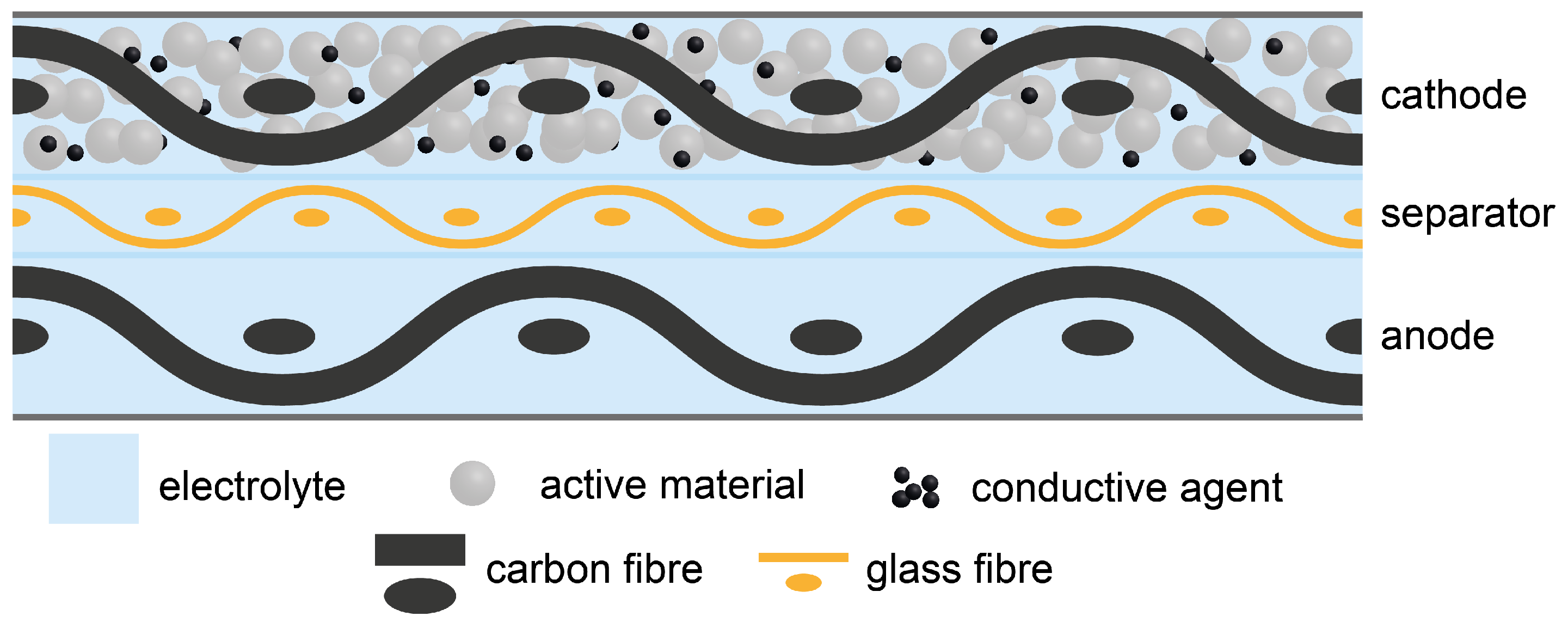
3.1. Cathode Half Cells
Structural cathodes were prepared as described in
Section 2.2. After coating, drying, and compression of the structural cathodes, the mass fraction of the carbon fibre in the cathode is approximately 53
. Taking into account the density of the carbon fibres and the coated cathode materials, this corresponds to a fibre volume content of 64%. No difference of the fibre volume content could be detected between the cathodes compressed at 500
and 5000
. According to
Section 2.2 cathode half cells have been built using a polymer film separator and lithium metal anode, to evaluate the electrochemical performance of the structural cathodes. The specific discharge capacity of the cathode half cells is presented in
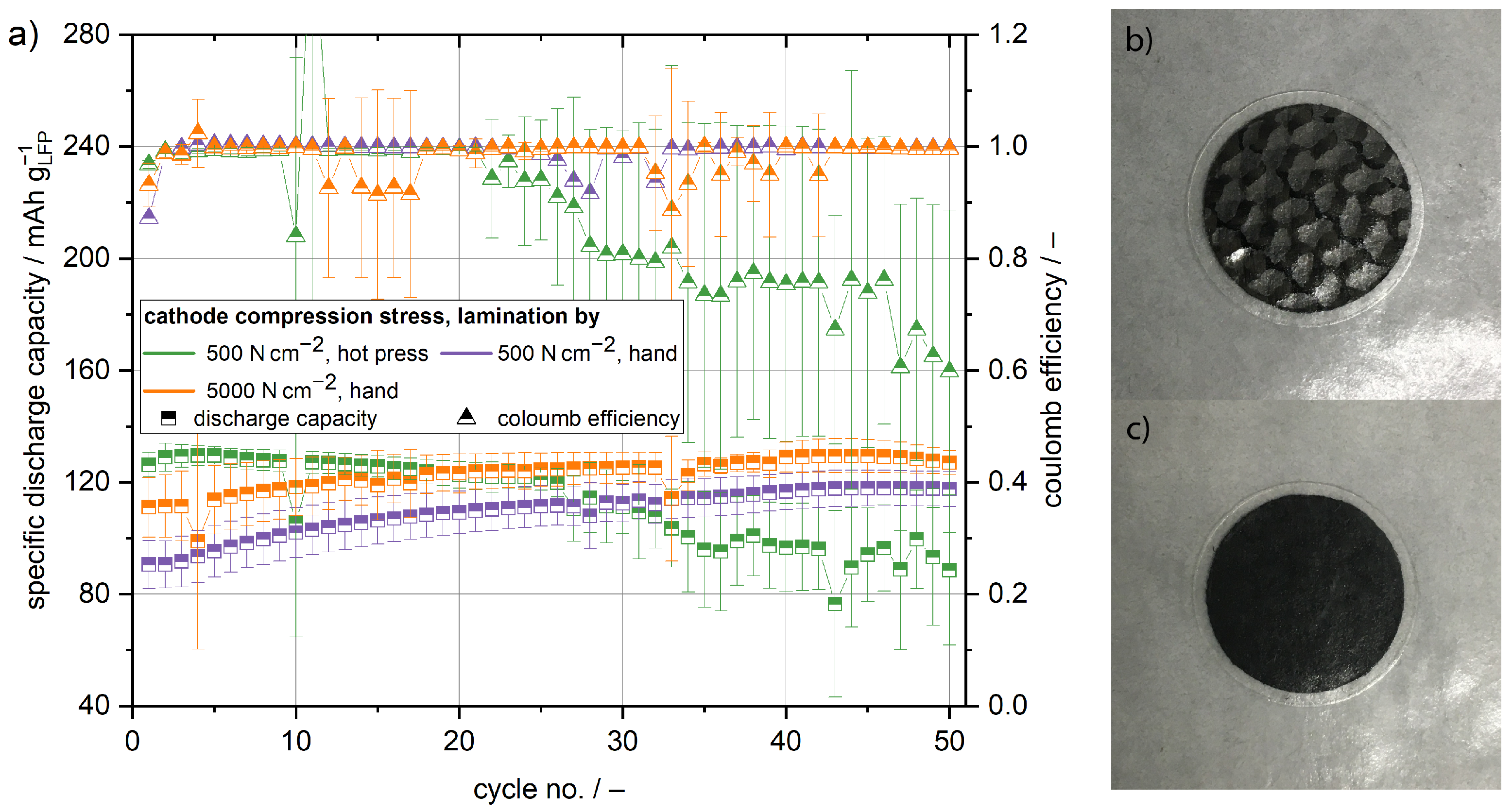
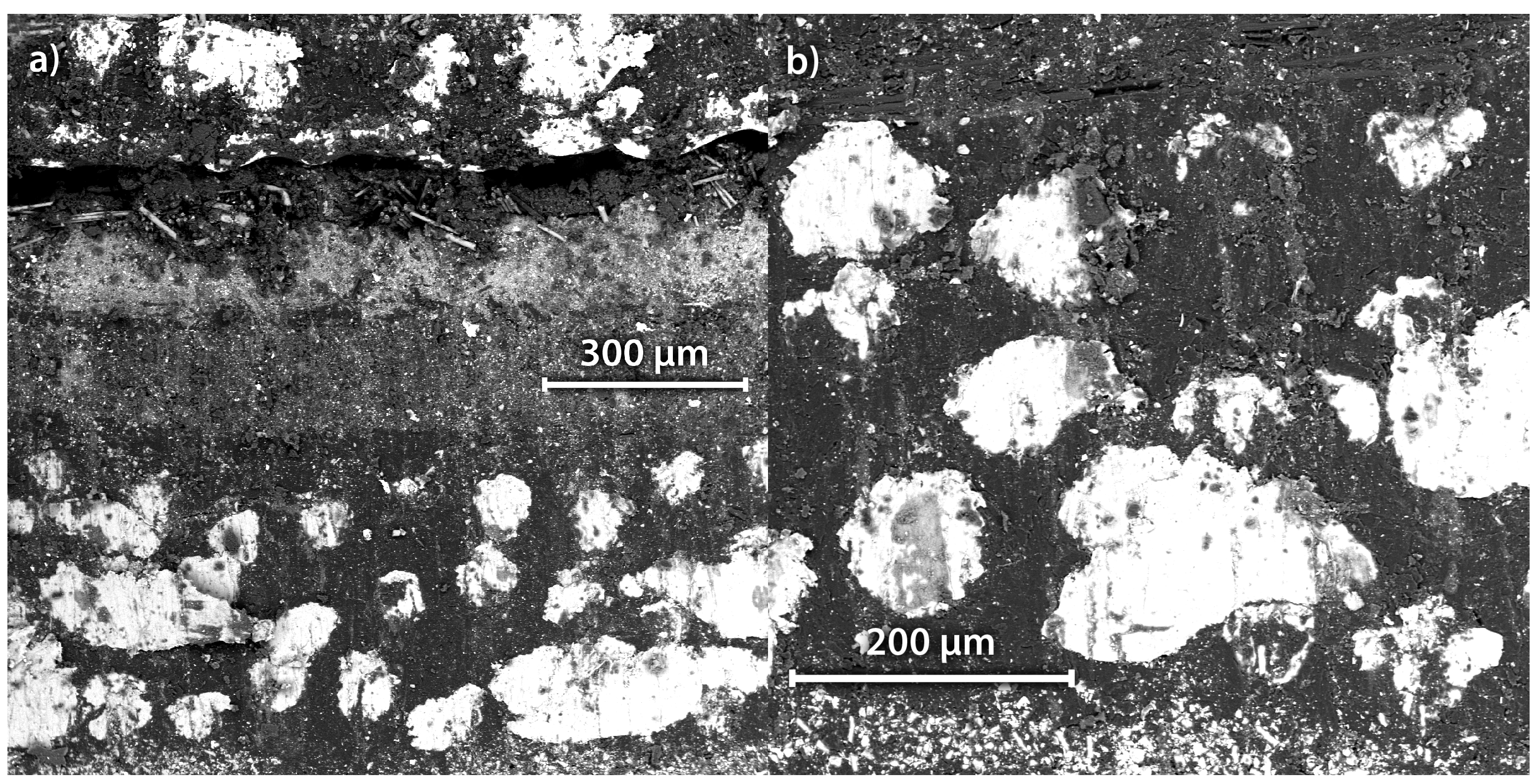
Figure 2. Lamination and compression pressure directly influence the cycling capacity of the cathode half cells. Comparing the initial cycle of the manually laminated cells, the cathodes compressed with 5000
exhibit higher capacities than the cathodes compressed at 500
( 113
compared to 91
). With the increasing cycle number the capacity of both cathodes increases. After 50 cycles, a capacity of 125
and 118
was reached for the cathode compressed at 5000
and 500
, respectively. The increasing capacity is most probably a result of better active material utilisation due to the improved contact of the cathode and the polymer film separator. From
Figure 2b,c, it is obvious that the manual lamination results in insufficient contact between the structural cathode and the polymer film separator. The regions of insufficient contact are visible as brighter spots. Due to the pressure in the coin cell housing and the cyclisation temperature of 80 °C, the contact between the structural cathode and polymer film separator improves over time. This is seconded by the voltage profiles of different cycles shown in
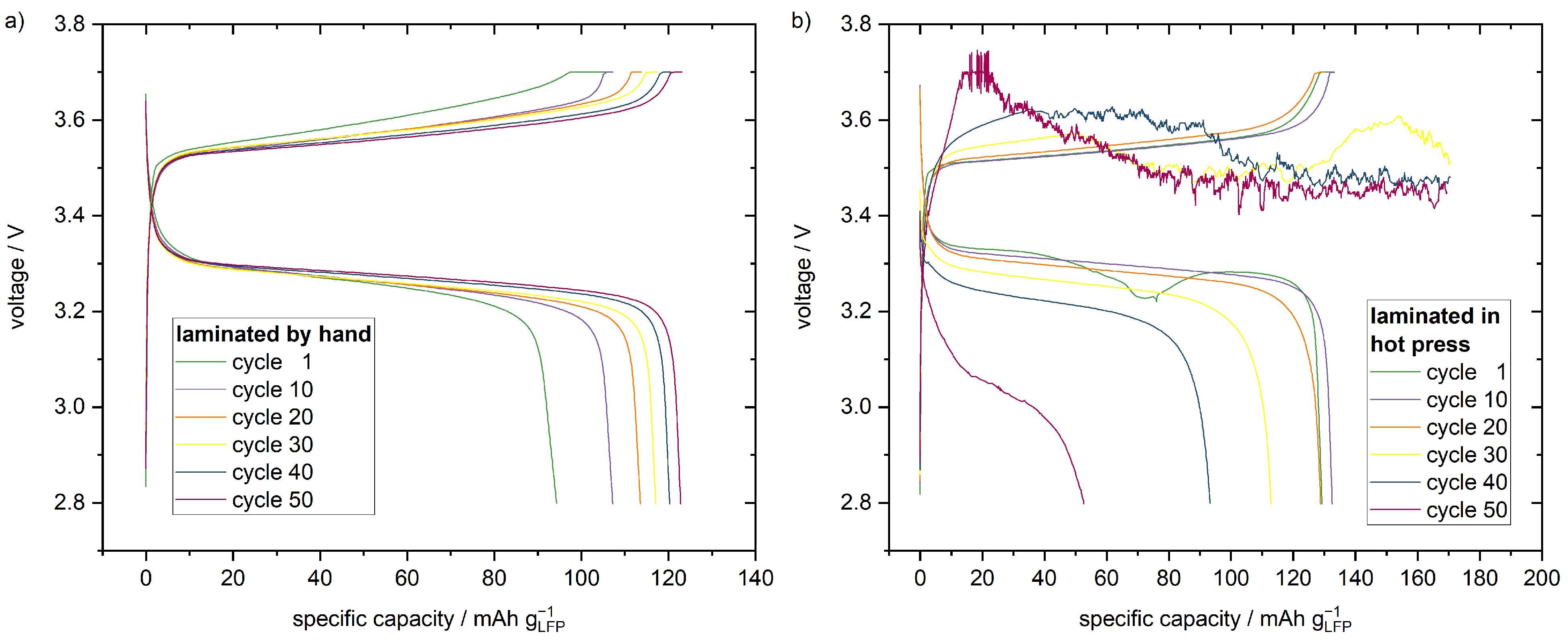
Figure 3. The specific charge that is extracted at the plateau voltage of around 3.3 V during discharge increases with the increasing cycle number and, therefore, the active material utilisation increases, as well. Furthermore, the plateau voltage during charging slightly decreases and increases during discharging with increasing cycle number. This is another indicator for a reduction of the cell resistance, due to the improved contact between cathode and separator.
In order to achieve better initial active material utilisation, cells were produced by using a hot press to laminate the electrodes and separator. In
Figure 2c, no brighter spots are visible, indicating proper contact between the cathode and the separator. This is supported by the polarisation of the cells during cycling. At a state of charge equalling to 20
, the cells laminated in the hot press exhibit a cell polarisation of 210 mV in the first cycle, which is smaller compared to the 300 mV polarisation of the manually laminated cells, as shown in
Figure 3. Therefore, the inner resistance of the cells laminated in the hot press is smaller. It is reasonable to assume the decrease in cell resistance is a result of better interface contact after hot pressing in comparison to manual lamination. Due to the initially better interface contact, the cells laminated in the hot press exhibit with 126
the highest capacity in the first cycle. However, in contrast to the manually laminated cells, the capacity fades with increasing cycle number, but during the first 25 cycles only slightly. This observed small capacity fading is in good agreement with previous studies researching solid state batteries with LFP as a cathode active material and a PEO based polymer electrolyte [
25,
26,
27]. In the later cycles, much more pronounced capacity fading occurs. In general, the coulomb efficiency is well above 99% during the first 20 cycles, but subsequently decreases rapidly. Until cycle 50, the coulomb efficiency drops to around 60%. The accelerated decrease of the capacity and the coulomb efficiency can not be explained by the usual degradation of the material system. A likely explanation for the faster degradation are micro short circuits over the separator due to lithium dendrite growth. The voltage profile reveals that the cells do not reach their cutoff potential in the region of rapid capacity fading and most probably, the current is conducted over the separator. This explanation is in accordance with to Homann et al. [
28], who reported noisy voltages during the galvanostatic cycling of LFP-based cathodes with a PEO-based separator. In the case of the structural cathodes, the lamination process plays a critical role for this behaviour. During the lamination in the hot press, higher pressure is applied to the cathode compared to the lamination by hand. Because of the inhomogeneous thickness of woven carbon textiles, the separator is locally subject to even higher pressure during the lamination in the hot press, which results in weak points vulnerable to dendrite growth and short circuits.
The cathodes were further cycled with a structural separator, which is a glass fibre textile that is impregnated with a electrolyte composed of PEO and LiTFSI. The mass fraction of the glass fibres in the structural separator is found to be approximately 17
. Therefore, the fibre volume content is 10% and, thus, much lower than in the cathode. The reduced fibre volume content corresponds to a lower amount of filler material in the separator and ensures good ionic conductivity, which significantly decreases with the increasing volume of non-conducting phases [
29]. The structural separator has a thickness of approximately 90
, which is slightly thinner than the polymer film separator. Measured by electrochemical impedance spectroscopy (EIS) at a temperature of 80 °C, the structural and polymer film separator exhibit comparable ionic conductivities of 0.46
and 0.44
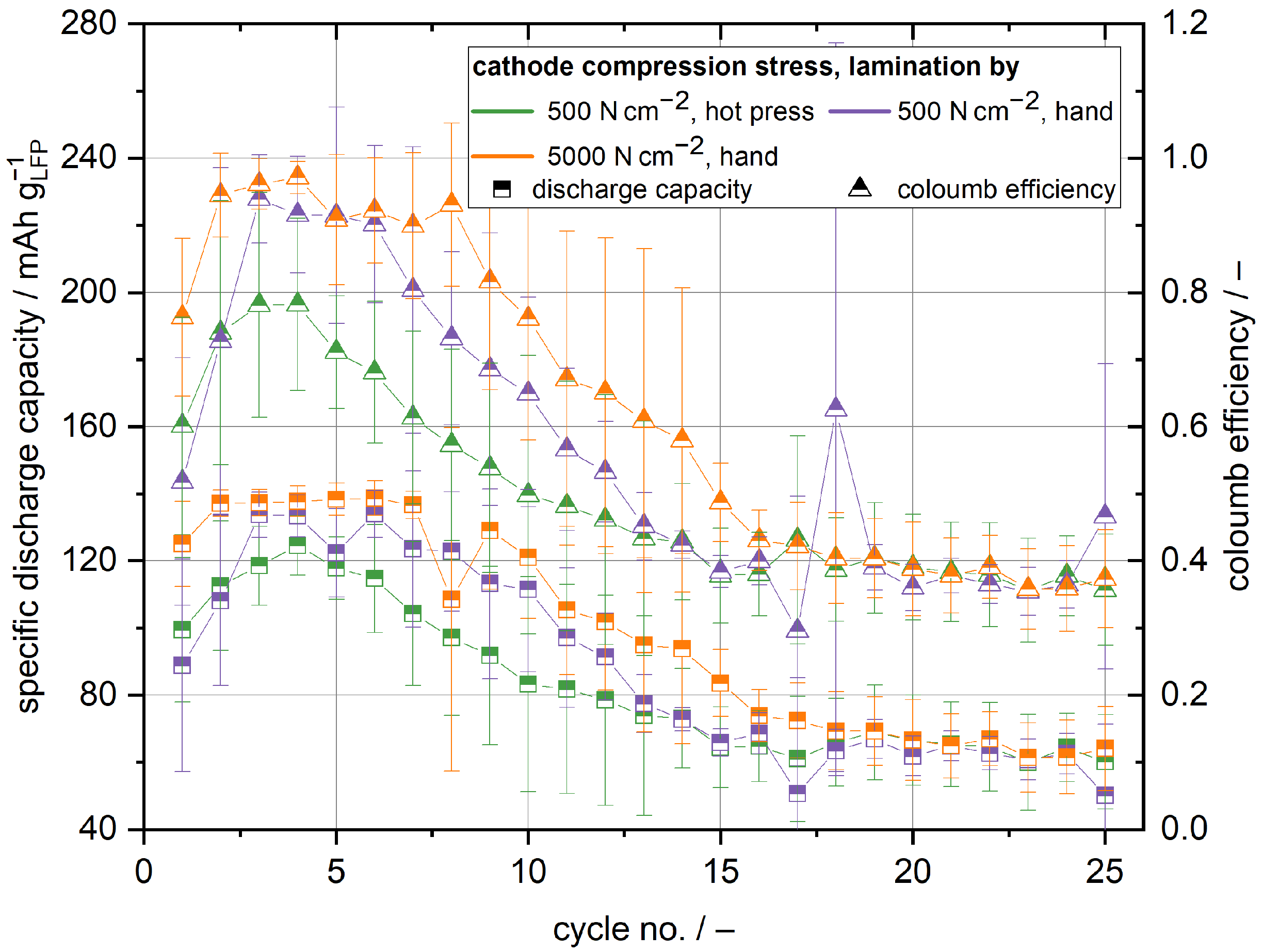
, respectively. Nevertheless, the cycling behaviour of the cathode half cells with the structural separator is found to be very different compared to cells with the polymer film separator. The cells with the cathode and separator compressed at a higher pressure achieve capacities of maximum 139
and, therefore, the best capacities of all investigated cells, see
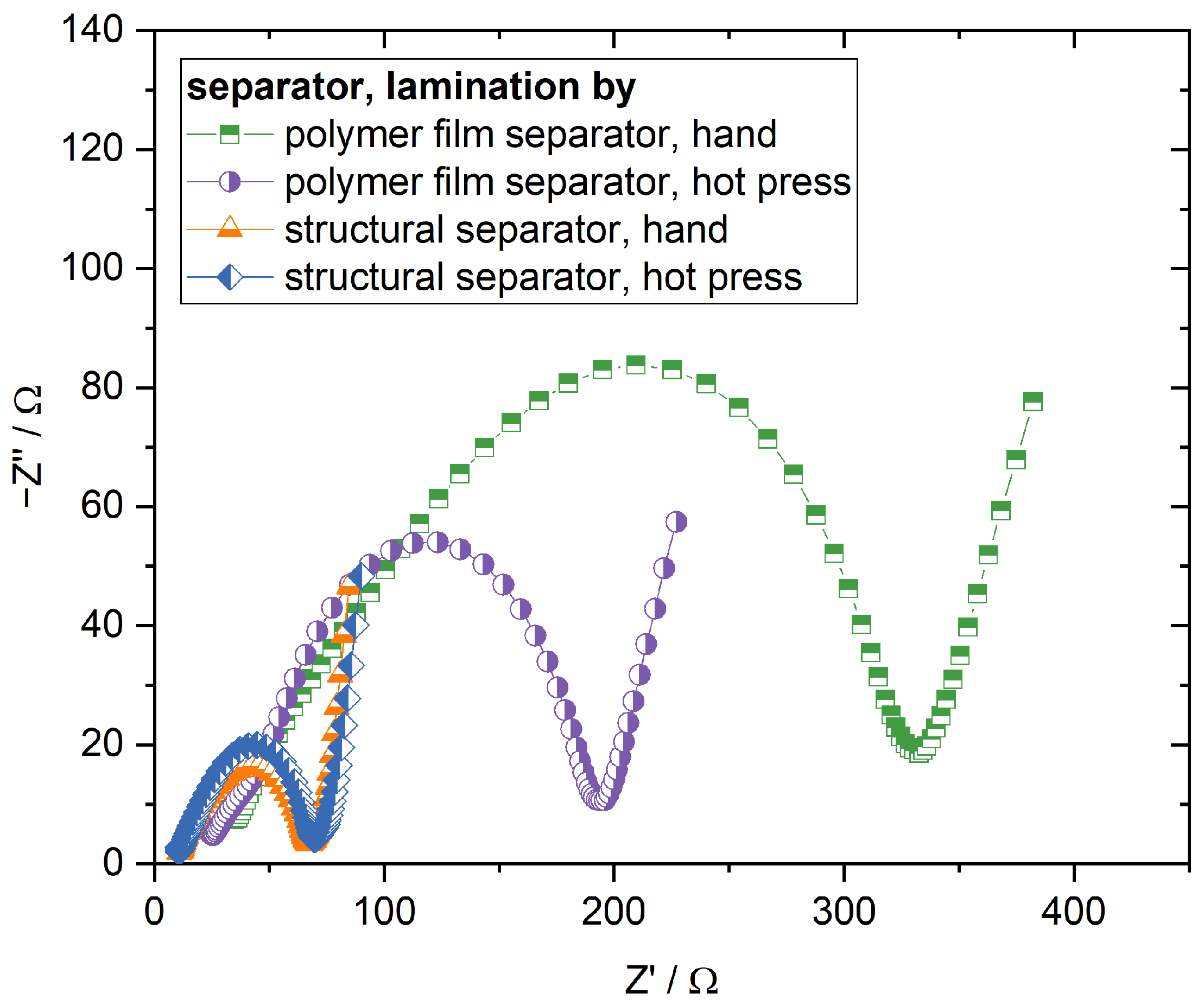
Figure 4. Because the ionic conductivity of the structural and polymer film separator are similar, the higher initial capacity in comparison to the cells with the polymer film separator can be mainly accounted for better contact between structural separator and cathode and the slightly lesser thickness of the structural separator. The discharge capacity of the manually laminated cells approaches its maximum after the first or second cycle, respectively, and remains stable for a few cycles thereafter. If one also takes into account that the maximum capacity is higher in comparison to the cells with the polymer film separator laminated in the hot press, it becomes clear that manual lamination leads to sufficient contact between the structural cathode and separator. This is verified by the EIS measurements of the cathode half cells shown in
Figure 5. While there is a significant difference in cell impedance for the cells with the polymer film separator between the manual lamination and lamination in the hot press, there is no meaningful difference when the cells with the structural separator are compared against each other. The impedance of the different cells with the structural separator is smaller than the impedance of the cells with the polymer film separator laminated in the hot press, further supporting that better interface contact is the main reason for the observed higher capacities. Moreover, the polarisation of the manually laminated cells with the structural separator at a state of charge equalling to 20
is with 200 mV smaller than the polarisation of the cells with the polymer film separator laminated in the hot press, as well. The comparison of the different lamination techniques shows again that lamination in the hot press does not have any beneficial influence in comparison to the cells with the polymer film separator. While the cell polarisation slightly decreases to 170 mV, the initial capacity is even worse compared to the manually laminated cells. Unlike for the cells with the polymer film separator, it is evident that the capacity already degrades after a few cycles significantly, independent of the lamination process. The pronounced capacity fading is accompanied by a decrease in coulomb efficiency. After 15 cycles, no coulomb efficiency higher than 50% was observed. The decrease in capacity and coulomb efficiency can be explained by micro short circuits caused by lithium dendrite growth similar to the polymer film separator [
28]. It can be concluded that the structural separator does not withstand lithium dendrite growth as well as the polymer film separator, because the degradation happens much faster and is more pronounced. This is supported by a maximum coulomb efficiency of only 97% observed for the manually laminated cells, which is relatively poor for this type of system [
26,
30]. The coulomb efficiency of the hot press-laminated cells does not reach any values above 78%. This indicates that the structural separator is like the polymer film separator locally weakened by the lamination in the hot press, because of the inhomogeneous pressure distribution due to the inhomogeneous thickness of the fibre textiles. Therefore, micro short circuits as a result of lithium dendrite growth occur even faster in the cells laminated in the hot press. This is supported by the voltage profiles depicted in
Figure 6. The manually laminated cells reach the cut-off voltage for the charging step in the first cycles, but during the successive cycles the voltage becomes noisy in the charging step. Similar to the cathode half cells with the polymer film separator, which have been laminated in the hot press, the voltage noise is accompanied by a degradation of the discharge capacity. The cells that have been laminated in the hot press never reach the cut-off voltage in the charging step and exhibit even faster capacity degradation.
3.2. Anode Half Cells
In order to assess the specific capacity of the carbon fibre anode, half cells have been built. The fibre mass fraction of the structural anode is approximately 62
. This is equivalent to a fibre volume content of 67%. From
Figure 7, it is evident that the capacity changes significantly with the cycle number. During the first fifteen cycles, the capacity increases from 17
to 92
. During further cycling, the capacity decreases again. The anode half cells have been laminated in the hot press. Therefore, the increasing capacity is not related to an improvement of the interface contact, but must be explained by structural changes of the carbon fibres and, thus, the structural anode. In accordance to Snyder et al. [
10] the formation of small fractures during the cycling is suspected to be the reason for the increasing capacity. Small fractures result in the partial exfoliation of some graphene sheets and locally damaged sizing of the carbon fibres. Both make the carbon fibres more accessible for the intercalation of lithium ions. With 92
, the maximum discharge capacity is quite poor compared to the cycling data of carbon fibres found in the literature, especially considering the rate of 0.01C with respect to the 372
capacity of graphite [
10,
31,
32,
33,
34]. No further anode half cell tests with the structural separator have been conducted, because significant problems with lithium dendrite formation were observed in the cathode half cell tests. In contrast to the findings known in literature, the carbon fibres were coated with an all-solid-state electrolyte. The wetting of the carbon fibres with an all-solid-state electrolyte seems to be insufficient compared to an electrolyte with liquid components. The cross section of a structural anode is depicted in
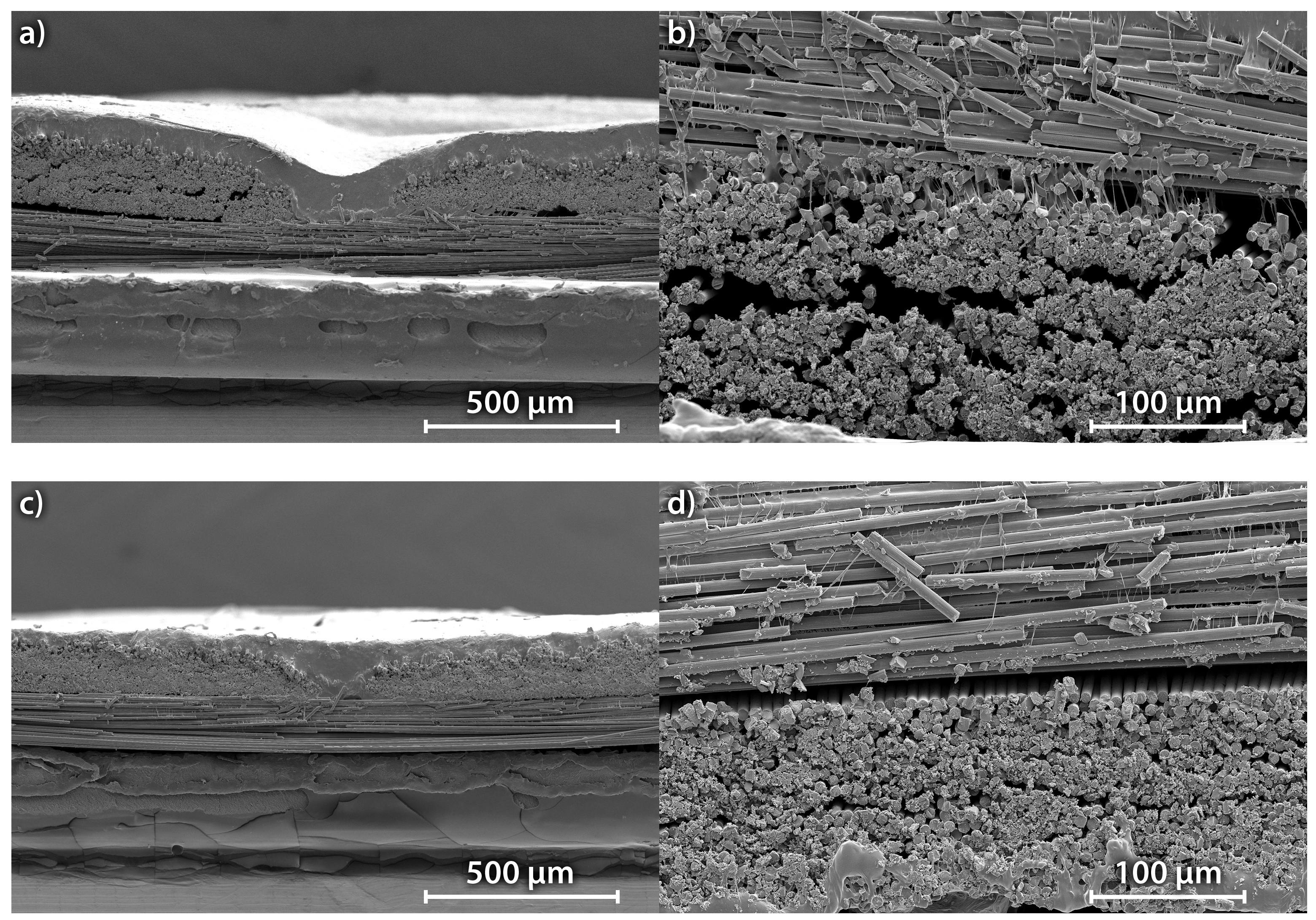
Figure 8. The electrolyte is visible as small white filaments connecting the carbon fibres. It is obvious that the wetting of the carbon fibres is insufficient, because the electrolyte covers only a small fraction of the carbon fibre surface. A large part of the electrolyte is located on top of the carbon fibre weave, especially before compression in the hot press. The compression reduces the roughness of the surface, but does not improve the wetting of the carbon fibres. Even after compression, big pores can be detected in the scanning electron microscope (SEM) images. Therefore, the accessibility of the available carbon is worse compared to the usage of a liquid electrolyte, which perfectly wets all the carbon fibres. Serious improvements of the distribution of the solid electrolyte in the carbon fibres are necessary to make a viable carbon fibre-based all-solid state anode.
The small coulomb efficiency of 56% in the first cycle reveals that a significant amount of lithium ions is irreversibly lost. Previous studies have revealed that even with the liquid electrolyte, significant amounts of lithium are irreversibly trapped in the carbon fibres [
31,
32,
33]. It is reasonable to assume that the lithium diffuses into the deeper layers of the carbon fibres and cannot be accessed anymore upon discharge. This is a major issue in this study, because of the poor wetting of the structural anodes with the solid electrolyte. The carbon fibres are only partially in contact with the electrolyte and are, therefore, only partially lithiated during charging. The partial lithiation results in a concentration gradient of lithium within the fibres and, therefore, in the diffusion of lithium within the fibres. Improving the wetting of the carbon fibres with the solid electrolyte is crucial to improve the capacity and coulomb efficiency of structural anodes. As lithium is trapped too, when a liquid electrolyte is used, further prelithiation of the carbon fibres might be necessary to reduce initial lithium losses and achieve sufficient capacities in the future. With the increasing cycle number, the coulomb efficiency increases as more of the theoretical capacity of the carbon fibres can be accessed. From
Figure 7, it is evident that the overpotentials decrease and the plateau region increases with the increasing cycle number. During the first charge cycle (intercalation of lithium into the carbon fibre), a small second plateau region around 0.8 V is visible. This vanishes in the consecutive cycles and, thus, is a result of irreversible processes contributing to the poor coulomb efficiency, especially in the first cycle.
3.3. Full Cells
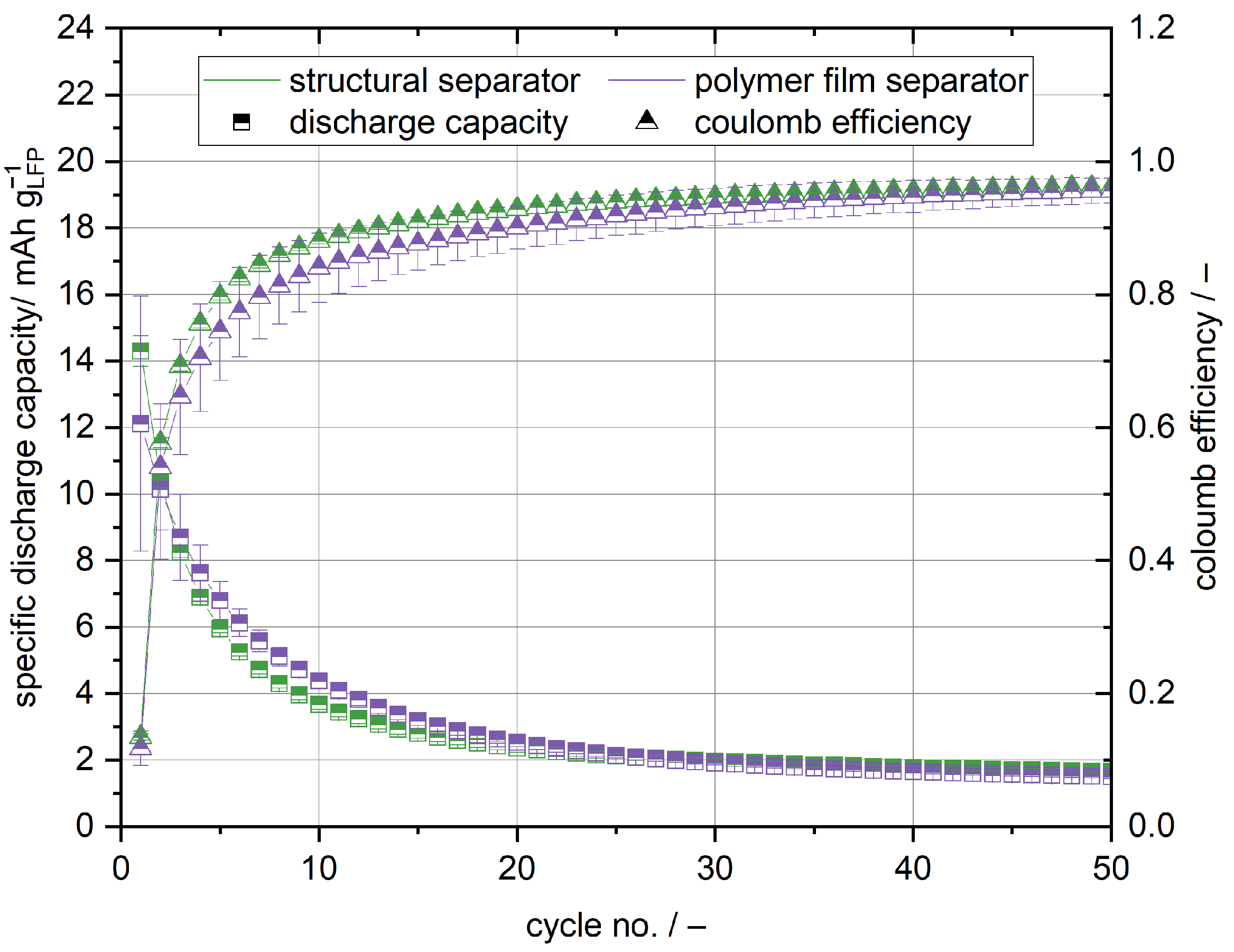
The cells with the structural cathode and anode exhibit no significant capacity regardless of the used separator, as shown in
Figure 9. The highest capacity of 14
can be accessed in the initial cycle. In the later cycles, the capacity significantly drops to below 2
. Similarly to the anode half cells, the coulomb efficiency is very low in the initial cycle, but increases to over 95% after 50 cycles. The general trend of capacity and coulomb efficiency is in accordance with the cycling data of the anode half cells. Especially in the first cycles, lithium is trapped in carbon fibres and is lost due to irreversible side reactions [
31,
32,
33]. However, in contrast to the half cell setup, only the lithium in the active material is available for the energy storage and conversion, so that the lithium supply is limited. Lithium depletes and the capacity decreases with increasing cycle number.
On the contrary to the cathode half cell setup, no decrease of the coulomb efficiency with cycle life is apparent even though the cell stacks have been laminated in the hot press and regardless of the used separator. This supports the hypothesis that in the cathode half cell setup lithium dendrite growth is responsible for capacity fading and the decrease of the coulomb efficiency. Lithium dendrites are much less of an issue in the full cell setup, as no lithium metal anode is used [
35].
In summary, the anode is the bottle neck of this structural battery setup. In order to increase the capacity, it is necessary to prevent the high initial lithium losses and, therefore, to increase the lithium availability. To cope with this issue, the wetting of the carbon fibres in the structural anode needs to be improved to reduce the diffusion of lithium into non-contacted areas of the carbon fibre due to concentration gradients arising from inhomogeneous lithiation, as shown in
Section 3.2. However, since initial lithium losses can even be observed with liquid electrolyte, which perfectly wets the carbon fibres, prelithiation of the carbon fibres might additionally be necessary to increase the lithium availability. This issue needs to be addressed in further research.
3.4. Structural Battery Demonstrator
In addition to the previously described coin cells, a structural battery in the pouch cell format (cathode:
, separator:
, anode:
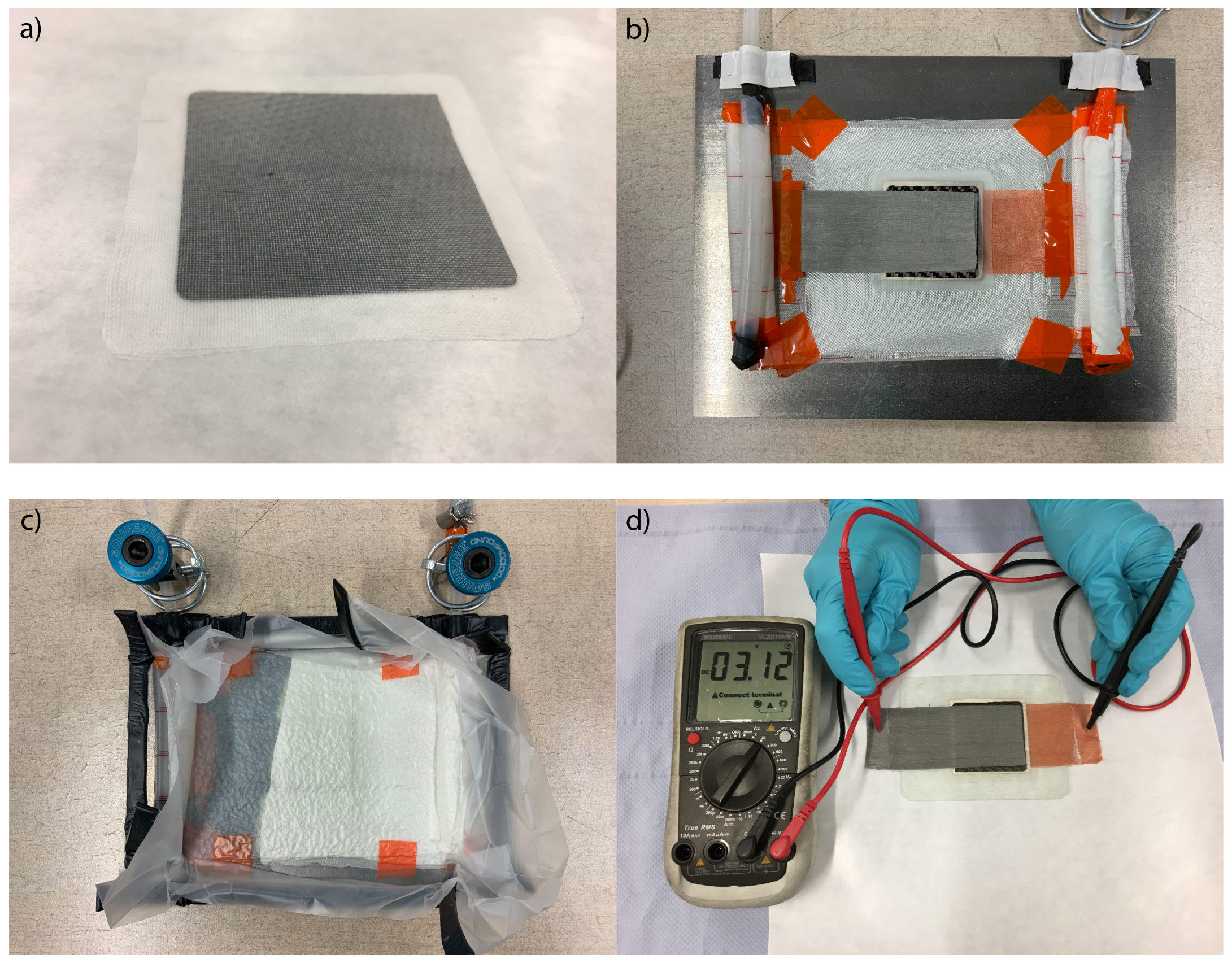
) was built. For the structural battery demonstrator, a lithium metal anode was used, because, as discussed before, lithium depletion is an issue when using the presented structural carbon fibre anode. The preparation of the demonstrator is shown in
Figure 10. All constituents were dried for at least 4 h at 40 °C in a vacuum climate chamber before use. All work was conducted in a dry room with a dew point better than −40 °C. Structural cathode, structural separator, and lithium metal have been laminated in a hot press applying a pressure of 100
at 80 °C. The lay up for the composite integration was prepared on a metal plate coated with chemlease 255 release agent. The laminated cell stack was sandwiched between four sheets of Interglas 91111 glass fibre. A nickel mesh was used as current collector for the cathode and a copper mesh was used for the anode. Additional layers of peel ply and fleece have been used, to ensure a good vacuum and homogeneous epoxy infiltration. On two opposing sides, spiral tubes were fixated, one for the connection to the vacuum pump and one for the supply of epoxy resin. The whole set up was sealed airtight with vacuum bagging film and sealing tape. After the evacuation of residual air, the set up was infiltrated with epoxy resin L and the hardener EPH 161 in a 100:25 mixture by weight. After 24 h curing at 20 °C, the structural battery was demolded.
Initially, the same cycling protocol as for the cathode half cells and full cells was tested for the demonstrator. Because of the very high overpotentials, the demonstrator could not be charged, as the charging step was instantly terminated in
Section 2.5, by the described voltage and current criteria. Therefore, the potential window for the cycling procedure had to be increased. The cutoff voltage for charging was increased to 4 V and the cut off voltage for discharging was decreased to 2.5 V. Furthermore, the charge and discharge rate were reduced to 0.02C in an effort to cope with the high overpotentials and the dendrite growth observed during the cycling of the cathode half cells with the structural separator. From
Figure 11, it is obvious that the cell voltage nearly instantly reaches the cut off voltage of 4 V during the charge and decreases to approximately 2.9 V during discharge. For comparison with the corresponding cathode half cell setup, the voltage plateaus were found to be around 3.5 V for the charge and 3.3 V for discharge. A likely reason for the observed overpotentials is found in the infiltration procedure with the epoxy resin. It cannot be ruled out that epoxy resin leaked in between some of the functional components, although the electrodes and separator have been laminated before the infiltration. A higher inner resistance and, therefore, overpotential would be the logical consequence. As the metal meshes used as a current collector were only mechanically connected to the electrodes due to the vacuum applied during infiltration, it is highly reasonable that epoxy resin partially isolated both from each other. To further investigate this issue, the demonstrator was cut under ambient atmosphere with a scroll saw and SEM pictures of the cut were taken. In
Figure 12, the different functional layers of the structural battery demonstrator can be clearly identified. The visible layers are from top to bottom: the copper mesh current collector, a fracture as a consequence of the degradation of the lithium metal anode during the cutting under ambient atmosphere, the structural separator, the structural cathode, and the nickel mesh current collector. It is clearly visible that epoxy resin is partially isolating the strands of the metal mesh current collectors from each other. It is suspected that the connection of the metal mesh current collectors can be optimised by the use of an electrical conducting silver glue.
As shown in
Figure 11, the maximum specific capacity of 124
is reached in the first cycle. Considering the weight of the electrodes and the separator, a maximum specific energy of 64
is achieved. The metal mesh current tab, surrounding the glass fibres and epoxy resin, have been neglected in this calculation. Similar to the cathode, half cells with a glass fibre separator capacity fading occurs. After 10 cycles, the capacity decreased to 111
. Much more pronounced is the capacity fading visible for the later cycles. The coulomb efficiency reaches no value higher than 89%. In accordance with the cathode half cells with the glass fibre separator, dendrite growth is the suspected reason for poor coulomb efficiency and pronounced capacity fading. The current profiles in
Figure 11 support that micro short circuits occur in the structural battery demonstrator, as well. Since the charging is reduced to the constant voltage step due to the high overpotentials, the characteristic noise is visible in the current during charging and not the voltage. Moreover, it is obvious that the resistance of the structural battery demonstrator increases with the cycle life as the current decreases. Most likely different polarisation processes such as concentration-, activation- and interface-polarisation contribute to the increase in cell resistance. To quantify the contribution of the different processes, in-depth EIS and distribution of relaxation times (DRT) analyses would be necessary, but this is beyond the scope of this study. The current decreases with cycle life, but, because of the micro short circuits, it is never below 50% of its nominal value, which is one the cutoff criteria of the charging step; therefore, the charging step is always terminated by the time criterion described in
Section 2.5. As a result, the absolute charge capacity decreases with the decreasing current. To overcome the issue of dendrite formation, it is necessary to improve the mechanical properties of the solid state electrolyte [
35]. For the current system, a reduction of the cyclisation temperature could improve the mechanical properties. The mechanical properties of polymers are strongly dependant on the temperature [
36]. In general, it is desirable to reduce the temperature requirements of the structural battery composites to enable a less restricted use. However, in contrast to the mechanical properties, the ionic conductivity of a polymer electrolyte decreases with decreasing temperature [
37]. To date, there is no all-solid-state electrolyte that fully satisfies the requirements of structural battery composites with respect to the mechanical properties, ionic conductivity, stability, and processability. Once new polymer electrolyte generations become available, the described fabrication processes can be easily adapted to greatly enhance the properties of the proposed structural battery composites.