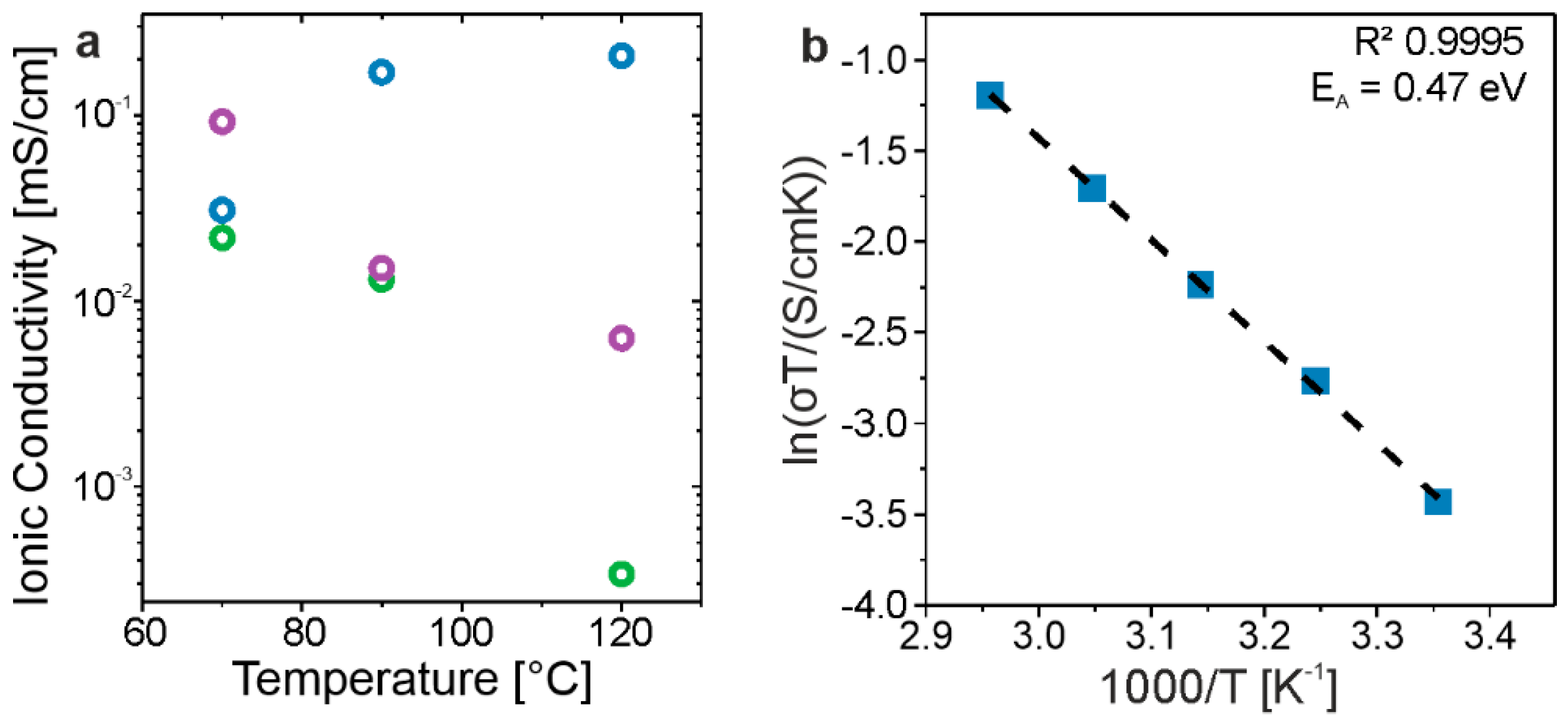
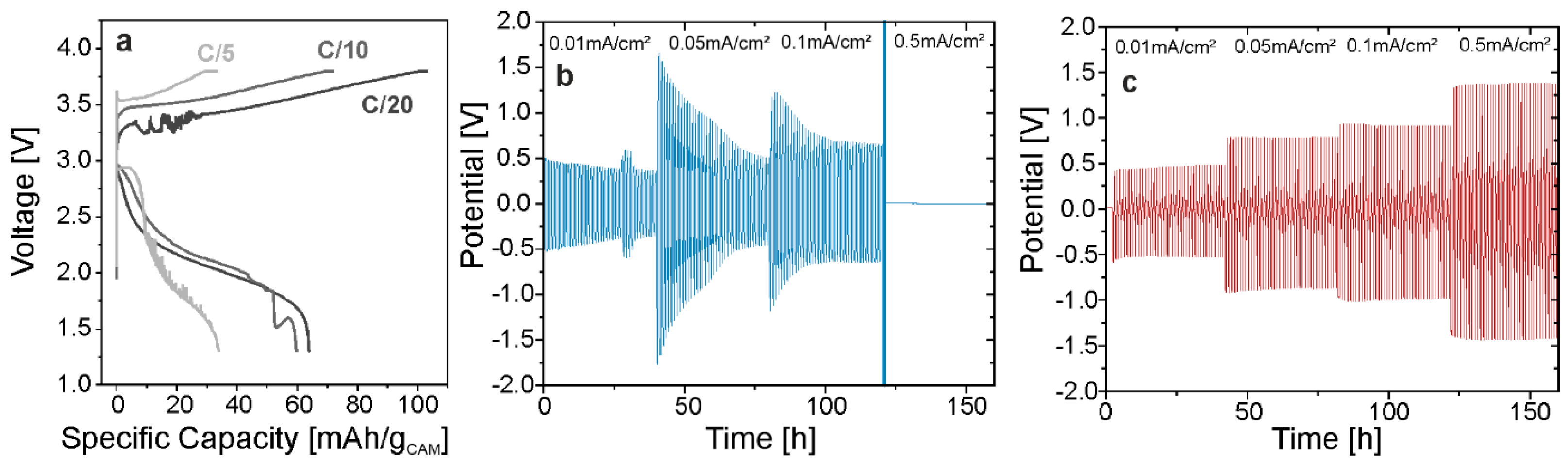
| Sample | Assumed Stoichiometry | Drying Temperature [°C] | Unit-Cell Volume [nm3] | Conductivity [mS/cm] |
|---|---|---|---|---|
| Li-ZHF-70 | Li2Zn3[Fe(CN)6]2 | 70 | 4.434(2) | 0.022 |
| Li-ZHF-90 | Li2Zn3[Fe(CN)6]2 | 90 | 4.422(2) | 0.013 |
| Li-ZHF-120 | Li2Zn3[Fe(CN)6]2 | 120 | 4.459(6) | 0.00034 |
| Na-ZHF-70 | Na2Zn3[Fe(CN)6]2 | 70 | 4.376(2) | 0.031 |
| Na-ZHF-90 | Na2Zn3[Fe(CN)6]2 | 90 | 4.332(2) | 0.17 |
| Na-ZHF-120 | Na2Zn3[Fe(CN)6]2 | 120 | 4.445(1) | 0.21 |
| K-ZHF-70 | K2Zn3[Fe(CN)6]2 | 70 | 4.425(2) | 0.092 |
| K-ZHF-90 | K2Zn3[Fe(CN)6]2 | 90 | 4.292(2) | 0.015 |
| K-ZHF-120 | K2Zn3[Fe(CN)6]2 | 120 | 4.364(4) | 0.0063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karger, L.; Murugan, S.; Wang, L.; Zhao-Karger, Z.; Kondrakov, A.; Strauss, F.; Brezesinski, T. Garnet-Type Zinc Hexacyanoferrates as Lithium, Sodium, and Potassium Solid Electrolytes. Batteries 2024, 10, 365. https://doi.org/10.3390/batteries10100365
Karger L, Murugan S, Wang L, Zhao-Karger Z, Kondrakov A, Strauss F, Brezesinski T. Garnet-Type Zinc Hexacyanoferrates as Lithium, Sodium, and Potassium Solid Electrolytes. Batteries. 2024; 10(10):365. https://doi.org/10.3390/batteries10100365
Chicago/Turabian StyleKarger, Leonhard, Saravanakumar Murugan, Liping Wang, Zhirong Zhao-Karger, Aleksandr Kondrakov, Florian Strauss, and Torsten Brezesinski. 2024. "Garnet-Type Zinc Hexacyanoferrates as Lithium, Sodium, and Potassium Solid Electrolytes" Batteries 10, no. 10: 365. https://doi.org/10.3390/batteries10100365
APA StyleKarger, L., Murugan, S., Wang, L., Zhao-Karger, Z., Kondrakov, A., Strauss, F., & Brezesinski, T. (2024). Garnet-Type Zinc Hexacyanoferrates as Lithium, Sodium, and Potassium Solid Electrolytes. Batteries, 10(10), 365. https://doi.org/10.3390/batteries10100365








