Boosting Capacity Performance of Bio-Waste Lignin-Derived Hierarchical Porous Carbon with Self-Doped Oxygen-Heteroatoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Preparations of OHPCs
2.3. Material Characterizations
2.4. Electrochemical Characterizations
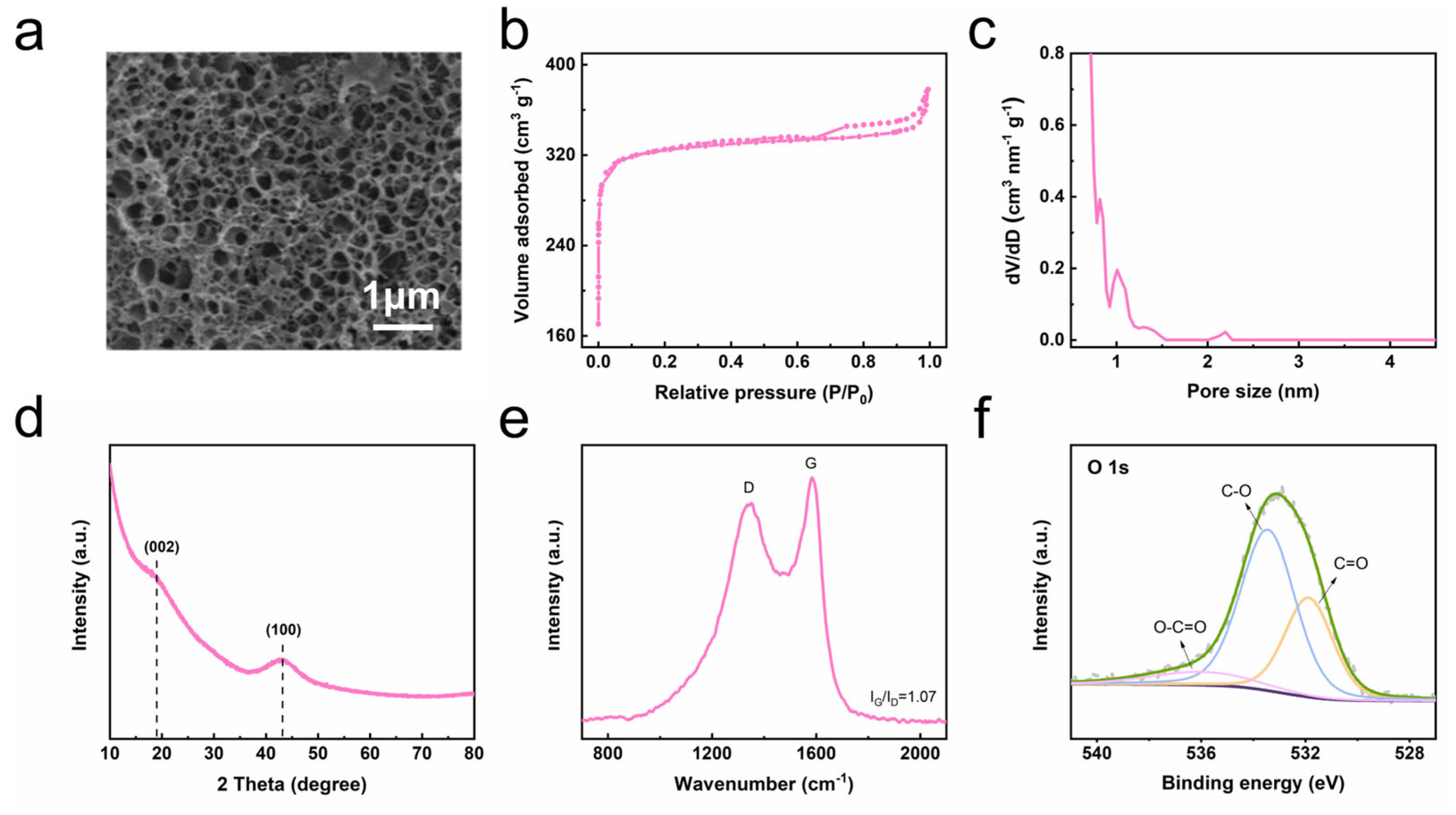
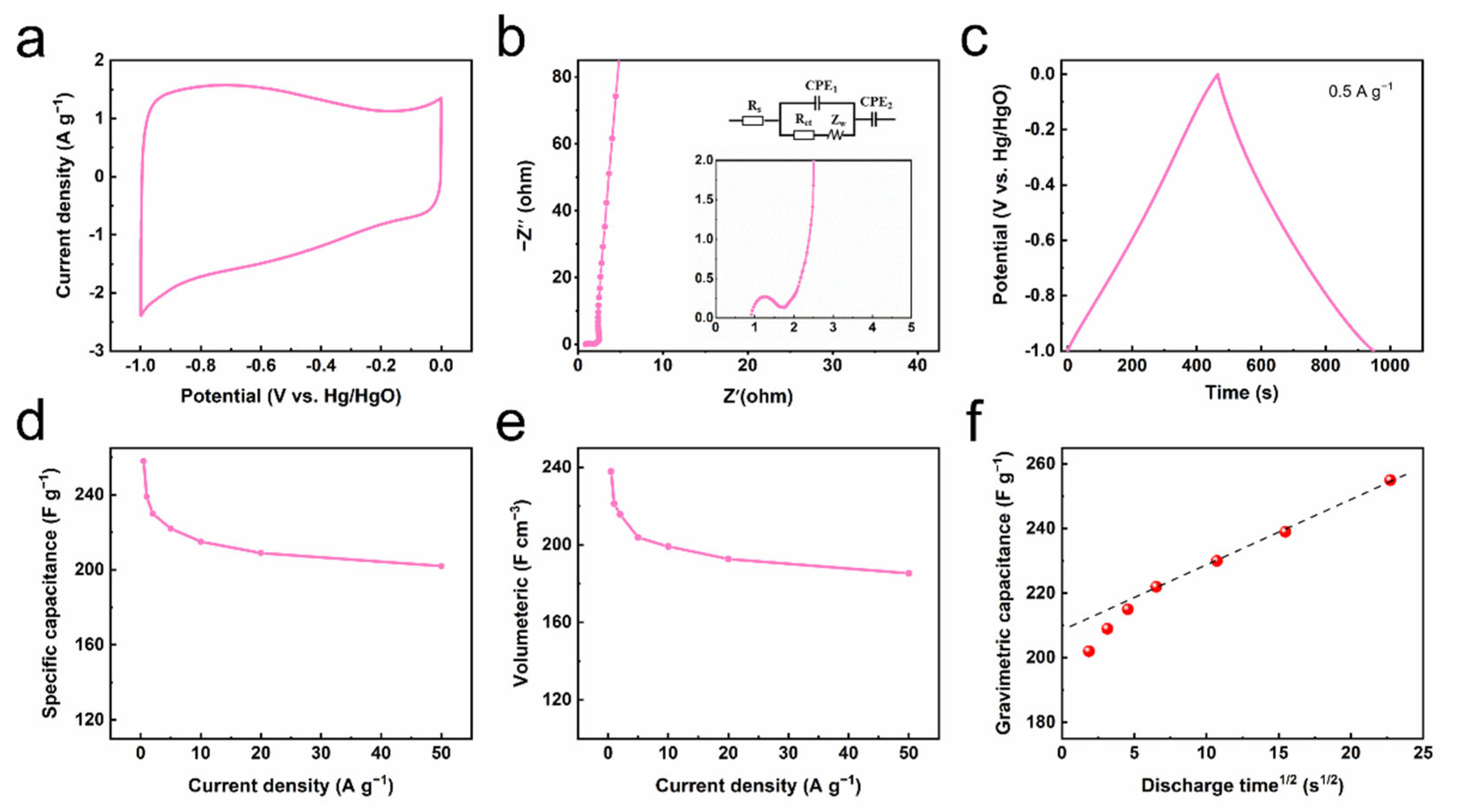
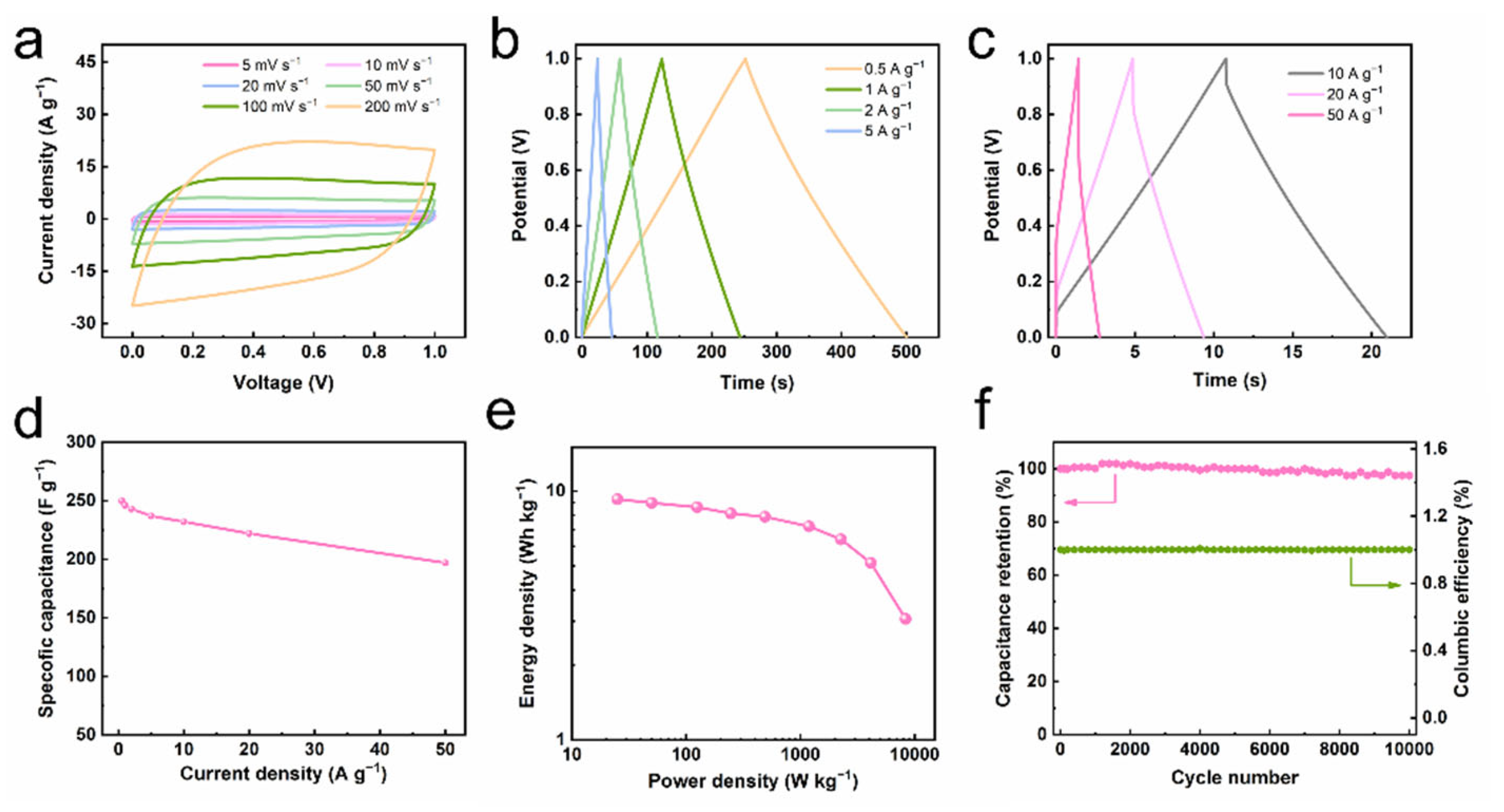
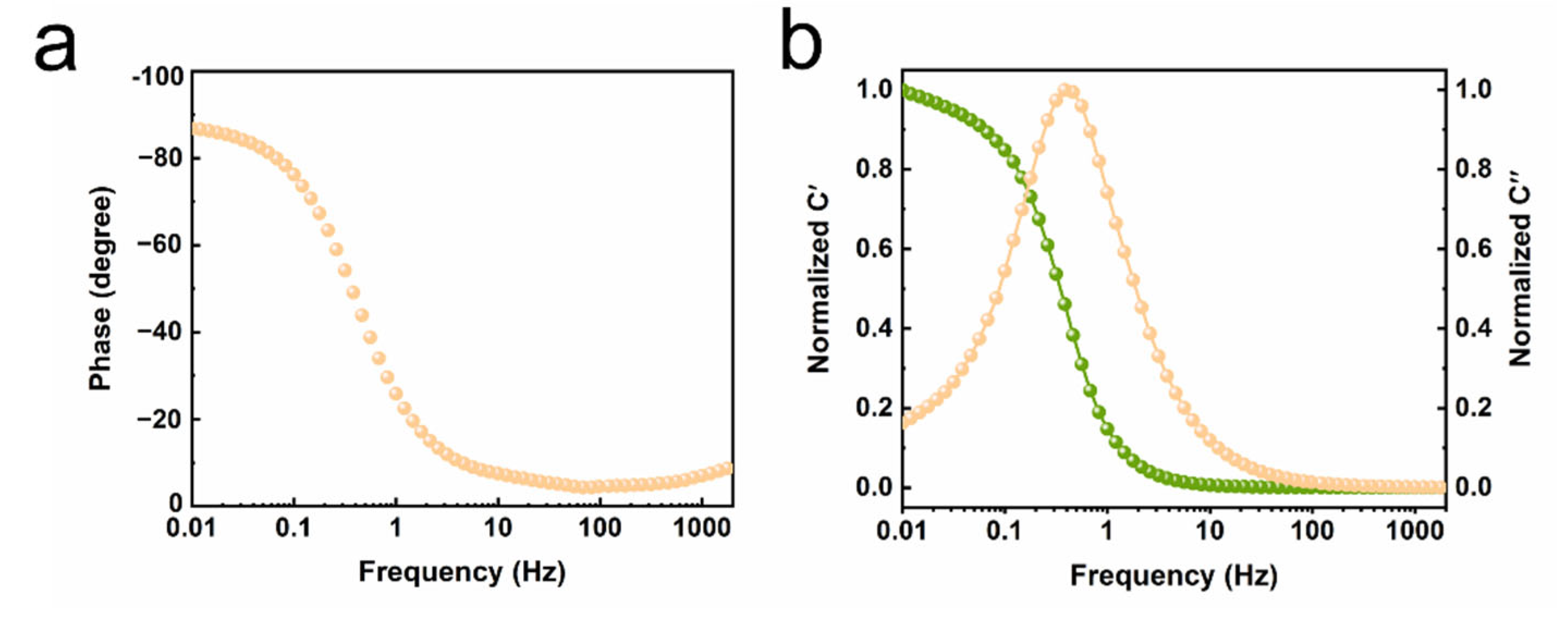
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Swain, N.; Tripathy, A.; Thirumurugan, A.; Saravanakumar, B.; Schmidt-Mende, L.; Ramadoss, A. A brief review on stretchable, compressible, and deformable supercapacitor for smart devices. Chem. Eng. J. 2022, 446, 136876. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Tao, X.; Liang, Y.; Yang, S.J.; Huang, J.Q.; Yuan, T.Q.; Titirici, M.M.; Zhang, Q. Recent progress on biomass-derived ecomaterials toward advanced rechargeable lithium batteries. EcoMat 2020, 2, e12019. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Liu, M.; Niu, J.; Zhang, Z.; Dou, M.; Wang, F. Potassium compound-assistant synthesis of multi-heteroatom doped ultrathin porous carbon nanosheets for high performance supercapacitors. Nano Energy 2018, 51, 366–372. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T.S. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Koda, K.; Taira, S.; Kubota, A.; Isozaki, T.; You, X.; Uraki, Y.; Sugimura, K.; Nishio, Y. Development of Lignin-Based Terpolyester Film and Its Application to Separator Material for Electric Double-Layer Capacitor. J. Wood Chem. Technol. 2019, 39, 199–214. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis Strategies of Porous Carbon for Supercapacitor Applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Schuetter, C.; Pohlmann, S.; Balducci, A. Industrial Requirements of Materials for Electrical Double Layer Capacitors: Impact on Current and Future Applications. Adv. Energy Mater. 2019, 9, 1900334. [Google Scholar] [CrossRef]
- Athanasiou, M.; Yannopoulos, S.N.; Ioannides, T. Biomass-derived graphene-like materials as active electrodes for supercapacitor applications: A critical review. Chem. Eng. J. 2022, 446, 137191. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef]
- Divyashree, A.; Hegde, G. Activated carbon nanospheres derived from bio-waste materials for supercapacitor applications—A review. RSC Adv. 2015, 5, 88339–88352. [Google Scholar] [CrossRef]
- Shaker, M.; Ghazvini, A.A.S.; Cao, W.; Riahifar, R.; Ge, Q. Biomass-derived porous carbons as supercapacitor electrodes—A review. New Carbon Mater. 2021, 36, 546–568. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Raju, T.D.; Badhulika, S. Green synthesis of nitrogen, sulfur-co-doped worm-like hierarchical porous carbon derived from ginger for outstanding supercapacitor performance. Carbon 2020, 168, 209–219. [Google Scholar] [CrossRef]
- Gang, X.; Krishnamoorthy, M.; Jiang, W.; Pan, J.; Pan, Z.; Liu, X. A novel in-situ preparation of N-rich spherical porous carbon as greatly enhanced material for high-performance supercapacitors. Carbon 2021, 171, 62–71. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zhou, J.; Liang, T. Biomass-Derived Porous Carbon Materials for Supercapacitor. Front. Chem. 2019, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Badhulika, S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.; Xu, C.; Ren, Y.; Tang, D.; Zhang, C.; Li, F.; Wei, X.; Zhang, R. Oxygen-nitrogen-sulfur self-doping hierarchical porous carbon derived from lotus leaves for high-performance supercapacitor electrodes. J. Power Sources 2020, 479, 228799. [Google Scholar] [CrossRef]
- Shen, F.; Zhu, L.; Qi, X. Nitrogen Self-Doped Hierarchical Porous Carbon from Myriophyllum Aquaticum for Supercapacitor Electrode. ChemistrySelect 2018, 3, 11350–11356. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable activated carbons from dead ginkgo leaves for supercapacitor electrode active materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Fan, H.; Wang, G. Naturally nitrogen doped porous carbon derived from waste shrimp shells for high-performance lithium ion batteries and supercapacitors. Micropor. Mesopor. Mat. 2017, 246, 72–80. [Google Scholar] [CrossRef]
- Huang, J.; Wu, J.; Dai, F.; Li, C.M. 3D honeycomb-like carbon foam synthesized with biomass buckwheat flour for high-performance supercapacitor electrodes. Chem. Commun. 2019, 55, 9168–9171. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; He, J.; Wang, Y.; Zhang, X.; Zhang, Y.; Liu, X.; Wang, K.; Wang, Y. Hierarchical porous carbon nanosheet derived from waste engine oil for high-performance supercapacitor application. Sustain. Energ Fuels 2019, 3, 499–507. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Wang, C.; Zhao, L.; Jian, W.; Lu, K.; Lin, H.; Qiu, X.; Alshareef, H.N. Lignin Derived Porous Carbons: Synthesis Methods and Supercapacitor Applications. Small Methods 2021, 5, e2100896. [Google Scholar] [CrossRef]
- Zhang, R.; Du, Q.; Wang, L.; Zheng, Z.; Guo, L.; Zhang, X.; Yang, X.; Yu, H. Unlocking the response of lignin structure for improved carbon fiber production and mechanical strength. Green Chem. 2019, 21, 4981–4987. [Google Scholar] [CrossRef]
- Khamnantha, P.; Homla-or, C.; Suttisintong, K.; Manyam, J.; Raita, M.; Champreda, V.; Intasanta, V.; Butt, H.-J.; Berger, R.; Pangon, A. Stable Lignin-Rich Nanofibers for Binder-Free Carbon Electrodes in Supercapacitors. ACS Appl. Nano Mater. 2021, 4, 13099–13111. [Google Scholar] [CrossRef]
- Thongsai, N.; Hrimchum, K.; Aussawasathien, D. Carbon fiber mat from palm-kernel-shell lignin/polyacrylonitrile as intrinsic-doping electrode in supercapacitor. Sustain. Mater. Technol. 2021, 30, e00341. [Google Scholar] [CrossRef]
- Fu, F.; Wang, H.; Yang, D.; Qiu, X.; Li, Z.; Qin, Y. Lamellar hierarchical lignin-derived porous carbon activating the capacitive property of polyaniline for high-performance supercapacitors. J. Colloid Interface Sci. 2022, 617, 694–703. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Sui, W.; Xu, T.; Li, Z.; Parvez, A.M.; Si, C. Facile and scalable preparation of cage-like mesoporous carbon from lignin-based phenolic resin and its application in supercapacitor electrodes. Carbon 2022, 196, 819–827. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, M.; Zhang, T.; Min, X.; Wang, Z.; Chai, L.; Shi, Y. High-performance supercapacitor energy storage using a carbon material derived from lignin by bacterial activation before carbonization. J. Mater. Chem. A 2019, 7, 26838–26848. [Google Scholar] [CrossRef]
- Chen, W.; Luo, M.; Yang, K.; Zhou, X. Microwave-assisted KOH activation from lignin into hierarchically porous carbon with super high specific surface area by utilizing the dual roles of inorganic salts: Microwave absorber and porogen. Micropor. Mesopor. Mater. 2020, 300, 110178. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.-G.; He, W.; Hou, X.; Ren, F.; Zhang, Q.; Jin, Y.-L. Boosting the electrochemical performance of nitrogen-oxygen co-doped carbon nanofibers based supercapacitors through esterification of lignin precursor. Renew. Energ 2020, 162, 613–623. [Google Scholar] [CrossRef]
- Taer, E.; Taslim, R.; Apriwandi, A. Ultrahigh Capacitive Supercapacitor Derived from Self-Oxygen Doped Biomass-Based 3D Porous Carbon Sources. ChemNanoMat 2022, 8, e202100388. [Google Scholar] [CrossRef]
- Shi, F.; Tong, Y.; Li, H.; Li, J.; Cong, Z.; Zhai, S.; An, Q.; Wang, K. Synthesis of oxygen/nitrogen/sulfur codoped hierarchical porous carbon from enzymatically hydrolyzed lignin for high-performance supercapacitors. J. Energy Storage 2022, 52, 104992. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Li, S.; Song, Y.; Wen, G. 3D porous oxygen-enriched graphene hydrogels with well-balanced volumetric and gravimetric performance for symmetric supercapacitors. J. Mater. Sci. 2020, 55, 12214–12231. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Highly porous and conductive functional carbon fibers from electrospun phosphorus-containing lignin fibers. Carbon 2022, 200, 134–148. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Hu, L.; Guo, K.; Li, H.; Zhai, T. Highly Porous Carbon with Graphene Nanoplatelet Microstructure Derived from Biomass Waste for High-Performance Supercapacitors in Universal Electrolyte. Adv. Sustain. Syst. 2017, 1, 1600011. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing functionalized graphene as electrodes for high-performance supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Zhai, D.D.; Chen, X.Y.; Zhang, Z.J. In-situ synthesis of highly nitrogen, sulfur co-doped carbon nanosheets from melamine-formaldehyde-thiourea resin with improved cycling stability and energy density for supercapacitors. J. Power Sources 2019, 416, 79–88. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, C.; Yu, D.; Sun, L.; Yang, C.; Zhang, H.; Sun, Y.; Besenbacher, F.; Yu, M. One-step production of O-N-S co-doped three-dimensional hierarchical porous carbons for high-performance supercapacitors. Nano Energy 2018, 47, 547–555. [Google Scholar] [CrossRef]
- Wan, X.; Shen, F.; Hu, J.; Huang, M.; Zhao, L.; Zeng, Y.; Tian, D.; Yang, G.; Zhang, Y. 3-D hierarchical porous carbon from oxidized lignin by one-step activation for high-performance supercapacitor. Int. J. Biol. Macromol. 2021, 180, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.A.; Lin, L.-T.; Ko, F.; Renneckar, S. Carbon Aerogels From Softwood Kraft Lignin for High Performance Supercapacitor Electrodes. Front. Mater. 2022, 9, 894061. [Google Scholar] [CrossRef]
- Tian, J.; Liu, C.; Lin, C.; Ma, M. Constructed nitrogen and sulfur codoped multilevel porous carbon from lignin for high-performance supercapacitors. J. Alloys Compd. 2019, 789, 435–442. [Google Scholar] [CrossRef]
- Du, B.; Zhu, H.; Chai, L.; Cheng, J.; Wang, X.; Chen, X.; Zhou, J.; Sun, R.-C. Effect of lignin structure in different biomass resources on the performance of lignin-based carbon nanofibers as supercapacitor electrode. Ind. Crop. Prod. 2021, 170, 113745. [Google Scholar] [CrossRef]
- Du, B.; Wang, X.; Chai, L.; Wang, X.; Pan, Z.; Chen, X.; Zhou, J.; Sun, R.C. Fabricating lignin-based carbon nanofibers as versatile supercapacitors from food wastes. Int. J. Biol. Macromol. 2022, 194, 632–643. [Google Scholar] [CrossRef]
- Thielke, M.W.; Lopez Guzman, S.; Victoria Tafoya, J.P.; García Tamayo, E.; Castro Herazo, C.I.; Hosseinaei, O.; Sobrido, A.J. Full Lignin-Derived Electrospun Carbon Materials as Electrodes for Supercapacitors. Front. Mater. 2022, 9, 859872. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, X.; Qi, X. Hierarchical nanoarchitectonics of ordered mesoporous carbon from lignin for high-performance supercapacitors. Int. J. Biol. Macromol. 2022, 213, 610–620. [Google Scholar] [CrossRef]
- Perera Jayawickramage, R.A.; Ferraris, J.P. High performance supercapacitors using lignin based electrospun carbon nanofiber electrodes in ionic liquid electrolytes. Nanotechnology 2019, 30, 155402. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Zhang, J.; Guo, H.; Feng, X.; Chen, Y. Synthesis of porous carbon spheres derived from lignin through a facile method for high performance supercapacitors. J. Mater. Sci. Technol. 2018, 34, 2189–2196. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Ong, W.K.; Lu, X. Ultrafast-Freezing-Assisted Mild Preparation of Biomass-Derived, Hierarchically Porous, Activated Carbon Aerogels for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 7, 403–411. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.; Tsang, D.C.W. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Mei, X.; Peng, F. Boosting Capacity Performance of Bio-Waste Lignin-Derived Hierarchical Porous Carbon with Self-Doped Oxygen-Heteroatoms. Batteries 2022, 8, 286. https://doi.org/10.3390/batteries8120286
Liu J, Mei X, Peng F. Boosting Capacity Performance of Bio-Waste Lignin-Derived Hierarchical Porous Carbon with Self-Doped Oxygen-Heteroatoms. Batteries. 2022; 8(12):286. https://doi.org/10.3390/batteries8120286
Chicago/Turabian StyleLiu, Jia, Xiuwen Mei, and Feng Peng. 2022. "Boosting Capacity Performance of Bio-Waste Lignin-Derived Hierarchical Porous Carbon with Self-Doped Oxygen-Heteroatoms" Batteries 8, no. 12: 286. https://doi.org/10.3390/batteries8120286
APA StyleLiu, J., Mei, X., & Peng, F. (2022). Boosting Capacity Performance of Bio-Waste Lignin-Derived Hierarchical Porous Carbon with Self-Doped Oxygen-Heteroatoms. Batteries, 8(12), 286. https://doi.org/10.3390/batteries8120286






