Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Electrolyte Solutions
2.2. Measurement of Electrolyte Solutions
3. Result and Discussion
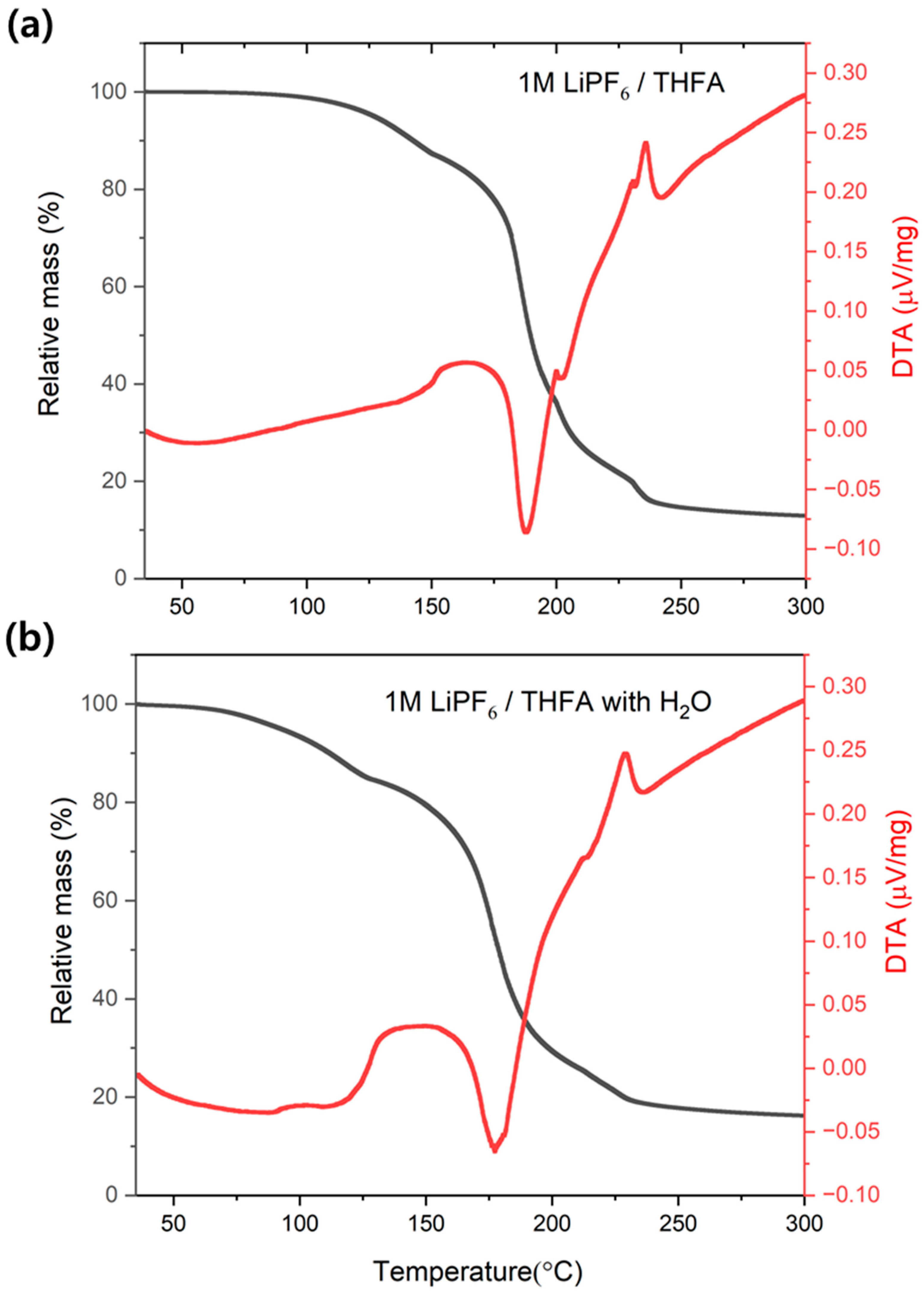
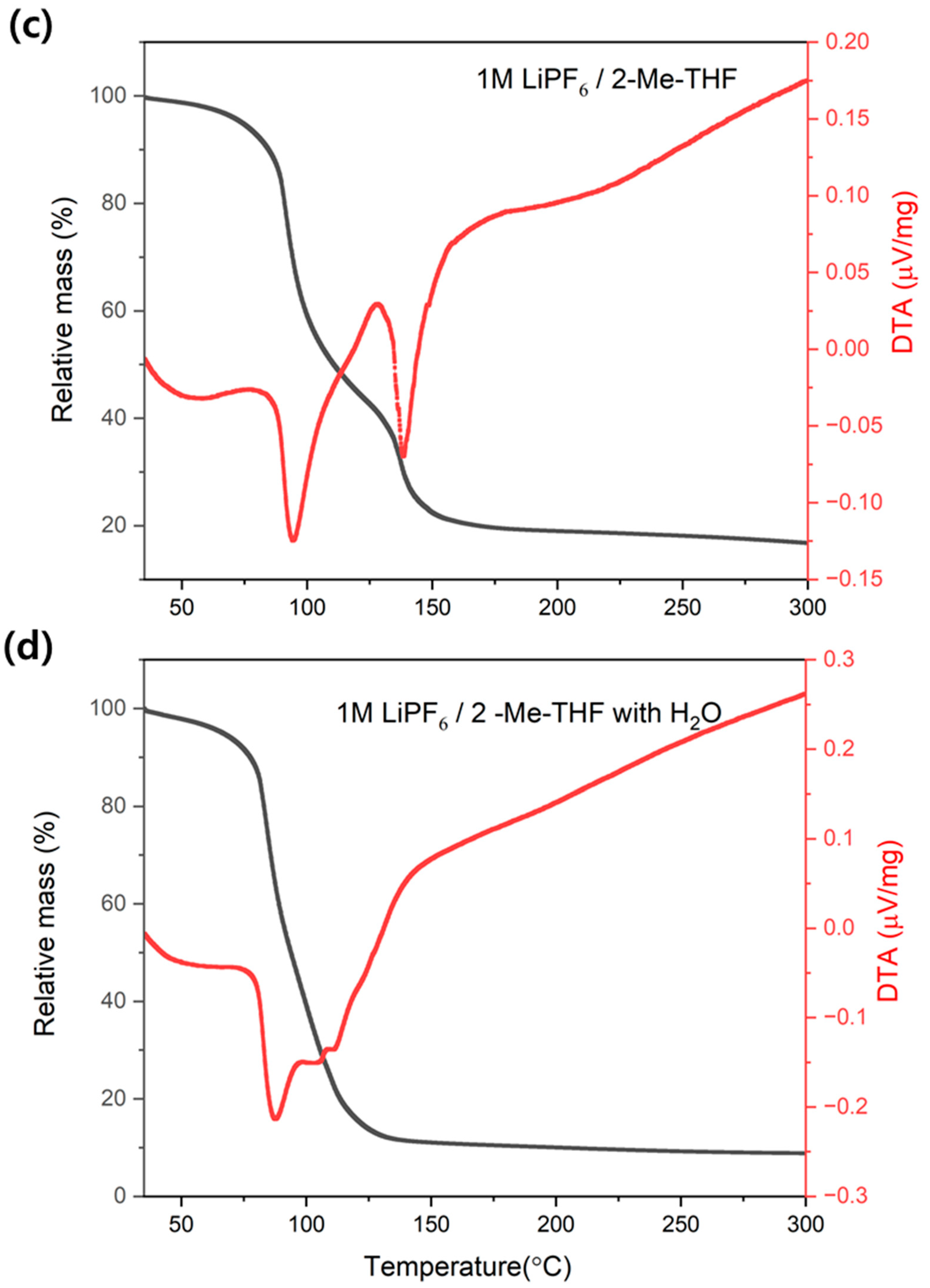
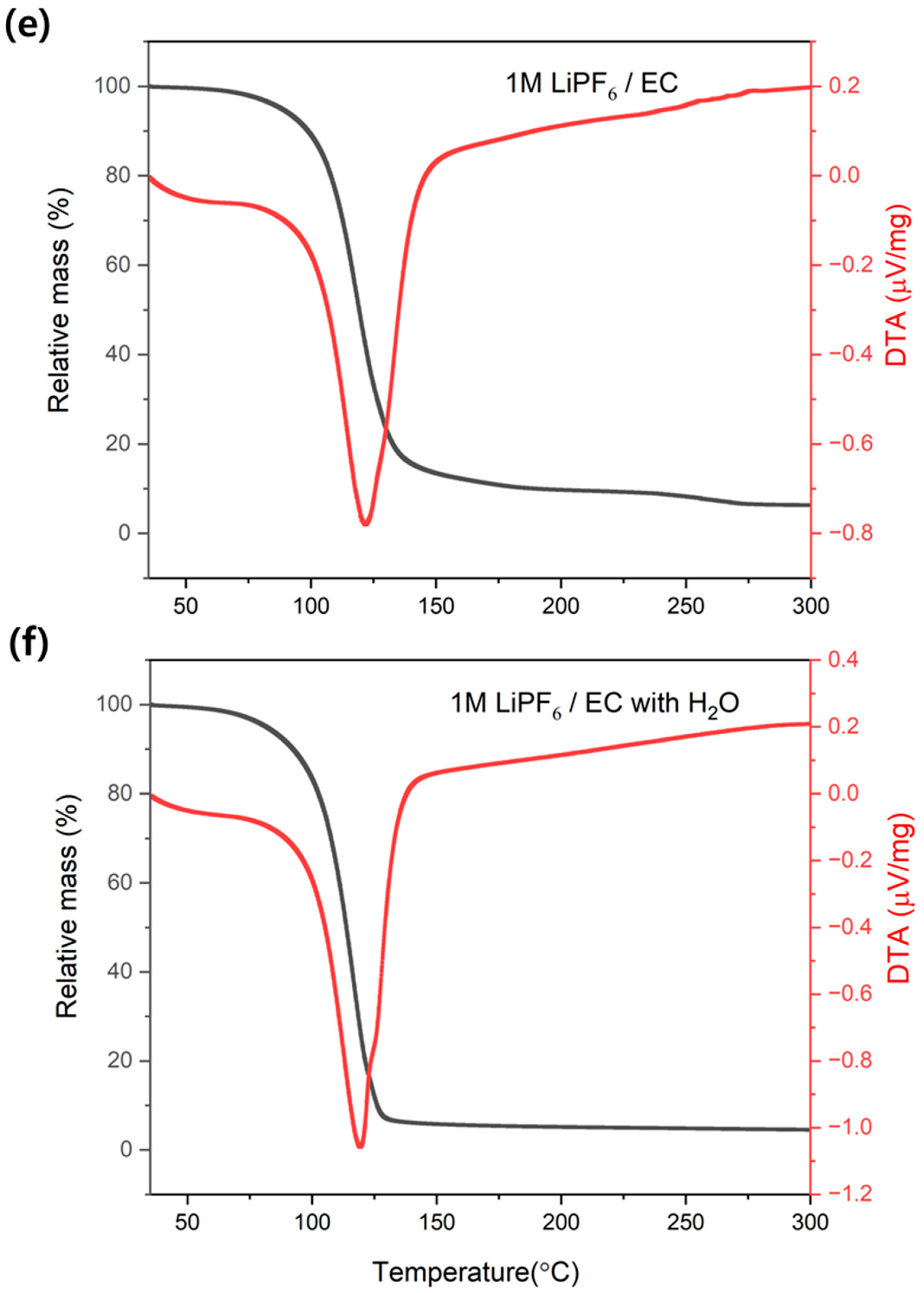
3.1. Effect of Water on Accelerating Electrolyte Decomposition
3.2. Analysis of Gases Generated from Electrolyte Decomposition
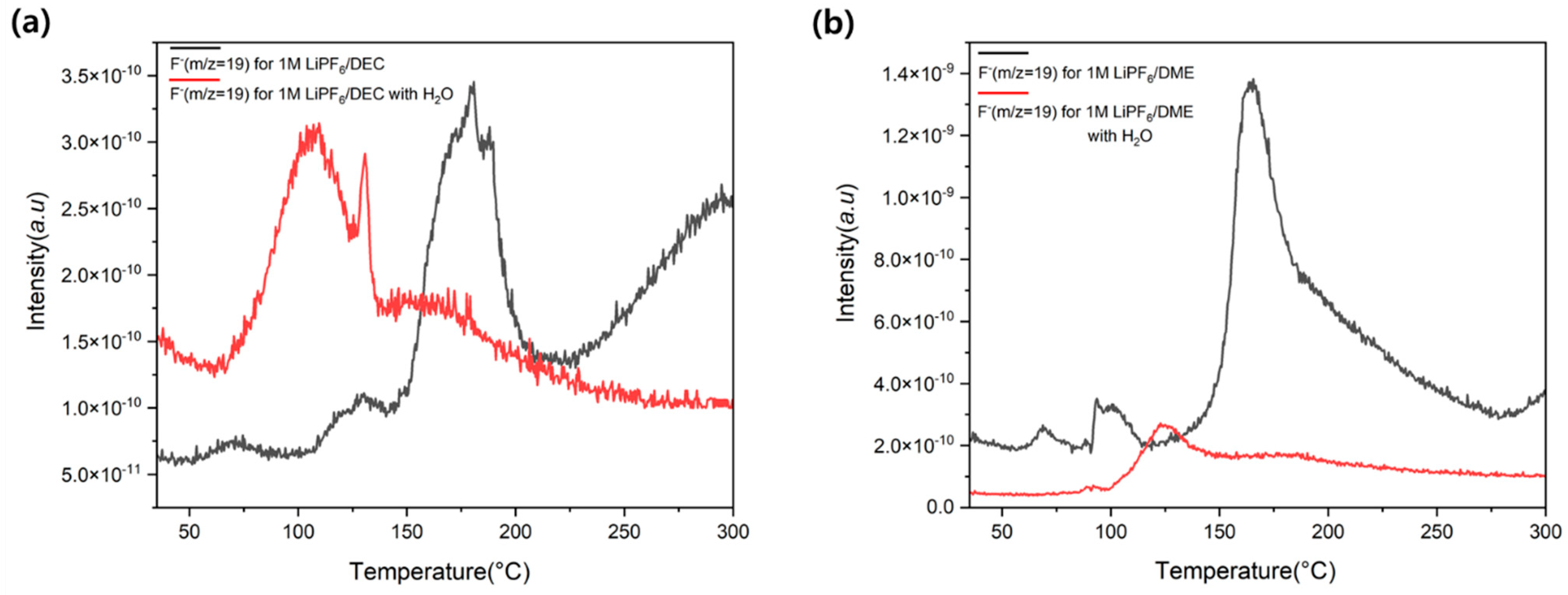
3.2.1. Hydrogen Cation (H+) and Fluoride Ion (F−)
3.2.2. Hydrogen Fluoride (HF)
3.2.3. Cyanogen Fluoride (FCN)
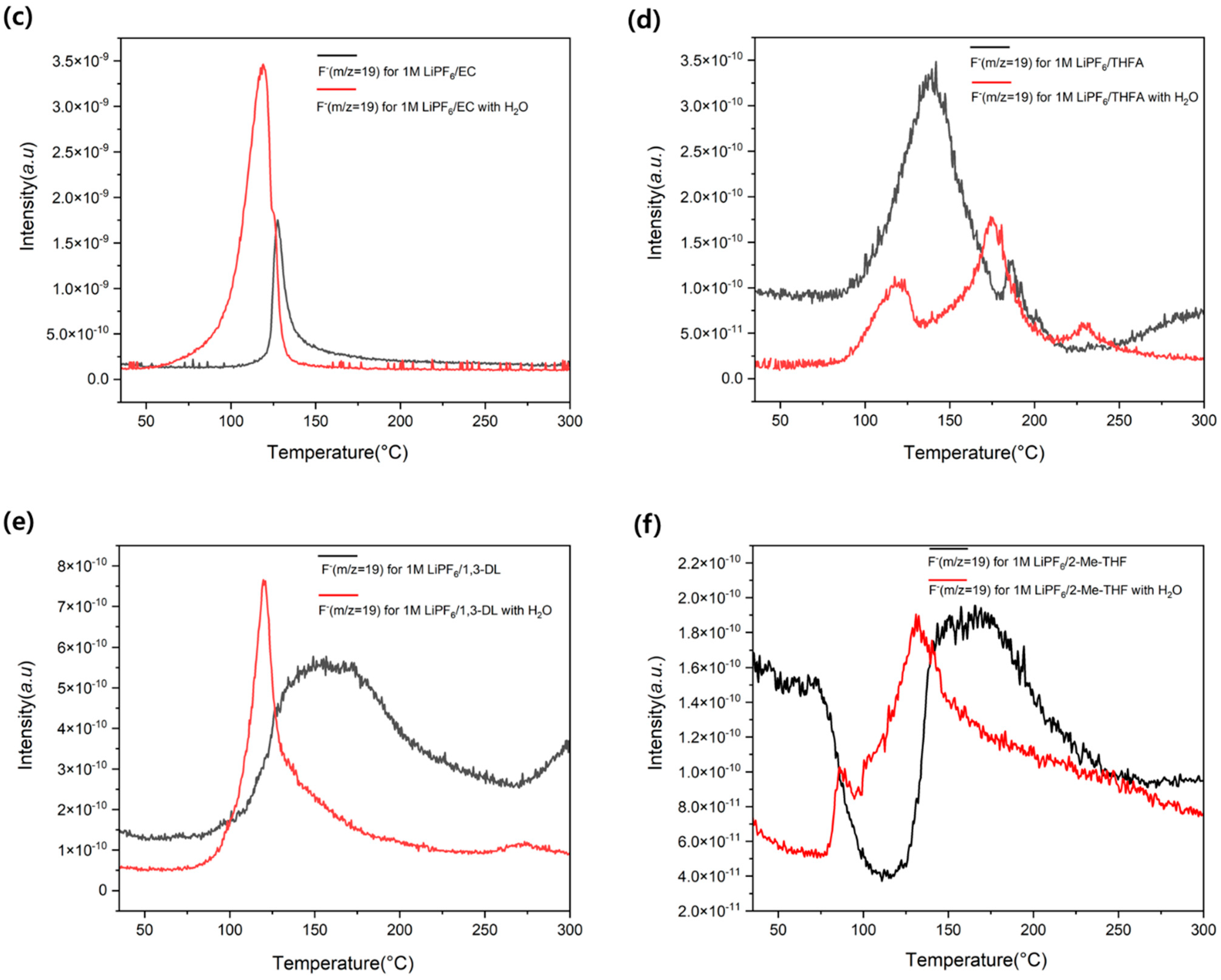
3.3. Effect of Water on F− Generation Temperature Shift
3.4. Effect of Water on HF Generation Temperature Shift
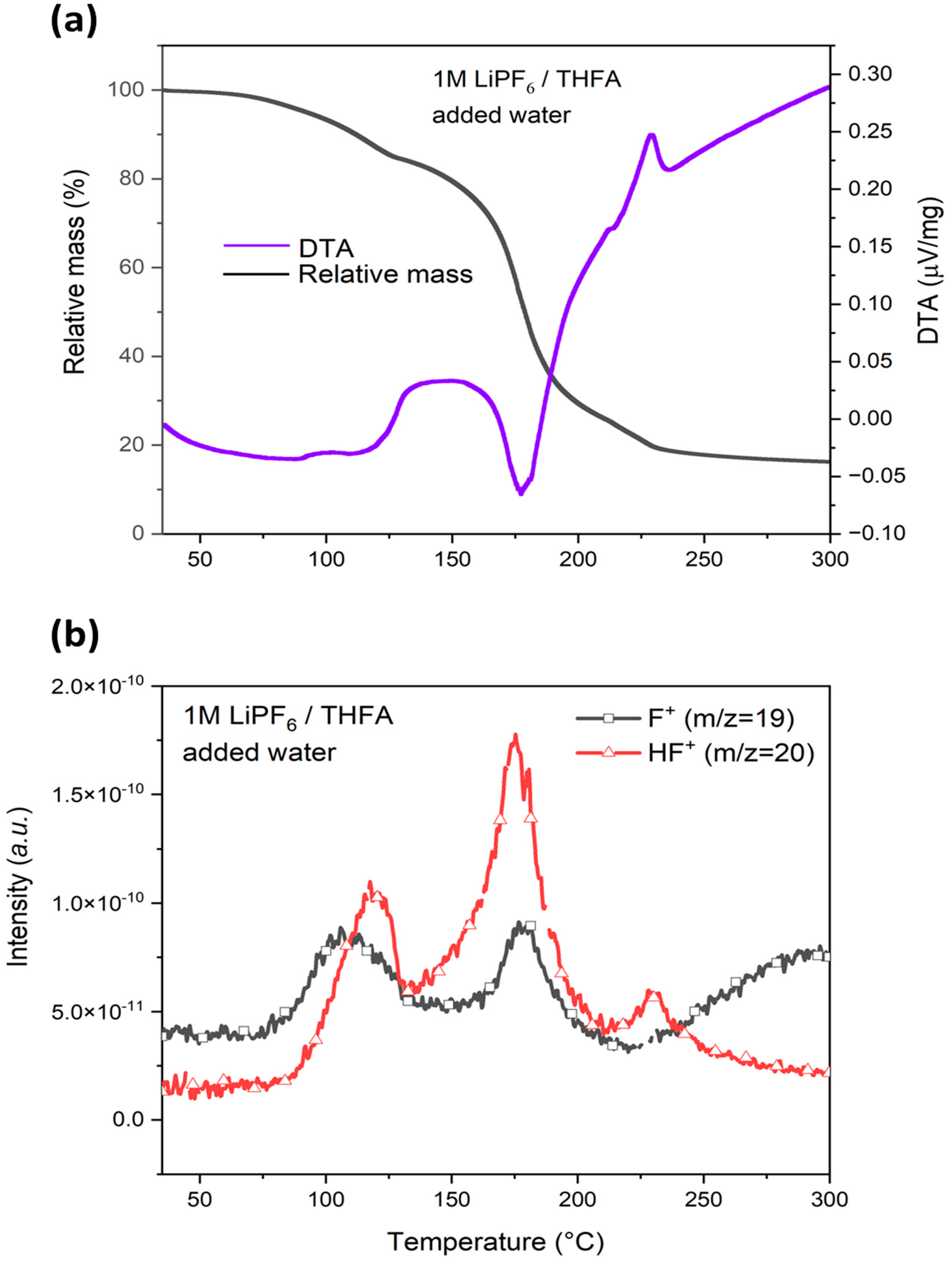
3.4.1. Effect of Water on HF Generation Temperature in LiPF6/THFA Electrolyte
3.4.2. Effect of Water on HF Generation Temperature in LiPF6/2-Me-THF Electrolyte
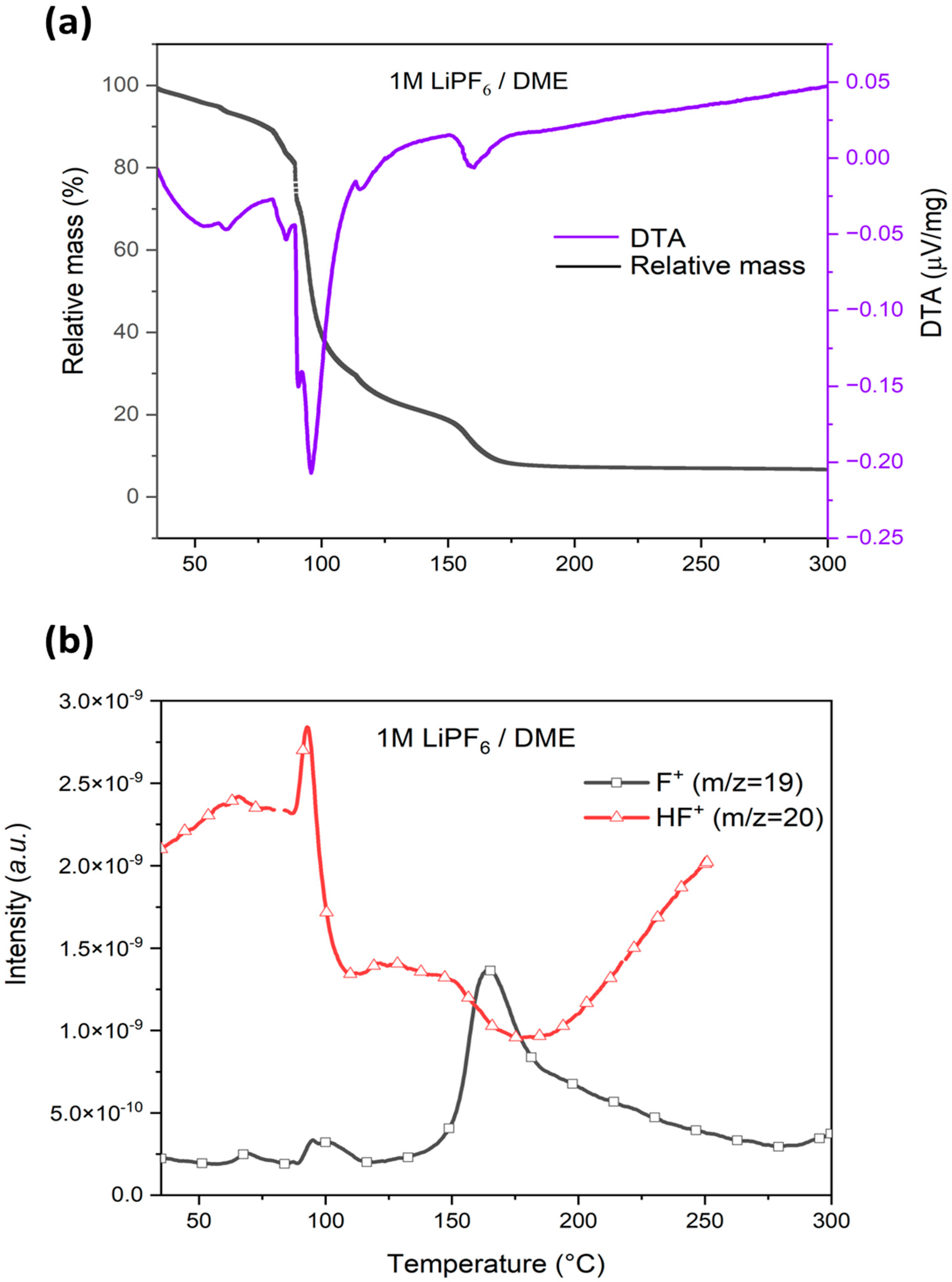
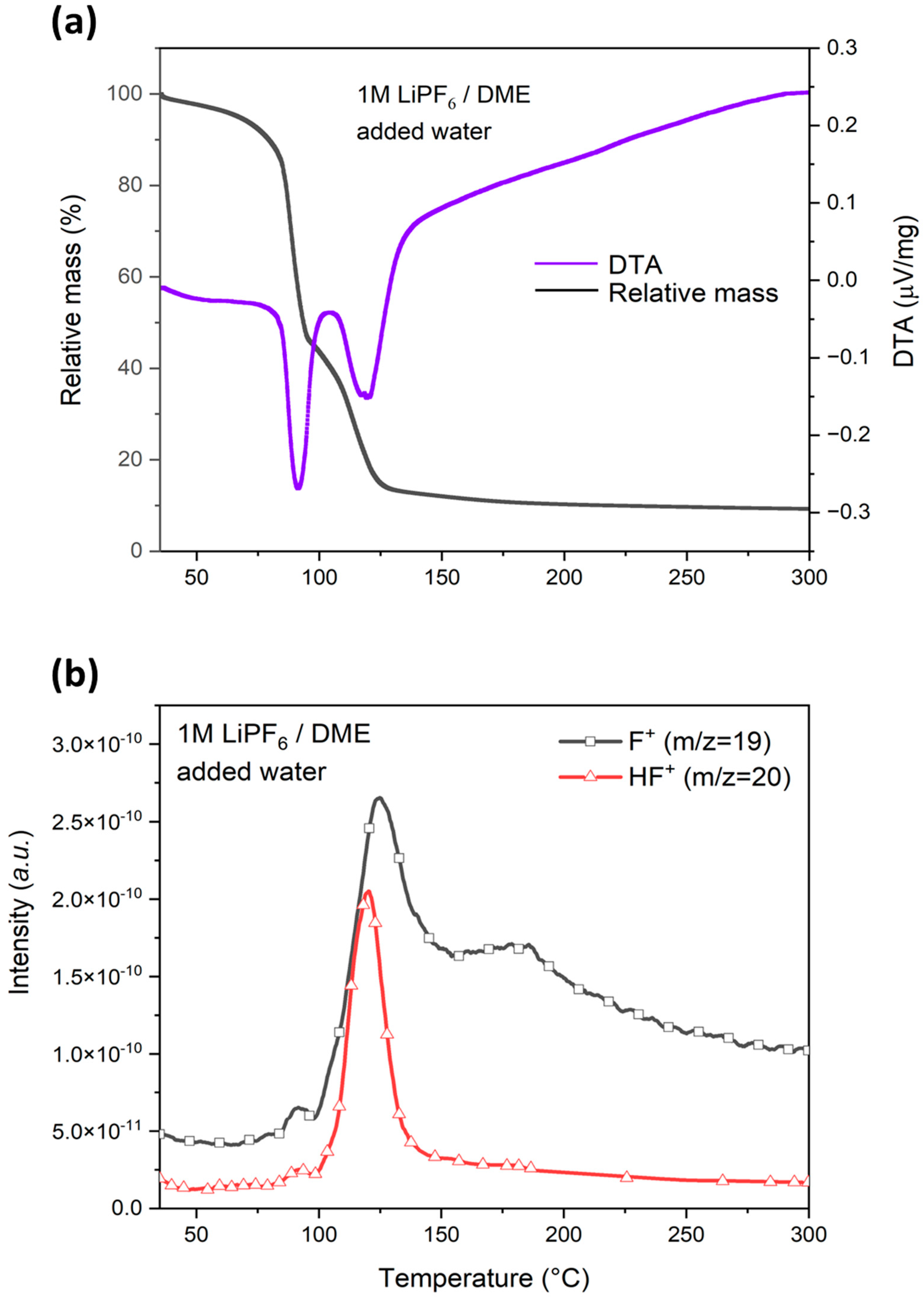
3.4.3. Effect of Water on HF Generation Temperature in LiPF6/DME Electrolyte
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-ion Battery Safety Concerns: The Issues, Strategies and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in Electrolytes for Rechargeable Li-based Batteries and Beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Schultz, C.; Vedder, S.; Winter, M.; Nowak, S. Investigation of the Decomposition of Organic Solvent-based Lithium Ion Battery Electrolytes with Liquid Chromatography-mass Spectrometry. Spectroscopyeurope 2016, 28, 21–24. [Google Scholar]
- Wang, J.; Zheng, Q.; Fang, M.; Ko, S.; Yamada, Y.; Yamada, A. Concentrated Electrolytes Widen the Operating Temperature Range of Lithium-Ion Batteries. Adv. Sci. 2021, 8, 2101646. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Ji, S.; Wang, Z.; Liu, Z.; Shen, J.; Hu, R.; Liu, J.; Zhu, M. Solvent-Free Method Prepared a Sandwich-like Nanofibrous Membrane-Reinforced Polymer Electrolyte for High-Performance All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 21586–21595. [Google Scholar] [CrossRef]
- Tasak, K.; Kanda, K.; Nakamura, S.; Ue, M. Decomposition of LiPF6 and Stability of PF5 in Li-Ion Battery Electrolytes: Density Functional Theory and Molecular Dynamics Studies. J. Electrochem. Soc. 2003, 150, A1628. [Google Scholar] [CrossRef]
- Roth, E.P.; Orendorff, C.J. How Electrolytes Influence Battery Safety. Electrochem. Soc. Interface 2012, 21, 45. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, L.; Fan, W.; Liu, J.; Yang, L.; Yu, L.; Zhu, M. Citraconic Anhydride as an Electrolyte Additive to Improve the High Temperature Performance of LiNi0·6Co0·2Mn0·2O2/Graphite Pouch Batteries. J. Alloys Compd. 2019, 805, 757–766. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Hao, J.; Xu, X.; Ouyang, L.; Fan, W.; Ye, J.; Liu, J.; Li, J.; Mei, A.; et al. The Electrolyte Additive Effects on Commercialized Ni-Rich LiNixCoyMnzO2 (x + y + z = 1) Based Lithium-Ion Pouch Batteries at High Temperature. ACS Appl. Energy Mater. 2021, 4, 2292–2299. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Li, W.; Liu, X.; Zhang, J. Safety-Reinforced Succinonitrile-Based Electrolyte with Interfacial Stability for High-Performance Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 29820–29828. [Google Scholar] [CrossRef] [PubMed]
- Ghiji, M.; Edmonds, S.; Moinuddin, K. A Review of Experimental and Numerical Studies of Lithium Ion Battery Fires. Appl. Sci. 2021, 11, 1247. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A Review of Gas Evolution in Lithium Ion Batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.V., Jr. Thermal Stability of LiPF6 Salt and Li-ion Battery Electrolytes Containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Lux, S.F.; Lucas, I.T.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. The Mechanism of HF Formation in LiPF6 Based Organic Carbonate Electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.E. Toxic Fluoride Gas Emissions from Lithium-ion Battery Fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Jie, Y.; Hu, F.; Li, Y.; Wilson, B.P.; Xi, Y.; Lai, Y.; Yang, S. Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 18228–18235. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef] [Green Version]
- Juba, B.W.; Mowry, C.D.; Fuentes, R.S.; Pimentel, A.S.; Román-Kustas, J.K. Lessons Learned—Fluoride Exposure and Response. ACS Chem. Health Saf. 2021, 28, 129–133. [Google Scholar] [CrossRef]
- Solchenbach, S.; Metzger, M.; Egawa, E.; Beyer, H.; Gasteiger, H.A. Quantification of PF5 and POF3 from Side Reactions of LiPF6 in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3022–A3028. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Duan, Q.; Zhao, C.; Huang, Z.; Wang, Q. Experimental Investigation on the Thermal Runaway and Its Propagation in the Large Format Battery Module with Li(Ni1/3Co1/3Mn1/3)O2 as Cathode. J. Hazard. Mater. 2019, 375, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Mukai, K. Roles of Positive or Negative Electrodes in the Thermal Runaway of Lithium-ion Batteries: Accelerating Rate Calorimetry Analyses with an All-inclusive Microcell. Electrochem. Commun. 2017, 77, 28–31. [Google Scholar] [CrossRef]
- Teng, X.; Zhan, C.; Bai, Y.; Ma, L.; Liu, Q.; Wu, C.; Wu, F.; Yang, Y.; Lu, J.; Amine, K. In Situ Analysis of Gas Generation in Lithium-ion Batteries with Different Carbonate-based Electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 22751–22755. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.P.; Boon-Brett, L. Considerations on the Chemical Toxicity of Contemporary Li-ion Battery Electrolytes and Their Components. J. Electrochem. Soc. 2016, 163, A821. [Google Scholar] [CrossRef]
- Gaulupeau, B.; Delobel, B.; Cahen, S.; Fontana, S.; Hérold, C. Real-time Mass Spectroscopy Analysis of Li-ion Battery Electrolyte Degradation under Abusive Thermal Conditions. J. Power Sources 2017, 342, 808–815. [Google Scholar] [CrossRef]
- Faigle, J.F.G.; Isolani, P.C.; Riveros, J.M. The Gas Phase Reaction of Fluoride(1-) and Hydroxide(1-) Ions with Alkyl Formats. J. Am. Chem. Soc. 1976, 98, 2049–2052. [Google Scholar] [CrossRef]
- Paul, G.K.; Petre, N.; Erik, J.B. Influence of Water Contamination on the SEI Formation in Li-Ion Cells: An Operando EQCM-D Study. ACS Appl. Mater. Interface 2020, 12, 15934–15942. [Google Scholar]
- Ribiere, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on The Fire-induced Hazards of Li-ion Battery Cells by Fire Calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Xu, M.; Xiao, A.; Li, W.; Lucht, B.L. Investigation of Lithium Tetrafluorooxalatophosphate LiPF4(C2O4) as a Lithium-Ion Battery Electrolyte for Elevated Temperature Performance. J. Electrochem. Soc. 2010, 157, A115. [Google Scholar] [CrossRef]





| Chemical Name | Chemical Structure | CAS No. | Molecular Weight | Boiling Point | Flash Point |
|---|---|---|---|---|---|
| (g/mol) | (°C) | (°C) | |||
| Diethyl carbonate (DEC) | 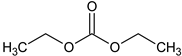 | 105-58-8 | 118.13 | 126–128 | 33 |
| 1,2-Dimethoxyethane (DME) |  | 110-71-4 | 90.12 | 85 | −2 |
| Ethylene carbonate (EC) | 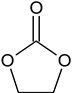 | 96-49-1 | 88.06 | 248 | 145.5 |
| 1,3-Dioxolane (1,3-DL) | 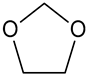 | 646-06-0 | 74.08 | 74 | −3 |
| Tetrahydrofurfuryl alcohol (THFA) | 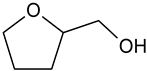 | 97-99-4 | 102.13 | 178 | 75 |
| 2-Methyl-tetrahydro furan (2-Me-THF) | 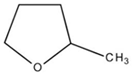 | 96-47-9 | 86.14 | 78–80 | −10 |
| Lithium hexafluorophosphate (LiPF6) * | 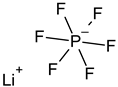 | 21324- 40-3 | 151.91 | - | - |
| Solvent | Water | Step | TG a (°C) | Mass Loss (%) | F+ Ion Detection Temperature b | HF Detection Temperature c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Tp | Tf | TiF | TpF | TfF | TiHF | TpHF | TfHF | ||||
| THFA | without water | I | 75 | 118 | 150 | 13 | 83 - | 139 186 | 179 - | - | - | - |
| II | 150 | 187 | 300 | 74 | - | - | - | 167 228 | 186 236 | 228 300 | ||
| added water | I | 68 | 88 | 126 | 15 | 75 148 | 108 178 | 148 220 | 84 | 118 | 131 | |
| II | 126 | 177 | 300 | 69 | - | - | - | 131 213 | 175 230 | 213 300 | ||
| 2-Me-THF | without water | I | 35 | 94 | 128 | 60 | 94 | 118 | - | - | - | - |
| II | 128 | 138 | 300 | 23 | 118 | 138 | 300 | - | 140 weak | - | ||
| added water | 35 - | 88 110 | 300 - | 91 | 75 96 | 88 131 | 96 300 | 76 | 112 | 165 | ||
| DME | without water | I | 35 | 96 | 152 | 83 | 89 | 96 | 117 | 35 | 93 | - |
| II | 152 | 161 | 300 | 10 | 117 | 164 | 300 | - | - | >250 | ||
| added water | I | 35 | 91 | 96 | 54 | - | 91 weak | - | - | 91 weak | - | |
| II | 96 | 119 | 300 | 37 | 91 | 124 | 300 | 91 | 120 | 150 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.Y.; Jung, S. Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6. Batteries 2022, 8, 61. https://doi.org/10.3390/batteries8070061
Han JY, Jung S. Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6. Batteries. 2022; 8(7):61. https://doi.org/10.3390/batteries8070061
Chicago/Turabian StyleHan, Ji Yun, and Seungho Jung. 2022. "Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6" Batteries 8, no. 7: 61. https://doi.org/10.3390/batteries8070061
APA StyleHan, J. Y., & Jung, S. (2022). Thermal Stability and the Effect of Water on Hydrogen Fluoride Generation in Lithium-Ion Battery Electrolytes Containing LiPF6. Batteries, 8(7), 61. https://doi.org/10.3390/batteries8070061







