Agglomeration-Free and Air-Inert Garnet for Upgrading PEO/Garnet Composite Solid State Electrolyte
Abstract
:1. Introduction
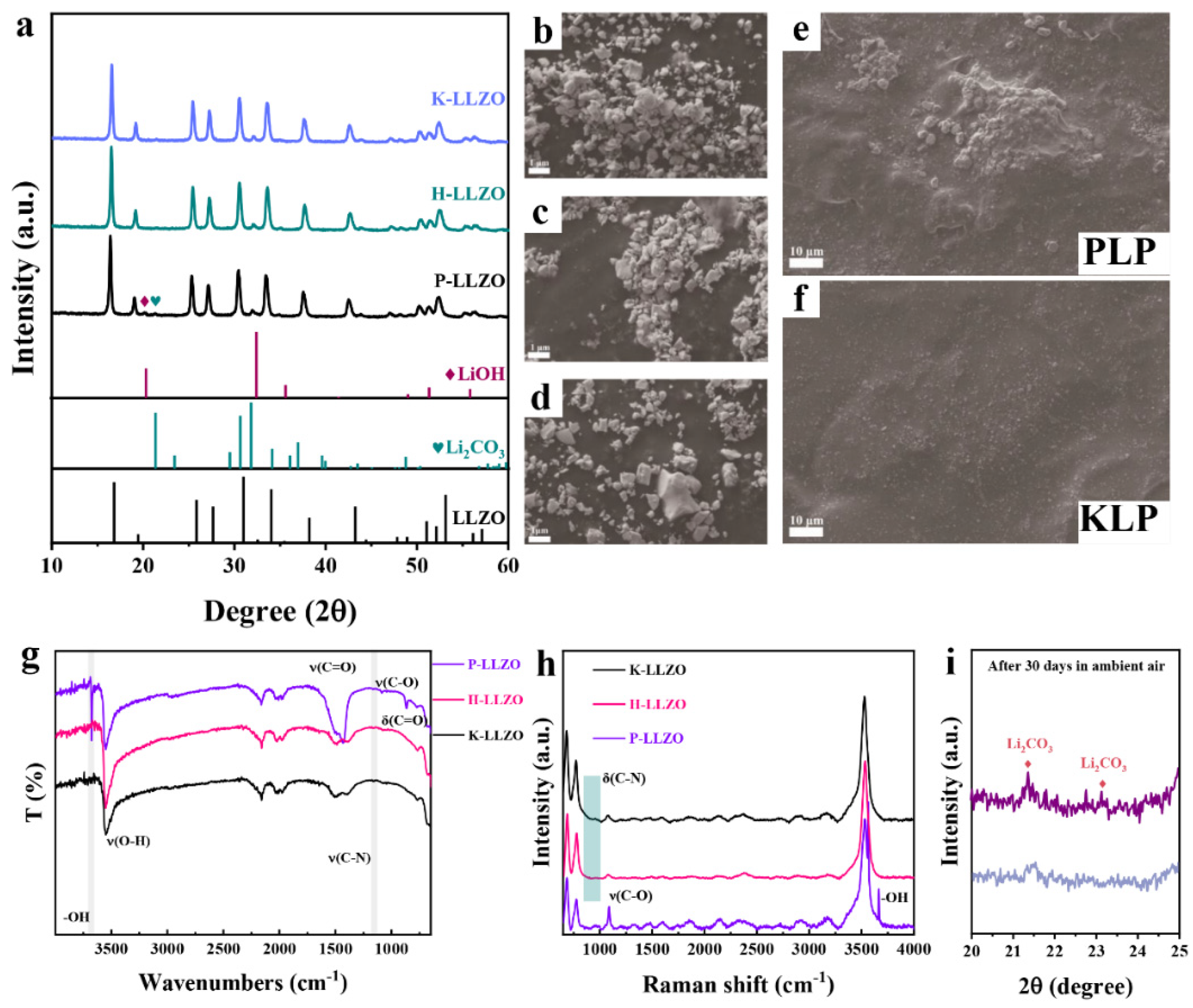
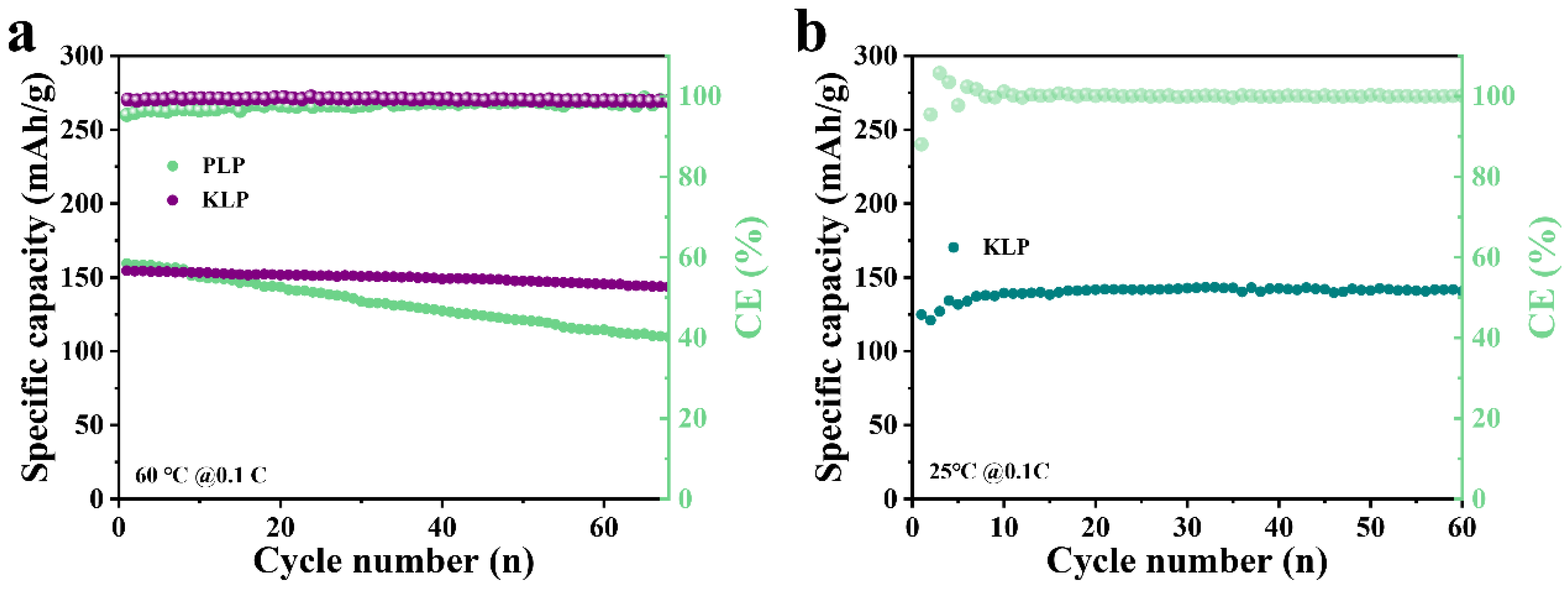
2. Results and Discussion
3. Conclusions
3.1. Experimental
3.1.1. Hydrochloric Acid Treatment of P−LLZO
3.1.2. The Preparation of K−LLZO
3.1.3. Fabrication of the Composite Electrolytes
3.1.4. Characterizations of Materials Properties
3.1.5. Electrochemical Characterization of Composite Electrolytes
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Sarkar, S.; Thangadurai, V. Critical Current Densities for High-Performance All-Solid-State Li-Metal Batteries: Fundamentals, Mechanisms, Interfaces, Materials, and Applications. ACS Energy Lett. 2022, 7, 1492–1527. [Google Scholar] [CrossRef]
- Schnell, J.; Knörzer, H.; Imbsweiler, A.J.; Reinhart, G. Solid versus Liquid—A Bottom-Up Calculation Model to Analyze the Manufacturing Cost of Future High-Energy Batteries. Energy Technol. 2020, 8, 1901237. [Google Scholar] [CrossRef]
- Tan, S.J.; Wang, W.P.; Tian, Y.F.; Xin, S.; Guo, Y.G. Advanced Electrolytes Enabling Safe and Stable Rechargeable Li-Metal Batteries: Progress and Prospects. Adv. Funct. Mater. 2021, 31, 2105253. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, Z.; Cheng, J.; Yamamoto, O.; Imanishi, N.; Chi, B.; Pu, J.; Li, J. Solid state lithium ionic conducting thin film Li1.4Al0.4Ge1.6(PO4)3 prepared by tape casting. J. Alloys Compd. 2014, 590, 147–152. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Chen, Y.T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.M.; Li, W.; Lu, B.; Ham, S.Y.; Sayahpour, B.; et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Yoshima, K.; Harada, Y.; Takami, N. Thin hybrid electrolyte based on garnet-type lithium-ion conductor Li7La3Zr2O12 for 12 V-class bipolar batteries. J. Power Sources 2016, 302, 283–290. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.-B.; Kang, F.; Li, B. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Naseer, M.A.; Tufail, M.K.; Ali, A.; Hussain, S.; Khan, U.; Jin, H.B. Review on Computational-Assisted to Experimental Synthesis, Interfacial Perspectives of Garnet-Solid Electrolytes for All-Solid-State Lithium Batteries. J. Electrochem. Soc. 2021, 168, 060529. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Ding, L.; Liu, Y.-Z.; Xiong, L.-P.; Chen, J.; Luo, X.-L. Room-temperature ionic conductivity of Ba, Y, Al co-doped Li7La3Zr2O12 solid electrolyte after sintering. Rare Met. 2021, 40, 2301–2306. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Seki, J.; Asaoka, T. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J. Power Sources 2012, 202, 332–335. [Google Scholar] [CrossRef]
- Kim, K.J.; Rupp, J.L.M. All ceramic cathode composite design and manufacturing towards low interfacial resistance for garnet-based solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 4930–4945. [Google Scholar] [CrossRef]
- Liu, T.; Ren, Y.; Shen, Y.; Zhao, S.-X.; Lin, Y.; Nan, C.-W. Achieving high capacity in bulk-type solid-state lithium ion battery based on Li6.75La3Zr1.75Ta0.25O12 electrolyte: Interfacial resistance. J. Power Sources 2016, 324, 349–357. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Nie, L.; Hu, X.; Huang, Y.; Yu, Y.; Liu, W. All-Solid-State Batteries with a Limited Lithium Metal Anode at Room Temperature using a Garnet-Based Electrolyte. Adv. Mater. 2020, 33, 2002325. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Chen, W.P.; Fan, M.; Wang, W.P.; Yu, L.; Tan, S.J.; Chen, X.; Zhang, Q.; Xin, S.; Wan, L.J.; et al. Building an Air Stable and Lithium Deposition Regulable Garnet Interface from Moderate-Temperature Conversion Chemistry. Angew. Chem. Int. Ed. Engl. 2020, 59, 12069–12075. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, A.; Kazyak, E.; Davis, A.L.; Yu, S.; Thompson, T.; Siegel, D.J.; Dasgupta, N.P.; Sakamoto, J. Surface Chemistry Mechanism of Ultra-Low Interfacial Resistance in the Solid-State Electrolyte Li7La3Zr2O12. Chem. Mater. 2017, 29, 7961–7968. [Google Scholar] [CrossRef]
- Rosen, M.; Ye, R.; Mann, M.; Lobe, S.; Finsterbusch, M.; Guillon, O.; Fattakhova-Rohlfing, D. Controlling the lithium proton exchange of LLZO to enable reproducible processing and performance optimization. J. Mater. Chem. A 2021, 9, 4831–4840. [Google Scholar] [CrossRef]
- Huo, H.; Luo, J.; Thangadurai, V.; Guo, X.; Nan, C.-W.; Sun, X. Li2CO3: A Critical Issue for Developing Solid Garnet Batteries. ACS Energy Lett. 2019, 5, 252–262. [Google Scholar] [CrossRef]
- Lopez, J.; Pei, A.; Oh, J.Y.; Wang, G.-J.N.; Cui, Y.; Bao, Z. Effects of Polymer Coatings on Electrodeposited Lithium Metal. J. Am. Chem. Soc. 2018, 140, 11735–11744. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Sun, Y.; Lin, X.; Doyle-Davis, K.; Liang, J.; Gao, X.; Li, R.; Huang, H.; Guo, X.; et al. Li2CO3 effects: New insights into polymer/garnet electrolytes for dendrite-free solid lithium batteries. Nano Energy 2020, 73, 104836. [Google Scholar] [CrossRef]
- Gupta, A.; Sakamoto, J. Controlling Ionic Transport through the PEO-LiTFSI/LLZTO Interface. Electrochem. Soc. Interface 2019, 28, 63–69. [Google Scholar] [CrossRef]
- Lee, M.J.; Shin, D.O.; Kim, J.Y.; Oh, J.; Kang, S.H.; Kim, J.; Kim, K.M.; Lee, Y.M.; Kim, S.O.; Lee, Y.-G. Interfacial barrier free organic-inorganic hybrid electrolytes for solid state batteries. Energy Storage Mater. 2021, 37, 306–314. [Google Scholar] [CrossRef]
- Jia, M.; Zhao, N.; Bi, Z.; Fu, Z.; Xu, F.; Shi, C.; Guo, X. Polydopamine-Coated Garnet Particles Homogeneously Distributed in Poly(propylene carbonate) for the Conductive and Stable Membrane Electrolytes of Solid Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 46162–46169. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, W.; Liang, P.; Jin, Z.; Grundish, N.; Li, Y.; Wang, C.-A. A dopamine modified Li6.4La3Zr1.4Ta0.6O12/PEO solid-state electrolyte: Enhanced thermal and electrochemical properties. J. Mater. Chem. A 2019, 7, 16425–16436. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, P.; Jia, H.; Du, Z.; Zhu, J.; Orenstein, R.; Cheng, H.; Wu, N.; Dirican, M.; Zhang, X. Garnet-rich composite solid electrolytes for dendrite-free, high-rate, solid-state lithium-metal batteries. Energy Storage Mater. 2020, 26, 448–456. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, H.; Duan, H.; Lu, X.; Xin, S.; Zhou, W.; Xue, L.; Fu, G.; Manthiram, A.; et al. Hybrid Polymer/Garnet Electrolyte with a Small Interfacial Resistance for Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2017, 56, 753–756. [Google Scholar] [CrossRef]
- Lv, J.-S.; Guo, S.-K.; He, Y.-B. Modification strategies of Li7La3Zr2O12 ceramic electrolyte for high-performance solid-state batteries. Tungsten 2021, 3, 260–278. [Google Scholar] [CrossRef]
- Huo, H.; Chen, Y.; Zhao, N.; Lin, X.; Luo, J.; Yang, X.; Liu, Y.; Guo, X.; Sun, X. In-situ formed Li2CO3-free garnet/Li interface by rapid acid treatment for dendrite-free solid-state batteries. Nano Energy 2019, 61, 119–125. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, T.; Zhao, B.; Shen, F.; Jin, H.; Han, X. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Energy Storage Mater. 2021, 34, 388–416. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Horowitz, Y.; Lifshitz, M.; Greenbaum, A.; Feldman, Y.; Greenbaum, S.; Sokolov, A.P.; Golodnitsky, D. Review—Polymer/Ceramic Interface Barriers: The Fundamental Challenge for Advancing Composite Solid Electrolytes for Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 160514. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Huo, H.; Wu, B.; Zhang, T.; Zheng, X.; Ge, L.; Xu, T.; Guo, X.; Sun, X. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Storage Mater. 2019, 18, 59–67. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, J.; Zeng, Z.; Li, Y.; Zhang, H.; Li, D.; Ci, L. Li2CO3: Insights into Its Blocking Effect on Li-Ion Transfer in Garnet Composite Electrolytes. ACS Appl. Energy Mater. 2022, 5, 2853–2861. [Google Scholar] [CrossRef]
- Cheng, J.; Hou, G.; Chen, Q.; Li, D.; Li, K.; Yuan, Q.; Wang, J.; Ci, L. Sheet-like garnet structure design for upgrading PEO-based electrolyte. Chem. Eng. J. 2022, 429, 132343. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Zhang, H.; Li, D.; Li, Y.; Zeng, Z.; Ji, F.; Wei, Y.; Xu, X.; Sun, Q.; Wang, S.; et al. Agglomeration-Free and Air-Inert Garnet for Upgrading PEO/Garnet Composite Solid State Electrolyte. Batteries 2022, 8, 141. https://doi.org/10.3390/batteries8100141
Cheng J, Zhang H, Li D, Li Y, Zeng Z, Ji F, Wei Y, Xu X, Sun Q, Wang S, et al. Agglomeration-Free and Air-Inert Garnet for Upgrading PEO/Garnet Composite Solid State Electrolyte. Batteries. 2022; 8(10):141. https://doi.org/10.3390/batteries8100141
Chicago/Turabian StyleCheng, Jun, Hongqiang Zhang, Deping Li, Yuanyuan Li, Zhen Zeng, Fengjun Ji, Youri Wei, Xiao Xu, Qing Sun, Shang Wang, and et al. 2022. "Agglomeration-Free and Air-Inert Garnet for Upgrading PEO/Garnet Composite Solid State Electrolyte" Batteries 8, no. 10: 141. https://doi.org/10.3390/batteries8100141
APA StyleCheng, J., Zhang, H., Li, D., Li, Y., Zeng, Z., Ji, F., Wei, Y., Xu, X., Sun, Q., Wang, S., Lu, J., & Ci, L. (2022). Agglomeration-Free and Air-Inert Garnet for Upgrading PEO/Garnet Composite Solid State Electrolyte. Batteries, 8(10), 141. https://doi.org/10.3390/batteries8100141












