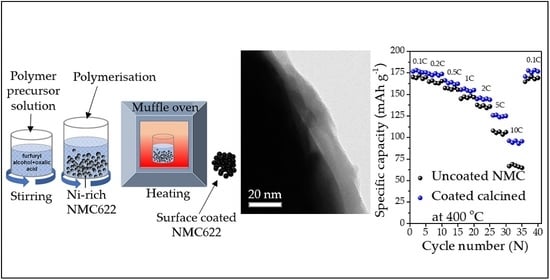
High-Performance Amorphous Carbon Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results
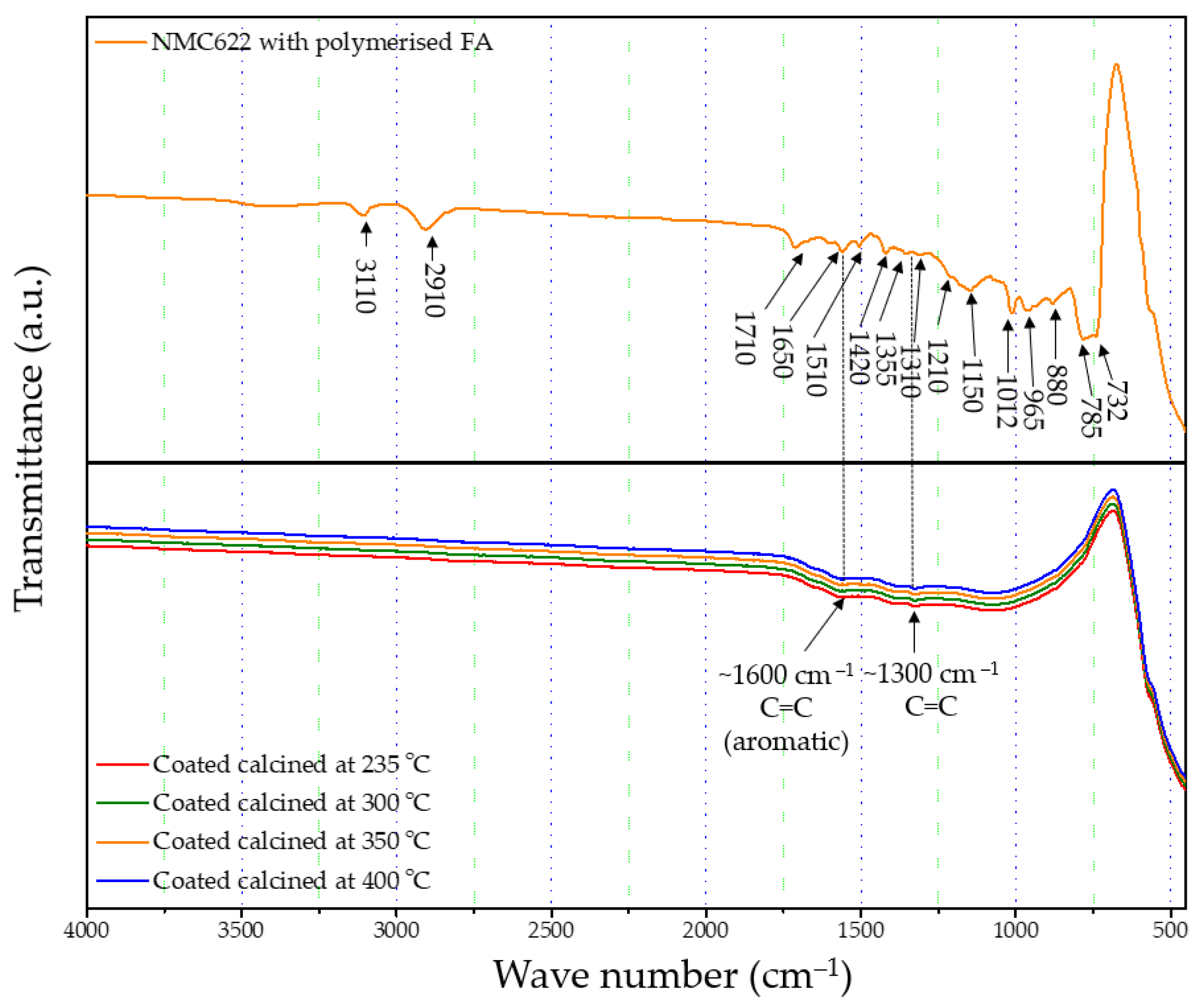
2.1. Polymer Formation
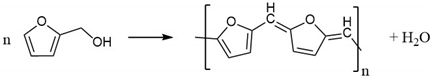
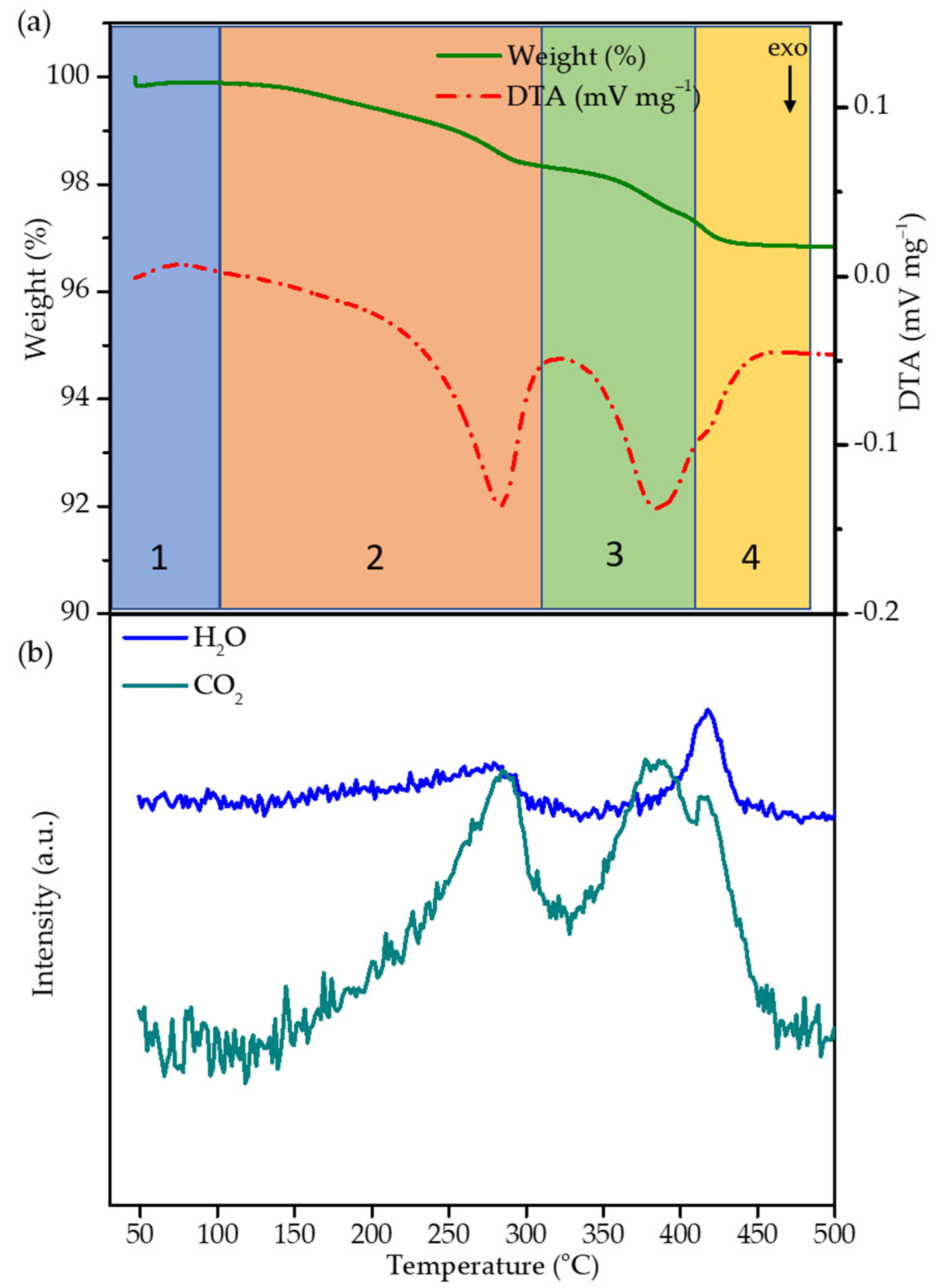
2.2. Thermal Decomposition of the FA Polymer
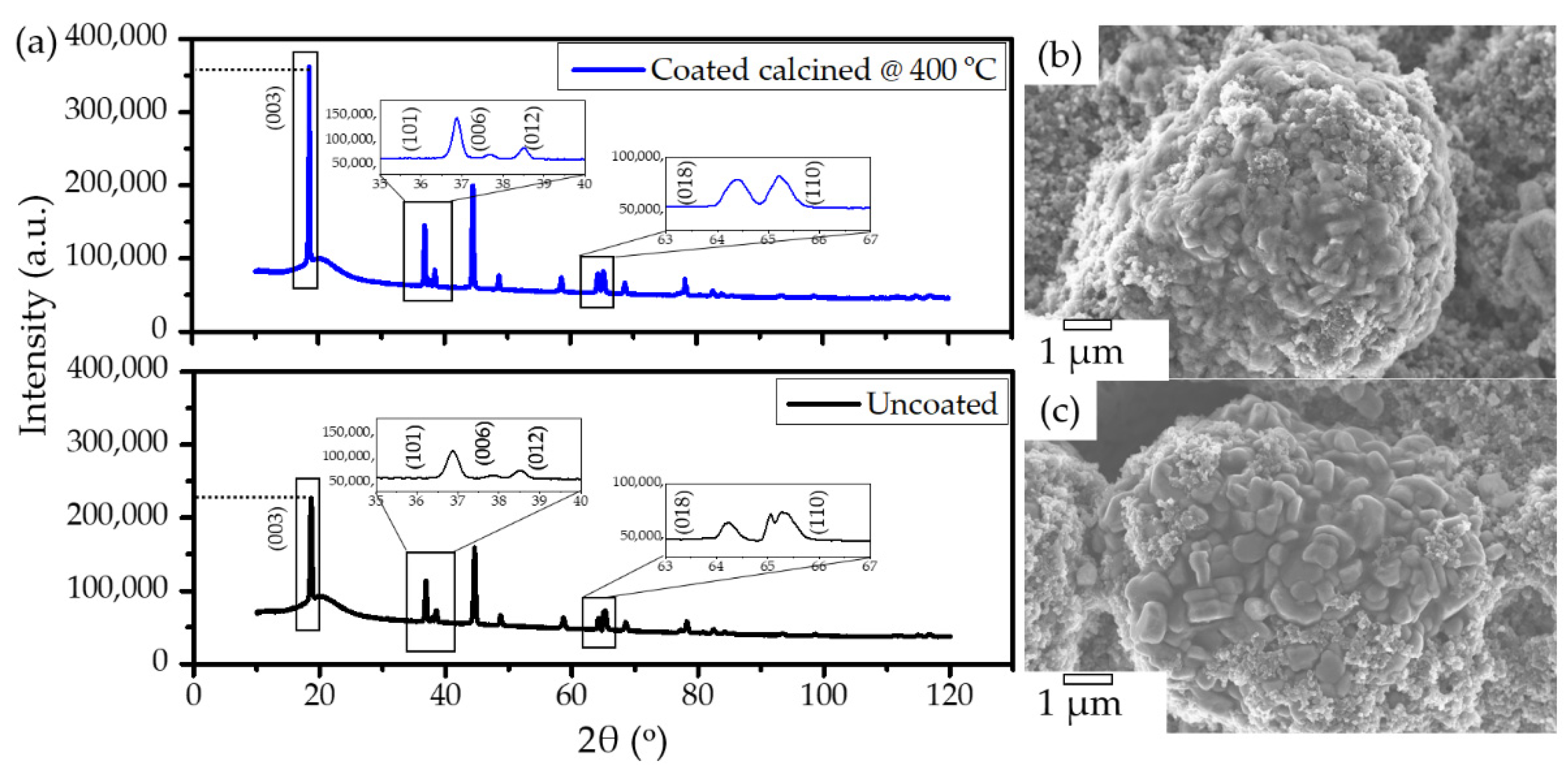
2.3. Structural Stability of Coated NMC622
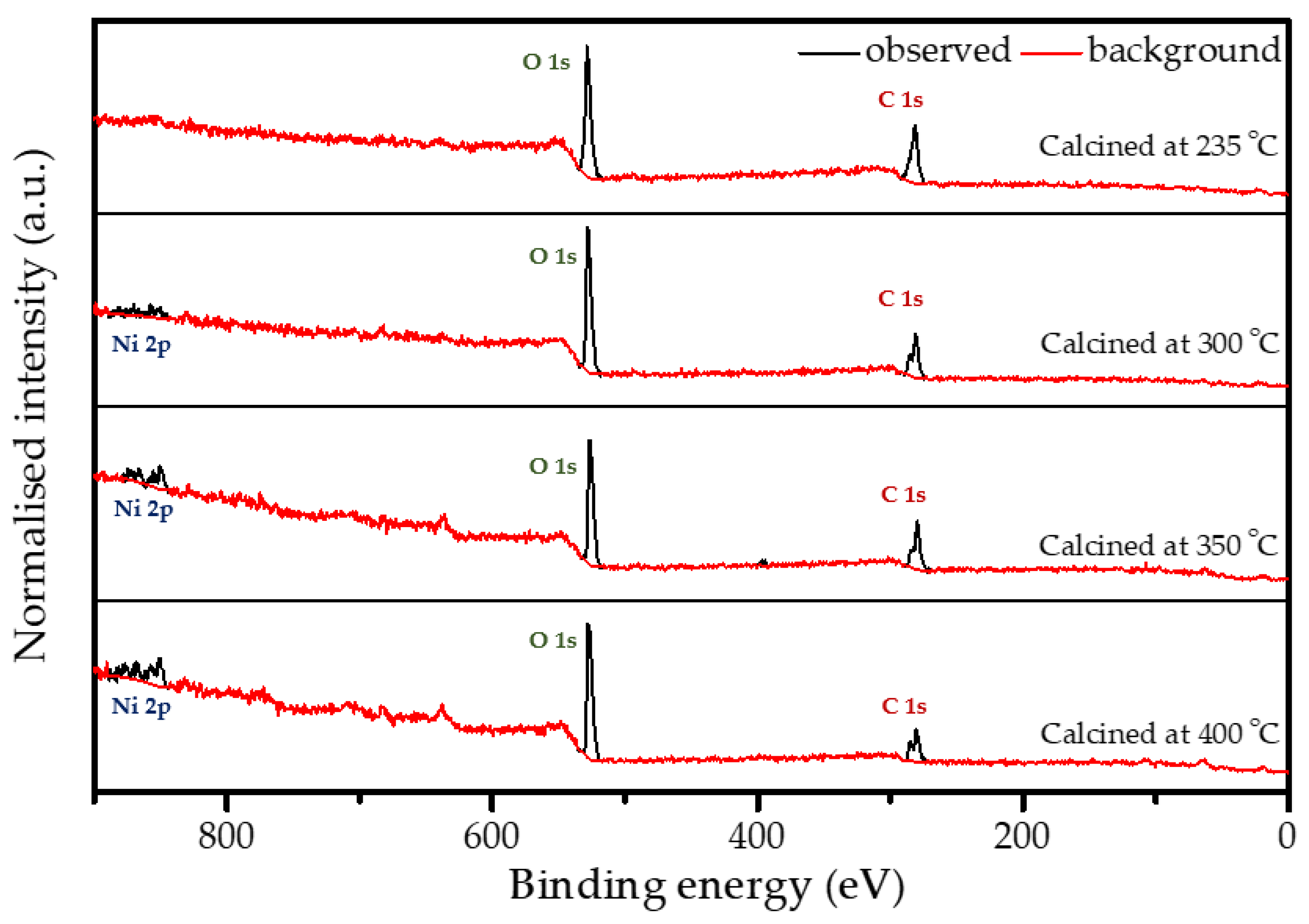
2.4. Structure of the Polymer Coating
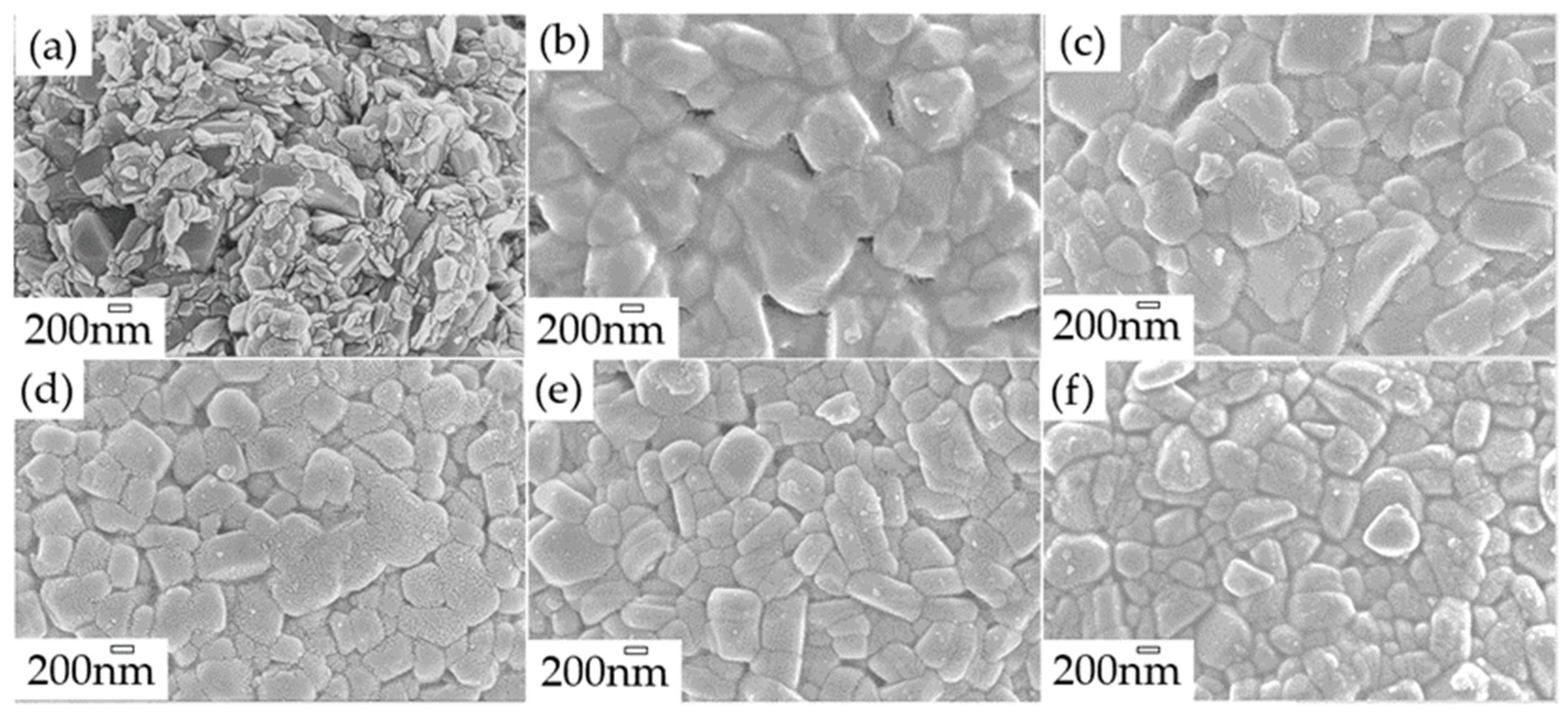
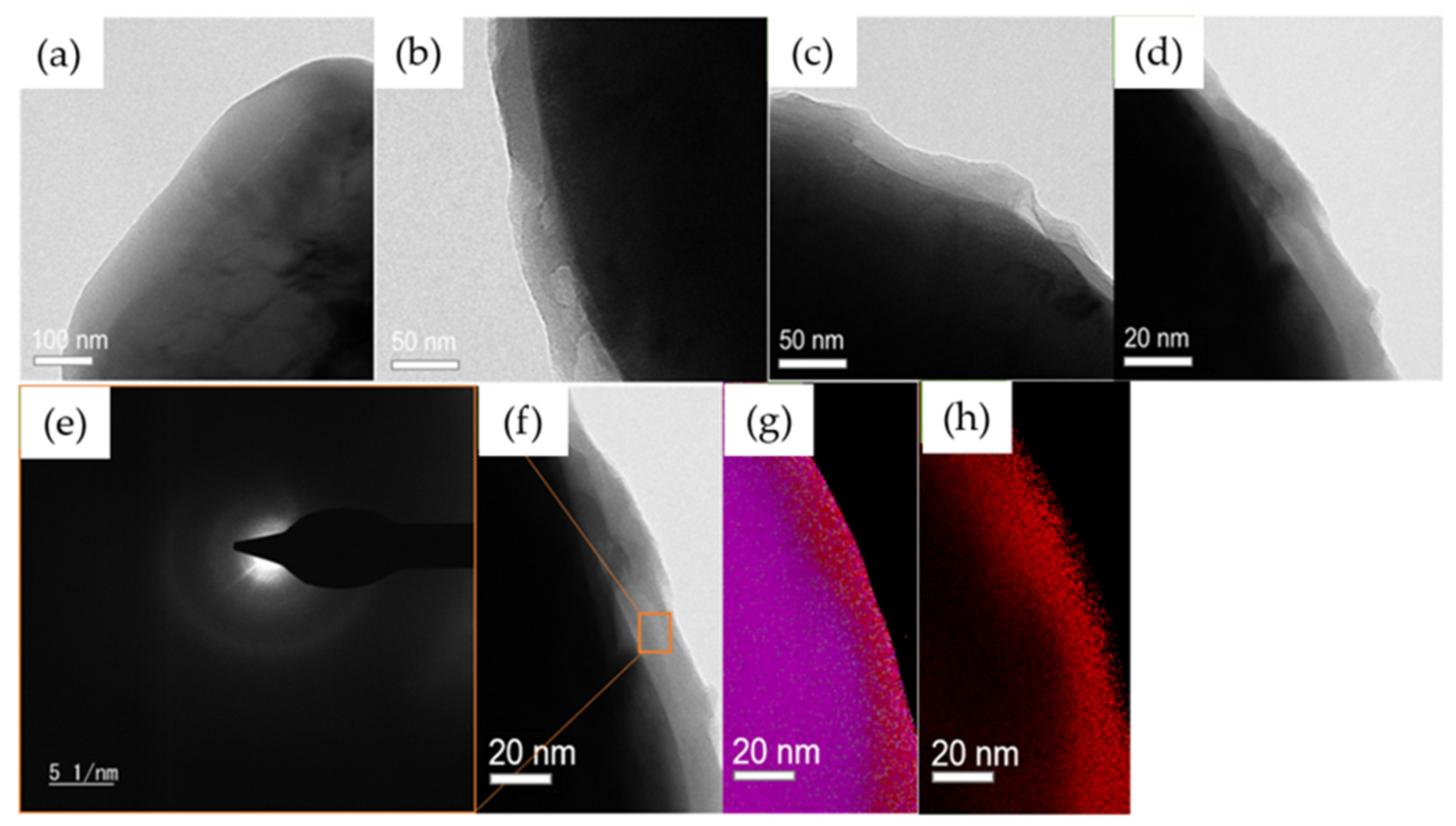
2.5. Morphology of the Polymer Coating
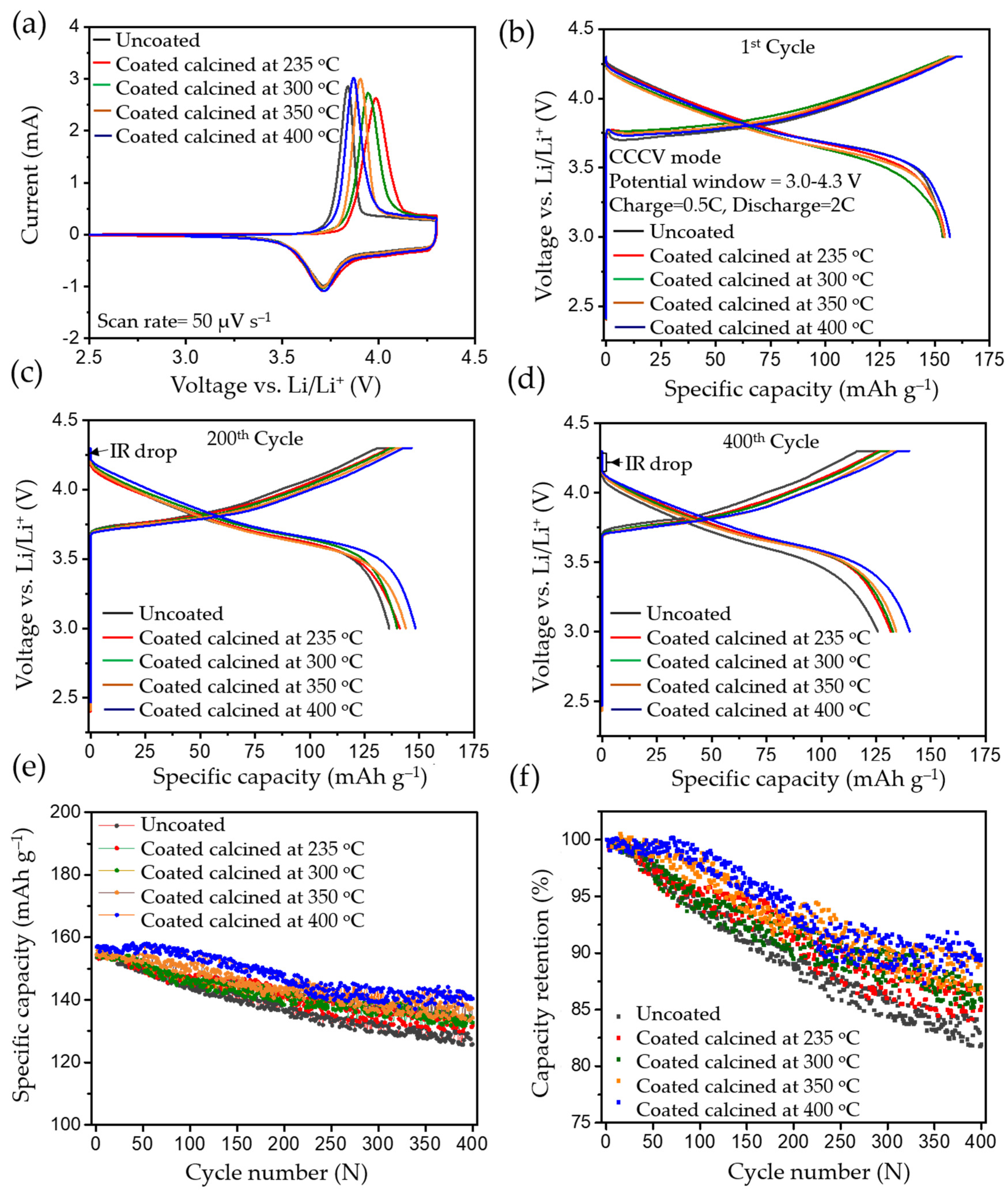
2.6. Electrochemical Performance
2.6.1. Cyclic Voltammetry
2.6.2. CCCV Charge and CC Discharge
2.6.3. Cycling Experiments of Heat Treated Uncoated Pristine NMC622
2.6.4. Rate Capacity and Electrochemical Impedance Spectroscopy Test
2.7. Post-Mortem Analysis
3. Materials and Methods
3.1. Material Preparation
3.2. Physico-Chemical Characterisation
3.3. Cell Preparation and Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered Li [NixCoyMnz] O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2016, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 2018, 8, 1–25. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.J.; Gauquelin, N.; Esken, D.; Verbeeck, J.; Janek, J. Increased Performance Improvement of Lithium-Ion Batteries by Dry Powder Coating of High-Nickel NMC with Nanostructured Fumed Ternary Lithium Metal Oxides. ACS Appl. Energy Mater. 2021, 4, 8832–8848. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem. Energy Rev. 2019, 31, 43–80. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 Coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges. Coatings 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Du, C.; Yan, C.; He, X.; Song, B.; Yin, G.; Zuo, P.; Cheng, X. Al2O3 coated concentration-gradient Li[Ni0.73Co0.12Mn0.15]O2 cathode material by freeze drying for long-life lithium ion batteries. Electrochim. Acta 2015, 174, 1185–1191. [Google Scholar] [CrossRef]
- Jian, Z.; Wang, W.; Wang, M.; Wang, Y.; AuYeung, N.; Liu, M.; Feng, Z. Al2O3 coated LiCoO2 as cathode for high-capacity and long-cycling Li-ion batteries. Chin. Chem. Lett. 2018, 29, 1768–1772. [Google Scholar] [CrossRef]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Tao, T.; Chen, C.; Yao, Y.; Liang, B.; Lu, S.; Chen, Y. Enhanced electrochemical performance of ZrO2 modified LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. Ceram. Int. 2017, 43, 15173–15178. [Google Scholar] [CrossRef]
- Laskar, M.R.; Jackson, D.H.K.; Xu, S.; Hamers, R.J.; Morgan, D.; Kuech, T.F. Atomic layer deposited MgO: A lower overpotential coating for Li[Ni0.5Mn0.3Co0.2]O2 cathode. ACS Appl. Mater. Interfaces 2017, 9, 11231–11239. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Zhang, J. Surface-coated LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode materials by Al2O3, ZrO2, and Li2O-2B2O3 thin-layers for improving the performance of lithium ion batteries. Front. Mater. 2019, 6, 309. [Google Scholar] [CrossRef]
- Hildebrand, S.; Vollmer, C.; Winter, M.; Schappacher, F.M. Al2O3, SiO2 and TiO2 as coatings for safer LiNi0.8Co0.15Al0.05O2 cathodes: Electrochemical performance and thermal analysis by accelerating rate calorimetry. J. Electrochem. Soc. 2017, 164, A2190–A2198. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, N.; Jahn, M.; Pfleging, W.; Seifert, H.J. Improved capacity retention of SiO2 -coated lithium-ion batteries. Appl. Sci. 2019, 9, 3671. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.-Z.; Ren, C.; Tai, G.-A.; Zhang, X.; Li, A.-D.; Wu, D.; Li, H.; Zhou, F. Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material. J. Power Sources 2014, 266, 433–439. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Y.; Yuan, A.; Xu, J. Effects of lithium excess and SnO2 surface coating on the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries. J. Alloys Compd. 2019, 787, 429–439. [Google Scholar] [CrossRef]
- Loghavi, M.M.; Mohammadi-Manesh, H.; Eqra, R. Y2O3-decorated LiNi0.8Co0.15Al0.05O2 cathode material with improved electrochemical performance for lithium-ion batteries. J. Electroanal. Chem. 2019, 848, 113326. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Liu, Q.; Wang, L.; Yin, G.; Zuo, P.; Ma, Y.; Cheng, X.; Du, C.; Gao, Y. Mixed lithium ion and electron conducting LiAlPO3.93F1.07-coated LiCoO2 cathode with improved electrochemical performance. Electrochem. commun. 2017, 83, 106–109. [Google Scholar] [CrossRef]
- Chen, X.; Ma, F.; Li, Y.; Liang, J.; Matthews, B.; Sokolowski, J.; Han, J.; Wu, G.; Lu, X.; Li, Q. Nitrogen-doped carbon coated LiNi0.6Co0.2Mn0.2O2 cathode with enhanced electrochemical performance for Li-Ion batteries. Electrochim. Acta 2018, 284, 526–533. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C. Surface modifications of electrode materials for lithium-ion batteries: Status and trends. Ionics 2014, 20, 751–787. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Liu, P.; Wang, S.; Zhang, W.; Chen, D. Facile preparation of carbon-LiNi1/3Co1/3Mn1/3O2 with enhanced stability and rate capability for lithium-ion batteries. J. Alloys Compd. 2019, 780, 643–652. [Google Scholar] [CrossRef]
- Guo, R.; Shi, P.; Cheng, X.; Du, C. Synthesis and characterization of carbon-coated LiNi1/3Co1/3Mn1/3O2 cathode material prepared by polyvinyl alcohol pyrolysis route. J. Alloys Compd. 2009, 473, 53–59. [Google Scholar] [CrossRef]
- Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Pejovnik, S.; Jamnik, J. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites. J. Electrochem. Soc. 2005, 152, A607–A610. [Google Scholar] [CrossRef]
- Sides, C.R.; Croce, F.; Young, V.Y.; Martin, C.R.; Scrosati, B. A high-rate, nanocomposite LiFePO4/carbon cathode. Electrochem. Solid-State Lett. 2005, 8, 484–487. [Google Scholar] [CrossRef]
- Singh, S.; Raj, A.K.; Sen, R.; Johari, P.; Mitra, S. Impact of Cl doping on electrochemical performance in orthosilicate (Li2FeSiO4): A density functional theory supported experimental approach. ACS Appl. Mater. Interfaces 2017, 9, 26885–26896. [Google Scholar] [CrossRef]
- Singh, S.; Panda, M.R.; Sen, R.; Johari, P.; Sinha, A.K.; Meena, S.S.; Mitra, S. Study of higher discharge capacity, phase transition, and relative structural stability in Li2FeSiO4 cathode upon lithium extraction using an experimental and theoretical approach and full cell prototype study. ACS Appl. Energy Mater. 2019, 2, 6584–6598. [Google Scholar] [CrossRef]
- Wang, L.-P.; Zhang, X.-D.; Wang, T.-S.; Yin, Y.-X.; Shi, J.-L.; Wang, C.-R.; Guo, Y.-G. Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers. Adv. Energy Mater. 2018, 8, 1801528. [Google Scholar] [CrossRef]
- Babbar, P.; Niehoff, P.; Schappacher, F.; Winter, M. Studying the effects of carbon coatings on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2. Meet. Abstr. 2018, MA2018-01, 359. [Google Scholar]
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Guan, H.; Wang, X. In situ polymerization of furfuryl alcohol with ammonium dihydrogen phosphate in poplar wood for improved dimensional stability and flame retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Lascovich, J.C.; Giorgi, R.; Scaglione, S. Evaluation of the sp2/sp3 ratio in amorphous carbon structure by XPS and XAES. Appl. Surf. Sci. 1991, 47, 17–21. [Google Scholar] [CrossRef]
- Tondi, G.; Cefarin, N.; Sepperer, T.; D’Amico, F.; Berger, R.J.F.; Musso, M.; Birarda, G.; Reyer, A.; Schnabel, T.; Vaccari, L. Understanding the polymerization of poly-furfuryl alcohol: Ring opening and diels-alder reactions. Polymers 2019, 11, 2126. [Google Scholar] [CrossRef] [Green Version]
- Janus, R.; Wach, A.; Kuśtrowski, P.; Dudek, B.; Drozdek, M.; Silvestre-Albero, A.M.; Rodríguez-Reinoso, F.; Cool, P. Investigation on the low-temperature transformations of poly (furfuryl alcohol) deposited on MCM-41. Langmuir 2013, 29, 3045–3053. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Qilu; Gendron, F.; Julien, C.M. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Lu, Z.; Beaulieu, L.Y.; Donaberger, R.A.; Thomas, C.L.; Dahn, J.R. Synthesis structure, and electrochemical behavior of Li [NixLi1/3-2x/3Mn2/3-x/3]O2. J. Electrochem. Soc. 2002, 149, A778–A791. [Google Scholar] [CrossRef]
- Julien, C. Local cationic environment in lithium nickel–cobalt oxides used as cathode materials for lithium batteries. Solid State Ionics 2000, 136–137, 887–896. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T. Comparative study of LiCoO2 surface modified with different oxides. J. Power Sources 2007, 174, 959–964. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-catalyzed polycondensation of furfuryl alcohol: Mechanisms of chromophore formation and cross-linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Verdejo, R.; Lamoriniere, S.; Cottam, B.; Bismarck, A.; Shaffer, M. Removal of oxidation debris from multi-walled carbon nanotubes. Chem. Commun. 2007, 106, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Corsaro, C.; Frusteri, F.; Magazù, V.; Mangione, A.; Migliardo, F.; Wanderlingh, U. Structural and vibrational properties of carbon nanotubes by TEM and infrared spectroscopy. Diam. Relat. Mater. 2004, 13, 1249–1253. [Google Scholar] [CrossRef]
- Bertarione, S.; Bonino, F.; Cesano, F.; Jain, S.; Zanetti, M.; Scarano, D.; Zecchina, A. Micro-FTIR and micro-raman studies of a carbon film prepared from furfuryl alcohol polymerization. J. Phys. Chem. B 2009, 113, 10571–10574. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mitra, S. Improved electrochemical activity of nanostructured Li2FeSiO4/MWCNTs composite cathode. Electrochim. Acta 2014, 123, 378–386. [Google Scholar] [CrossRef]
- De Almeida Filho, C.; Zarbin, A.J.G. Porous carbon obtained by the pyrolysis of TiO2/poly (furfuryl alcohol) nanocomposite: Preparation, characterization and utilization for adsorption of reactive dyes from aqueous solution. J. Braz. Chem. Soc. 2006, 17, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Czigány, Z.; Brunell, I.F.; Neidhardt, J.; Hultman, L.; Suenaga, K. Growth of fullerene-like carbon nitride thin solid films consisting of cross-linked nano-onions. Appl. Phys. Lett. 2001, 79, 2639–2641. [Google Scholar] [CrossRef]
- Wang, C.; Appleby, A.J.; Little, F.E. Electrochemical impedance study of initial lithium ion intercalation into graphite powders. Electrochim. Acta 2001, 46, 1793–1813. [Google Scholar] [CrossRef]
- Xu, K.; Lam, Y.; Zhang, S.S.; Jow, T.R.; Curtis, T.B. Solvation Sheath of Li + in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J. Phys. Chem. C 2007, 111, 7411–7421. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectros. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Tougaard, S. Universality classes of inelastic electron scattering cross-sections. Surf. Interface Anal. 1997, 25, 137–154. [Google Scholar] [CrossRef]

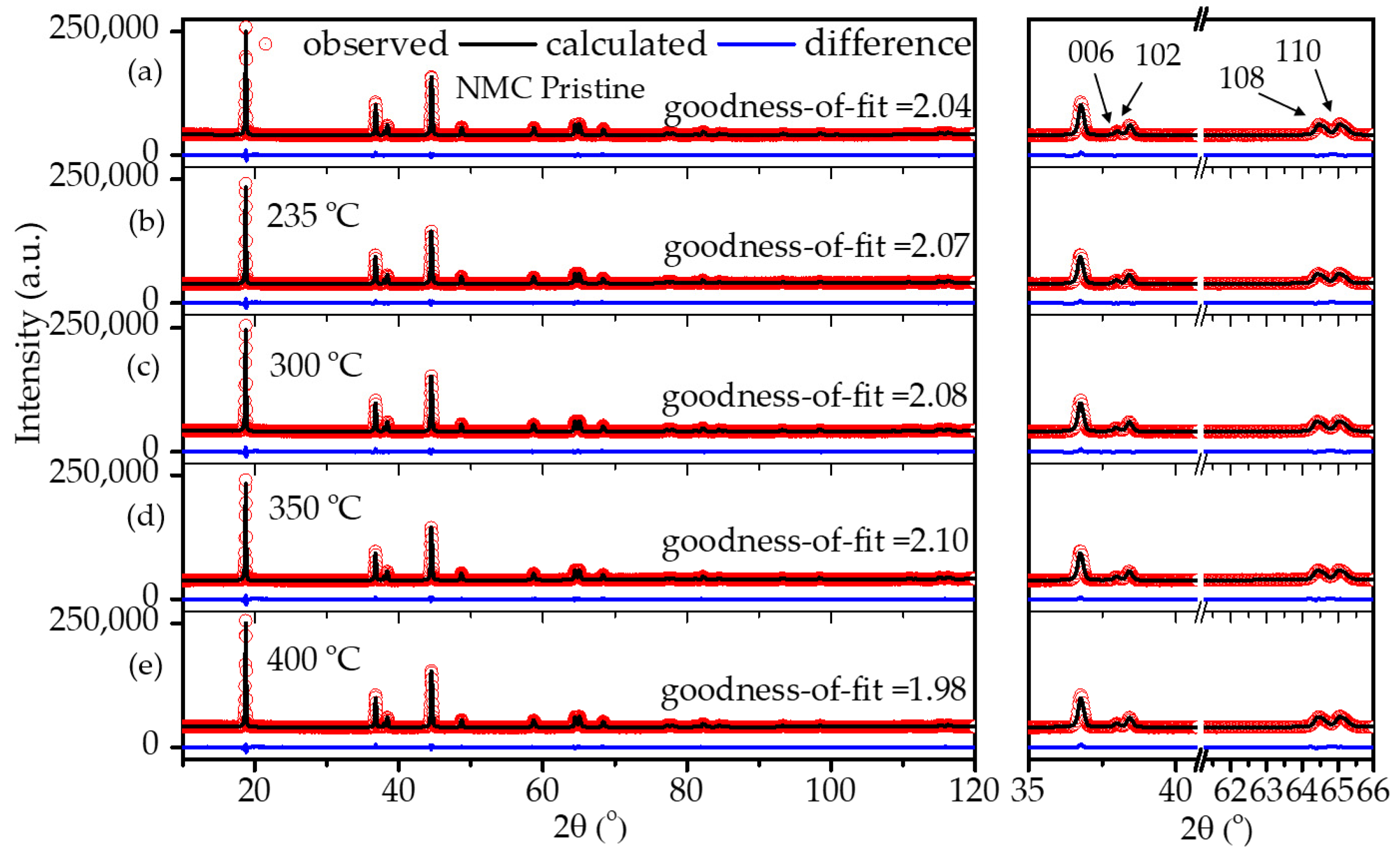

| Sample/Calcination Temperature | a | c | c/a | I(003)/I(104) | Rwp | Goodness of Fit (χ2) |
|---|---|---|---|---|---|---|
| (°C) | (Å) | (Å) | ||||
| Uncoated pristine | 2.8681(2) | 14.2230(4) | 4.95 | 1.62 | 0.99 | 2.04 |
| 235 | 2.8689(4) | 14.2317(8) | 4.96 | 1.65 | 1.03 | 2.07 |
| 300 | 2.8692(3) | 14.2377(3) | 4.96 | 1.66 | 1.01 | 2.08 |
| 350 | 2.8684(5) | 14.2329(4) | 4.96 | 1.64 | 1.05 | 2.10 |
| 400 | 2.8680(2) | 14.2294(5) | 4.96 | 1.65 | 0.96 | 1.98 |
| Calcination Temperature | C | Ni | O |
|---|---|---|---|
| (°C) | (at%) | (at%) | (at%) |
| 235 | 52.3 | <1 | 47.7 |
| 300 | 43.2 | 1.1 | 55.7 |
| 350 | 45.2 | 1.6 | 53.2 |
| 400 | 36.6 | 2.8 | 60.7 |
| Calcination Temperature | Carbon Quantity |
|---|---|
| (°C) | (wt.%) |
| 235 | 0.93 ± 0.03 |
| 300 | 0.83 ± 0.03 |
| 350 | 0.80 ± 0.02 |
| 400 | 0.76 ± 0.02 |
| Calcination Temperature | Initial Capacity | Final Capacity | Capacity Retention |
|---|---|---|---|
| (°C) | (mAh g−1) | (mAh g−1) | (%) |
| Uncoated pristine | 153.59 | 125.80 | 81.38 |
| 235 | 154.51 | 131.73 | 85.29 |
| 300 | 154.60 | 132.67 | 85.56 |
| 350 | 154.41 | 134.23 | 86.93 |
| 400 | 156.98 | 140.36 | 89.42 |
| Calcination Temperature | Initial Capacity | Final Capacity | Capacity Retention |
|---|---|---|---|
| (°C) | (mAh g−1) | (mAh g−1) | (%) |
| Uncoated pristine | 153.59 | 125.80 | 81.38 |
| 235 | 155.18 | 128.89 | 83.06 |
| 300 | 155.05 | 130.68 | 84.28 |
| 350 | 155.80 | 131.45 | 84.37 |
| 400 | 156.46 | 134.27 | 85.81 |
| Untreated Pristine | 235 °C | 300 °C | 350 °C | 400 °C | |
|---|---|---|---|---|---|
| Current Rate | (mAh g−1) | (mAh g−1) | (mAh g−1) | (mAh g−1) | (mAh g−1) |
| 0.1 C | 169.68 | 170.61 | 171.93 | 174.35 | 176.40 |
| 0.2 C | 164.28 | 165.60 | 167.66 | 170.41 | 173.20 |
| 0.5 C | 156.48 | 157.21 | 158.60 | 161.38 | 163.37 |
| 1 C | 146.46 | 147.77 | 149.51 | 152.61 | 155.01 |
| 2 C | 136.02 | 138.33 | 140.65 | 143.11 | 144.69 |
| 5 C | 100.60 | 118.27 | 120.96 | 123.48 | 124.99 |
| 10 C | 63.41 | 87.23 | 91.66 | 92.65 | 94.97 |
| Calcination Temperature | Uncoated Pristine | 235 °C | 300 °C | 350 °C | 400 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Units | Value | Error (%) | Value | Error (%) | Value | Error (%) | Value | Error (%) | Value | Error (%) |
| Rsol | (Ω) | 2.443 | 0.002 | 2.312 | 0.002 | 2.291 | 0.002 | 2.157 | 0.002 | 2.265 | 0.001 |
| CPEsei-T | (Fsα−1) | 4.47 × 10−4 | 0.006 | 4.10 × 10−4 | 0.006 | 4.04 × 10−4 | 0.006 | 0.000 | 0.007 | 4.04 × 10−4 | 0.006 |
| CPEsei-P | (/) | 0.698 | 0.001 | 0.705 | 0.001 | 0.710 | 0.001 | 0.704 | 0.001 | 0.725 | 0.001 |
| Rsei | (Ω) | 8.502 | 0.001 | 8.402 | 0.001 | 8.413 | 0.001 | 8.090 | 0.002 | 8.020 | 0.001 |
| CPEdl-T | (Fsα−1) | 3.35 × 10−3 | 0.003 | 4.35 × 10−3 | 0.004 | 4.22 × 10−3 | 0.005 | 0.004 | 0.005 | 3.99 × 10−3 | 0.005 |
| CPEdli-P | (/) | 0.923 | 0.001 | 0.871 | 0.001 | 0.863 | 0.001 | 0.860 | 0.001 | 0.879 | 0.001 |
| Rct | (Ω) | 54.110 | 0.007 | 20.110 | 0.005 | 17.910 | 0.005 | 16.340 | 0.005 | 15.010 | 0.004 |
| W-R | (Ω) | 70.550 | 0.001 | 19.560 | 0.025 | 18.120 | 0.021 | 16.770 | 0.019 | 14.870 | 0.014 |
| W-T | (s) | 5.899 | 0.002 | 10.900 | 0.056 | 9.801 | 0.048 | 8.180 | 0.041 | 6.862 | 0.030 |
| W-P | (/) | 0.419 | 0.007 | 0.419 | 0.010 | 0.420 | 0.009 | 0.421 | 0.009 | 0.429 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathribail, A.R.; Rezqita, A.; Lager, D.; Hamid, R.; Surace, Y.; Berecibar, M.; Van Mierlo, J.; Hubin, A.; Jahn, M.; Kahr, J. High-Performance Amorphous Carbon Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries. Batteries 2021, 7, 69. https://doi.org/10.3390/batteries7040069
Kathribail AR, Rezqita A, Lager D, Hamid R, Surace Y, Berecibar M, Van Mierlo J, Hubin A, Jahn M, Kahr J. High-Performance Amorphous Carbon Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries. Batteries. 2021; 7(4):69. https://doi.org/10.3390/batteries7040069
Chicago/Turabian StyleKathribail, Anish Raj, Arlavinda Rezqita, Daniel Lager, Raad Hamid, Yuri Surace, Maitane Berecibar, Joeri Van Mierlo, Annick Hubin, Marcus Jahn, and Jürgen Kahr. 2021. "High-Performance Amorphous Carbon Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries" Batteries 7, no. 4: 69. https://doi.org/10.3390/batteries7040069
APA StyleKathribail, A. R., Rezqita, A., Lager, D., Hamid, R., Surace, Y., Berecibar, M., Van Mierlo, J., Hubin, A., Jahn, M., & Kahr, J. (2021). High-Performance Amorphous Carbon Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries. Batteries, 7(4), 69. https://doi.org/10.3390/batteries7040069