Effects of Different Charging Currents and Temperatures on the Voltage Plateau Behavior of Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
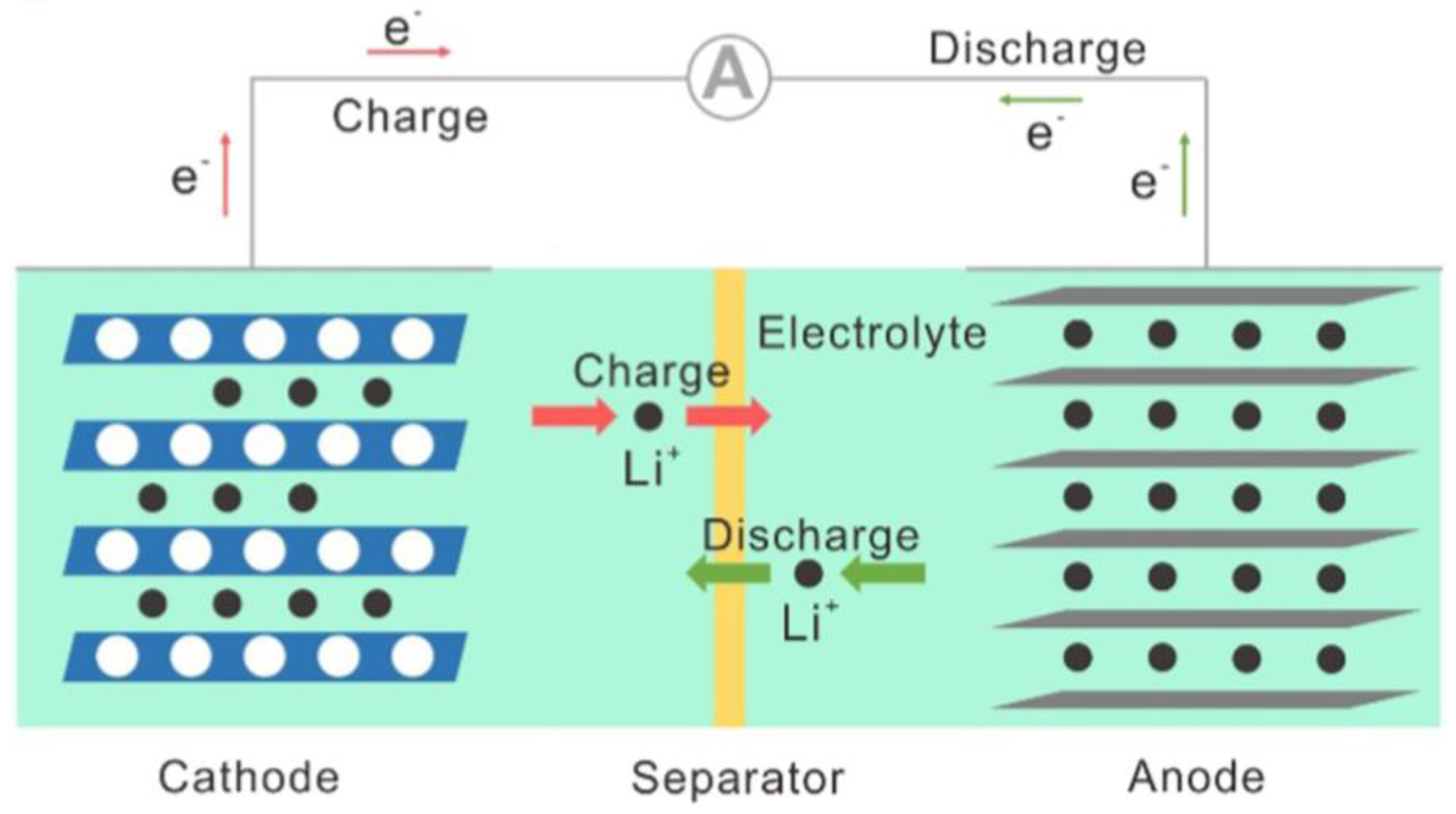
2.1. Object
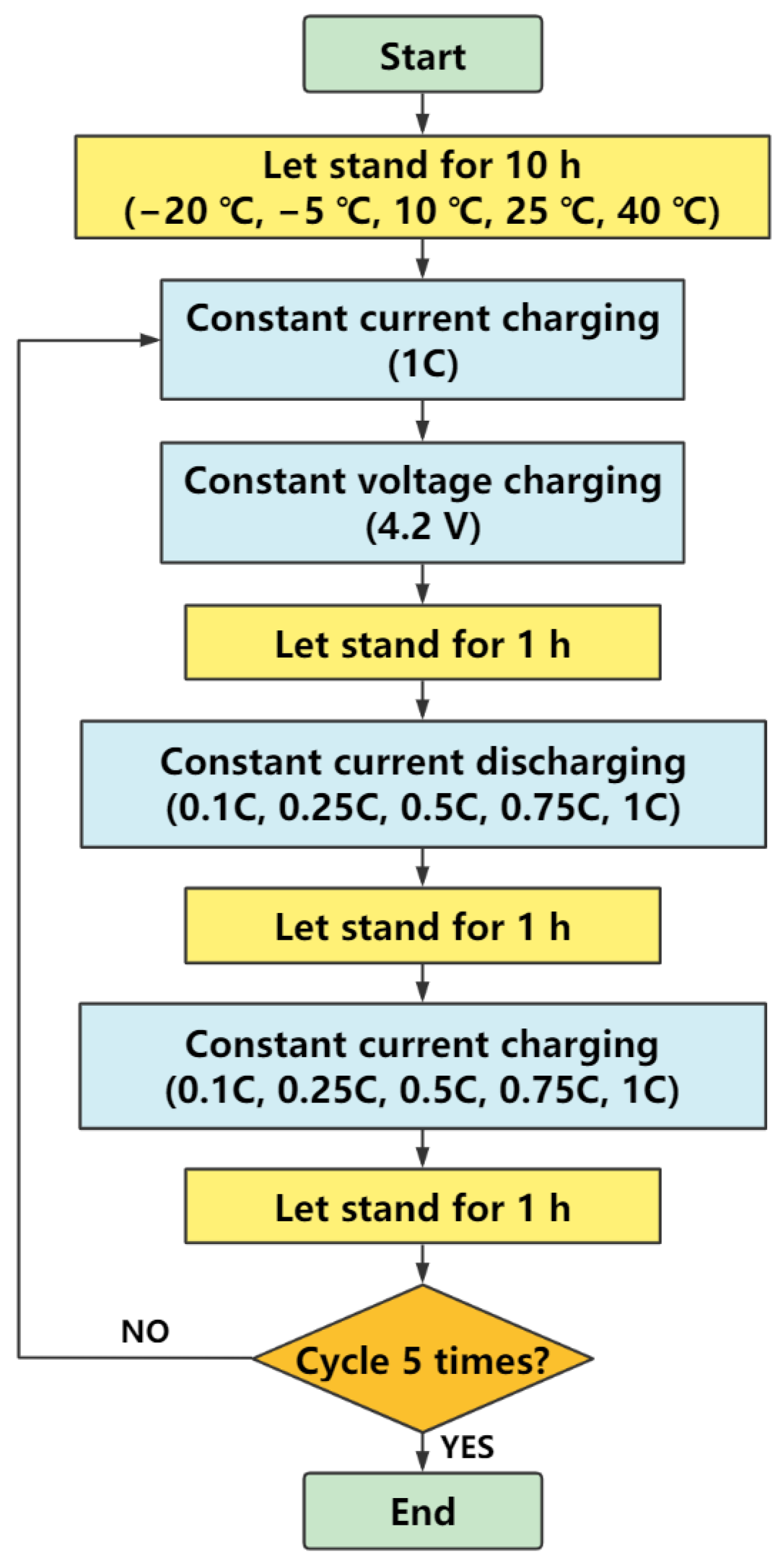
2.2. Method
2.3. Platform
3. Results
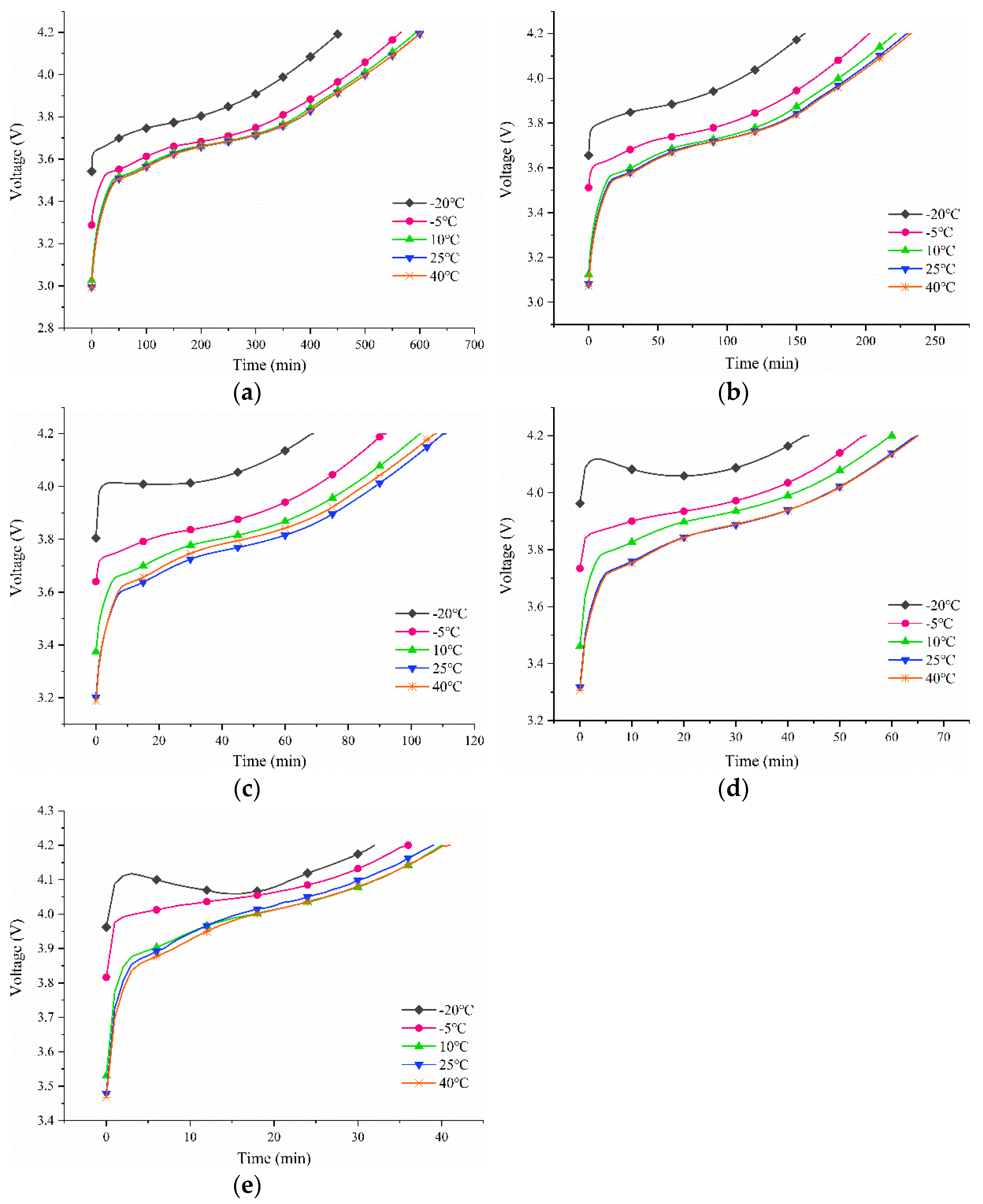
3.1. Variations in the Test Temperature’s Impact on the Battery Charge Voltage
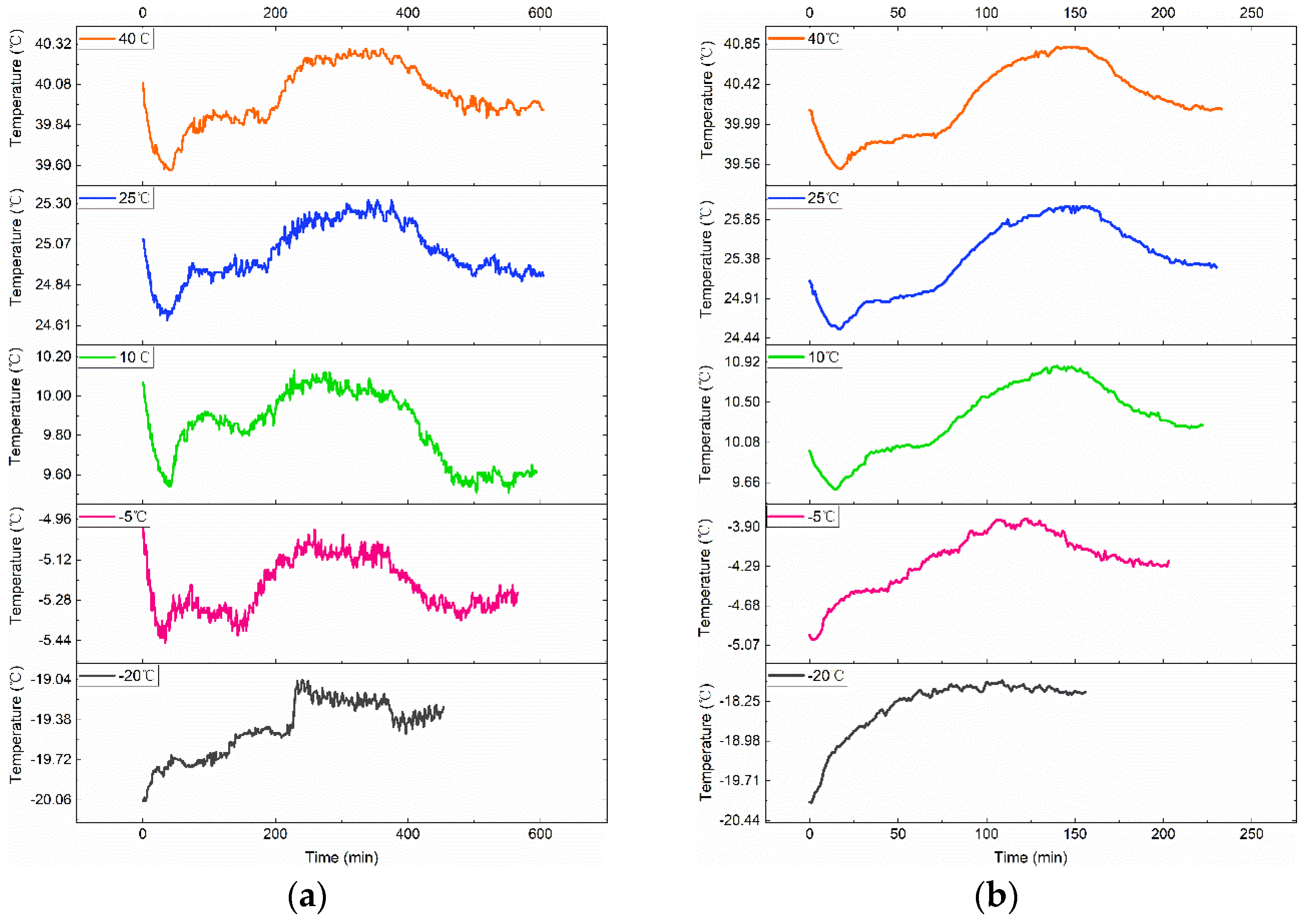
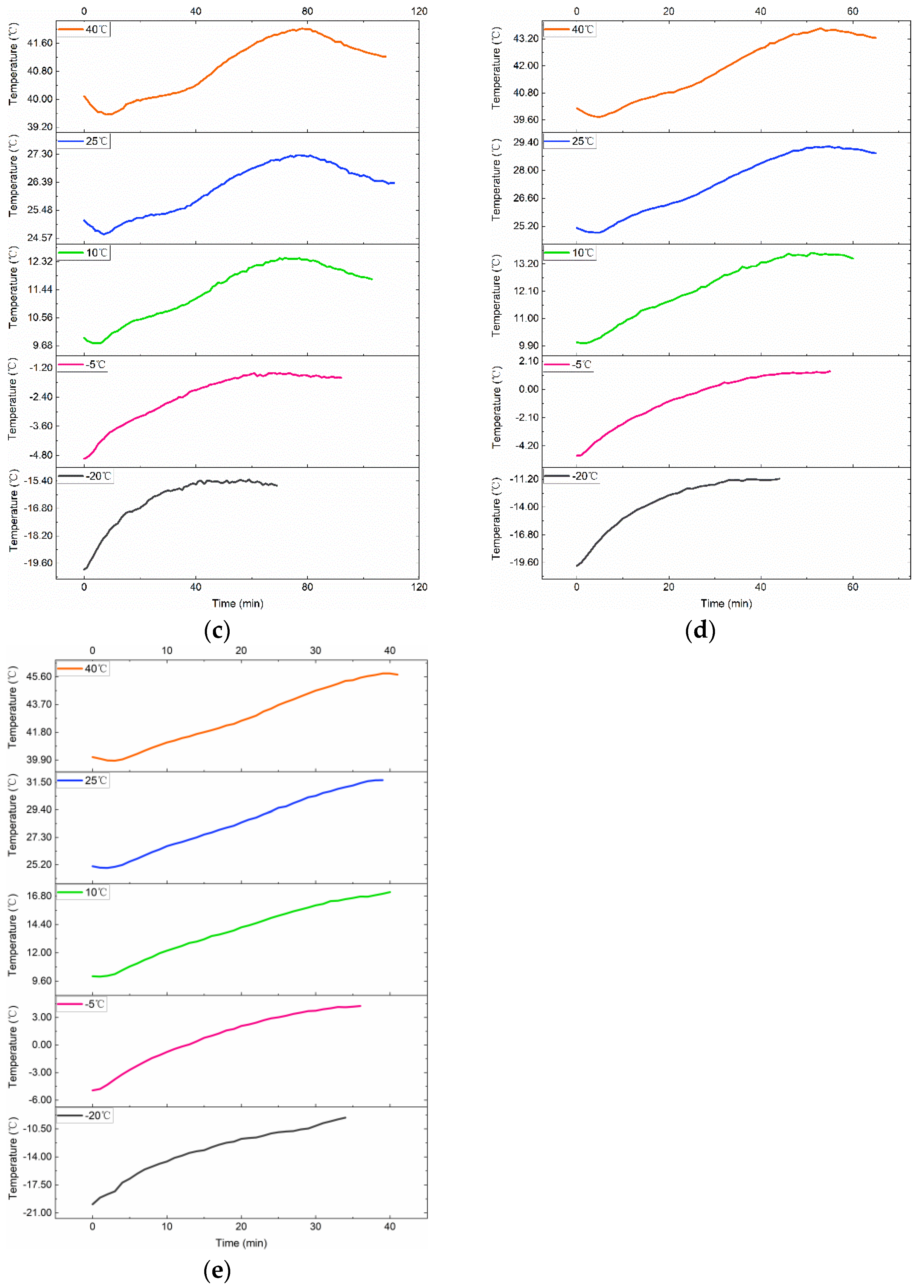
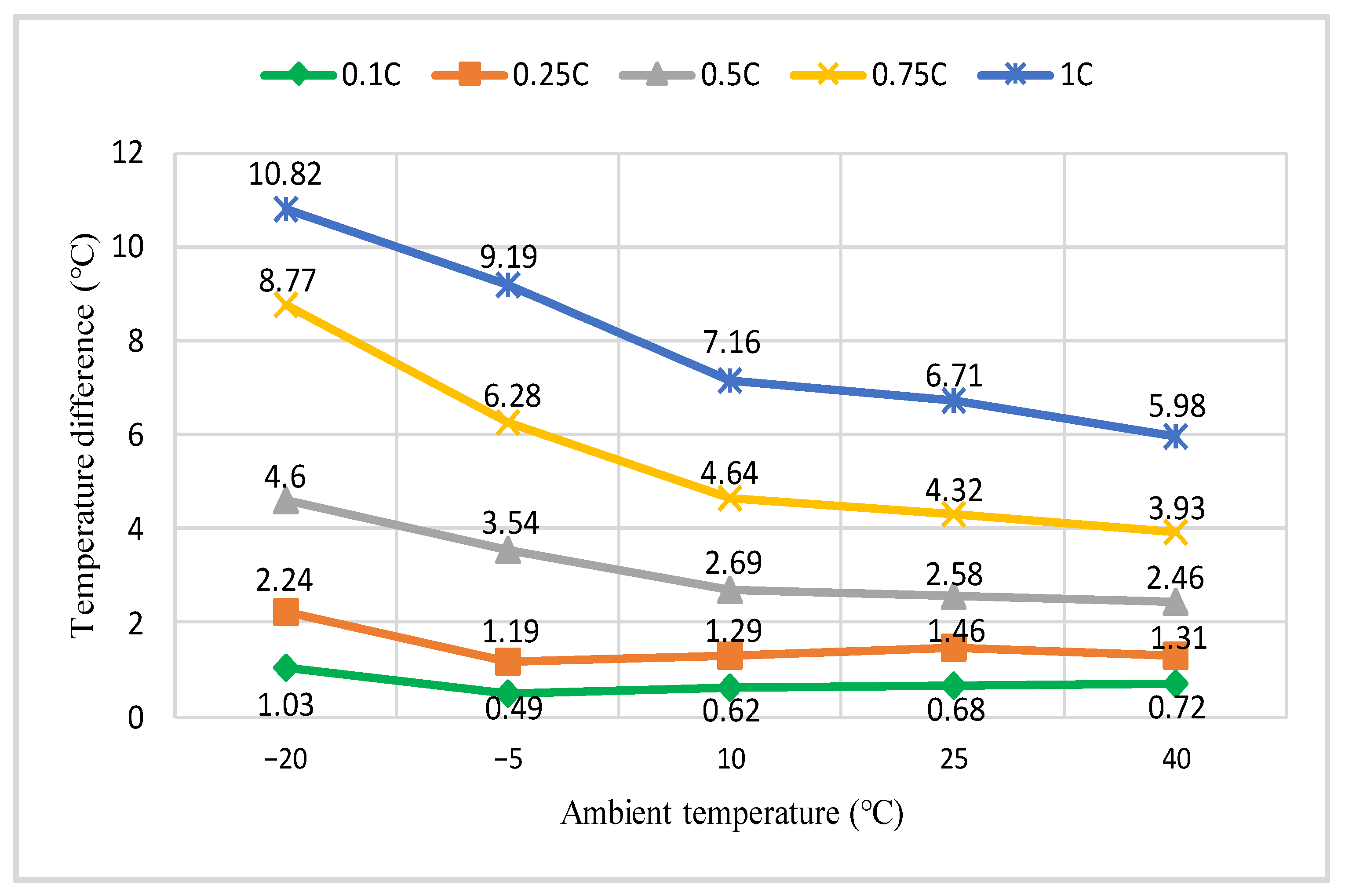
3.2. Various Test Temperatures’ Effects on the Rise in Temperature
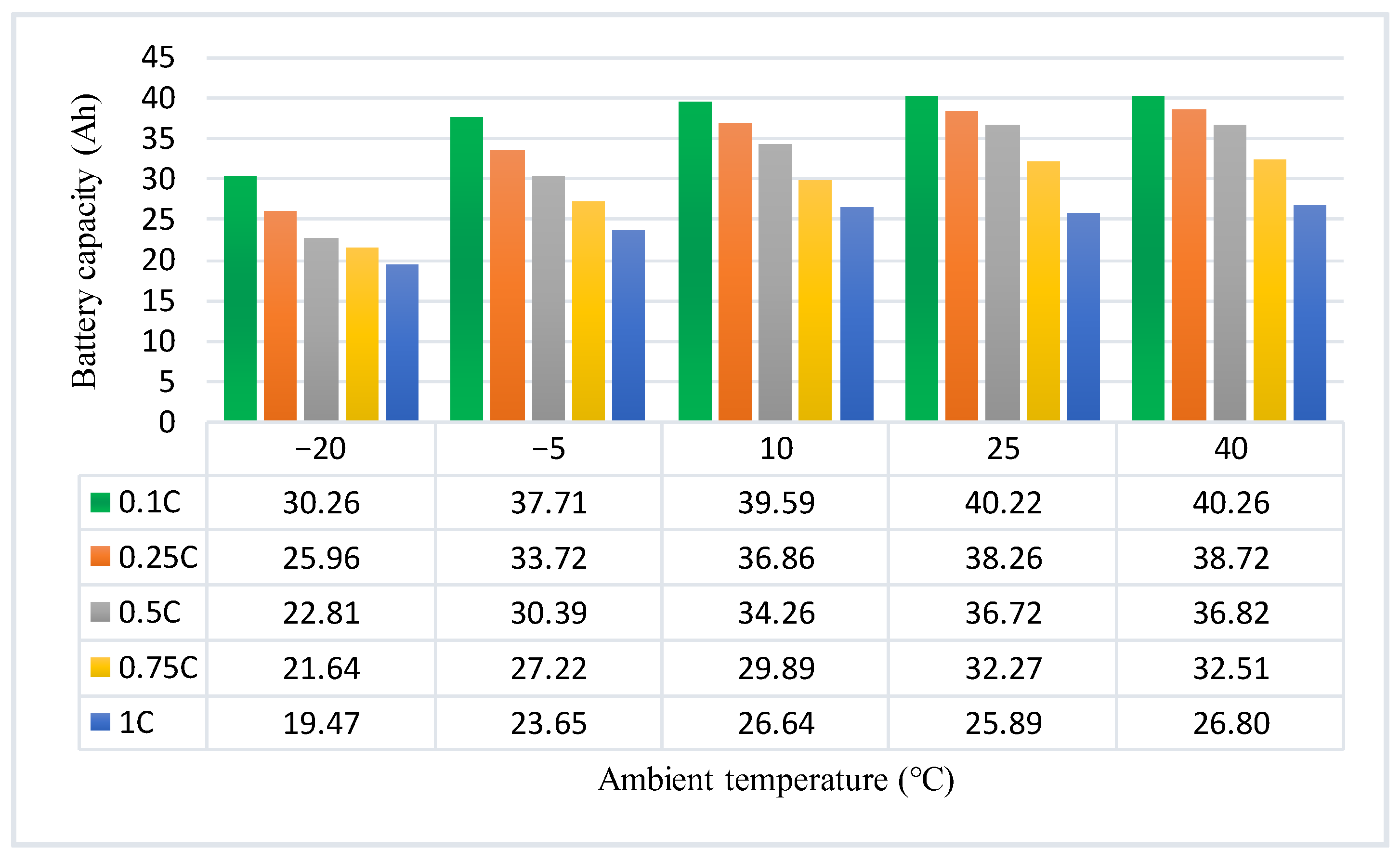
3.3. Effects of Various Test Temperatures and Charge Currents on the Capacity
4. Discussion
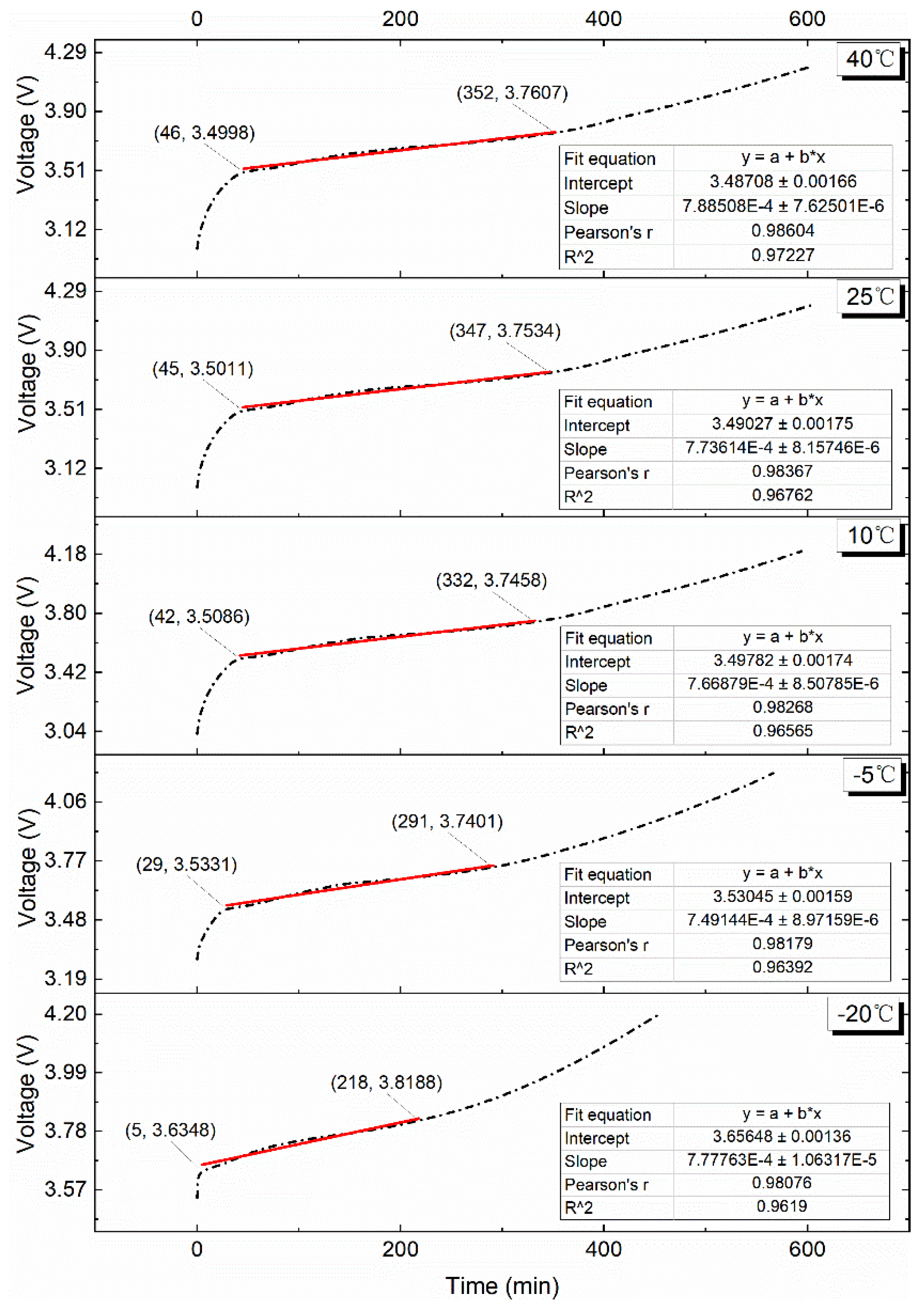
4.1. Brief Introduction of VPP and Its Division Method
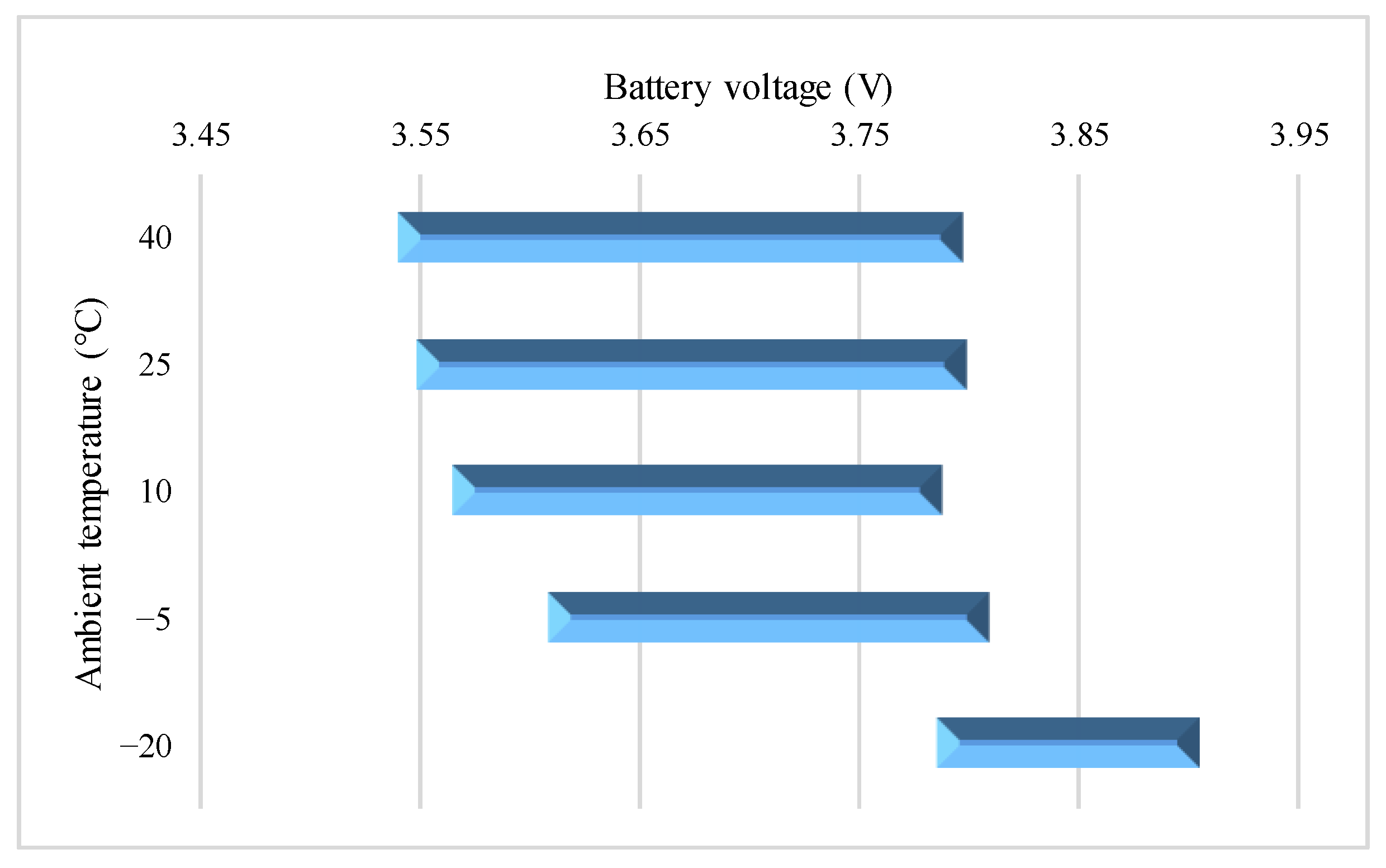
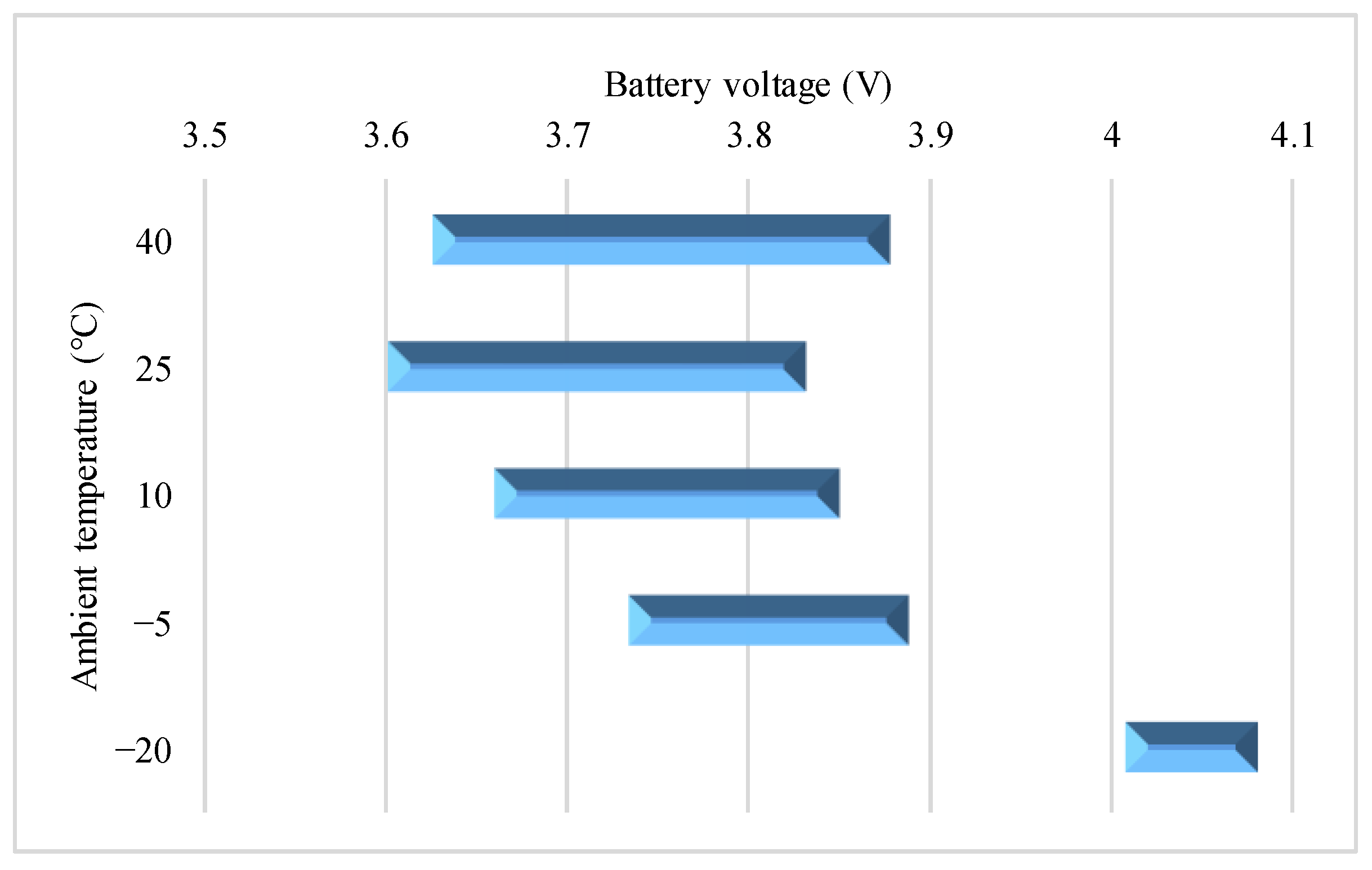
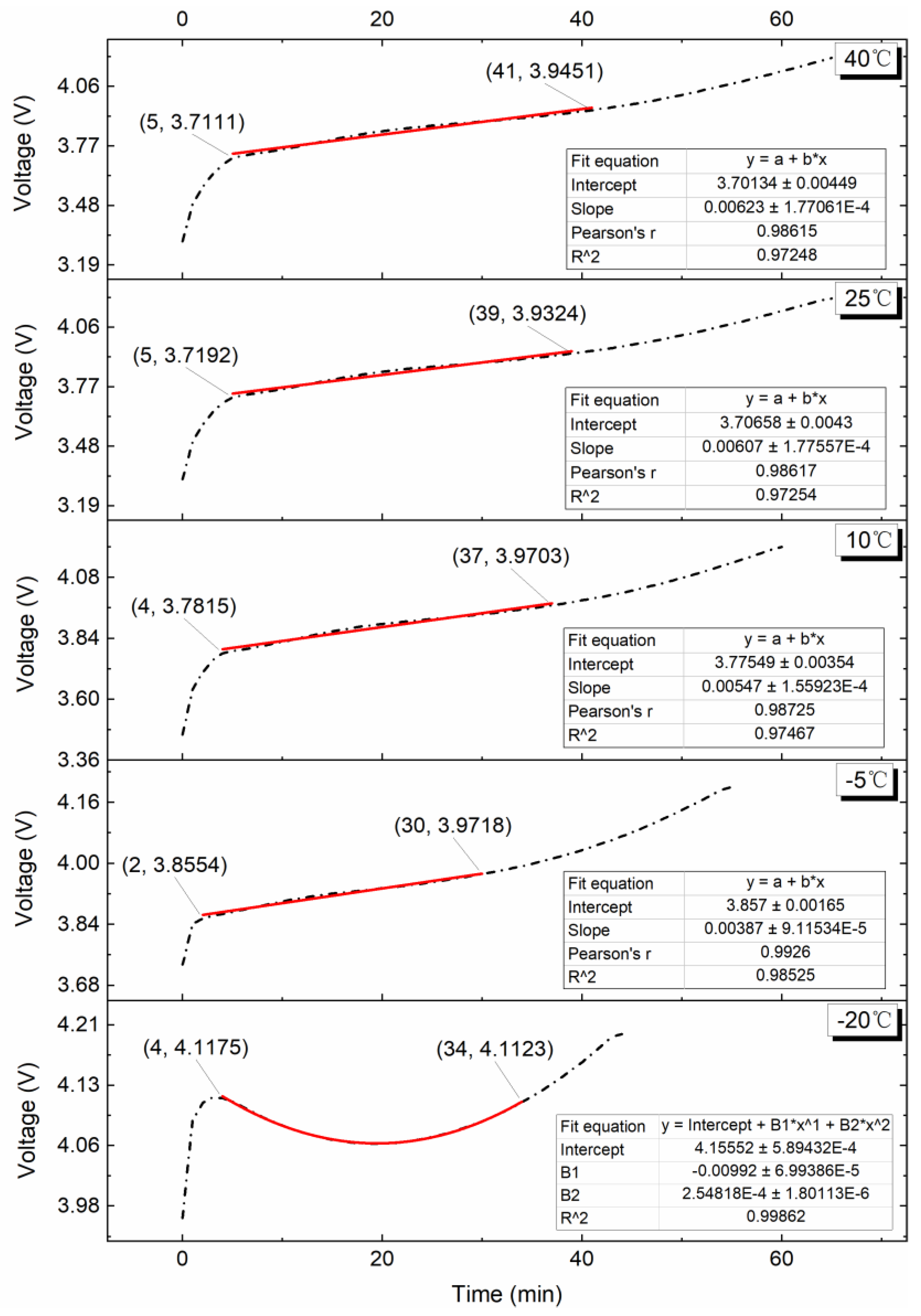
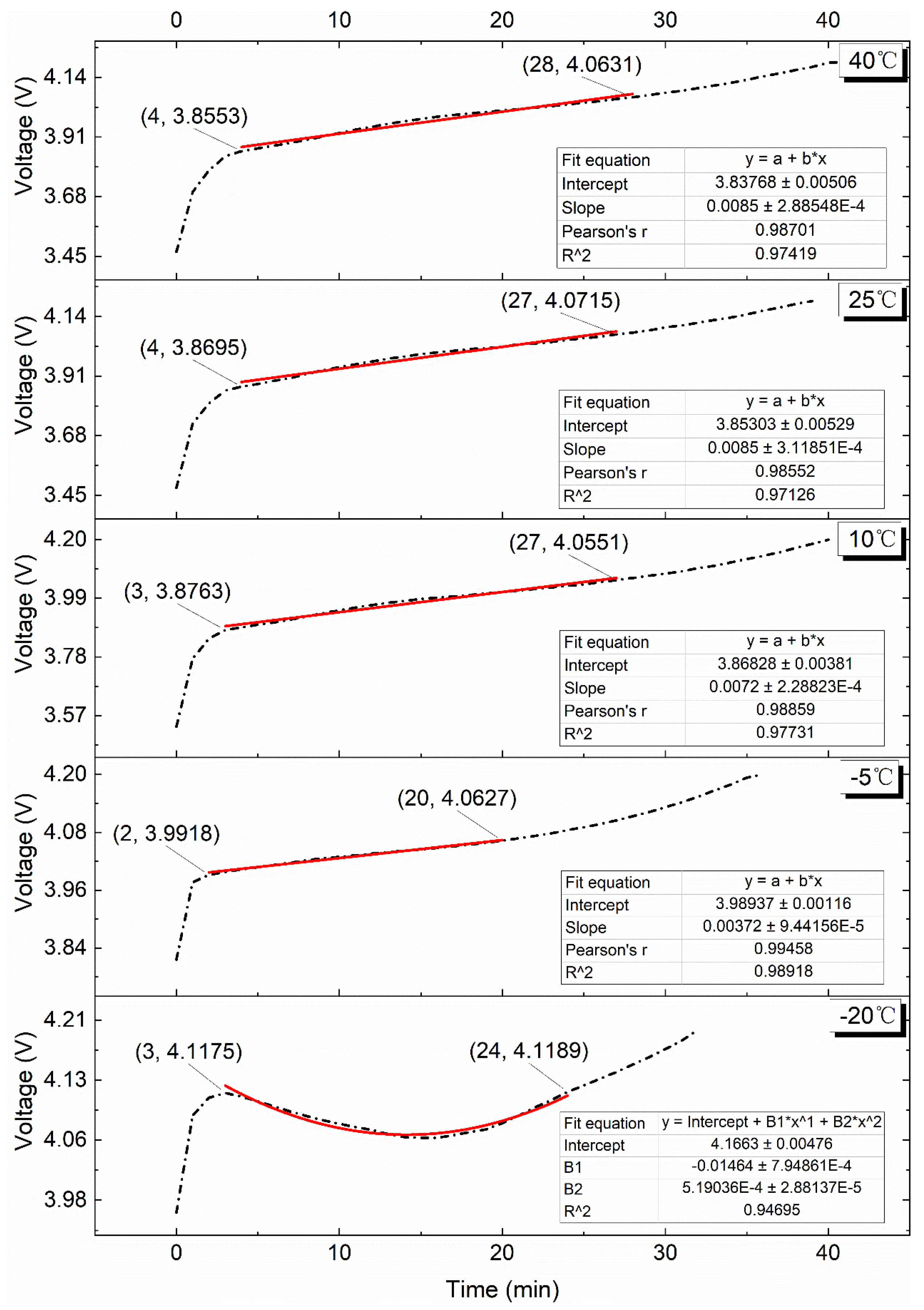
4.2. Effect of Various Test Temperatures on the VPP
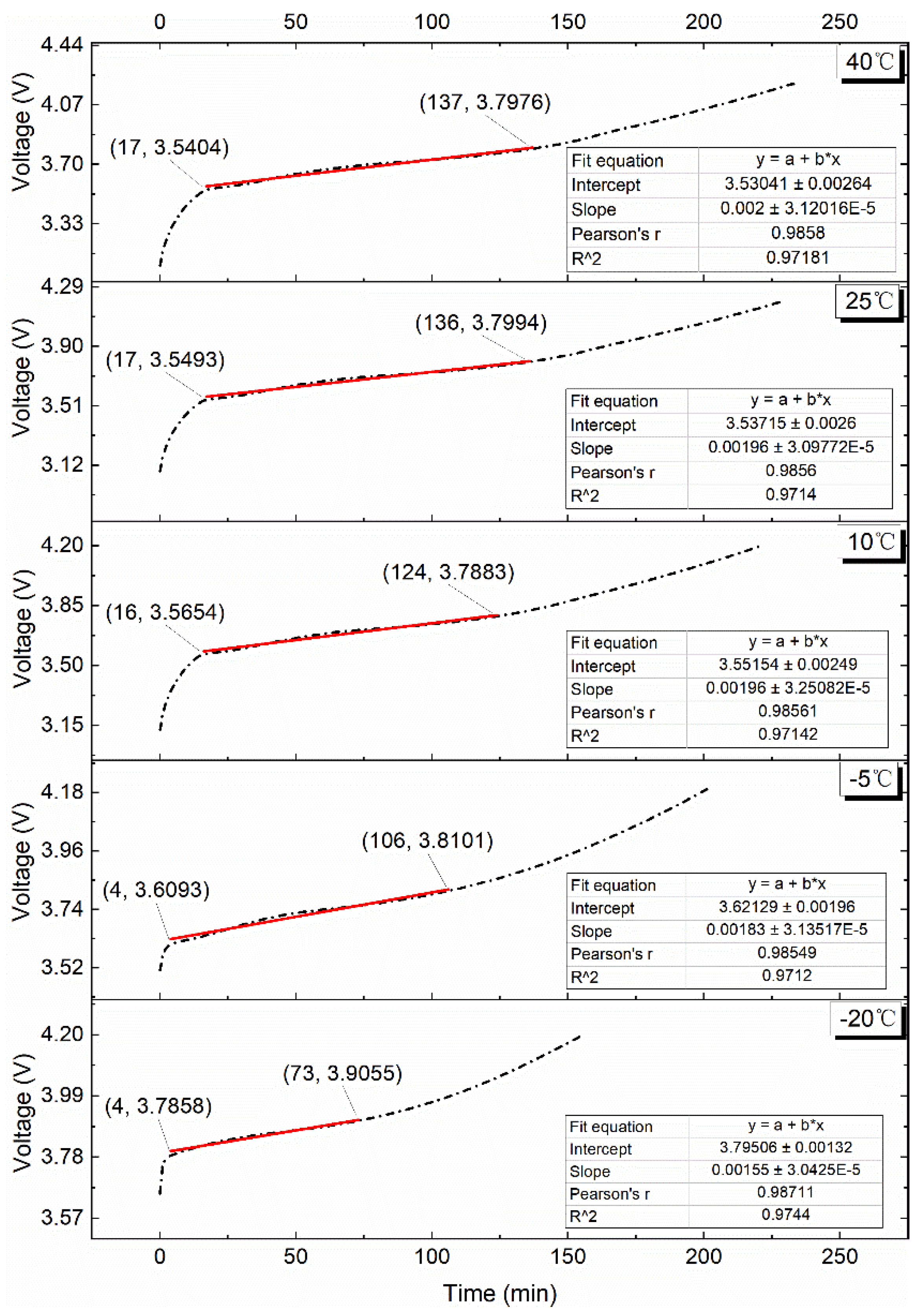
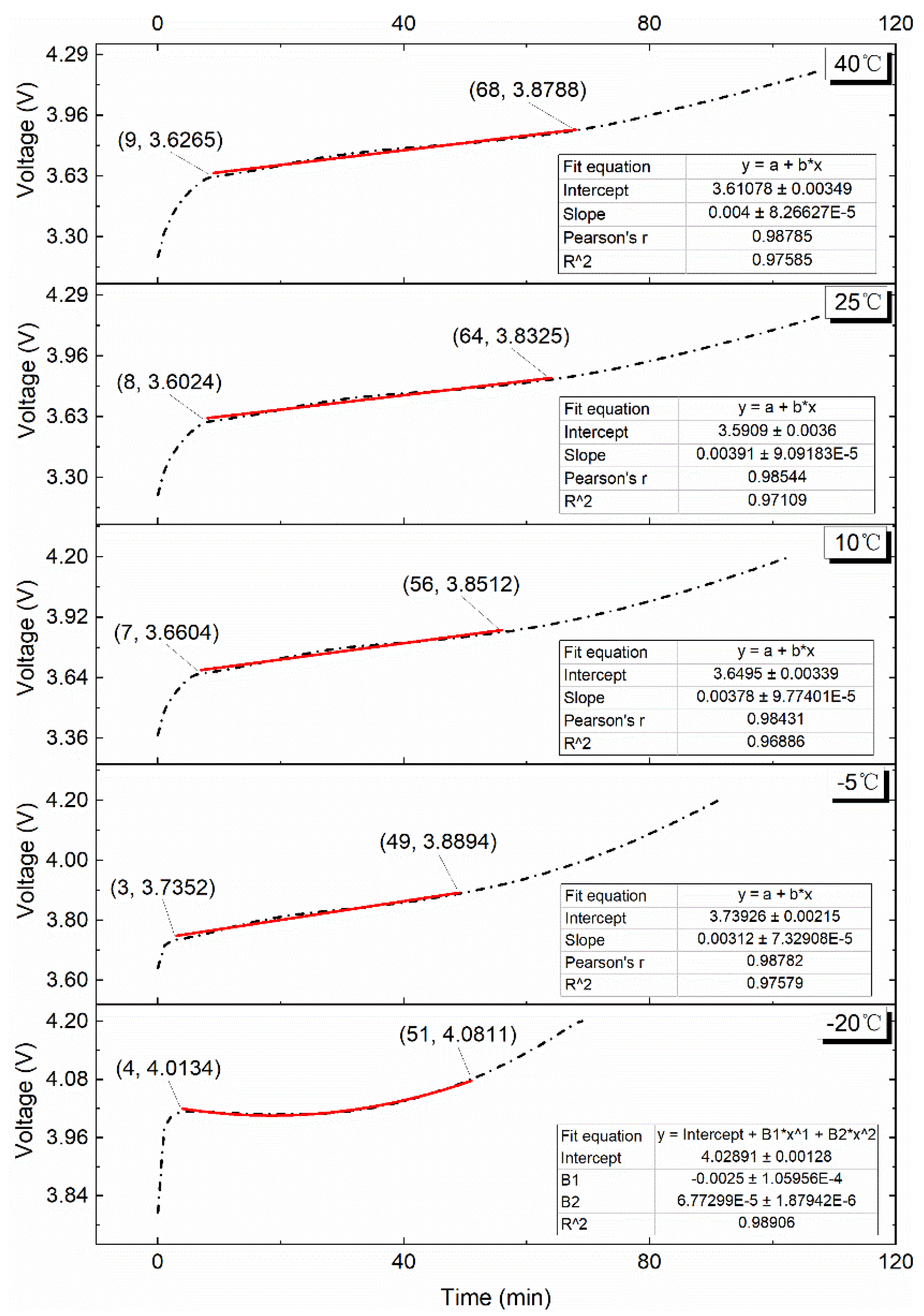
4.2.1. 0.10C Charge
4.2.2. 0.25C Charge
4.2.3. 0.50C Charge
4.2.4. 0.75C Charge
4.2.5. 1.00C Charge
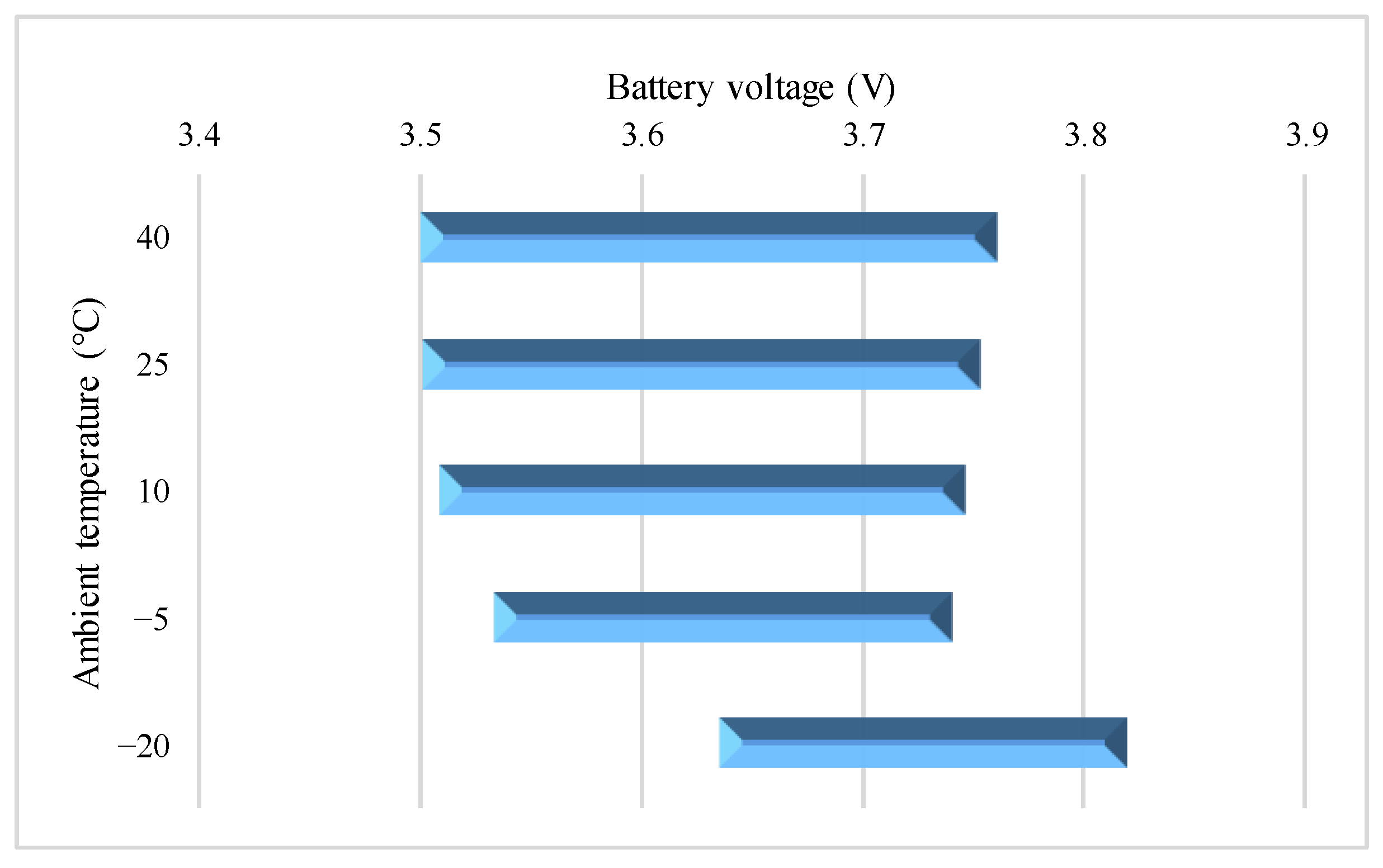
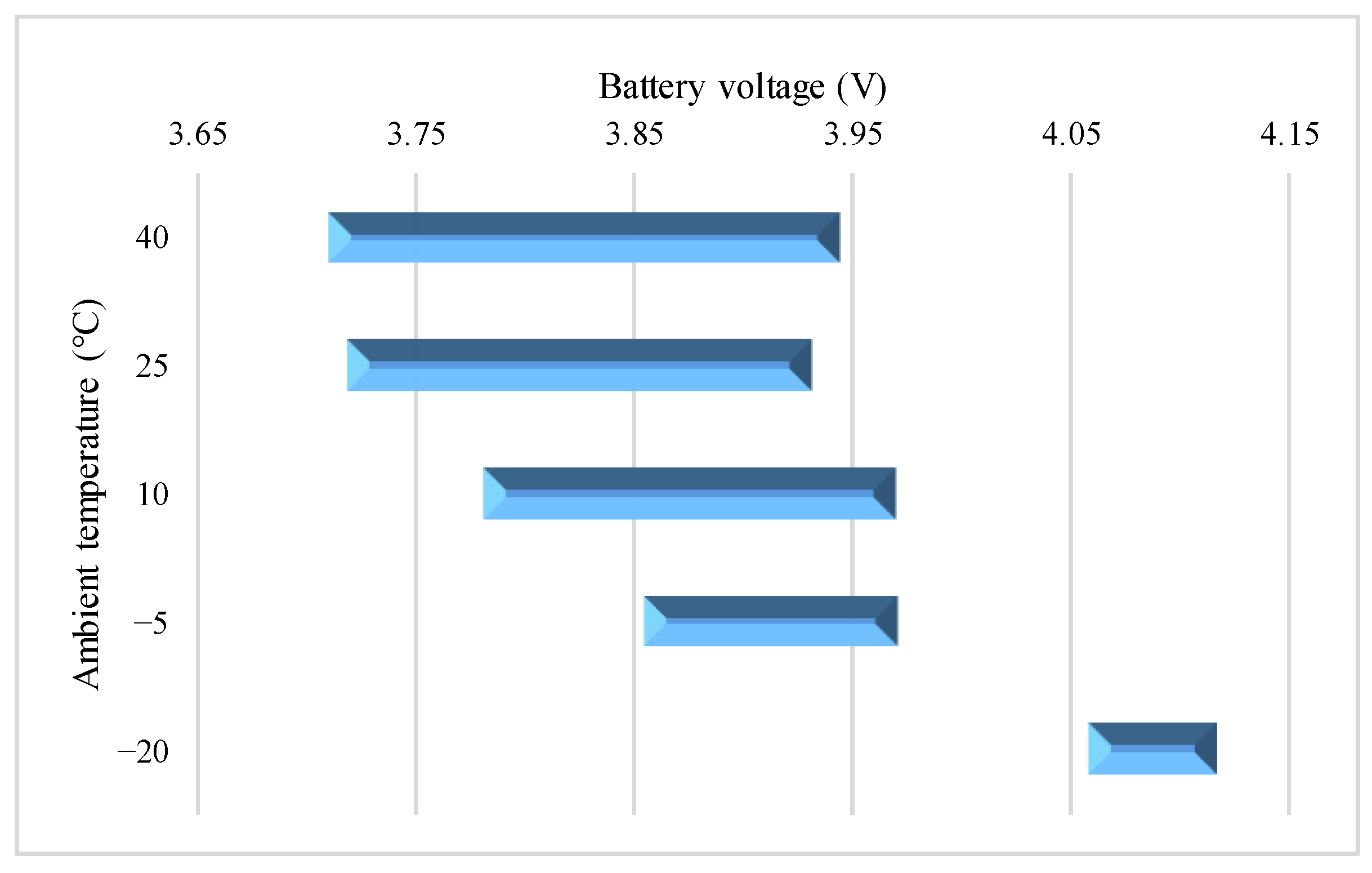
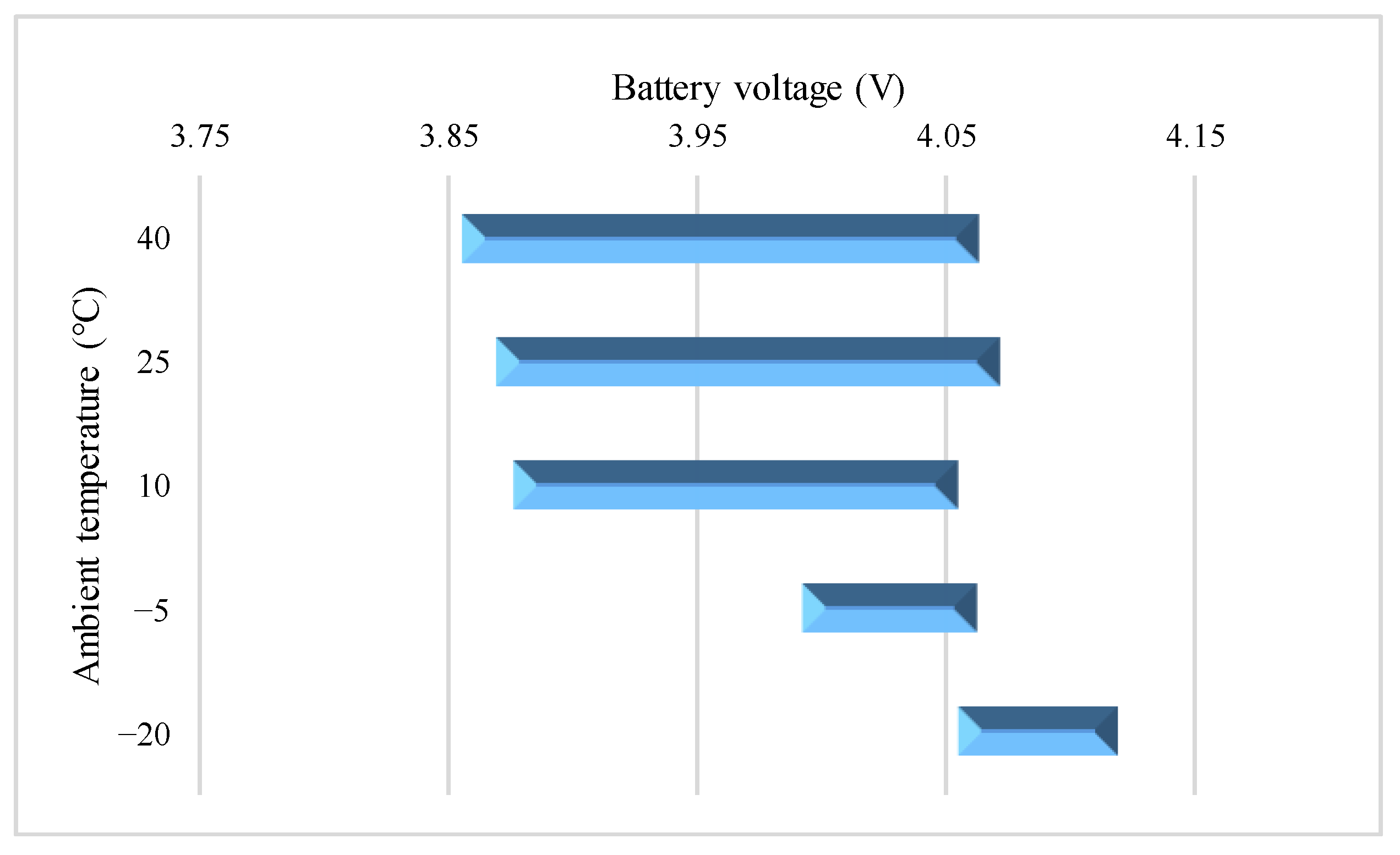
4.3. Study on Surface Temperature Range during the VPP
4.4. Charge Energy Comparison during the VPP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knez, M.; Zevnik, G.K.; Obrecht, M. A review of available chargers for electric vehicles: United States of America, European Union, and Asia. Renew. Sustain. Energy Rev. 2019, 109, 284–293. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Khajepour, A.; Song, J.C. A comprehensive review of the key technologies for pure electric vehicles. Energy 2019, 182, 824–839. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, J.L.; Kintner-Meyer, M.C.W.; Lu, X.C.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Liu, L.; Lin, C.J.; Fan, B.; Wang, F.; Lao, L.; Yang, P.X. A new method to determine the heating power of ternary cylindrical lithium ion batteries with highly repeatable thermal runaway test characteristics. J. Power Sources 2020, 472, 228503. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Wood, V. On the use of electrochemical impedance spectroscopy to characterize and model the aging phenomena of lithium-ion batteries: A critical review. J. Power Sources 2021, 505, 229860. [Google Scholar] [CrossRef]
- Wang, X.X.; Liu, S.R.; Zhang, Y.J.; Lv, S.S.; Ni, H.J.; Deng, Y.L.; Yuan, Y.N. A review of the power battery thermal management system with different cooling, heating and coupling system. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- Philippot, M.; Alvarez, G.; Ayerbe, E.; Van Mierlo, J.; Messagie, M. Eco-efficiency of a lithium-ion battery for electric vehicles: Influence of manufacturing country and commodity prices on GHG emissions and costs. Batteries 2019, 5, 23. [Google Scholar] [CrossRef]
- Ohzuku, T.; Brodd, R.J. An overview of positive-electrode materials for advanced lithium-ion batteries. J. Power Sources 2007, 174, 449–456. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Liu, P.; Song, L.B.; Cao, Z.; Du, J.L.; Zhou, C.F.; Jiang, P. The correlation between structure and thermal properties of nickel-rich ternary cathode materials: A review. Ionics 2021, 27, 3207–3217. [Google Scholar] [CrossRef]
- Shao, Y.J.; Huang, B.; Liu, Q.B.; Liao, S.J. Preparation and modification of Ni-Co-Mn ternary cathode materials. Prog. Chem. 2018, 30, 410–419. [Google Scholar]
- Williard, N.; He, W.; Hendricks, C.; Pecht, M. Lessons learned from the 787 Dreamliner issue on lithium-ion battery reliability. Energies 2013, 6, 4682–4695. [Google Scholar] [CrossRef]
- Huang, H. Cycle life fading of LiFePO4 lithium-ion battery and its life prediction. Chin. J. Power Sources 2022, 46, 376–379. [Google Scholar]
- Chen, X.; Lv, S.S.; Pei, Y.; Chen, L.; Ni, H.J. Influence of temperature on the LiFePO4 power batteries’ discharge voltage values. New Chem. Mater. 2015, 43, 202–203. [Google Scholar]
- Ye, Y.; Saw, L.H.; Shi, Y.; Tay, A.A.O. Numerical analyses on optimizing a heat pipe thermal management system for lithium-ion batteries during fast charging. Appl. Therm. Eng. 2015, 86, 281–291. [Google Scholar] [CrossRef]
- An, Z.J.; Jia, L.; Ding, Y.; Dang, C.; Li, X.J. A review on lithium-ion power battery thermal management technologies and thermal safety. J. Therm. Sci. 2017, 26, 391–412. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Shi, X.; Wang, H.; Lv, H.; Zheng, L.; Chen, J.; Qi, L.; Wu, T.; Wang, C. The influence of cutoff charge-voltage on performance of lithium manganite/lithium titanate lithium-ion battery for power applications. Mater. Technol. 2016, 31, 642–645. [Google Scholar] [CrossRef]
- Wang, C.X. Study on Internal Thermal Strain Characteristics of Lithium Batteries at High C Rates under Low Temperature. Master’s Thesis, Civil Aviation Flight University of China, Guanghan, China, 2020. [Google Scholar]
- Liu, Z.W.; Liu, X.L.; Hao, H.; Zhao, F.Q.; Amer, A.A.; Babiker, H. Research on the critical issues for power battery reusing of new energy vehicles in China. Energies 2020, 13, 1932. [Google Scholar] [CrossRef]
- Gantenbein, S.; Schönleber, M.; Weiss, M.; Ivers-Tiffée, E. Capacity fade in lithium-ion batteries and cyclic aging over various state-of-charge ranges. Sustainability 2019, 11, 6697. [Google Scholar] [CrossRef]
- Situ, W.F.; Yang, X.Q.; Li, X.X.; Zhang, G.Q.; Rao, M.M.; Wei, C.; Huang, Z. Effect of high temperature environment on the performance of LiNi0.5Co0.2Mn0.3O2 battery. Int. J. Heat Mass Transf. 2017, 104, 743–748. [Google Scholar] [CrossRef]
- Bloom, I.; Walker, L.K.; Basco, J.K.; Abraham, D.P.; Christophersen, J.P.; Ho, C.D. Differential voltage analyses of high-power lithium-ion cells. 4. Cells containing NMC. J. Power Sources 2010, 195, 877–882. [Google Scholar] [CrossRef]
- Jia, X.L. The Study on Estimation Method about the Capacity of Power Lithium-Ion Battery. Master’s Thesis, Shenzhen Graduate School, Harbin Institute of Technology, Shenzhen, China, 2016. [Google Scholar]
- Zhang, Y.P.; Wu, L.L.; Zhao, J.B.; Yu, W.Y. A facile precursor-separated method to synthesize nano-crystalline LiFePO4/C cathode materials. J. Electroanal. Chem. 2014, 719, 1–6. [Google Scholar] [CrossRef]
- Li, C.L.; Cui, N.X.; Wang, C.Y.; Zhang, C.H. Simplified electrochemical lithium-ion battery model with variable solid-phase diffusion and parameter identification over wide temperature range. J. Power Sources 2021, 497, 229900. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.D.; Tao, P.; Song, C.Y.; Wu, J.B.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, S.L.; Wu, B.; Fernandez, C.; Xiong, X.; Coffie-Ken, J. A state-of-charge estimation method of the power lithium-ion battery in complex conditions based on adaptive square root extended Kalman filter. Energy 2021, 219, 119603. [Google Scholar] [CrossRef]
- Wang, S.L.; Stroe, D.; Fernandez, C.; Yu, C.M.; Zou, C.Y.; Li, X.X. A novel energy management strategy for the ternary lithium batteries based on the dynamic equivalent circuit modeling and differential Kalman filtering under time-varying conditions. J. Power Sources 2020, 450, 227652. [Google Scholar] [CrossRef]
- Liu, X. Test Bench Establishing and Nonlinear Modeling of Lithium-Ion Battery and Supercapacitor. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2019. [Google Scholar]
- Li, B.; Xia, D.G. Anionic redox in rechargeable lithium batteries. Adv. Mater. 2017, 29, 1701054. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Lux, S.F.; Schmuck, M.; Appetecchi, G.B.; Passerini, S.; Winter, M.; Balducci, A. Lithium insertion in graphite from ternary ionic liquid–lithium salt electrolytes: II. Evaluation of specific capacity and cycling efficiency and stability at room temperature. J. Power Sources 2009, 192, 606–611. [Google Scholar] [CrossRef]
- Gholami, J.; Barzoki, M.F. Electrochemical modeling and parameter sensitivity of lithium-ion battery at low temperature. J. Energy Storage 2021, 43, 103189. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, Y.J.; Ni, H.J.; Lv, S.S.; Zhang, F.B.; Zhu, Y.; Yuan, Y.N.; Deng, Y.L. Influence of different ambient temperatures on the discharge performance of square ternary lithium-ion batteries. Energies 2022, 15, 5348. [Google Scholar] [CrossRef]
- Lei, Z.G.; Zhang, Y.W.; Lei, X.G. Temperature uniformity of a heated lithium-ion battery cell in cold climate. Appl. Therm. Eng. 2018, 129, 148–154. [Google Scholar] [CrossRef]

| Type | Specific Energy | Platform Voltage | Advantages | Disadvantages |
|---|---|---|---|---|
| LiFePO4 battery | 120–180 Wh kg−1 | 3.2 V | High temperature resistance, low cost, impact resistance | Poor consistency, poor low temperature performance |
| NCM ternary Li-ion battery | 200–300 Wh kg−1 | 3.7 V | High specific energy, low temperature resistance, good discharge linearity | Poor thermal stability |
| Performance | Unit | Parameter |
|---|---|---|
| Nominal voltage | V | 3.65 |
| Working voltage | V | 2.75–4.2 |
| Rated capacity | Ah | 40 |
| Standard internal resistance | mΩ | 0.7 |
| Specific energy | Wh kg−1 | 206 |
| Size | mm | 148 × 91 × 27 |
| Weight | kg | 0.7 |
| Charge Rate | Ambient Temperature (°C) | σ (Wh) | Fitting Accuracy (%) | ||
|---|---|---|---|---|---|
| 0.1C | 40 | 74.32 | 74.40 | 0.0566 | 99.8925 |
| 25 | 73.29 | 73.32 | 0.0212 | 99.9591 | |
| 10 | 70.41 | 70.44 | 0.0212 | 99.9574 | |
| −5 | 63.69 | 63.75 | 0.0424 | 99.9059 | |
| −20 | 53.15 | 53.20 | 0.0354 | 99.9060 | |
| 0.25C | 40 | 73.73 | 73.68 | 0.0354 | 99.9321 |
| 25 | 73.20 | 73.18 | 0.0141 | 99.9727 | |
| 10 | 66.41 | 66.37 | 0.0283 | 99.9397 | |
| −5 | 63.34 | 63.25 | 0.0636 | 99.8577 | |
| −20 | 44.34 | 44.39 | 0.0354 | 99.8874 | |
| 0.5C | 40 | 70.98 | 71.03 | 0.0354 | 99.9296 |
| 25 | 69.62 | 69.64 | 0.0141 | 99.9713 | |
| 10 | 61.60 | 61.56 | 0.0283 | 99.9350 | |
| −5 | 58.57 | 58.59 | 0.0141 | 99.9659 | |
| −20 | 63.05 | 63.06 | 0.0071 | 99.9841 | |
| 0.75C | 40 | 66.21 | 66.18 | 0.0212 | 99.9547 |
| 25 | 65.32 | 65.34 | 0.0141 | 99.9694 | |
| 10 | 64.16 | 64.22 | 0.0424 | 99.9066 | |
| −5 | 54.88 | 54.91 | 0.0212 | 99.9454 | |
| −20 | 61.18 | 61.24 | 0.0424 | 99.9020 | |
| 1C | 40 | 63.61 | 63.62 | 0.0071 | 99.9843 |
| 25 | 61.09 | 61.05 | 0.0283 | 99.9345 | |
| 10 | 63.62 | 63.65 | 0.0212 | 99.9529 | |
| −5 | 48.43 | 48.37 | 0.0424 | 99.8760 | |
| −20 | 57.44 | 57.21 | 0.1626 | 99.5980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Y.; Deng, Y.; Yuan, Y.; Zhang, F.; Lv, S.; Zhu, Y.; Ni, H. Effects of Different Charging Currents and Temperatures on the Voltage Plateau Behavior of Li-Ion Batteries. Batteries 2023, 9, 42. https://doi.org/10.3390/batteries9010042
Wang X, Zhang Y, Deng Y, Yuan Y, Zhang F, Lv S, Zhu Y, Ni H. Effects of Different Charging Currents and Temperatures on the Voltage Plateau Behavior of Li-Ion Batteries. Batteries. 2023; 9(1):42. https://doi.org/10.3390/batteries9010042
Chicago/Turabian StyleWang, Xingxing, Yujie Zhang, Yelin Deng, Yinnan Yuan, Fubao Zhang, Shuaishuai Lv, Yu Zhu, and Hongjun Ni. 2023. "Effects of Different Charging Currents and Temperatures on the Voltage Plateau Behavior of Li-Ion Batteries" Batteries 9, no. 1: 42. https://doi.org/10.3390/batteries9010042
APA StyleWang, X., Zhang, Y., Deng, Y., Yuan, Y., Zhang, F., Lv, S., Zhu, Y., & Ni, H. (2023). Effects of Different Charging Currents and Temperatures on the Voltage Plateau Behavior of Li-Ion Batteries. Batteries, 9(1), 42. https://doi.org/10.3390/batteries9010042








