Abstract
Aqueous zinc ion batteries (ZIBs) with cost—effectiveness, air stability, and remarkable energy density have attracted increasing attention for potential energy storage system applications. The unique electrical properties and competitive layer spacing of transition metal dichalcogenides (TMDs) provide dramatical freedom for facilitating ion diffusion and intercalation, making TMDs suitable for ZIB cathode materials. The recently updated advance of TMDs for high−performance ZIB cathode materials have been summarized in this review. In particular, the key modification strategies of TMDs for realizing the full potential in ZIBs are highlighted. Finally, the insights for further development of TMDs as ZIB cathodes are proposed, to guide the research directions related to the design of aqueous ZIBs while approaching the theoretical performance metrics.
1. Introduction
In order to overcome the energy crisis and environmental degradation in today’s society, developing reliable, renewable, and clean energy resources have been considered as a crucial strategy [1]. Lithium ion batteries (LIBs) are the most deeply researched energy storage systems in the commercialized market [2]. However, the widely used organic solvent electrolytes of LIBs may lead to safety concerns and increased costs. Compared with LIBs, aqueous zinc ion batteries (ZIBs) have some advantages, such as cost−effectiveness, air stability, environmental friendliness, and high theoretical energy density, and they have been considered an ideal candidate for next−generation energy devices [3,4,5]. Thus far, vanadium−based oxides [6], manganese−based oxides [7], and Prussian blue analogs [8] have exhibited remarkable Zn2+ storage capabilities, making them suitable for ZIB cathode materials [9]. However, the reversible Zn2+ storage behavior exhibited by these materials is not superior enough. For instance, Mn−based or V−based oxides as ZIB cathode materials suffer from poor cycling performance and unsatisfactory conductivity [7]. For Prussian blue analogues, the unstable crystal structure induced phase transformation and limited the capacity [3]. Therefore, exploiting a cathode with a large specific capacity and long−time cycling stability to further increase the Zn2+ storage capabilities of ZIBs is highly desired.
Transition metal dichalcogenides (TMDs), such as MoS2, WSe2, and VS2, have been considered as another promising cathode material in energy storage systems, benefitting from unique electrical properties and competitive layer spacing [10]. Compared with the above−mentioned cathode materials, TMDs process the unique hexagonal crystal structure and chemical stability, which may make TMDs exhibit better cycling performance. In particular, the appealing property of TMDs, the wide interlayer distance, is feasible for ion diffusion and intercalation, which is ideal for high−capacity aqueous ZIBs [11]. However, the inserted Zn2+−induced electrostatic interactions and the poor electrical conductivity hinder the capacity of bulk TMDs. As a result, bulk MoS2 and WS2 exhibit unsatisfactory capacity of 18 and 22 mAh·g−1 as the aqueous ZIB cathode materials, respectively [12]. Hence, efficient strategies for improving the Zn2+ storage ability of TMD cathodes are highly desired. In this review, recent advanced approaches to optimize the Zn2+ storage ability of TMDs, including interlayer engineering, phase engineering, defect engineering, metallic 1T TMDs, oxidation, and hybridization, have been comprehensively summarized (Scheme 1), which would provide clear guidance to design high−performance TMD−based aqueous ZIBs.
Scheme 1.
Schematic of the effective strategies for optimizing the Zn2+ storage ability and cycling performance of TMD−based ZIB cathodes. Reprinted with permission from Refs. [13,14,15,16,17,18]. Copyright 2021, Wiley-VCH GmbH, Weinheim. Copyright 2020, Elsevier. Copyright 2021, Wiley-VCH GmbH, Weinheim. Copyright 2020, Elsevier. Copyright 2021, Elsevier. Copyright 2021, Elsevier.
2. Basic Structural Characteristics of TMDs
These TMDs are a class of materials with the formula MX2, where M is a transition metal element of group IV, group V or group VI, while X is a brass element. X−M−X is the form of layered TMD materials, in which the brass atoms are located in two hexagonal planes separated by transition metal atoms [19], as shown in Figure 1a,b. In energy−related applications, the most representative TMD is MoS2.
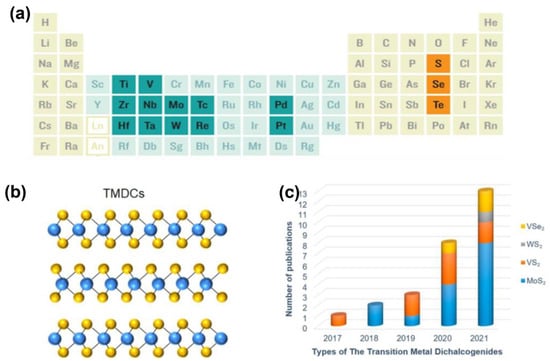
Figure 1.
Structure of TMD materials. (a) Chemical elements composition of TMD materials. (b) Three−dimensional atomic structures of typical TMDs. Reprinted with permission from Ref. [19]. Copyright 2021, Springer. (c) The trend of publications on TMDs as ZIB cathode materials. Reprinted with permission from Ref. [10]. Copyright 2022, MDPI (Basel, Switzerland).
The bulk MoS2 usually consists of monolayer MoS2 van der Waals stacked together. MoS2 is the extensively researched electronic material for various energy−related devices. In general, MoS2 has three different crystalline phases: 2H (hexagonal symmetry), 3R (rhombohedral symmetry) and 1T (tetragonal symmetry) [20]. For different phases, the arrangement of MoS2 monolayers is different, which may lead to different physical and chemical properties and which may provide flexibility for material design. More importantly, due to the large interlayer spacing (0.62 nm), TMDs can ensure the diffusion of a larger radius of cations favorable as ZIB cathode materials. The year−on−year increase in the number of published articles is evidence of the growing interest in TMD materials for ZIBs (Figure 1c) [10].
3. Proposed Modifying Strategies for the TMDs Cathode of Aqueous ZIBs
In general, the specific capacity of pristine MoS2 as the ZIB cathode is about 1–40 mAh·g−1 [12]. In order to accommodate hydrated Zn2+, the host material must embrace a layer spacing larger than its radius (0.40–0.43 nm). Although the layer spacing of bulk MoS2 (≈0.62 nm) is larger than the radius of hydrated Zn ions, the poor electrochemical activity and low electrical conductivity of intrinsic MoS2 are not feasible for reversible intercalation of Zn2+. When pristine MoS2 and WS2 obtained in nature were tested in aqueous ZnSO4 solutions, no observable redox peaks were observed, suggesting that the intrinsic MoS2 and WS2 cannot store reasonable amounts of Zn2+ to show acceptable capacity [12].
Based on the above discussion, modification strategies become important in order to obtain outstanding performance from TMDs for functional ZIB cathodes. In this section, six effective methods to overcome the high Zn2+ intercalation energy barriers of TMDs and to ensure the structural stability of TMDs are proposed: (1) interlayer engineering, (2) phase engineering, (3) defect engineering, (4) metallic 1T TMDs, (5) oxidation, and (6) hybridization.
3.1. Interlayer Engineering
The intercalation of small guest layers, such as ions and molecules, can tune the interlayer spacing of TMDs due to the unique van der Waals stacking. Therefore, interlayer engineering is a widely used means to modulate the electrochemical properties of TMDs. Via interlayer engineering, TMDs can not only achieve larger interlayer spacing but can also obtain more stable structures. Meanwhile, the energy barrier of Zn2+ intercalation can be lowered, and the storage of Zn2+ can also be enhanced in the TMDs framework with larger interlayer spacing. Furthermore, the interlayer engineering becomes more critical if the diffused hydrated Zn2+ is to be separated through a narrow gap in MoS2. As the layer spacing of MoS2 increases, the effective energies can be lowered at similar hydration levels [21]. Based on these benefits, there are many researchers exploiting the various intercalators to modify the MoS2 interlayer spacing to functionalize the ZIBs.
Zhang et al. synthesized MoS2−nH2O nanoflowers for storing Zn2+ via hydrothermal synthesis [22], and the layer spacing of MoS2 can be enlarged by water (Figure 2a). In addition, the electrostatic interaction between Zn2+ and the host material can be shielded, which further increases the Zn2+ diffusion channel. MoS2−nH2O exhibited a high reversible capacity of 165 mAh·g−1 at 0.1 A·g−1 (Figure 2b,c). The capacity retention was 88% after 800 cycles at 2 A·g−1. Moreover, the insertion of highly conductive carbon material between MoS2 layers is another approach often used in interlayer engineering [13,15,23]. The synergistic effect of expanding the layer spacing and enhancing the conductivity can effectively improve the performance of MoS2−based ZIBs. Liu et al. [13] used an electrostatic self−assembly strategy to successfully insert graphene between molybdenum disulfide layers, leading to the enlarged layer spacing from 0.62 to 1.16 nm (Figure 2d), and the reduced graphene oxide and 1T−rich phase MoS2 gave the material better ionic/electronic conductivity. The “sandwich” structure of MoS2/graphene composite nanosheets self−assembled into a nanoflower structure can effectively suppress the stacking of molybdenum disulfide and graphene layers, which is conducive to sufficient electrolyte infiltration and rapid diffusion of zinc ions to maintain structural stability. Compared with bulk molybdenum disulfide (21.9 mAh·g−1 at 0.05 A·g−1), the material has more than 10 times higher zinc storage capacity (285.4 and 141.6 mAh·g−1 at 0.05 and 5 A·g−1, respectively) and remarkable cycling stability, as displayed in Figure 2e. Except for the interlayer engineering, the highly flexible and reversible transition of MoS2 between the 2H−phase and 1T−phase during Zn2+ insertion/extraction also contributed to the remarkable storage capacity. The cycling stability is remarkable (about 88.2% capacity retention after 1800 cycles), which benefits from the large spacing−induced low Zn ion migration barriers that are also verified by density function theory calculation (Figure 2f). By combining interlayer polymerization with template−assisted insertion of N−doped carbon between MoS2 layers, Li et al. produced MoS2 nanocages with multilevel structures with expanded interlayer spacing, as displayed in Figure 2g [23]. The cage structure suppresses the accumulation of nanosheets. Meanwhile, the ion migration induces the volume expansion of alleviates during (non)charging. As a result, the layer spacing−enhanced MoS2 exhibits high zinc storage capacity (247.8 mAh·g−1 at 0.1 A·g−1) and remarkable capacity retention (85.6% after 3200 cycles at 1.0 A g−1) (Figure 2h).
3.2. Phase Engineering
Phase engineering has been verified as a potential technique to tune the electrochemical properties of materials [24]. Monolayer TMDs can exist in three different types according to the atomic configuration, namely 1T, 2H, and 3R. Monolayer TMDs can be composed of either a trigonal phase (2H) or an octahedral phase (1T). Phase control may be key to the electronic properties modification of TMDs. This is because the TMD phase is strongly related to the electron d−orbital density of the transition metal. When the Zn diffusion pathway was simulated, 2H MoS2 exhibits a higher Zn energy barrier than 1T MoS2, regardless of the interlayer spacing [14], indicating that the reversible Zn2+ storage can be effectively activated via phase control. Interleaving alkaline metals is the most common method for controlling TMDs phase transitions. Taking Li ion batteries as an example, electrons are transferred to the TMD via the insertion of an alkaline metal, leading to an increase in the d−orbital electron density. As a result, the phase transition of TMDs from 2H to 1T can be accomplished through increasing the d−orbital electron density [25]. Lithium−containing compounds, such as n−butyllithium diluted in hexane, were used to achieve the phase transition of TMDs [26]. Furthermore, 1T MoS2 with increased interlayer spacing can be obtained by intercalation of Na+ into 2H MoS2 as demonstrated by Zeng et al. Therefore, alkali metal intercalation−induced phase transition of TMDs has been verified as a synergistic method to activate MoS2 and TMDs for Zn2+ storage [27].
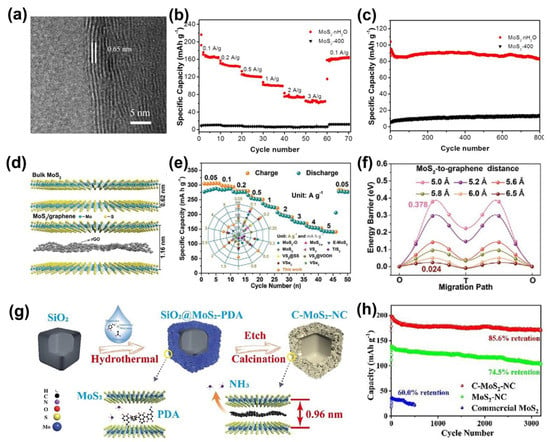
Figure 2.
Interlayer engineering for optimized MoS2−based ZIBs. (a) HRTEM images of MoS2·nH2O. (b) Rate performance of Zn/MoS2·nH2O and Zn/MoS2−400 battery at 2 A·g−1. Reprinted with permission from Ref. [22]. Copyright 2021, Elsevier. (c) Cycling performance of Zn/MoS2·nH2O and Zn/MoS2−400 battery. (d) Crystal structures of bulk MoS2 and graphene−enlarged MoS2. (e) Rate capability of the graphene−enlarged MoS2 at 0.05−5 A·g−1. (f) Migration barriers associated with MoS2 and graphene distances. Reprinted with permission from Ref. [13]. Reprinted with permission from Ref.Copyright 2021, Wiley-VCH GmbH, Weinheim. (g) Illustration of the synthetic process of C−MoS2−NC. (h) The capacity retention performance comparison for the various MoS2 cathodes. Reprinted with permission from Ref. [23]. Copyright 2022, Elsevier.
For the first time, Liu et al. prepared defective MoS2 with different phases as ZIB cathode materials (Figure 3a). 1T−MoS2 performed more remarkable specific capacity and more remarkable capacity retention than 2H−MoS2 in aqueous ZIBs. The combination of experiments and DFT calculations reveals that 1T−MoS2 can effectively lower the Zn2+ diffusion energy barrier (Figure 3b). Tang et al. demonstrated that 1T−WS2 nanosheets obtained via phase engineering can be used as a ZIB cathode candidate. Benefiting from the interlayer expanding (Figure 3c) and conductivity increasing of the 1T phase, the reversible discharge capacities of the synthesized WS2−200 at current densities of 50, 100 and 200 mA·g−1 were 233.26, 206.25 and 179.99 mAh·g−1, respectively, with 179.99 mAh·g−1 far exceeding the discharge capacity of the commercial 2H−WS2 (22 mAh·g−1 at 200 mA·g−1), as displayed in Figure 3d.
To further enhance the transfer kinetics of zinc ions, the vertically aligned 1T phase MoS2 was fabricated by Liu et al., as displayed in Figure 3e [28]. The vertically aligned freestanding structure of the 1T−MoS2 contributed to a remarkable specific capacity of 198 mA h·g−1 at 0.1 A·g−1 (Figure 3f). More importantly, it also exhibits remarkable cycling stability with a specific capacity retention of 97.2% after 1000 cycles at a current density of 2 A·g−1 (Figure 3g).
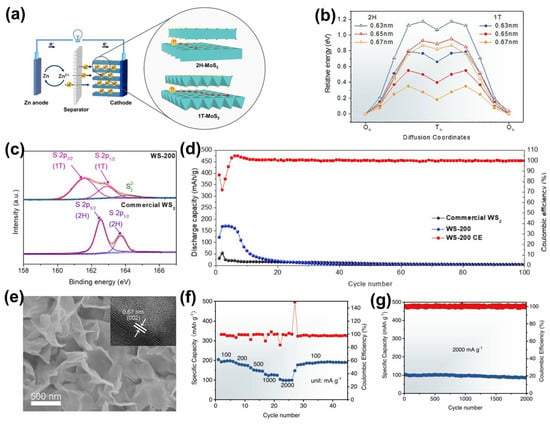
Figure 3.
Phase−engineered TMDs for ZIB cathode. (a) Illustration of the rechargeable Zn/MoS2 battery system. (b) Relative Zn2+ diffusion energies associated with the interlayer spacing in 2H and 1T MoS2. Reprinted with permission from Ref. [14]. Copyright 2020, Elsevier. (c) High−resolution XPS spectra of S2p in the commercial WS2 and WS2−200 (1T−WS2) samples. (d) Galvanostatic charge/discharge curves of WS2−200 with various current densities. Reprinted with permission from Ref. [29]. Copyright 2022, Elsevier. (e) SEM images of vertically aligned 1T−MoS2. Inset: Section TEM images of 1T−MoS2. (f) Specific capacity of 1T−MoS2 from 0.1 to 2 A·g−1. (g) Capacity retention of 1T−MoS2 at 2A·g−1. Reprinted with permission from Ref. [28]. Copyright 2022, Elsevier.
3.3. Defect Engineering
Defect engineering is another widely studied method to change the physical and chemical properties of TMDs [15,30,31,32,33]. Defect engineering causes structural changes in TMDs, which facilitate the optimization of Zn2+ ion diffusion and charge transfer processes [34]. In addition, defect engineering can increase the number of ion storage sites and can achieve higher charge capacity. Defect engineering has been verified by many studies that can help improve the kinetics of reactions and electrochemical properties due to the active sites increasing [35]. Therefore, the implementation of defect engineering in TMDs is a feasible approach to tuning electrochemical properties for functional cathodes.
Vacancy is one of the most common structural defect of materials, such as sulfur vacancies, transition metal vacancies, edges, and holes in TMDs lattice, as shown in Figure 4a [34,36]. These vacancy defects are particularly attractive in two−dimensional monolayer TMDs because the transport of charge carriers in the two−dimensional plane is significantly enhanced. Moreover, the creation of vacancies exposes sites in the TMDs that can then be used for chemical functionalization [37,38]. It has been reported by Liu et al. that the formation energy of S vacancies in MoS2 (2.35 eV) is lower than that of Mo vacancies (8.02 eV) [39], indicating that it is easier to generate S vacancies than Mo vacancies, which is also verified by Koh et al. [40]. In particular, S vacancy is critical for the storage mechanism due to the ability to facilitate ion transit through the MoS2 layer [41]. Xu et al. investigated defect−engineered MoS2−x nanosheets as cathodes for zinc ion batteries [42]. Compared with defect−free MoS2, the defect−rich MoS2−x nanosheets provided more intercalation sites for zinc ions, resulting in higher capacity (Figure 4b). More importantly, it also exhibits remarkable cycling stability with a specific capacity retention of 87.8% after 1000 cycles at a current density of 1 A·g−1 (Figure 4c).
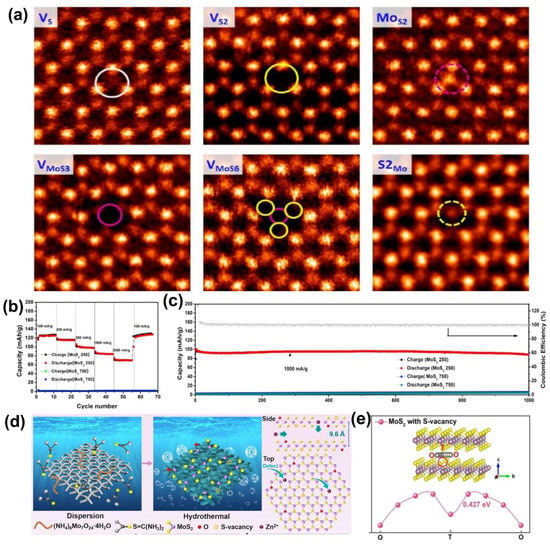
Figure 4.
Defect−tailored MoS2 for ZIB cathode. (a) Characterization of various point defects in single−layer MoS2, including VS, VS2, MoS2, VMoS3, VMoS6, and S2Mo. Reprinted with permission from Ref. [34]. Copyright 2013, American Chemical Society. (b) Rate performance of defect−rich MoS2−x at various currents densities. (c) Long−term cyclic properties of MoS2−x at specific current of 1 A·g−1. Reprinted with permission from Ref. [42]. Copyright 2019, Elsevier. (d) Illustration of the preparation process and the crystal structure of defect−engineered MoS2−O. (e) Zn ion migration behaviors along the ab plane in O−doped MoS2 with S−vacancy. Reprinted with permission from Ref. [15]. Copyright 2021, Wiley-VCH GmbH, Weinheim.
Recently, Liu et al. successfully unlocked the molybdenum disulfide substrate by implanting structural defects and lattice oxygen (D−MoS2−O) in molybdenum disulfide through in situ molecular engineering (Figure 4d) [15], expanding the layer spacing from 6.2 to 9.6 Å. The enriched 1T phase gives the material better electrical conductivity and hydrophilicity and achieves efficient three−dimensional transport of zinc ions along the ab−plane and c−axis of molybdenum disulfide. The prepared molybdenum disulfide nanosheets containing structural defects and lattice oxygen are grown vertically on a carbon cloth substrate, which effectively suppresses the stacking of molybdenum disulfide layers and facilitates sufficient electrolyte infiltration and rapid diffusion of zinc ions. Benefiting from the synergistic engineering (structural defects and 1T−phase transition) that induces interlayer expanding, the material exhibits remarkable multiplicative performance (reversible capacities of 261 and 102.4 mAh·g−1 at 0.1 and 10 A·g−1, respectively) and outstanding cycling stability (90.5% capacity retention after 1000 cycles). Electrochemical tests of the system indicate that the material has a high zinc ion diffusion coefficient, while DFT theoretical calculations demonstrate a low Zn2+ migration energy barrier and a feasible three−dimensional zinc ion transport mechanism in the material (Figure 4e).
3.4. Metallic 1T TMDs
Beyond the 1T phase transition of 2H MoS2 and WS2, the direct selection of metallic 1T phase TMDs is another effective strategy to ensure large layer spacing and high electric conductivity, while the structure is also more easily stabilized compared to that of 1T−MoS2/WS2 during the phase transition process.
Li et al. developed metallic 1T− VS2 directly grown on stainless steel (VS2@SS) mesh cathode for an aqueous ZIB [43]. The cell exhibited a remarkable Zn ion storage capacity (198 mAh·g−1) and stable cycling performance (more than 80% capacity retention over 2000 cycles at 2 A·g−1), which suggests that the electrode is promising for practical applications. Wu et al. observed the remarkable zinc ion storage properties in ultrathin VSe2 nanosheets (Figure 5a) [44]. The X−ray photoelectron spectrum (XPS) unveiled the two−step intercalation/de−intercalation reaction mechanism of VSe2 (Figure 5b). VSe2 nanosheets exhibited a specific capacity of 131.8 mAh·g−1 at 0.1 A·g−1 and a high energy density of 107.3 Wh·kg−1 (power density of 81.2 W·kg−1). Moreover, the ZIBs with this material exhibit remarkable cycling stability (80.8% capacity retention after 500 cycles) (Figure 5c).
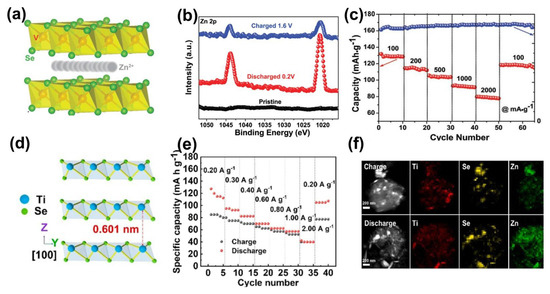
Figure 5.
Metallic 1T TMDs for high−performance ZIBs. (a) Atomic structure of VSe2. (b) Ex situ high−resolution XPS of the Zn 2p of the cathode at different states. (c) Rate performance of VSe2 electrodes from 0.10 to 2.00 A·g−1. Reprinted with permission from Ref. [44]. Copyright 2020, Wiley-VCH GmbH, Weinheim. (d) Schematic atomic structure of TiSe2. (e) Rate capability at various current densities. (f) Elemental distribution characterization of discharged/charged TiSe2. Reprinted with permission from Ref. [16]. Copyright 2021, Elsevier.
Recently, TiSe2 was verified to be an ideal candidate for aqueous ZIBs by Wen et al., as shown in Figure 5d [16]. The TiSe2 with 0.601 nm layer spacing and good electron conduction properties make it suitable for efficient (de)intercalation of zinc ions. As a result, the TiSe2 electrode showed a capacity of 128.0 mAh·g−1 at 0.20 A·g−1 (Figure 5e) and recoverability of 70.0% after 300 cycles. Especially, the high concentration distribution of zinc ions in the fully discharged state demonstrates the accomplished intercalation of zinc ions (Figure 5f).
3.5. Oxidation
The use of in situ electrochemistry to oxidize the inserted ions to the corresponding oxides without changing the ion insertion position of the TMD, not only increases the active site but also enhances the hydrophilicity of the ions, leading to an effective diffusion of Zn2+ [45,46].
To verify the effectiveness of in situ oxidation, Yu et al. constructed VS2/VOx composite structures with porous VOx nanosheets uniformly distributed on VS2 using electrochemical pretreatment [47]. In particular, the lattice fringes of 0.295 and 0.345 nm correspond to the (400) and (100) planes of V6O13 (Figure 6a). Benefiting from the synergy of the high conductivity of VS2 and the remarkable stability of VOx, the VS2/VOx heterostructure facilitates the regulation of guest ion intercalation and improves the chemical stability of the VS2 backbone. The buffer volume was changed by the gradual insertion of Zn2+ while accelerating the reversible reaction kinetics. The VS2/VOx electrode maintained a high capacity of 75% after 3000 cycles at 1 A∙g−1. (Figure 6b). In addition, MoS2−O was prepared as a cathode for ZIBs by a simple solvothermal method by Jia et al. [17]. EDS and XPS characterization verifies the existence of O insertion in MoS2 (Figure 6c,d). MoS2−O reduced the ion diffusion resistance and maintained the stability of the MoS2 layer. The optimized MoS2−O electrode exhibited a high specific capacity of 206.7 mAh·g−1 at a current density of 0.1 A·g−1 (Figure 6e) and remarkable cycling stability after 300 cycles, further confirming the effectiveness of the oxidation strategy.
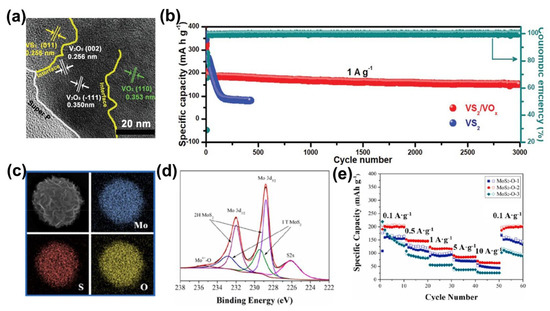
Figure 6.
Modification of TMD−based ZIBs by electrochemical oxidation. (a) The TEM image of in situ formed VS2/VOx. (b) The cycling performance of VS2/VOx heterostructure at 1 A·g−1. Reprinted with permission from Ref. [47]. Copyright 2021, Wiley-VCH GmbH, Weinheim. (c) Elemental characterization in MoS2−O. (d) XPS survey spectra of MoS2−O. (e) Rate performance of MoS2−O at various current densities. Reprinted with permission from Ref. [17]. Copyright 2021, Elsevier.
3.6. Hybridization
Hybridization is another well−proven method for altering the properties of a material or for giving it new properties [48]. Two−dimensional TMDs can be compounded with other materials to obtain new properties. In general, DMTs have low electrical conductivity, which can considerably limit the specific capacity that they can achieve. To overcome the challenge, the researchers demonstrated that the electrochemical properties of the entire composite can be improved via hybridizing the host TMDs with other materials, such as other TMDs, carbon materials, and so on [49].
The hybridization strategy is particularly applicable between TMD materials because the host and guest TMDs usually have similar structures and the lattice mismatch can be neglected. Generally, the host and guest TMDs can be hybridized by epitaxial growth, and the composites are often able to exhibit stronger intrinsic properties, multifunctionality, or even new properties. For example, a MoS2−MoSe2 heterostructure assembled by liquid−phase ultrasound−assisted exfoliation was reported by Yang et al. [50]. Benefiting from the unique van der Waals interlayer coupling, the MoS2−MoSe2 heterostructure is able to provide more electrochemical active sites. Moreover, the fluffy construction of the MoS2−MoSe2 heterostructure enhances ion transport. Pu et al. fabricated VS2@VOOH via hydrothermal synthesis (Figure 7a) [51]. The presence of V−O−V and O−H vibrational was demonstrated by FT−IR spectroscopic characterization at typical 480 and 1610 cm−1 adsorption peaks, respectively (Figure 7b), which further solidifies the existence of VOOH in VS2. The O−H in VOOH not only improved the wettability of the electrode and electrolyte, but also reduced vanadium dissolution and improved the electrochemical performance. This composite displayed a remarkable capacity of 107.5 mAh·g−1 after 350 cycles at 1.5 A·g−1. In particular, after 400 cycles at a high current density of 2.5 A·g−1, it maintains a capacity of 91.4 mAh·g−1 (Figure 7c). In the hybridization of cathode materials for energy storage systems, both carbon nanotubes and graphene are widely used for their remarkable electrical and mechanical properties [52,53], and by hybridizing TMDs and CNT/graphene, the conductivity of the whole composite can be effectively improved while maintaining a large layer spacing colleague. A novel two−dimensional layered composite composed of graphene sheets were grown vertically on metallic 1T−VS2 nanosheets by a one−step solvothermal strategy by Chen et al. [54], as shown in Figure 7d. The rGO−VS2 was confirmed by Raman spectroscopy (Figure 7e). Due to the advantageous synergy between the layered VS2 and graphene nanosheets, the rGO−VS2 composites demonstrated a high capacity of 238 mAh·g−1 at 0.1 A·g−1 and capacity retention of more than 93% after 1000 cycles at 5 A·g−1 as ZIB cathodes (Figure 7f).
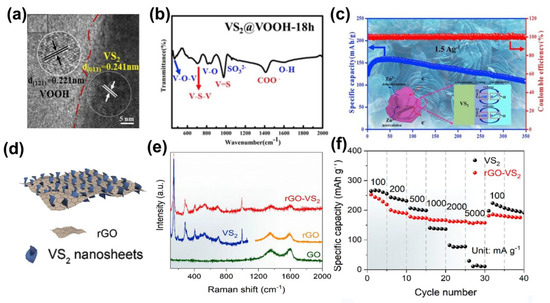
Figure 7.
Hybridization of TMDs for high−performance ZIBs. (a) HRTEM images of the VS2@VOOH. (b) FTIR spectra of VS2@VOOH. (c) Cycling retention at 1.5 A·g−1 of VS2@VOOH. Reprinted with permission from Ref. [51]. Copyright 2019, Elsevier. (d) Illustration of hierarchical rGO−VS2 composites. (e) Raman characterization of the GO, rGO, VS2, and rGO−VS2. (f) Rate capability of rGO−VS2 and VS2 Reprinted with permission from Ref. [54]. Copyright 2020, Elsevier.
Yan et al. synthesized marble−like rGO−VSe2 nanobridges [18]. Benefiting from the inhibition of electrostatic buildup of VSe2 nanosheets by the strongly conducting rGO, the electrolyte diffusion path is shortened, while the highly conductive rGO network provided a three−dimensional highway for electron transport and improved reaction kinetics. The rGO−VSe2 nanohybrids were used as the ZIB cathode candidate, exhibiting high capacity 221.5 mAh·g−1 at a current density of 0.5 A·g−1, and performed 91.6% capacity retention after 150 cycles. Wang et al. combined metal 1T phase MoS2 with multi−walled carbon nanotubes (MWCNT) to construct ZIB cathode materials by the hydrothermal method [55]. The synergistic effect of phase engineering and hybridization was utilized to accelerate the zinc ion transfer kinetics and to cost−effectively improve electrochemical properties at room temperature and low temperature. The hybrid electrode exhibited a high reversible capacity of 161.5 mAh·g−1 with good cycling stability after 100 cycles at 0.1 A·g−1 and capacity retention of 84.6% after 500 cycles at 1 A·g−1.
4. Conclusions and Perspective
In this review, potential ZIB cathode materials, TMDs, are systematically discussed. The timeline and electrochemical performance of TMD materials as ZIB cathode is summarized in Figure 8 and Table 1. In order to effectively reduce the diffusion potential of Zn2+ in TMDs and maintain the stability of the material over multiple cycles, six possible strategies for eliciting satisfactory electrochemical performance of TMDs, including (1) interlayer engineering, (2) phase engineering, (3) defect engineering, (4) metallic 1T TMDs, (5) oxidation, and (6) hybridization are comprehensively summarized in this review. Although these modification strategies have only been initially validated in some TMD materials, more members of the TMDs family will definitely become excellent ZIB cathode materials as the research continues, driving the flourishing development of aqueous ZIBs. To further the continuous upgrade of these modification strategies, we also conclude by listing a few key breakthrough directions:
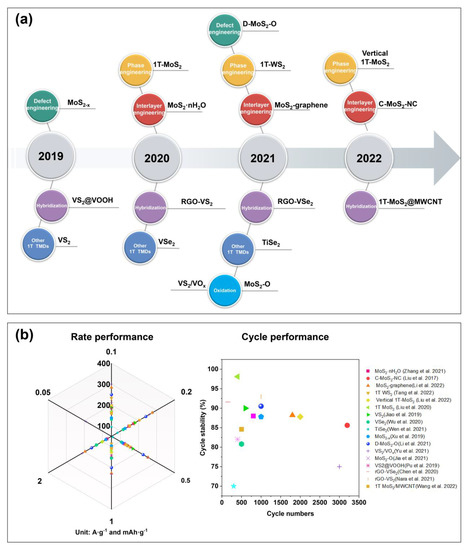
Figure 8.
Summary of modified TMDs for high−performance ZIBs. (a) Timeline of developed strategies for TMD−based ZIB cathode. (b) The radar chart of the electrochemical performance of TMD materials as ZIB cathode. Refs. [12,14,16,17,18,22,23,28,29,42,43,44,47,51,54,55].

Table 1.
Summary of the electrochemical performance of TMDs as ZIB cathode.
- (1)
- Focusing on mechanism exploration behind the ZIBs technology:
Although previous studies have analyzed the electrochemical properties of TMD−based ZIBs and their energy storage mechanisms, the link between the lattice structure, crystal defects and properties of cathode materials needs to be deeply resolved. Fully revealing the effects of various strategies, engineered TMDs on Zn2+ storage behavior is crucial to achieving higher performance ZIB batteries.
- (2)
- Increasing synergistic engineering between different strategies:
Each of the strategies mentioned above addresses a specific performance enhancement of TMD−based ZIBs. To achieve the overall enhancement, the interplay between different modified strategies is required, such as the synergy of defect engineering and phase engineering increasing the layer spacing [15], reducing the Zn2+ transport barriers, and enhancing the cycling characteristics. The hybridization of metallic 1T−TMD materials and graphene enhances the stability of the material while guaranteeing conductivity. More strategy combinations provide new freedom to design high−performance ZIBs.
- (3)
- Solving cyclic stability from a system perspective:
In ZIBs, the storage capacity can approach theoretical values by material selection and modifications. However, the storage capacity suffers from rapid fading upon long−term cycling, which is more crucial for the commercialization of ZIBs. For TMD−based ZIBs, improving material structure and chemical stability are the basic guarantees. In addition, in−depth exploration of the underlying and overall correlation between TMD cathode materials, electrolytes, and Zn anodes is the further direction.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All collected data are presented in the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, Y.; Hou, Y.; Liu, L.; Wu, Y.; Loh, K.P.; Zhang, H.; Zhu, K. Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energy Environ. Sci. 2013, 6, 2093–2104. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Mo, F.; Ji, X.; Zhi, C. Non-metallic charge carriers for aqueous batteries. Nat. Rev. Mater. 2021, 6, 109–123. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, J.; Yan, B.; Li, Q.; Chen, A.; Chen, Z.; Wang, X.; Xiong, B.; Fan, J.; Xu, J. Gradient fluorinated alloy to enable highly reversible Zn-metal anode chemistry. Energy Environ. Sci. 2022, 15, 1086–1096. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Ji, D.; Zhao, K.; Wang, S.; Cheng, F. Vanadium-based cathodes for aqueous zinc-ion batteries: From crystal structures, diffusion channels to storage mechanisms. J. Mater. Chem. A 2021, 9, 5258–5275. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Fang, G.; Zhang, C.; Zhang, H.; Guo, X.; Cao, X.; Zhou, J.; Pan, A.; Liang, S. Electrochemical activation of manganese-based cathode in aqueous zinc-ion electrolyte. Adv. Funct. Mater. 2020, 30, 2002711. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, F.; Chen, M.; Shao, T.; Li, Z.; Xu, Q.; Cheng, D.; Liu, H.; Xia, Y. Cubic Manganese Potassium Hexacyanoferrate Regulated by Controlling of the Water and Defects as a High-Capacity and Stable Cathode Material for Rechargeable Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 26924–26935. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Yuan, J.; Xia, Y.; Liu, Y.; Sun, A. Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries. Materials 2022, 15, 2654. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and Transition Metal Dichalcogenides as Functional Zinc-Ion Battery Cathode: A Perspective. Small Methods 2021, 5, 2000815. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hao, J.; Xu, C.; Mou, J.; Dong, L.; Jiang, F.; Kang, Z.; Wu, J.; Jiang, B.; Kang, F. Investigation of zinc ion storage of transition metal oxides, sulfides, and borides in zinc ion battery systems. Chem. Commun. 2017, 53, 6872–6874. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-Like Heterostructures of MoS2/Graphene with Enlarged Interlayer Spacing and Enhanced Hydrophilicity as High-Performance Cathodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef]
- Liu, J.; Xu, P.; Liang, J.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Boosting aqueous zinc-ion storage in MoS2 via controllable phase. Chem. Eng. J. 2020, 389, 124405. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Cui, K.; Shen, Q.; Li, P.; Qu, X.; Jiao, L. Molecular Engineering on MoS2 Enables Large Interlayers and Unlocked Basal Planes for High-Performance Aqueous Zn-Ion Storage. Angew. Chem. 2021, 133, 20448–20455. [Google Scholar] [CrossRef]
- Wen, L.; Wu, Y.; Wang, S.; Shi, J.; Zhang, Q.; Zhao, B.; Wang, Q.; Zhu, C.; Liu, Z.; Zheng, Y. A Novel TiSe2 (De) Intercalation Type Anode for Aqueous Zinc-Based Energy Storage. Nano Energy 2021, 93, 106896. [Google Scholar] [CrossRef]
- Jia, H.; Qiu, M.; Tawiah, B.; Liu, H.; Fu, S. Interlayer-expanded MoS2 hybrid nanospheres with superior zinc storage behavior. Compos. Commun. 2021, 27, 100841. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Hu, L.; Kirubasankar, B.; Liu, Z.; Angaiah, S.; Yan, C. Nanohybrid engineering of the vertically confined marigold structure of rGO-VSe2 as an advanced cathode material for aqueous zinc-ion battery. J. Alloys Compd. 2021, 882, 160704. [Google Scholar] [CrossRef]
- Liu, B.; Du, J.; Yu, H.; Hong, M.; Kang, Z.; Zhang, Z.; Zhang, Y. The coupling effect characterization for van der Waals structures based on transition metal dichalcogenides. Nano Res. 2021, 14, 1734–1751. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous zinc-ion storage in MoS2 by tuning the intercalation energy. Nano Lett. 2019, 19, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Wang, R.; Li, H.; Yan, J.; Jin, Q.; Feng, P.; Wang, K.; Jiang, K. Crystal water assisting MoS2 nanoflowers for reversible zinc storage. J. Alloys Compd. 2021, 872, 159599. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Wang, Y.; Lu, Y.; Zhu, L.; Sun, T. Drastically-enlarged interlayer-spacing MoS2 nanocages by inserted carbon motifs as high performance cathodes for aqueous zinc-ion batteries. Energy Storage Mater. 2022, 49, 144–152. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.T.; Zhou, W.; Loh, K.P. Phase restructuring in transition metal dichalcogenides for highly stable energy storage. ACS Nano 2016, 10, 9208–9215. [Google Scholar] [CrossRef] [Green Version]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Fujita, T.; Ito, Y.; Tan, Y.; Yamaguchi, H.; Hojo, D.; Hirata, A.; Voiry, D.; Chhowalla, M.; Chen, M. Chemically exfoliated ReS2 nanosheets. Nanoscale 2014, 6, 12458–12462. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. 2011, 123, 11289–11293. [Google Scholar] [CrossRef]
- Liu, J.; Gong, N.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Vertically aligned 1T phase MoS2 nanosheet array for high-performance rechargeable aqueous Zn-ion batteries. Chem. Eng. J. 2022, 428, 130981. [Google Scholar] [CrossRef]
- Tang, B.; Tian, N.; Jiang, J.; Li, Y.; Yang, J.; Zhu, Q. Investigation of zinc storage capacity of WS2 nanosheets for rechargeable aqueous Zn-ion batteries. J. Alloys Compd. 2022, 894, 162391. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Z.; Han, C.; He, J.; Ni, Z.; Chen, W. Two-dimensional transition metal dichalcogenides: Interface and defect engineering. Chem. Soc. Rev. 2018, 47, 3100–3128. [Google Scholar] [CrossRef]
- Liang, G.; Gan, Z.; Wang, X.; Jin, X.; Xiong, B.; Zhang, X.; Chen, S.; Wang, Y.; He, H.; Zhi, C. Reconstructing Vanadium Oxide with Anisotropic Pathways for a Durable and Fast Aqueous K-Ion Battery. ACS Nano 2021, 15, 17717–17728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Q.; Liu, S.; Kang, Z.; Zhang, Z.; Du, J.; Li, F.; Zhang, S.; Xiao, J.; Liu, B. Poly(4-styrenesulfonate)-induced sulfur vacancy self-healing strategy for monolayer MoS2 homojunction photodiode. Nat. Commun. 2017, 8, 15881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Q.; Kang, Z.; Liu, B.; Liu, X.; Ou, Y.; Xiao, J.; Du, J.; Liu, Y.; Gao, L. Hidden vacancy benefit in monolayer 2D semiconductors. Adv. Mater. 2021, 33, 2007051. [Google Scholar] [CrossRef]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.-C. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Carvalho, B.R.; Kahn, E.; Lv, R.; Rao, R.; Terrones, H.; Pimenta, M.A.; Terrones, M. Defect Engineering of Two-Dimensional Transition Metal Dichalcogenides. 2D Mater. 2016, 3, 022002. [Google Scholar] [CrossRef]
- Chee, S.S.; Lee, W.J.; Jo, Y.R.; Cho, M.K.; Chun, D.; Baik, H.; Kim, B.J.; Yoon, M.H.; Lee, K.; Ham, M.H. Atomic vacancy control and elemental substitution in a monolayer molybdenum disulfide for high performance optoelectronic device arrays. Adv. Funct. Mater. 2020, 30, 1908147. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, H.; Zhang, G.; Zhang, Y.-W. Modulating carrier density and transport properties of MoS2 by organic molecular doping and defect engineering. Chem. Mater. 2016, 28, 8611–8621. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.; Fang, L.; Robertson, J. Sulfur vacancies in monolayer MoS2 and its electrical contacts. Appl. Phys. Lett. 2013, 103, 183113. [Google Scholar] [CrossRef]
- Koh, G.; Zhang, Y.-W.; Pan, H. First-principles study on hydrogen storage by graphitic carbon nitride nanotubes. Int. J. Hydrogen Energy 2012, 37, 4170–4178. [Google Scholar] [CrossRef]
- Yao, K.; Xu, Z.; Huang, J.; Ma, M.; Fu, L.; Shen, X.; Li, J.; Fu, M. Bundled defect-rich MoS2 for a high-rate and long-life sodium-ion battery: Achieving 3D diffusion of sodium ion by vacancies to improve kinetics. Small 2019, 15, 1805405. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, C.; Zhao, K.; Cheng, X.; Rawal, S.; Xu, Y.; Wang, Y. Defect engineering activating (Boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019, 16, 527–534. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L. Binder-free hierarchical VS2 electrodes for high-performance aqueous Zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330–16338. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, C.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Gu, Q.; Sun, Z.; Hu, L. Ultrathin VSe2 Nanosheets with Fast Ion Diffusion and Robust Structural Stability for Rechargeable Zinc-Ion Battery Cathode. Small 2020, 16, 2000698. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, B.; Wang, F.; Yang, J.; Wu, F.; Ning, Y.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Anodic oxidation strategy toward structure-optimized V2O3 cathode via electrolyte regulation for Zn-ion storage. ACS Nano 2020, 14, 7328–7337. [Google Scholar] [CrossRef]
- Cao, Z.; Chu, H.; Zhang, H.; Ge, Y.; Clemente, R.; Dong, P.; Wang, L.; Shen, J.; Ye, M.; Ajayan, P.M. An in situ electrochemical oxidation strategy for formation of nanogrid-shaped V3O7· H2O with enhanced zinc storage properties. J. Mater. Chem. A 2019, 7, 25262–25267. [Google Scholar] [CrossRef]
- Yu, D.; Wei, Z.; Zhang, X.; Zeng, Y.; Wang, C.; Chen, G.; Shen, Z.X.; Du, F. Boosting Zn2+ and NH4+ Storage in Aqueous Media via In-Situ Electrochemical Induced VS2/VOx Heterostructures. Adv. Funct. Mater. 2021, 31, 2008743. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. MoSe2 Nanosheet Array with Layered MoS2 Heterostructures for Superior Hydrogen Evolution and Lithium Storage Performance. ACS Appl. Mater. Interfaces 2017, 9, 44550–44559. [Google Scholar] [CrossRef]
- Pu, X.; Song, T.; Tang, L.; Tao, Y.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Rose-like vanadium disulfide coated by hydrophilic hydroxyvanadium oxide with improved electrochemical performance as cathode material for aqueous zinc-ion batteries. J. Power Sources 2019, 437, 226917. [Google Scholar] [CrossRef]
- Kumar, N.A.; Dar, M.A.; Gul, R.; Baek, J.-B. Graphene and molybdenum disulfide hybrids: Synthesis and applications. Mater. Today 2015, 18, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhu, X.; Chen, X.; Zhang, Q.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. VS2 nanosheets vertically grown on graphene as high-performance cathodes for aqueous zinc-ion batteries. J. Power Sources 2020, 477, 228652. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Zhang, Z.-Z.; Li, M. One-pot synthesis of 1T MoS2/MWCNT hybrids for enhanced zinc-ion storage. Nano Futures 2022, 6, 025001. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).