Two-Dimensional Materials for Dendrite-Free Zinc Metal Anodes in Aqueous Zinc Batteries
Abstract
1. Introduction
2. Construction Strategies of 2D Material/Zn Hybrid Anodes
2.1. Blade Coating
2.2. Langmuir-Blodgett Method and Dipping Coating
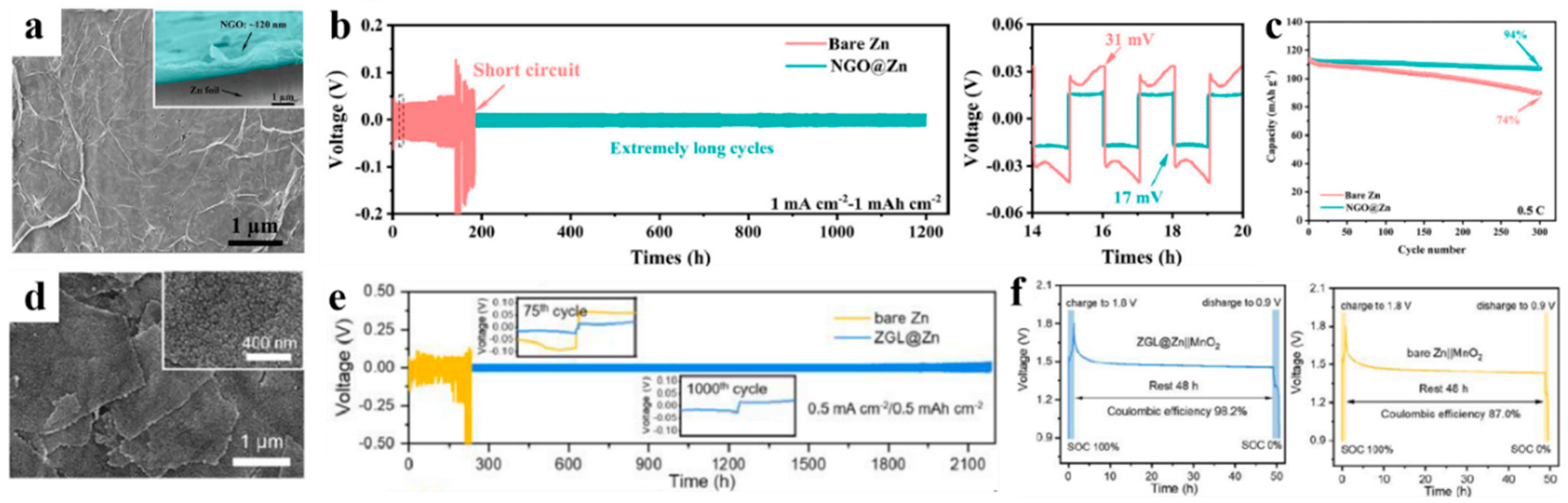
2.3. Electrochemical Deposition
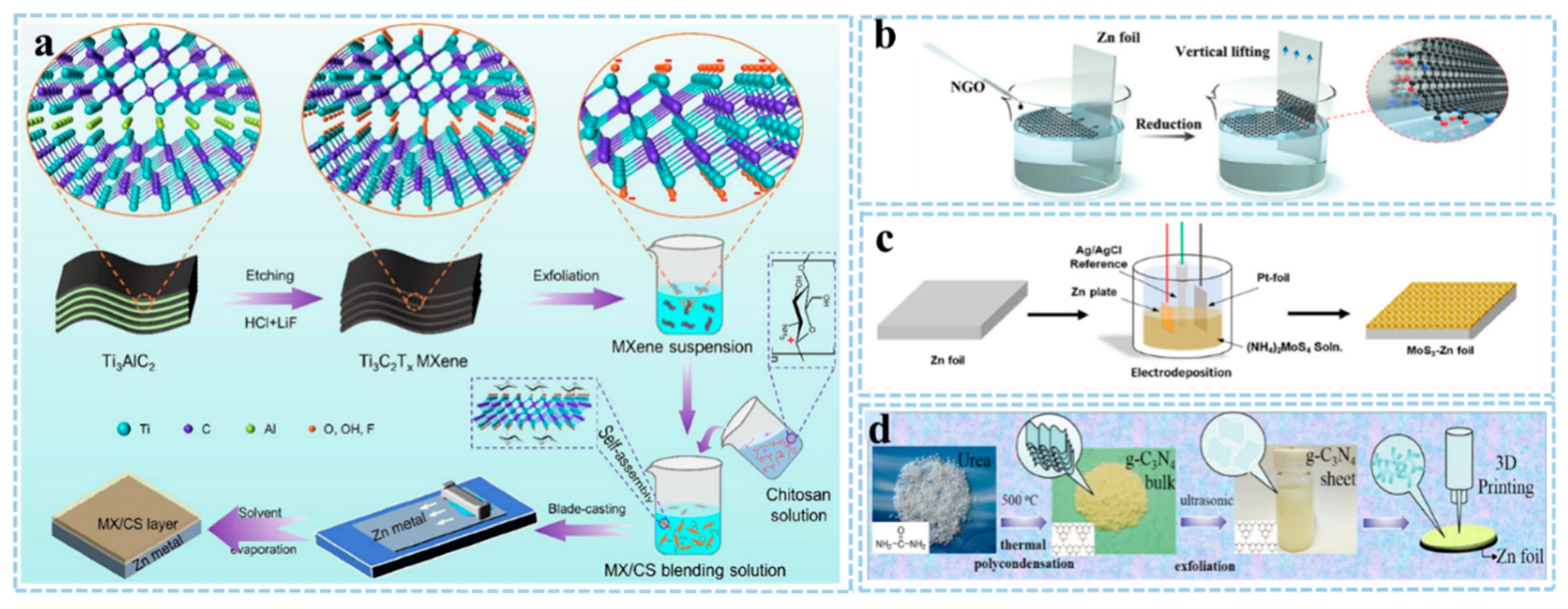
2.4. 3D Printing
2.5. Other Methods
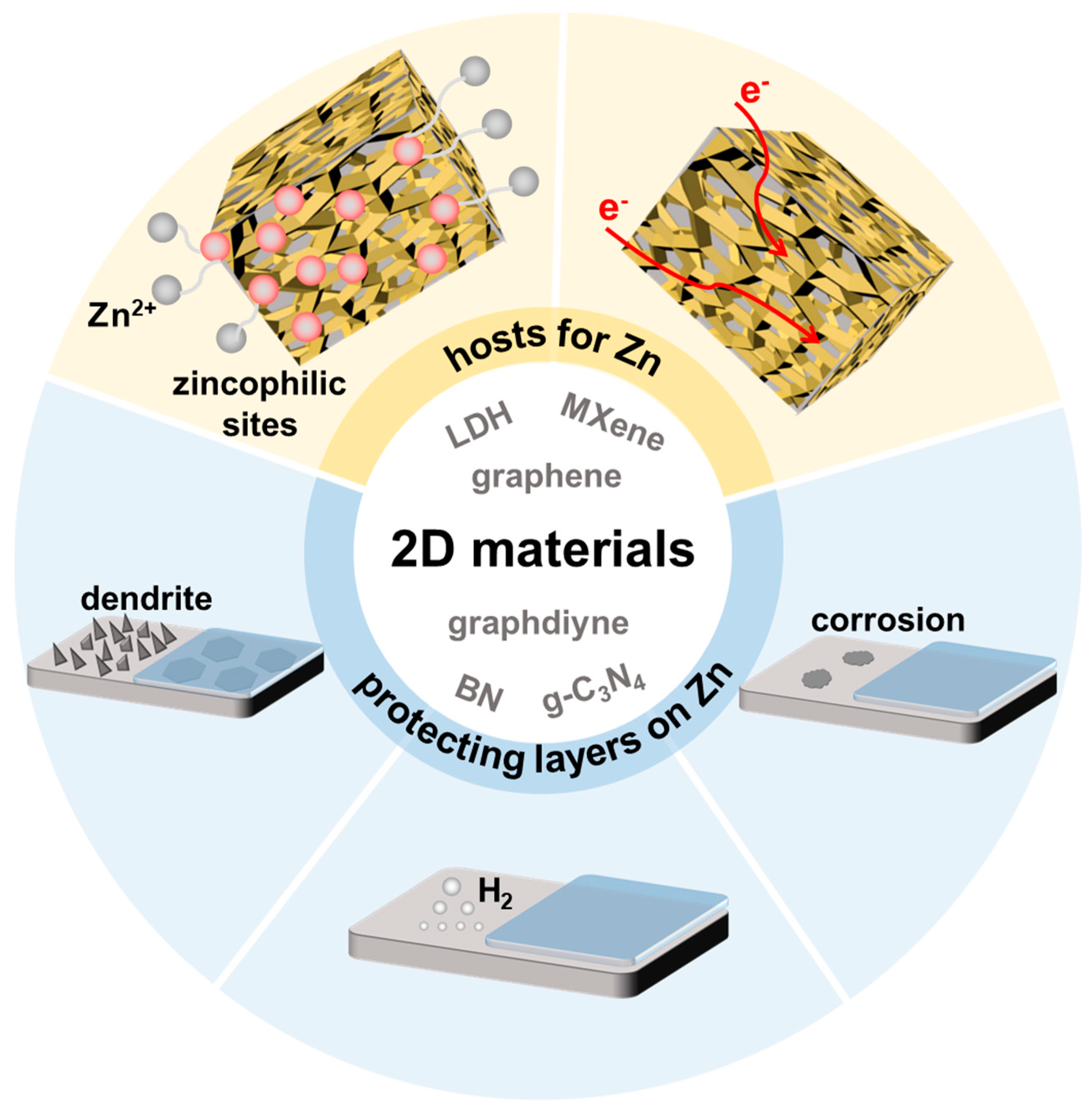
3. Application of 2D Materials in Zn Anodes
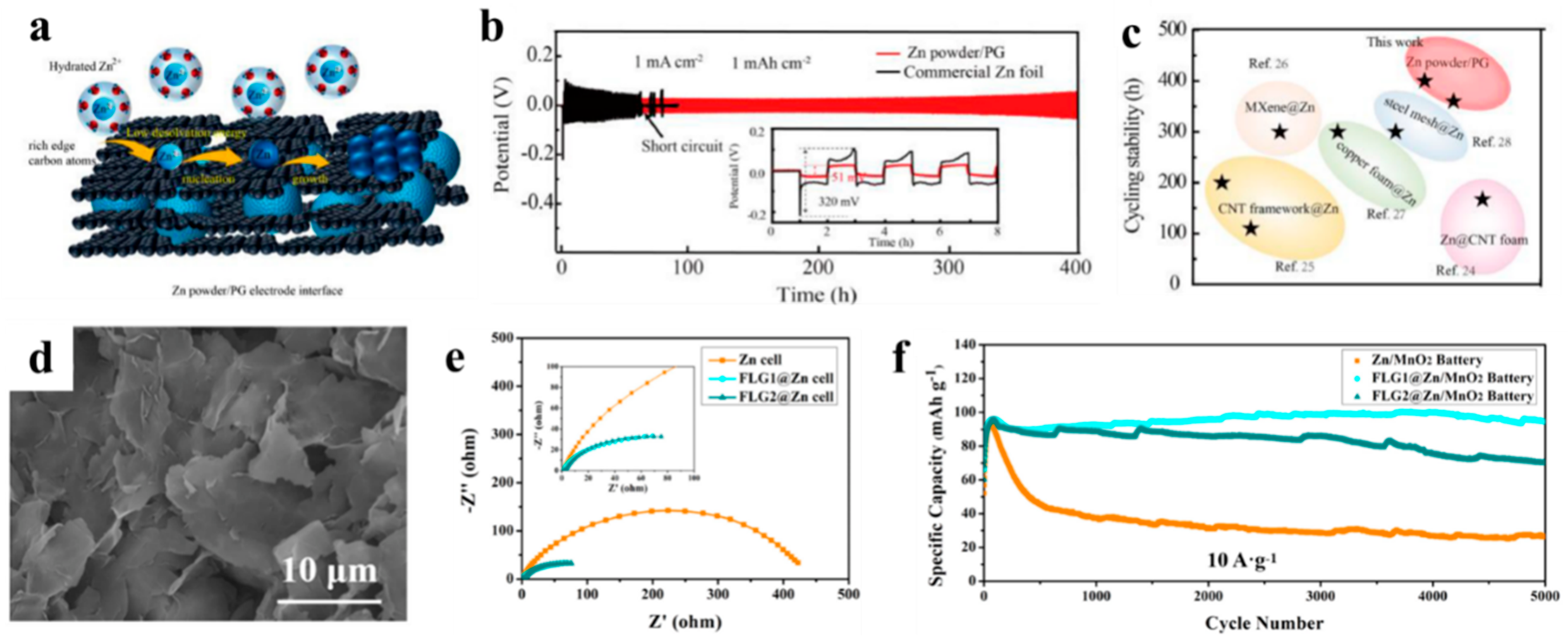
3.1. Graphene for Zn Anodes
3.2. MXene for Zn Anodes
3.2.1. MXene Hosts for Zn
3.2.2. MXene Coatings on Zn
3.3. Other 2D Materials for Zn Anodes
4. Conclusions and Prospects
- (1)
- New strategies remain to be developed to precisely construct ultra-thin and stable 2D material-based protecting layers on Zn. At present, 2D materials are usually mixed with polymer binders, and then coated on Zn foils to construct 2D material/Zn hybrid anodes, in which the thickness of protecting layers is relatively thick. Meanwhile, the interfaces between the protecting layers and Zn anodes are usually weak, seriously deteriorating their cycling stability. In this regard, physical vapor deposition and atomic layer deposition may be the feasible strategies since the deposition time and other parameters can be precisely controlled, and the interfaces between deposited films and substrates are intimate [100]. Moreover, other advanced strategies can also be considered, such as 3D printing [101,102], magnetron sputtering [87,103,104], and laser etching.
- (2)
- To fabricate flexible and wearable battery devices, it is indispensable to construct Zn host materials with high mechanical strength and innoxious and zincophilic features. In this regard, some 2D materials can be ideal materials for Zn anode supports. However, the research on 2D material-based hosts for Zn is still in its infancy, because only graphene and MXene nanosheets have been reported to serve as Zn host materials so far. Therefore, other 2D materials with unique physicochemical properties are highly needed to be exploited and evaluated their potentials for high-performance Zn metal anodes in the near future; in this regard, controllable synthesis and assembly of 2D materials with desirable structures and properties (e.g., flexibility) are of great importance to construct flexible AZBs.
- (3)
- Two-dimensional material/Zn hybrid anodes should be evaluated in harsh conditions for practical applications. At present, most reported works on 2D materials for Zn anodes conduct electrochemical tests under gentle conditions. To meet the requirements of practical application, 2D material/Zn hybrid anodes should be evaluated in harsh conditions, such as high-capacity Zn deposition, high areal current density, and even extreme temperatures. In this regard, 2D materials will definitely play key roles in improving the reversibility of Zn anodes under such harsh conditions since it is feasible to rationally select one 2D material with specific properties from the large pool of candidates.
- (4)
- Advanced characterizations are highly needed to understand the real roles of 2D materials for enhanced electrochemical performance. The understanding of the mechanism of Zn dendrite formation is still in the initial stage. The issues of Zn dendrite formation and HER can be suppressed at the source only by truly exploring the root causes of them. Therefore, advanced technologies (e.g., in situ synchrotron radiation and spherical aberration-corrected electron microscope) are needed to insightfully disclose the structural evolution of 2D materials, the interfacial interactions between 2D materials and Zn, and the mechanisms of 2D materials on the de-solvation behaviors of Zn2+ ions. Meanwhile, theoretical calculations and simulations should be combined to better understand the relative results in terms of thermodynamic and dynamic views.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Xia, H.; Xue, D.F.; Lu, L. Double-Shelled Nanocapsules of V2O5-Based Composites as High-Performance Anode and Cathode Materials for Li Ion Batteries. J. Am. Chem. Soc. 2009, 131, 12086–12087. [Google Scholar] [CrossRef]
- Liu, Z.X.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X.L.; Huang, Z.D.; Zhi, C.Y. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232. [Google Scholar] [CrossRef]
- Dong, Y.; Miao, L.C.; Ma, G.Q.; Di, S.L.; Wang, Y.Y.; Wang, L.B.; Xu, J.B.; Zhang, N. Nonconcentrated aqueous electrolytes with organic solvent additives for stable zinc batteries. Chem. Sci. 2021, 12, 5843–5852. [Google Scholar] [CrossRef]
- Chao, D.L.; Zhou, W.H.; Ye, C.; Zhang, Q.H.; Chen, Y.G.; Gu, L.; Davey, K.; Qiu, S.Z. An Electrolytic Zn-MnO2 Battery for High-Voltage and Scalable Energy Storage. Angew. Chem. Int. Ed. 2019, 58, 7905–7910. [Google Scholar] [CrossRef]
- Xue, H.R.; Gong, H.; Yamauchi, Y.; Sasaki, T.; Ma, R.Z. Photo-enhanced rechargeable high-energy-density metal batteries for solar energy conversion and storage. Nano Res. Energy 2022, 1, e9120007. [Google Scholar] [CrossRef]
- Tian, Y.D.; Chen, S.; He, Y.L.; Chen, Q.W.; Zhang, L.L.; Zhang, J.T. A highly reversible dendrite-free Zn anode via spontaneous galvanic replacement reaction for advanced zinc-iodine batteries. Nano Res. Energy 2022, 1, e9120025. [Google Scholar] [CrossRef]
- Sonigara, K.K.; Zhao, J.W.; Machhi, H.K.; Cui, G.L.; Soni, S.S. Self-Assembled Solid-State Gel Catholyte Combating Iodide Diffusion and Self-Discharge for a Stable Flexible Aqueous Zn–I2 Battery. Adv. Energy Mater. 2020, 10, 2001997. [Google Scholar] [CrossRef]
- Zhao, J.W.; Li, Y.Q.; Peng, X.; Dong, S.M.; Ma, J.; Cui, G.L.; Chen, L.Q. High-voltage Zn/LiMn0.8Fe0.2PO4 aqueous rechargeable battery by virtue of “water-in-salt” electrolyte. Electrochem. Commun. 2016, 69, 6–10. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.L.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Ma, G.Q.; Miao, L.C.; Dong, Y.; Yuan, W.T.; Nie, X.Y.; Di, S.L.; Wang, Y.Y.; Wang, L.B.; Zhang, N. Reshaping the electrolyte structure and interface chemistry for stable aqueous zinc batteries. Energy Storage Mater. 2022, 47, 203–210. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhang, Y.; Hu, H.L.; Shi, H.-Y.; Song, Y.; Guo, D.; Liu, X.X.; Sun, X.Q. A Zn(ClO4)2 Electrolyte Enabling Long-Life Zinc Metal Electrodes for Rechargeable Aqueous Zinc Batteries. ACS Appl. Mater. Inter. 2019, 11, 42000–42005. [Google Scholar] [CrossRef]
- Zhan, R.M.; Wang, X.C.; Chen, Z.H.; Seh, Z.W.; Wang, L.; Sun, Y.M. Promises and Challenges of the Practical Implementation of Prelithiation in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2101565. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.L.; Sun, W.; Han, F.D.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C.S. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Chao, D.L.; Zhu, C.R.; Song, M.; Liang, P.; Zhang, X.; Tiep, N.H.; Zhao, H.F.; Wang, J.; Wang, R.M.; Zhang, H.; et al. A High-Rate and Stable Quasi-Solid-State Zinc-Ion Battery with Novel 2D Layered Zinc Orthovanadate Array. Adv. Mater. 2018, 30, 1803181. [Google Scholar] [CrossRef]
- Qiu, H.Y.; Hu, R.X.; Du, X.F.; Chen, Z.; Zhao, J.W.; Lu, G.L.; Jiang, M.F.; Kong, Q.Y.; Yan, Y.Y.; Du, J.Z.; et al. Eutectic Crystallization Activates Solid-State Zinc-Ion Conduction. Angew. Chem. Int. Ed. 2022, 61, e202113086. [Google Scholar] [CrossRef]
- Han, D.L.; Cui, C.J.; Zhang, K.Y.; Wang, Z.X.; Gao, J.C.; Guo, Y.; Zhang, Z.C.; Wu, S.C.; Weng, Z.; Yang, Q.H.; et al. A non-flammable hydrous organic electrolyte for sustainable zinc batteries. Nat. Sustain. 2022, 5, 205–213. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Kumar, V.; Zhang, Y.W.; Luo, J.M.; Wang, W.Y.; Sun, Y.M.; Li, W.Y.; Seh, Z.W. Room-Temperature Sodium–Sulfur Batteries and Beyond: Realizing Practical High Energy Systems through Anode, Cathode, and Electrolyte Engineering. Adv. Energy Mater. 2021, 11, 2003493. [Google Scholar] [CrossRef]
- Recent Advances and Perspectives for Zn-Based Batteries: Zn Anode and Electrolyte. Available online: https://www.sciopen.com/article/10.26599/NRE.2023.9120039?utm_source=TrendMD&utm_medium=cpc&utm_campaign=Nano_Research_Energy_TrendMD_1 (accessed on 8 November 2022).
- Mu, Y.L.; Zhou, T.Y.; Zhai, Z.Y.; Zhang, S.B.; Li, D.X.; Chen, L.; Ge, G.L. Metal organic complexes as an artificial solid-electrolyte interface with Zn-ion transfer promotion for long-life zinc metal batteries. Nanoscale 2021, 13, 20412–20416. [Google Scholar] [CrossRef]
- Wu, K.; Yi, J.; Liu, X.Y.; Sun, Y.; Cui, J.; Xie, Y.H.; Liu, Y.Y.; Xia, Y.Y.; Zhang, J.J. Regulating Zn Deposition via an Artificial Solid-Electrolyte Interface with Aligned Dipoles for Long Life Zn Anode. Nano-Micro. Lett. 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Hannig, M.; Carrasco-Carmona, Á.; Osorio, M.T.; García-Godoy, F.; Cabello, I.; Osorio, R. Zn-containing Adhesives Facilitate Collagen Protection and Remineralization at the Resin-Dentin Interface: A Narrative Review. Polymers 2022, 14, 642. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.W.; Du, Z.G.; Duan, H.P.; Yang, S.B. Zinc anode with artificial solid electrolyte interface for dendrite-free Ni-Zn secondary battery. J. Colloid Interface Sci. 2019, 555, 174–179. [Google Scholar] [CrossRef]
- Zhao, K.N.; Wang, C.X.; Yu, Y.H.; Yan, M.Y.; Wei, Q.L.; He, P.; Dong, Y.F.; Zhang, Z.Y.; Wang, X.D.; Mai, L.Q. Ultrathin Surface Coating Enables Stabilized Zinc Metal Anode. Adv. Mater. Interfaces 2018, 5, 1800848. [Google Scholar] [CrossRef]
- Xie, C.L.; Li, Y.H.; Wang, Q.; Sun, D.; Tang, Y.G.; Wang, H.Y. Issues and solutions toward zinc anode in aqueous zinc-ion batteries: A mini review. Carbon Energy 2020, 2, 540–560. [Google Scholar] [CrossRef]
- Yi, Z.H.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2020, 11, 2003065. [Google Scholar] [CrossRef]
- Hu, W.; Ju, J.G.; Deng, N.P.; Liu, M.Y.; Liu, W.C.; Zhang, Y.X.; Fan, Y.Y.; Kang, W.M.; Cheng, B.W. Recent progress in tackling Zn anode challenges for Zn ion batteries. J. Mater. Chem. A 2021, 9, 25750–25772. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Liu, Z.X.; Wang, D.H.; Guo, Y.; Li, X.L.; Tang, Y.C.; Li, H.F.; Dong, B.B.; Zhi, C.Y. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, 2001854. [Google Scholar] [CrossRef]
- Li, H.F.; Ma, L.T.; Han, C.; Wang, Z.F.; Liu, Z.F.; Tang, Z.J.; Zhi, C.Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Du, W.C.; Ang, E.H.; Yang, Y.; Zhang, Y.F.; Ye, M.H.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Zhang, T.S.; Tang, Y.; Guo, S.; Cao, X.X.; Pan, A.Q.; Fang, G.Z.; Zhou, J.; Liang, S.Q. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Zhou, J.H.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.T.; Huang, R.L.; Wei, J.L.; Liu, A.N.; Li, L.; Chen, R.J. Ultrathin Surface Coating of Nitrogen-Doped Graphene Enables Stable Zinc Anodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, e2101649. [Google Scholar] [CrossRef]
- Lin, H.C.; Jian, Q.F.; Bai, X.Y.; Li, D.Q.; Huang, Z.; Huang, W.T.; Feng, S.S.; Cheng, Z.Y. Recent advances in thermal conductivity and thermal applications of graphene and its derivatives nanofluids. Appl. Therm. Eng. 2023, 218, 119176. [Google Scholar] [CrossRef]
- Dyck, O.; Yeom, S.; Dillender, S.; Lupini, A.R.; Yoon, M.; Jesse, S. The role of temperature on defect diffusion and nanoscale patterning in graphene. Carbon 2023, 201, 212–221. [Google Scholar] [CrossRef]
- Nasir, A.; Raza, A.; Tahir, M.; Yasin, T.; Nadeem, M.; Ahmad, B. Synthesis and study of polyaniline grafted graphene oxide nanohybrids. Mater. Res. Bull. 2023, 157, 112006. [Google Scholar] [CrossRef]
- Low, T.; Chaves, A.; Caldwell, J.; Avouris, P.; Heinz, T.F.; Guinea, F.; Martin-Moreno, L.; Koppens, F. Polaritons in layered two-dimensional materials. Nat. Mater. 2017, 16, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Parlak, O.; İncel, A.; Uzun, L.; Turner, A.; Tiwari, A. Structuring Au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens. Bioelectron. 2017, 89, 545–550. [Google Scholar] [CrossRef]
- Janowska, I.; Chizari, K.; Ersen, O.; Zafeiratos, S.; Soubane, D.; Costa, V.D.; Speisser, V.; Boeglin, C.; Houllé, M.; Bégin, D.; et al. Microwave synthesis of large few-layer graphene sheets in aqueous solution of ammonia. Nano Res. 2010, 3, 126–137. [Google Scholar] [CrossRef]
- Safaei, J.; Wang, G.X. Progress and prospects of two-dimensional materials for membrane-based osmotic power generation. Nano Res. Energy 2022, 1, e9120008. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.Y.; Yu, M.; Niu, Z.Q.; Cheng, F.Y.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z.Q. Design Strategies for Vanadium-based Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 16508–16517. [Google Scholar] [CrossRef]
- Mostafavi, E.; Iravani, S. MXene-Graphene Composites: A Perspective on Biomedical Potentials. Nano-Micro Lett. 2022, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.F.; Lu, J.Y.; He, R.F.; Jiang, G.Y.; Jiang, X.; Wang, B.B.; Song, L.; Hu, Y. Octopus sucker-inspired hierarchical structure MXene@carbon nanotubes enhancing the mechanical properties and fire safety of thermoplastic polyurethane composites through the interfacial engineering. Chem. Eng. J. 2022, 450, 138184. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.X.; Shi, X.Y.; Zhu, Y.Y.; Wu, Z.S. Recent status and future perspectives of ultracompact and customizable micro-supercapacitors. Nano Res. Energy 2022, 1, e9120018. [Google Scholar] [CrossRef]
- Zhang, N.N.; Huang, S.; Yuan, Z.S.; Zhu, J.C.; Zhao, Z.F.; Niu, Z.Q. Direct Self-Assembly of MXene on Zn Anodes for Dendrite-Free Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kwak, M.J.; Hwang, C.; Kang, K.N.; Liu, N.; Jang, J.H.; Grzybowski, B.A. Self-Assembling Films of Covalent Organic Frameworks Enable Long-Term, Efficient Cycling of Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2101726. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.D.; Yue, Y.L.; Wang, X.; Pakornchote, T.; Bovornratanaraks, T.; Zhang, X.Y.; Wu, Z.S.; Qin, J.Q. Oxygen defect enriched (NH4)2V10O25·8H2O nanosheets for superior aqueous zinc-ion batteries. Nano Energy 2021, 84, 105876. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.G.; Wang, S.; Zhou, F.; Das, P.; Sun, C.L.; Zheng, S.H.; Wu, Z.S. 2D Amorphous V2O5/Graphene Heterostructures for High-Safety Aqueous Zn-Ion Batteries with Unprecedented Capacity and Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 2000081. [Google Scholar] [CrossRef]
- Li, Y.; Lu, P.F.; Shang, P.; Wu, L.S.; Wang, X.; Dong, Y.F.; He, R.H.; Wu, Z.S. Pyridinic nitrogen enriched porous carbon derived from bimetal organic frameworks for high capacity zinc ion hybrid capacitors with remarkable rate capability. J. Energy Chem. 2021, 56, 404–411. [Google Scholar] [CrossRef]
- Dong, Y.F.; Li, Y.; Shi, H.D.; Qin, J.Q.; Zheng, S.H.; He, R.H.; Wu, Z.S. Graphene encapsulated iron nitrides confined in 3D carbon nanosheet frameworks for high-rate lithium ion batteries. Carbon 2020, 159, 213–220. [Google Scholar] [CrossRef]
- Liu, X.X.; Wei, W.; Yang, Y.F.; Li, Y.J.; Li, Y.; Xu, S.C.; Dong, Y.F.; He, R.H. A porous membrane electrolyte enabled by poly(biphenyl piperidinium triphenylmethane) for dendrite-free zinc anode with enhanced cycling life. Chem. Eng. J. 2022, 437, 135409. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Hussain, T.; Wang, D.H.; Hui, L.; Guo, Y.; Liang, G.J.; Li, X.L.; Chen, Z.; Huang, Z.D.; et al. Stabilizing Interface pH by N-Modified Graphdiyne for Dendrite-Free and High-Rate Aqueous Zn-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202112304. [Google Scholar] [CrossRef]
- Wu, L.S.; Zhang, Y.; Shang, P.; Dong, Y.F.; Wu, Z.-S. Redistributing Zn ion flux by bifunctional graphitic carbon nitride nanosheets for dendrite-free zinc metal anodes. J. Mater. Chem. A 2021, 9, 27408–27414. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.D.; Gu, C.; Wang, X.; Wang, S.M.; Zhang, X.Y.; Qin, J.Q.; Wu, Z.S. Manipulating Crystallographic Orientation of Zinc Deposition for Dendrite-free Zinc Ion Batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Liu, K.; Huang, X.B.; Wang, H.Y.; Li, F.Z.; Tang, Y.G.; Li, J.S.; Shao, M.H. Co3O4–CeO2/C as a Highly Active Electrocatalyst for Oxygen Reduction Reaction in Al–Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 34422–34430. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Liu, S.Q.; Wang, H.Y.; Wang, X.W.; Zhang, X.; Jin, G.H. A novel solvothermal synthesis of Mn3O4/graphene composites for supercapacitors. Electrochim. Acta. 2013, 90, 210–218. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Lai, Z.Z.; Han, Y.; Zhang, H.Z.; Xie, S.L.; Lu, X.H. Oxygen-Vacancy and Surface Modulation of Ultrathin Nickel Cobaltite Nanosheets as a High-Energy Cathode for Advanced Zn-Ion Batteries. Adv. Mater. 2018, 30, 1802396. [Google Scholar] [CrossRef]
- Hao, Y.T.; Zhou, J.H.; Wei, G.L.; Liu, A.N.; Zhang, Y.X.; Mei, Y. Artificial N-doped Graphene Protective Layer Enables Stable Zn Anode for Aqueous Zn-ion Batteries. ACS Appl. Energy Mater. 2021, 4, 6364–6373. [Google Scholar] [CrossRef]
- Li, X.L.; Li, M.; Luo, K.; Hou, Y.; Li, P.; Yang, Q.; Huang, Z.D.; Liang, G.J.; Chen, Z.; Du, S.Y.; et al. Lattice Matching and Halogen Regulation for Synergistically Induced Uniform Zinc Electrodeposition by Halogenated Ti3C2 MXenes. ACS Nano 2022, 16, 813–822. [Google Scholar] [CrossRef]
- Liu, H.Q.; Qiu, M.H.; Tang, C.X.; Xu, J.L.; Jia, H. Enhance interfacial deposition kinetics to achieve dendrite-free zinc anodes by graphene oxide modified boron nitride nanosheets. Electrochim. Acta. 2022, 405, 139776. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Zhang, Y.M.; Chen, P.; Wu, Y.T.; Yang, H.C.; Ding, H.R.; Zhang, Y.; Wang, Z.Z.; Du, X.; Liu, N. Graphene oxide-modified zinc anode for rechargeable aqueous batteries. Chem. Eng. Sci. 2019, 194, 142–147. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.L.; Yang, Y.J.; Xu, B.G. Robust nitrogen/selenium engineered MXene/ZnSe hierarchical multifunctional interfaces for dendrite-free zinc-metal batteries. Energy Storage Mater. 2022, 49, 122–134. [Google Scholar] [CrossRef]
- Tan, L.W.; Wei, C.L.; Zhang, Y.C.; An, Y.L.; Xiong, S.L.; Feng, J.K. Long-life and dendrite-free zinc metal anode enabled by a flexible, green and self-assembled zincophilic biomass engineered MXene based interface. Chem. Eng. J. 2022, 431, 134277. [Google Scholar] [CrossRef]
- Wang, N.; Wu, Z.T.; Long, Y.; Chen, D.R.; Geng, C.N.; Liu, X.C. MXene-assisted polymer coating from aqueous monomer solution towards dendrite-free zinc anodes. J. Energy. Chem. 2022, 73, 277–284. [Google Scholar] [CrossRef]
- Zhu, X.D.; Li, X.Y.; Essandoh, M.L.K.; Tan, J.; Cao, Z.Y.; Zhang, X.; Dong, P.; Ajayan, P.M.; Ye, M.X.; Shen, J.F. Interface engineering with zincophilic MXene for regulated deposition of dendrite-free Zn metal anode. Energy Storage Mater. 2022, 50, 243–251. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Wang, M.C.; Tao, Y.; Zhang, K.; Cai, M.L.; Ding, Y.; Liu, X.P.; Hayat, T.W.; Alsaedi, A.; Dai, S.Y. Electrochemically Derived Graphene-Like Carbon Film as a Superb Substrate for High-Performance Aqueous Zn-Ion Batteries. Adv. Funct. Mater. 2019, 30, 1907120. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mhin, S.; Jeon, J.E.; Park, K.; Kim, J.; Choi, W. Stable and High-Energy-Density Zn-Ion Rechargeable Batteries Based on a MoS2-Coated Zn Anode. ACS Appl. Mater. Interfaces 2020, 12, 27249–27257. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.G.; Zhang, Z.Y.; Hao, R.; Huang, Y.P.; Liu, W.F.; Tan, Y.Y.; Li, P.L.; Yan, J.; Liu, K.Y. Ultra-highly stable zinc metal anode via 3D-printed g-C3N4 modulating interface for long life energy storage systems. Chem. Eng. J. 2021, 403, 126425. [Google Scholar] [CrossRef]
- Manipulating Horizontal Zn Deposition with Graphene Interpenetrated Zn Hybrid Foils for Dendrite-Free Aqueous Zinc Ion Batteries. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/eem2.12423 (accessed on 28 April 2022).
- Huang, J.H.; Guo, Z.W.; Ma, Y.Y.; Bin, D.; Wang, Y.G.; Xia, Y.Y. Recent Progress of Rechargeable Batteries Using Mild Aqueous Electrolytes. Small Methods 2019, 3, 1800272. [Google Scholar] [CrossRef]
- Konarvo, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.K.; Myung, S.T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Fang, G.Z.; Zhou, J.; Pan, A.Q.; Liang, S.Q. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Du, W.C.; Huang, S.; Zhang, Y.F.; Ye, M.H.; Li, C.C. Enable commercial Zinc powders for dendrite-free Zinc anode with improved utilization rate by pristine graphene hybridization. Energy Storage Mater. 2022, 45, 465–473. [Google Scholar] [CrossRef]
- Xia, A.L.; Pu, X.M.; Tao, Y.Y.; Liu, H.M.; Wang, Y.G. Graphene oxide spontaneous reduction and self-assembly on the zinc metal surface enabling a dendrite-free anode for long-life zinc rechargeable aqueous batteries. Appl. Surf. Sci. 2019, 481, 852–859. [Google Scholar] [CrossRef]
- Qiu, M.H.; Jia, H.; Liu, H.Q.; Tawiah, B.; Fu, S.H. Realizing Long-life Zn Anode by Few-layer Graphene Ion-oriented Interface. J. Alloys Compd. 2022, 891, 161886. [Google Scholar] [CrossRef]
- Sun, B.; Wang, P.P.; Yang, L.W.; Wei, X.L.; Jin, Y.; Wu, H. Rational Design of an Interfacial Bilayer for Aqueous Dendrite-Free Zinc Anodes. ACS Appl. Mater. Interfaces 2022, 14, 954–960. [Google Scholar] [CrossRef]
- Qiu, M.H.; Wang, D.; Tawiah, B.J.M.; Jia, H.; Fei, B.; Fu, S.H. Constructing PEDOT:PSS/Graphene sheet nanofluidic channels to achieve dendrite-free Zn anode. Compos. Part B Eng. 2021, 215, 108798. [Google Scholar] [CrossRef]
- Gan, H.; Wu, J.; Li, R.; Huang, B.W.; Liu, H.B. Ultra-stable and deeply rechargeable zinc metal anode enabled by a multifunctional protective layer. Energy Storage Mater. 2022, 47, 602–610. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.Y.; Li, S.; Liu, C.; Li, H.Y.; Zou, G.Q.; Hu, J.G.; Hou, H.S.; Ji, X.B. Graphene quantum dots enable dendrite-free zinc ion battery. Nano Energy 2022, 92, 106752. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.L.; Wei, C.L.; Xi, B.J.; Xiong, S.L.; Feng, J.K.; Qian, Y.T. Flexible and Free-Standing Ti3C2Tx MXene@Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Gu, J.A.; Tao, Y.; Chen, H.; Cao, Z.J.; Zhang, Y.Z.; Du, Z.G.; Cui, Y.L.S.; Yang, S.B. Stress-Release Functional Liquid Metal-MXene Layers toward Dendrite-Free Zinc Metal Anodes. Adv. Energy Mater. 2022, 12, 2200115. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, P.F.; Ye, K.; Zhu, K.; Yan, J.; Wang, G.L.; Chen, G.H.; Cao, D.X. MXene-modified conductive framework as a universal current collector for dendrite-free lithium and zinc metal anode. J. Colloid. Interface Sci. 2022, 625, 700–710. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fang, Y.Z.; Liang, P.C.; Xu, J.H.; Xing, B.; Zhu, K.; Liu, Y.Y.; Zhang, J.J.; Yi, J. Surface-tuned two-dimension MXene scaffold for highly reversible zinc metal anode. Chin. Chem. Lett. 2021, 32, 2899–2903. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.L.; Liu, C.K.; Xiong, S.L.; Feng, J.K.; Qian, Y.T. Reversible zinc-based anodes enabled by zincophilic antimony engineered MXene for stable and dendrite-free aqueous zinc batteries. Energy Storage Mater. 2021, 41, 343–353. [Google Scholar] [CrossRef]
- An, Y.L.; Tian, Y.; Liu, C.K.; Xiong, S.L.; Feng, J.K.; Qian, Y.T. Rational Design of Sulfur-Doped Three-Dimensional Ti3C2Tx MXene/ZnS Heterostructure as Multifunctional Protective Layer for Dendrite-Free Zinc-Ion Batteries. ACS Nano 2021, 15, 15259–15273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Cao, Z.J.; Liu, S.J.; Du, Z.G.; Cui, Y.L.S.; Gu, J.A.; Shi, Y.Z.; Li, B.; Yang, S.B. Charge-Enriched Strategy Based on MXene-Based Polypyrrole Layers Toward Dendrite-Free Zinc Metal Anodes. Adv. Energy Mater. 2022, 12, 2103979. [Google Scholar] [CrossRef]
- Jia, H.; Qiu, M.H.; Tang, C.X.; Liu, H.Q.; Fu, S.H.; Zhang, X.W. Nano-scale BN interface for ultra-stable and wide temperature range tolerable Zn anode. EcoMat 2022, 4, e12190. [Google Scholar] [CrossRef]
- Negatively Charged Insulated Boron Nitride Nanofibers Directing Subsurface Zinc Deposition for Dendrite-Free Zinc Anodes. Available online: https://link.springer.com/article/10.1007/s12274-022-4619-5 (accessed on 15 June 2022).
- Qiu, M.H.; Jia, H.; Lan, C.T.; Liu, H.Q.; Fu, S.H. An enhanced kinetics and ultra-stable zinc electrode by functionalized boron nitride intermediate layer engineering. Energy Storage Mater. 2022, 45, 1175–1182. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.Y.; Lv, Z.H.; Yang, H.; Cheng, X.; Zhang, S.Z.; Ye, M.H.; Zhang, Y.F.; Chen, L.B.; Zhao, J.B.; et al. Redistributing Zn-ion flux by interlayer ion channels in Mg-Al layered double hydroxide-based artificial solid electrolyte interface for ultra-stable and dendrite-free Zn metal anodes. Energy Storage Mater. 2021, 41, 230–239. [Google Scholar] [CrossRef]
- Yin, C.; Zhu, M.; Kong, Y.; Wang, Q.; Zhou, H.H.; Qi, L.M.; Tong, L.M.; Zhang, J. Rapid synthesis of few-layer graphdiyne using radio frequency heating and its application for dendrite-free zinc anodes. 2D Mater. 2021, 8, 044003. [Google Scholar] [CrossRef]
- An, G.H.; Hong, J.; Park, S.; Cho, Y.; Lee, S.; Hou, B.; Cha, S.N. 2D Metal Zn Nanostructure Electrodes for High-Performance Zn Ion Supercapacitors. Adv. Energy Mater. 2020, 10, 1902981. [Google Scholar] [CrossRef]
- Cao, Z.J.; Zhang, Y.Z.; Cui, Y.L.S.; Gu, J.N.; Du, Z.G.; Shi, Y.Z.; Shen, K.; Chen, H.; Li, B.; Yang, S.B. Harnessing the Unique Features of 2D Materials toward Dendrite-free Metal Anodes. Energy Environ. Mater. 2022, 5, 45–67. [Google Scholar] [CrossRef]
- Dong, Y.F.; Wu, Z.S.; Ren, W.C.; Cheng, H.M.; Bao, X.H. Graphene: A promising 2D material for electrochemical energy storage. Sci. Bull. 2017, 62, 724–740. [Google Scholar] [CrossRef]
- Khamsanga, S.; Uyama, H.; Nuanwat, W.; Pattananuwat, P. Polypyrrole/reduced graphene oxide composites coated zinc anode with dendrite suppression feature for boosting performances of zinc ion battery. Sci. Rep. 2022, 12, 8689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Ji, C.C.; Meng, Q.Q.; Mi, H.Y.; Yang, Q.; Li, Z.C.X.; Yang, N.J.; Qiu, J.S. Freeze-Tolerant Hydrogel Electrolyte with High Strength for Stable Operation of Flexible Zinc-Ion Hybrid Supercapacitors. Small 2022, 18, 2200055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Xu, W.; Wu, L.S.; Dong, Y.F. Recent progress in MXene-based nanomaterials for highperformance aqueous zinc-ion hybrid capacitors. New Carbon Mater. 2022, 37, 508–526. [Google Scholar] [CrossRef]
- Liu, F.F.; Jin, S.; Xia, Q.X.; Zhou, A.G.; Fan, L.Z. Research progress on construction and energy storage performance of MXene heterostructures. J. Energy Chem. 2021, 62, 220–242. [Google Scholar] [CrossRef]
- Dong, Y.F.; Shi, H.D.; Wu, Z.S. Recent Advances and Promise of MXene-Based Nanostructures for High-Performance Metal Ion Batteries. Adv. Funct. Mater. 2020, 30, 2000706. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Yan, B.X.; Wang, C.D.; Liu, Z.X.; Huang, Z.D.; Wang, Y.K.; Li, Y.R.; Li, H.F.; Song, L.; et al. Hydrogen-Substituted Graphdiyne Ion Tunnels Directing Concentration Redistribution for Commercial-Grade Dendrite-Free Zinc Anodes. Adv. Mater. 2020, 32, 2001755. [Google Scholar] [CrossRef]
- He, H.B.; Tong, H.; Song, X.Y.; Song, X.P.; Liu, J. Highly stable Zn metal anodes enabled by atomic layer deposited Al2O3 coating for aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 7836–7846. [Google Scholar] [CrossRef]
- Gómez, I.J.; Alegret, N.; Dominguez-Alfaro, A.; Vázquez Sulleiro, M. Recent Advances on 2D Materials towards 3D Printing. Chemistry 2021, 3, 1314–1343. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, J.Z.; Yang, C.; Qiu, L.; Cheng, H.M. Recent Progress in 3D Printing of 2D Material-Based Macrostructures. Adv. Mater. Technol. 2020, 5, 1901066. [Google Scholar] [CrossRef]
- Zheng, J.X.; Huang, Z.H.; Zeng, Y.; Liu, W.Q.; Wei, B.B.; Qi, Z.B.; Wang, Z.C.; Xia, C.; Liang, H.F. Electrostatic Shielding Regulation of Magnetron Sputtered Al-Based Alloy Protective Coatings Enables Highly Reversible Zinc Anodes. Nano Lett. 2022, 22, 1017–1023. [Google Scholar] [CrossRef] [PubMed]

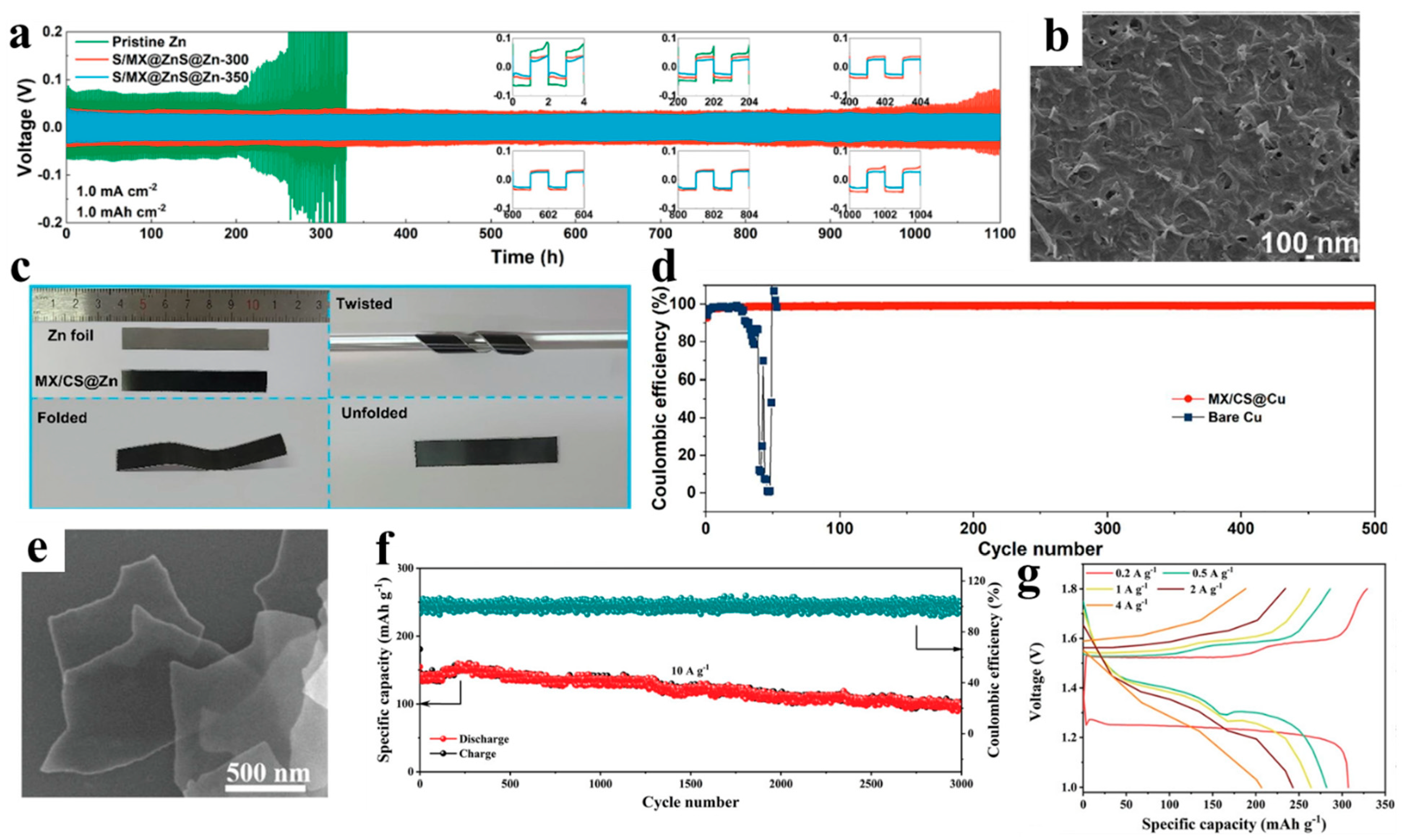
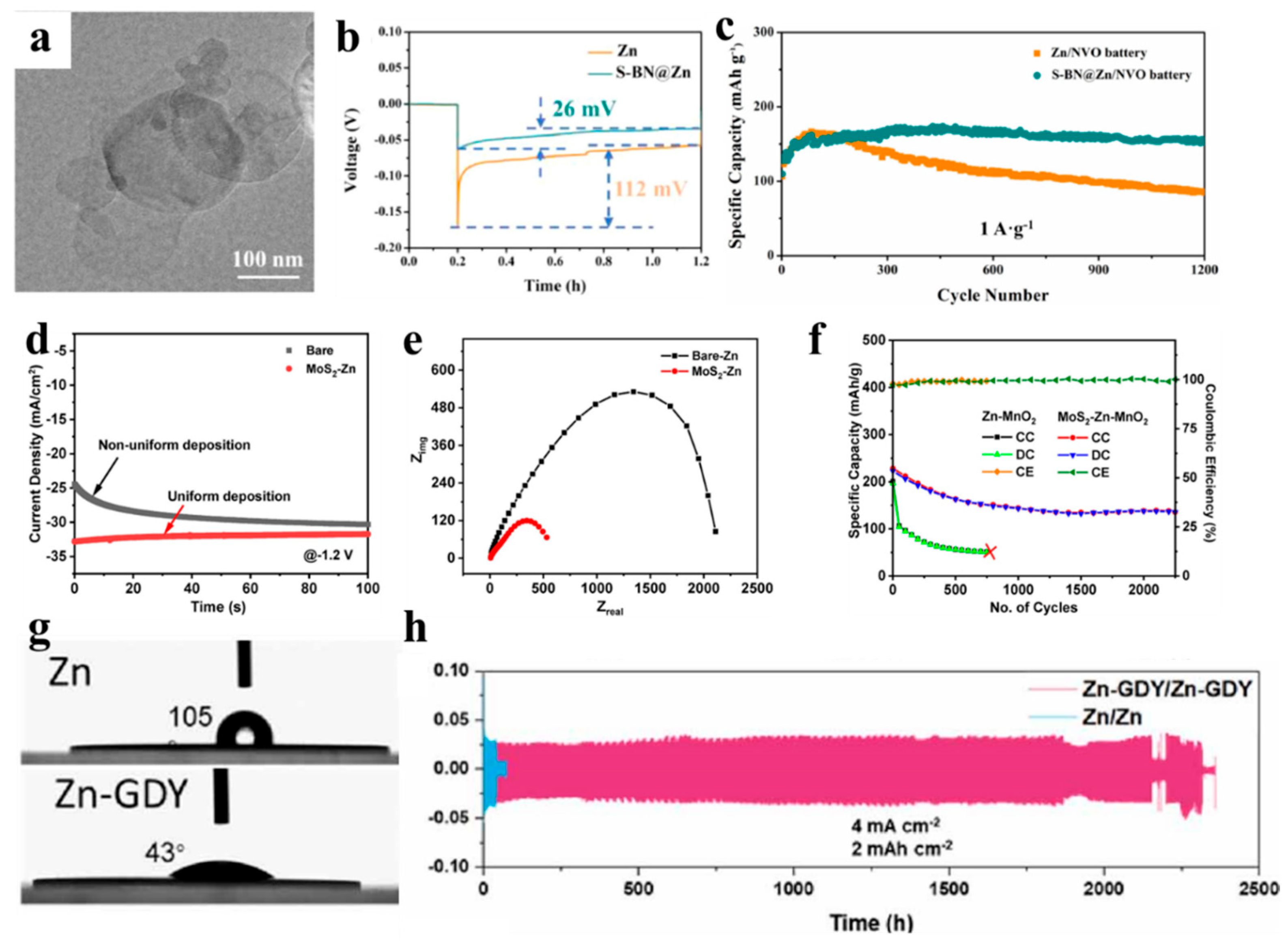
| Type of 2D Material | Anode Name | Voltage Hysteresis | Nucleation Overpotential | Life Span | Average CE | Refs. |
|---|---|---|---|---|---|---|
| Graphene | Zn@PG | 58 mV (1 mA cm−2) | 40 mV | 400 h (1 mA cm−2) | 97.37%/100 | [73] |
| Zn@rGO | – | ~20 mV | 300 h (1 mA cm−2) | – | [74] | |
| FLG@Zn | 69 mV (1 mA cm−2) | 54 mV | 500 h (1 mA cm−2) | 98%/100 | [75] | |
| NG@Zn | 37.2 mV (1 mA cm−2) | 84.3 mV | 500 h (1 mA cm−2) | – | [58] | |
| NGO@Zn | 17 mV (1 mA cm−2) | 1200 h (1 mA cm−2) | 99.5%/250 | [31] | ||
| MZn | – | 11 mV | 2000 h (0.5 mA cm−2) | – | [76] | |
| PEDOT:PSS/GS@Zn | – | 66 mV | 500 h (1 mA cm−2) | 98%/100 | [77] | |
| ZGL@Zn | 42 mV | 108 mV | >2180 h (0.5 mA cm−2) | 99.6%/1450 | [78] | |
| GiZn | 30.4 mV (0.5 mA cm−2) | 35 mV | 792 h (0.5 mA cm−2) | 96.8%/200 | [69] | |
| Zn/GCF | – | – | 100 h (1 mA cm−2) | ~100%/100 | [66] | |
| GQDs | 50 mV (0.8 mA cm−2) | 36.5 mV | 2200 h (0.8 mA cm−2) | – | [79] | |
| MXene | Ti3C2Tx MXene@Zn | 75 mV (1 mA cm−2) | – | 300 h (1 mA cm−2) | 94.13%/400 | [80] |
| ZnGaIn//MXene | 25 mV (1 mA cm−2) | ~0 mV | 600 h (1 mA cm−2) | – | [81] | |
| MCF@Zn | 63 mV (10 mA cm−2) | 14 mV | 300 h (10 mA cm−2) | 97.72%/500 | [82] | |
| LF-MXene | 63 mV (5 mA cm−2) | 32 mV | 280 h (5 mA cm−2) | – | [83] | |
| MXene@Sb | – | – | 1000 h (0.5 mA cm−2) | 97.2%/1500 | [84] | |
| MZn | 47 mV (0.2 mA cm−2) | 16 mV | >800 h (0.2 mA cm−2) | – | [45] | |
| Ti3C2Cl2-Zn | <103 mV (2 mA cm−2) | 40.7 mV | >840 h (2 mA cm−2) | 99.3%/200 | [59] | |
| S/MX@ZnS@Zn | 27 mV (1 mA cm−2) | – | 1100 h (1 mA cm−2) | – | [85] | |
| N/Se-MXene@ZnSe@Zn | 35 mV (1 mA cm−2) | 20 mV | 2500 h (1 mA cm−2) | – | [62] | |
| MX-TMA@Zn | ~60 mV (2 mA cm−2) | – | 3600 h (2 mA cm−2) | – | [65] | |
| MX/CS@Zn | 76 mV (0.5 mA cm−2) | 31 mV | 2100 h (1 mA cm−2) | >99%/500 | [63] | |
| PASM-Zn | – | – | >1900 h (0.2 mA cm−2) | – | [64] | |
| MXene-mPPy/Zn | 29 mV (1 mA cm−2) | 10 mV | 2500 h (0.2 mA cm−2) | – | [86] | |
| Other 2D materials | BN@Zn | 25.4 mV (1 mA cm−2) | 27 mV | 3000 h (1 mA cm−2) | 99.3%/150 | [87] |
| BNNFs@Zn | 51 mV (1 mA cm−2) | – | 1600 h (1 mA cm−2) | – | [88] | |
| S-BN@Zn | 45 mV | 26 mV | 2500 h (2 mA cm−2) | 99.5%/60 | [89] | |
| MoS2@Zn | – | 120 mV | >150 h (2.5 mA cm−2) | – | [67] | |
| LDH@Zn | 37.7 mV (1 mA cm−2) | – | 400 h (1 mA cm−2) | 99.2%/2000 | [90] | |
| g-C3N4@Zn | 80 mV (2 mA cm−2) | ~55 mV | 500 h (2 mA cm−2) | – | [68] | |
| GDY@Zn | 25 mV (4mA cm−2) | – | >400 h (4 mA cm−2) | 98%/200 | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhang, M.; Dong, Y.; Zhao, J. Two-Dimensional Materials for Dendrite-Free Zinc Metal Anodes in Aqueous Zinc Batteries. Batteries 2022, 8, 293. https://doi.org/10.3390/batteries8120293
Xu W, Zhang M, Dong Y, Zhao J. Two-Dimensional Materials for Dendrite-Free Zinc Metal Anodes in Aqueous Zinc Batteries. Batteries. 2022; 8(12):293. https://doi.org/10.3390/batteries8120293
Chicago/Turabian StyleXu, Wen, Minghui Zhang, Yanfeng Dong, and Jingwen Zhao. 2022. "Two-Dimensional Materials for Dendrite-Free Zinc Metal Anodes in Aqueous Zinc Batteries" Batteries 8, no. 12: 293. https://doi.org/10.3390/batteries8120293
APA StyleXu, W., Zhang, M., Dong, Y., & Zhao, J. (2022). Two-Dimensional Materials for Dendrite-Free Zinc Metal Anodes in Aqueous Zinc Batteries. Batteries, 8(12), 293. https://doi.org/10.3390/batteries8120293









