Design of Cuboidal FeNi2S4-rGO-MWCNTs Composite for Lithium-Ion Battery Anode Showing Excellent Half and Full Cell Performances
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of Cuboidal Shaped Porous FeNi2S4@rGO/MWCNTs (FNS@GC)
2.3. Material Characterisation
2.4. Electrochemical Characterisation
3. Results and Discussion
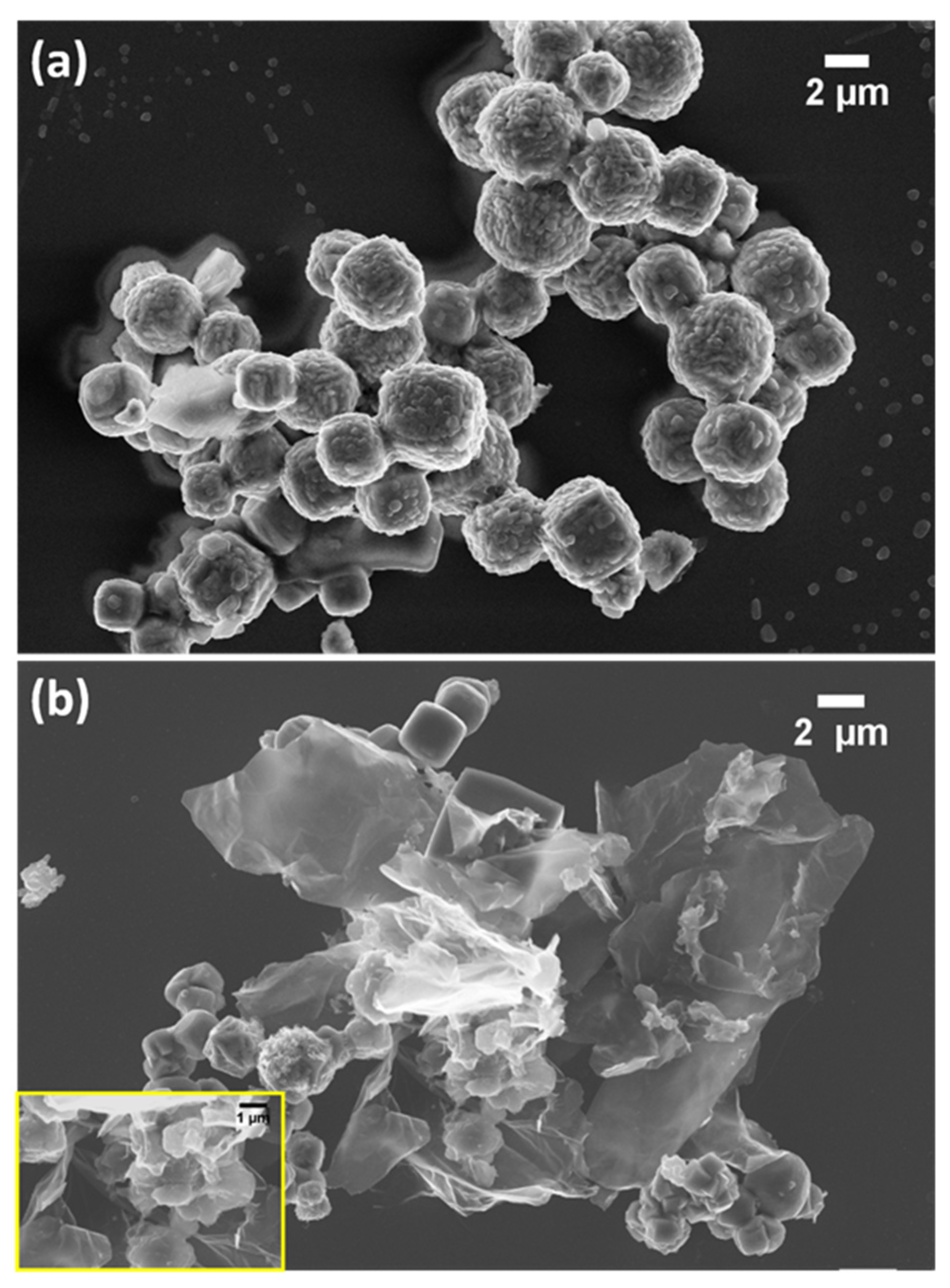
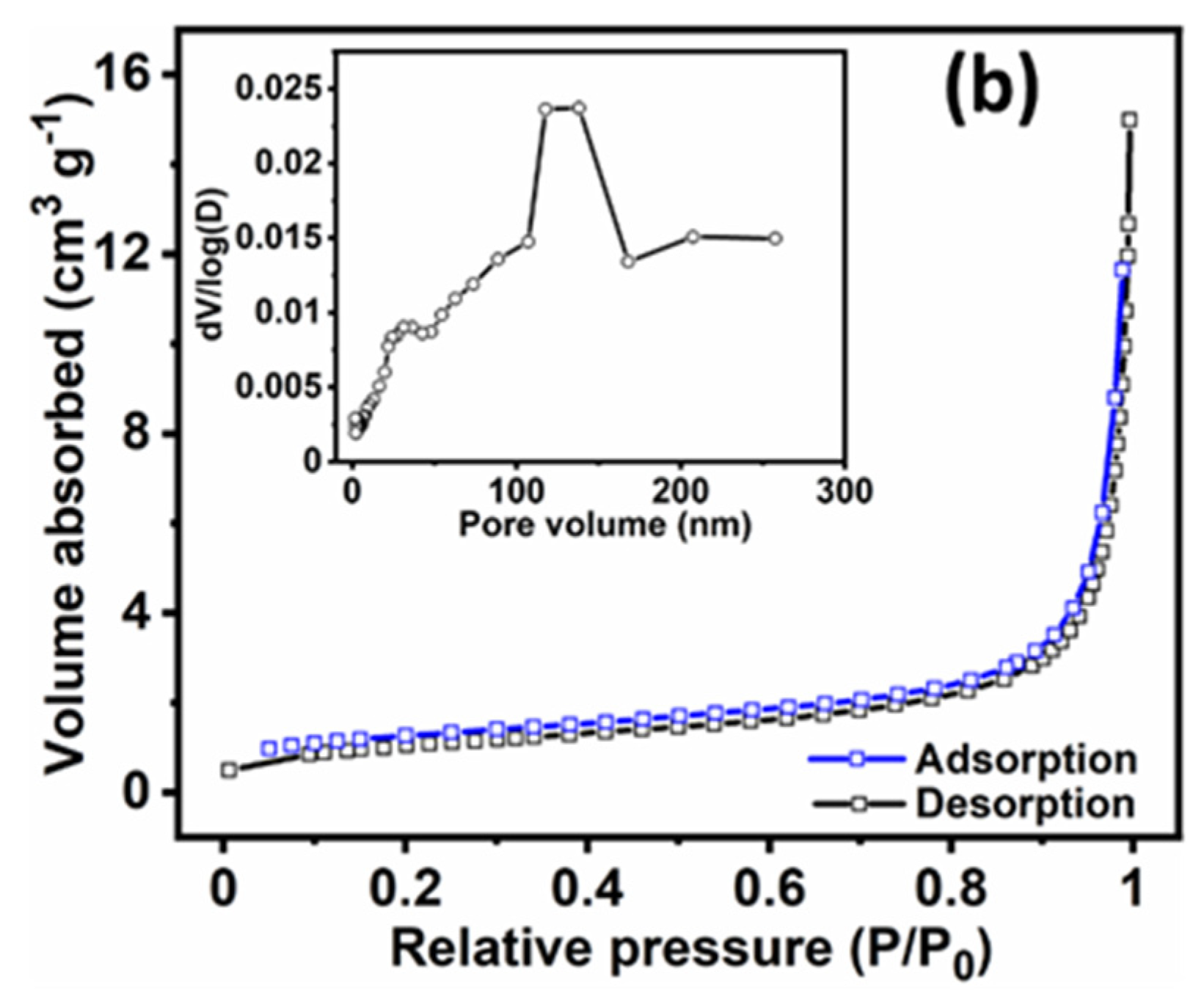
3.1. Physical Characterisation
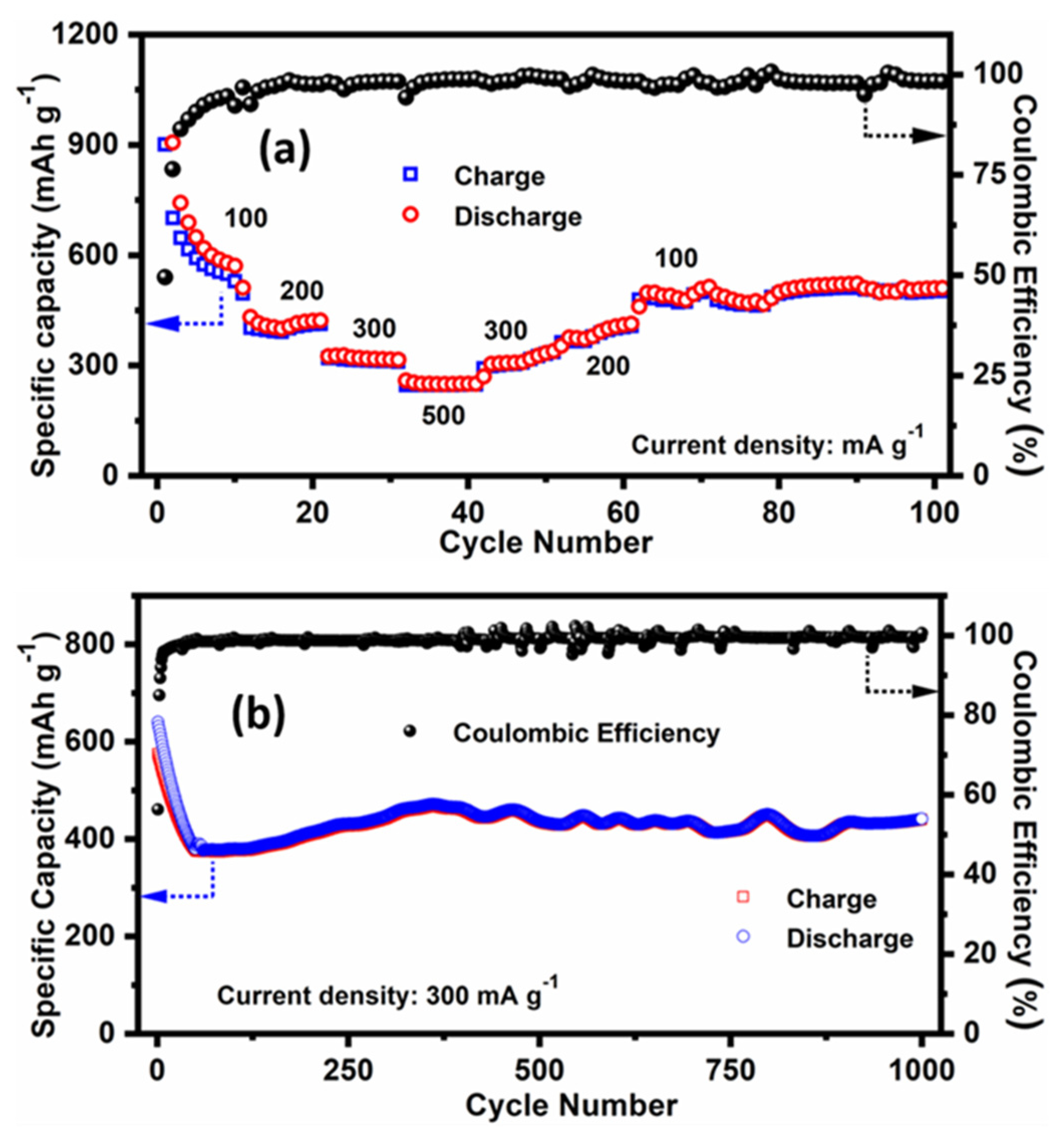
3.2. Electrochemical Characterisation as Half Cell Lithium-Ion Battery (LIB) Anode
| Materials | Morphology/Electrode Composition | Voltage Window (V) | Current Density (mA g−1) | Specific Capacity (mAh g−1)/Cycle Number | Ref. |
|---|---|---|---|---|---|
| NiCo2S4 | hexagonal nanosheets@rGO | 0.01–3.0 | 2000 | 607/800 | [20] |
| NiCo2S4 | hollow spheres | 0.01–3.0 | 200 | 696/100 | [41] |
| NiCo2S4 | hollow nanowires@ carbon | 0.005–3.0 | 500 | 1198/500 | [21] |
| NiCo2S4 | nanosheet array/carbon cloth | 0.01–3.0 | 100 | 1137/100 | [16] |
| NiCo2S4 | nanocores in-situ encapsulated in graphene sheets | 0.01–3.0 | 200 | 535/1000 | [42] |
| NiCo2S4 | multi-shelled hollow polyhedrons | 0.0–3.0 | 100 | 745.5/100 | [15] |
| CuCo2S4 | agglomerated non-uniform nanoparticles/MWCNTs | 0.01–3.0 | 500 | 1300/200 | [17] |
| CuCo2S4 | CuCo2S4/reduced graphene oxide nanocomposites | 0.01–2.5 | 100 | 433/50 | [18] |
| CuCo2S4 | Sphere | 0.01–3.0 | 1000 | 773.7/1000 | [19] |
| NiTi2S4 | nanoparticles | 0.01–3.0 | 1000 | 635/50 | [6] |
| ZnCo2S4/NiCo2S4 | Flakes/@carbon cloth | 0.01–3.0 | 1.5 mA cm−2 | 2.4 mAh cm−2/100 | [25] |
| FeNi2S4 | Agglomerated particle/FeNi2S4 quantum dot @C composites | 0.005–3.0 | 500 | ~750/700 | [22] |
| (Fe0.5Ni0.5)S2/rGO | 0D/2D-sheet like nanocomposite | 0.03–3.0 | 200 | 830/100 | [43] |
| FNS@GCFNS@GC//LiFePO4(Full cell) | Cuboidal shaped FNS particle/with 1D/2D carbon composite | 0.01–3.0 | 100 | 901/600 | This work |
| 300 | 440/1000 | ||||
| 1.0–3.6 | 50 | 77/60 |
3.3. Electrochemical Performance of Full Cell LIB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, C.; Wan, C.; Wang, R.; Dai, X.; Wei, J.; Xia, H.; Li, W.; Zhang, W.; Cao, S.; et al. Strain-Driven Auto-Detachable Patterning of Flexible Electrodes. Adv. Mater. 2022, 34, 2202877. [Google Scholar] [CrossRef] [PubMed]
- Grey, C.P.; Hall, D.S. Prospects for Lithium-Ion Batteries and beyond—a 2030 Vision. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode Materials for Lithium-Ion Batteries: A Review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, K.-H.; Lee, J.; Hong, S.-H. Electrochemical Properties and Reaction Mechanism of NiTi2S4 Ternary Metal Sulfide as an Anode for Lithium Ion Battery. ACS Sustain. Chem. Eng. 2021, 9, 9680–9688. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The Application of Nanostructured Transition Metal Sulfides as Anodes for Lithium Ion Batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef]
- Yi, T.F.; Pan, J.J.; Wei, T.T.; Li, Y.; Cao, G. NiCo2S4-Based Nanocomposites for Energy Storage in Supercapacitors and Batteries. Nano Today 2020, 33, 100894. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Dhawa, T.; Sreemany, M.; Mahanty, S. High Faradaic Charge Storage in ZnCo2S4 Film on Ni-Foam with a Hetero-Dimensional Microstructure for Hybrid Supercapacitor. Mater. Today Energy 2018, 9, 416–427. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Sreemany, M.; Mahanty, S. Carbon Doped MnCo2S4 Microcubes Grown on Ni Foam as High Energy Density Faradaic Electrode. Electrochim. Acta 2016, 213, 672–679. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Chattopadhyay, S.; De, G.; Mahanty, S. ‘Cotton-Ball’ Shaped Porous Iron-Nickel Sulfide: A High-Rate Cathode for Long-Life Aqueous Rechargeable Battery. Mater. Res. Bull. 2021, 140, 111307. [Google Scholar] [CrossRef]
- Venugopal, B.; Shown, I.; Samireddi, S.; Syum, Z.; Krishnamoorthy, V.; Wu, H.L.; Chu, C.W.; Lee, C.H.; Chen, L.C.; Chen, K.H. Microstructural Intra-Granular Cracking in Cu2ZnSnS4@C Thin-Film Anode Enhanced the Electrochemical Performance in Lithium-Ion Battery Applications. Mater. Adv. 2021, 2, 5672–5685. [Google Scholar] [CrossRef]
- Zuo, X.; Song, Y.; Zhen, M. Carbon-Coated NiCo2S4 Multi-Shelled Hollow Microspheres with Porous Structures for High Rate Lithium Ion Battery Applications. Appl. Surf. Sci. 2020, 500, 144000. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Ye, L.W.; Zhang, D.; Chen, F.; Zhu, M.; Wang, L.N.; Yin, S.M.; Cai, G.S.; Guo, S.Y. NiCo2S4 Multi-Shelled Hollow Polyhedrons as High-Performance Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2019, 299, 289–297. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, Z.; Yuen, M.F.; Sun, M.; Hu, J.; Lee, C.S.; Zhang, W. Three-Dimensional-Networked NiCo2S4 Nanosheet Array/Carbon Cloth Anodes for High-Performance Lithium-Ion Batteries. NPG Asia Mater. 2015, 7, 1–8. [Google Scholar] [CrossRef]
- Muralidharan, N.; Nallathamby, K. CuCo2S4: Versatile Anode for High Capacity and High Rate for Lithium and Sodium Ion Battery Application. J. Phys. Chem. Solids 2021, 151, 109902. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, J.; Wang, H.; Xu, J. CuCo2S4/Reduced Graphene Oxide Nanocomposites Synthesized by One-Step Solvothermal Method as Anode Materials for Sodium Ion Batteries. Electrochim. Acta 2018, 292, 895–902. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.; Meng, X.; Li, S.; Ren, M. Porous Core–Shell CuCo2S4 Nanospheres as Anode Material for Enhanced Lithium-Ion Batteries. Chem. A Eur. J. 2019, 25, 885–891. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Z.; Li, Y.; Wang, Q.; Fang, F.; Zhou, Y.N.; Hu, L.; Sun, D. Pseudocapacitance-Tuned High-Rate and Long-Term Cyclability of NiCo2S4 Hexagonal Nanosheets Prepared by Vapor Transformation for Lithium Storage. J. Mater. Chem. A 2017, 5, 9022–9031. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Wang, B.; Liu, J.; Yu, M. Long-Term Cycling Stability of NiCo2S4 Hollow Nanowires Supported on Biomass-Derived Ultrathin N-Doped Carbon 3D Networks as an Anode for Lithium-Ion Batteries. Chem. Commun. 2021, 57, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Song, H.; Liu, Y.; Wang, C. FeNi2S4 QDs @C Composites as a High Capacity and Long Life Anode Material for Lithium Ion Battery and Ex Situ Investigation of Electrochemical Mechanism. Electrochim. Acta 2017, 258, 1173–1181. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Shi, X.L.; Suo, G.; Chen, H.; Noman, M.; Tao, X.; Chen, Z.G. Hierarchical SnS2/Carbon Nanotube@reduced Graphene Oxide Composite as an Anode for Ultra-Stable Sodium-Ion Batteries. Chem. Eng. J. Adv. 2020, 4, 100053. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Mahanty, S. Reduced Graphene Oxide Anchored Cu(OH)2 as a High Performance Electrochemical Supercapacitor. Dalt. Trans. 2015, 44, 14604–14612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Lin, X.; Han, T.; Cheng, M.; Long, J.; Li, J. A Novel Binary Metal Sulfide Hybrid Li-Ion Battery Anode: Three-Dimensional ZnCo2S4/NiCo2S4 Derived from Metal-Organic Foams Enables an Improved Electron Transfer and Ion Diffusion Performance. J. Alloys Compd. 2020, 817, 153293. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Sreemany, M.; Mahanty, S. Rock-Salt-Templated Mn3O4 Nanoparticles Encapsulated in a Mesoporous 2D Carbon Matrix: A High Rate 2 V Anode for Lithium-Ion Batteries with Extraordinary Cycling Stability. ChemistrySelect 2017, 2, 7854–7864. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, S.; Wang, W.K.; Huang, G.X.; Huang, B.C.; Zhang, F.; Zhang, Y.J.; Yu, H.Q. Ultrahigh Electrocatalytic Oxygen Evolution by Iron-Nickel Sulfide Nanosheets/Reduced Graphene Oxide Nanohybrids with an Optimized Autoxidation Process. Nano Energy 2018, 43, 300–309. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, P.; Dai, E.; Liu, J.; Tian, Z.; Liang, C.; Shao, G. A Novel Reduction Approach to Fabricate Quantum-Sized SnO 2-Conjugated Reduced Graphene Oxide Nanocomposites as Non-Enzymatic Glucose Sensors. Phys. Chem. Chem. Phys. 2014, 16, 8801–8807. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, L.; Zhang, C.; Huang, S.; Liu, T. Facile Fabrication of Functionalized Graphene Sheets (FGS)/ZnO Nanocomposites with Photocatalytic Property. ACS Appl. Mater. Interfaces 2011, 3, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Maiti, S.; Mahanty, S. Superior Lithium Storage Properties of Fe2(MoO4)3/MWCNT Composite with a Nanoparticle (0D)–Nanorod (1D) Hetero-Dimensional Morphology. Chem. Eng. J. 2017, 307, 239–248. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, Y.; Rui, X.; Xiao, N.; Zhu, J.; Zhang, W.; Chen, J.; Liu, W.; Tan, H.; Hoon Hng, H.; et al. Controlled Soft-Template Synthesis of Ultrathin C@FeS Nanosheets with High-Li-Storage Performance. ACS Nano 2012, 6, 4713–4721. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Maiti, S.; Mahanty, S. Metal Hydroxides as a Conversion Electrode for Lithium-Ion Batteries: A Case Study with a Cu(OH)2 Nanoflower Array. J. Mater. Chem. A 2014, 2, 18515–18522. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Sreemany, M.; Mahanty, S. High Electrochemical Energy Storage in Self-Assembled Nest-like CoO Nanofibers with Long Cycle Life. J. Nanoparticle Res. 2016, 18, 93. [Google Scholar] [CrossRef]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dollé, M.; Dupont, L.; Tarascon, J.-M. On the Origin of the Extra Electrochemical Capacity Displayed by MO/Li Cells at Low Potential. J. Electrochem. Soc. 2002, 149, A627. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.S.; Wen, L.; Lu, G.Q.; Cheng, H.M. Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, T.; Cao, L.; Luo, K. High-Capacity Anode Material for Lithium-Ion Batteries with a Core-Shell NiFe2O4/Reduced Graphene Oxide Heterostructure. ACS Omega 2021, 6, 25269–25276. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Yu, G.; Xu, W.; Yin, H.; Hou, Z. Multifunctional Sandwich-Structured Double-Carbon-Layer Modified SnS Nanotubes with High Capacity and Stability for Li-Ion Batteries. Mater. Adv. 2022, 3, 3631. [Google Scholar] [CrossRef]
- Jin, R.; Liu, D.; Liu, C.; Liu, G. Hierarchical NiCo2S4 Hollow Spheres as a High Performance Anode for Lithium Ion Batteries. RSC Adv. 2015, 5, 84711–84717. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Shen, P.K. NiCo2S4 Nanocores In-Situ Encapsulated in Graphene Sheets as Anode Materials for Lithium-Ion Batteries. Chem. Eng. J. 2019, 364, 167–176. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Zhang, L.; Cheng, B.; You, W.; Yu, J. 0D/2D (Fe0.5Ni0.5)S2/RGO Nanocomposite with Enhanced Supercapacitor and Lithium Ion Battery Performance. J. Power Sources 2019, 426, 266–274. [Google Scholar] [CrossRef]
- Liu, D.H.; Lü, H.Y.; Wu, X.L.; Wang, J.; Yan, X.; Zhang, J.P.; Geng, H.; Zhang, Y.; Yan, Q. A New Strategy for Developing Superior Electrode Materials for Advanced Batteries: Using a Positive Cycling Trend to Compensate the Negative One to Achieve Ultralong Cycling Stability. Nanoscale Horizons 2016, 1, 496–501. [Google Scholar] [CrossRef]
- Hu, L.-H.; Wu, F.Y.; Lin, C.T.; Khlobystov, A.N.; Li, L.J. Graphene-Modified LiFePO4 Cathode for Lithium Ion Battery beyond Theoretical Capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef]
- Yuan, L.X.; Wang, Z.H.; Zhang, W.X.; Hu, X.L.; Chen, J.T.; Huang, Y.H.; Goodenough, J.B. Development and Challenges of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Nag, S.; Pramanik, A.; Shyamal Roy, S.M. Enhancement of Li+ Ion Kinetics in Boehmite Nanofiber Coated Polypropylene Separator in LiFePO4 Cells. J. Solid State Chem. 2022, 312, 123214. [Google Scholar] [CrossRef]
- Kakarla, A.K.; Narsimulu, D.; Yu, J.S. Two-dimensional porous NiCo2O4 nanostructures for use as advanced high-performance anode material in lithium-ion batteries. J. Alloys Compd. 2021, 886, 161224. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Wang, Z.; Guo, H.; Li, Y. A novel NiCo2O4 anode morphology for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 11970. [Google Scholar] [CrossRef]
- Kang, Y.; Shi, H.; Zhang, Y.-H.; Shi, F.-N. High-performance ZnCo2O4 microsheets as an anode for lithium-ion batteries. Chem. Commun. 2021, 57, 10723. [Google Scholar] [CrossRef]
- Zhu, L.; Li, F.; Yao, T.; Liu, T.; Wang, J.; Li, Y.; Lu, H.; Qian, R.; Liu, Y.; Wang, H. Electrospun MnCo2O4 Nanotubes as High-Performance Anode Materials for Lithium-Ion Batteries. Energy Fuels 2020, 34, 11574–11580. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Design of Cuboidal FeNi2S4-rGO-MWCNTs Composite for Lithium-Ion Battery Anode Showing Excellent Half and Full Cell Performances. Batteries 2022, 8, 261. https://doi.org/10.3390/batteries8120261
Pramanik A, Chattopadhyay S, De G, Mahanty S. Design of Cuboidal FeNi2S4-rGO-MWCNTs Composite for Lithium-Ion Battery Anode Showing Excellent Half and Full Cell Performances. Batteries. 2022; 8(12):261. https://doi.org/10.3390/batteries8120261
Chicago/Turabian StylePramanik, Atin, Shreyasi Chattopadhyay, Goutam De, and Sourindra Mahanty. 2022. "Design of Cuboidal FeNi2S4-rGO-MWCNTs Composite for Lithium-Ion Battery Anode Showing Excellent Half and Full Cell Performances" Batteries 8, no. 12: 261. https://doi.org/10.3390/batteries8120261
APA StylePramanik, A., Chattopadhyay, S., De, G., & Mahanty, S. (2022). Design of Cuboidal FeNi2S4-rGO-MWCNTs Composite for Lithium-Ion Battery Anode Showing Excellent Half and Full Cell Performances. Batteries, 8(12), 261. https://doi.org/10.3390/batteries8120261






