High-Performance Full Sodium Cells Based on MgO-Treated P2-Type Na0.67(Mn0.5Fe0.5)1−xCoxO2 Cathodes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
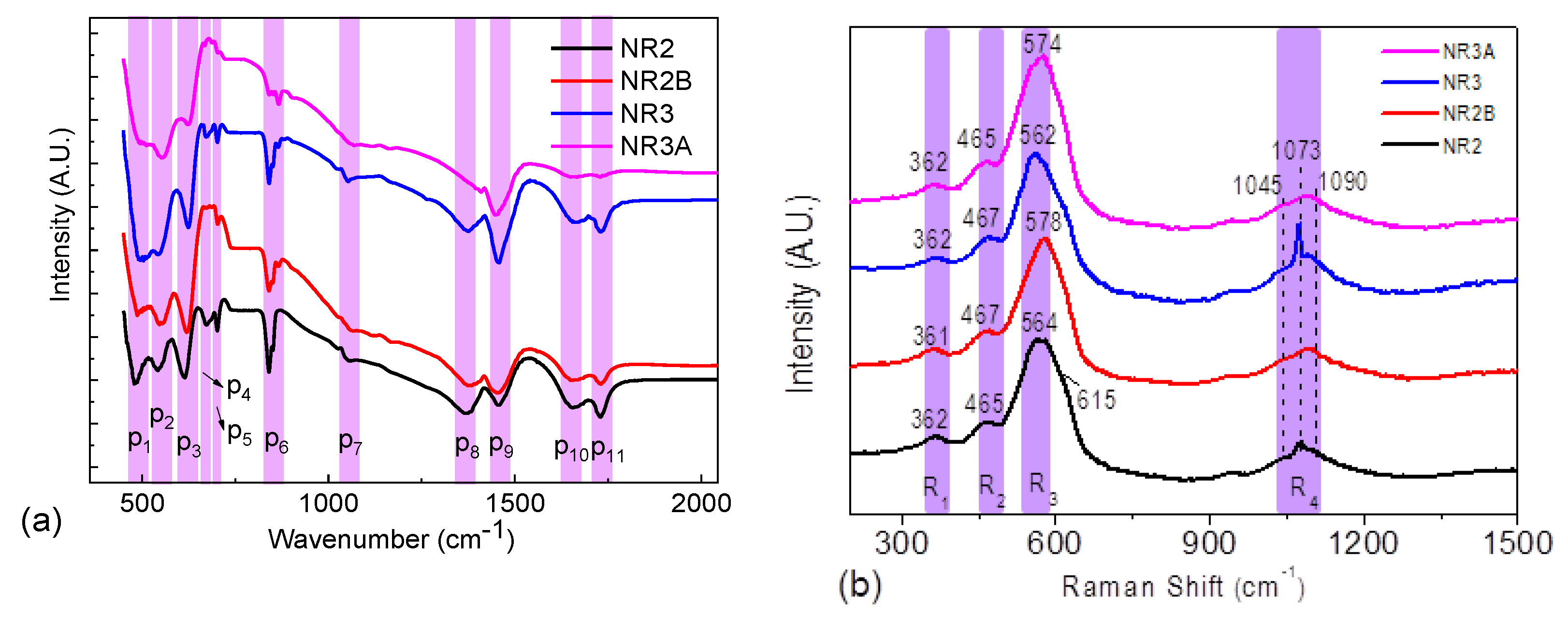
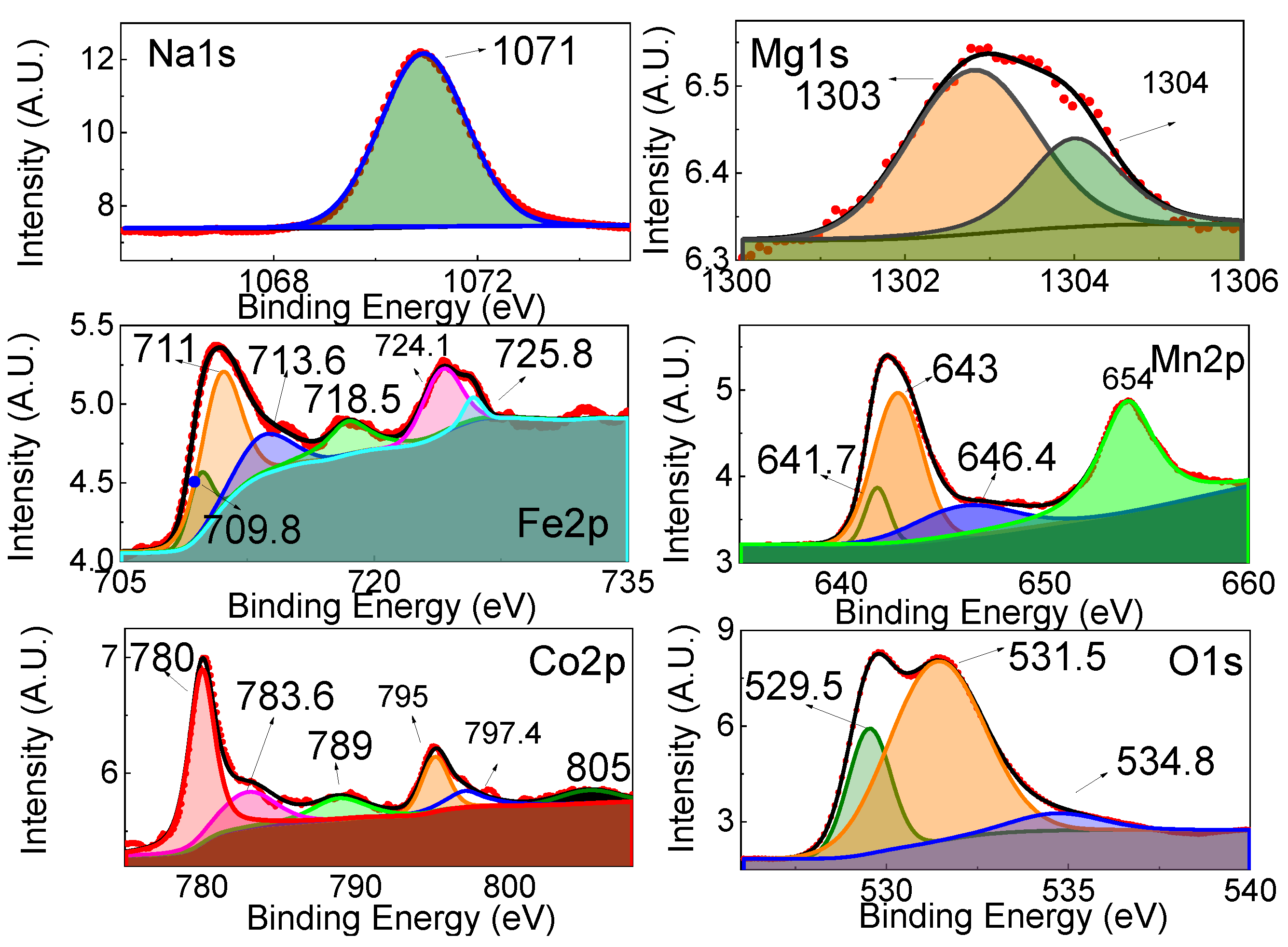
3.1. Structural Properties
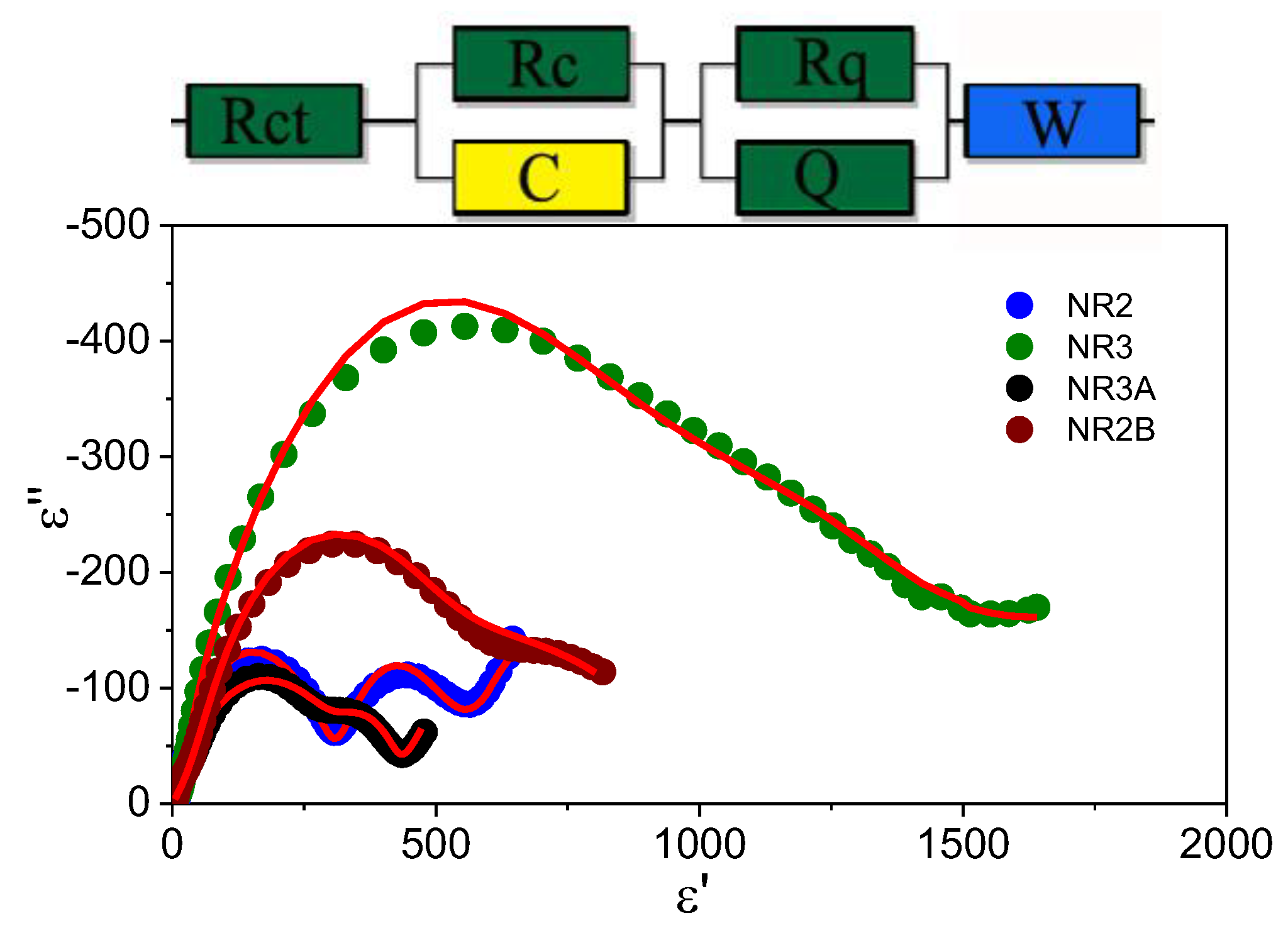
3.2. Electrochemical Analysis for Half Cells
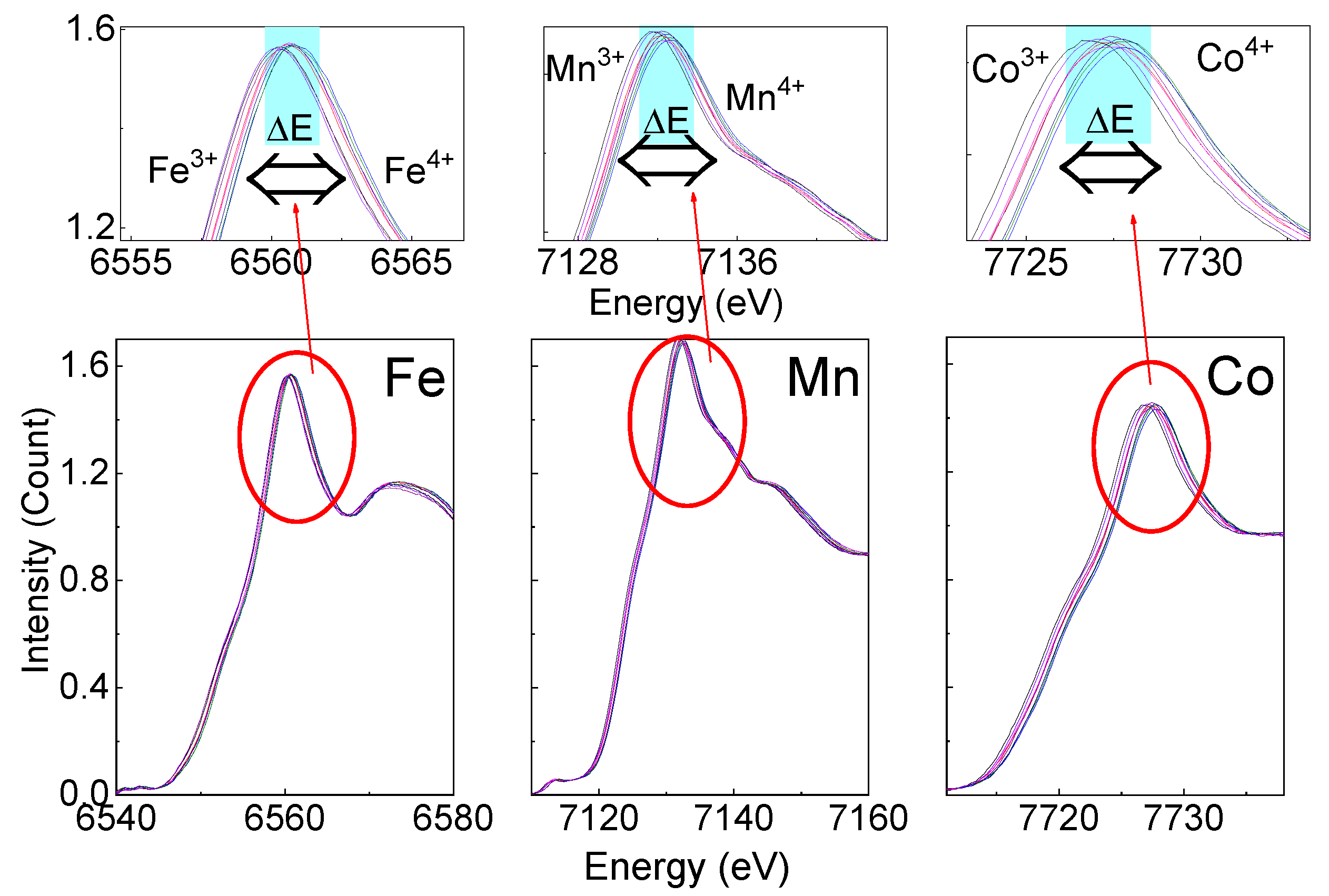
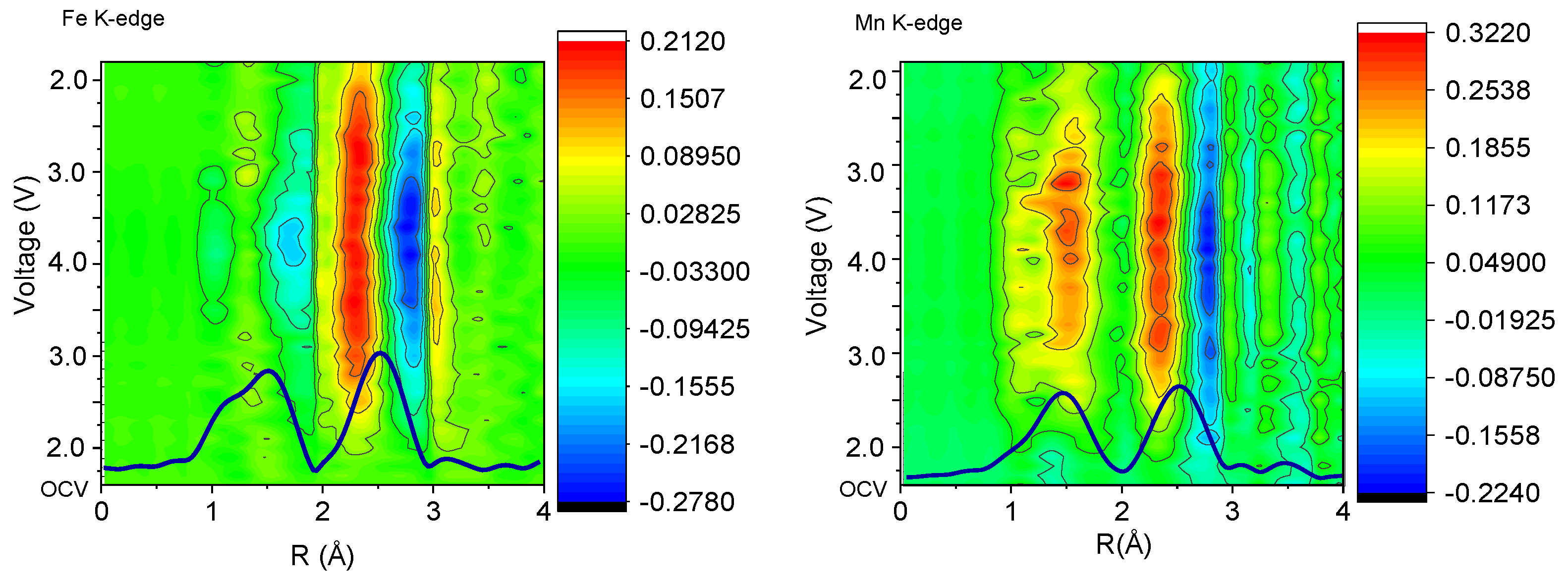
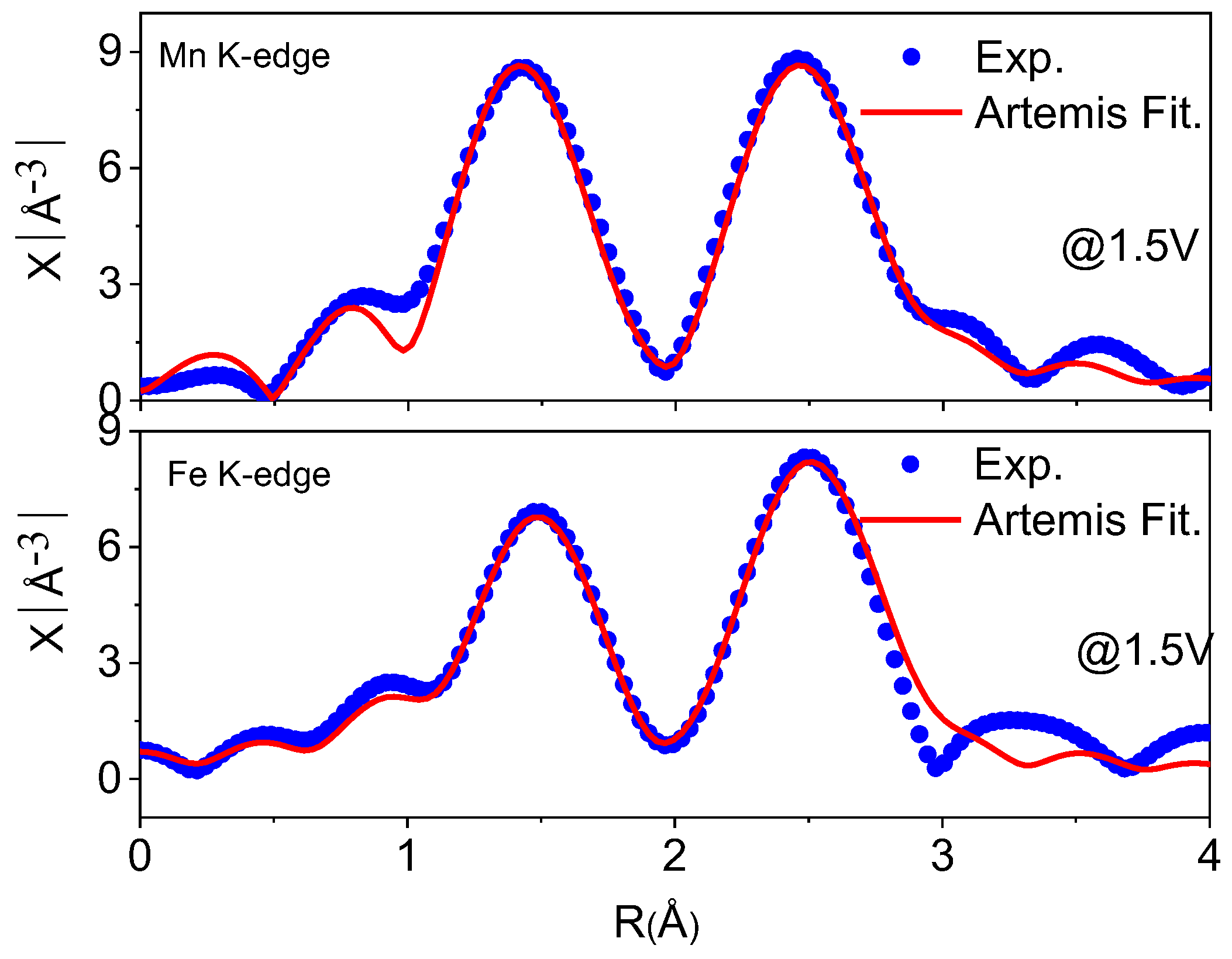
3.3. Operando Study of NR2B
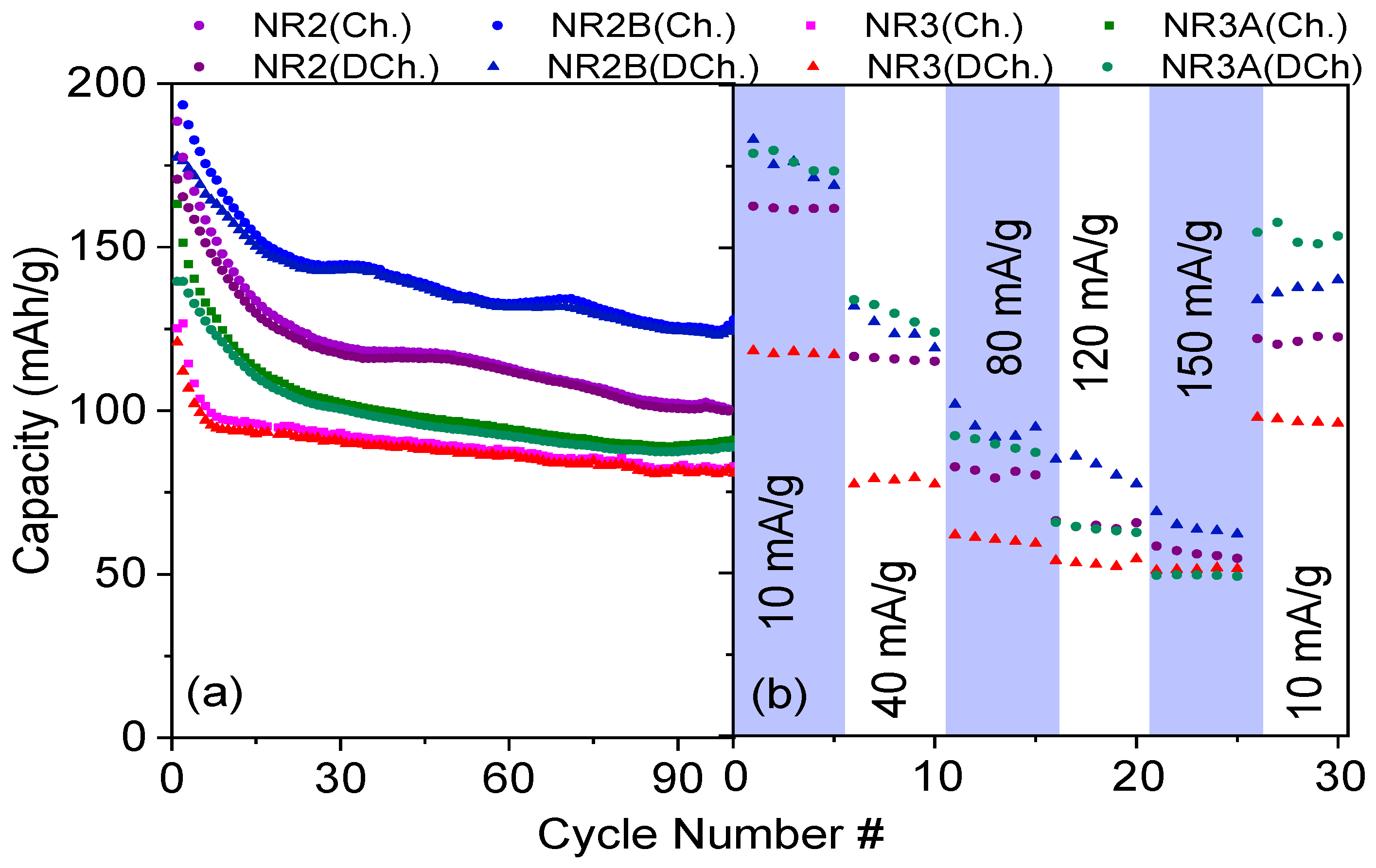
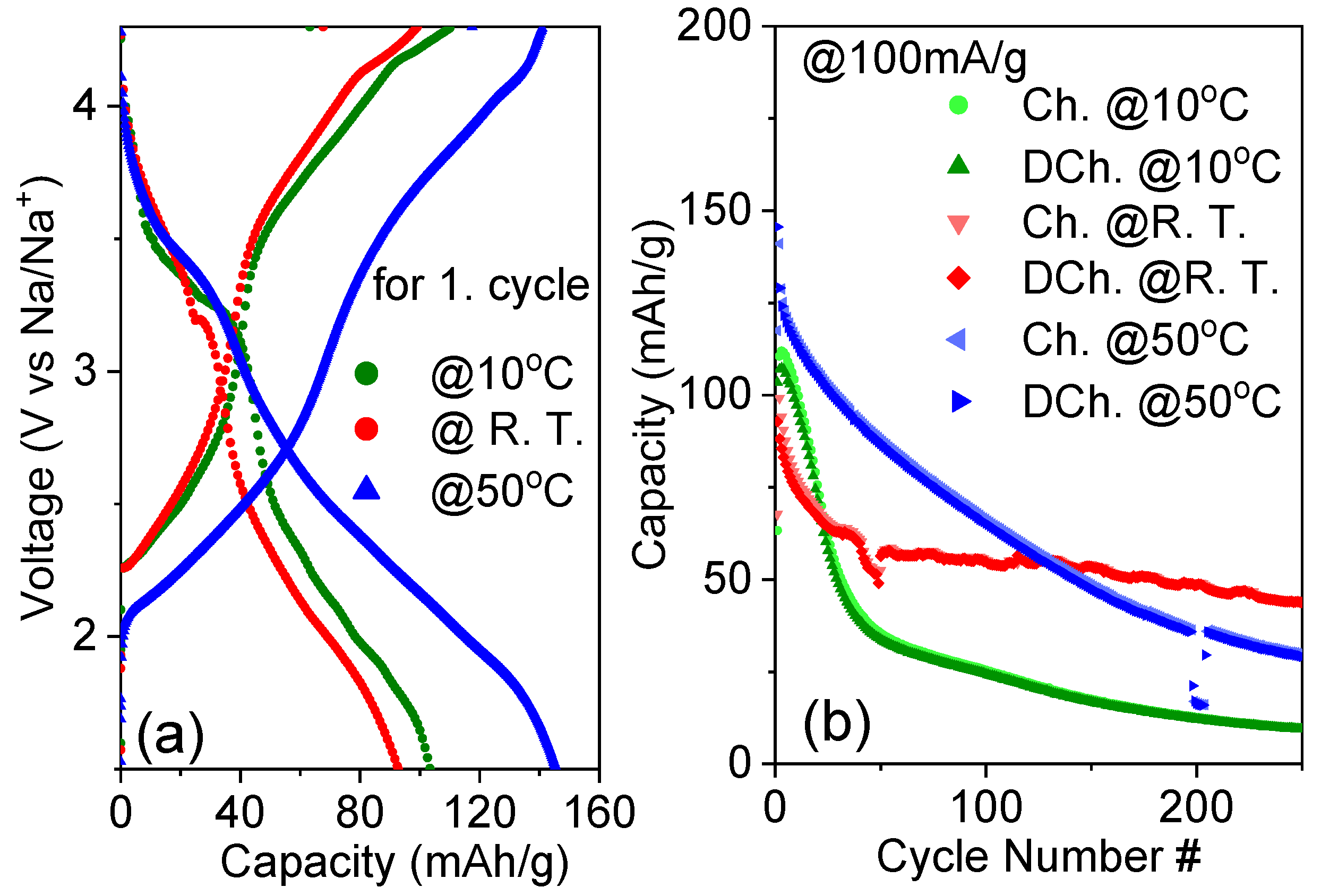
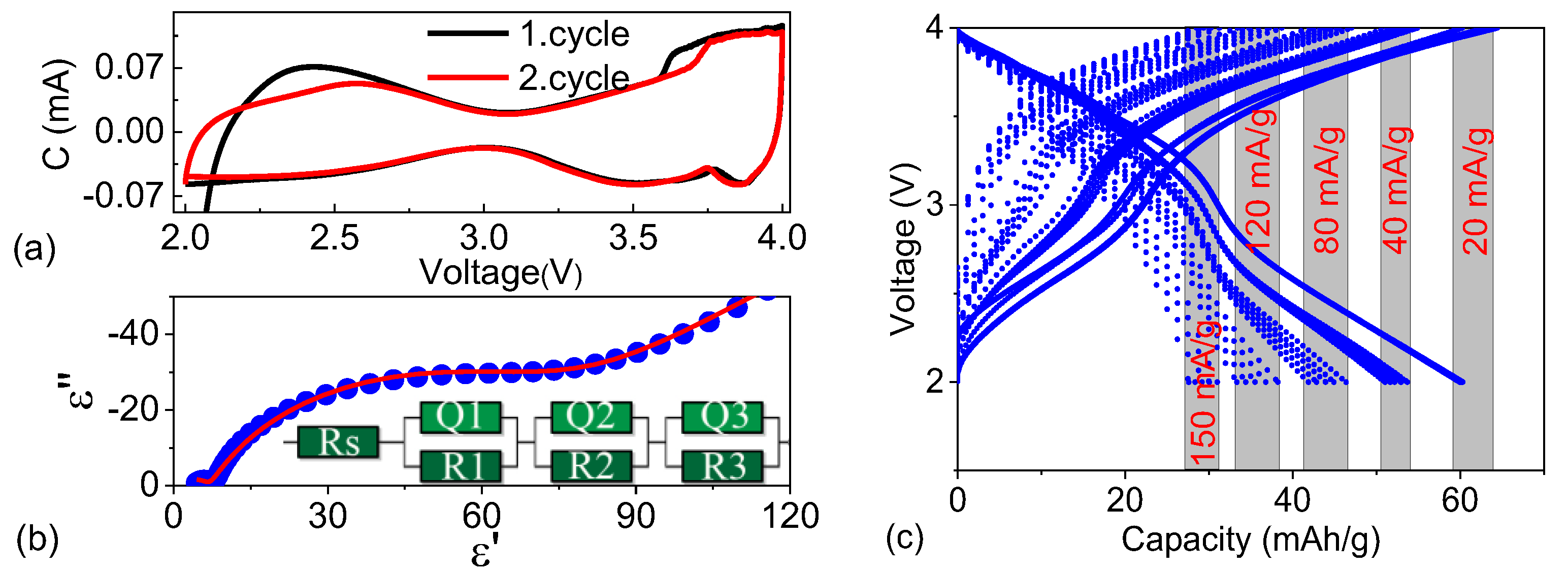
3.4. Electrochemical Analysis of Full Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariyappan, S.; Wang, Q.; Tarascon, J.M. Will Sodium Layered Oxides Ever Be Competitive for Sodium-Ion Battery Applications? J. Electrochem. Soc. 2018, 165, A3714–A3722. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Li, W.; Zou, C.; Jin, H.; Wang, S.; Chou, S.; Dou, S.-X. Sodium transition metal oxides: The preferred cathode choice for future sodium-ion batteries? Energy Environ. Sci. 2021, 14, 158–179. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Q.; Zhang, P.; Tian, W.; Dai, K.; Zhang, L.; Mao, J.; Shao, G. Review—Research Progress on Layered Transition Metal Oxide Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2021, 168, 050524. [Google Scholar] [CrossRef]
- Chen, T.; Ouyang, B.; Fan, X.; Zhou, W.; Liu, W.; Liu, K. Oxide cathodes for sodium-ion batteries: Designs, challenges, and perspectives. Carbon Energy 2022, 4, 170–199. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, S.; Seng, K.H.; Wei, K.P.; Guo, Z.; Dou, S. Electrospun P2-type Na2/3(Fe1/2Mn1/2)O2 hierarchical nanofibers as cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 8953–8958. [Google Scholar] [CrossRef] [PubMed]
- Mortemard De Boisse, B.; Carlier, D.; Guignard, M.; Bourgeois, L.; Delmas, C. P2-NaxMn1/2Fe1/2O2 phase used as positive electrode in Na batteries: Structural changes induced by the electrochemical (De)intercalation process. Inorg. Chem. 2014, 53, 11197–11205. [Google Scholar] [CrossRef]
- Zarrabeitia, M.; Nobili, F.; Lakuntza, O.; Carrasco, J.; Rojo, T.; Casas-Cabanas, M.; Muñoz-Márquez, M.A. Role of the voltage window on the capacity retention of P2-Na2/3[Fe1/2Mn1/2]O2 cathode material for rechargeable sodium-ion batteries. Commun. Chem. 2022, 5, 11. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, M.; Sun, X.; Dong, W.; Huang, F. Recent Advances on High-Capacity Sodium Manganese-Based Oxide Cathodes for Sodium-ion Batteries. Chem.—Eur. J. 2023, 29, e202202997. [Google Scholar] [CrossRef]
- Konarov, A.; Kim, H.J.; Voronina, N.; Bakenov, Z.; Myung, S.T. P2-Na2/3MnO2 by Co Incorporation: As a Cathode Material of High Capacity and Long Cycle Life for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 28928–28933. [Google Scholar] [CrossRef] [PubMed]
- Bucher, N.; Hartung, S.; Franklin, J.B.; Wise, A.M.; Lim, L.Y.; Chen, H.Y.; Weker, J.N.; Toney, M.F.; Srinivasan, M. P2-NaxCoyMn1-yO2 (y = 0, 0.1) as Cathode Materials in Sodium-Ion Batteries—Effects of Doping and Morphology to Enhance Cycling Stability. Chem. Mater. 2016, 28, 2041–2051. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Bo, S.-H.; Wang, Y.; Chen, H.; Twu, N.; Wu, D.; Ceder, G. High-performance P2-Type Na2/3(Mn1/2Fe1/4Co1/4)O2 cathode material with superior rate capability for Na-ion batteries. Adv. Energy Mater. 2015, 5, 1500944. [Google Scholar] [CrossRef]
- Yoda, Y.; Kubota, K.; Kuroki, K.; Suzuki, S.; Yamanaka, K.; Yaji, T.; Amagasa, S.; Yamada, Y.; Ohta, T.; Komaba, S. Elucidating Influence of Mg- and Cu-Doping on Electrochemical Properties of O3-Nax[Fe,Mn]O2 for Na-Ion Batteries. Small 2020, 16, 2006483. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Hu, E.; Liu, J.; Zhang, Y.; Yang, X.-Q.; Nanda, J.; Huq, A.; Page, K. A novel P3-type Na2/3Mg1/3Mn2/3O2 as high capacity sodium-ion cathode using reversible oxygen redox. J. Mater. Chem. A 2019, 7, 1491–1498. [Google Scholar] [CrossRef]
- Maitra, U.; House, R.A.; Somerville, J.W.; Tapia-Ruiz, N.; Lozano, J.G.; Guerrini, N.; Hao, R.; Luo, K.; Jin, L.; Pérez-Osorio, M.A.; et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72] O2. Nat. Chem. 2018, 10, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liua, L.; Jamil, S.; Xie, J.; Liu, W.; Xia, J.; Nie, S.; Wang, X. Al2O3 coated Na0.44MnO2 as high-voltage cathode for sodium ion batteries. Appl. Surf. Sci. 2019, 494, 1156–1165. [Google Scholar] [CrossRef]
- Yu, Y.; Kong, W.; Li, Q.; Ning, D.; Schuck, G.; Schumacher, G.; Su, C.; Liu, X. Understanding the Multiple Effects of TiO2 Coating on NaMn0.33Fe0.33Ni0.33O2 Cathode Material for Na-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 933–942. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Yua, T.-Y.; Suna, Y.-K. Simultaneous MgO coating and Mg doping of Na[Ni0.5Mn0.5]O2 cathode: Facile and customizable approach to high-voltage sodium-ion batteries. J. Mater. Chem. A 2018, 6, 16854–16862. [Google Scholar] [CrossRef]
- Kong, W.; Wang, H.; Sun, L.; Su, C.; Liu, X. Understanding the synergic roles of MgO coating on the cycling and rate performance of Na0.67Mn0.5Fe0.5O2 cathode. Appl. Surf. Sci. 2019, 497, 143814. [Google Scholar] [CrossRef]
- Xue, L.; Bao, S.; Yan, L.; Zhang, Y.; Lu, J.; Yin, Y. MgO-Coated Layered Cathode Oxide With Enhanced Stability for Sodium-Ion Batteries. Front. Energy Res. 2022, 10, 847818. [Google Scholar] [CrossRef]
- Wang, J.; Teng, Y.; Su, G.; Bao, S.; Lu, J. A dual-modification strategy for P2-type layered oxide via bulk Mg/Ti cosubstitution and MgO surface coating for sodium ion batteries. J. Colloid Interface Sci. 2022, 608, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, A.; Kubota, K.; Igarashi, D.; Youn, A.; Tateyama, Y.; Ando, H.; Gotoh, K.; Komaba, S. MgO-Template Synthesis of Extremely High Capacity Hard Carbon for Na-Ion Battery. Angew. Chem. Int. Ed. 2021, 60, 5114–5120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Huang, Y. Air-Stable NaxTMO2 Cathodes for Sodium Storage. Front. Chem. 2019, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Ginell, K.; Horn, C.; Von Dreele, R.B.; Toby, B.H. Materials for Learning Use of GSAS-II. Powder Diffr. 2019, 34, 184–188. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Wu, Z.; Pang, W.K.; Chen, L.; Johannessen, B.; Guo, Z. In Situ Synchrotron X-Ray Absorption Spectroscopy Studies of Anode Materials for Rechargeable Batteries. Batter. Supercaps 2021, 4, 1547–1566. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Landrot, G. FASTOSH: A Software to Process XAFS Data for Geochemical & Environmental Applications. In Proceedings of the Goldschmidt Abstracts, Boston, MA, USA, 12–17 August 2018; p. 1402. [Google Scholar]
- Maddukuri, S.; Valerie, P.; Upadhyayula, V.V. Synthesis and Electrochemical Study of New P3 Type Layered Na0.6Ni0.25Mn0.5Co0.25O2 for Sodium-Ion Batteries. ChemistrySelect 2017, 2, 5660–5666. [Google Scholar] [CrossRef]
- Sendova-Vassileva, M.; Stoyanova, R.; Carlier, D.; Yoncheva, M.; Zhecheva, E.; Delmas, C. Raman Spectroscopy Study on Na2/3Mn1-xFexO2 Oxides. Adv. Sci. Technol. 2010, 74, 60–65. [Google Scholar]
- Lu, Z.-H.; Dahn, J.R. The effect of Co substitution for Ni on the structure and electrochemical behaviour of T2 and O2 structure Li2/3 (Cox Ni1/3-x Mn2/3) O2. J. Electrochem. Soc. 2001, 148, A237. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Hou, J.G.; Zhu, Q. First-principles lattice dynamics of NaCoO2. Phys. Rev. B 2004, 70, 144518. [Google Scholar] [CrossRef]
- Zhao, L.; Ni, J.; Wang, H.; Gao, L. Na0.44MnO2–CNT electrodes for non-aqueous sodium batteries. RSC Adv. 2013, 3, 6650–6655. [Google Scholar] [CrossRef]
- Singh, K.; Kirubasankar, B.; Angaiah, S. Synthesis and electrochemical performance of P2-Na0.67AlxCo1-xO2 (0.0 ≤ × ≤ 0.5) nanopowders for sodium-ion capacitors. Ionics 2017, 23, 731–739. [Google Scholar] [CrossRef]
- Xie, M.; Luo, R.; Lu, J.; Chen, R.; Wu, F.; Wang, X.; Zhan, C.; Wu, H.; Albishri, H.M.; Al-Bogami, A.S.; et al. Synthesis-Microstructure-Performance Relationship of Layered Transition Metal Oxides as Cathode for Rechargeable Sodium Batteries Prepared by High-Temperature Calcination. ACS Appl. Mater. Interfaces 2014, 6, 17176–17183. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; You, Y.; Yin, Y.X.; Wang, Y.S.; Wan, L.J.; Gu, L.; Guo, Y.G. Suppressing the P2-O2 Phase Transition of Na0.67Mn0.67Ni0.33O2 by Magnesium Substitution for Improved Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 7445–7449. [Google Scholar] [CrossRef] [PubMed]
- Altin, S.; Oz, E.; Altin, E.; Demirel, S.; Bayria, A.; Avci, S. Investigations of the capacity fading mechanism of Na0.44MnO2 via ex situ XAS and magnetization measurements. Dalton Trans. 2018, 47, 17102. [Google Scholar] [CrossRef]
- Darbar, D.; Muralidharan, N.; Hermann, R.; Nanda, J.; Bhattacharya, I. Evaluation of electrochemical performance and redox activity of Fe in Ti doped layered P2-Na0.67Mn0.5Fe 0.5O2 cathode for sodium ion batteries. Electrochim. Acta 2021, 380, 138156. [Google Scholar] [CrossRef]
- Rahmawati, F.; Kusumaningtyas, A.A.; Saraswati, T.E.; Prasetyo, A.; Suendo, V. Mn-doped NaFeO2 from a low purity-Fe precursor and its performance as cathode for Sodium-Ion Battery. Inorg. Nano-Met. Chem. 2021, 51, 383–390. [Google Scholar] [CrossRef]
- Su, C.; Suarez, D.L. In situ infrared speciation of adsorbed carbonate on aluminum and iron oxides. Clays Clay Miner. 1997, 45, 814–825. [Google Scholar] [CrossRef]
- Coluccia, S.; Marchese, L.; Martra, G. Characterisation of microporous and mesoporous materials by the adsorption of molecular probes: FTIR and UV–Vis studies. Microporous Mesoporous Mater. 1999, 30, 43–56. [Google Scholar] [CrossRef]
- Stoilova, D.; Koleva, V.; Vassileva, V. Infrared study of some synthetic phases of malachite (Cu2(OH)2CO3)–hydrozincite (Zn5(OH)6(CO3)2) series. Spectrochim. Acta Part A 2002, 58, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Spratt, H.J.; Palmer, S.J. Infrared and near-infrared spectroscopic study of synthetic hydrotalcites with variable divalent/trivalent cationic ratios. Spectrochim. Acta A 2009, 72, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Sathiya, M.; Hemalatha, K.; Ramesha, K.; Tarascon, J.M.; Prakash, A.S. Synthesis, Structure, and Electrochemical Properties of the Layered Sodium Insertion Cathode Material: NaNi1/3Mn1/3Co1/3O2. Chem. Mater. 2012, 24, 1846–1853. [Google Scholar] [CrossRef]
- Duffort, V.; Talaie, E.; Black, R.; Nazar, L.F. Uptake of CO2 in Layered P2-Na0.67Mn0.5Fe0.5O2: Insertion of Carbonate Anions. Chem. Mater. 2015, 27, 2515–2524. [Google Scholar] [CrossRef]
- Zuo, W.; Qiu, J.; Liu, X.; Ren, F.; Liu, H.; He, H.; Luo, C.; Li, J.; Ortiz, G.F.; Duan, H.; et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres. Nat. Commun. 2020, 11, 3544. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cai, H.; Chen, Q.; Kong, X.; Pan, H.; Hu, Y.-S. Origin of Air-Stability for Transition Metal Oxide Cathodes in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 5338–5345. [Google Scholar] [CrossRef] [PubMed]
- Kalapsazova, M.; Kukeva, R.; Harizanova, S.; Markov, P.; Nihtyanova, D.; Zhecheva, E.; Stoyanova, R. High-Performance Layered Oxides for Sodium-Ion Batteries Achieved through Combined Aluminum Substitution and Surface Treatment. Batteries 2023, 9, 144. [Google Scholar] [CrossRef]
- Peng, B.; Sun, Z.; Zhao, L.; Li, J.; Zhang, G. Dual-Manipulation on P2-Na0.67Ni0.33Mn0.67O2 Layered Cathode toward Sodium-Ion Full Cell with Record Operating Voltage Beyond. Energy Storage Mater. 2021, 35, 620–629. [Google Scholar] [CrossRef]
- Song, T.; Chen, L.; Gastol, D.; Dong, B.; Marco, J.F.; Berry, F.; Slater, P.; Reed, D.; Kendrick, E. High-Voltage Stabilization of O3-Type Layered Oxide for Sodium-Ion Batteries by Simultaneous Tin Dual Modification. Chem. Mater. 2022, 34, 4153–4165. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Monson, P.A. Understanding adsorption/desorption hysteresis for fluids in mesoporous materials using simple molecular models and classical density functional theory. Microporous Mesoporous Mater. 2012, 160, 47–66. [Google Scholar] [CrossRef]
- Lu, X.; Sun, S.; Fan, Q.; Pei, X.; Dun, Y.; Feng, X.; Zou, C.; Lu, W. Investigation of Protective Performance of a Mg-Rich Primer Containing Aluminum Tri-Polyphosphate on AZ91D Magnesium Alloy in Simulated Acid Rain. Coatings 2019, 9, 649. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Hussain, S.; Jia, L.; Lin, D.; Yin, X.; Lin, Y.; Cheng, Z.L. Wang, FeS2/carbon hybrids on carbon cloth: A highly efficient and stable counter electrode for dye-sensitized solar cells. Sustain. Energy Fuels 2019, 3, 1749–1756. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, G.; Wang, L.; Huang, T.; Qin, L. Highly Active S-Modified ZnFe2O4 Heterogeneous Catalyst and Its Photo-Fenton Behavior under UV-Visible Irradiation. Ind. Eng. Chem. Res. 2011, 50, 7219–7227. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Gutkin, V.; Driess, M. Boosting electrochemical water oxidation throughreplacement of Oh Co sites in cobalt oxide spinelwith manganese. Chem. Commun. 2017, 53, 8018–8021. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Gonzalez-Flores, D.; Sahraie, N.R.; Zaharieva, I.; Schwarze, M.; Strasser, P.; Dau, H.; Driess, M. HighPerformance Oxygen Redox Catalysis with Multifunctional Cobalt Oxide Nanochains: Morphology-Dependent Activity. ACS Catal. 2015, 5, 2017–2027. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Hossain, M.A.; Uchida, K.; Tamura, T.; Sugawa, K.; Mochida, T.; Otsuki, J.; Mohiuddin, T.; Boby, M.A.; Alam, M.S. Graphene oxide/carbon nanoparticle thin film based IR detector: Surface properties and device characterization. AIP Adv. 2015, 5, 107228. [Google Scholar] [CrossRef]
- Altin, S.; Altundag, S.; Altin, E.; Oz, E.; Harfouche, M.; Bayri, A. Investigation of Ti-substitution effects on structural andelectrochemical properties of Na0.67Mn0.5Fe0.5O2 battery cells. Int. J. Energy Res. 2020, 44, 11794–11806. [Google Scholar] [CrossRef]
- Zhao, W.; Tsuchiya, Y.; Yabuuchi, N. Influence of Synthesis Conditions on Electrochemical Properties of P2-Type Na2/3Fe2/3Mn1/3O2 for Rechargeable Na Batteries. Small Methods 2018, 3, 1800032. [Google Scholar] [CrossRef]
- Massaro, A.; Langella, A.; Muñoz-García, A.-B.; Pavone, M. First-principles insights on anion redox activity in NaxFe1/8Ni1/8Mn3/4O2: Toward efficient high-energy cathodes for Na-ion batteries. J. Am. Chem. Soc. 2023, 106, 109–119. [Google Scholar] [CrossRef]
- Altin, S.; Altundağ, S.; Altin, E.; Harfouche, M.; Bayri, A. An investigation of the improvement in energy storage performance of Na2/3Mn1/2Fe1/2O2 by systematic Al-substitution. J. Mater. Sci. Mater. Electron. 2020, 31, 14784–14794. [Google Scholar] [CrossRef]
- Wang, H.; Gao, R.; Li, Z.; Sun, L.; Hu, Z.; Liu, X. Different Effects of Al Substitution for Mn or Fe on the Structure and Electrochemical Properties of Na0.67Mn0.5Fe0.5O2 as a Sodium Ion Battery Cathode Material. Inorg. Chem. 2018, 57, 5249–5257. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Kulka, A.; Molenda, J. Alluaudite-Na1.47Fe3(PO4)3: Structural and electrochemical properties of potential cathode material for Na-ion Batteries. Solid State Sci. 2019, 87, 21–26. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wu, X.-L.; Zhang, X.-H.; Lü, H.-Y.; Wang, G.; Guo, J.-Z.; Wan, F.; Wang, R.-S. Romanechite-structured Na0.31MnO1.9 nanofibers as high-performance cathode material for a sodium-ion battery. Chem. Commun. 2015, 51, 14848–14851. [Google Scholar] [CrossRef] [PubMed]
- Griese, J.J.; Kositzki, R.; Schrapers, P.; Rui, X.; Branca, M.M.; Nordström, X.A.; Lehtiö, J.; Haumann, M.; Högbom, X.M. Structural Basis for Oxygen Activation at a Heterodinuclear Manganese/Iron Cofactor. J. Biol. Chem. 2015, 290, 25254–25272. [Google Scholar] [CrossRef]
- Gholam, T.; Zheng, L.R.; Wang, J.O.; Qian, H.J.; Wu, R.; Wang, H.Q. Synchrotron X-ray Absorption Spectroscopy Study of Local Structure in Al-Doped BiFeO3 Powders. Nanoscale Res. Lett. 2019, 14, 137. [Google Scholar] [CrossRef]
- Taylor, N.T.; Davies, F.H.; Rudkin, I.E.M.; Price, C.J.; Chan, T.H.; Hepplestone, S.P. ARTEMIS: Ab initio restructuring tool enabling the modelling of interface structures. Comput. Phys. Commun. 2020, 257, 107515. [Google Scholar] [CrossRef]
- Raut, A.V.; Agrawal, A.; Bagde, A.; Fulzele, P.; Syed, Z.Q. 3-D Bioprinting in cartilage tissue engineering for bioinks-short review. Mater. Today Proceeding 2021, 44, 3296. [Google Scholar] [CrossRef]
- Yang, L.; López del Amo, J.M.; Shadike, Z.; Bak, S.-M.; Bonilla, F.; Galceran, M.; Nayak, P.K.; Buchheim, J.R.; Yang, X.-Q.; Rojo, T.; et al. A Co- and Ni-Free P2/O3 Biphasic Lithium Stabilized LayeredOxide for Sodium-Ion Batteries and its Cycling Behavior. Adv. Funct. Mater. 2020, 30, 2003364. [Google Scholar] [CrossRef]
- Meyer-Klaucke, W.; Gnida, M.; Henkel, G. X-ray Absorption Spectroscopy in Biology. Mol. Sci. Chem. Eng. 2014. [Google Scholar] [CrossRef]
- Moeez, I.; Jung, H.G.; Lim, H.-D.; Chung, K.-Y. Pre-Sodiation Strategies and Their Effect on Electrode-Electrolyte Interphases for High-Performance Electrodes for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 41394–41401. [Google Scholar] [CrossRef]




| Co-Substituted Oxides | Notation | MgO-Treated Oxides | Notation |
|---|---|---|---|
| Na0.67(Mn0.5Fe0.5)0.95Co0.05O2 | NR1 | ||
| Na0.67(Mn0.5Fe0.5)0.9Co0.1O2 | NR2 | 1%MgO/Na0.67(Mn0.5Fe0.5)0.9Co0.1O2 | NR2A |
| 2%MgO/Na0.67(Mn0.5Fe0.5)0.9Co0.1O2 | NR2B | ||
| 3%MgO/Na0.67(Mn0.5Fe0.5)0.9Co0.1O2 | NR2C | ||
| Na0.67(Mn0.5Fe0.5)0.8Co0.2O2 | NR3 | 1%MgO/Na0.67(Mn0.5Fe0.5)0.8Co0.2O2 | NR3A |
| 2%MgO/Na0.67(Mn0.5Fe0.5)0.8Co0.2O2 | NR3B | ||
| 3%MgO/Na0.67(Mn0.5Fe0.5)0.8Co0.2O2 | NR3C | ||
| Na0.67(Mn0.5Fe0.5)0.7Co0.3O2 | NR4 |
| Sample Code | 1st Cycle (mAh/g) | 100th Cycle (mAh/g) | Capacity Loss (%) |
|---|---|---|---|
| NR-1 | 130 | 67 | 48.5 |
| NR-2 | 169 | 96 | 43.2 |
| NR-3 | 121 | 81 | 33.1 |
| NR-4 | 51 | 53 | ~0 |
| NR-2A | 131 | 100 | 23.6 |
| NR-2B | 175 | 125.5 | 28.3 |
| NR-2C | 96 | 41.5 | 43.8 |
| NR-3A | 138 | 88 | 36.2 |
| NR-3B | 123 | 86 | 30 |
| NR-3C | 118 | 80 | 32.2 |
| EXAFS Region | Voltage→ | 1.5 V | 2.5 | 3.5 | 4.3 | 3.5 | 1.5 V |
|---|---|---|---|---|---|---|---|
| Parameter↓ | |||||||
| RMn-O (Å) | 1.926 | 1.940 | 1.937 | 1.902 | 1.933 | 1.931 | |
| RMn-TM (Å) | 2.913 | 2.909 | 2.904 | 2.899 | 2.915 | 2.921 | |
| Mn K-edge | σ2Mn-O × 10−3 | 3.22 | 2.71 | 2.35 | 2.71 | 2.68 | 2.19 |
| σ2Mn-TM × 10−3 | 5.18 | 4.34 | 5.02 | 4.16 | 4.90 | 4.73 | |
| NMn | 5.504 | 5.370 | 5.246 | 5.155 | 5.137 | 4.864 | |
| R-factor | 0.155 | 0.234 | 0.247 | 0.028 | 0.162 | 0.137 | |
| RFe-O (Å) | 2.005 | 2.039 | 1.960 | 1.995 | 1.988 | 2.001 | |
| RFe-TM (Å) | 2.943 | 2.961 | 2.926 | 2.935 | 2.933 | 2.948 | |
| Fe K-edge | σ2Fe-O × 10−3 | 7.78 | 7.66 | 8.18 | 8.28 | 7.68 | 7.00 |
| σ2Fe-TM × 10−3 | 8.84 | 9.38 | 9.28 | 8.78 | 8.60 | 8.55 | |
| NFe | 7.697 | 7.675 | 7.757 | 7.697 | 6.377 | 6.219 | |
| R-factor | 0.012 | 0.089 | 0.081 | 0.009 | 0.021 | 0.007 |
| Rs | Qy1 | Qa1 | R1 | Qy2 | Qa2 | R2 | Qy3 | Qa3 | R3 |
|---|---|---|---|---|---|---|---|---|---|
| 0.0002 | 0.0132 | 0.538 | 701 | 4.6 × 10−5 | 0.566 | 6.98 | 9.6 × 10−4 | 0.789 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskiran, N.; Altundag, S.; Koleva, V.; Altin, E.; Arshad, M.; Avci, S.; Ates, M.N.; Altin, S.; Stoyanova, R. High-Performance Full Sodium Cells Based on MgO-Treated P2-Type Na0.67(Mn0.5Fe0.5)1−xCoxO2 Cathodes. Batteries 2023, 9, 497. https://doi.org/10.3390/batteries9100497
Taskiran N, Altundag S, Koleva V, Altin E, Arshad M, Avci S, Ates MN, Altin S, Stoyanova R. High-Performance Full Sodium Cells Based on MgO-Treated P2-Type Na0.67(Mn0.5Fe0.5)1−xCoxO2 Cathodes. Batteries. 2023; 9(10):497. https://doi.org/10.3390/batteries9100497
Chicago/Turabian StyleTaskiran, Nermin, Sebahat Altundag, Violeta Koleva, Emine Altin, Muhammad Arshad, Sevda Avci, Mehmet Nurullah Ates, Serdar Altin, and Radostina Stoyanova. 2023. "High-Performance Full Sodium Cells Based on MgO-Treated P2-Type Na0.67(Mn0.5Fe0.5)1−xCoxO2 Cathodes" Batteries 9, no. 10: 497. https://doi.org/10.3390/batteries9100497
APA StyleTaskiran, N., Altundag, S., Koleva, V., Altin, E., Arshad, M., Avci, S., Ates, M. N., Altin, S., & Stoyanova, R. (2023). High-Performance Full Sodium Cells Based on MgO-Treated P2-Type Na0.67(Mn0.5Fe0.5)1−xCoxO2 Cathodes. Batteries, 9(10), 497. https://doi.org/10.3390/batteries9100497