1. Introduction
Aging investigations on Li-ion cells are time and cost intensive. The rate of aging depends on many factors, such as electrode materials; electrolyte composition; cell geometry; and the operational conditions SOC, C-rate, depth of discharge (DOD), and temperature. Thus, for each specific Li-ion cell type, many tests have to be carried out in order to create and parametrize a cell model, which is able to predict the aging behavior of the Li-ion cell. Therefore, electrode-specific aging studies may be helpful for developing more accurate aging models. By using the three-electrode-cells PAT-Cells by EL-CELL GmbH (hereinafter referred to as coin cell), the electrode potentials can be measured during cell testing and serve as additional information to understand the aging behavior.
Cycling the coin cells may reveal additional aging information; e.g., the influence of geometry on the cell performance: volume expansion during intercalation can cause stress in bent electrode layers in Li-ion cells [
1] and the current distribution over the planar electrode area can be influenced by the current tabs positions, especially at higher C-rates [
2,
3]. In contrast, the electrodes used in PAT-Cells are not bent and the current collector contacted to the back of the electrodes should ensure a homogeneous current distribution. The PAT-Cells are assumed to be isothermal due to their limited electrode area and their thermal capacity. A more generalized, geometry-independent cell model, applicable to a wide field of cells of the same chemistry, might lower the needed number of measurements to adjust the battery model in the future. This work compares the aging behavior of the original Li-ion cell with the coin cells and is therefore a step towards this future goal.
Moreover, high-capacity Li-ion cells need a sophisticated and thus expensive experimental setup which is capable of providing high currents conforming to the safety regulations. As an alternative, aging the cell material in coin cells can tremendously lower both the required current and the safety regulations. Since only a small electrode area is required for building a coin cell, many coin cells can be assembled out of one original Li-ion cell. Hence, aging tests could be highly parallelized.
A prerequisite for this coin cell approach is a reproducible and minimum invasive extraction procedure. Our preparation method demonstrates a high reproducibility with a coefficient of variation in capacity of
of 62 reassembled coin cells at beginning of life (BOL) [
4]. The electrodes have not been washed during preparation [
4] so that the electrolyte of the built coin cells is as identical as possible to the original electrolyte. Further information about our preparation and other extraction concepts is given in our previous study [
4].
The aging process of Li-ion cells can have many causes [
5,
6]. NMC-based cathode materials can be degraded by structural deformation [
7,
8], manganese dissolution [
5,
9,
10,
11], corrosion of the current collector [
5,
9], the decomposition of the binder and the electrolyte, particle cracking, and the growth of a cathode interface layer [
12,
13,
14]. On the graphite (GR) side, SEI growth [
15,
16], particle cracking [
17,
18], Li-Plating [
19,
20,
21,
22], and the incorporation of Mn-Ions into the SEI [
5,
10,
23,
24] can take place. The assignment of each single cause of aging to a specific electrical response behavior of a Li-ion cell is almost impossible [
6]. Therefore, the causes which have similar electrical impacts on the cell are clustered together to loss of lithium inventory (LLI), loss of active material at the positive electrode (LAM
), and loss of active material at the negative (LAM
) electrode [
6,
25,
26]. For instance, LLI is an indication for SEI growth which is the main cause of calendar aging [
15,
16,
27,
28,
29] because Li is consumed to build up the SEI [
15]. Once the cell is cycled, the changes in the volume of the GR can lead to cracks in the SEI structure (LLI) [
17,
18] and to a minor extent in the particle (LAM
) [
5]. Loss of electrical contact to the particles and structural disordering (e.g. in the positive electrode) are the typical causes for LAM.
In order to compare the cell aging behavior of the commercial Li-ion cells and the coin cells, the cell aging mechanisms have to be identified. Several methods have been introduced to detect the aging mechanisms [
6,
25,
27,
30,
31,
32,
33]. Therefore, the characterization methods incremental capacity (IC), differential voltage (DV), maximum/minimum electrode potentials, electrochemical impedance spectroscopy (EIS), and pulse current measurements (PCM) are used and introduced in
Section 2. After the experimental part in
Section 3,
Section 4 compares the 1C cyclic aging behavior of commercially manufactured 40 Ah Li-ion pouch cells to their extracted coin cells at the three specified temperatures
C. In case of cycling at 1C, an aggravated capacity loss due to an inhomogeneous temperature distribution inside the 40 Ah cells can be neglected [
34]. Finally, the aging mechanisms of both cell types are estimated.
The coin cells used for comparing to the original cells have an identical anode to cathode planar area ratio
without any anode overhang which is different to the 40 Ah cells with
. The electrochemical performance of Li-ion cells is sensitive to the anode overhang [
35,
36,
37,
38]. Therefore, an additional experiment in
Section 5 is carried out to test the influence of the
factor on the aging performance of the coin cells.
Traces of moisture and oxygen can hardly be avoided in reusable coin cell constructions. But some contamination by water is tolerable in the cell assembly process [
39,
40]. The PF
from the dissociated LiPF
reacts with water the following way:
Kawamura et al. measured the reaction rates of LiPF
with water in an ethylene carbonate (EC):dimethyl carbonate (DMC) 50:50 vol.% solution at several temperatures [
41]. The reaction rate is mainly driven by the viscosity and the polarization of the electrolyte [
41]. The viscosities of DMC (
at
C) and ethyl methyl carbonate (EMC) (
at
C) and their relative permittivities (
and
) are similar [
42,
43]. Thus, it is possible to anticipate the reaction rate of water in the electrolyte used in this study (
in EC:EMC 30:70 wt.%). Therefore, another aging experiment in
Section 6 shows the influence of the entry of moisture on the capacity loss of the coin cells with the help of EDX measurements.
Finally, a path dependency aging test is conducted for both cell types in
Section 7. This investigation addresses the question of whether the capacity loss
, consisting of a calendar
and a cyclic aging part
at certain time intervals
and
,
is commutative. Two studies detected that the capacity loss during calendar aging is commutative [
44,
45]; however, the question arises of whether this independence of direction also applies to cyclic aging. A commutative aging behavior could lower the necessary number of measurements to predict the cell aging: If Equation (
3) was valid, one side of this Equation could be determined without any measurement. Hence, cost intensive measurement time would be lowered. Additionally, this investigation is used to further prove the transferability of the proposed coin cell aging method to commercial cells.
5. Anode Overhang
Table 8 presents the results of the two cell groups during formation.
A larger delithiated anode compared to the cathode results in a higher loading ratio
. Thus, the maximum and minimum anode potentials are shifted. Since the cell voltage as
is controlled during the experiment, a larger delithiated anode at cell assembly leads to a slightly higher cathode potential
at the end of charging the asymmetric cells. Additionally, the current density increases at the edge of the cathode due to the anode overhang [
36]. However, oxidation of the cathode should not happen in case of an anode overhang
[
36]. Only in the first cycle is the Coulomb efficiency (CE) of the asymmetric cells significantly lower: the larger anode area leads to a higher LLI to form the SEI, as seen by the lower discharge capacity for the asymmetric cells in
Table 8.
The coefficient of variation
is slightly higher for the symmetric cells than for the asymmetric cells. This result is contrary to the results of Long et al. [
64]. They described a higher reproducibility of building their 2032-type Hohsen coin cells by using a larger anode to avoid misaligned electrodes [
64]. The COVs of the two cell subgroups during formation were low (
; see
Table 8) compared to the
in the third cycle of the work in Long et al. [
64]. The PAT-Cell from EL-CELL GmbH used in this work enables precisely aligned electrodes even for
.
After subtracting the two worst performing cells in each group, the averaged capacity curves cannot be distinguished from each other as seen in
Figure 14.
Hence, the capacity degradation of the coin cells is not sensitive to the anode overhang within the range
for the specific coin cell structure. The distance between the electrodes, defined by the separator thickness, influences the impact of an anode overhang [
36]. In case of an asymmetric assembly (
;
), a larger distance between the anode and cathode can enlarge the charging time until Li plating occurs [
36]. The inhomogeneity in current density from the edge to the middle of the probe lowers due to the longer pathway of the Li-ions [
36]. However, the thicker separator
used in the coin cells minimally influences the aging results since we used an extension length of only
. Furthermore, the porosity of the coin cell separator is with
significantly higher compared to
of the original separator [
4]. The MacMullin number is directly linked to the porosity [
65]
[
66]. The MacMullin number should be minimized in order to optimize the high rate performance of the cell [
66]. The influence of the thicker separator used in the coin cells on the anode overhang results is therefore considered to be low.
7. Path Dependent Cyclic Aging
The capacity results of this test are summarized in
Figure 17a–d.
Figure 17a,b disproves the hypothesis of Equation (
3) and instead reveals a path dependency
for both cell types. There is a short increase in capacity when changing from calendar to cyclic aging, whereas in the opposite direction the C/3 capacity is dropping instantaneously. The sudden increases in
in
Figure 17c,d after calendar aging (CAL CYC) indicates a temporary recovery effect of Li during the first cycles for both cell types. In contrast, the phase widths
in the opposite mode (CYC CAL) decrease immediately after changing the mode from CYC to CAL.
The reason for this temporary recovery effect may be explained by the inhomogeneous lithiation in the GR electrodes: Concentration gradients in lithiation are balanced during relaxation periods [
67]. In general, electrode particles are unevenly distributed in the electrode. Some particles are in passive areas—for instance, particles in the anode overhang or particles connected to adjacent particles but not directly in contact with electrolyte—which means a lower current density of Li-ions at the particle interface due to higher over-potentials. Diffusion and migration processes compensate for the inhomogeneous lithiation over time. Relatively long diffusion times are needed to distribute the charges equally. The 40 Ah cells had an initial SOC of about 16% after shipping. It is assumed that the cells are homogeneously lithiated at BOL. Then, while cyclic aging at 1C, the Li stored in the passive electrode particles is not or is less-so electrochemically active. The cycle test procedure, as introduced in
Section 3.5, includes a break of
at 0% SOC after each
cycles to cool down the 40 Ah cell for the C/3 capacity test. This ensures similar temperatures between the 40 Ah cells and the coin cells and between the cyclic and calendar aging modes (mean 40 Ah cell temperatures during C/3 capacity tests at the set temperature
C:
C;
C). The LLI during cycling causes concentration gradients in Li distribution between active and passive particles. When the cell’s SOC is held at
for
after cyclic aging, the remaining higher concentration of Li-ions in the passive areas causes a flow of Li-ions to homogenize the electrode parts. This migration is relatively high at low SOCs due to the big differences of the GR open-circuit potential at low SOCs [
68]. This means a consumption of Li-ions from the passive areas during the cyclic aging procedure carried out in this work.
There is no need for such a relaxation period for the calendar aging procedure. The cells are immediately charged with C/3 after discharging with C/3 to 0% SOC. Between each C/3 reference cycle, the cells are aged for five days at
. These five days are not sufficient to homogenize the Li distribution. It takes approximately 400 days at
to balance the electrode at a SOC of 42% of a prismatic 25 Ah NMC111-GR Li-ion cell [
38]. The Li stored in the passive areas during and after calendar aging is then released when changing to cyclic aging mode. Eddahech et al. report a similar recovery effect of the cell capacity after cyclic aging due to an enhanced movement of charge carriers [
69].
The cell impedances are also dependent on the aging direction, as seen in
Table 10. Thus, Equation (
12) is also valid for the impedance increase
of both the 40 Ah cells and the coin cells.
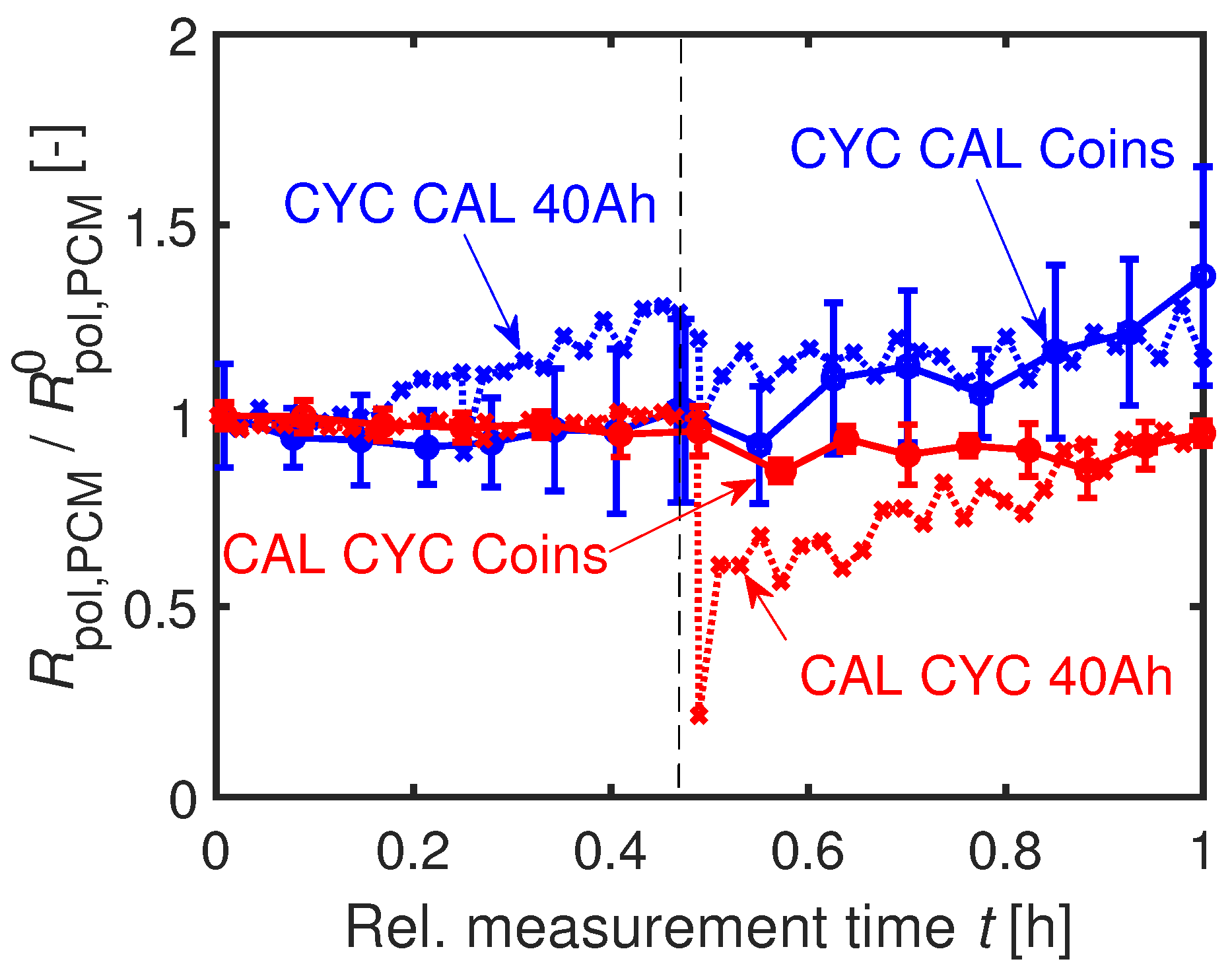
The time-dependent developments of the normed resistances
are illustrated in
Figure 18. The polarization resistance
increases more strongly for CYC CAL compared to CAL CYC. In this case, even the absolute values of the coin cells agree with the values of the 40 Ah cells at end of investigation, as seen in
Figure 18.
An explanation for the stronger increase in resistance and the faster capacity fading of the CYC CAL cells is an increased surface area caused by particle cracking at the beginning of the cycling. This assumption is underlined by a stronger decrease of the coin cells’
during cyclic aging (
) compared to calendar aging (
) at
after the start of the path dependency experiment. The charge transfer resistance
is reciprocally proportional to the surface area
S of the exchange current density
[
70]. The variable
z is the number of contributed electrons,
F the Faraday constant, and R the gas constant. Thus, for CYC CAL cells, the reaction rate of the calendar aging, which is also dependent on the surface area, may be higher than for the CAL CYC cells due to the previously enlarged surface area during cyclic aging. In addition, the SEI growth of the CAL CYC cells during the calendar aging might reduce the particle cracking caused by the subsequent cyclic aging. The stronger increase in the surface-dependent double-layer capacitance
for the CYC CAL original cell (see
Table 10) supports this theory. The trends in
of the coin cells strongly scatter and therefore do not allow any interpretation.
8. Conclusions
Aging Li-ion cell material in coin cells offers an orders-of-magnitude-lower power requirement per channel to the battery tester. Significantly cheaper testing and simplifications in the safety precautions of the test setup are the consequences.
The preparation procedure used in this work enables building coin cells in a reproducible manner [
4]. This work is based on 40 Ah NMC111-graphite Li-ion pouch cells. The original 40 Ah cells and the assembled coin cells (PAT-Cells) are cycled with 1C at three different temperatures
. Incremental capacity analysis, differential voltage analysis, maximum/minimum electrode potentials, electrochemical impedance spectroscopy, and pulse current measurements are taken into account to determine the cell aging mechanisms. The results show the same basic aging mechanisms in the coin cells compared to the 40 Ah cells. The almost identical activation energy in cyclic aging between the 40 Ah cells
(
) and the averaged coin cells
(
) confirms this. A trend of increasing loss of active material toward higher temperatures can be detected for both cell types.
The differences in anode overhang do not significantly influence the development of remaining capacity. Post-mortem analyses prove diffusion of moisture into the cell housing. However, the increasing concentration of fluor by measurement time on the graphite anodes of the coin cells is not directly linked to the loss of capacity. Hence, the diffusion of moisture is a superposed aging effect. The temperature-dependent aging mechanisms of the 40 Ah cells are still transferable to coin cell level. Finally, an exemplary path dependency test of both cell types has been carried out. A cyclically and then calendrically aged 40 Ah cell degrades faster compared to a 40 Ah cell, which was calendrically aged before cyclic aging. The observed path dependency of the 40 Ah cell can be transferred to the coin cell format. The developments in capacity and resistance are comparable for both cell types.
This paper serves as orientation for further coin cell preparation work in laboratories. The proposed coin cell aging method proves the possibility of detecting the aging mechanisms happening in cycled commercial Li-ion cells, making it a useful tool to analyze the cell material. However, the gradients in capacity degradation of the coin cells and the original cells are different in this work. Therefore, quantitative aging prediction of commercial Li-ion cells by aging the cell material in coin cells is probably not suitable. Nevertheless, further experiments could reveal the impact of the geometry on aging in Li-ion cells by analyzing coin cells of different sizes.