Impact of Full Prelithiation of Si-Based Anodes on the Rate and Cycle Performance of Li-Ion Capacitors
Abstract
:1. Introduction
2. Results and Discussion
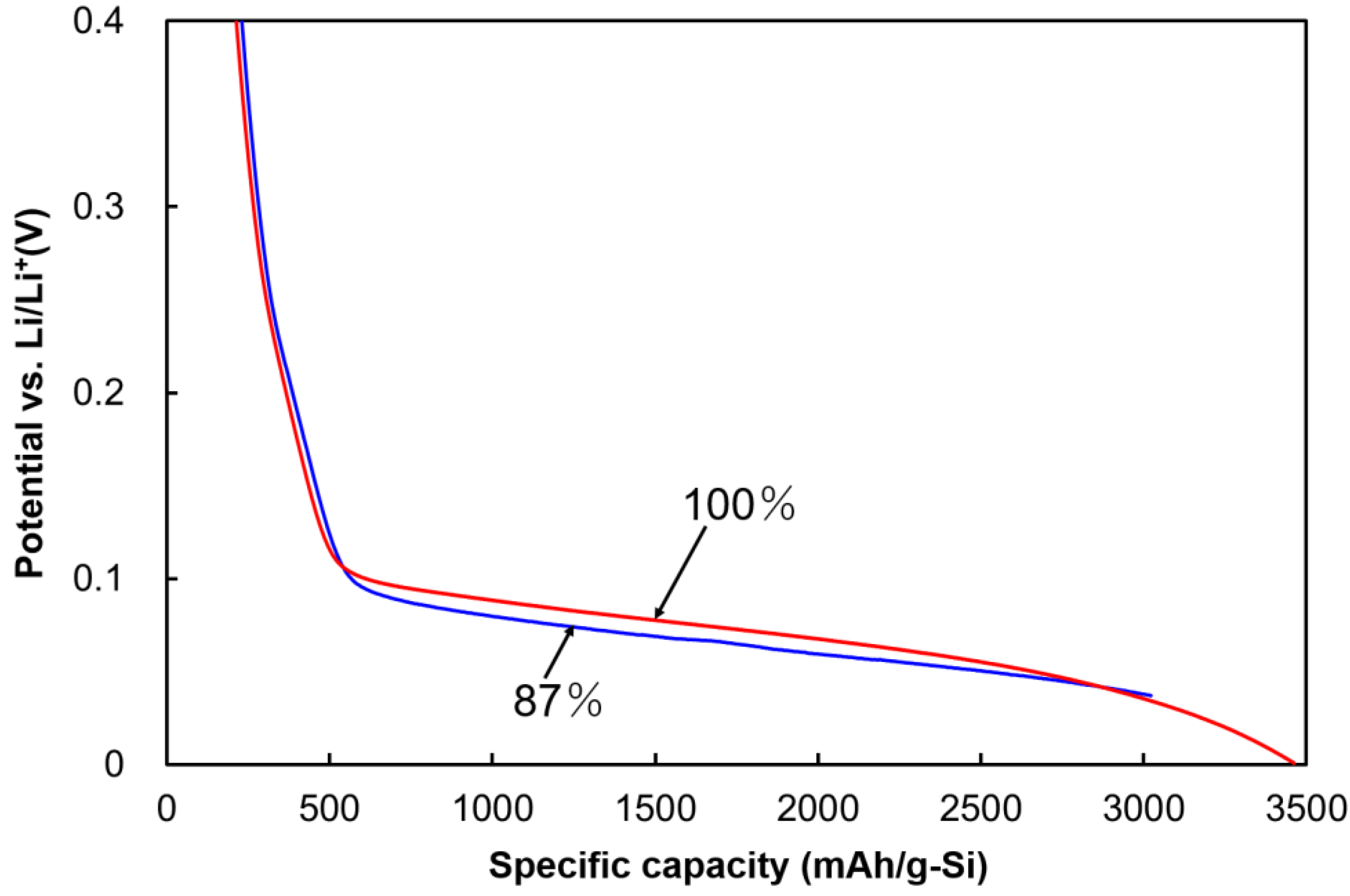
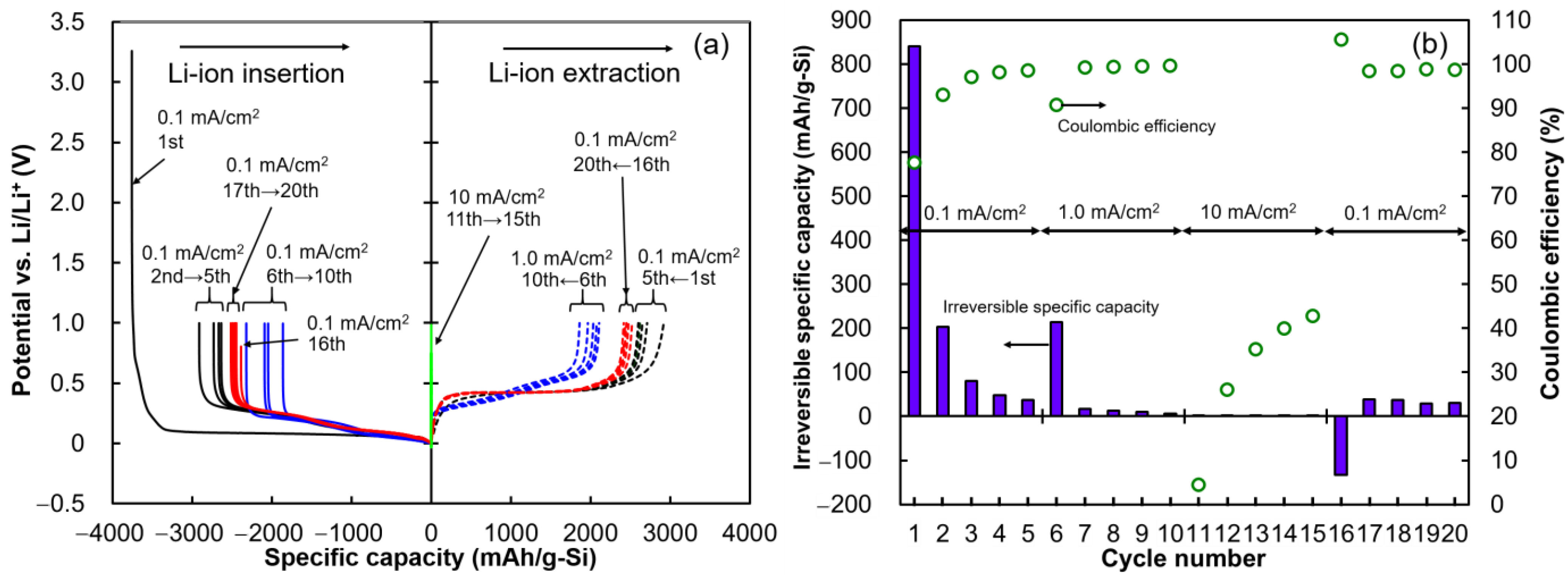
2.1. Prelithiation of Si Anode
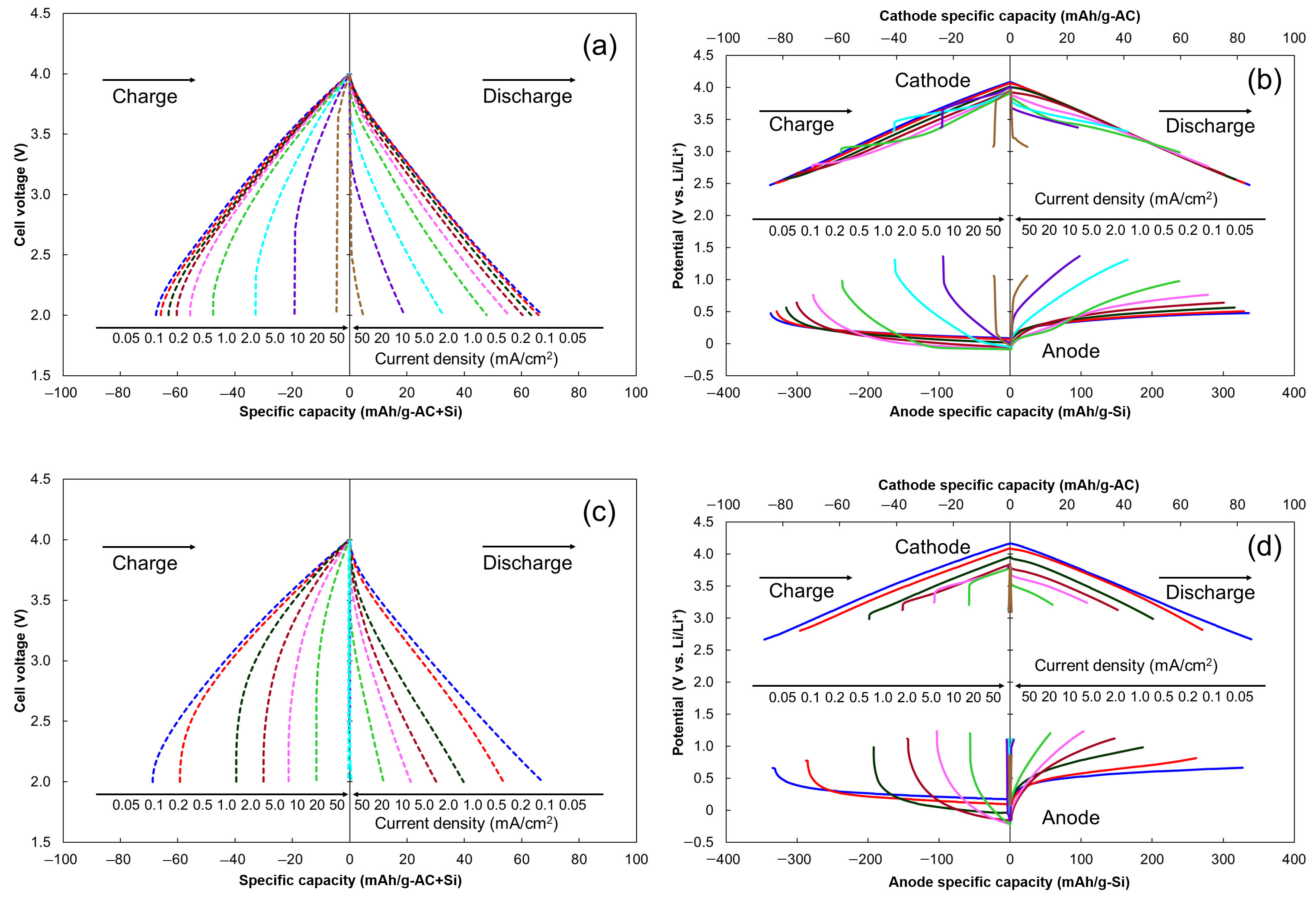
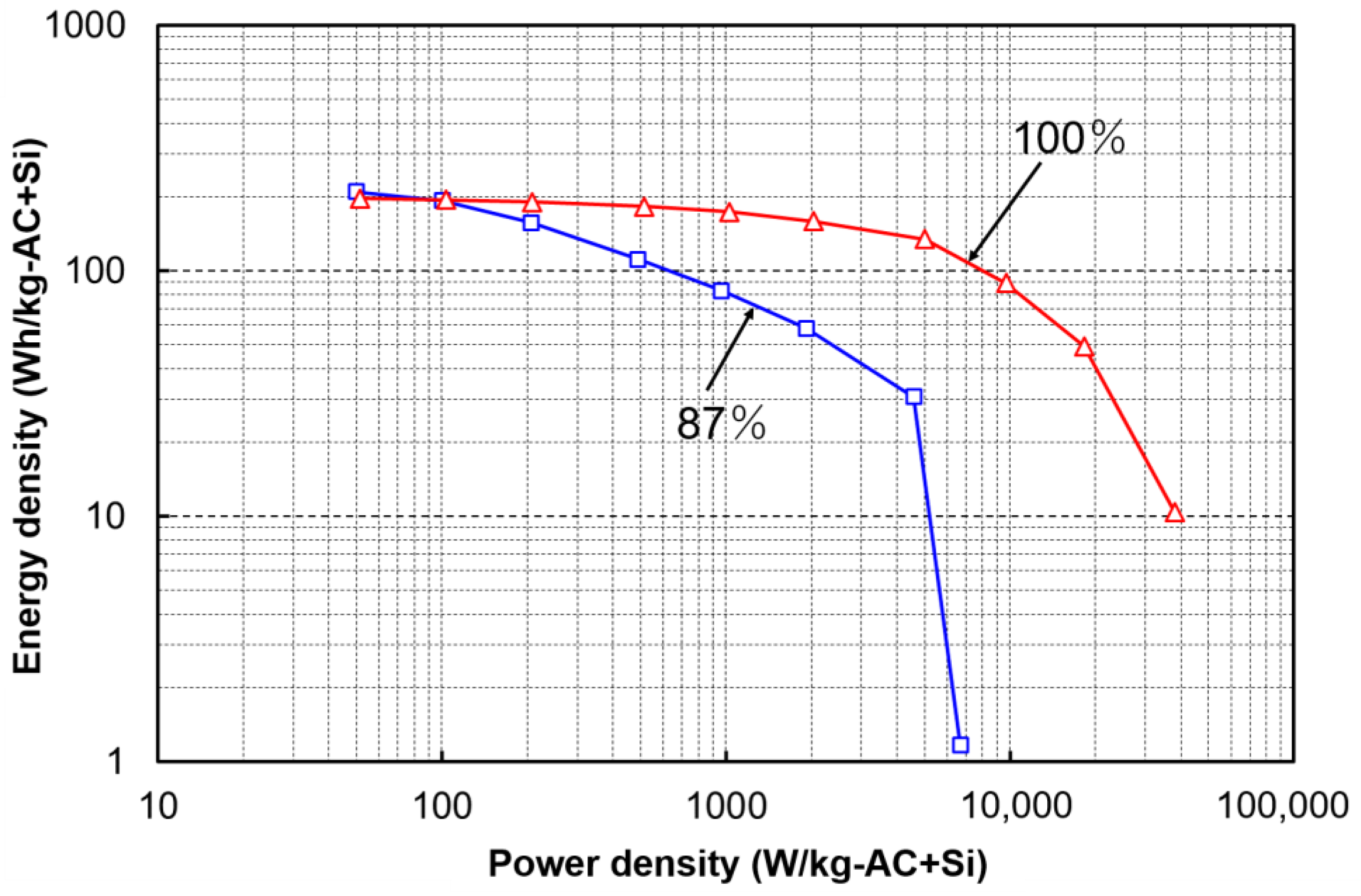
2.2. Rate Capability of LIC Cells
2.3. Impedance Analysis
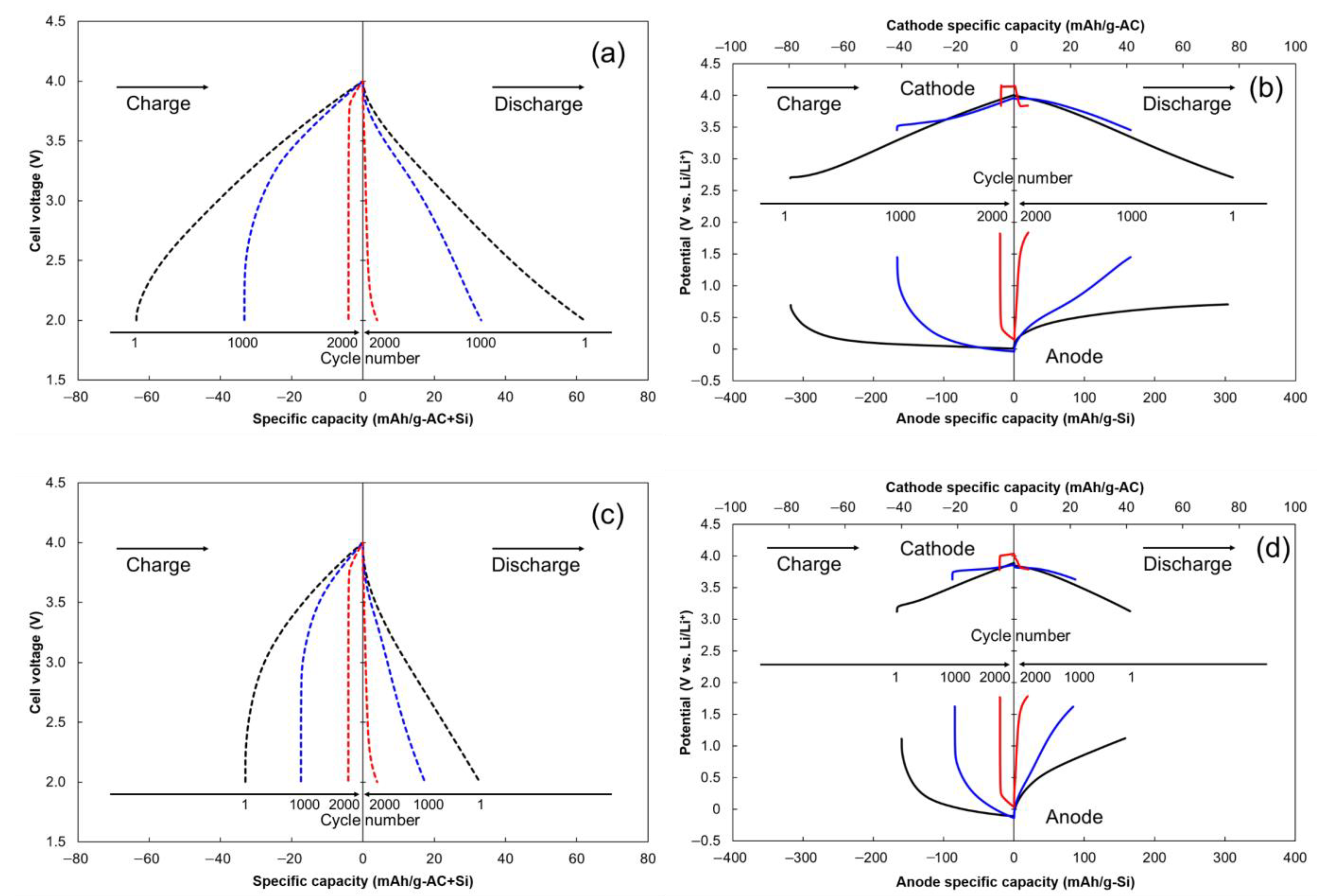
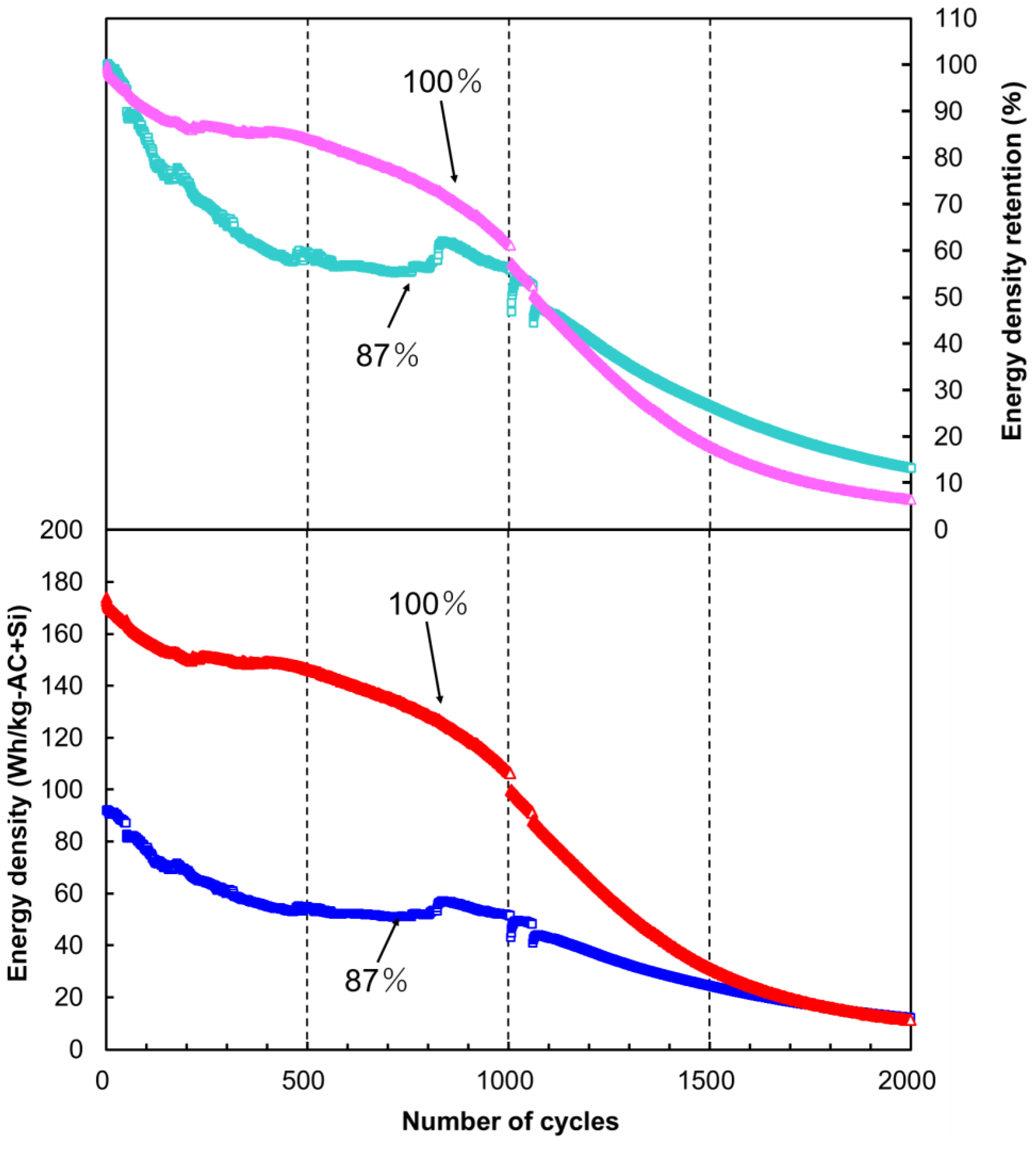
2.4. Cycle Stability of LIC Cells
2.5. Impact of Anode Full Prelithiation in Si-Based LICs
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Gong, R.; Qin, N.; Jin, L.; Zheng, J.; Wu, Q.; Zheng, J.P. The influence of electrode matching on capacity decaying of hybrid lithium ion capacitor. J. Electroanal. Chem. 2019, 845, 84–91. [Google Scholar] [CrossRef]
- Madabattula, G.; Wu, B.; Marinescu, M.; Offer, G. How to design lithium ion capacitors: Modelling, mass ratio of electrodes and pre-lithiation. J. Electrochem. Soc. 2020, 167, 013527. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, W.; Zhu, Y.; Xu, Y. Electrochemical performance of pre-lithiated graphite as negative electrode in lithium-ion capacitors. Russ. J. Electrochem. 2014, 50, 1050–1057. [Google Scholar] [CrossRef]
- Holtsiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-lithiation strategies for rechargeable energy storage technologies: Concepts, promises and challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Lamb, J.J.; Burheim, O.S. Lithium-ion capacitors: A review of design and active materials. Energies 2021, 14, 979. [Google Scholar] [CrossRef]
- Cao, W.J.; Zheng, J.P. Li-ion capacitors with carbon cathode and hard carbon/stabilized lithium metal powder anode electrodes. J. Power Sources 2012, 213, 180–185. [Google Scholar] [CrossRef]
- Cai, M.; Sun, X.; Nie, Y.; Chen, W.; Qiu, Z.; Chen, L.; Liu, Z.; Tang, H. Electrochemical performance of lithium-ion capacitors using pre-lithiated multiwalled carbon nanotubes as anode. Nano 2017, 12, 1750051. [Google Scholar] [CrossRef] [Green Version]
- Sugiawati, V.A.; Vacandio, F.; Yitzhack, N.; Ein-Eli, Y.; Djenizian, T. Direct pre-lithiation of electropolymerized carbon nanotubes for enhanced cycling performance of flexible Li-ion micro-batteries. Polymers 2020, 12, 406. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, A.; Maiti, S.; Sreemany, M.; Mahanty, S. Rock-salt-templated Mn3O4 nanoparticles encapsulated in a mesoporous 2D carbon matrix: A high rate 2 V anode for lithium-ion batteries with extraordinary cycling stability. Chem. Select 2017, 2, 7854–7864. [Google Scholar]
- Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Efficient energy storage in mustard husk derived porous spherical carbon nanostructures. Mater. Adv. 2021, 2, 7463–7472. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-based materials for lithium-ion hybrid supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Wang, J.; Shi, J.; Shi, Z. Different types of pre-lithiated hard carbon as negative electrode material for lithium-ion capacitors. Electrochim. Acta 2016, 187, 134–142. [Google Scholar] [CrossRef]
- Rauhala, T.; Leis, J.; Kallio, T.; Vuorilehto, K. Lithium-ion capacitors using carbide-derived carbon as the positive electrode—A comparison of cells with graphite and Li4Ti5O12 as the negative electrode. J. Power Sources 2016, 331, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, H.; Wang, J.; Shi, J.; Shi, Z. Pre-lithiation design and lithium ion intercalation plateaus utilization of mesocarbon microbeads anode for lithium-ion capacitors. Electrochim. Acta 2015, 182, 156–164. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Wang, C. Effect of pre-lithiation degrees of mesocarbon microbeads anode on the electrochemical performance of lithium-ion capacitors. Electrochim. Acta 2014, 125, 22–28. [Google Scholar] [CrossRef]
- Xu, N.; Sun, X.; Zhao, F.; Jin, X.; Zhang, X.; Wang, K.; Huang, K.; Ma, Y. The role of pre-lithiation in activated carbon/Li4Ti5O12 asymmetric capacitors. Electrochim. Acta 2017, 236, 443–450. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Shen, C.; Qin, N.; Zheng, J.; Wu, Q.; Zhang, C.; Zheng, J.P. A universal matching approach for high power-density and high cycling-stability lithium ion capacitor. J. Power Sources 2019, 441, 227211. [Google Scholar] [CrossRef]
- Seo, H.; Yang, H.-R.; Yang, Y.; Kim, K.; Kim, S.H.; Lee, H.; Kim, J.-H. Scalable synthesis and electrochemical properties of porous Si-CoSi2-C composites as an anode for Li-ion batteries. Materials 2021, 14, 5397. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Yeh, F.-H.; Yu, I.-S. A commercial carbonaceous anode with a-Si layers by plasma enhanced chemical vapor deposition for lithium ion batteries. J. Compos. Sci. 2020, 4, 72. [Google Scholar] [CrossRef]
- Ahn, S.; Momma, T.; Sugimoto, W.; Osaka, T. Electrodeposited Si˗O˗C as a high-rate performance anode for Li˗ion capacitor. J. Electrochem. Soc. 2019, 166, A2683–A2688. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.-M.; Zhang, Y.-C.; Song, Y.; Wang, G.-K.; Liu, Y.-X.; Wu, Z.-G.; Zhong, B.-H.; Zhong, Y.-J.; Guo, X.-D. A review of rational design and investigation of binders applied in silicon-based anodes for lithium-ion batteries. J. Power Sources 2021, 485, 229331. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/carbon composite anode materials for lithium-ion batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Pollak, E.; Salitra, G.; Baranchugov, V.; Aurbach, D. In situ conductivity, impedance spectroscopy, and ex situ Raman spectra of amorphous silicon during the insertion/extraction of lithium. J. Phys. Chem. C 2007, 111, 11437–11444. [Google Scholar] [CrossRef]
- Eguchi, T.; Sawada, K.; Tomioka, M.; Kumagai, S. Energy density maximization of Li-ion capacitor using highly porous activated carbon cathode and micrometer-sized Si anode. Electrochim. Acta 2021, 394, 139115. [Google Scholar] [CrossRef]
- Kumagai, S.; Abe, Y.; Saito, T.; Eguchi, T.; Tomioka, M.; Kabir, M.; Tashima, D. Lithium-ion capacitor using rice husk-derived cathode and anode active materials adapted to uncontrolled full-pre-lithiation. J. Power Sources 2019, 437, 226924. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Gong, R.; Zheng, J.; Xiang, Z.; Zhang, C.; Zheng, J.P. Target-oriented electrode constructions toward ultra-fast and ultra-stable all-graphene lithium ion capacitors. Energy Storage Mater. 2019, 23, 409–417. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]

| Cell | Active Material | Cathode-to-Anode Mass Ratio | |||||
|---|---|---|---|---|---|---|---|
| Mass Loading 1 (mg) | Surface Area 2 (m2) | Bulk Density 3 (g/cm3) | |||||
| Cathode | Anode | Cathode | Anode | Cathode | Anode | ||
| 100% Prelithiation Cell | 3.95 | 0.99 | 12.0 × 103 | 4.95 | 0.28 | 0.93 | 3.99 |
| 87% Prelithiation Cell | 4.00 | 1.04 | 12.2 × 103 | 5.20 | 0.25 | 0.84 | 3.85 |
| Current Density (mA/cm2) | Number of Cycles | Cycle Selected for Performance Evaluation |
|---|---|---|
| 0.05 | 3 | 2nd |
| 0.1 | 5 | 3rd |
| 0.2 | 5 | 3rd |
| 0.5 | 10 | 5th |
| 1.0 | 10 | 5th |
| 2.0 | 25 | 13th |
| 5.0 | 50 | 25th |
| 10 | 100 | 50th |
| 20 | 100 | 50th |
| 50 | 100 | 50th |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguchi, T.; Sugawara, R.; Abe, Y.; Tomioka, M.; Kumagai, S. Impact of Full Prelithiation of Si-Based Anodes on the Rate and Cycle Performance of Li-Ion Capacitors. Batteries 2022, 8, 49. https://doi.org/10.3390/batteries8060049
Eguchi T, Sugawara R, Abe Y, Tomioka M, Kumagai S. Impact of Full Prelithiation of Si-Based Anodes on the Rate and Cycle Performance of Li-Ion Capacitors. Batteries. 2022; 8(6):49. https://doi.org/10.3390/batteries8060049
Chicago/Turabian StyleEguchi, Takuya, Ryoichi Sugawara, Yusuke Abe, Masahiro Tomioka, and Seiji Kumagai. 2022. "Impact of Full Prelithiation of Si-Based Anodes on the Rate and Cycle Performance of Li-Ion Capacitors" Batteries 8, no. 6: 49. https://doi.org/10.3390/batteries8060049
APA StyleEguchi, T., Sugawara, R., Abe, Y., Tomioka, M., & Kumagai, S. (2022). Impact of Full Prelithiation of Si-Based Anodes on the Rate and Cycle Performance of Li-Ion Capacitors. Batteries, 8(6), 49. https://doi.org/10.3390/batteries8060049







