Deciphering Electrolyte Degradation in Sodium-Based Batteries: The Role of Conductive Salt Source, Additives, and Storage Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Sample Preparation
2.2. Instrumentation
3. Results and Discussion
3.1. NaPF6 with 99.5% Purity
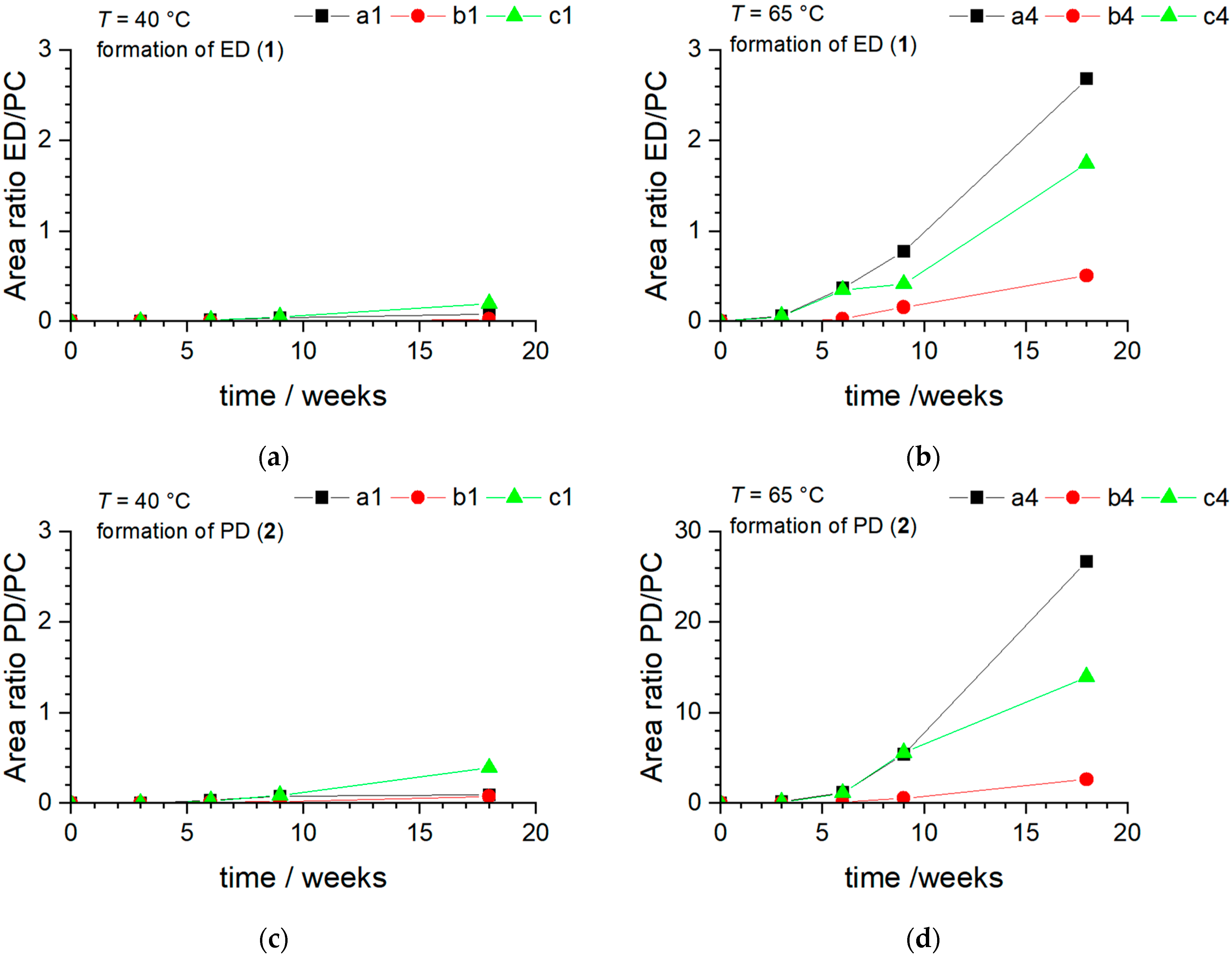
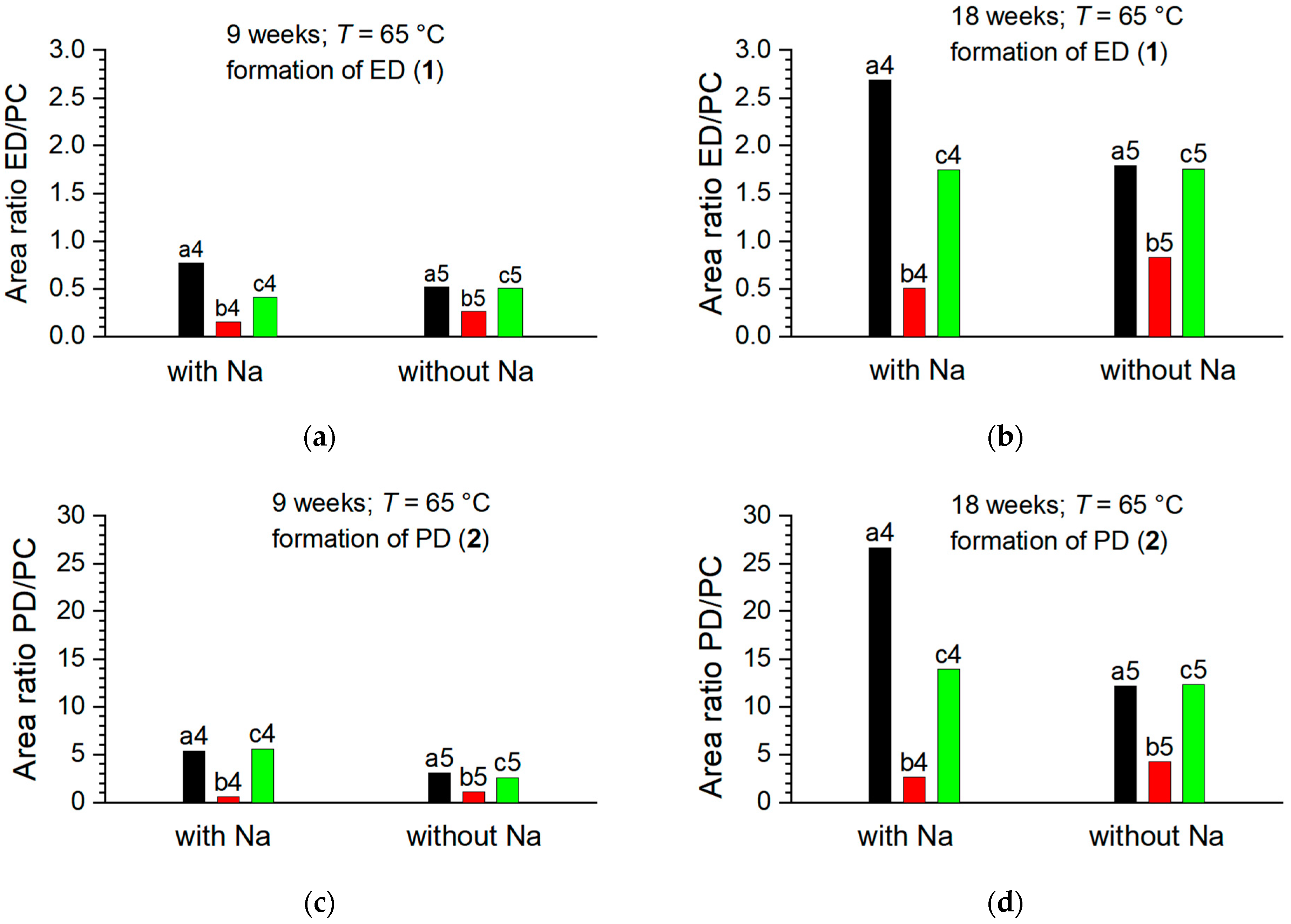
3.1.1. Degradation Product Formation
3.1.2. Degradation Product Formation in the Presence of NaDFOB and FEC
3.1.3. Analysis of Diol Formation
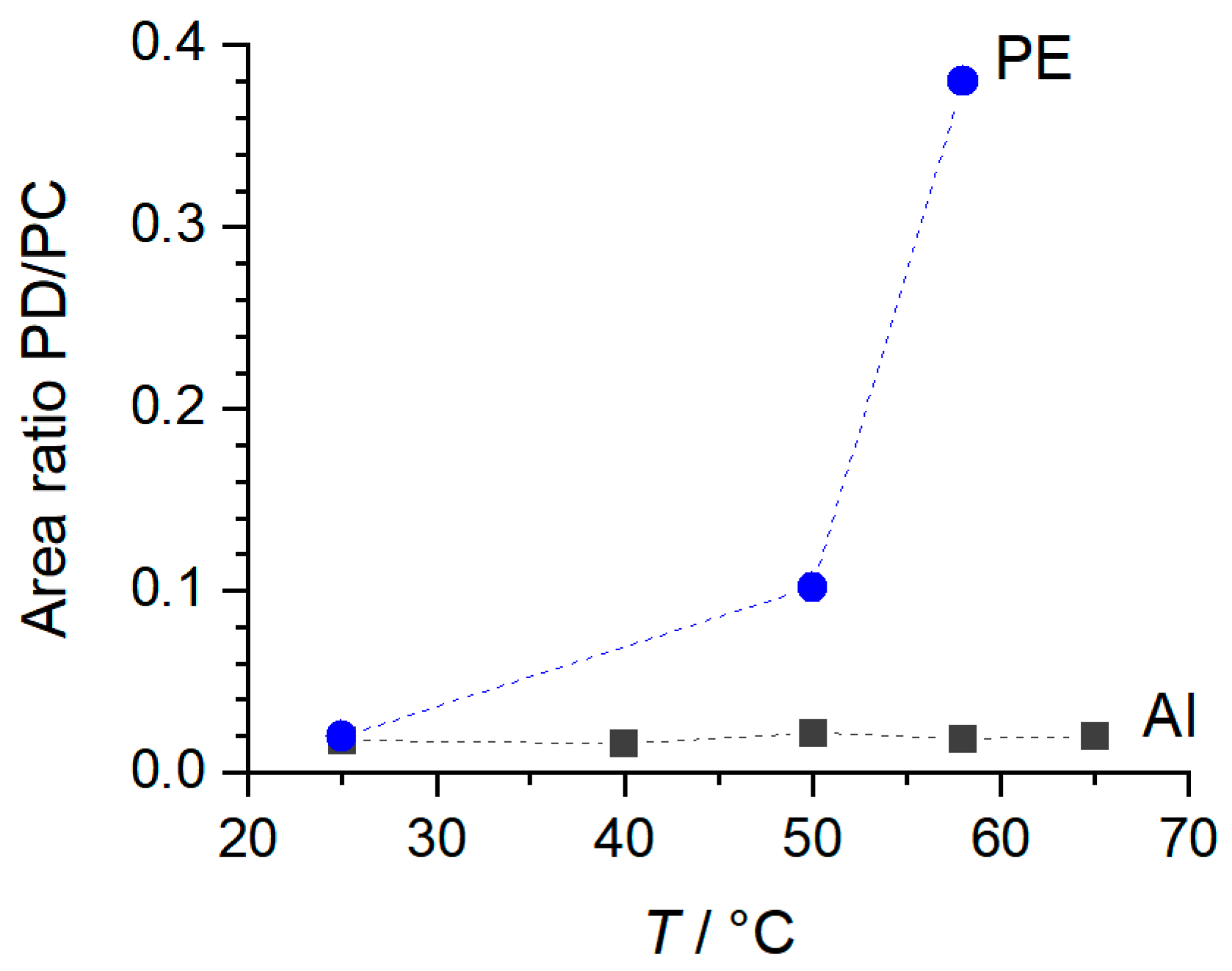
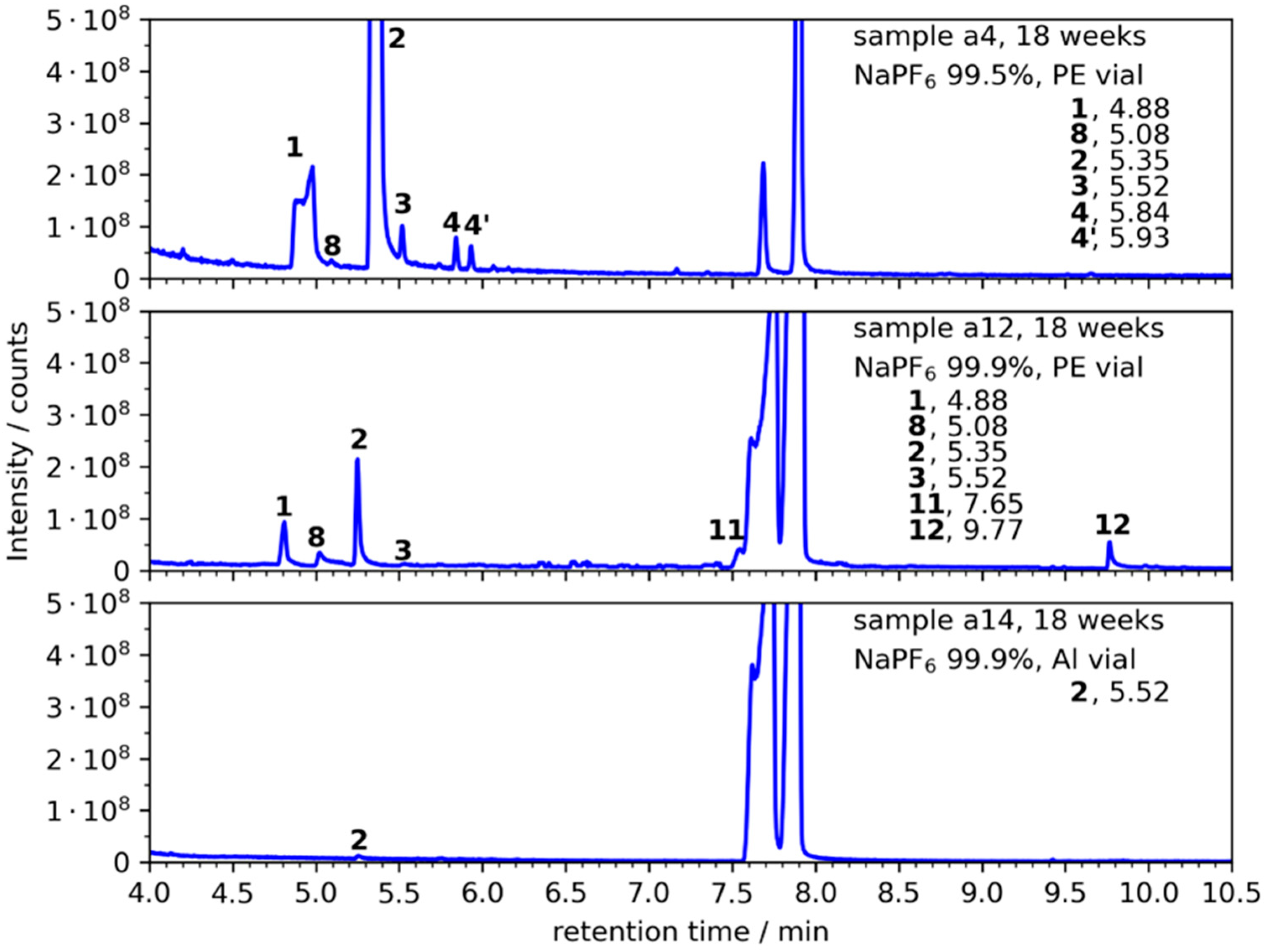
3.2. NaPF6 with 99.9% Purity
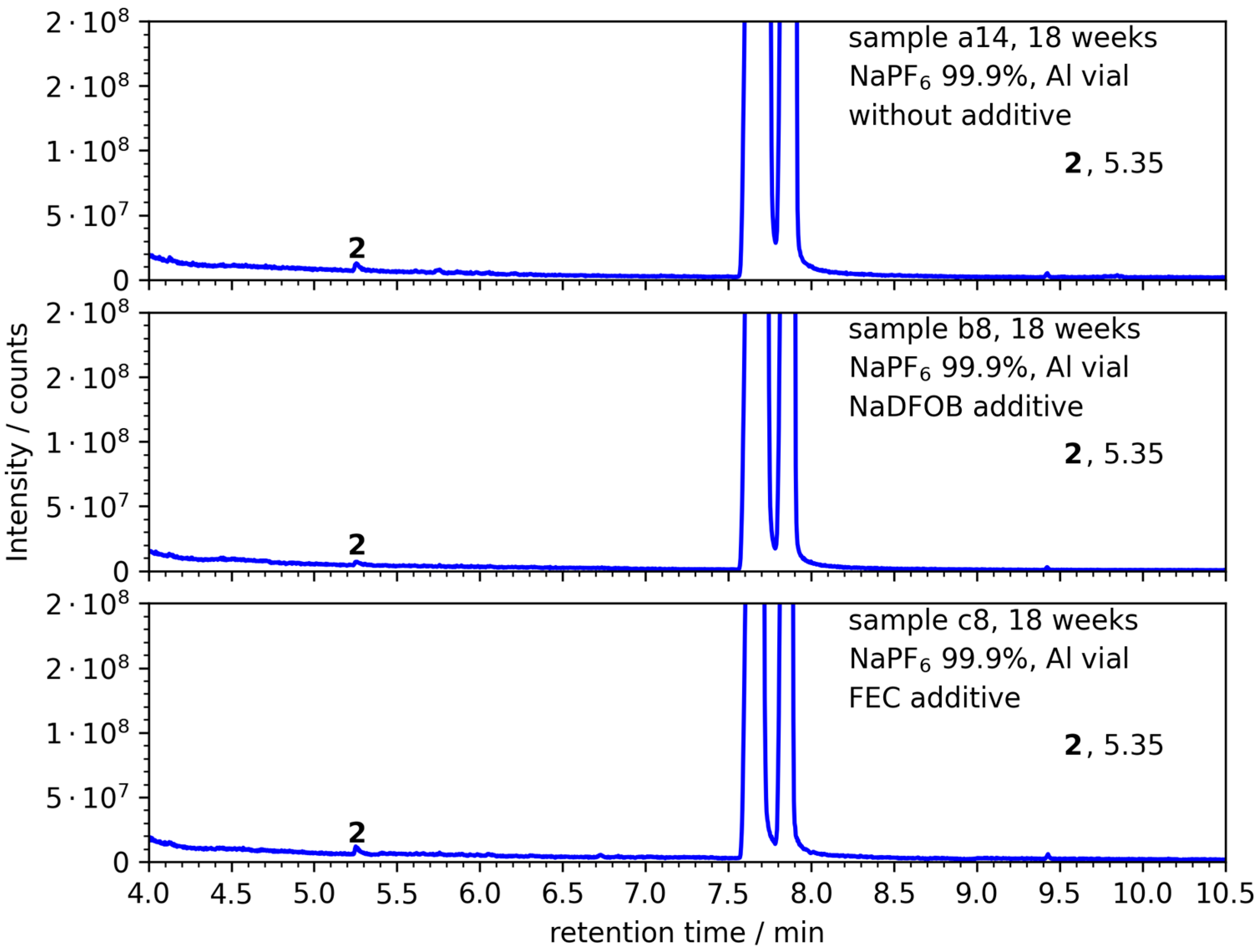
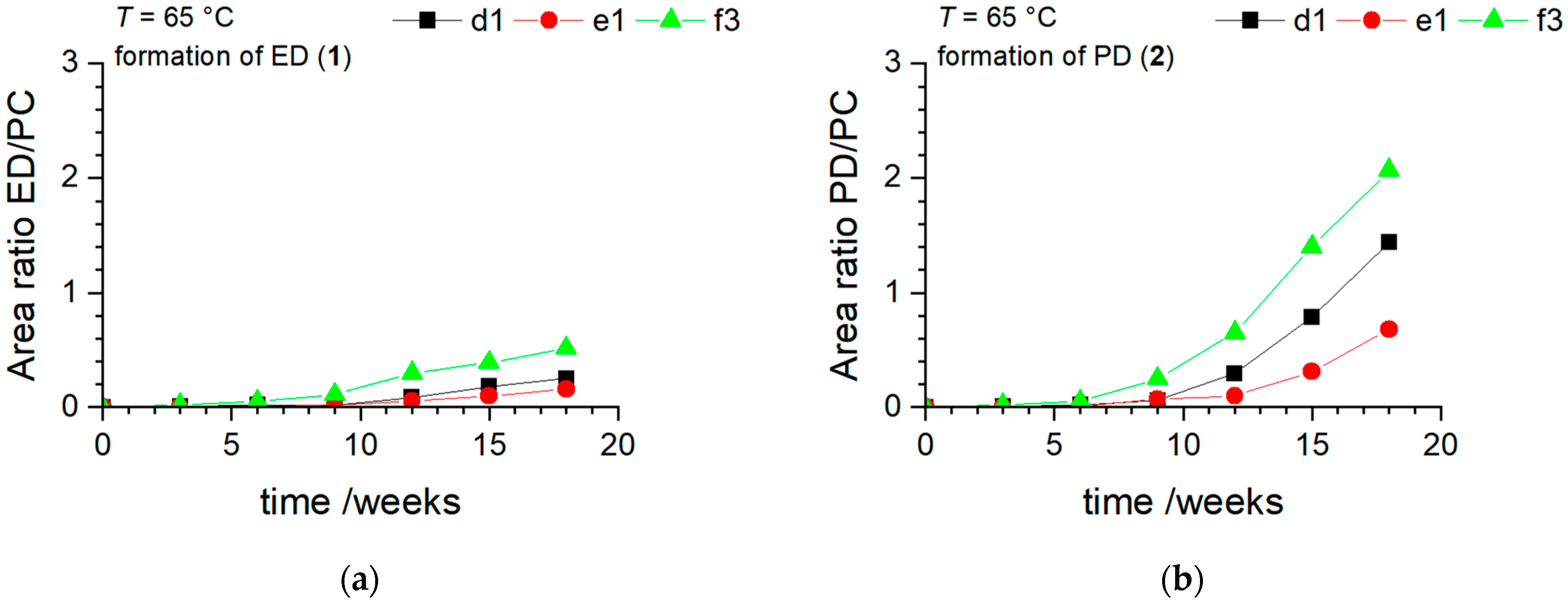
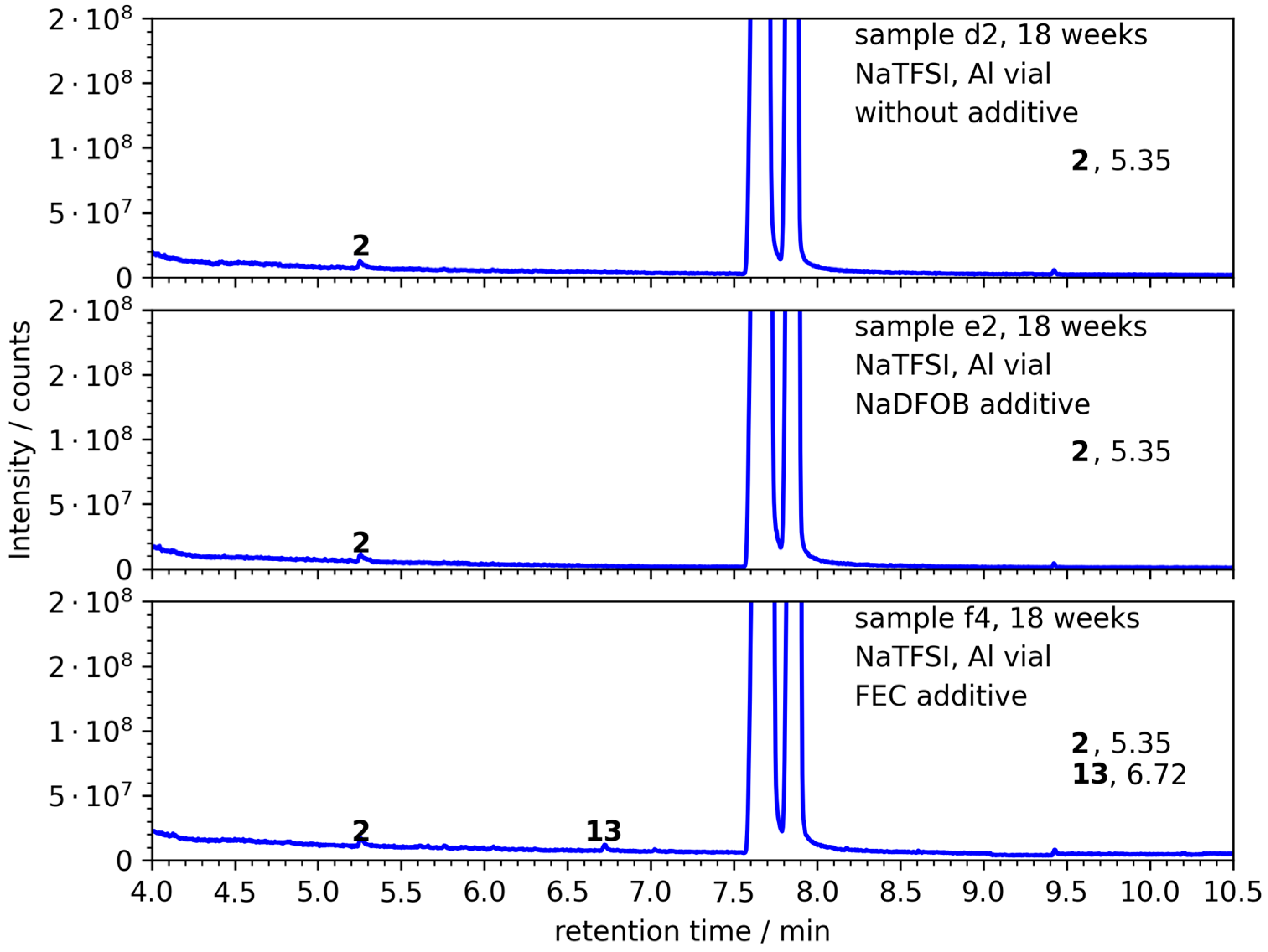
3.3. NaTFSI with 99.9% Purity
3.4. FEC Reactivity and Color Changes
- In PE vials at 40 °C, FEC disappears after 3 weeks when sodium metal is present (c1) but remains stable over 18 weeks when sodium metal is absent (c2).
- In aluminum vials at 40 °C, regardless of the presence (c7) or absence (c3) of sodium metal, FEC remains stable over the 18 weeks duration.
- At 65 °C in PE vials, FEC disappears after 3 weeks when sodium metal is present (c4), and after 6 weeks when sodium metal is absent (c5).
- In aluminum vials at 65 °C, FEC remains stable over 18 weeks when sodium metal is absent (c6), but it disappears after 12 weeks when sodium metal is present (c8).
- In PE vials at 40 °C, FEC disappears after 9 weeks when sodium metal is present (f1) but remains stable over 18 weeks when sodium metal is absent (f5).
- In aluminum vials at 40 °C, regardless of the presence (f2) or absence (f6) of sodium metal, FEC remains stable over the 18 weeks duration.
- At 65 °C in PE vials, FEC disappears after 3 weeks when sodium metal is present (f3), and after 12 weeks when sodium metal is absent (f4).
- In aluminum vials at 65 °C, FEC remains stable over 18 weeks, irrespective of the presence (f8) or absence (f7) of sodium metal.
4. Conclusions
- Temperature effects: In both sets of samples, FEC appears to degrade faster at higher temperatures. This behavior aligns with the general principle of chemical kinetics that reaction rates typically increase with temperature. This is usually explained by the Arrhenius equation, which states that a higher temperature increases the fraction of molecules possessing energy greater than the activation energy, leading to an increased rate of reaction. In the context of our study, higher temperatures could facilitate degradation reactions involving FEC, thereby causing its faster consumption.
- Effects of sodium metal presence: The presence of sodium metal seems to accelerate the disappearance of FEC. This suggests that FEC reacts with sodium, possibly through a reductive decomposition mechanism. The resulting products could contribute to the formation of a stable SEI layer, which could help improve the overall stability of the electrolyte system.
- Effects of vial material: FEC tends to last longer in aluminum vials than in PE vials, indicating that the material of the storage vial can impact the stability of the additive. The reason behind this observation could be the better thermal conductivity of aluminum compared to PE, which could help dissipate heat more efficiently, thereby reducing the rate of degradation reactions.
- Effects of conductive salt: Although both types of salts studied (NaPF6 and NaTFSI) are sodium salts, differences in the reactivity of FEC were observed between them. This suggests that the anion part of the salt might play a role in the reactions involving FEC. Some reports in the literature suggest that is less reactive and more thermally stable than , which could explain the observed behavior [76,77].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, J.; Park, K.Y.; Kim, H.; Kim, S.W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of solid-state electrolytes for sodium-ion battery—A short review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Bin, D.; Wang, F.; Tamirat, A.G.; Suo, L.M.; Wang, Y.G.; Wang, C.S.; Xia, Y.Y. Progress in Aqueous Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703008–1703038. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.X.; Tan, D.H.S.; Zhang, M.H.; Zhao, E.Y.; Meng, Y.S. Sodium-Ion Batteries Paving the Way for Grid Energy Storage. Adv. Energy Mater. 2020, 10, 2001274–2001281. [Google Scholar] [CrossRef]
- Tian, Z.; Zou, Y.; Liu, G.; Wang, Y.; Yin, J.; Ming, J.; Alshareef, H.N. Electrolyte Solvation Structure Design for Sodium Ion Batteries. Adv. Sci. 2022, 9, e2201207. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Z.; Guo, S.; Yang, Q.H.; Zhou, H. Toward High Performance Anodes for Sodium-Ion Batteries: From Hard Carbons to Anode-Free Systems. ACS Cent. Sci. 2023, 9, 1076–1087. [Google Scholar] [CrossRef]
- Rafie, A.; Kim, J.W.; Sarode, K.K.; Kalra, V. A review on the use of carbonate-based electrolytes in Li-S batteries: A comprehensive approach enabling solid-solid direct conversion reaction. Energy Stor. Mater. 2022, 50, 197–224. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Zhang, Q.; Song, X.; Lu, X.; Zhang, Z.; Onyianta, A.J.; Wang, M.; Titirici, M.M.; Eichhorn, S.J. Stable Sodium-Metal Batteries in Carbonate Electrolytes Achieved by Bifunctional, Sustainable Separators with Tailored Alignment. Adv. Mater. 2022, 34, e2206367. [Google Scholar] [CrossRef]
- Qin, M.; Zeng, Z.; Cheng, S.; Xie, J. Challenges and strategies of formulating low-temperature electrolytes in lithium-ion batteries. Interdiscip. Mater. 2023, 2, 308–336. [Google Scholar] [CrossRef]
- Barnes, P.; Smith, K.; Parrish, R.; Jones, C.; Skinner, P.; Storch, E.; White, Q.; Deng, C.J.; Karsann, D.; Lau, M.L.; et al. A non-aqueous sodium hexafluorophosphate-based electrolyte degradation study: Formation and mitigation of hydrofluoric acid. J. Power Sources 2020, 447, 227363–227370. [Google Scholar] [CrossRef]
- Mosallanejad, B.; Malek, S.S.; Ershadi, M.; Daryakenari, A.A.; Cao, Q.; Ajdari, F.B.; Ramakrishna, S. Cycling degradation and safety issues in sodium-ion batteries: Promises of electrolyte additives. J. Electroanal. Chem. 2021, 895, 115505–115522. [Google Scholar] [CrossRef]
- Eom, J.Y.; Jung, I.H.; Lee, J.H. Effects of vinylene carbonate on high temperature storage of high voltage Li-ion batteries. J. Power Sources 2011, 196, 9810–9814. [Google Scholar] [CrossRef]
- Genieser, R.; Loveridge, M.; Bhagat, R. Practical high temperature (80 °C) storage study of industrially manufactured Li-ion batteries with varying electrolytes. J. Power Sources 2018, 386, 85–95. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Q.; Tian, J.; Chen, L.; Li, N.; Su, Y.; Bao, L.; Lu, Y.; Cao, D.; Yan, K.; et al. High-Temperature Storage Deterioration Mechanism of Cylindrical 21700-Type Batteries Using Ni-Rich Cathodes under Different SOCs. ACS Appl. Mater. Interfaces 2021, 13, 6286–6297. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.; Weber, W.; Grutzke, M.; Winter, M.; Nowak, S. Study of decomposition products by gas chromatography-mass spectrometry and ion chromatography-electrospray ionization-mass spectrometry in thermally decomposed lithium hexafluorophosphate-based lithium ion battery electrolytes. RSC Adv. 2015, 5, 80150–80157. [Google Scholar] [CrossRef]
- Gachot, G.; Ribiere, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.B.; Pilard, S.; Laruelle, S. Gas chromatography/mass spectrometry as a suitable tool for the Li-ion battery electrolyte degradation mechanisms study. Anal. Chem. 2011, 83, 478–485. [Google Scholar] [CrossRef]
- Horsthemke, F.; Friesen, A.; Monnighoff, X.; Stenzel, Y.P.; Grutzke, M.; Andersson, J.T.; Winter, M.; Nowak, S. Fast screening method to characterize lithium ion battery electrolytes by means of solid phase microextraction-gas chromatography-mass spectrometry. RSC Adv. 2017, 7, 46989–46998. [Google Scholar] [CrossRef]
- Peschel, C.; Horsthemke, F.; Leissing, M.; Wiemers-Meyer, S.; Henschel, J.; Winter, M.; Nowak, S. Analysis of Carbonate Decomposition During Solid Electrolyte Interphase Formation in Isotope-Labeled Lithium Ion Battery Electrolytes: Extending the Knowledge about Electrolyte Soluble Species. Batter. Supercaps 2020, 3, 1183–1192. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2327–A2334. [Google Scholar] [CrossRef]
- Gauthier, N.; Courrèges, C.; Demeaux, J.; Tessier, C.; Martinez, H. Impact of the cycling temperature on electrode/electrolyte interfaces within Li4Ti5O12 vs LiMn2O4 cells. J. Power Sources 2020, 448, 227573–227583. [Google Scholar] [CrossRef]
- Ravdel, B.; Abraham, K.M.; Gitzendanner, R.; DiCarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119–121, 805–810. [Google Scholar] [CrossRef]
- Lee, H.H.; Wan, C.C.; Wang, Y.Y. Thermal Stability of the Solid Electrolyte Interface on Carbon Electrodes of Lithium Batteries. J. Electrochem. Soc. 2004, 151, A542–A547. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sust. Energ. Rev. 2016, 64, 106–128. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Hofmann, A.; Uhlmann, N.; Ziebert, C.; Wiegand, O.; Schmidt, A.; Hanemann, T. Preventing Li-ion cell explosion during thermal runaway with reduced pressure. Appl. Therm. Eng. 2017, 124, 539–544. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Wang, C.; Porter, S.; Wang, B.; Lie, W.; Liu, H.K. Sodium-difluoro(oxalato)borate (NaDFOB): A new electrolyte salt for Na-ion batteries. Chem. Commun. 2015, 51, 9809–9812. [Google Scholar] [CrossRef]
- Purushotham, U.; Takenaka, N.; Nagaoka, M. Additive effect of fluoroethylene and difluoroethylene carbonates for the solid electrolyte interphase film formation in sodium-ion batteries: A quantum chemical study. RSC Adv. 2016, 6, 65232–65242. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, L.; Ye, X.; Zhang, J.; Min, F.; Luo, W.; Huang, Y. Critical effects of electrolyte recipes for Li and Na metal batteries. Chem 2021, 7, 2312–2346. [Google Scholar] [CrossRef]
- Dahbi, M.; Nakano, T.; Yabuuchi, N.; Fujimura, S.; Chihara, K.; Kubota, K.; Son, J.-Y.; Cui, Y.-T.; Oji, H.; Komaba, S. Effect of Hexafluorophosphate and Fluoroethylene Carbonate on Electrochemical Performance and the Surface Layer of Hard Carbon for Sodium-Ion Batteries. ChemElectroChem 2016, 3, 1856–1867. [Google Scholar] [CrossRef]
- Dugas, R.; Ponrouch, A.; Gachot, G.; David, R.; Palacin, M.R.; Tarascon, J.M. Na Reactivity toward Carbonate-Based Electrolytes: The Effect of FEC as Additive. J. Electrochem. Soc. 2016, 163, A2333–A2339. [Google Scholar] [CrossRef]
- Cheng, Z.; Mao, Y.; Dong, Q.; Jin, F.; Shen, Y.; Chen, L. Fluoroethylene Carbonate as an Additive for Sodium-Ion Batteries: Effect on the Sodium Cathode. Acta Phys. Chim. Sin. 2019, 35, 868–875. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Investigation of the Effect of Fluoroethylene Carbonate Additive on Electrochemical Performance of Sb-Based Anode for Sodium-Ion Batteries. Electrochim. Acta 2016, 190, 402–408. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Martinez-Ibañez, M.; Sánchez-Diez, E.; Gracia, I.; Li, C.; Rodriguez-Martinez, L.M.; Rojo, T.; Zhang, H.; Armand, M. Electrolyte Additives for Room-Temperature, Sodium-Based, Rechargeable Batteries. Chem. Asian J. 2018, 13, 2770–2780. [Google Scholar] [CrossRef]
- Bouibes, A.; Takenaka, N.; Kubota, K.; Komaba, S.; Nagaoka, M. Development of advanced electrolytes in Na-ion batteries: Application of the Red Moon method for molecular structure design of the SEI layer. RSC Adv. 2021, 12, 971–984. [Google Scholar] [CrossRef]
- Hou, X.; Li, T.; Qiu, Y.; Jiang, M.; Zheng, Q.; Li, X. Weak coulomb interaction between anions and Na+ during solvation enabling desirable solid electrolyte interphase and superior kinetics for HC-based sodium ion batteries. Chem. Eng. J. 2023, 453, 139932. [Google Scholar] [CrossRef]
- Shin, H.; Park, J.; Sastry, A.M.; Lu, W. Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces at Elevated Temperatures. J. Electrochem. Soc. 2015, 162, A1683–A1692. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093–2000133. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Liu, Y.; Yamauchi, Y.; Huang, Z.; Kong, X. Revealing the chemistry of an anode-passivating electrolyte salt for high rate and stable sodium metal batteries. J. Mater. Chem. A 2018, 6, 12012–12017. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Chen, Q.; Kong, X. The chemical evolution of solid electrolyte interface in sodium metal batteries. Sci. Adv. 2022, 8, eabm4606. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wu, F.; Huang, Y. Sodium-Ion Batteries: Advanced Technology and Applications; De Gruyter: Berlin, Germany, 2022. [Google Scholar]
- Chen, X.; Shen, X.; Hou, T.-Z.; Zhang, R.; Peng, H.-J.; Zhang, Q. Ion-Solvent Chemistry-Inspired Cation-Additive Strategy to Stabilize Electrolytes for Sodium-Metal Batteries. Chem 2020, 6, 2242–2256. [Google Scholar] [CrossRef]
- Sun, Z.; Fu, W.; Liu, M.Z.; Lu, P.; Zhao, E.; Magasinski, A.; Liu, M.; Luo, S.; McDaniel, J.; Yushin, G. A nanoconfined iron(iii) fluoride cathode in a NaDFOB electrolyte: Towards high-performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 4091–4098. [Google Scholar] [CrossRef]
- Law, H.M.; Yu, J.; Kwok, S.C.T.; Zhou, G.; Robson, M.J.; Wu, J.; Ciucci, F. A hybrid dual-salt polymer electrolyte for sodium metal batteries with stable room temperature cycling performance. Energy Stor. Mater. 2022, 46, 182–191. [Google Scholar] [CrossRef]
- Metzger, M.; Strehle, B.; Solchenbach, S.; Gasteiger, H.A. Hydrolysis of Ethylene Carbonate with Water and Hydroxide under Battery Operating Conditions. J. Electrochem. Soc. 2016, 163, A1219–A1225. [Google Scholar] [CrossRef]
- Caracciolo, L.; Madec, L.; Gachot, G.; Martinez, H. Impact of the Salt Anion on K Metal Reactivity in EC/DEC Studied Using GC and XPS Analysis. ACS Appl. Mater. Interfaces 2021, 13, 57505–57513. [Google Scholar] [CrossRef]
- Laruelle, S.; Pilard, S.; Guenot, P.; Grugeon, S.; Tarascon, J.M. Identification of Li-based electrolyte degradation products through DEI and ESI high-resolution mass spectrometry. J. Electrochem. Soc. 2004, 151, A1202–A1209. [Google Scholar] [CrossRef]
- Grützke, M.; Weber, W.; Winter, M.; Nowak, S. Structure determination of organic aging products in lithium-ion battery electrolytes with gas chromatography chemical ionization mass spectrometry (GC-CI-MS). RSC Adv. 2016, 6, 57253–57260. [Google Scholar] [CrossRef]
- Stenzel, Y.P.; Horsthemke, F.; Winter, M.; Nowak, S. Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art. Separations 2019, 6, 26. [Google Scholar] [CrossRef]
- Fang, C.; Tran, T.N.; Zhao, Y.Z.; Liu, G. Electrolyte decomposition and solid electrolyte interphase revealed by mass spectrometry. Electrochim Acta 2021, 399, 139362–139371. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Ma, L.A.; Naylor, A.J.; Nyholm, L.; Younesi, R. Strategies for Mitigating Dissolution of Solid Electrolyte Interphases in Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 4855–4863. [Google Scholar] [CrossRef]
- Single, F.; Horstmann, B.; Latz, A. Dynamics and morphology of solid electrolyte interphase (SEI). Phys. Chem. Chem. Phys. 2016, 18, 17810–17814. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Xu, G.-L.; Amine, R.; Abouimrane, A.; Che, H.; Dahbi, M.; Ma, Z.-F.; Saadoune, I.; Alami, J.; Mattis, W.L.; Pan, F.; et al. Challenges in Developing Electrodes, Electrolytes, and Diagnostics Tools to Understand and Advance Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702403–1702465. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Chang, Z.; Qiao, Y.; Deng, H.; Yang, H.; He, P.; Zhou, H. A stable high-voltage lithium-ion battery realized by an in-built water scavenger. Energy Environ. Sci. 2020, 13, 1197–1204. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, S.-S.; Shin, S.C.; Choi, N.-S. Effect of SEI on Capacity Losses of Spinel Lithium Manganese Oxide/Graphite Batteries Stored at 60 °C. Electrochem. Solid-State Lett. 2010, 13, A168–A172. [Google Scholar] [CrossRef]
- Sinha, N.N.; Burns, J.C.; Dahn, J.R. Storage Studies on Li/Graphite Cells and the Impact of So-Called SEI-Forming Electrolyte Additives. J. Electrochem. Soc. 2013, 160, A709–A714. [Google Scholar] [CrossRef]
- Müller, C.; Wang, Z.; Hofmann, A.; Stüble, P.; Liu-Théato, X.; Klemens, J.; Smith, A. Influences on Reliable Capacity Measurements of Hard Carbon in Highly Loaded Electrodes. Batter. Supercaps 2023, e202300322. [Google Scholar] [CrossRef]
- Hofmann, A.; Müller, F.; Schöner, S.; Jeschull, F. Revealing the Formation of Dialkyl Dioxahexane Dioate Products from Ethylene Carbonate based Electrolytes on Lithium and Potassium Surfaces. Batter. Supercaps 2023, e202300325. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, Y.; Wozny, J.; Lu, J.; Du, H.; Li, C.; Li, T.; Kang, F.; Tavajohi, N.; Li, B. Recycling of sodium-ion batteries. Nat. Rev. Mater. 2023, 8, 623–634. [Google Scholar] [CrossRef]
- Pfeiffer, L.F.; Li, Y.; Mundszinger, M.; Geisler, J.; Pfeifer, C.; Mikhailova, D.; Omar, A.; Baran, V.; Biskupek, J.; Kaiser, U.; et al. Origin of Aging of a P2-NaxMn3/4Ni1/4O2 Cathode Active Material for Sodium-Ion Batteries. Chem. Mater. 2023, 35, 8065–8080. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zhou, J.; Li, X.; Zhou, W.; Zhang, D.; Mao, J.; Dai, K. Optimizing Electrochemical Performance in Sodium-Ion Batteries using O3-type Na0.90Cu0.22Fe0.30Mn0.48O2 and Hard Carbon. J. Electrochem. Soc. 2023, 170, 70518–70533. [Google Scholar] [CrossRef]
- Xia, X.; Lamanna, W.M.; Dahn, J.R. The Reactivity of Charged Electrode Materials with Sodium Bis(trifluoromethanesulfonyl)imide (NaTFSI) Based-Electrolyte at Elevated Temperatures. J. Electrochem. Soc. 2013, 160, A607–A609. [Google Scholar] [CrossRef]
- Ding, M.S.; Xu, K.; Jow, T.R. Liquid-Solid Phase Diagrams of Binary Carbonates for Lithium Batteries. J. Electrochem. Soc. 2000, 147, 1688–1694. [Google Scholar] [CrossRef]
- Nanbu, N.; Suzuki, K.; Yagi, N.; Sugahara, M.; Takehara, M.; Ue, M.; Sasaki, Y. Use of Fluoroethylene Carbonate as Solvent for Electric Double-Layer Capacitors. Electrochemistry 2007, 75, 607–610. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes, Interfaces and Interphases: Fundamentals and Applications in Batteries; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Hofmann, A.; Wang, Z.; Bautista, S.P.; Weil, M.; Müller, F.; Löwe, R.; Schneider, L.; Mohsin, I.U.; Hanemann, T. Comprehensive characterization of propylene carbonate based liquid electrolyte mixtures for sodium-ion cells. Electrochim. Acta 2022, 403, 139670–139687. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Q.; Wang, Y.; Zhao, C.L.; Liu, S.B.; Xu, S.D. Electrochemical Performance of Sodium Difluoro(oxalato)borate as the Additive of Non-Aqueous Electrolytes for Sodium-Ion Batteries. J. Electrochem. 2017, 23, 473–479. [Google Scholar]
- Eshetu, G.G.; Grugeon, S.; Kim, H.; Jeong, S.; Wu, L.; Gachot, G.; Laruelle, S.; Armand, M.; Passerini, S. Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions. ChemSusChem 2016, 9, 462–471. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, P.; Xiang, H.; Liang, X.; Yu, Y. High-Safety Nonaqueous Electrolytes and Interphases for Sodium-Ion Batteries. Small 2019, 15, 1805479–1805494. [Google Scholar] [CrossRef]





| Solvent | Mw [g·mol−1] | ρ at 25 °C [g·cm−3] | Tb [°C] | ε at 40 °C | η at 40 °C [mPa·s] | Refs. |
|---|---|---|---|---|---|---|
| EC | 88.06 | 1.321 a | 248 | 89.78 | 1.93 | [70] |
| PC | 102.09 | 1.205 b | 242 | 66.14 b | 2.53 c | [70] |
| FEC | 106.05 | 1.454 | 212 | 78.4 | 4.10 | [71,72] |
| Salt | Structure of Anion | Mw [g·mol−1] | Tm [°C] | σ in 1 M PC Solution [mS·cm−1] | Anodic Stability/V vs. Na+/Na0 | Refs. |
|---|---|---|---|---|---|---|
| NaPF6 |  | 167.95 | dec. 300 | 7.98 | 5 a | [41,73] |
| NaTFSI | 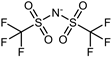 | 303.13 | 257 | 6.20 | 3.4/5 a,b | [41,73] |
| NaDFOB | 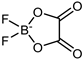 | 159.82 | - | 4.27 | 5.51 c | [30,41] |
| Solvent | EC/PC | |||||||||||||||||
| Salt | NaPF6; 99.5% | |||||||||||||||||
| Additive | - | NaDFOB | FEC | |||||||||||||||
| T [°C] | 40 | 65 | 40 | 65 | 40 | 65 | ||||||||||||
| Vial | PE | PE | Al | PE | PE | Al | PE | PE | Al | PE | PE | Al | PE | PE | Al | PE | PE | Al |
| Na | + | - | - | + | - | - | + | - | - | + | - | - | + | - | - | + | - | - |
| name/code | a1 | a2 | a3 | a4 | a5 | a6 | b1 | b2 | b3 | b4 | b5 | b6 | c1 | c2 | c3 | c4 | c5 | c6 |
| Solvent | EC/PC | |||||||||||
| Salt | NaPF6; 99.9% | |||||||||||
| Additive | - | NaDFOB | FEC | |||||||||
| T [°C] | 25 | 40 | 50 | 58 | 65 | 40 | 65 | 40 | 65 | |||
| Vial | PE | Al | Al | PE | Al | PE | Al | Al | Al | Al | Al | Al |
| Na | + | + | + | + | + | + | + | + | + | + | + | + |
| name/code | a7 | a8 | a9 | a10 | a11 | a12 | a13 | a14 | b7 | b8 | c7 | c8 |
| Solvent | EC/PC | |||||||||||
| Salt | NaTFSI | |||||||||||
| Additive | - | NaDFOB | FEC | |||||||||
| T [°C] | 65 | 65 | 40 | 65 | ||||||||
| Vial | PE | Al | PE | Al | PE | Al | PE | Al | PE | Al | PE | Al |
| Na | + | + | + | + | + | + | - | - | + | + | - | - |
| name/code | d1 | d2 | e1 | e2 | f1 | f2 | f5 | f6 | f3 | f4 | f7 | f8 |
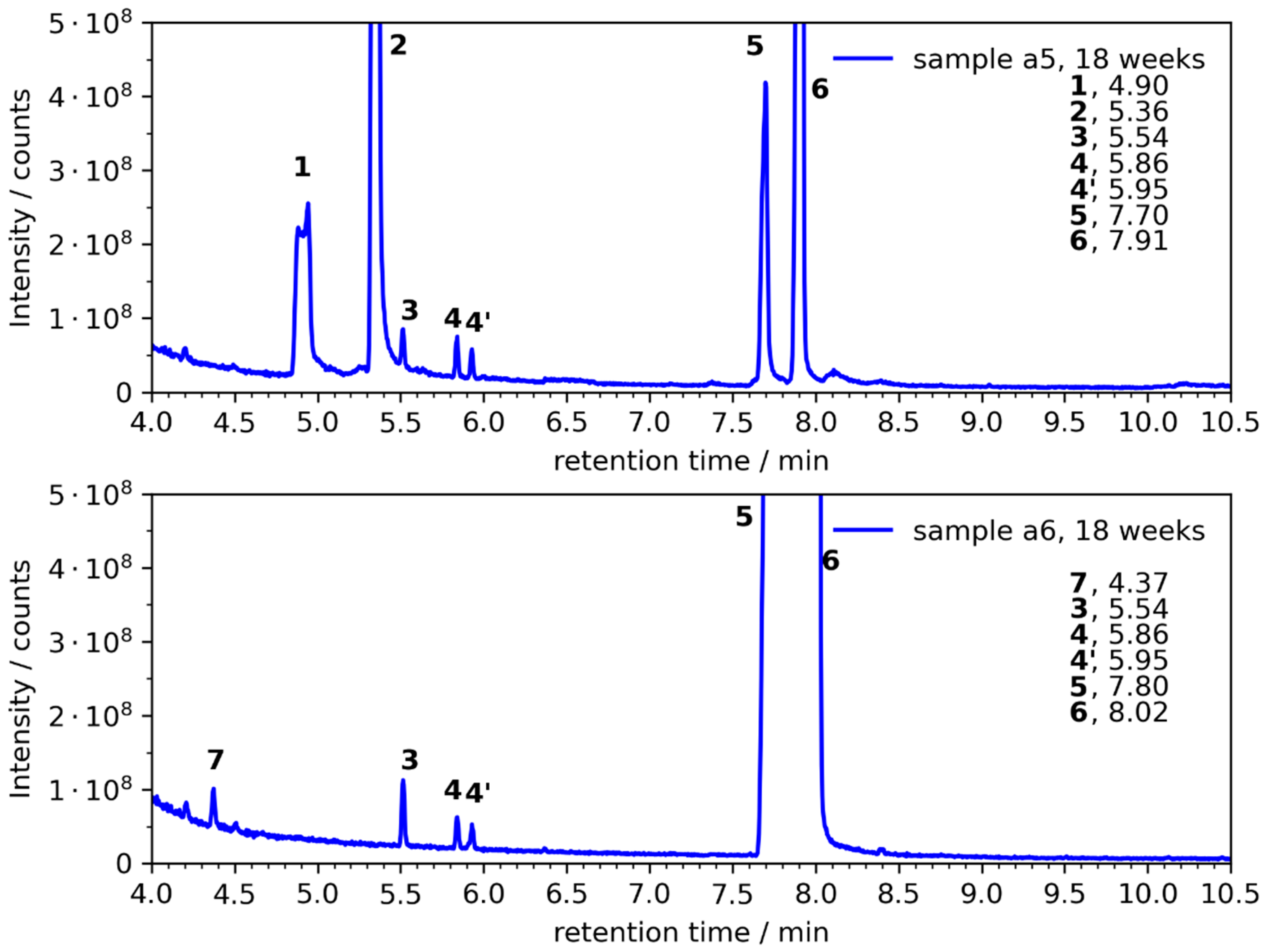
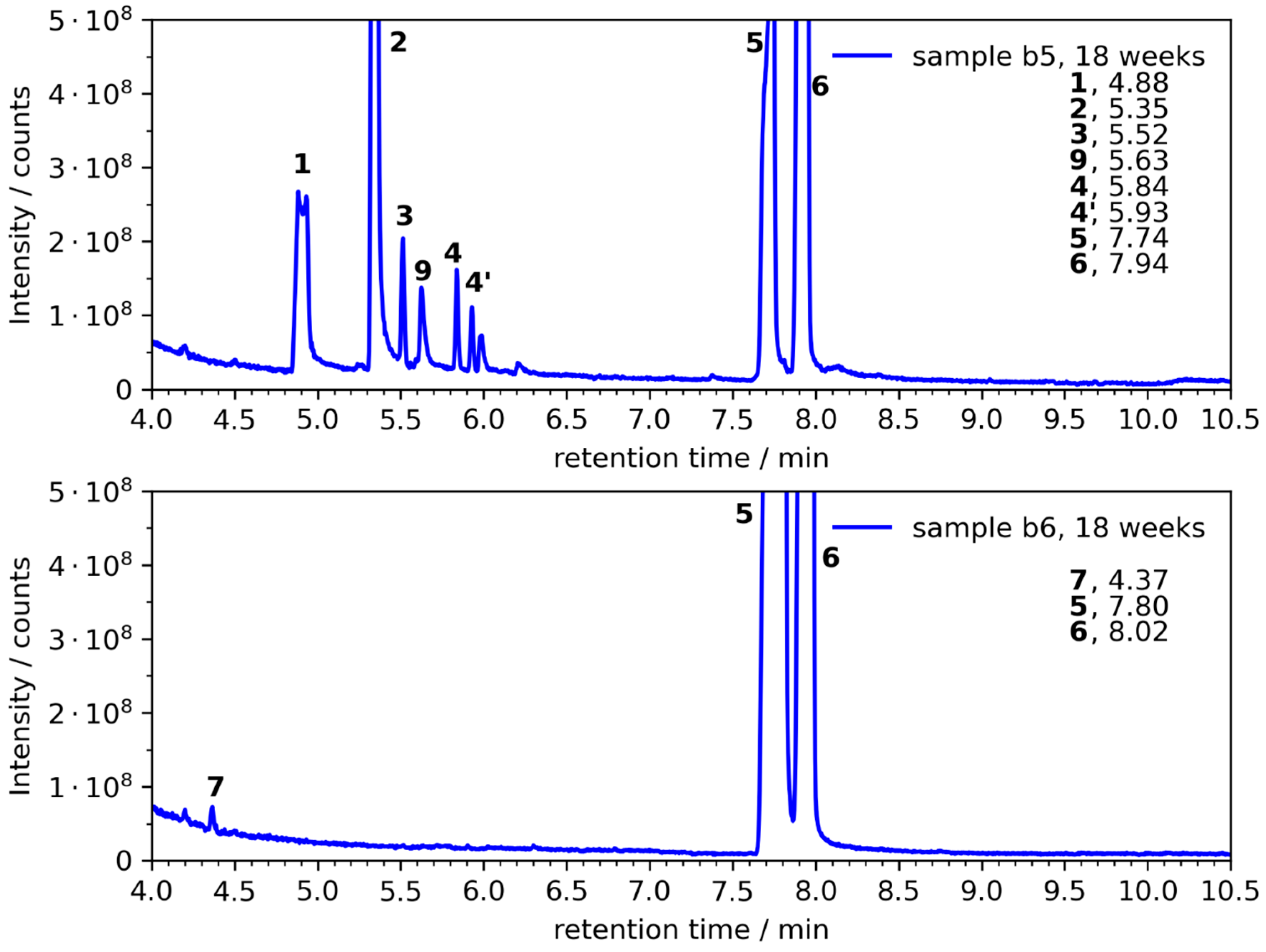
| № | Compound | CAS | Ret. Time a [min] | RI b | RI c NIST | Mass Frag. m/z | Confirmed d |
|---|---|---|---|---|---|---|---|
| 1 | 1,2-ethanediol | 107-21-1 | 4.90 | 692 | 702 ± 10 | 43, 33, 42, 62, 61 | yes |
| 2 | 1,2-propanediol | 57-55-6 | 5.35 | 739 | 740 ± 18 | 45, 43, 61, 75, 76 | yes |
| 3 | 2-ethyl-1,3-dioxolane | 2568-96-9 | 5.54 | 753 | 780 ± 7 | 73, 45, 57, 72, 101 | yes |
| 4 | 2-ethyl-4-methyl-1,3-dioxolane | 4359-46-0 | 5.86, 5.95 | 790, 799 | NA | 87, 59, 41, 57, 72, 115 | yes |
| 5 | EC | 96-49-1 | 7.70 | 978 | NA | 43, 88, 44, 58, 42 | yes |
| 6 | PC | 108-32-7 | 7.91 | 1000 | NA | 57, 43, 87, 58, 42 | yes |
| 7 | amylene hydrate | 75-85-4 | 4.37 | 637 | 615 ± 16 | 59, 73, 55, 43, 41 | yes |
| 8 | 1,4-dioxane | 123-91-9 | 5.08 | 710 | 675 ± 19 | 88, 58, 57, 87, 89 | yes |
| 9 | ethanediol monoformate | 628-35-3 | 5.63 | 764 | NA | 60, 44, 43, 45, 61 | yes |
| 10 | FEC | 1144335-02-8 | 6.55 | 859 | NA | 62, 44, 58, 106, 73 | yes |
| 11 | diethylene glycol | 111-46-6 | 7.65 | 978 | 980 | 45, 75, 76, 43, 44 | yes |
| 12 | triethylene glycol | 112-27-6 | 9.77 | 1256 | 1255 | 45, 89, 58, 75, 43 | yes |
| 13 | 1,3-dioxolane-2-methanol | 5694-68-8 | 6.72 | 883 | 881 | 73, 45, 43, 44, 74 | yes |
| Sample | Weeks | |||
|---|---|---|---|---|
| 3 | 6 | 9 | 18 | |
| a1 | --- | (1), (2) | 1, 2 | 1, 2, (8) |
| a2 | --- | --- | 7 | 3, 4/4′, 7 |
| a3 | --- | --- | (7) | 7 |
| a4 | 1, 2 | 1, 2, (3), (4/4′) | 1, 2, (3), (4/4′) | 1, 2, 3, 4/4′, (8) |
| a5 | 3, 4/4′ | 1, 2, 3, 4/4′ | 1, 2, 3, 4/4′ | 1, 2, 3, 4/4′, (8) |
| a6 | --- | (4/4′), (7) | (3), (4/4′), 7 | 3, 4/4′, 7 |
| Sample | Weeks | |||
|---|---|---|---|---|
| 3 | 6 | 9 | 18 | |
| b1 | --- | --- | (1), (2) | 1, 2 |
| b2 | (7) | (7) | 7, (9) | 1, 2, 3, 4/4′, 7, 9 |
| b3 | --- | (7) | (7) | 7 |
| b4 | --- | (1), 2, (3), (4/4′), 9 | 1, 2, 3, 4/4′, 9 | 1, 2, 3, 4/4′, 9 |
| b5 | (3) | 1, 2, 3, 4/4′, 9 | 1, 2, 3, 4/4′, 9 | 1, 2, 3, 4/4′, 9 |
| b6 | --- | --- | 7 | 7 |
| c1 | --- | (1), (2) | 1, 2 | 1, 2 |
| c2 | 10 | 10 | 7, 10 | 3, 4/4′, 7, 10 |
| c3 | 10 | 10 | (7), 10 | 7, 10 |
| c4 | 1, 2 | 1, 2 | 1, 2, 3, 4/4′ | 1, 2, 3, 4/4′ |
| c5 | 10 | 1, 2, 3, 4/4′, (10) | 1, 2, 3, 4/4′ | 1, 2, 3, 4/4′ |
| c6 | 10 | 10 | 7, 10 | 3, (4/4′), 7, 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimov, M.; Hofmann, A. Deciphering Electrolyte Degradation in Sodium-Based Batteries: The Role of Conductive Salt Source, Additives, and Storage Condition. Batteries 2023, 9, 530. https://doi.org/10.3390/batteries9110530
Hashimov M, Hofmann A. Deciphering Electrolyte Degradation in Sodium-Based Batteries: The Role of Conductive Salt Source, Additives, and Storage Condition. Batteries. 2023; 9(11):530. https://doi.org/10.3390/batteries9110530
Chicago/Turabian StyleHashimov, Mahir, and Andreas Hofmann. 2023. "Deciphering Electrolyte Degradation in Sodium-Based Batteries: The Role of Conductive Salt Source, Additives, and Storage Condition" Batteries 9, no. 11: 530. https://doi.org/10.3390/batteries9110530
APA StyleHashimov, M., & Hofmann, A. (2023). Deciphering Electrolyte Degradation in Sodium-Based Batteries: The Role of Conductive Salt Source, Additives, and Storage Condition. Batteries, 9(11), 530. https://doi.org/10.3390/batteries9110530






