Water-Soluble Conductive Composite Binder for High-Performance Silicon Anode in Lithium-Ion Batteries
Abstract
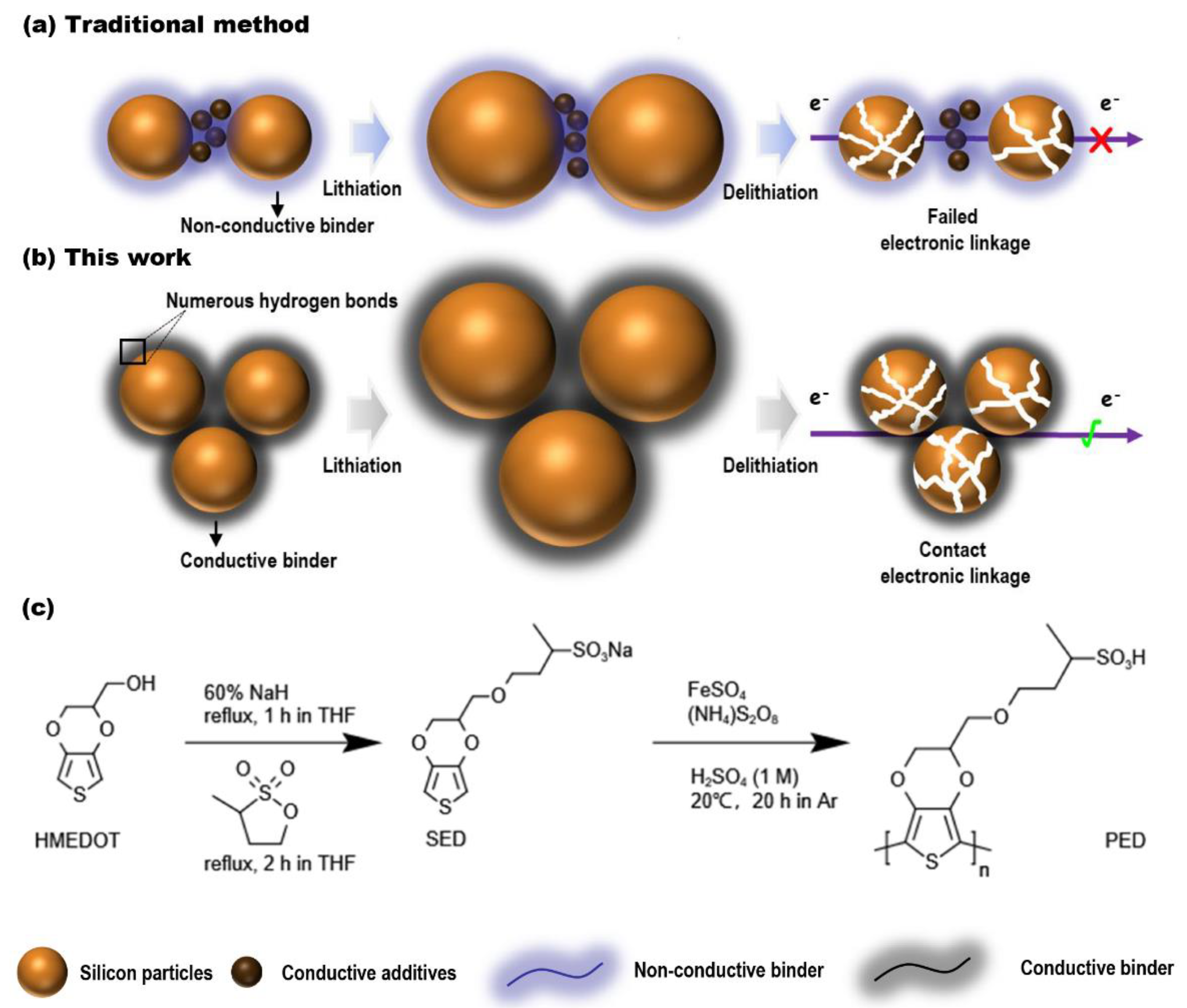
:1. Introduction
2. Material and Methods
2.1. Synthesis of the Conductive Polymer
2.1.1. Synthesis of Sodium 4-[(2,3-dihydrothieno [3,4-b] [1,4] dioxin-2-yl) methoxy] butane-2-sulfonate (SED Monomer)
2.1.2. Synthesis of PED
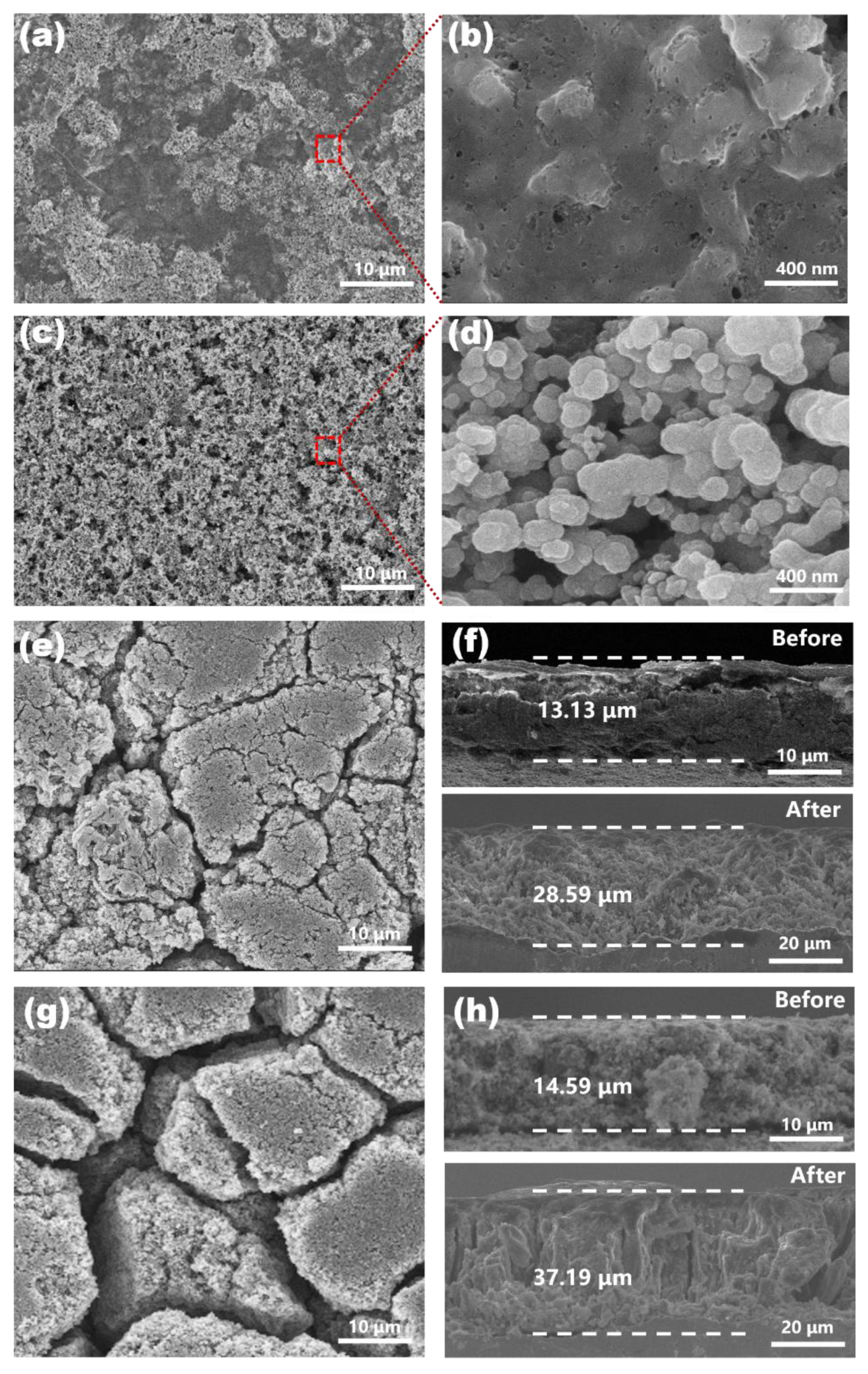
2.2. Material Characterization
2.3. Swelling Ability Test of Binders
2.4. Electrode Preparation
2.5. Adhesion Performance of the Binders
2.6. Electrochemical Measurements
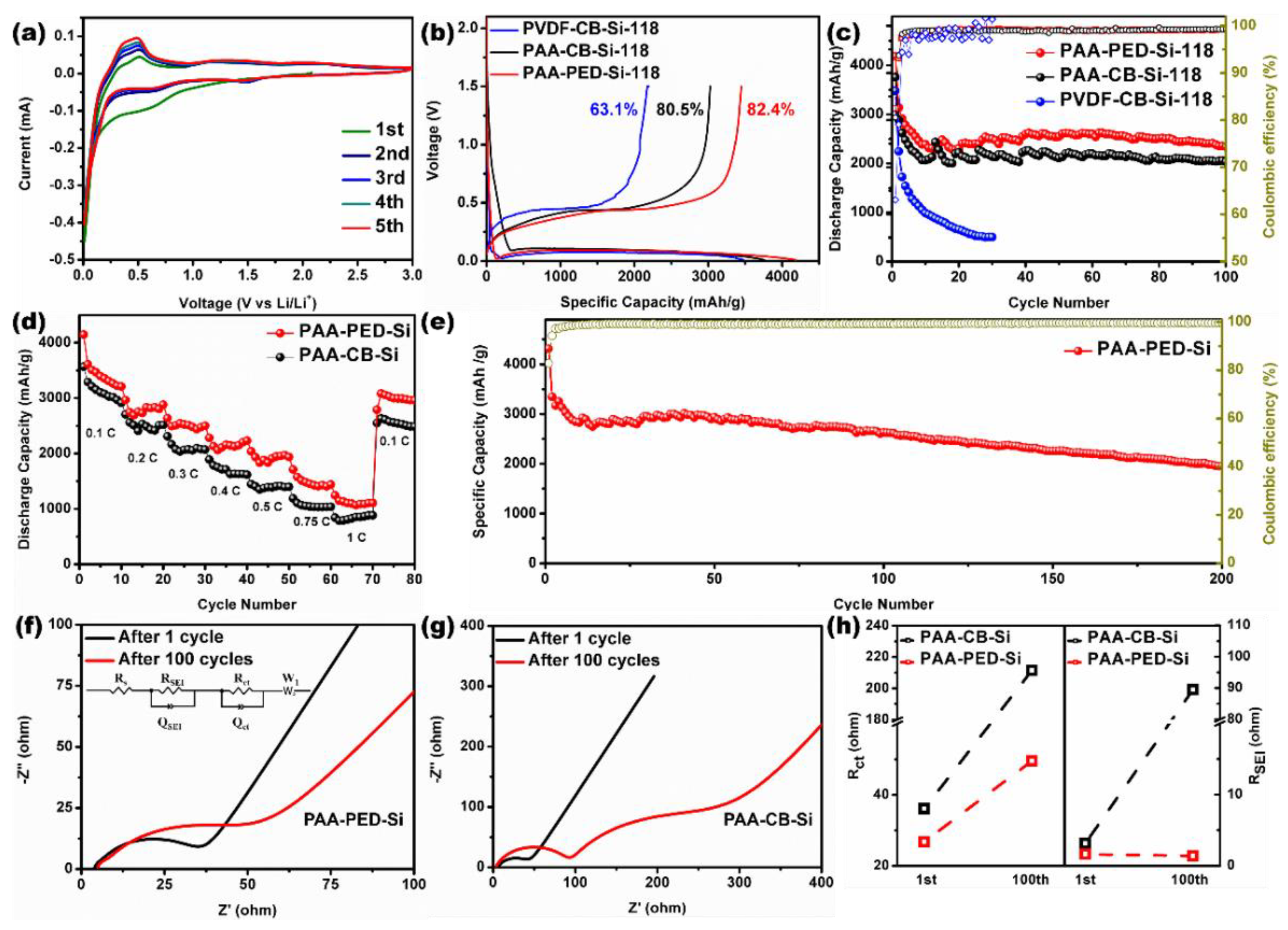
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Ma, Z.; Pan, F.; Curtiss, L.A.; Amine, K. The role of nanotechnology in the development of battery materials for electric vehicles. Nat. Nanotechnol. 2016, 11, 1031–1038. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics tuning of li-ion diffusion in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, T.; Hu, Z.; Wei, Y.; Song, X.; Ren, Y.; Wang, W.; Rao, M.; Lin, Y.; Chen, Z.; et al. Tuning of thermal stability in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2016, 138, 13326–13334. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Minnici, K.; Park, J.J.; Lee, S.R.; Zhang, G.; Takeuchi, E.S.; Takeuchi, K.J.; Marschilok, A.C.; Reichmanis, E. SWNT anchored with carboxylated polythiophene “links” on high-capacity li-ion battery anode materials. J. Am. Chem. Soc. 2018, 140, 5666–5669. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Tsumura, T.; Dimov, N. Electrochemical behaviors of silicon based anode material. J. Power Sources 2005, 146, 10–14. [Google Scholar] [CrossRef]
- Kim, H.; Seo, M.; Park, M.H.; Cho, J. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 2010, 49, 2146–2149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Chemomechanical modeling of lithiation-induced failure in high-volume-change electrode materials for lithium ion batteries. NPJ Comput. Mater. 2017, 3, 8986. [Google Scholar] [CrossRef]
- Li, J.; Dahn, J.R. An in situ x-ray diffraction study of the reaction of li with crystalline si. J. Electrochem. Soc. 2007, 154, A156. [Google Scholar] [CrossRef]
- Mazouzi, D.; Lestriez, B.; Roueé, L.; Guyomard, D. Silicon composite electrode with high capacity and long cycle life. Electrochem. Solid ST. 2009, 12, A215. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, G.; Wang, K.; Li, M.; Yang, J. Encapsulation of core-satellite silicon in carbon for rational balance of the void space and capacity. Chem. Commun. 2019, 55, 10531–10534. [Google Scholar] [CrossRef]
- Michan, A.L.; Parimalam, B.S.; Leskes, M.; Kerber, R.N.; Yoon, T.; Grey, C.P.; Lucht, B.L. Fluoroethylene carbonate and vinylene carbonate reduction: Understanding lithium-ion battery electrolyte additives and solid electrolyte interphase formation. Chem. Mater. 2016, 28, 8149–8159. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, S.; Ha, J.H.; Kim, S.W.; Lee, J.; Park, S.; Cho, B.-W.; Choi, H.-J. Enhancing the stability of silicon nanosheets electrodes by fluoroethylene carbonate. Chem. Phys. Lett. 2017, 684, 383–389. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Zou, F.; Manthiram, A. A review of the design of advanced binders for high-performance batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Li, J.; Lewis, R.B.; Dahn, J.R. Sodium carboxymethyl cellulose. Electrochem. Solid ST 2007, 10, A17. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for li-ion battery si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.-K. Progress of 3d network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Ling, M.; Xu, Y.; Zhao, H.; Gu, X.; Qiu, J.; Li, S.; Wu, M.; Song, X.; Yan, C.; Liu, G.; et al. Dual-functional gum arabic binder for silicon anodes in lithium ion batteries. Nano Energy 2015, 12, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.-T.; Huang, L.; Sun, S.-G. A robust ion-conductive biopolymer as a binder for Si anodes of lithium-ion batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S.-I. Silicon microparticle anodes with self-healing multiple network binder. Joule 2018, 2, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Mu, T.; Shen, B.; Meng, Q.; Lu, G.; Lou, S.; Zuo, P.; Ma, Y.; Du, C.; Yin, G. Stable silicon anodes realized by multifunctional dynamic cross-linking structure with self-healing chemistry and enhanced ionic conductivity for lithium-ion batteries. Nano Energy 2022, 99, 107334. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, D.; Xie, H.; Wang, D.; Hu, C.; Dai, L. In-situ construction of chemically bonded conductive polymeric network for high-performance silicon microparticle anodes in lithium-ion batteries. J. Power Sources 2022, 539, 231591. [Google Scholar] [CrossRef]
- Liu, G.; Xun, S.; Vukmirovic, N.; Song, X.; Olalde-Velasco, P.; Zheng, H.; Battaglia, V.S.; Wang, L.; Yang, W. Polymers with tailored electronic structure for high capacity lithium battery electrodes. Adv. Mater. 2011, 23, 4679–4683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-M.; Kim, M.H.; Choi, S.Y.; Lee, J.G.; Jang, J.; Lee, J.B.; Ryu, J.H.; Hwang, S.S.; Park, J.-H.; Shin, K.; et al. Poly(phenanthrenequinone) as a conductive binder for nano-sized silicon negative electrodes. Energy Environ. Sci. 2015, 8, 1538–1543. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.-H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G. A commercial conducting polymer as both binder and conductive additive for silicon nanoparticle-based lithium-ion battery negative electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Peng, X.; Zeng, W.; Jin, Z.; Tian, W.; Gao, B.; Zhou, Y.; Chu, P.K.; Huo, K. Highly stretchable conductive glue for high-performance silicon anodes in advanced lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1704858. [Google Scholar] [CrossRef]
- Jonas, F.; Krafft, W.; Muys, B. Poly(3, 4-ethylenedioxythiophene): Conductive coatings, technical applications and properties. Macromol. Symp. 1995, 100, 169–173. [Google Scholar] [CrossRef]
- Hohnholz, D.; Okuzaki, H.; MacDiarmid, A.G. Plastic electronic devices through line patterning of conducting polymers. Adv. Funct. Mater. 2005, 15, 51–56. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective approaches to improve the electrical conductivity of Pedot:Pss: A review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Horii, T.; Hikawa, H.; Katsunuma, M.; Okuzaki, H. Synthesis of highly conductive Pedot:Pss and correlation with hierarchical structure. Polymer 2018, 140, 33–38. [Google Scholar] [CrossRef]
- Ashizawa, S.; Horikawa, R.; Okuzaki, H. Effects of solvent on carrier transport in poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate). Synth. Met. 2005, 153, 5–8. [Google Scholar] [CrossRef]
- Yano, H.; Kudo, K.; Marumo, K.; Okuzaki, H. Fully soluble self-doped poly(3,4-ethylenedioxythiophene) with an electrical conductivity greater than 1000 s cm−1. Sci. Adv. 2019, 5, eaav9492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhao, Y.; Tan, R.; Tian, L.-L.; Liu, Y.; Chen, H.; Pan, F. Novel conductive binder for high-performance silicon anodes in lithium ion batteries. Nano Energy 2017, 36, 206–212. [Google Scholar] [CrossRef]
- Jiao, X.; Yin, J.; Xu, X.; Wang, J.; Liu, Y.; Xiong, S.; Zhang, Q.; Song, J. Highly energy-dissipative, fast self-healing binder for stable si anode in lithium-ion batteries. Adv. Funct. Mater. 2020, 31, 5699. [Google Scholar] [CrossRef]
- Zeng, X.; Shi, Y.; Zhang, Y.; Tang, R.; Wei, L. Vinyltriethoxysilane crosslinked poly(acrylic acid sodium) as a polymeric binder for high performance silicon anodes in lithium ion batteries. RSC Adv. 2018, 8, 29230–29236. [Google Scholar] [CrossRef] [Green Version]
- Hays, K.A.; Ruther, R.E.; Kukay, A.J.; Cao, P.; Saito, T.; Wood, D.L.; Li, J. What makes lithium substituted polyacrylic acid a better binder than polyacrylic acid for silicon-graphite composite anodes? J. Power Sources 2018, 384, 136–144. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Thenamirtham, P.; Kalai Selvan, R. Structural and electrochemical properties of polythiophene. Appl. Surf. Sci. 2011, 257, 9063–9067. [Google Scholar] [CrossRef]
- Jang, J.; Chang, M.; Yoon, H. Chemical sensors based on highly conductive poly (3,4-ethylenedioxythiophene) nanorods. Adv. Mater. 2005, 17, 1616–1620. [Google Scholar] [CrossRef]
- Granström, M.; Inganäs, O. Electrically conductive polymer fibres with mesoscopic diameters: 1. Studies of structure and electrical properties. Polymer 1995, 36, 2867–2872. [Google Scholar] [CrossRef]
- Aasmundtveit, K.E.; Samuelsen, E.J.; Pettersson, L.A.A.; Inganäs, O.; Johansson, T.; Feidenhans’l, R. Structure of thin films of poly(3,4-ethylenedioxythiophene). Synth. Met. 1999, 101, 561–564. [Google Scholar] [CrossRef]
- Niu, L.; Kvarnström, C.; Fröberg, K.; Ivaska, A. Electrochemically controlled surface morphology and crystallinity in poly(3,4-ethylenedioxythiophene) films. Synth. Met. 2001, 122, 425–429. [Google Scholar] [CrossRef]
- Okuzaki, H.; Ishihara, M. Spinning and characterization of conducting microfibers. Macromol. Rapid Commun. 2003, 24, 261–264. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.-M.; Zhang, Y.-C.; Song, Y.; Wang, G.-K.; Liu, Y.-X.; Wu, Z.-G.; Zhong, B.-H.; Zhong, Y.-J.; Guo, X.-D. A review of rational design and investigation of binders applied in silicon-based anodes for lithium-ion batteries. J. Power Sources 2021, 485, 229331. [Google Scholar] [CrossRef]
- Liu, B.; Soares, P.; Checkles, C.; Zhao, Y.; Yu, G. Three-dimensional hierarchical ternary nanostructures for high-performance li-ion battery anodes. Nano Lett. 2013, 13, 3414–3419. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Guo, A.; Liu, D. Water-Soluble Conductive Composite Binder for High-Performance Silicon Anode in Lithium-Ion Batteries. Batteries 2022, 8, 54. https://doi.org/10.3390/batteries8060054
Li Z, Guo A, Liu D. Water-Soluble Conductive Composite Binder for High-Performance Silicon Anode in Lithium-Ion Batteries. Batteries. 2022; 8(6):54. https://doi.org/10.3390/batteries8060054
Chicago/Turabian StyleLi, Zikai, Anru Guo, and Dong Liu. 2022. "Water-Soluble Conductive Composite Binder for High-Performance Silicon Anode in Lithium-Ion Batteries" Batteries 8, no. 6: 54. https://doi.org/10.3390/batteries8060054
APA StyleLi, Z., Guo, A., & Liu, D. (2022). Water-Soluble Conductive Composite Binder for High-Performance Silicon Anode in Lithium-Ion Batteries. Batteries, 8(6), 54. https://doi.org/10.3390/batteries8060054





