High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes
Abstract
1. Introduction
2. Results and Discussion
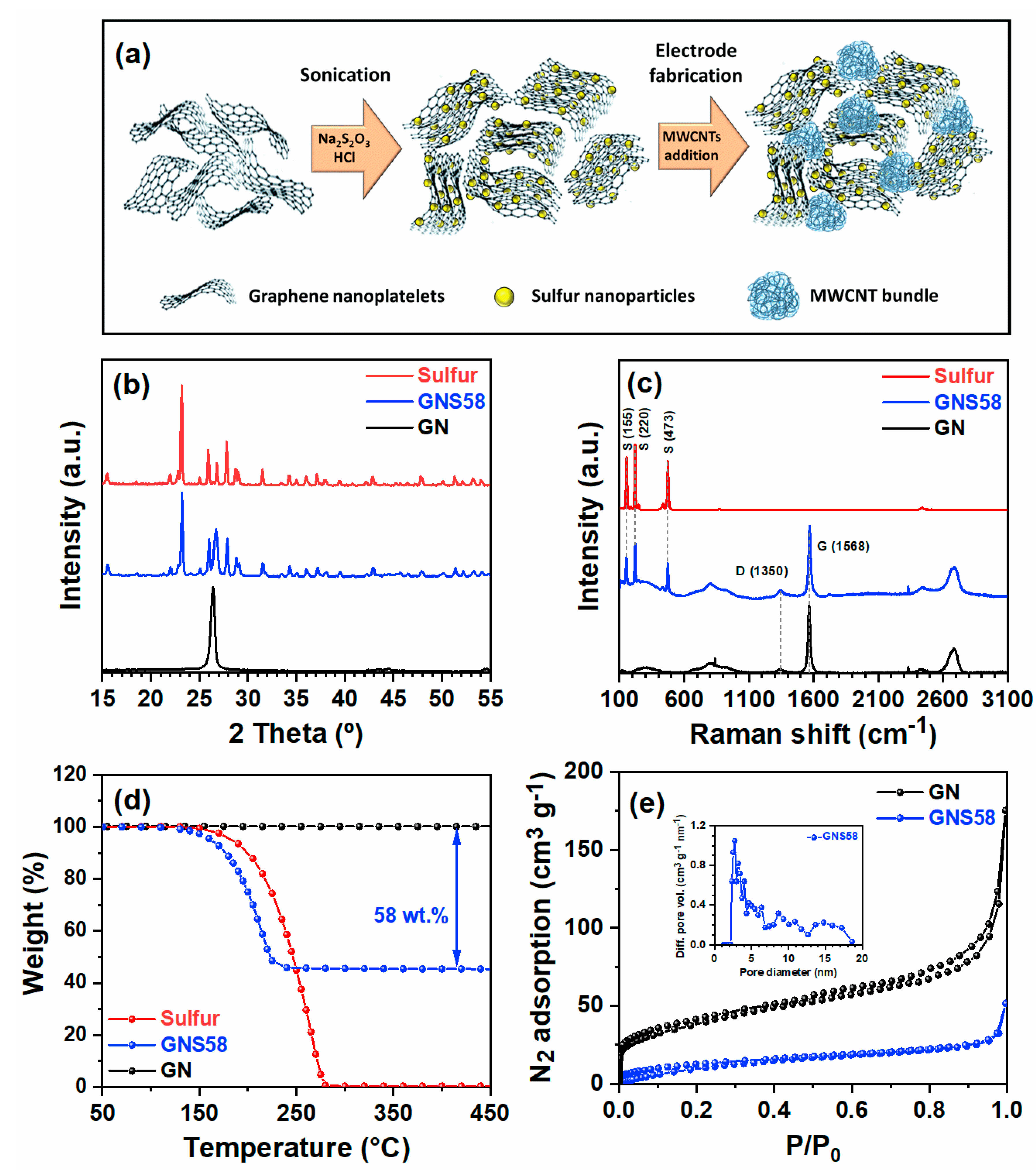
2.1. Synthesis and Characterization of Graphene-CNT-Nanosulfur Hybrid Cathode
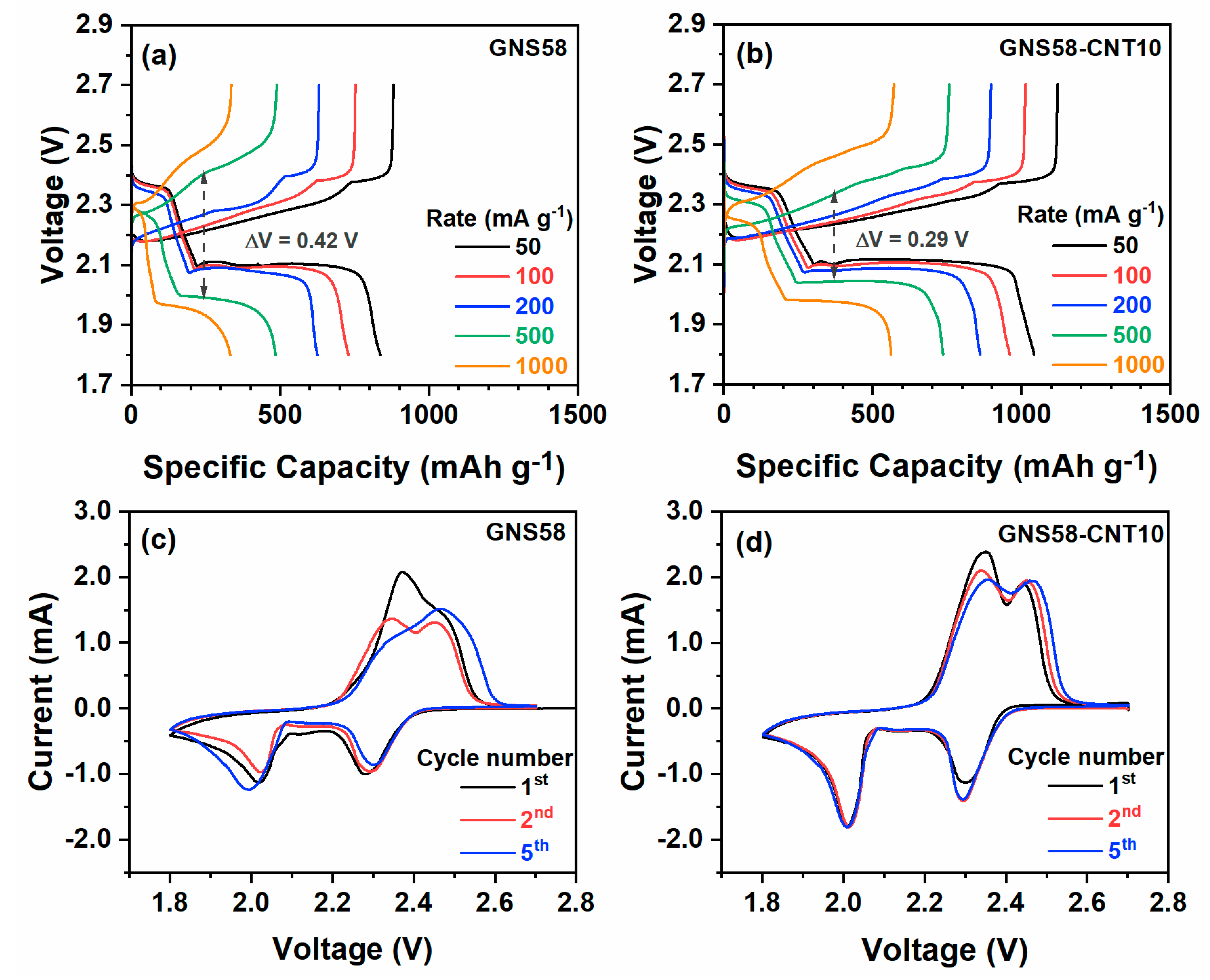
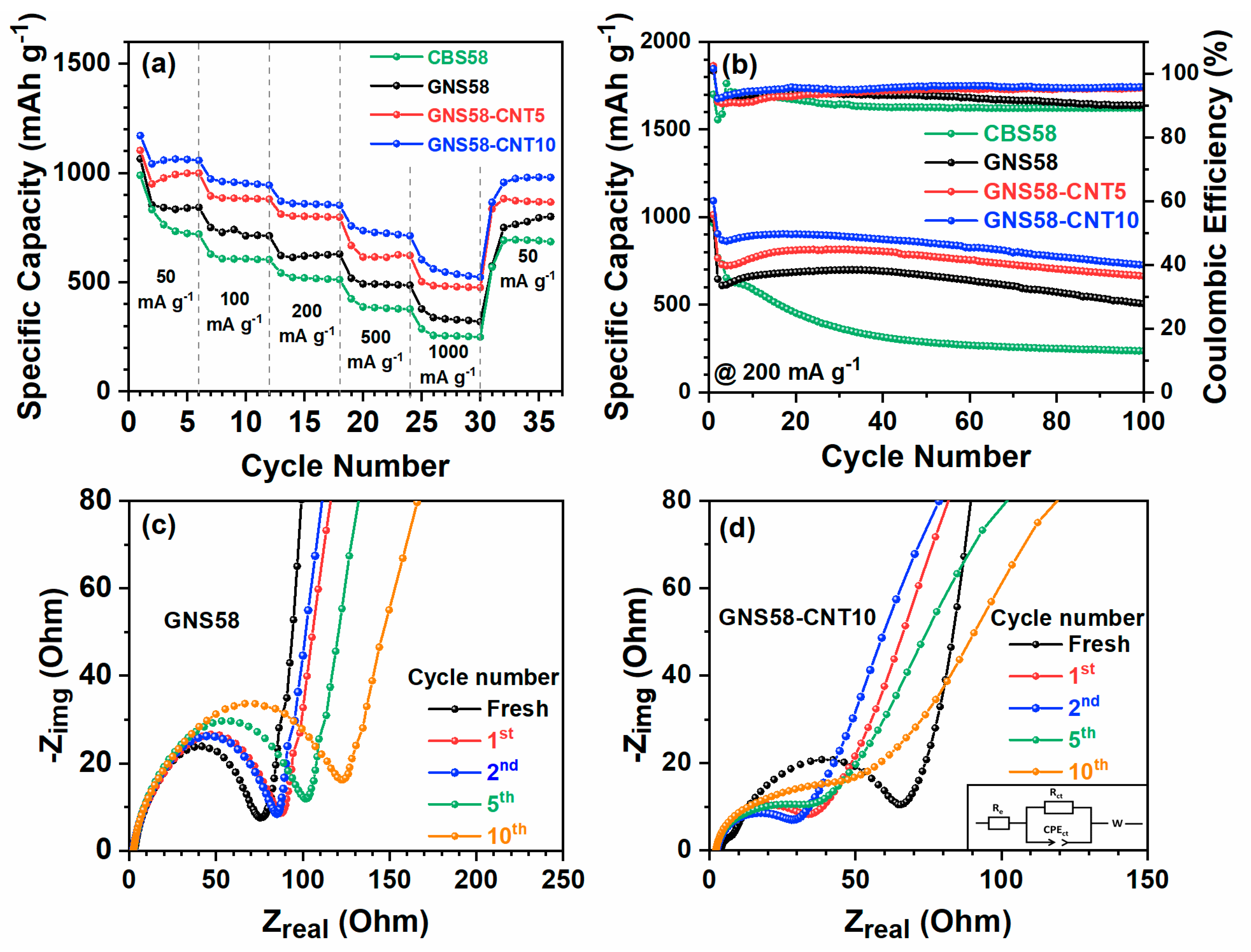
2.2. Electrochemical Performance Evaluation of Hybrid Cathode
2.3. Post-Cycling Analysis of Hybrid Cathode
3. Materials and Methods
3.1. Material Synthesis
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, S.C.N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Wild, M. A review on electric vehicle battery modelling: From Lithium-ion toward Lithium–Sulphur. Renew. Sustain. Energy Rev. 2016, 56, 1008–1021. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, e1800561. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nat. Cell Biol. 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Bini, M.; Capsoni, D.; Ferrari, S.; Quartarone, E.; Mustarelli, P. Rechargeable lithium batteries: Key scientific and technological challenges. In Rechargeable Lithium Batteries from Fundamentals to Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–17. [Google Scholar]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Accounts Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium-Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.-H.; Zu, C. Lithium-Sulfur Batteries: Progress and Prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Haag, M.R. Handbook of Chemistry, 3rd ed.; McGraw-Hill: New York, NY, USA, 1950. [Google Scholar]
- Chen, L.; Shaw, L.L. Recent advances in lithium–sulfur batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-Sulfur Battery Systems: An Integral Perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Su, Y.-S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef]
- Suo, L.; Hu, Y.-S.; Li, H.; Armand, M.; Chen, L. A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Cai, W.; Wang, C.; Li, G.; Li, Z.P.; Mao, W.; Qian, Y. A novel class of functional additives for cyclability enhancement of the sulfur cathode in lithium sulfur batteries. Inorg. Chem. Front. 2018, 5, 2013–2017. [Google Scholar] [CrossRef]
- Hong, C.N.; Kye, D.K.; Mane, A.U.; Elam, J.W.; Etacheri, V.; Pol, V.G. Blocking Polysulfides in Graphene–Sulfur Cathodes of Lithium–Sulfur Batteries through Atomic Layer Deposition of Alumina. Energy Technol. 2019, 7, 1900621. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, S.; Tao, D.; Wang, M. Sulfur/Co3O4 nanotube composite with high performances as cathode materials for lithium sulfur batteries. Funct. Mater. Lett. 2014, 7, 1450020. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2on a nitrogen-doped graphene/sulfur electrode for high performance lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Ding, B.; Yuan, C.; Shen, L.; Xu, G.; Nie, P.; Zhang, X. Encapsulating Sulfur into Hierarchically Ordered Porous Carbon as a High-Performance Cathode for Lithium-Sulfur Batteries. Chem. A Eur. J. 2012, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, Q.; Tao, Z.; Chen, J. Composite of sulfur impregnated in porous hollow carbon spheres as the cathode of Li-S batteries with high performance. Nano Res. 2013, 6, 38–46. [Google Scholar] [CrossRef]
- Li, N.-W.; Yin, Y.-X.; Guo, Y.-G. Three-dimensional sandwich-type graphene@microporous carbon architecture for lithium–sulfur batteries. RSC Adv. 2015, 6, 617–622. [Google Scholar] [CrossRef]
- Wei, W.; Wang, J.; Zhou, L.; Yang, J.; Schumann, B.; Nuli, Y. CNT enhanced sulfur composite cathode material for high rate lithium battery. Electrochem. Commun. 2011, 13, 399–402. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. Graphene-enveloped sulfur in a one pot reaction: A cathode with good coulombic efficiency and high practical sulfur content. Chem. Commun. 2012, 48, 1233–1235. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Jozwiuk, A.; Sommer, H.; Janek, J.; Brezesinski, T. Fair performance comparison of different carbon blacks in lithium–sulfur batteries with practical mass loadings—Simple design competes with complex cathode architecture. J. Power Sources 2015, 296, 454–461. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, C.; Zhou, Z.; Gao, X. Preparation and electrochemical properties of sulfur–acetylene black composites as cathode materials. Electrochim. Acta 2009, 54, 3708–3713. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Qi, W.; Saraf, L.V.; Xiao, J.; Nie, Z.; Mietek, J.; Zhang, J.-G.; Schwenzer, B.; Liu, J. Optimization of mesoporous carbon structures for lithium–sulfur battery applications. J. Mater. Chem. 2011, 21, 16603–16610. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Liu, X.-F.; Zhang, Q.; Chen, C.-M.; Zhao, M.-Q.; Zhang, S.-M.; Zhu, W.; Qian, W.-Z.; Wei, F. Entrapment of sulfur in hierarchical porous graphene for lithium–sulfur batteries with high rate performance from −40 to 60 °C. Nano Energy 2013, 2, 314–321. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Lee, J.H.; Koo, H.Y.; Shakoor, R.A.; Kahraman, R.; Jo, Y.N.; Park, M.-S.; Choi, J.W. Hierarchical Porous Carbon by Ultrasonic Spray Pyrolysis Yields Stable Cycling in Lithium–Sulfur Battery. Nano Lett. 2014, 14, 4418–4425. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Zhang, C.; Bin Wu, H.; Yuan, C.; Guo, Z.; Lou, X.W. (David). Confining Sulfur in Double-Shelled Hollow Carbon Spheres for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2012, 51, 9592–9595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.-W.; Li, F.; Hou, P.-X.; Yin, L.; Liu, C.; Lu, G.Q.; Gentle, I.R.; Cheng, H.-M. A flexible nanostructured sulphur–carbon nanotube cathode with high rate performance for Li-S batteries. Energy Environ. Sci. 2012, 5, 8901–8906. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Zhao, M.-Q.; Wei, F. Aligned carbon nanotube/sulfur composite cathodes with high sulfur content for lithium–sulfur batteries. Nano Energy 2014, 4, 65–72. [Google Scholar] [CrossRef]
- Han, S.-C.; Song, M.-S.; Lee, H.; Kim, H.-S.; Ahn, H.-J.; Lee, J.-Y. Effect of Multiwalled Carbon Nanotubes on Electrochemical Properties of Lithium/Sulfur Rechargeable Batteries. J. Electrochem. Soc. 2003, 150, A889–A893. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, H.; Qiu, X.; Chen, L.; Zhu, W. Improvement of cycle property of sulfur-coated multi-walled carbon nanotubes composite cathode for lithium/sulfur batteries. J. Power Sources 2009, 189, 1141–1146. [Google Scholar] [CrossRef]
- Zhou, G.; Pei, S.; Li, L.; Wang, D.-W.; Wang, S.; Huang, K.; Yin, L.-C.; Li, F.; Cheng, H.-M. A Graphene-Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Lu, L.; Choucair, M.; Stride, J.A.; Xu, X.; Liu, H.-K. Sulfur-graphene composite for rechargeable lithium batteries. J. Power Sources 2011, 196, 7030–7034. [Google Scholar] [CrossRef]
- Park, M.-S.; Yu, J.-S.; Kim, K.J.; Jeong, G.; Kim, J.-H.; Jo, Y.-N.; Hwang, U.; Kang, S.; Woo, T.; Kim, Y.-J. One-step synthesis of a sulfur-impregnated graphene cathode for lithium–sulfur batteries. Phys. Chem. Chem. Phys. 2012, 14, 6796–6804. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium–Sulfur Battery Cathode Material with High Capacity and Cycling Stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef]
- Kumar, G.G.; Chung, S.-H.; Kumar, T.R.; Manthiram, A. Three-Dimensional Graphene–Carbon Nanotube–Ni Hierarchical Architecture as a Polysulfide Trap for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 20627–20634. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, P.; Fu, F.; Wang, Z.; Zhang, W. Three-dimensional CNT/graphene–sulfur hybrid sponges with high sulfur loading as superior-capacity cathodes for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 18605–18610. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q.; Zhao, M.-Q.; Huang, J.-Q.; Cheng, X.-B.; Tian, G.-L.; Peng, H.-J.; Wei, F. Nitrogen-Doped Aligned Carbon Nanotube/Graphene Sandwiches: Facile Catalytic Growth on Bifunctional Natural Catalysts and Their Applications as Scaffolds for High-Rate Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 6100–6105. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Zhao, M.-Q.; Zhang, Q.; Cheng, X.-B.; Liu, X.-Y.; Qian, W.-Z.; Wei, F. Nanoarchitectured Graphene/CNT@Porous Carbon with Extraordinary Electrical Conductivity and Interconnected Micro/Mesopores for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 2772–2781. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Liu, X.-F.; Zhang, Q.; Tian, G.-L.; Huang, J.-Q.; Zhu, W.; Wei, F. Graphene/Single-Walled Carbon Nanotube Hybrids: One-Step Catalytic Growth and Applications for High-Rate Li–S Batteries. ACS Nano 2012, 6, 10759–10769. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Zhao, Z.; Niu, J.; Xia, Z. Synthesis, properties and applications of 3D carbon nanotube–graphene junctions. J. Phys. D Appl. Phys. 2016, 49, 443001. [Google Scholar] [CrossRef]
- Gao, F. Graphene/Carbon Nanotubes Composite as a Polysulfide Trap for Lithium-Sulfur Batteries. Int. J. Electrochem. Sci. 2019, 14, 3301–3314. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Yuan, L.; Hao, Z.; Huang, Y. Status and prospects in sulfur–carbon composites as cathode materials for rechargeable lithium–sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Cao, Y.; Li, M.; Liu, J.; Amine, K. Bridging the academic and industrial metrics for next-generation practical batteries. Nat. Nanotechnol. 2019, 14, 200–207. [Google Scholar] [CrossRef]
- Hagen, M.; Dörfler, S.; Fanz, P.; Berger, T.; Speck, R.; Tübke, J.; Althues, H.; Hoffmann, M.; Scherr, C.; Kaskel, S. Development and costs calculation of lithium–sulfur cells with high sulfur load and binder free electrodes. J. Power Sources 2013, 224, 260–268. [Google Scholar] [CrossRef]
- Weng, W.; Pol, V.G.; Amine, K. Ultrasound Assisted Design of Sulfur/Carbon Cathodes with Partially Fluorinated Ether Electrolytes for Highly Efficient Li/S Batteries. Adv. Mater. 2013, 25, 1608–1615. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- McNamara, W.B.; Didenko, Y.T.; Suslick, K.S. Sonoluminescence temperatures during multi-bubble cavitation. Nat. Cell Biol. 1999, 401, 772–775. [Google Scholar] [CrossRef]
- Awati, P.; Awate, S.; Shah, P.; Ramaswamy, V. Photocatalytic decomposition of methylene blue using nanocrystalline anatase titania prepared by ultrasonic technique. Catal. Commun. 2003, 4, 393–400. [Google Scholar] [CrossRef]
- Dysart, A.D.; Burgos, J.C.; Mistry, A.; Chen, C.-F.; Liu, Z.; Hong, C.N.; Balbuena, P.B.; Mukherjee, P.P.; Pol, V.G. Towards Next Generation Lithium-Sulfur Batteries: Non-Conventional Carbon Compartments/Sulfur Electrodes and Multi-Scale Analysis. J. Electrochem. Soc. 2016, 163, A730–A741. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode–Electrolyte Interfaces in Lithium–Sulfur Batteries with Liquid or Inorganic Solid Electrolytes. Accounts Chem. Res. 2017, 50, 2653–2660. [Google Scholar] [CrossRef]
- Yu, M.; Li, R.; Wu, M.; Shi, G. Graphene materials for lithium–sulfur batteries. Energy Storage Mater. 2015, 1, 51–73. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, G.; Wang, H.; Bai, J. A novel three-dimensional sulfur/graphene/carbon nanotube composite prepared by a hydrothermal co-assembling route as binder-free cathode for lithium–sulfur batteries. J. Nanoparticle Res. 2015, 17, 36. [Google Scholar] [CrossRef]
- Papandrea, B.; Xu, X.; Xu, Y.; Chen, C.-Y.; Lin, Z.; Wang, G.; Luo, Y.; Liu, M.; Huang, Y.; Mai, L.; et al. Three-dimensional graphene framework with ultra-high sulfur content for a robust lithium–sulfur battery. Nano Res. 2016, 9, 240–248. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Wu, X.; Liu, J. Flexible Carbon Nanotube–Graphene/Sulfur Composite Film: Free-Standing Cathode for High-Performance Lithium/Sulfur Batteries. J. Phys. Chem. C 2015, 119, 10288–10294. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, L.-L.; Liu, S.; Li, G.-R.; Gao, X.-P. A High-Efficiency Sulfur/Carbon Composite Based on 3D Graphene Nanosheet@Carbon Nanotube Matrix as Cathode for Lithium-Sulfur Battery. Adv. Energy Mater. 2017, 7, 1602543. [Google Scholar] [CrossRef]
- Rehman, S.; Khan, K.; Zhao, Y.; Hou, Y. Nanostructured cathode materials for lithium–sulfur batteries: Progress, challenges and perspectives. J. Mater. Chem. A 2017, 5, 3014–3038. [Google Scholar] [CrossRef]
- Warren, B.E.; Burwell, J.T. The Structure of Rhombic Sulphur. J. Chem. Phys. 1935, 3, 6–8. [Google Scholar] [CrossRef]
- Iijima, S. Electron Microscopy of Small Particles. QJM Int. J. Med. 1985, 34, 249–265. [Google Scholar] [CrossRef]
- Siegel, R.W.; Trigg, G.L. Nanophase Materials. In Encyclopedia of Applied Physics; Trigg, G.L., Ed.; Wiley-VCH: Weinheim, Germany, 1994; pp. 1–27. [Google Scholar]
- Popova, A.N. Crystallographic analysis of graphite by X-Ray diffraction. Coke Chem. 2017, 60, 361–365. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Res. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Ward, A.T. Raman spectroscopy of sulfur, sulfur-selenium, and sulfur-arsenic mixtures. J. Phys. Chem. 1968, 72, 4133–4139. [Google Scholar] [CrossRef]
- Sing, K.S. Characterization of porous solids: An introductory survey. Adv. Pharmacol. 1991, 62, 1–9. [Google Scholar] [CrossRef]
- Fan, X.; Sun, W.; Meng, F.; Xing, A.; Liu, J. Advanced chemical strategies for lithium–sulfur batteries: A review. Green Energy Environ. 2018, 3, 2–19. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, W.; Li, X.; Zhuang, J.; Zhou, H.; Wang, H. High-performance Li–S battery cathode with catalyst-like carbon nanotube-MoP promoting polysulfide redox. Nano Res. 2017, 10, 3698–3705. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doñoro, Á.; Muñoz-Mauricio, Á.; Etacheri, V. High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries 2021, 7, 26. https://doi.org/10.3390/batteries7020026
Doñoro Á, Muñoz-Mauricio Á, Etacheri V. High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries. 2021; 7(2):26. https://doi.org/10.3390/batteries7020026
Chicago/Turabian StyleDoñoro, Álvaro, Álvaro Muñoz-Mauricio, and Vinodkumar Etacheri. 2021. "High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes" Batteries 7, no. 2: 26. https://doi.org/10.3390/batteries7020026
APA StyleDoñoro, Á., Muñoz-Mauricio, Á., & Etacheri, V. (2021). High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries, 7(2), 26. https://doi.org/10.3390/batteries7020026






