Comparative Study of Kilowatt-Scale Vanadium Redox Flow Battery Stacks Designed with Serpentine Flow Fields and Split Manifolds
Abstract
1. Introduction
2. Details of Materials and Methods
2.1. Stacks Fabricated
2.2. Electrochemical Studies
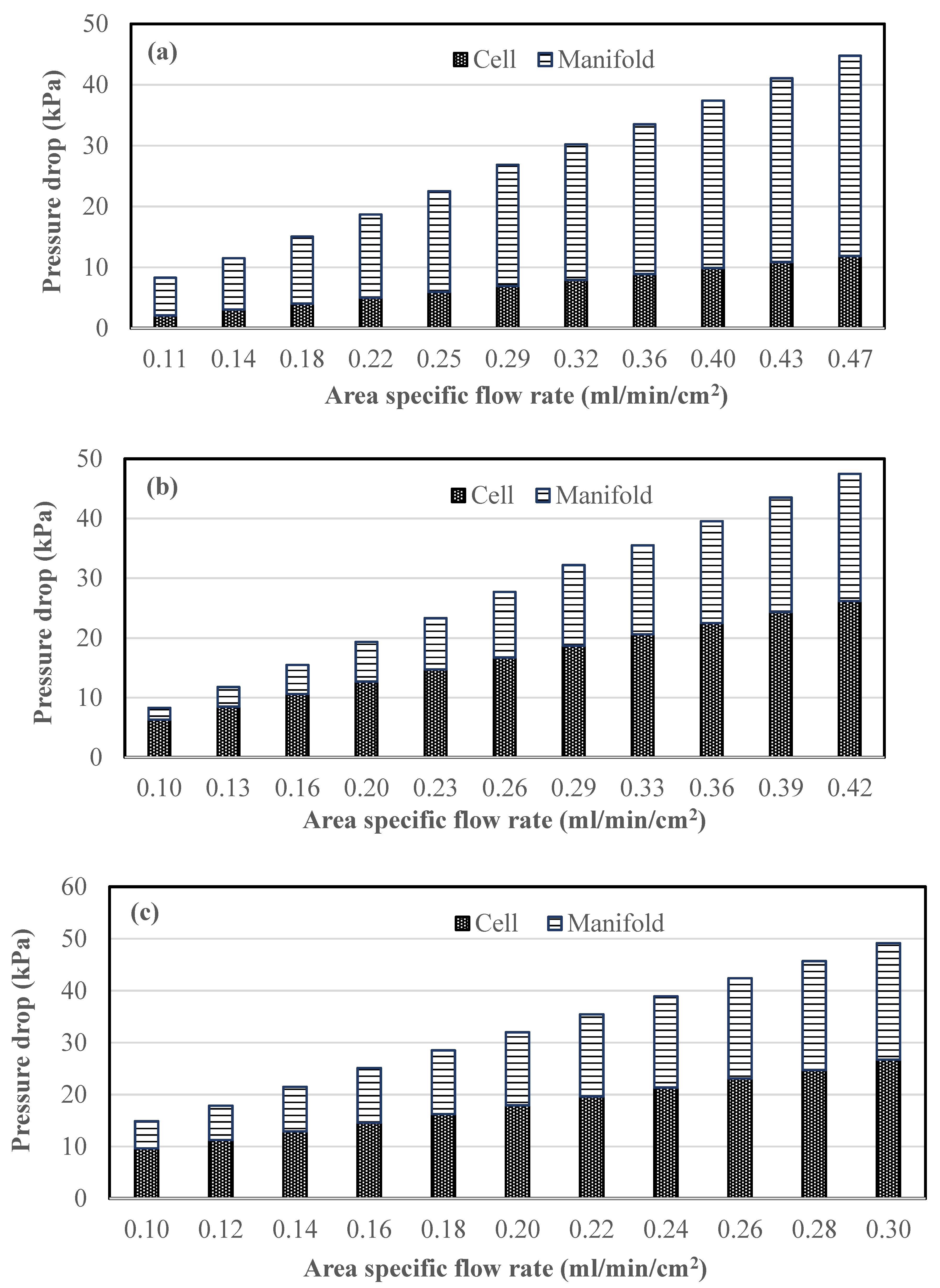
2.3. Pressure Drop Studies
3. Results and Discussions
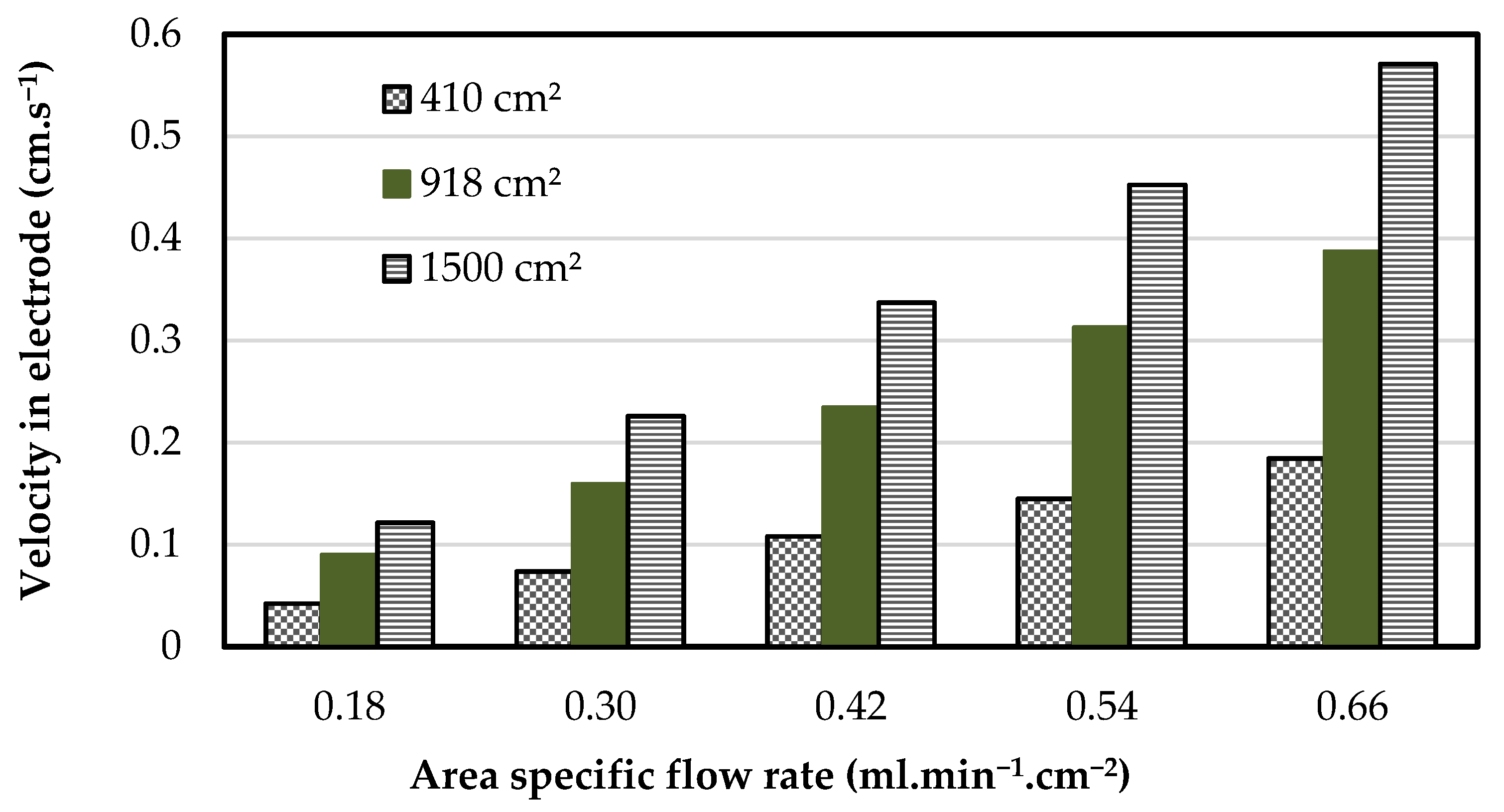
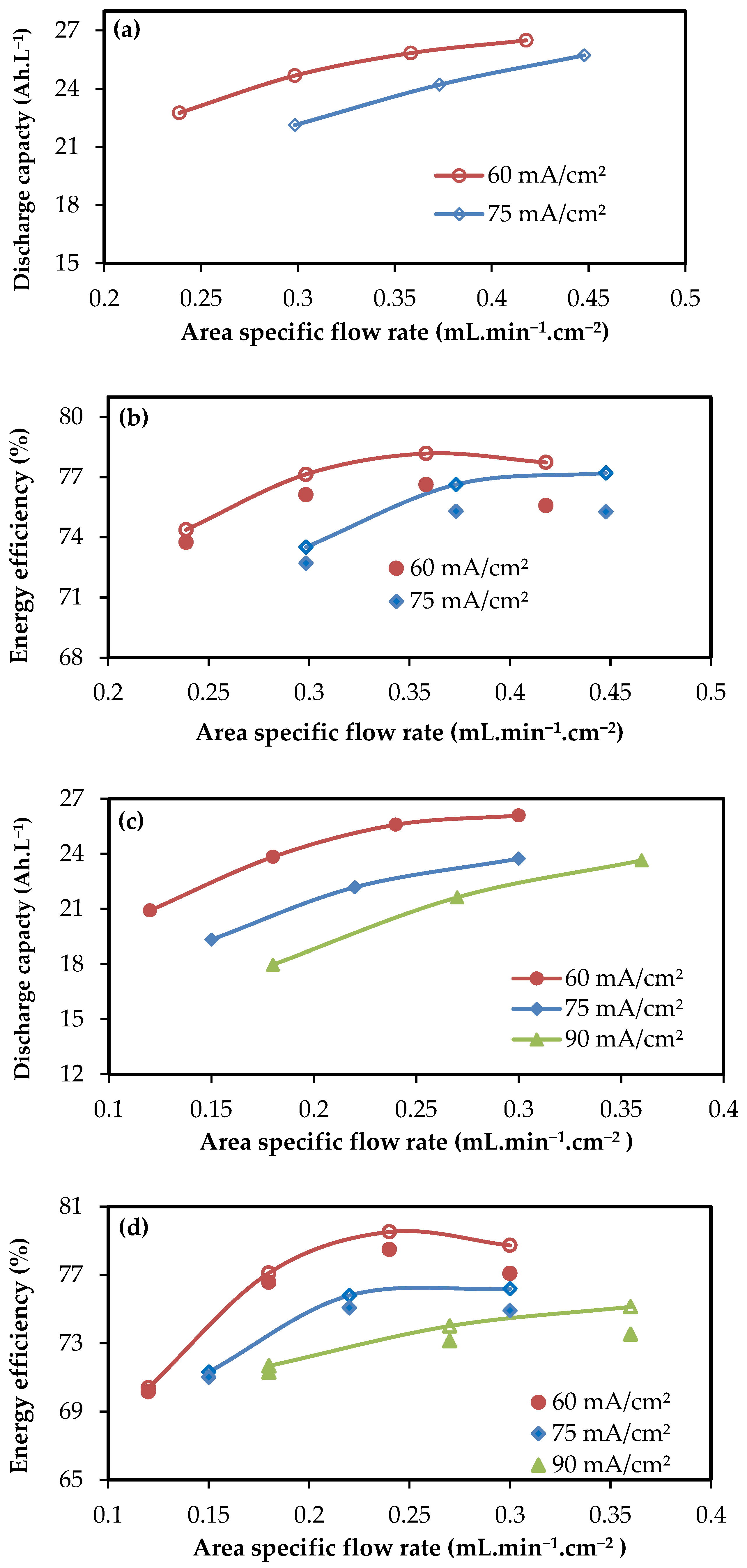
3.1. Operating Flow Rate
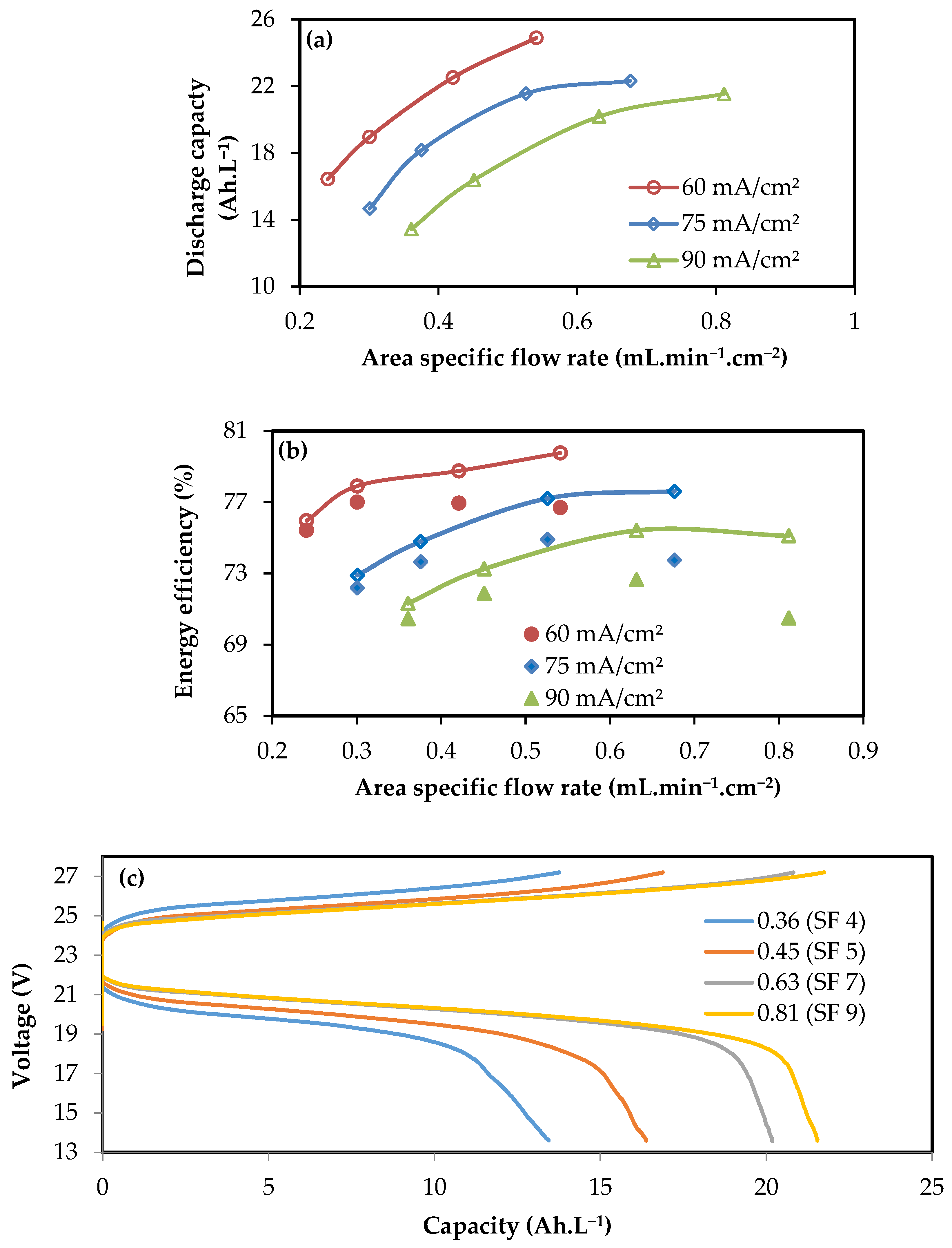
3.2. Performance of 4-Cell Stacks
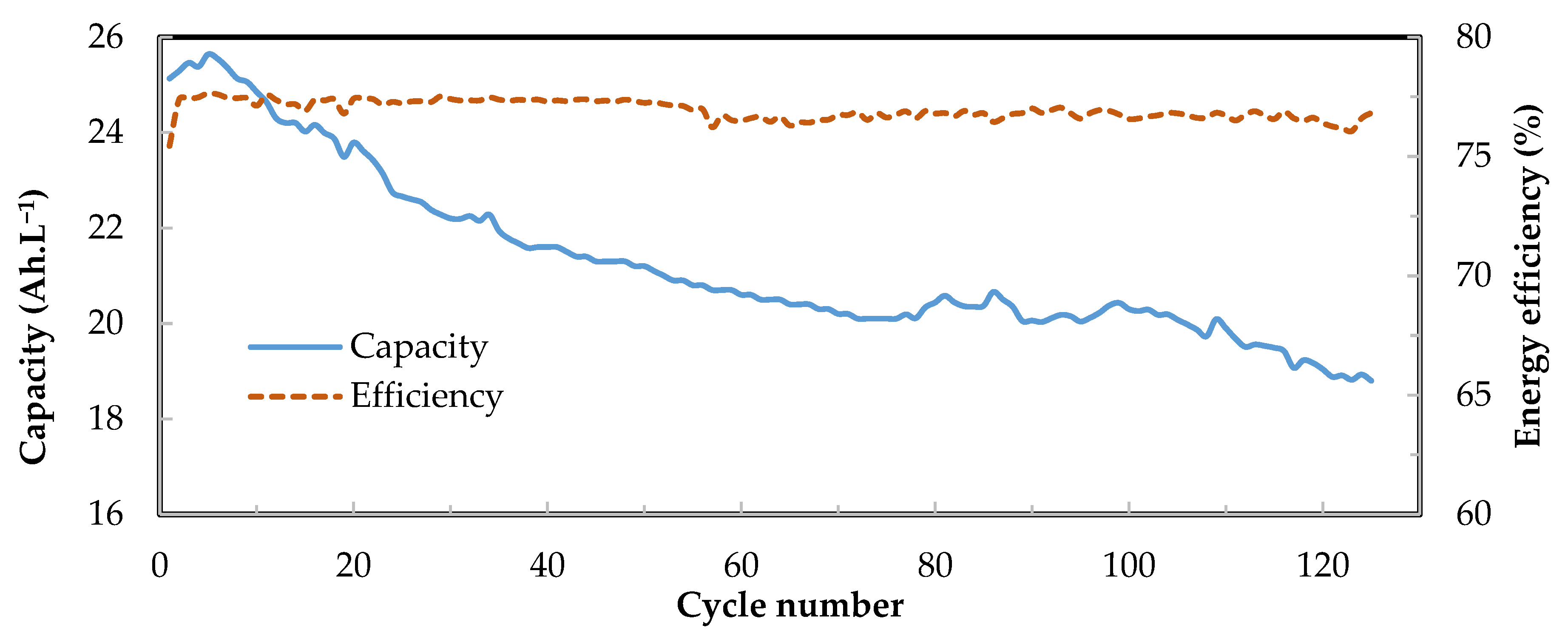
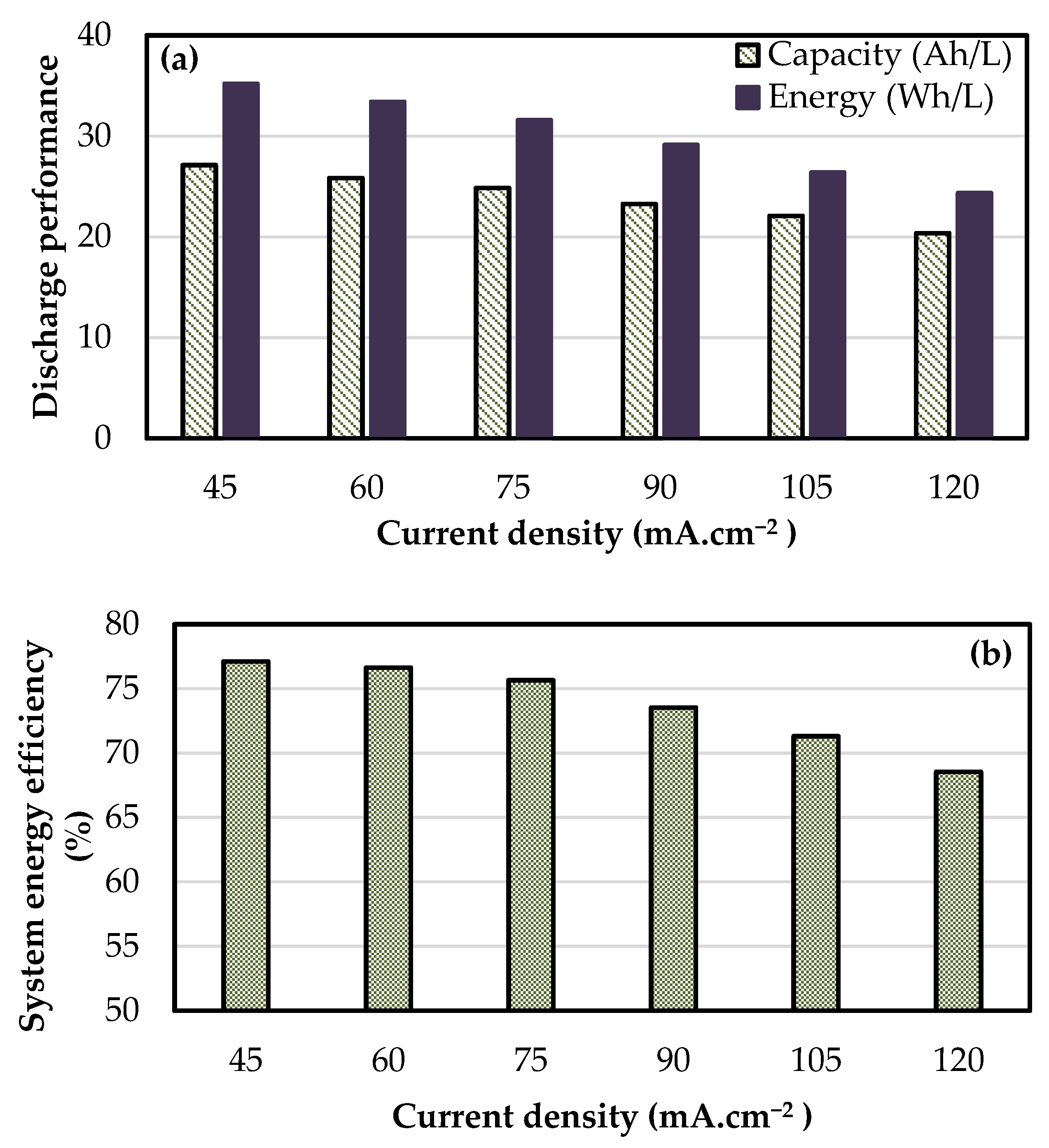
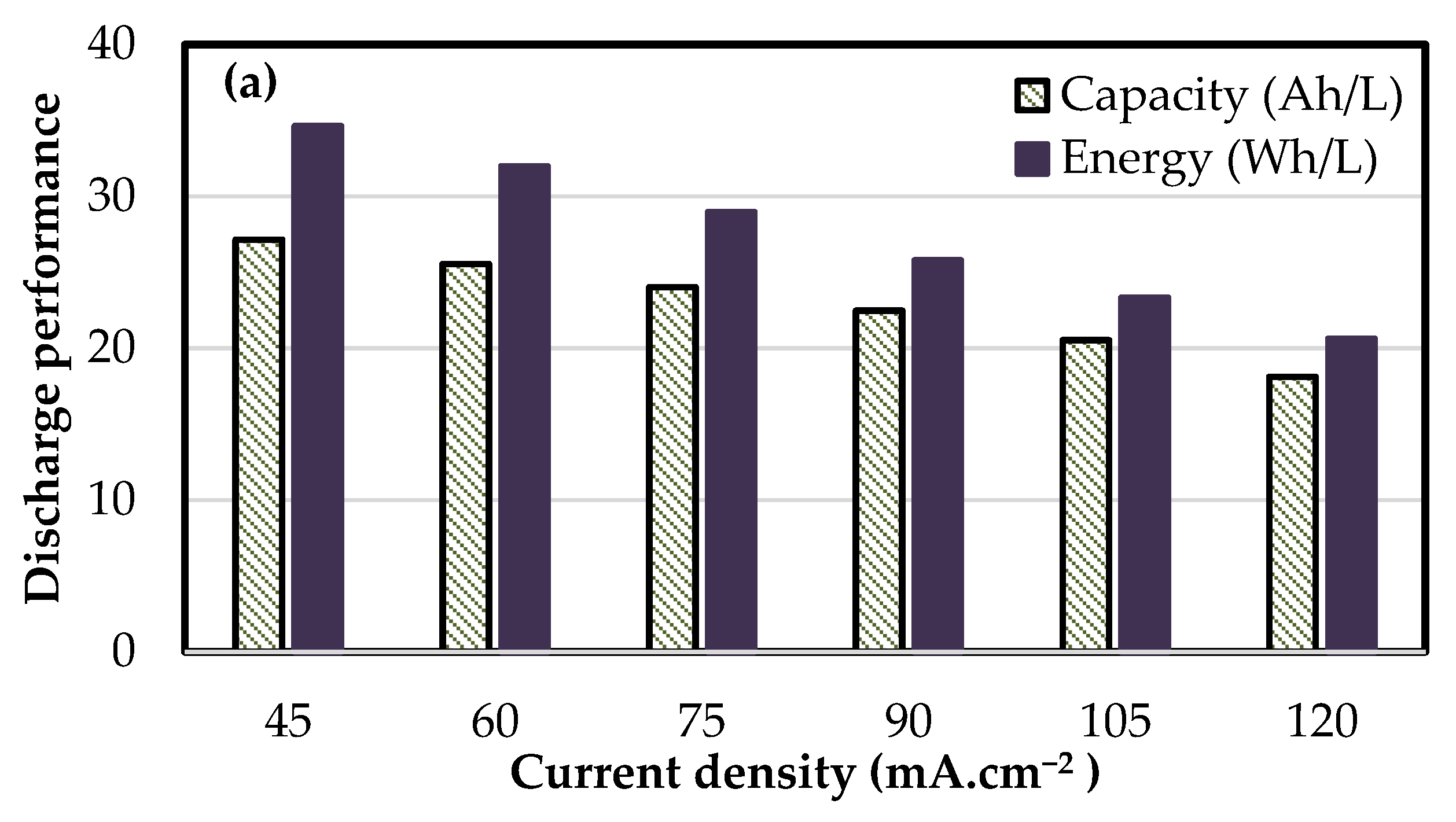
3.3. Performance of kW Scale Stacks
3.4. Cell and Stack Resistance
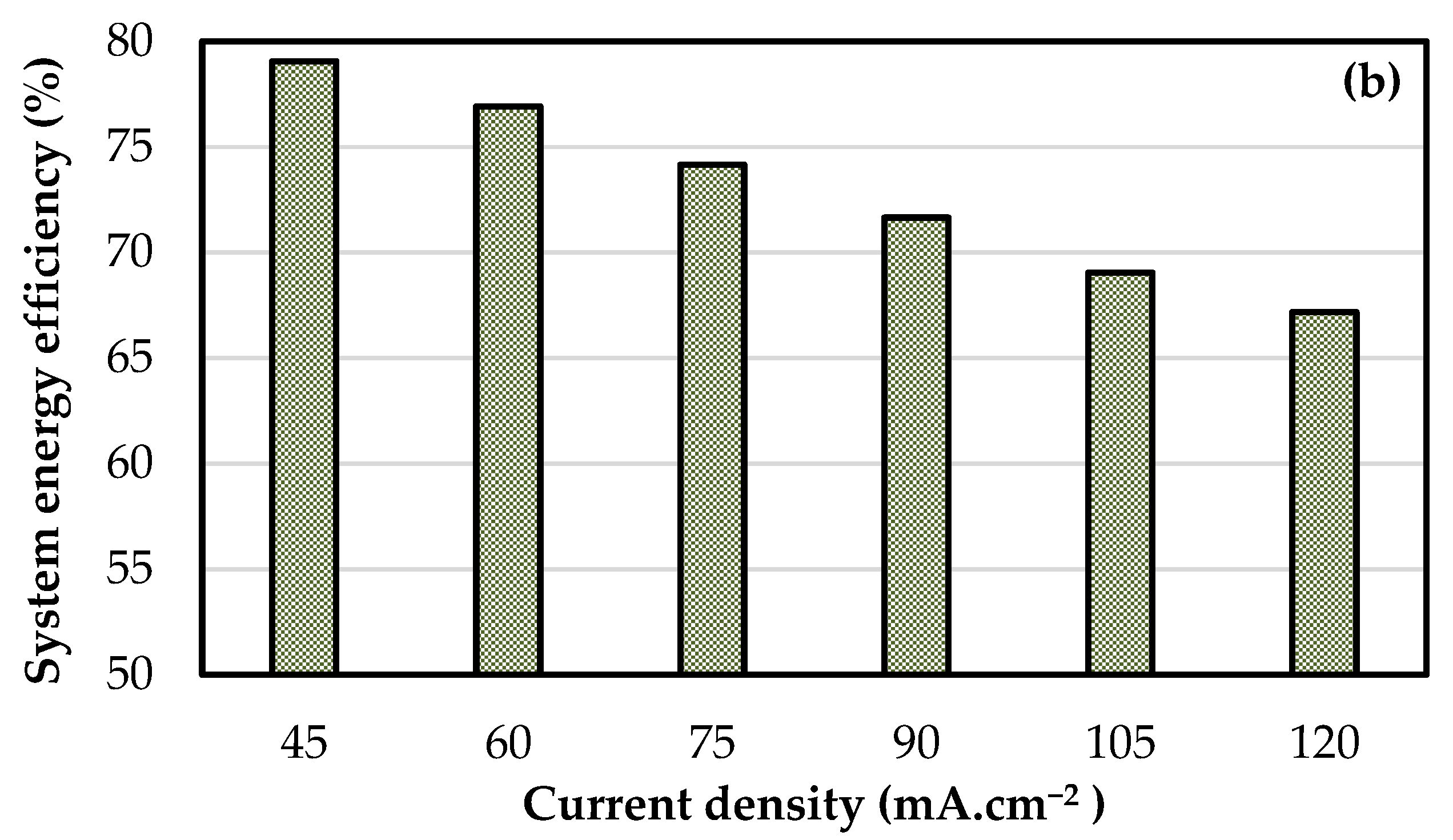
4. Fixed Flow Rate Operation
5. Conclusions
- Stacks of different cell sizes have different optimal flow rate conditions. Under their optimal flow conditions, stacks of all three cell sizes exhibit good and similar electrochemical performance of discharge energy density >30 Wh per liter and energy efficiency >75%. This can be attributed to the use of optimized serpentine flow field.
- Manifold pressure losses constitute a significant part of the total pumping power consumption. Careful design of split manifolds can reduce pressure losses, while maintaining uniform distribution to all cells.
- Stacks having larger cell sizes have the advantage of being able to operate at lower stoichiometric flow factors in obtaining good electrochemical performance. This reduces the power spent on pumping compared to smaller cell size stacks.
- Stacks designed with serpentine flow fields can be operated at a fixed flow rate for power variations of ±25%, with no significant changes in discharge capacity and efficiency. A wider window of operation of up to ±50% can be executed with minor changes in the electrochemical perfor-mance.
- Stacks of the present design can be operated at considerably lower pressure drops for a 2 kW stack, resulting in efficiency loss of 1 to 2% only when pumping power is considered. This is in contrast to a loss of 5 to 7% for flow frame-supported thin graphite sheet design.
- Cells of 1500 cm2 active area can be used to develop kilowatt-scale VRFB stacks with serpentine flow fields.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Skyllaskazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in flow battery research and development. J. Electrochem. Soc. 2011, 158, R55–R79. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new all-vandium redox flow battery. J. Power Sources 1988, 22, 59–61. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, Financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Menictas, C.; Lim, T. Redox flow batteries for medium- to large-scale energy storage. In Electricity Transmission, Distribution and Storage Systems; Melhem, Z., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 398–441. Available online: http://dx.doi.org/10.1533/9780857097378.3.398 (accessed on 29 April 2021).
- Ma, X.; Zhang, H.; Sun, C.; Zou, Y.; Zhang, T. An optimal strategy of electrolyte flow rate for vanadium redox flow battery. J. Power Sources 2012, 203, 153–158. [Google Scholar] [CrossRef]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- Park, D.-J.; Jeon, K.-S.; Ryu, C.-H.; Hwang, G.-J. Performance of the all-vanadium redox flow battery stack. J. Ind. Eng. Chem. 2017, 45, 387–390. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, X.; Wang, Z.; Liu, J.; Hu, Y.; Deng, Q.; Yin, X.; Zhou, Q.; Zhou, W.; Wu, Y. Electrochemical performance of 5 kW all-vanadium redox flow battery stack with a flow frame of multi-distribution channels. J. Solid State Electrochem. 2017, 21, 429–435. [Google Scholar] [CrossRef]
- García-Quismondo, E.; Almonacid, I.; Martínez, M.; Ángeles, C.; Miroslavov, V.; Serrano, E.; Palma, J.; Salmerón, J.P.A. Operational experience of 5 kW/5 kWh all-vanadium flow batteries in photovoltaic grid applications. Batteries 2019, 5, 52. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Bao, J.; Skyllas-Kazacos, M. Studies on optimal charging conditions for vanadium redox flow batteries. J. Energy Storage 2017, 11, 191–199. [Google Scholar] [CrossRef]
- Wang, T.; Fu, J.; Zheng, M.; Yu, Z. Dynamic control strategy for the electrolyte flow rate of vanadium redox flow batteries. Appl. Energy 2018, 227, 613–623. [Google Scholar] [CrossRef]
- Guarnieri, M.; Trovo, A.; Picano, F. Enhancing the efficiency of kW-class vanadium redox flow batteries by flow factor modulation: An experimental method. Appl. Energy 2020, 262, 114532. [Google Scholar] [CrossRef]
- Guarnieri, M.; Trovò, A.; Marini, G.; Sutto, A.; Alotto, P. High current polarization tests on a 9 kW vanadium redox flow battery. J. Power Sources 2019, 431, 239–249. [Google Scholar] [CrossRef]
- Trovò, A.; Picano, F.; Guarnieri, M. Comparison of energy losses in a 9 kW vanadium redox flow battery. J. Power Sources 2019, 440, 227144. [Google Scholar] [CrossRef]
- Xing, F.; Zhang, H.; Ma, X. Shunt current loss of the vanadium redox flow battery. J. Power Sources 2011, 196, 10753–10757. [Google Scholar] [CrossRef]
- Kim, S.; Thomsen, E.; Xia, G.; Nie, Z.; Bao, J.; Recknagle, K.; Wang, W.; Viswanathan, V.; Luo, Q.; Wei, X.; et al. 1 kW/1 kWh advanced vanadium redox flow battery utilizing mixed acid electrolytes. J. Power Sources 2013, 237, 300–309. [Google Scholar] [CrossRef]
- Yin, C.; Guo, S.; Fang, H.; Liu, J.; Li, Y.; Tang, H. Numerical and experimental studies of stack shunt current for vanadium redox flow battery. Appl. Energy 2015, 151, 237–248. [Google Scholar] [CrossRef]
- Ye, Q.; Hu, J.; Cheng, P.; Ma, Z. Design trade-offs among shunt current, pumping loss and compactness in the piping system of a multi-stack vanadium flow battery. J. Power Sources 2015, 296, 352–364. [Google Scholar] [CrossRef]
- Fink, H.; Remy, M. Shunt currents in vanadium flow batteries: Measurement, modelling and implications for efficiency. J. Power Sources 2015, 284, 547–553. [Google Scholar] [CrossRef]
- Arenas, L.; de León, C.P.; Walsh, F. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef]
- Chalamala, B.R.; Soundappan, T.; Fisher, G.R.; Anstey, M.R.; Viswanathan, V.V.; Perry, M.L. Redox flow batteries: An engineering perspective. Proc. IEEE 2014, 102, 976–999. [Google Scholar] [CrossRef]
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Wainright, J.S.; Zawodzinski, T.A.; Savinell, R.F. Rechargeable redox flow batteries: Flow fields, stacks and design considerations. Chem. Soc. Rev. 2018, 47, 8721–8743. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Kumar, S.; Jayanti, S. Stack design considerations for vanadium redox flow battery. INAE Lett. 2018, 3, 149–157. [Google Scholar] [CrossRef]
- Moro, F.; Trovò, A.; Bortolin, S.; Del Col, D.; Guarnieri, M. An alternative low-loss stack topology for vanadium redox flow battery: Comparative assessment. J. Power Sources 2017, 340, 229–241. [Google Scholar] [CrossRef]
- Gurieff, N.; Cheung, C.; Timchenko, V.; Menictas, C. Performance enhancing stack geometry concepts for redox flow battery systems with flow through electrodes. J. Energy Storage 2019, 22, 219–227. [Google Scholar] [CrossRef]
- Whitehead, A.; Rabbow, T.; Trampert, M.; Pokorny, P. Critical safety features of the vanadium redox flow battery. J. Power Sources 2017, 351, 1–7. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Case studies of operational failures of VRFB stacks, diagnosis and remedial actions. J. Energy Storage 2020, 33, 102078. [Google Scholar] [CrossRef]
- Schreiber, M.; Harrer, M.; Whitehead, A.; Bucsich, H.; Dragschitz, M.; Seifert, E.; Tymciw, P. Practical and commercial issues in the design and manufacture of vanadium flow batteries. J. Power Sources 2012, 206, 483–489. [Google Scholar] [CrossRef]
- Minke, C.; Kunz, U.; Turek, T. Techno-economic assessment of novel vanadium redox flow batteries with large-area cells. J. Power Sources 2017, 361, 105–114. [Google Scholar] [CrossRef]
- Li, M.-J.; Zhao, W.; Chen, X.; Tao, W.-Q. Economic analysis of a new class of vanadium redox-flow battery for medium- and large-scale energy storage in commercial applications with renewable energy. Appl. Therm. Eng. 2017, 114, 802–814. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Clement, J.T.; Veith, G.M.; Zawodzinski, T.A.; Mench, M.M. High performance electrodes in vanadium redox flow batteries through oxygen-enriched thermal activation. J. Power Sources 2015, 294, 333–338. [Google Scholar] [CrossRef]
- Monteiro, R.; Leirós, J.; Boaventura, M.; Mendes, A. Insights into all-vanadium redox flow battery: A case study on components and operational conditions. Electrochim. Acta 2018, 267, 80–93. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.; Jiang, Z.; Yang, W. Experimental study on the performance of a vanadium redox flow battery with non-uniformly compressed carbon felt electrode. Appl. Energy 2018, 213, 293–305. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lv, Y.; Li, Y.; Zhou, X. Binder-free carbon nano-network wrapped carbon felt with optimized heteroatom doping for vanadium redox flow batteries. J. Mater. Chem. A 2019, 7, 25132. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Lv, Y.; Lin, L.; Wu, Q. Nano-catalytic layer engraved carbon felt via copper oxide etching for vanadium redox flow batteries. Carbon 2019, 153, 674–681. [Google Scholar] [CrossRef]
- Wu, Q.; Lv, Y.; Lin, L.; Zhang, X.; Liu, Y.; Zhou, X. An improved thin-film electrode for vanadium redox flow batteries enabled by a dual layered structure. J. Power Sources 2019, 410–411, 152–161. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, J.; Wei, L.; Wu, M.; Shyy, W.; Zhao, T. A high power density and long cycle life vanadium redox flow battery. Energy Storage Mater. 2020, 24, 529–540. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, L.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. Effectively suppressing vanadium permeation in vanadium redox flow battery application with modified Nafion membrane with nacre-like nanoarchitectures. J. Power Sources 2017, 352, 111–117. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.; Zhang, C. Performance of a vanadium redox flow battery with and without flow fields. Electrochim. Acta 2014, 142, 61–67. [Google Scholar] [CrossRef]
- Jyothi Latha, T.; Jayanti, S. Hydrodynamic analysis of flow fields for redox flow battery applications. J. Appl. Electrochem. 2014, 44, 995–1006. [Google Scholar] [CrossRef]
- Knudsen, E.; Albertus, P.; Cho, K.; Weber, A.; Kojic, A. Flow simulation and analysis of high-power flow batteries. J. Power Sources 2015, 299, 617–628. [Google Scholar] [CrossRef]
- Kumar, S.; Jayanti, S. Effect of flow field on the performance of an all-vanadium redox flow battery. J. Power Sources 2016, 307, 782–787. [Google Scholar] [CrossRef]
- Kumar, S.; Jayanti, S. High energy efficiency with low-pressure drop configuration for an all-vanadium redox flow battery. J. Electrochem. Energy Convers. Storage 2016, 13, 041005. [Google Scholar] [CrossRef]
- You, X.; Ye, Q.; Cheng, P. Scale-up of high power density redox flow batteries by introducing interdigitated flow fields. Int. Commun. Heat Mass Transf. 2016, 75, 7–12. [Google Scholar] [CrossRef]
- Bhattarai, A.; Wai, N.; Schweiss, R.; Whitehead, A.; Lim, T.M.; Hng, H.H. Advanced porous electrodes with flow channels for vanadium redox flow battery. J. Power Sources 2017, 341, 83–90. [Google Scholar] [CrossRef]
- Maurya, S.; Nguyen, P.T.; Kim, Y.S.; Kang, Q.; Mukundan, R. Effect of flow field geometry on operating current density, capacity and performance of vanadium redox flow battery. J. Power Sources 2018, 404, 20–27. [Google Scholar] [CrossRef]
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Savinell, R.F. Redox flow batteries with serpentine flow fields: Distributions of electrolyte flow reactant penetration into the porous carbon electrodes and effects on performance. J. Power Sources 2018, 384, 295–302. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, F.; Lu, F.; Zhou, X.; Yuan, Y.; Cao, X.; Xiang, B. A hierarchical interdigitated flow field design for scale-up of high-performance redox flow batteries. Appl. Energy 2019, 238, 435–441. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, M.; Yang, Z.; Yu, Z. Flow field design pathways from lab-scale toward large-scale flow batteries. Energy 2019, 173, 637–646. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Effect of channel dimensions of serpentine flow fields on the performance of a vanadium redox flow battery. J. Energy Storage 2019, 23, 148–158. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Effect of electrode compression and operating parameters on the performance of large vanadium redox flow battery cells. J. Power Sources 2019, 427, 231–242. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Effect of electrolyte convection velocity in the electrode on the performance of vanadium redox flow battery cells with serpentine flow fields. J. Energy Storage 2020, 30, 101516. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Performance characteristics of several variants of interdigitated flow fields for flow battery applications. J. Power Sources 2020, 467, 228225. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Dataset on performance of large-scale vanadium redox flow batteries with serpentine flow fields. Data Brief 2021, 35, 106835. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.; Thomsen, E.; Li, B.; Wang, W.; Nie, Z.; Koeppel, B.; Kizewski, J.; Sprenkle, V. Stack developments in a kW class all vanadium mixed acid redox flow battery at the pacific northwest national laboratory. J. Electrochem. Soc. 2016, 163, A5211–A5219. [Google Scholar] [CrossRef]
- Reed, D.; Thomsen, E.; Li, B.; Wang, W.; Nie, Z.; Koeppel, B.; Sprenkle, V. Performance of a low cost interdigitated flow design on a 1 kW class all vanadium mixed acid redox flow battery. J. Power Sources 2016, 306, 24–31. [Google Scholar] [CrossRef]
- Bhattarai, A.; Wai, N.; Schweiss, R.; Whitehead, A.; Scherer, G.G.; Ghimire, P.C.; Lim, T.M.; Hng, H.H. Vanadium redox flow battery with slotted porous electrodes and automatic rebalancing demonstrated on a 1 kW system level. Appl. Energy 2019, 236, 437–443. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.; Leung, P. Numerical investigations of flow field designs for vanadium redox flow batteries. Appl. Energy 2013, 105, 47–56. [Google Scholar] [CrossRef]
- Dennison, C.R.; Agar, E.; Akuzum, B.; Kumbur, E.C. Enhancing Mass Transport in Redox Flow Batteries by Tailoring Flow Field and Electrode Design. J. Electrochem. Soc. 2016, 163, A5163–A5169. [Google Scholar] [CrossRef]
- Park, S.-K.; Shim, J.; Yang, J.H.; Jin, C.-S.; Lee, B.S.; Lee, Y.-S.; Shin, K.-H.; Jeon, J.-D. The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery. Electrochim. Acta 2014, 116, 447–452. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, M. State of charge monitoring methods for vanadium redox flow battery control. J. Power Sources 2011, 196, 8822–8827. [Google Scholar] [CrossRef]
- Kim, J.; Park, H. Electrokinetic parameters of a vanadium redox flow battery with varying temperature and electrolyte flow rate. Renew. Energy 2019, 138, 284–291. [Google Scholar] [CrossRef]
- Jayanti, S.; Gundlapalli, R.; Chetty, R.; Jeevandoss, C.R.; Ramanujam, K.; Monder, D.S.; Rengaswamy, R.; Suresh, P.V.; Swarup, K.S.; Varadaraju, U.V.; et al. Characteristics of an indigenously developed 1 kW vanadium redox flow battery stack. In Proceedings of the 7th International Conference on Advances in Energy Research. Springer Proceedings in Energy; Bose, M., Modi., A., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Zhang, C.; Zhao, T.; Xu, Q.; An, L.; Zhao, G. Effects of operating temperature on the performance of vanadium redox flow batteries. Appl. Energy 2015, 155, 349–353. [Google Scholar] [CrossRef]
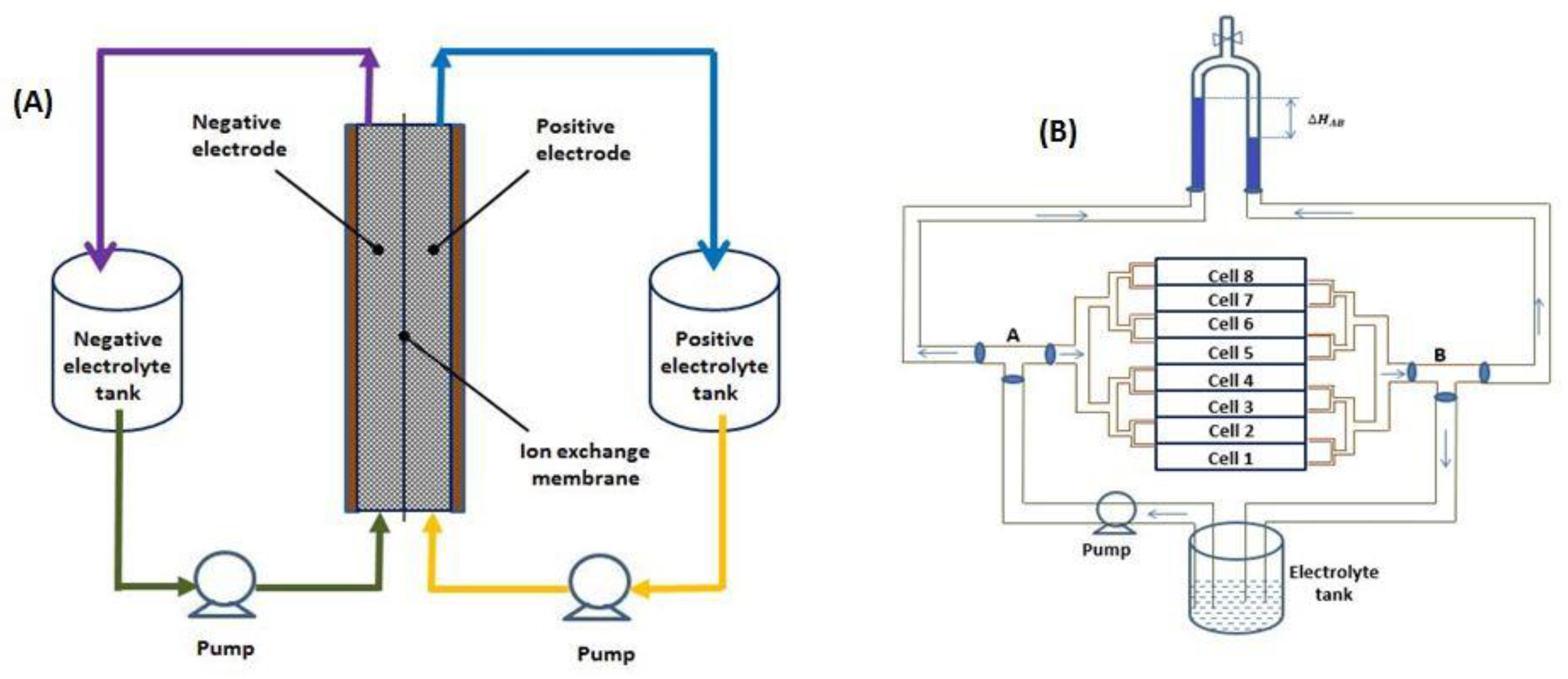


| Component | Description |
|---|---|
| Electrode | Graphite felt (GFA 6EA SGL Carbon) |
| Electrolyte | 1.61 M Vanadyl sulfate in 5 M Sulfuric acid |
| Membrane | Nafion 117 |
| Gasket | Silicone |
| Tubing | Silicone, Viton and Tygon |
| Graphite plate | SGL Carbon (R7650), Thickness-1.5 cm |
| Current collector | Copper pates of thickness 3 mm |
| Diameter (cm) of manifold components | T1-19.05, T2-12.7, T3-9.53 and T4-6 E1-12.7, E2-7.4, E3-4.5 and E4-3.5 (E-Elbow, T-split) |
| Preliminary Stacks | |||
|---|---|---|---|
| Average Power Rating (kW) | Number of Cells | Electrode Area (cm2) | Volume of Electrolyte (L) |
| 0.25 | 4 | 410 (20.5 × 20) | 1 |
| 0.50 | 4 | 918 (30.6 × 30) | 2.25 |
| Kilowatt-scale stacks | |||
| 1 | 16 | 410 (20.5 × 20) | 8 |
| 1 | 8 | 918 (30.6 × 30) | 8 |
| 2 | 8 | 1500 (30 × 50) | 8 |
| Stack | 16 Cell—410 cm2 | 8 Cell—918 cm2 | 8 Cell—1500 cm2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J (mA/cm2) | Flow Rate (mL/min/cm2) | CE (%) | VE (%) | Flow Rate (mL/min/cm2) | CE (%) | VE (%) | Flow Rate (mL/min/cm2) | CE (%) | VE (%) |
| 60 | 0.24 | 97.2 | 78.2 | 0.24 | 95.4 | 78.0 | 0.12 | 93.9 | 75.0 |
| 60 | 0.30 | 97.1 | 80.2 | 0.30 | 96.7 | 79.8 | 0.18 | 95.9 | 80.4 |
| 60 | 0.42 | 95.2 | 82.7 | 0.36 | 95.6 | 81.8 | 0.24 | 95.1 | 83.6 |
| 60 | 0.54 | 95.1 | 83.1 | 0.42 | 96.2 | 80.8 | 0.30 | 95.6 | 82.3 |
| 75 | 0.30 | 97.1 | 75.1 | 0.30 | 95.8 | 76.7 | 0.15 | 95.7 | 74.5 |
| 75 | 0.38 | 97.0 | 77.1 | 0.37 | 96.0 | 79.8 | 0.22 | 95.5 | 79.4 |
| 75 | 0.53 | 96.3 | 80.1 | 0.45 | 96.5 | 80.0 | 0.30 | 96.7 | 78.8 |
| 75 | 0.68 | 96.4 | 80.5 | ||||||
| 90 | 0.36 | 97.7 | 73.0 | 0.18 | 97.3 | 73.6 | |||
| 90 | 0.45 | 97.0 | 75.5 | 0.27 | 97.1 | 76.2 | |||
| 90 | 0.63 | 96.9 | 77.8 | 0.36 | 97.5 | 77.0 | |||
| 90 | 0.81 | 96.6 | 77.8 | ||||||
| Stack Configuration | Power Rating (kW) | Optimal Flow Rate (mL/min/cm2) | Pressure Drop (kPa) | Coulombic Efficiency (%) | Energy Efficiency (%) | Discharge | ||
|---|---|---|---|---|---|---|---|---|
| Without Pump | With Pump | Capacity (Ah/L) | Energy (Wh/L) | |||||
| 16 cell—410 cm2 | 1 | 0.54 | 51.6 | 94.8 | 79.8 | 76.7 | 26.3 | 32.8 |
| 8 cell—918 cm2 | 1 | 0.36 | 39.4 | 95.6 | 78.2 | 76.6 | 25.8 | 33.4 |
| 8 cell—1500 cm2 | 2 | 0.24 | 38.7 | 95.1 | 79.5 | 78.5 | 25.6 | 33.0 |
| Configuration | Cell Area (cm2) | ASR from Polarization Data | ASR from Voltage at 50% Capacity |
|---|---|---|---|
| Single-cell studies | 410 | 2.08 | 1.83 |
| 918 | 2.32 | 2.08 | |
| 1500 | 2.41 | 2.25 | |
| Stack-level studies | 4 cell—410 cm2 | 1.90 | 1.95 |
| 4 cell—918 cm2 | 1.86 | 2.08 | |
| 16 cell—410 cm2 | 2.17 | 1.93 | |
| 8 cell—918 cm2 | 2.14 | 2.05 | |
| 8 cell—1500 cm2 | 2.10 | 2.02 |
| Stack Configuration | Flow Field | Operating Flow Rate (mL/min/cm2) | Total Pressure Drop (kPa) | Current Density (mA/cm2) | Discharge Capacity (Ah/L) | EE (%) | System Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 15 Cell—875 cm2 [9] | No | Constant-0.90 | 75 | 18.4 | 77.9 | 58.5 | |
| Step-up flow rate | 18.4 | 73.7 | 66.5 | ||||
| 40 Cell—1500 cm2 [10] | No | Constant-0.60 | 108 | 50 | 76.0 | 69.0 | |
| Variable | 76.0 | 73.0 | |||||
| 31 Cell—2714 cm2 [11] | No | Constant-1.0 | 60 | 76.0 | |||
| 40 Cell—1800 cm2 [12] | No | Constant-0.70 | 154 (Cell level) | 50 | 29.6 | 82.8 | |
| 80 | 26.0 | 78.4 | |||||
| 40 Cell—1800 cm2 [13] | No | Constant-0.21 | 75 | 73.7 | 64.0 | ||
| Variable | 73.7 | 65.5 | |||||
| 40 Cell—600 cm2 [16,17,18] | No | Constant-0.63 | 50 | 24.5 | 75.8 | 70.3 | |
| Variable | 68.3 | ||||||
| 3 Cell—780 cm2 [61] * | Interdigitated on bipolar plate | Constant-0.51 | 15 | 160 | 17.5 | 78.0 | |
| 20 Cell—625 cm2 [62] * | Interdigitated on electrode | Constant-0.64 | 47 (Cell level #) | 80 | 29.0 | 80.0 | |
| 8 Cell—1500 cm2 (Present) | Serpentine on bipolar plate | Constant-0.30 | 49 | 60 | 25.5 | 78.6 | 76.9 |
| 90 | 22.5 | 72.6 | 71.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundlapalli, R.; Jayanti, S. Comparative Study of Kilowatt-Scale Vanadium Redox Flow Battery Stacks Designed with Serpentine Flow Fields and Split Manifolds. Batteries 2021, 7, 30. https://doi.org/10.3390/batteries7020030
Gundlapalli R, Jayanti S. Comparative Study of Kilowatt-Scale Vanadium Redox Flow Battery Stacks Designed with Serpentine Flow Fields and Split Manifolds. Batteries. 2021; 7(2):30. https://doi.org/10.3390/batteries7020030
Chicago/Turabian StyleGundlapalli, Ravendra, and Sreenivas Jayanti. 2021. "Comparative Study of Kilowatt-Scale Vanadium Redox Flow Battery Stacks Designed with Serpentine Flow Fields and Split Manifolds" Batteries 7, no. 2: 30. https://doi.org/10.3390/batteries7020030
APA StyleGundlapalli, R., & Jayanti, S. (2021). Comparative Study of Kilowatt-Scale Vanadium Redox Flow Battery Stacks Designed with Serpentine Flow Fields and Split Manifolds. Batteries, 7(2), 30. https://doi.org/10.3390/batteries7020030







