PEI/Super P Cathode Coating: A Pathway to Superior Lithium–Sulfur Battery Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Unmodified Standard Sulfur Cells
2.3. Preparation of Sulfur Cathode Modified with PEI/Super P and PVDF/Super P
2.4. Structural and Elemental Analysis of the Electrode
2.5. Li–S Coin Cell Assembly and Electrochemical Analysis
2.6. Assembly and Electrochemical Characterization of Symmetric Cells
3. Results and Discussion
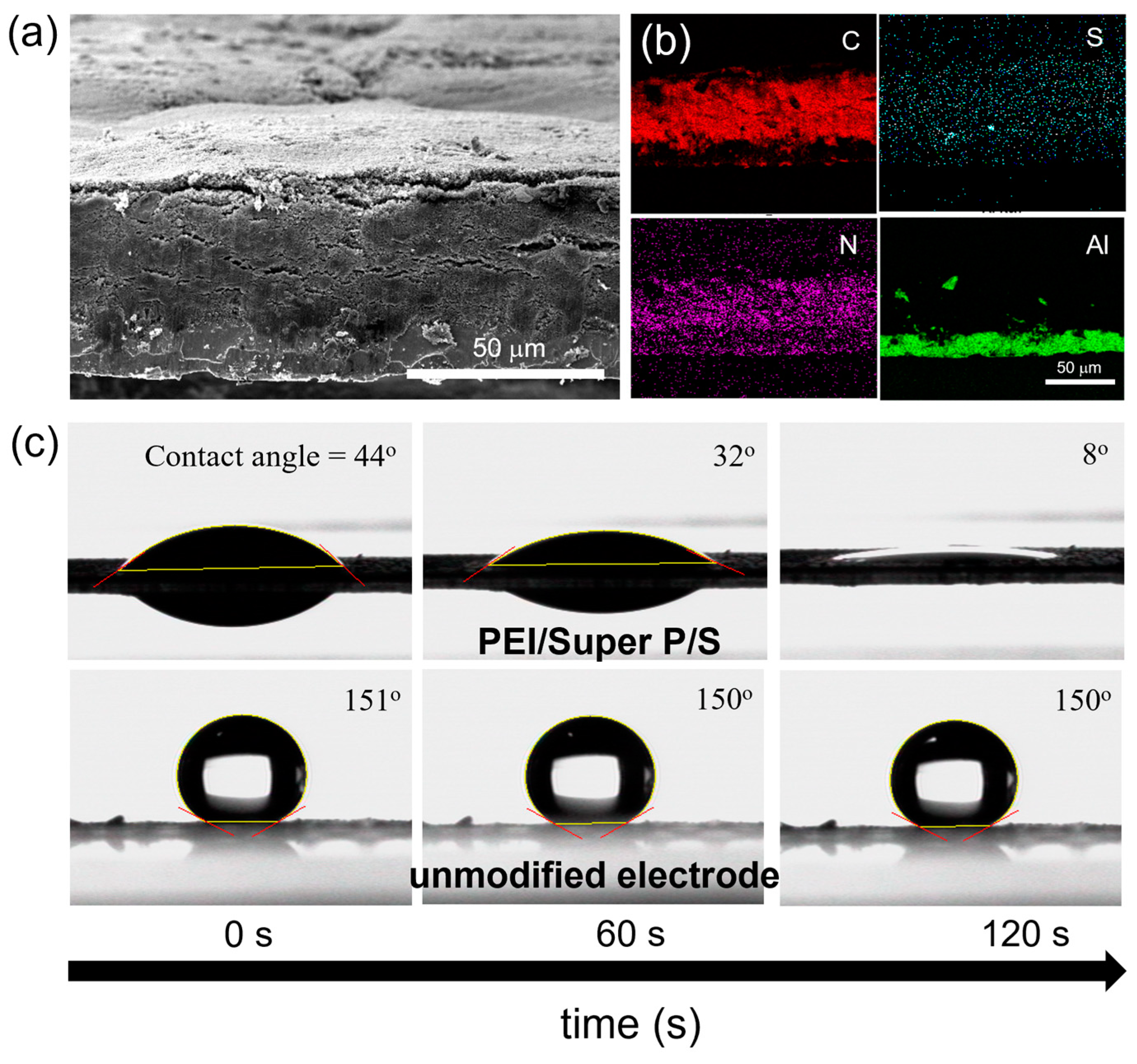
3.1. Characterization of the Electrode Structure and Surface Properties of PEI/SuperP/S Electrodes
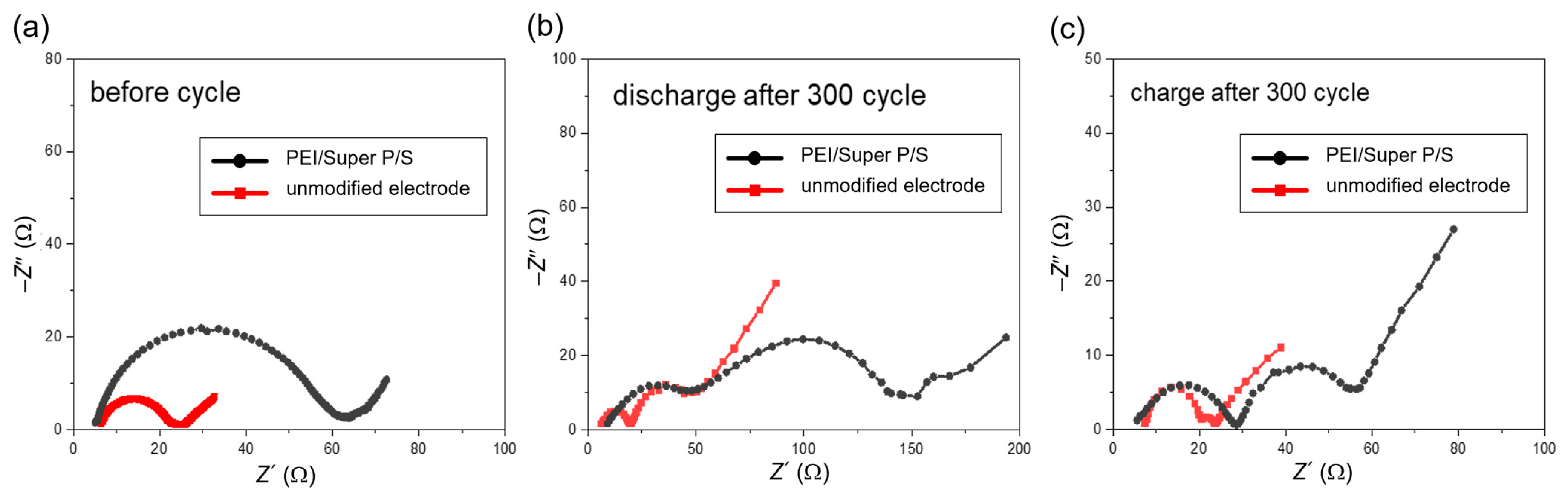
3.2. Symmetric Cell Experiment to Investigate the Impact of PEI/Super P
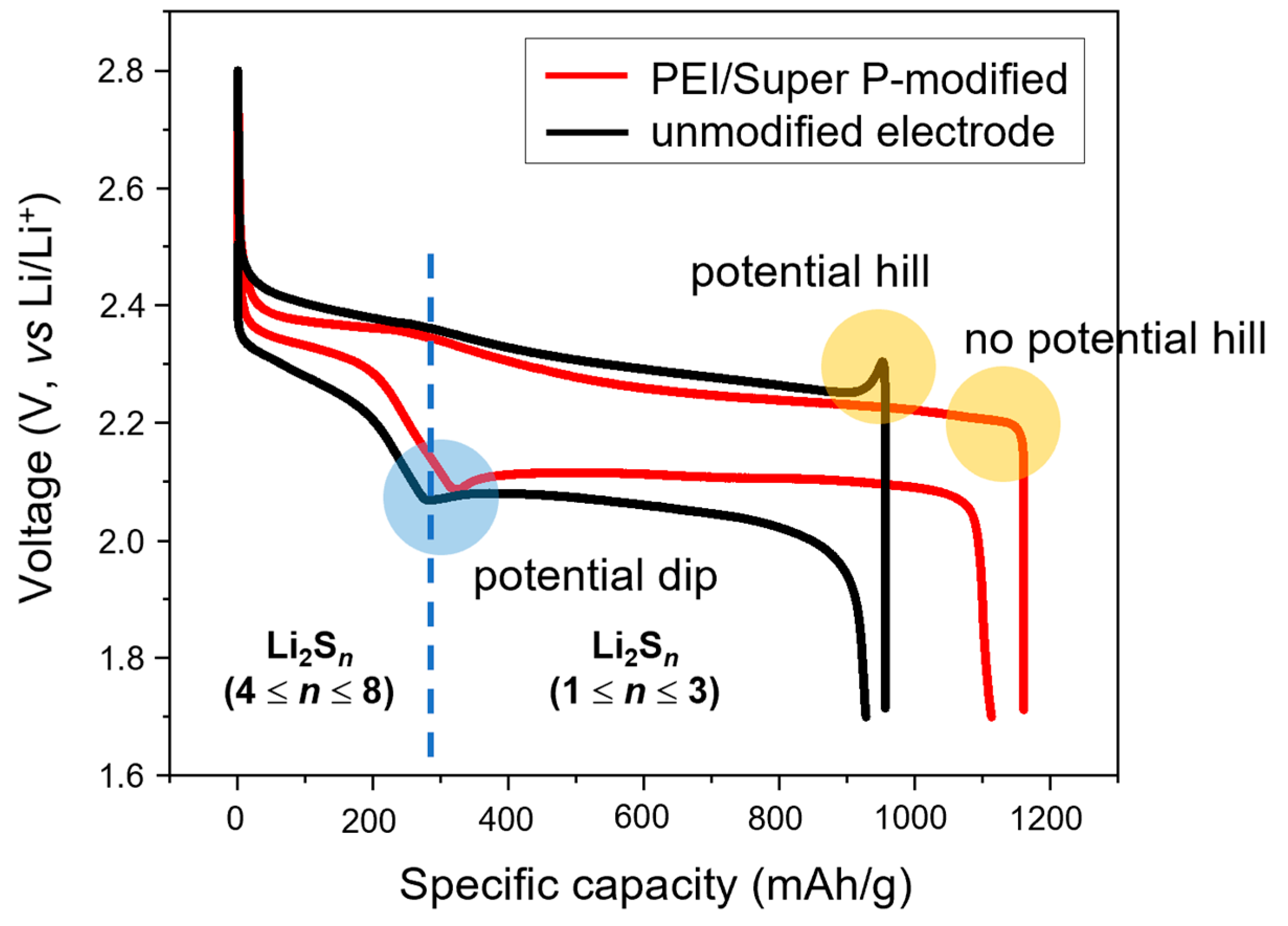
3.3. Assessment of the Electrochemical Behavior of PEI/Super P/S Electrodes
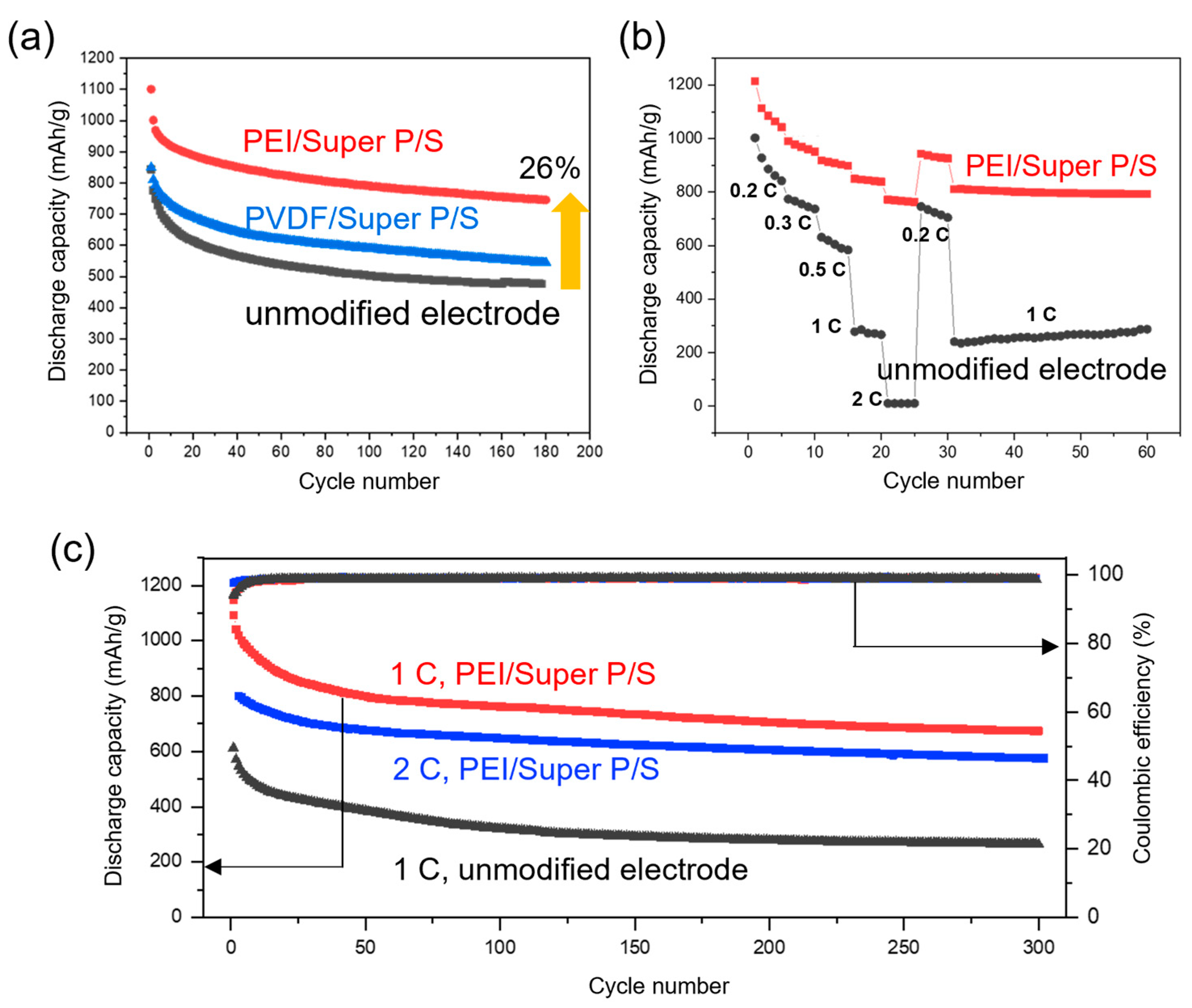
3.4. Cycling Performance and Rate Capability of PEI/Super P/S Electrode
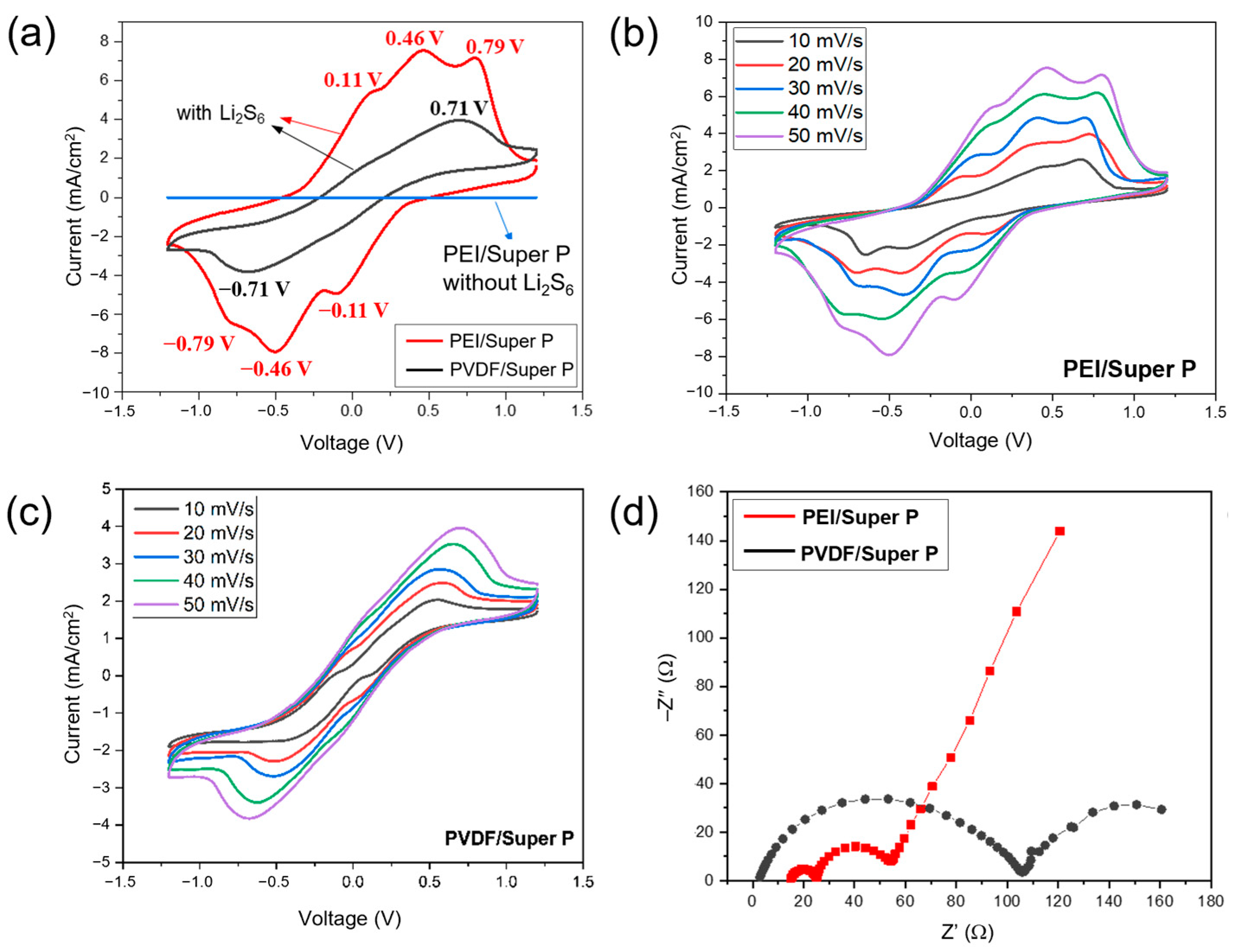
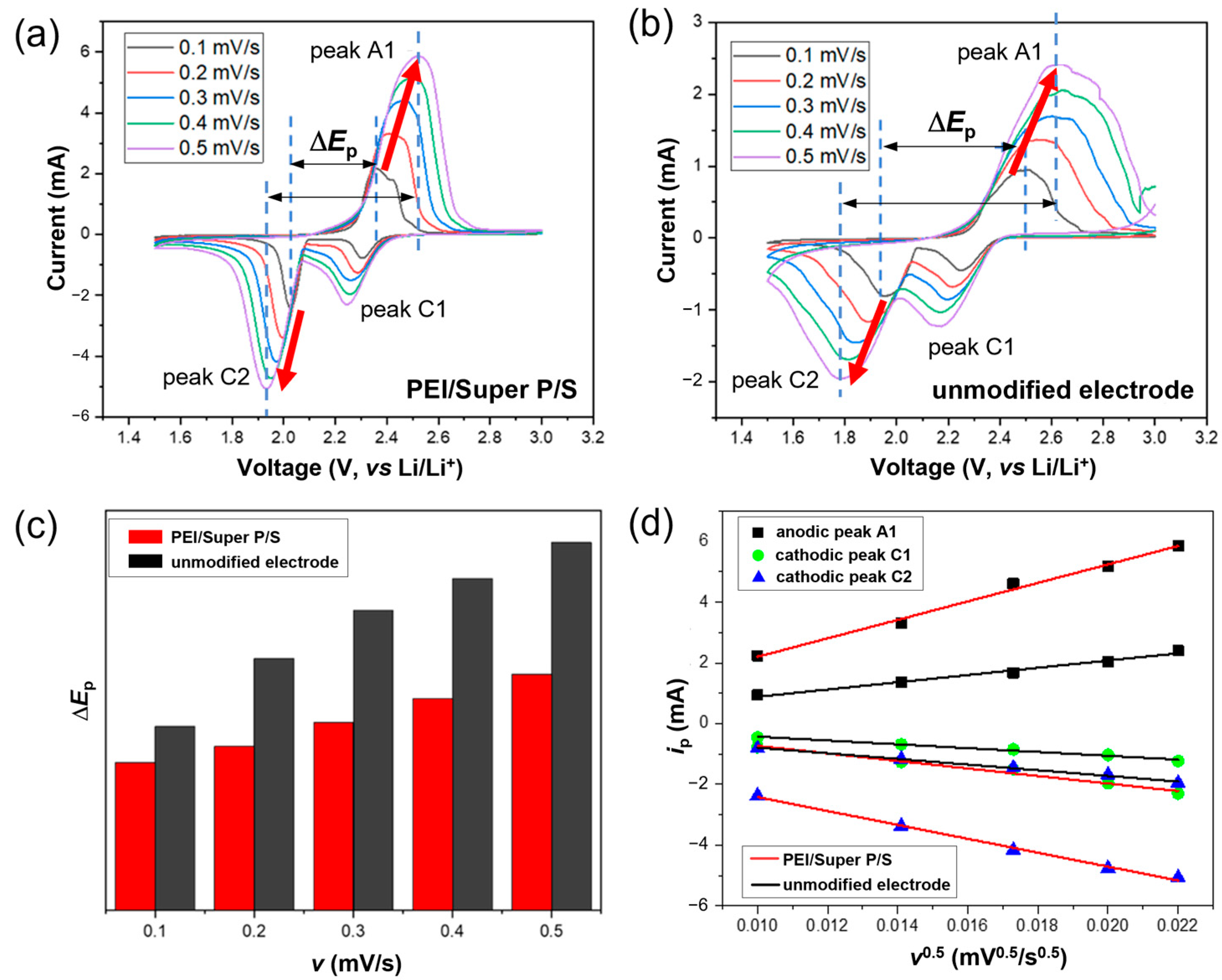
3.5. Cyclic Voltammetry Studies of the PEI/Super P/S Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ma, L.; Hendrickson, K.E.; Wei, S.; Archer, L.A. Nanomaterials: Science and applications in the lithium-sulfur battery. Nano Today 2015, 10, 315–338. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.H.; Zu, C. Lithium-sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Urbonaite, S.; Poux, T.; Novák, P. Progress towards commercially viable Li-S battery cells. Adv. Energy Mater. 2015, 5, 1500118. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Liu, B.; Li, Y.; Shen, S.; Deng, S.; Lu, C.; Zhang, W.; Xia, Y.; Pan, G.; et al. Electrode design for lithium-sulfur batteries: Problems and solutions. Adv. Funct. Mater. 2020, 30, 1910375. [Google Scholar] [CrossRef]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-sulfur battery systems: An integral perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef]
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon nanotube-based materials for lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 17204–17241. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef]
- He, G.; Ji, X.; Nazar, L. High “C” rate Li-S cathodes: Sulfur imbibed bimodal porous carbons. Energy Environ. Sci. 2011, 4, 2878–2883. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Nazar, L.F. New approaches for high energy density lithium-sulfur battery cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-G.; Mun, Y.; Cho, A.; Jo, C.; Lee, S.; Han, J.W.; Lee, J. Synergistic effect of molecular-type electrocatalysts with ultrahigh pore volume carbon microspheres for lithium-sulfur batteries. ACS Nano 2018, 12, 6013–6022. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-Y.; Yuan, H.-Y.; Li, H.-H.; Wang, H.-F.; Li, W.-L.; Sun, H.-Z.; Wu, X.-L.; Zhang, J.-P. The effective design of a polysulfide-trapped separator at the molecular level for high energy density Li-S batteries. ACS Appl. Mater. Interfaces 2016, 8, 16108–16115. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef]
- Pu, J.; Shen, Z.; Zheng, J.; Wu, W.; Zhu, C.; Zhou, Q.; Zhang, H.; Pan, F. Multifunctional Co3S4@sulfur nanotubes for enhanced lithium-sulfur battery performance. Nano Energy 2017, 37, 7–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Li, M.; Liu, J.; Wu, Y. Sulfur-deficient MoS2−x promoted lithium polysulfides conversion in lithium-sulfur battery: A first-principles study. Appl. Surf. Sci. 2019, 487, 452–463. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Wang, H.-Y.; Lu, L.; Wang, C.; Zhong, X.-B.; Wang, J.-G.; Jiang, Q.-C. Hierarchical TiO2 spheres as highly efficient polysulfide host for lithium-sulfur batteries. Sci. Rep. 2016, 6, 22990. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium-sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817–8819. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Chung, S.-H.; Manthiram, A.; Kalra, V. A free-standing carbon nanofiber interlayer for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 4530–4538. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhuang, T.-Z.; Zhang, Q.; Peng, H.-J.; Chen, C.-M.; Wei, F. Permselective graphene oxide membrane for highly stable and anti-self-discharge lithium-sulfur batteries. ACS Nano 2015, 9, 3002–3011. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Y.; Zhang, Z.; Zhang, K.; Li, J. Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochim. Acta 2014, 129, 55–61. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Qin, X.; Liang, G.; Han, W.; Zhou, D.; He, Y.-B.; Li, B.; Kang, F. Suppressing self-discharge and shuttle effect of lithium-sulfur batteries with V2O5-decorated carbon nanofiber interlayer. Small 2017, 13, 1602539. [Google Scholar] [CrossRef]
- Li, X.; Banis, M.; Lushington, A.; Yang, X.; Sun, Q.; Zhao, Y.; Liu, C.; Li, Q.; Wang, B.; Xiao, W.; et al. A high-energy sulfur cathode in carbonate electrolyte by eliminating polysulfides via solid-phase lithium-sulfur transformation. Nat. Commun. 2018, 9, 4509. [Google Scholar] [CrossRef]
- Lim, S.; Lilly Thankamony, R.; Yim, T.; Chu, H.; Kim, Y.-J.; Mun, J.; Kim, T.-H. Surface modification of sulfur electrodes by chemically anchored cross-linked polymer coating for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2015, 7, 1401–1405. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, Q.; Cha, J.J.; Yang, Y.; Li, W.; Seh, Z.W.; Cui, Y. Amphiphilic surface modification of hollow carbon nanofibers for improved cycle life of lithium sulfur batteries. Nano Lett. 2013, 13, 1265–1270. [Google Scholar] [CrossRef]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Sulfur-carbon nanocomposite cathodes improved by an amphiphilic block copolymer for high-rate lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2012, 4, 6046–6052. [Google Scholar] [CrossRef]
- Wang, G.; Lai, Y.; Zhang, Z.; Li, J.; Zhang, Z. Enhanced rate capability and cycle stability of lithium-sulfur batteries with a bifunctional MCNT@PEG-modified separator. J. Mater. Chem. A 2015, 3, 7139–7144. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, J.; Choi, J.; Shin, D.; Carter, M.; Jeon, Y.; Wang, C.; Hu, L.; Paik, U. Synergistic ultrathin functional polymer-coated carbon nanotube interlayer for high performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 20092–20099. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Li, X.; Xiao, W.; Chen, Z.; Lu, J. Interlayer material selection for lithium-sulfur batteries. Joule 2019, 3, 361–386. [Google Scholar] [CrossRef]
- Laverde, J.; Rosero-Navarro, N.C.; Miura, A.; Buitrago-Sierra, R.; Tadanaga, K.; López, D. Impact of sulfur infiltration time and its content in an N-doped mesoporous carbon for application in Li-S batteries. Batteries 2022, 8, 58. [Google Scholar] [CrossRef]
- He, Z.; Dou, X.; Liu, W.; Zhang, L.; Lv, L.; Liu, J.; Meng, F. Yeast-derived sulfur host for the application of sustainable Li–S battery cathode. Batteries 2023, 9, 289. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, M.; Feng, J.; Liu, G.; Guo, J. Effective electrostatic confinement of polysulfides in lithium/sulfur batteries by a functional binder. Nano Energy 2017, 40, 559–565. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li-S batteries using amine-functionalized carbon nanotubes in the cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef]
- Li, W.; Hicks-Garner, J.; Wang, J.; Liu, J.; Gross, A.F.; Sherman, E.; Graetz, J.; Vajo, J.J.; Liu, P. V2O5 polysulfide anion barrier for long-lived li-s batteries. Chem. Mater. 2014, 26, 3403–3410. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-sulfur batteries: Attaining the critical metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.-J.; Hou, T.-Z.; Huang, J.-Q.; Chen, C.-M.; Wang, D.-W.; Cheng, X.-B.; Wei, F.; Zhang, Q. Powering lithium-sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium-sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H.; et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, L.; Li, K.; Wu, Z.; Zhang, F.; Wang, L. Unravel the catalytic effect of two-dimensional metal sulfides on polysulfide conversions for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2020, 12, 43560–43567. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, S.; Zhang, T.; Ye, H.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Elucidating the catalytic activity of oxygen deficiency in the polysulfide conversion reactions of lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1801868. [Google Scholar] [CrossRef]
- Lim, W.G.; Kim, S.; Jo, C.; Lee, J. A comprehensive review of materials with catalytic effects in li-s batteries: Enhanced redox kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746–18757. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, G.; Misra, S.; Nelson, J.; Toney, M.F.; Cui, Y. High-capacity micrometer-sized Li2S particles as cathode materials for advanced rechargeable lithium-ion batteries. J. Am. Chem. Soc. 2012, 134, 15387–15394. [Google Scholar] [CrossRef]
- Chung, S.-H.; Lai, K.-Y.; Manthiram, A. A facile, low-cost hot-pressing process for fabricating lithium-sulfur cells with stable dynamic and static electrochemistry. Adv. Mater. 2018, 30, 1805571. [Google Scholar] [CrossRef]
- Li, M.; Wahyudi, W.; Kumar, P.; Wu, F.; Yang, X.; Li, H.; Li, L.-J.; Ming, J. Scalable approach to construct free-standing and flexible carbon networks for lithium-sulfur battery. ACS Appl. Mater. Interfaces 2017, 9, 8047–8054. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: Modeling and analysis of capacity fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar] [CrossRef]
- Cañas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigations of lithium-sulfur batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar] [CrossRef]
- Waluś, S.; Barchasz, C.; Bouchet, R.; Alloin, F. Electrochemical impedance spectroscopy study of lithium-sulfur batteries: Useful technique to reveal the Li/S electrochemical mechanism. Electrochim. Acta 2020, 359, 136944. [Google Scholar] [CrossRef]
- Qu, L.; Liu, P.; Yi, Y.; Wang, T.; Yang, P.; Tian, X.; Li, M.; Yang, B.; Dai, S. Enhanced cycling performance for lithium-sulfur batteries by a laminated 2D g-C3N4/graphene cathode interlayer. ChemSusChem 2019, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Senthil, C.; Kim, S.-S.; Jung, H.Y. Flame retardant high-power Li-S flexible batteries enabled by bio-macromolecular binder integrating conformal fractions. Nat. Commun. 2022, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Vassilieva, S.Y.; Levina, E.E.; Nikitina, V.A. Kinetic analysis of lithium intercalating systems: Cyclic voltammetry. Electrochim. Acta 2016, 190, 1087–1099. [Google Scholar] [CrossRef]
- Vujković, M.; Mentus, S. Potentiodynamic and galvanostatic testing of NaFe0.95V0.05PO4/C composite in aqueous NaNO3 solution, and the properties of aqueous Na1.2V3O8/NaNO3/NaFe0.95V0.05PO4/C battery. J. Power Sources 2016, 325, 185–193. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Qiao, F.; Wang, J.; Li, H.; Eichel, R.-A.; Notten, P.H.L. Sulfur reduction reaction in lithium–sulfur batteries: Mechanisms, catalysts, and characterization. Adv. Energy Mater. 2022, 12, 2202094. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.; Min, G.; Lee, J.B.; Kim, P.J.; Shin, K.; Cheong, I.W.; Kang, H.; Yoon, S.; Lim, W.-G.; Lee, J.; et al. PEI/Super P Cathode Coating: A Pathway to Superior Lithium–Sulfur Battery Performance. Batteries 2023, 9, 531. https://doi.org/10.3390/batteries9110531
Heo J, Min G, Lee JB, Kim PJ, Shin K, Cheong IW, Kang H, Yoon S, Lim W-G, Lee J, et al. PEI/Super P Cathode Coating: A Pathway to Superior Lithium–Sulfur Battery Performance. Batteries. 2023; 9(11):531. https://doi.org/10.3390/batteries9110531
Chicago/Turabian StyleHeo, Junhee, Gyeonguk Min, Jae Bin Lee, Patrick Joohyun Kim, Kyuchul Shin, In Woo Cheong, Hyunchul Kang, Songhun Yoon, Won-Gwang Lim, Jinwoo Lee, and et al. 2023. "PEI/Super P Cathode Coating: A Pathway to Superior Lithium–Sulfur Battery Performance" Batteries 9, no. 11: 531. https://doi.org/10.3390/batteries9110531
APA StyleHeo, J., Min, G., Lee, J. B., Kim, P. J., Shin, K., Cheong, I. W., Kang, H., Yoon, S., Lim, W.-G., Lee, J., & Joo, J. (2023). PEI/Super P Cathode Coating: A Pathway to Superior Lithium–Sulfur Battery Performance. Batteries, 9(11), 531. https://doi.org/10.3390/batteries9110531








