Abstract
As the desired components and crystal structure of a transition metal oxide catalyst are selected, architecture is a dominating factor affecting its electrocatalytic performance for applications in lithium-sulfur (Li-S) batteries. Nano-compounds with a hollow architecture are undoubtedly the ideal catalysts for enhancing cathodic performance for more exposed active sites and shortened path lengths than are other architectures. Additionally, the internal stress in hollow architecture is favorable for further performance enhancement, due to its regulation effects of driving the d-band center of the transition metal in the active sites to migrate toward the Fermi level, which will promote the chemical adsorption and catalytic conversion of the polysulfides (PSs). To this point, we select hierarchical porous dual transition metal oxide CoNiO2 nano-boxes (CoNiO2(B)) as the conceptual model; meanwhile, CoNiO2 nano-flakes (CoNiO2(F)) with identical stoichiometry and crystal structure are also analyzed as a comparison. Li-S batteries based on CoNiO2(B) deliver superior energy storage features, including a reversible discharge capacity of 1232 mAh g−1 at 0.05 C and a stable cycle performance with decay rate of 0.1% each cycle even after 300 cycles at 1 C. This research presents an alternative scheme for booting the performance of Li-S batteries.
1. Introduction
Li-S batteries are generally accepted as the most attractive energy storage systems owing to their high specific capacity of 1675 mAh g−1 and theoretical energy density of 2600 Wh kg−1 [1,2]. Additionally, as an active substance, sulfur is ample on Earth and environmentally friendly [3,4]. However, several technical issues, including the intrinsic insulating properties of sulfur, the shuttle effect associated with soluble PSs, and the enormous variation in volume during electrochemical reaction, significantly constrain the industrial application of Li-S batteries [5,6].
In the last few decades, many kinds of sulfur hosts have been developed to tackle the problems mentioned above. Among each host, conductive porous carbon has been considered the most promising material [7,8,9]. The porous carbon host greatly increases the sulfur cathode’s electronic conductivity as well as provides sufficient empty space to contain volumetric alterations during charging and discharging reactions [10,11,12]. Nazar et al. prepared mesoporous carbon with a highly organized structure (CMK-3), and the S/CMK-3 cathode had a high initial capacity of 1005 mAh g−1 at sulfur loads of up to 70 wt% [13]. Despite the fact that their initial cycle-specific capacities for carbon/sulfur composites are extraordinarily high, capacity decays sharply in later cycles due to the repulsion between polar PSs and non-polar carbonaceous substrates.
In recent years, polar non-organic substances such as transition metal oxides [14,15,16,17], sulfides [18,19,20], carbides [21,22], and hydroxides [23,24] have been focused on effective sulfur hosts, which are not only an effective component to chemically capture lithium polysulfide (LiPSs) but also an excellent catalysis for facilitating LiPSs conversion kinetics [25]. Among them, the layered double hydroxides (LDHs) and their derived oxide are a class of prominent sulfur hosts. The abundant hydrophilic groups in LDHs have strong affinity for LiPSs, and the existence of numerous sulfophilic sites accelerates the transformation kinetics of LiPSs [26,27]. As the desired components and crystal structure are selected, architecture-tuning is another key factor affecting the electrocatalytic performance of the material. Nanostructures with hollow architecture, including nano-boxes, nano-cages, and hollowed-spheres, etc., are undoubtedly a series of ideal configuration in enhancing catalytic performance, exhibiting more exposed active sites than other morphological structures. Furthermore, tensile strain aroused from the smaller curvature radius in hollow architecture is another effective factor in performance enhancement, as the tensile strain regulates the electronic structure of materials and drives the d-band center of the transition metal in the active sites to migrate toward the Fermi level, which will enhance the chemical adsorption and catalytic conversion of the PSs [28,29]. Benefiting from multivariate designable morphologies and controllable synthesis, metal-organic frameworks (MOFs) are desired sacrificial templates for synthesis of hollowed-LDH-nanostructures. Through annealing at appropriate temperature, they can further be transformed to oxide-hollowed analogues. Due to the high aspect ratio and hierarchical porous structure inherited from MOFs, these hydroxide- or oxide-hollowed nanostructures are especially favorable for improving utilization of a sulfur cathode. For example, Zhao’s group prepared a hollow-structured NiCo-LDH, and the corresponding Li-S battery exhibited excellent capacity (1540 mAh g−1 at 0.1 C) and a high rate of electrical performance (485 mAh g−1 at 5.0 C) [30]. Ying et al. developed a MnO-TiO2 core-shell nano-box composite with a 0.05% decay rate each cycle after 500 cycles at 0.5 C [31]. These transition metal hydroxide and oxide sulfur hosts grown on MOFs exhibit extraordinary electrochemical activity for Li-S batteries.
Given its low cost and inherent corrosion resistance, bimetallic nickel cobalt oxide has great advantages as an excellent catalyst with dual roles for accelerating the slow redox kinetics [32]. In this paper, we adopt dual transition metal oxide CoNiO2(B) with a hierarchical porous structure as the conceptual model and CoNiO2(F) with identical stoichiometry and crystal structure as the comparative control sample. CoNiO2(B) was transformed from ZIF-67 through the ultrasonic solve-thermal method. The Li-S battery based on CoNiO2(B) delivers outstanding energy storage features, including a reversible discharge capacity of 1232 mAh g−1 at 0.05 C, and a stable cycle performance with a decay rate of 0.1% each cycle even after 300 cycles at 1 C.
2. Results and Discussion
The preparation process of the S/CoNiO2(B) cathode is schematically illustrated in Scheme 1. First, ZIF-67 is used as a sacrificial template. When nickel nitrate is added, ZIF-67 is etched by H+, which is produced by the hydrolysis of Ni2+. Then, the −NH2 falls off from ZIF-67 under ultrasonic vibration and hydrolyzes to generate OH−, which accelerates the internal collapse of ZIF-67 and causes Co2+ to move outward. Both free Co2+ and Ni2+ bind with OH− and co-deposit on the surface of the ZIF-67 framework to form NiCo-LDH nanosheets [33], which finally stack together to constitute a hollow NiCo-LDH nano-box (NiCo-LDH(B)). With further annealing, NiCo-LDH(B) is transformed into CoNiO2(B). Sulfur is injected into CoNiO2(B) by the melt-diffusion method; finally, formed S/CoNiO2(B), carbon black, and PVDF are mixed as the cathode to assemble the Li-S battery.
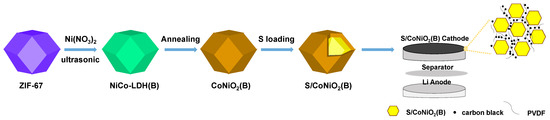
Scheme 1.
Scheme of the synthesis of the CoNiO2(B), S/CoNiO2(B), and S/CoNiO2(B) cathode.
Figure 1a is the scanning electron microscope (SEM) picture of ZIF-67. It can be observed that the formed ZIF-67 template exhibits a regular dodecahedron morphology. NiCo-LDH(B) (Figure 1b) constructed with nanosheets presents a hollow nano-box shape with uniform size. The finally transformed CoNiO2(B) in Figure 1c has almost the same regular polyhedral morphology as ZIF-67. By thermal removal of crystal water in NiCo-LDH nano-flakes (NiCo-LDH(F)) (Figure 1d) synthesized by hydrothermal procedure, CoNiO2(F) is also prepared as the comparative sample, whose two-dimensional morphology is inherited from NiCo-LDH(F), as shown in Figure 1e. Transmission electron microscopy (TEM) was employed to further analyze the morphology and crystal structure of the transition metal oxide nano-boxes and nano-flakes. The TEM image of CoNiO2(F) (Figure 1f) verifies the perfect crystallinity, and its inset exhibits distinct lattice fringes with a spacing of 0.24 nm corresponding to the (200) plane of CoNiO2. The transmission electron microscope image of CoNiO2(B) (Figure 1g) illustrates the hollow interior. Figure 1h is a high-magnification TEM image of CoNiO2(B), which indicates two set lattice fringes, with interplanar spacings of 0.24 and 0.21 nm, which correspond to the (200) and (111) planes of the CoNiO2 compound crystal, respectively. Figure 1f is the EDS mapping of CoNiO2(B), which displays the uniform elemental distribution of the Ni, Co, and O components.
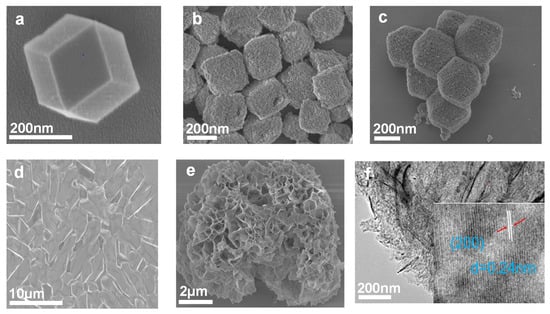
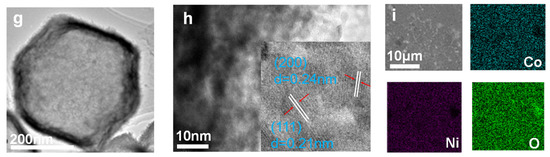
Figure 1.
The SEM and TEM images. The SEM image of (a) ZIF-67, (b) NiCo-LDH(B), (c) CoNiO2(B), (d) NiCo-LDH(F), and (e) CoNiO2(F). TEM images of (f) CoNiO2(F) and (g) CoNiO2(B). (h) HRTEM images of CoNiO2(B). (i) EDS elemental mapping of CoNiO2(B).
X-ray diffraction (XRD) measurements were conducted to investigate the crystallographic characteristics of the materials. Diffraction peaks of NiCo-LDH(B) in Figure 2a were located at 2θ = 11.2°, 22.3°, 34.1°, and 62.8°, corresponding to the (003), (006), (012), and (110) planes, respectively, of a standard NiCo-LDH crystal [34]. The two materials of CoNiO2(B) and CoNiO2(F) have identical XRD diffraction patterns as shown in Figure 2b. The diffraction peaks at 36.8°, 42.8°, 61.8°, 73.9°, and 77.9° exactly fit in with the crystal planes (111), (200), (220), (311), and (222), respectively, of the standard CoNiO2 bulk crystal (PDF#10-0188). Raman spectra also verified the detection results of XRD, as displayed in Figure 2c. CoNiO2(B) and CoNiO2(F) have two obvious Raman spectra peaks at 494 cm-1 and 1061 cm−1, which correspond to the fingerprint Raman peaks of CoNiO2 oxide compounds. After vulcanization, the Raman peak position of S/CoNiO2(B) and S/CoNiO2(F) remains unchanged, and the peak intensity becomes weak. The results of XRD diffraction and Raman characterization confirm that the two CoNiO2 nano-compounds have the identical crystal structures. In Figure 2d, S/CoNiO2(B) and S/CoNiO2(F) exhibited strong XRD diffraction peaks of sulfur, indicating that sulfur had penetrated into the interior of the two materials. The SEM image of S/CoNiO2(B) (Figure S1) displays that the vulcanized composite maintains the polyhedral morphology, and the corresponding element mapping explains that the injected sulfur is evenly distributed. To inspect the interface and pore characteristics of CoNiO2(B) and CoNiO2(F), Brunauer–Emmett–Teller (BET) tests were carried out. Figure 2e clearly reveals that CoNiO2(B) possesses a specific surface area of 204 m2 g−1, which is larger than CoNiO2(F) (137 m2 g−1), indicating that the architecture of the hollow nano-box is conducive to expansion of the specific surface area of the CoNiO2 nano-compound. Figure 2f shows that the average pore-sizes of CoNiO2(B) and CoNiO2(F) are 17.356 nm and 34.563 nm, respectively. Although both materials have a mesoporous structure, CoNiO2(B) has a larger specific surface area and smaller average pore-size, which can provide more active sites and efficient electron/ion transportation channels than CoNiO2(F), which is beneficial for electrochemical performance of the Li-S batteries.
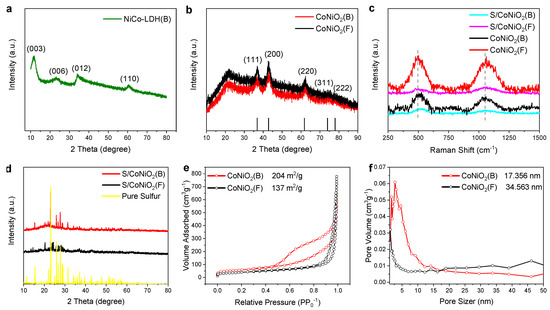
Figure 2.
The result of XRD, Raman and BET; (a) X-ray diffraction pattern of NiCo-LDH(B). (b) XRD pattern of CoNiO2(B) and CoNiO2(F). (c) Raman spectra of CoNiO2(B) and CoNiO2(F). (d) XRD pattern of S/CoNiO2(B) and S/CoNiO2(F). (e) The isotherms of N2 adsorption-desorption and (f) the distributions of pore sizes in CoNiO2(B) and CoNiO2(F).
Sulfur is injected into the host by melt-diffusion, and the mass proportion of sulfur was obtained from a thermogravimetric analysis (TGA) measurement. Due to sublimation of sulfur, the TGA curve has a large weight drop in the temperatures ranging from 200 °C to 350 °C, and the weight loss reflects the mass percentage of the loaded sulfur, as displayed in Figure 3a. S/CoNiO2(B) has a higher sulfur content (72.4 wt%) than S/CoNiO2(F) (69.9 wt%). This can be attributed to the large internal storage capacity of the hollow architecture of the CoNiO2(B) material. In order to characterize the lithium hexasulfide-adsorption of the materials, 8 mg of the obtained NiCo-LDH(B), CoNiO2(F), and CoNiO2(B) were respectively soaked into 4 mL of 5 mM Li2S6 solution with a mixture solvent of DOL and DOE (volume ratio V/V = 1/1); their corresponding containing bottles were labeled as bottle 2, bottle 3, and bottle 4, while the bottle containing pure Li2S6 solution was labeled as bottle 1. All the solutions rested for 10 h. Figure 3b shows the results of the visualized static Li2S6 adsorption. It is clearly observed that bottle 4 has the highest transparency, followed by bottle 3, and finally bottle 2. Among them, the solution containing CoNiO2(B) is almost colorless, indicating that CoNiO2(B) has an excellent adsorption capacity for PSs. UV-vis spectra also confirmed the same conclusion, as the solution containing CoNiO2(B) has the weakest absorption peak at the characteristic wavelength position of LiPSs [35].
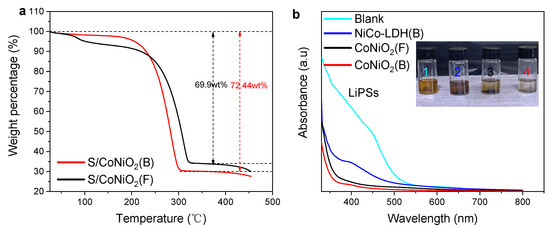
Figure 3.
TGA and adsorption measurement; (a) TGA curve of S/CoNiO2(B) and S/CoNiO2(F). (b) UV/vis adsorption spectra and static adsorption images.
X-ray photoelectron spectroscopy (XPS) surveys were performed to explore the chemical state of the materials. The XPS full range binding energy spectrum (Figure S2) shows that CoNiO2(B) and CoNiO2(F) all contain the four elements Co, Ni, O, and C; the residual C may come from the ZIF-67 template or from environmental contamination. Figure 4a–c shows the photoelectron spectra of Co 2p, O 1s and Ni 2p in CoNiO2(B). The Co 2p photoelectron spectroscopy in Figure 4a is split into two bands of Co 2p3/2 and Co 2p1/2. The three subpeaks of the Co 2p3/2 band at 781.52 eV, 779.50 eV, and 785.94 eV are attributed to Co2+, Co3+, and satellite peaks, respectively. The Co 2p1/2 band can be deconvoluted into three peaks at 796.94 eV, 795.36 eV, and 802.32 eV, arising from Co2+, Co3+, and satellite peaks, respectively [36]. In Figure 4b, the two fitting peaks of O 1s are located at 529.59 eV and 531.36 eV, which are severally related with O2− and hydroxide [37]. Doublet bands of Ni 2p3/2 and Ni 2p1/2 constitute the XPS spectrum of Ni 2p (Figure 4c). Ni 2p3/2 and Ni 2p1/2 can be deconvoluted into Ni3+ (855.70 eV, 873.47 eV), Ni2+ (853.80 eV, 871.74 eV), and satellite peaks (860.91 eV, 879.08 eV) [38]. Figure 4d–f reveals the photoelectron spectra of Co 2p, O 1s, and Ni 2p in CoNiO2(F). The Co 2p photoelectron spectrum in Figure 4d contains the two spin splitting peaks Co 2p3/2 (779.19 eV, 781.06 eV) and Co 2p1/2 (794.89 eV, 796.56 eV) as well as satellite peaks (785.59 eV, 801.97 eV), indicating the presence of Co2+ and Co3+ [36]. In Figure 4e, the two peaks (529.21 eV, 530.73 eV) in the XPS spectrum of O 1s individually correlated with O2− and the hydroxyl groups [37]. In the XPS spectrum of Ni 2p (Figure 4f), there are four peaks at 853.52 eV, 855.22 eV, 871.17 eV, and 872.83 eV, belonging to Ni3+ 2p3/2, Ni2+ 2p3/2, Ni2+ 2p1/2, and Ni3+ 2p1/2, while those positioned at 860.66 eV and 878.70 eV represent the satellite peaks of Ni 2p [38]. From the above results of the XPS analysis, it can be concluded that CoNiO2(B) and CoNiO2(F) possess almost the same chemical status except the upshift of the binding energy of the former. Due to the attenuation of the curvature radius from the nano-flake to the nano-box, the material surface will generate corresponding internal stress [39]. Deng et al. [40] found that compared with solid nanowires, hollow nanotubes exhibit higher tensile strength. The upshift of the XPS spectrum is a fingerprint signal of the occurrence of tensile strain inside the material. Zhou et al. [41] have proved that the existence of the tensile strain results in the lattice deformation of materials, which makes the spectrum of XPS shift toward the direction of the high binding energy. Although the lattice deformation is so trifling that XRD test did not detect its existence, the XPS spectra of Co, Ni, and O elements presented above are a shift toward the direction of high binding energy, which is circumstantial evidence of the existence of tensile strain in the hollow architecture of CoNiO2(B) [42].
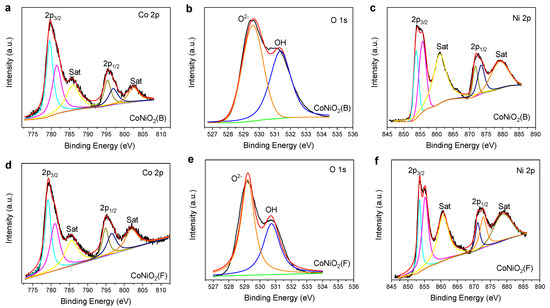
Figure 4.
XPS spectra of CoNiO2(B) and CoNiO2(F). (a) Co 2p, (b) O 1s, and (c) Ni 2p XPS spectra of CoNiO2(B). (d) Co 2p, (e) O 1s, and (f) Ni 2p XPS spectra of CoNiO2(F).
In order to detect the chemical valence of sulfur injected into the material, the S 2p spectra in S/CoNiO2(B) are shown in Figure S3a. The peaks at 163.4 eV and 164.56 eV can be assigned to the S 2p3/2 and S 2p1/2 of the chemical bonds towards S8. S-O species (168.37 eV, 169.53 eV) are observed in the S/CoNiO2(B) [43]. In Figure S3b, the S 2p peaks (163.18 eV and 164.34 eV) of S/CoNiO2(F) are also attributed to S 2p3/2 and S 2p1/2, and peaks (168.39 eV, 169.55 eV) are consistent with S-O species [43]. It can be concluded that the sulfur in S/CoNiO2(B) and S/CoNiO2(F) exists in two forms: pure sulfur and the combination between sulfur and CoNiO2.
During the charging and discharging process, a rapid conversion of PSs can significantly minimize the shuttle effect. The effects of CoNiO2(B) and CoNiO2(F) on the liquid–liquid and solid–liquid conversion of PSs was measured by a symmetric cell. The CoNiO2(B) symmetric cell without Li2S6 in Figure 5a produced nearly zero capacitive current, while the CoNiO2(B) symmetric cell with Li2S6 electrolyte exhibited more than twice the redox current than CoNiO2(F), indicating that CoNiO2(B) vigorously accelerates the redox kinetics of the liquid–liquid conversion of PSs [44]. EIS spectra (Figure 5b) showed that CoNiO2(B) symmetric cells exhibit lower charge transfer resistance (Rct), further confirming that CoNiO2(B) could promote redox reaction kinetics of PSs. The deposition of Li2S represents the conversion of soluble PSs to insoluble Li2S (liquid–solid). As exhibited in Figure 5c, the onset of Li2S deposition is located at the upward inflection point of the potentiostatic discharge curve. The initial deposition time of Li2S on CoNiO2(B) is 897 s, which is earlier than that of CoNiO2(F) at 1831.2 s. The peak current of CoNiO2(B) is 0.430 A g−1, which is larger than the 0.211 A g−1 of CoNiO2(F). The shorter Li2S deposition time and the larger peak current confirm that CoNiO2(B) can efficiently boost the transition of soluble PSs to solid Li2S [45]. The excellent kinetic promotion of CoNiO2(B) is predominantly attributed to the increased active site density and shortened path lengths for mass and ion transportation. Furthermore, the tensile strain that resulted from hollow architecture, as discussed above, is additionally beneficial for improvement of catalytic performance. Liu et al. [46] confirm that slight lattice deformation aroused from the tensile strain will result in lengthened chemical bonds, which will exert adjustment on the crystalline electronic structure. Wang et al. [47] demonstrated that, with tensile strain, the d-band center of the transition metal atom in the active sites shifts toward the Fermi level, thus improving the chemical adsorption and catalytic conversion of the PSs for further performance enhancement of Li-S batteries.
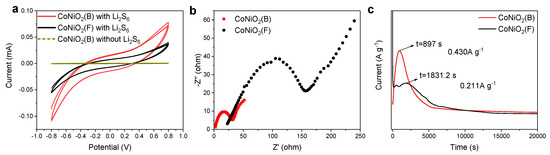
Figure 5.
Electrochemical Performance of Symmetric Battery; (a) CV cycle curves and (b) EIS curves of CoNiO2(B) and CoNiO2(F) symmetric cells. (c) Li2S deposition profiles on CoNiO2(B) and CoNiO2(F).
A coin battery was assembled for the cyclic voltammetry (CV) cycle testing to inspect the electrochemical reactivity of the Li-S battery. The CV curves of the S/CoNiO2(B) and S/CoNiO2(F) electrodes were examined at a scanning rate of 0.1 mV/s, and the results are displayed in Figure 6a. Within the voltage range of 1.7–2.8 V, there are two cathodic peaks (2.27 V, 2.01 V), which reflect the reaction that occurs during discharge—sulfur is reduced to soluble PSs, and soluble PSs are further reduced to solid state lithium sulfide. The anodic peak (2.42 V) signifies the oxidation of dilithium sulfide to sulfur during charging. The S/CoNiO2(B) electrode exhibits a significantly larger peak current and narrower peak width, which indicates that CoNiO2(B) enhances the charge–discharge reaction kinetics of PSs. In addition, charge–discharge reaction kinetics can be further analyzed by CV. The results of CV at five different scan rates are shown in Figure 6b–c. As the Randles–Sevcik principle points out, with the square root of the scanning rate and the peak current of the CV curve as variables, the slope of the fitted curve between them reflects the Li+ diffusion coefficient [48]. In general, the higher the slope, the faster the diffusion of Li+. Figure 6d–f show that the slopes of the fitted curves for the S/CoNiO2(B) cathode at peak A, peak C1, and peak C2 are numerically larger than those for the S/CoNiO2(F) cathode. The results verify that CoNiO2(B) provides more channels and reduced path lengths for mass and ion transportation in the redox reaction.
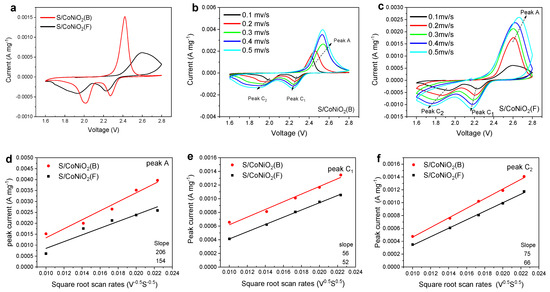
Figure 6.
CV curves and Li+ diffusion coefficient fitting. (a) CV curves of S/CoNiO2(B) and S/CoNiO2(F) electrode at 0.1 mV s−1. CV curves of (b) S/CoNiO2(B) and (c) S/CoNiO2(F) electrode at 0.1–0.5 mV s−1. (d–f) The peak current of the CV curve is linearly related to the square root of the corresponding scan rate.
All assembled coin batteries were aged at 50 °C for 48 h for activation before charge and discharge. Figure 7a shows galvanostatic charge–discharge profiles of the batteries with S/CoNiO2(B) and S/CoNiO2(F) cathodes for the initial three cycles at 0.05 C. The S/CoNiO2(B) cathode exhibits one plateau near 2.39 V related to sulfur oxidation and two stages at 2.26 V and 2.10 V correlated with sulfur reduction, while the S/CoNiO2(F) cathode exhibits one oxidation peak at 2.40 V and two reduction peaks at 2.24 V and 2.10 V. The charging–discharging overpotential of S/CoNiO2(B) is 0.13 V, lower than that of S/CoNiO2(F) (0.16 V), which denotes slighter polarization of the CoNiO2(B) host. The discharge specific capacities of S/CoNiO2(B) corresponding to the first, second, and third cycles are 1232 mAh g−1, 1215 mAh g−1, and 1190 mAh g−1, respectively, which are all larger than S/CoNiO2(F) (894 mAh g−1, 869 mAh g−1, 849 mAh g−1). The 0.5 C rate cycling characteristics of the two type cathodes were revealed in Figure 7b. The first 25 cycles are conducted at current density of 0.05, 0.1, 0.2, 0.5 and 1 C. The first discharge specific capacity of the S/CoNiO2(B) cathode decreased to 822 mAh g−1, while a stable value of 730 mAh g−1 remained after 150 cycles, which is 50% greater than the S/CoNiO2(F) cathode (486 mAh g−1). Figure 7c further reveals the rate performance of the S/CoNiO2(B) cathode, which delivered an initial specific capacity of 931.2 mAh g−1, 836.72 mAh g−1, 736.82 mAh g−1, 655 mAh g−1, and 502.1 mAh g−1 at a current density of 0.1, 0.2, 0.5, 1, and 2 C, respectively. When the current density suddenly decreased from 2 C to 0.2 C or 0.1 C, the reversible specific capacity can still be maintained at 757 mAh g−1 and 807.441 mAh g−1, respectively.
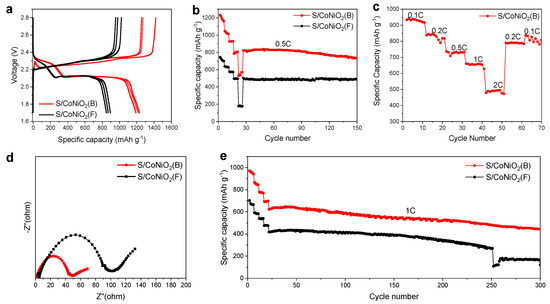
Figure 7.
The electrochemical measurement of S/CoNiO2(B) and S/CoNiO2(F); (a) The voltage plateau curve of the S/CoNiO2(B) electrode at 0.05 C in initial three cycles. (b) Cycle performances of the S/CoNiO2(B) and S/CoNiO2(F) electrode at 0.5 C. (c) Rate performances of the S/CoNiO2(B) electrode. (d) EIS curves and (e) cycle performances at 1 C for the S/CoNiO2(B) and S/CoNiO2(F) electrode.
The electrochemical impedance spectra (EIS) of S/CoNiO2(B) and S/CoNiO2(F) cathodes are presented in Figure 7d. At the interface between electrolyte and cathode, the resistance of charge transfer is equivalent to the length from the coordinate origin to the right inflection point of the semicircle. The smaller impedance shows that the CoNiO2(B) compound exhibits excellent electrochemical conductivity and diffusion kinetics. Long term cycle stability is an important evaluation index of Li-S battery performance. In order to study the effect of sulfur loading on battery performance, sulfur loading was accurately regulated at 1.6 mg cm−2. When operated under 1 C rate, the initial specific capacity of the CoNiO2(B) cathode maintained 631 mAh g−1, while the CoNiO2(F) cathode was only 421 mAh g−1, as represented in Figure 7e. For the first 20 cycles, the current density gradually increases from 0.05, 0.1, 0.2, to 0.5C. After 300 cycles, their specific capacities were maintained at 431 mAh g−1 and 121 mAh g−1, respectively, and the CoNiO2(B) was almost three times higher than CoNiO2(F). Within 300 cycles, the capacity decay rate of the S/CoNiO2(B) cathode was 0.1% per cycle, which is remarkably lower than that of the S/CoNiO2(F) cathode (0.26%). The cycling stability of the S/CoNiO2(B) electrode is preferable to the S/CoNiO2(F) cathode.
Except for the fact that CoNiO2(B) shows higher binding energy, CoNiO2(B) and CoNiO2(F) exhibit the same chemical status. However, the electrochemical performance of CoNiO2(B) is much better than that of CoNiO2(F). When material transforms from two-dimensional nano-flakes to three-dimensional nano-boxes, the specific surface area is enlarged, and slight internal tensile strain is generated [39]. The increase of the specific surface area not only exposes more active sites, but also provides more channels and reduced path lengths for mass and ion transportation. These effects are conducive to the performance improvement of Li-S batteries. In addition, due to the slight lattice deformation resulting from tensile strain in the material, chemical bonds are lengthened, which leads to the upshift of binding energy [46]. The lengthened chemical bonds will also exert effects on the electronic structure of the crystal; that is, the d-band center of the transition metal in the active sites will migrate toward the Fermi level, which will promote the chemical adsorption and catalytic conversion of the PSs for further performance enhancement of Li-S batteries [47]. It can be asserted that, for the transition metal oxide compound, the hollow architecture is more promising than the two-dimensional analogues for the applications as sulfur host in Li-S batteries.
3. Materials and Methods
3.1. Material Preparation
Cobalt nitrate hexahydrate and nickel nitrate hexahydrate were analytical grade and produced by Damao Chemical Reagent Factory. 2-methylimidazole(2-MeIM) and Li2S were purchased from Macklin Biochemical Co., Ltd. in Shanghai, China. Methanol, ethanol, and urea were purchased from XiLong Science Co., Ltd. in Guangdong, China. The sulfur was made by Heng Xing Chemical Reagent Manufacturing Co., Ltd. in Tianjin, China.
3.1.1. Synthesis of ZIF-67
2 mmol of cobalt nitrate hexahydrate was added to anhydrous methanol (50 mL), and the resulting solution after ultrasonic dispersion is solution A. 0.657 g of 2-MeIM (8 mmol) was completely dispersed in 50 mL of anhydrous methanol by sonication to get solution B. Solution A was forcefully stirred (>500 rpm), and solution B was rapidly added to solution A, then stirred for one minute and stewed 24 h. Finally, the purple product was rinsed with methanol 3–4 times and dried overnight in a 70 °C oven.
3.1.2. Synthesis of NiCo-LDH
20 mg of prepared ZIF-67 was dissolved in 20 mL ethanol to gain solution A. 100 mg of nickel nitrate hexahydrate was completely dispersed in 20 mL ethanol to get solution B. Solution A was stirred (>500 rpm), and solution B was quickly poured into solution A, continually stirred for 2 min. Then, the mixture was treated by ultrasonic treatment at room temperature with a power of 600 W for 1 h. Lastly, it was rinsed three to four times with ethanol before drying at 70 °C overnight.
3.1.3. Synthesis of NiCo-LDH(F)
Nickel nitrate hexahydrate (9 mmol), cobalt nitrate hexahydrate (4.5 mmol), and urea (31.5 mmol) were dissolved in 35 mL methanol. The mixture was stirred vigorously to form a transparent solution, then the transparent solution was poured into a 100 mL polytetrafluoroethylene lined stainless steel reaction kettle. Then, the reaction kettle was heated from room temperature to 120 °C at a rate of 5 °C/min and steeped for 10 h. Finally, NiCo-LDH(F) was obtained by washing and drying the mixture in the reaction kettle.
3.1.4. Synthesis of the CoNiO2(B)/CoNiO2(F)
The prepared 50 mg NiCo-LDH was annealed at 300 °C for 120 min with argon gas. The final product was CoNiO2(B). The CoNiO2(F) material was prepared from NiCo-LDH(F) by the same process.
3.1.5. Synthesis of the S/CoNiO2(B) and S/CoNiO2(F) Composite
CoNiO2(B) and sulfur were mixed in 1:3 weight ratio and uniformly stirred for 5 h. Then, the product was heated at 155 °C for 6 h in a sealed autoclave for the preparation of S/CoNiO2(B). S/CoNiO2(F) composites were also obtained by the identical procedure.
3.2. Visualized Adsorption of Li2S6
To create a 5 mM Li2S6 solution, S and Li2S powder (1:5 moles) were dissolved in a mixed solution of 1,3 dioxolane and 1,2-dimethoxyethane (1:1 volume) and stirred vigorously at 70 °C for 24 h. Then, 8 mg each of the NiCo-LDH, CoNiO2(F), and CoNiO2(B) were soaked in the prepared Li2S6 solution (4 mL) for 10 h.
3.3. Assembly of Symmetric Cells
The two hosts CoNiO2(B) and CoNiO2(F) were separately dissolved in absolute ethanol and sonicated for 60 min. The resultant homogeneous suspension was added dropwise to 12 mm discs of aluminum foil and dried quickly. The symmetrical battery was assembled by two identical electrodes into a 2025 coin battery with Celgard 2400 as the separator and an electrolyte containing 0.42 M Li2S6. The electrolyte also included 1.0 mol/L lithium bis-trifluoromethanesulfonimide and 2% lithium nitrate, and equal amounts of 1,3-dioxolane and 1,2-dimethoxyethane. The CV curve scanning voltage range of the symmetrical battery ranged from −0.8 to 0.8 V at 200 mV/s. The corresponding EIS curves were measured by a Corrtest CS350H electrochemical workstation at 10−2–105 Hz with an amplitude of 5 mV.
3.4. Nucleation of Li2S
The 2025 coin battery was assembled with CoNiO2((B) as the cathode (or CoNi02(F)), Li foil as the anode, and Celgard 2400 as the separator, and contained 0.3 M Li2S8 electrolyte. The electrolyte also contained 1.0 mol/L lithium bis-trifluoromethanesulfonimide and 2% lithium nitrate, and equal amounts of 1,3-dioxolane and 1,2-dimethoxyethane. The assembled battery was first discharged with constant current to 2.10 V at 0.110 mA and subsequently with the voltage at 2.09 V until the current was almost zero.
3.5. Material Characterization
The microscopic structure and morphological features of the composite material were characterized by cold field emission scanning electron microscope (SEM, ZEISS Sigma 300, 3 kv) and transmission electron microscopy (TEM, JEOL JEM 2100, 200 KV). The mass of sulfur in the cathode was measured by thermogravimetric analysis (TGA) in the N2 atmosphere from 20 to 500 °C. Raman spectroscopy was measured with a 532 nm laser. The N2 adsorption-desorption isotherm measurement was used to determine the Brunauer–Emmett–Teller (BET) surface area, and the diameter of pores was calculated from the BJH method (MicrotracBEL Corp). X-ray diffraction (XRD) spectra were measured by a D8 Advance diffractometer. X-ray photoelectron spectroscopy (XPSs, ESCALAB25Oxi) measurements were designed to detect the chemical forms of elements in materials, and Shirley fitting background type was selected for the XPS test data. UV/vis absorption spectra were measured by Agilent Technologies Cary 60 UV-Vis (G6860A).
3.6. Assembly of the Li-S Batteries and Measurements
The S/CoNiO2(B) (or S/CoNiO2(F)), carbon black, and polyvinylidene fluoride were mixed with a weight ratio of 7:2:1. The N-methylpyrrolidinone was added to the mixture, and the mixture turned into a slurry under prolonged stirring. The slurry was evenly coated on the dark side of aluminum foil and dried at 60 °C. 2025 Coin cells were constructed with the S cathode, lithium foil anode, and a Celgard 2400 separator. The electrolyte was 1.0 mol/L lithium bis-trifluoromethanesulfonimide and 2% lithium nitrate, which dissolved in equal parts 1,3-dioxolane and 1,2-dimethoxyethane. The cathode was loaded with 1.0–1.6 mg cm−2 of sulfur. The amount of electrolyte was controlled at E/S = 30 μL mg−1. The galvanostatic charge–discharge was measured by Neware battery test devices with the cut-off voltage of 1.7–2.8 V. EIS and CV were measured by a CorrTest CS350H Electrochemical Workstation. The amplitude of the EIS spectra was 5 mV, and the frequency was arranged from 10−1 Hz to 105 Hz. The CV cycling curves were scanned at 0.1 to 0.5 mV s−1.
4. Conclusions
In this work, we explored the effects of the catalyst architecture on the cathodic performance of Li-S batteries. Dual transition metal oxide CoNiO2 compound was selected as the conceptual material to synthesize three-dimensional hollow CoNiO2(B) and two-dimensional CoNiO2(F). As sulfur host, cells with a cathode comprised of nano-boxes delivered a of 1232 mAh g−1 and a low overpotential of 0.13 V at 0.05 C, as well as a cycle stability with decay rate of 0.1% per cycle even after 300 cycles at 1 C, which are superior to two-dimensional analogues. The experiments imply that CoNiO2(B) exhibits strong affinity toward PSs and excellent catalytic activity for the PSs redox kinetics compared with CoNiO2(F). The enlarged specific surface area that resulted from the hollow architecture of the nano-box affords not only more active sites but also more channels and reduced path lengths for fast mass and ion transportation, which are conducive to performance enhancement of Li-S batteries. Additionally, the tensile strain aroused from the attenuated curvature radius from nano-flake to nano-box is another advantageous factor for performance enhancement, as tensile strain drives the d-band center of the transition metal in the active sites to migrate toward the Fermi level, which will promote the chemical adsorption and catalytic conversion of the PSs. Our research has demonstrated that catalysts with hollow architecture are more promising than their two-dimensional analogues in practical application in the Li-S batteries.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/batteries8120262/s1. Figure S1: (a) The SEM image of S/CoNiO2(B), (b) EDS elemental mapping of S/CoNiO2(B); Figure S2: The XPS survey spectrum of (a) CoNiO2(B) and (b) CoNiO2(F); Figure S3: S 2p XPS spectra of (a) S/CoNiO2(B) and (b) S/CoNiO2(F).
Author Contributions
Conceptualization, Y.Z.; methodology, L.Q.; software, X.L.; formal analysis, Z.H.; investigation, F.L.; resources, Z.Z.; data curation, H.Y.; writing—original draft preparation, L.C.; funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Yong Zhao] grant number [No. 12175098]. And the APC was funded by [No. 12175098].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 12175098).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chung, S.; Chang, C.; Manthiram, A. Progress on the Critical Parameters for Lithium-Sulfur Batteries to be Practically Viable. Adv. Funct. Mater. 2018, 28, 1801188. [Google Scholar] [CrossRef]
- Fang, X.; Peng, H. A Revolution in Electrodes: Recent Progress in Rechargeable Lithium-Sulfur Batteries. Small 2015, 11, 1488–1511. [Google Scholar] [CrossRef]
- Salama, M.; Rosy; Attias, R.; Yemini, R.; Gofer, Y.; Aurbach, D.; Noked, M. Metal–Sulfur Batteries: Overview and Research Methods. ACS Energy Lett. 2019, 4, 436–446. [Google Scholar] [CrossRef]
- Tu, S.; Chen, X.; Zhao, X.; Cheng, M.; Xiong, P.; He, Y.; Zhang, Q.; Xu, Y. A Polysulfide-Immobilizing Polymer Retards the Shuttling of Polysulfide Intermediates in Lithium-Sulfur Batteries. Adv. Mater. 2018, 30, e1804581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Pang, Y.-C.; Chen, X.; Lang, J.; Xu, J.; Xiao, C.; Li, H.; Xi, K.; Ding, S. Carbon@titanium nitride dual shell nanospheres as multi-functional hosts for lithium sulfur batteries. Energy Storage Mater. 2019, 16, 228–235. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic Effects in Lithium-Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Y.; Ma, S.; Lian, Z.; Gu, X.; Li, J.; Li, Z.; Liu, Q. Optimizing CO2 reduction and evolution reaction mediated by o-phenylenediamine toward high performance Li-CO2 battery. Electrochim. Acta 2022, 419, 140424. [Google Scholar] [CrossRef]
- Zeng, L.; Zhu, J.; Liu, M.; Zhang, P. Sb nanosheet modified separator for Li-S batteries with excellent electrochemical performance. RSC Adv. 2021, 11, 6798–6803. [Google Scholar] [CrossRef]
- Yi, T.F.; Shi, L.; Han, X.; Wang, F.; Zhu, Y.; Xie, Y. Approaching High-Performance Lithium Storage Materials by Constructing Hierarchical CoNiO2 @CeO2 Nanosheets. Energy Environ. Mater. 2021, 4, 586–595. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.H.; Zhang, Z.; Chen, Y.; Xiang, Y.; Liu, X.; Chen, J.S.; Chen, P. Naturally derived honeycomb-like N, S-codoped hierarchical porous carbon with MS2 (M = Co, Ni) decoration for high-performance Li-S battery. Nanoscale 2020, 12, 5114–5124. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Guo, S.; Hou, Y. Rational Design of Si/SiO2@Hierarchical Porous Carbon Spheres as Efficient Polysulfide Reservoirs for High-Performance Li-S Battery. Adv. Mater. 2016, 28, 3167–3172. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lee, K.T.; Nazar, L. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Hart, C.J.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Lou, X.W. Hollow Carbon Nanofibers Filled with MnO2 Nanosheets as Efficient Sulfur Hosts for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2015, 54, 12886–12890. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Guan, B.; Wang, D.; Liu, L.-M.; Lou, X.W. A sulfur host based on titanium monoxide@carbon hollow spheres for advanced lithium-sulfur batteries. Nat. Commun. 2016, 7, 13065. [Google Scholar] [CrossRef]
- Li, Z.; Guan, B.Y.; Zhang, J.; Lou, X.W. A Compact Nanoconfined Sulfur Cathode for High-Performance Lithium-Sulfur Batteries. Joule 2017, 1, 576–587. [Google Scholar] [CrossRef]
- You, Y.; Ye, Y.; Wei, M.; Sun, W.; Tang, Q.; Zhang, J.; Chen, X.; Li, H.; Xu, J. Three-dimensional MoS2/rGO foams as efficient sulfur hosts for high-performance lithium-sulfur batteries. Chem. Eng. J. 2019, 355, 671–678. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.; Guo, C.; Li, D.; Vasileff, A.; Wang, H.; Qiao, S. A 3D Hybrid of Chemically Coupled Nickel Sulfide and Hollow Carbon Spheres for High Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1702524. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Nazar, L.F. A graphene-like metallic cathode host for long-life and high-loading lithium-sulfur batteries. Mater. Horizons 2016, 3, 130–136. [Google Scholar] [CrossRef]
- Peng, H.-J.; Zhang, G.; Chen, X.; Zhang, Z.-W.; Xu, W.-T.; Huang, J.-Q.; Zhang, Q. Enhanced Electrochemical Kinetics on Conductive Polar Mediators for Lithium-Sulfur Batteries. Angew. Chem. 2016, 128, 13184–13189. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; Zhou, G.; Lv, W.; Sun, P.; Kang, F.; Li, B.; Yang, Q.-H. An in-plane heterostructure of graphene and titanium carbide for efficient polysulfide confinement. Nano Energy 2017, 39, 291–296. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Chen, Y.; Gao, S.; Lou, X.W. Nickel–Iron Layered Double Hydroxide Hollow Polyhedrons as a Superior Sulfur Host for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 57, 10944–10948. [Google Scholar] [CrossRef]
- Qiu, W.; Li, G.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Wang, X.; Chen, Z. Hierarchical Micro-Nanoclusters of Bimetallic Layered Hydroxide Polyhedrons as Advanced Sulfur Reservoir for High-Performance Lithium-Sulfur Batteries. Adv. Sci. 2021, 8, 2003400. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.-J.; Hou, T.-Z.; Huang, J.Q.; Chen, C.-M.; Wang, D.-W.; Cheng, X.-B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, H.; Li, Z.; Lou, X.W.D. Double-Shelled Nanocages with Cobalt Hydroxide Inner Shell and Layered Double Hydroxides Outer Shell as High-Efficiency Polysulfide Mediator for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2016, 55, 3982–3986. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, F.; Cao, H.; Liu, L.; Wang, Y.; Niu, Z. Integration of Binary Active Sites: Co3V2O8 as Polysulfide Traps and Catalysts for Lithium-Sulfur Battery with Superior Cycling Stability. Small 2020, 16, e1907153. [Google Scholar] [CrossRef]
- Freund, L. Substrate curvature due to thin film mismatch strain in the nonlinear deformation range. J. Mech. Phys. Solids 2000, 48, 1159–1174. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, W.; Hong, X.; He, Q.; Lu, R.; Liao, X.; Zhao, Y. Accelerating conversion of LiPSs on strain-induced MXene for high-performance Li-S battery. Chem. Eng. J. 2022, 439, 135679. [Google Scholar] [CrossRef]
- Li, J.; Qiu, W.; Liu, X.; Zhang, Y.; Zhao, Y. NiCo-Layered Double Hydroxide to Composite with Sulfur as Cathodes for High-Performance Lithium-Sulfur Batteries. ChemElectroChem 2022, 9, e202101211. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Cheong, J.L.; Zaghib, K.; Trudeau, M.L.; Ying, J.Y. Nanoboxes with a porous MnO core and amorphous TiO2 shell as a mediator for lithium-sulfur batteries. J. Mater. Chem. A 2021, 9, 4952–4961. [Google Scholar] [CrossRef]
- An, L.; Huang, B.; Zhang, Y.; Wang, R.; Zhang, N.; Dai, T.; Xi, P.; Yan, C. Interfacial Defect Engineering for Improved Portable Zinc–Air Batteries with a Broad Working Temperature. Angew. Chem. Int. Ed. 2019, 58, 9459–9463. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Zhao, M.; Luo, R.; Yang, Y.; Peng, T.; Yan, H.; Liu, X.; Luo, Y. Controllable Tuning of Cobalt Nickel-Layered Double Hydroxide Arrays as Multifunctional Electrodes for Flexible Supercapattery Device and Oxygen Evolution Reaction. ACS Nano 2019, 13, 12206–12218. [Google Scholar] [CrossRef]
- Li, Z.; Mi, H.; Guo, F.; Ji, C.; He, S.; Li, H.; Qiu, J. Oriented Nanosheet-Assembled CoNi-LDH Cages with Efficient Ion Diffusion for Quasi-Solid-State Hybrid Supercapacitors. Inorg. Chem. 2021, 60, 12197–12205. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.-R.; Xue, F.; Jia, Y.-J.; Ye, J.-C.; Bai, C.-D.; Zheng, M.-S.; Dong, Q.-F. Co4N Nanosheet Assembled Mesoporous Sphere as a Matrix for Ultrahigh Sulfur Content Lithium-Sulfur Batteries. ACS Nano 2017, 11, 6031–6039. [Google Scholar] [CrossRef]
- Chen, F.-F.; Chen, J.; Li, L.; Peng, F.; Yu, Y. g-C3N4 microtubes@CoNiO2 nanosheets p–n heterojunction with a hierarchical hollow structure for efficient photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 579, 151997. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Yu, Y.; Ahmad, M.; Sun, H. Facile synthesis of single-crystal mesoporous CoNiO2 nanosheets assembled flowers as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 132, 404–409. [Google Scholar] [CrossRef]
- Rehman, S.U.; Wang, J.; Luo, Q.; Sun, M.; Jiang, L.; Han, Q.; Liu, J.; Bi, H. Starfish-like C/CoNiO2 heterostructure derived from ZIF-67 with tunable microwave absorption properties. Chem. Eng. J. 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Mézin, A. Coating internal stress measurement through the curvature method: A geometry-based criterion delimiting the relevance of Stoney’s formula. Surf. Coatings Technol. 2006, 200, 5259–5267. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, F.; Deng, C. Near-ideal strength in metal nanotubes revealed by atomistic simulations. Appl. Phys. Lett. 2013, 103, 231911. [Google Scholar] [CrossRef]
- Zhou, W.-P.; Lewera, A.; Bagus, P.S.; Wieckowski, A. Electrochemical and Electronic Properties of Platinum Deposits on Ru (0001): Combined XPS and Cyclic Voltammetric Study. J. Phys. Chem. C 2007, 111, 13490–13496. [Google Scholar] [CrossRef]
- Clavel, M.B.; Hudait, M.K. Band Offset Enhancement of a-Al2O3/Tensile-Ge for High Mobility Nanoscale pMOS Devices. IEEE Electron Device Lett. 2017, 38, 1196–1199. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Li, Y.; Luo, S.; Liu, S.; Hong, X.; Xie, K.; Liu, Y.; Tan, X.; Zheng, C. Rational Construction of Fe2N@C Yolk-Shell Nanoboxes as Multifunctional Hosts for Ultralong Lithium-Sulfur Batteries. ACS Nano 2019, 13, 12137–12147. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Bai, Y.; Luo, M.; Qu, M.; Wang, Z.; Sun, W.; Sun, K. Enhancing Polysulfide Confinement and Electrochemical Kinetics by Amorphous Cobalt Phosphide for Highly Efficient Lithium-Sulfur Batteries. ACS Nano 2021, 15, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, J.; Li, N.; Han, X.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Multifunctional LDH/Co9S8 heterostructure nanocages as high-performance lithium-sulfur battery cathodes with ultralong lifespan. Energy Storage Mater. 2020, 30, 187–195. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, S.; Jia, Y.; Xiong, X.; Yang, H.; Liu, S.; Tang, J.; Zhang, J.; Liu, D.; Zheng, L.; et al. NiFe Hydroxide Lattice Tensile Strain: Enhancement of Adsorption of Oxygenated Intermediates for Efficient Water Oxidation Catalysis. Angew. Chem. Int. Ed. 2019, 58, 736–740. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wang, J.; Sun, Z.; Cui, G.; Chen, Y.; Wang, T.; Zheng, L.; Zhao, Y.; Shui, L.; et al. Strain Engineering of a MXene/CNT Hierarchical Porous Hollow Microsphere Electrocatalyst for a High-Efficiency Lithium Polysulfide Conversion Process. Angew. Chem. Int. Ed. 2021, 60, 2371–2378. [Google Scholar] [CrossRef]
- He, J.; Hartmann, G.; Lee, M.; Hwang, G.S.; Chen, Y.; Manthiram, A. Freestanding 1T MoS2/graphene heterostructures as a highly efficient electrocatalyst for lithium polysulfides in Li-S batteries. Energy Environ. Sci. 2019, 12, 344–350. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).