Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments
Abstract
1. Introduction
- >70 °C
- >120 °C
- >130 °C
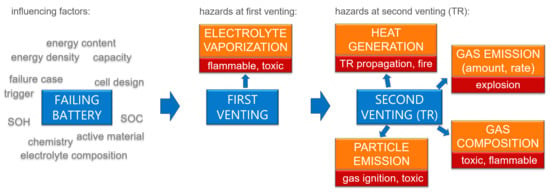
- Further gas develops and electrolyte vaporizes. The cell internal pressure increases until the cell casing opens at the weakest point. Accumulated gas vents from inside the cell into the battery pack (first venting). It can occur at about 120–220 °C cell surface temperature [18,19]. Separator melts between 130 °C–190 °C [6,20].
- ~160 °C
- Starting at about 160 °C the exothermic process inside the cell accelerates the self-heating and results in a TR. The TR is accompanied by violent gas and particle release (second venting). Electrolyte decomposes exothermally [5,21] between 200–300 °C [17]. At the TR, the cell temperature increases enormously due to chemical reactions inside the cell. Metal oxide cathodes decompose and produce oxygen (O2) [22,23]. O2 further reacts with electrolyte and produces CO2 and H2O [21,23].
1.1. Categorized Hazards from LIBs
1.1.1. Electrolyte Vaporization
1.1.2. Heat Generation
1.1.3. Gas Emission
1.1.4. Gas Composition
1.1.5. Particle Emission
- The vent gas amount: Koch et al. measured an average 1.96 L/Ah at 20‒81 Ah NMC cells in air (present O2) and refer to a gas emission in the range between 1.3 L/Ah up to 2.5 L/Ah for current batteries with regular electrolytes [34] for mainly small capacity cells. Zhao et al. [35] measured for 2 Ah NMC cells at extended volume accelerating rate calorimeter (EV-ARC) abuse 1.4 L/Ah. A detailed analysis of the gas amount produced at failing high capacity NMC/LMO cells in N2 is missing in current literature.
- The venting rate: Golubkov et al. published a characteristic venting rate of (0.8 ± 0.3) mol/s at heat ramp TR experiments of 50 Ah prismatic LMO cells [19]. A relevant analysis of the venting rate of NMC and NMC/LMO pouch cells is not available in accessible literature.
- A comprehensive gas composition analysis at heat triggered TR: Koch et al. conduct the experiments in air (present O2) [34] and does not quantify electrolyte components and H20. Zhang et al. set huge effort to quantify higher hydrocarbons (1.63% of total gas amount) [47] and does not analyze electrolyte components, HF and O2. A comprehensive gas analysis at heat triggered TR in N2 atmosphere including electrolyte quantification is missing.
- Vent particles emission at TR: published by Zhang et al. for a prismatic 50 Ah NMC cells in N2 atmosphere [38,47]. Since the investigated cells by Zhang et al. have a different cell design (metal can), electrolyte composition and energy density there is a need to further investigate the size and content of particles produced at TR with a nondestructive analysis method.
- A study of the five mentioned hazards including quantification of the safety relevant parameters for the same specific cell.
- Comprehensive gas composition analysis at the first venting or at abuse experiments of cells with low SOC, where no self-heating into TR can be triggered.
- Gas composition at first venting (gas concentrations including electrolyte vapor)
- Heat generation at TR (cell surface temperatures including maximum reached temperature)
- Gas emission at TR (amount of produced gas and venting rate)
- Gas composition at TR (gas concentrations)
- Particle emission at TR (particle size distribution and composition)
1.2. Structure of the Study
2. Investigated Cell
3. Experimental Setup and Test Methods
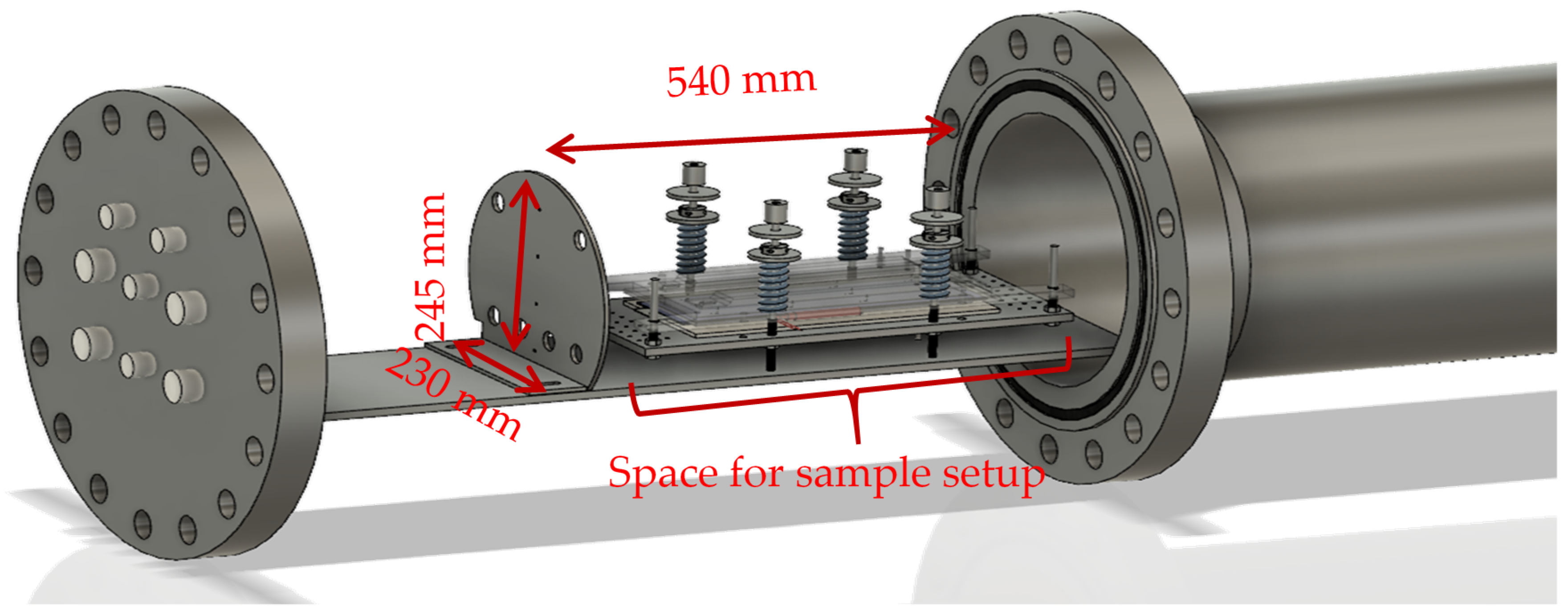
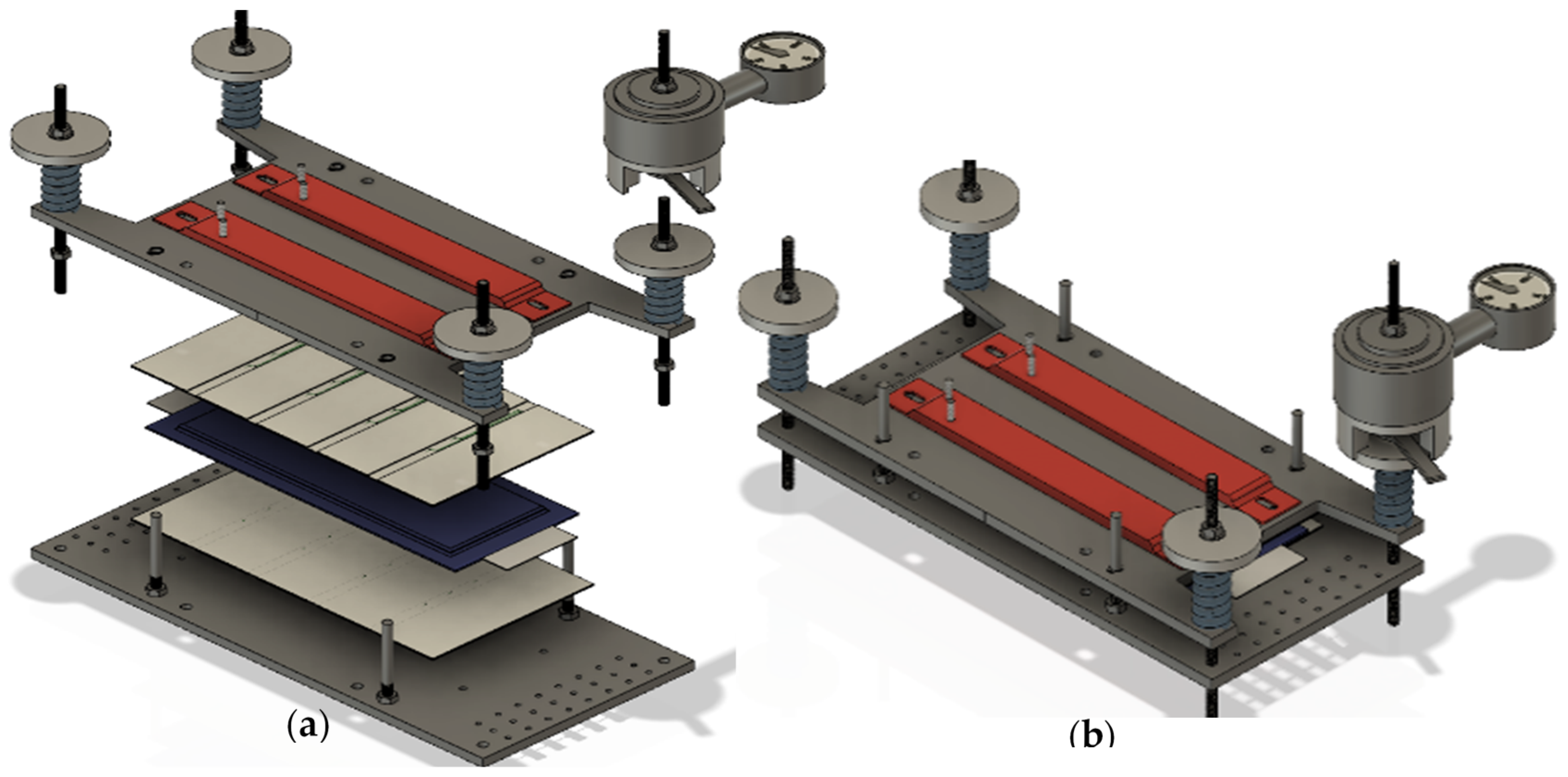
3.1. Reactor Setup
3.2. Experimental Method:
- 1.
- Insulating mica plates with thermocouples for temperature measurement are placed on the top and the bottom side of the cell (beige plates in Figure 4a).
- 2.
- The sample is fixed in the sample holder with a defined force of 3000 N (54 kPa).
- 3.
- Reactor is closed and evacuated.
- 4.
- N2 is added until ambient pressure. Step 3 and 4 are repeated at least 2 times.
- 5.
- All gas valves are closed (the reactor is hermetically sealed).
- 6.
- Sample is charged to the desired SOC (0%, 30% and 100%).
- 7.
- The data acquisition system is started: measurement of cell surface temperature, cell voltage, temperature and pressure inside the reactor. The cell is pulsed with a battery cycler (±1 A pulses) in order to get information on the cell resistance.
- 8.
- The desired TR trigger is chosen. Here: the cell is heated by the sample holder with a constant rate of temperature increase from both sides with a specified heat ramp (0.39 °C/min at 100% SOC; 0.36 °C/min at 30% SOC until 38,000 s, then increased rate; 0.33 °C/min at 0% SOC).
- 9.
- The sample exhibits the first venting and, after being heated to the critical temperatures, the TR.
- 10.
- After reaching the maximum temperature during the exothermic reaction, the TR, the heating is switched off. The cell starts to cool down. Wait 5 min to start the experiment after-treatment.
- 11.
- The valves to the gas analysis section are opened. The gas composition analysis is started.
- 12.
- After finishing the gas measurement series, the data acquisition is stopped.
- 13.
- Reactor is heated, evacuated and flushed with N2 several times before opening. Ejected particles are sampled, and the test cell is removed.
3.3. Heat Generation Analysis/Temperature Measurement
3.4. Gas Emission Analysis
3.5. Gas Composition Analysis
3.5.1. FTIR Spectrometer (FTIR)
3.5.2. Gas Chromatograph (GC)
3.5.3. Accuracy of the Gas Quantification
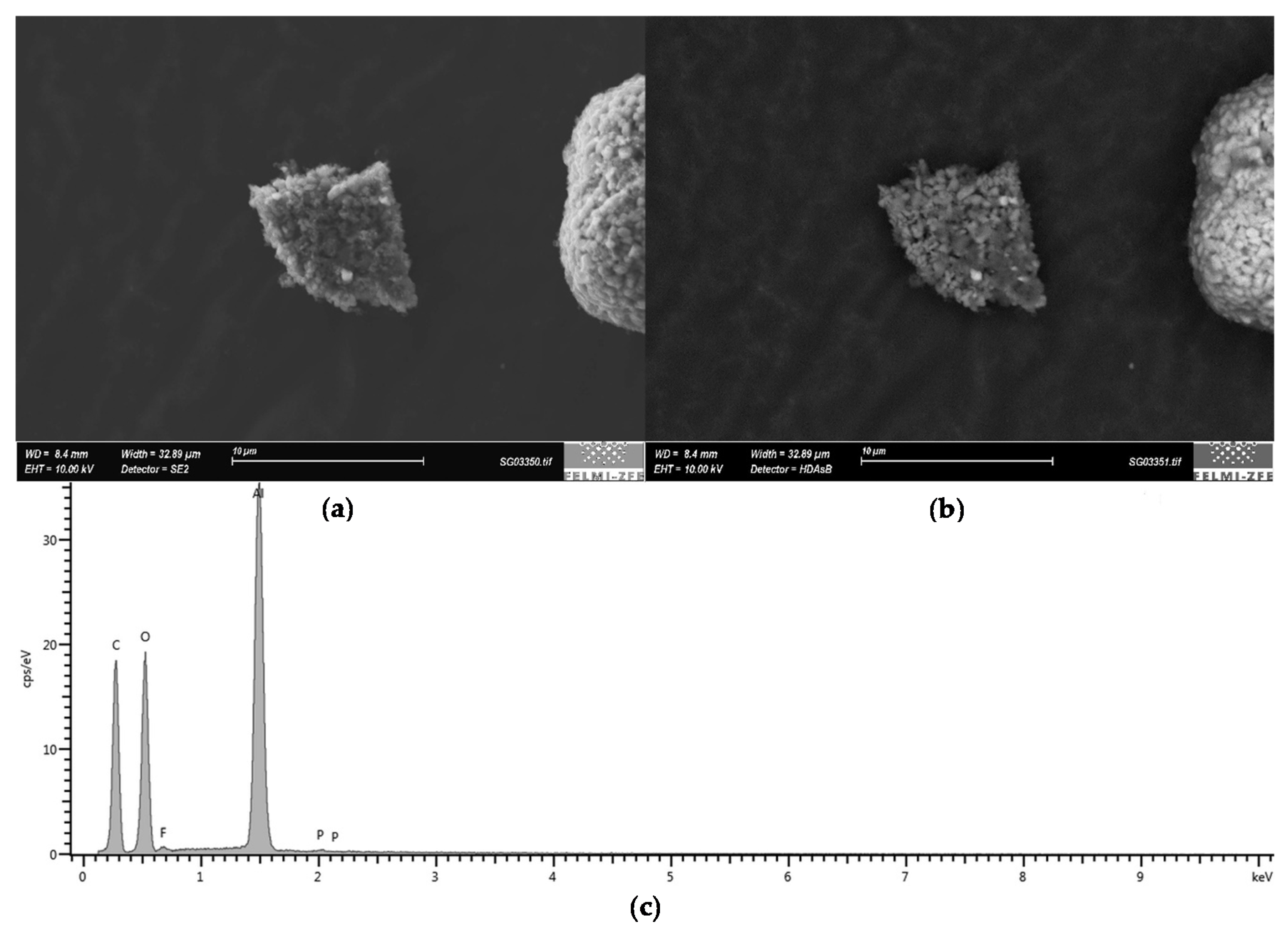
3.6. Particle Collection and Particle Analysis
- For material contrast: imaging with backscattered electron (BSE);
- For topographic contrast: imaging with secondary electrons (SE);
- For elemental analysis: energy dispersive X-ray spectroscopy (EDX).
3.7. Mass Reduction Analysis
4. Results
4.1. Heat Generation/Temperature Response
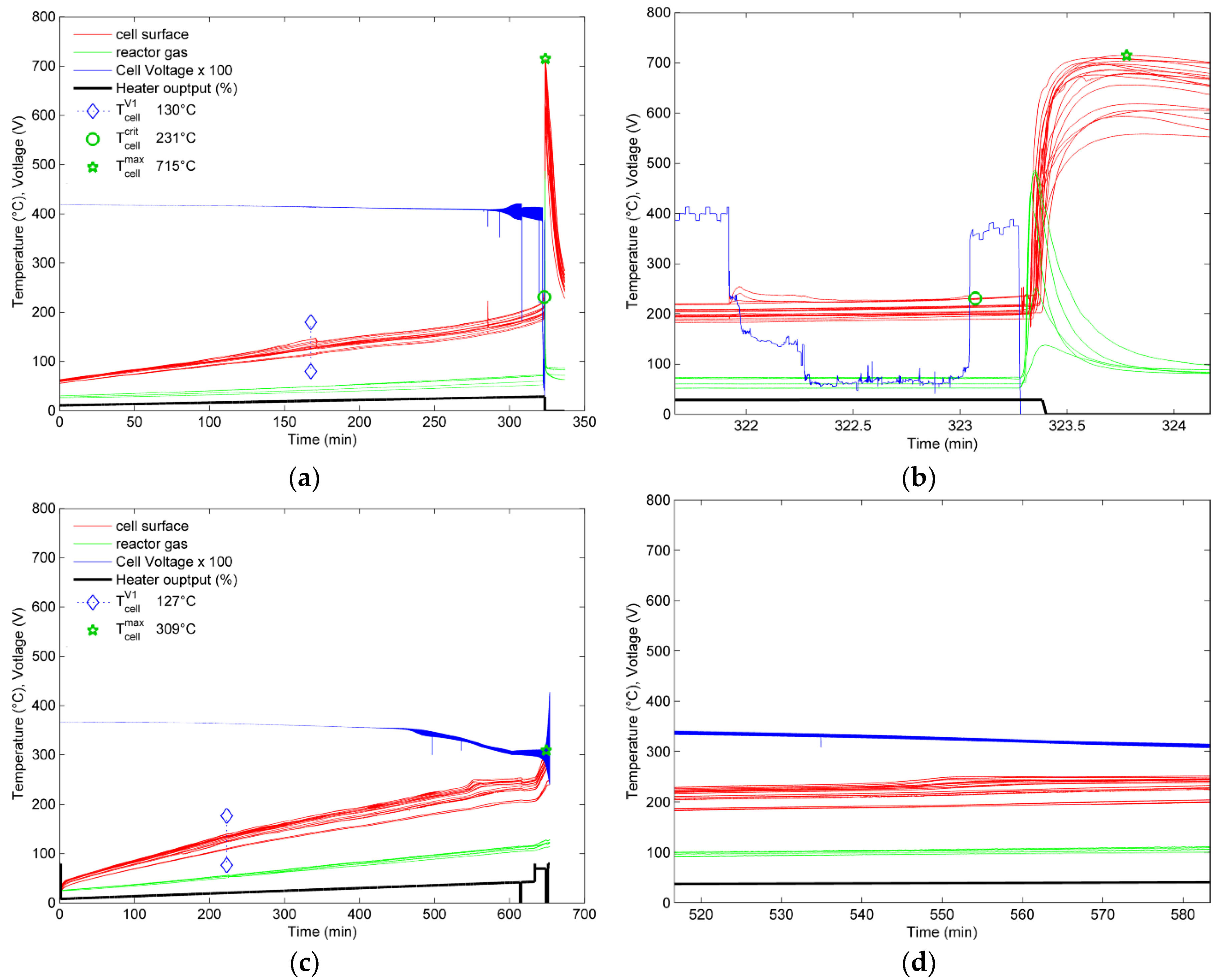
4.1.1. Experiment with the 100% SOC Cell
4.1.2. Experiment with the 30% and 0% SOC Cell
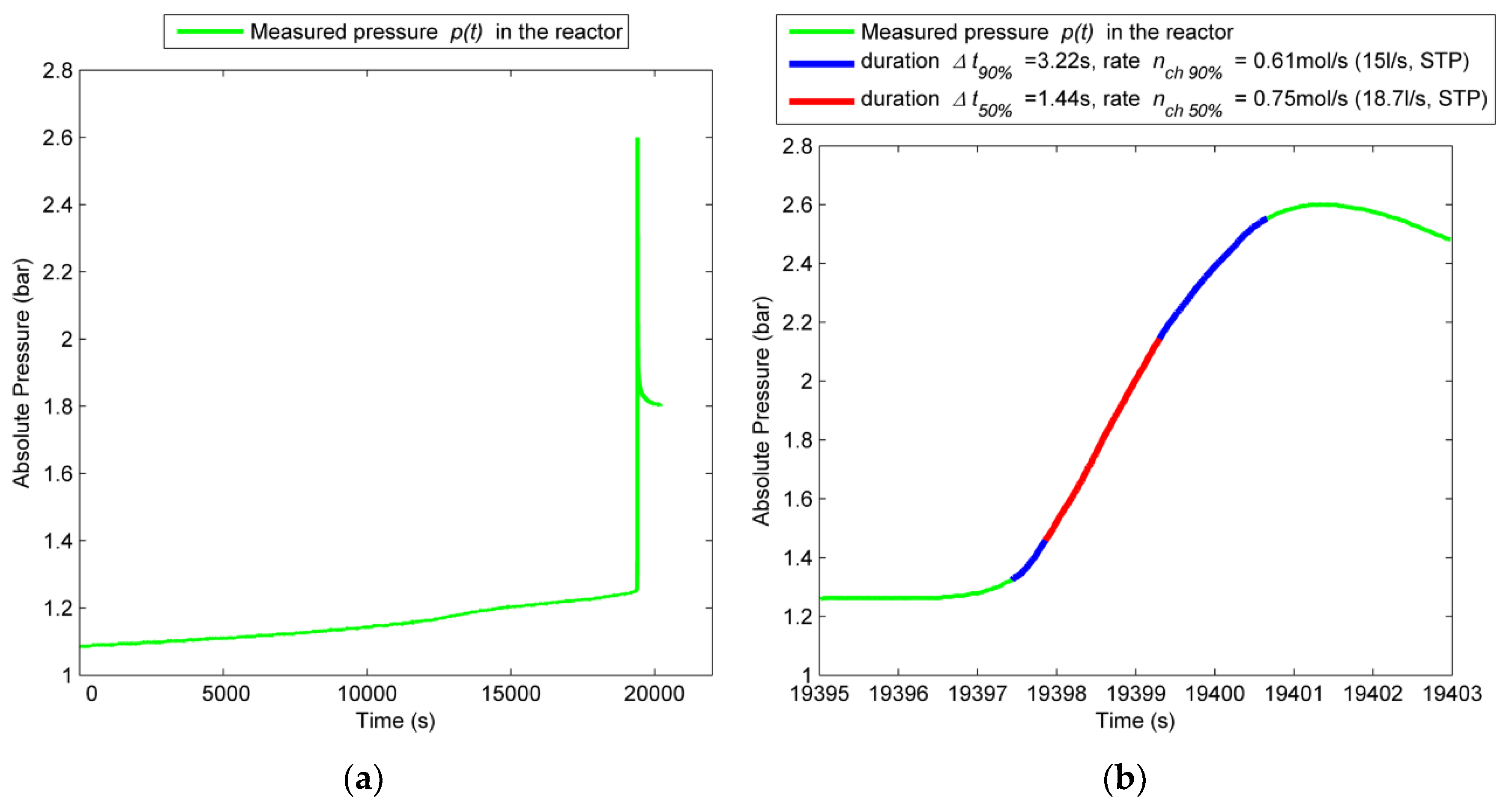
4.2. Gas Emission
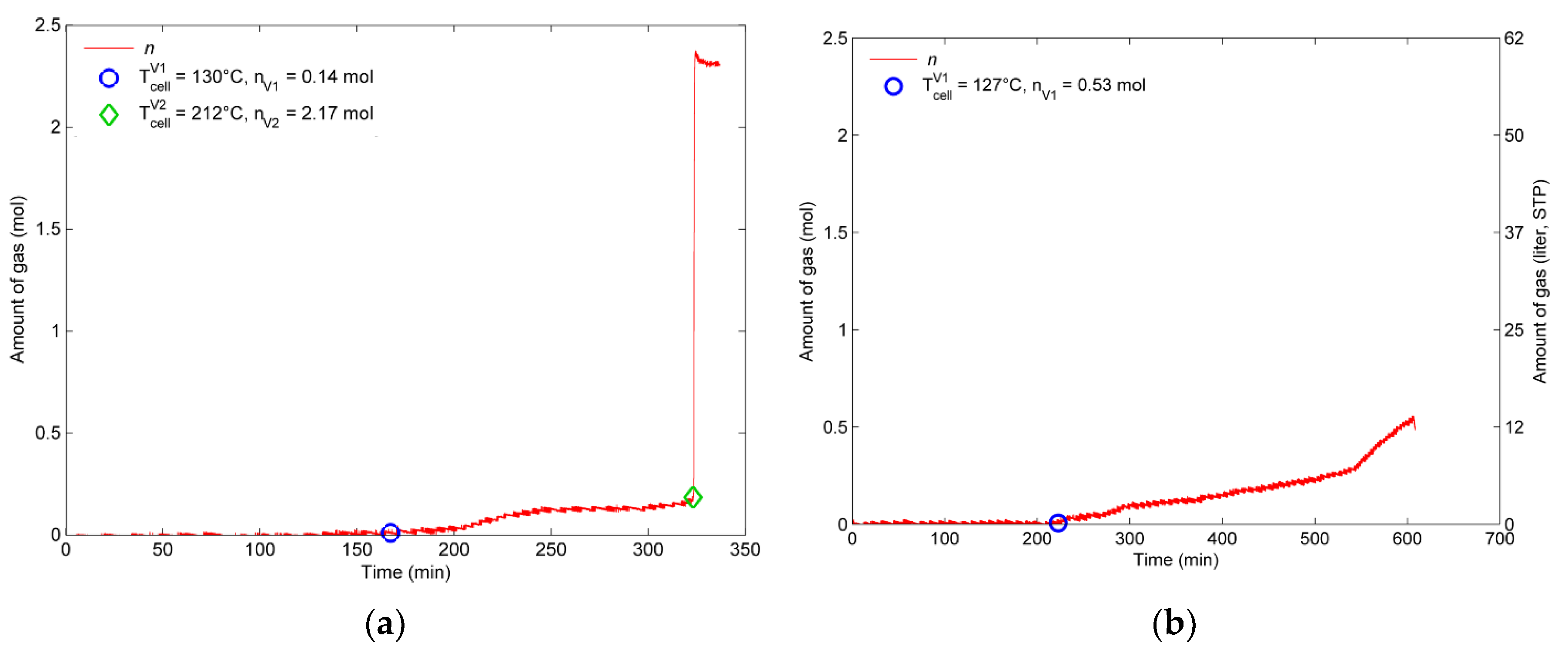
4.2.1. Experiment with the 100% SOC Cell
4.2.2. Experiment with the 30% and 0% SOC Cell
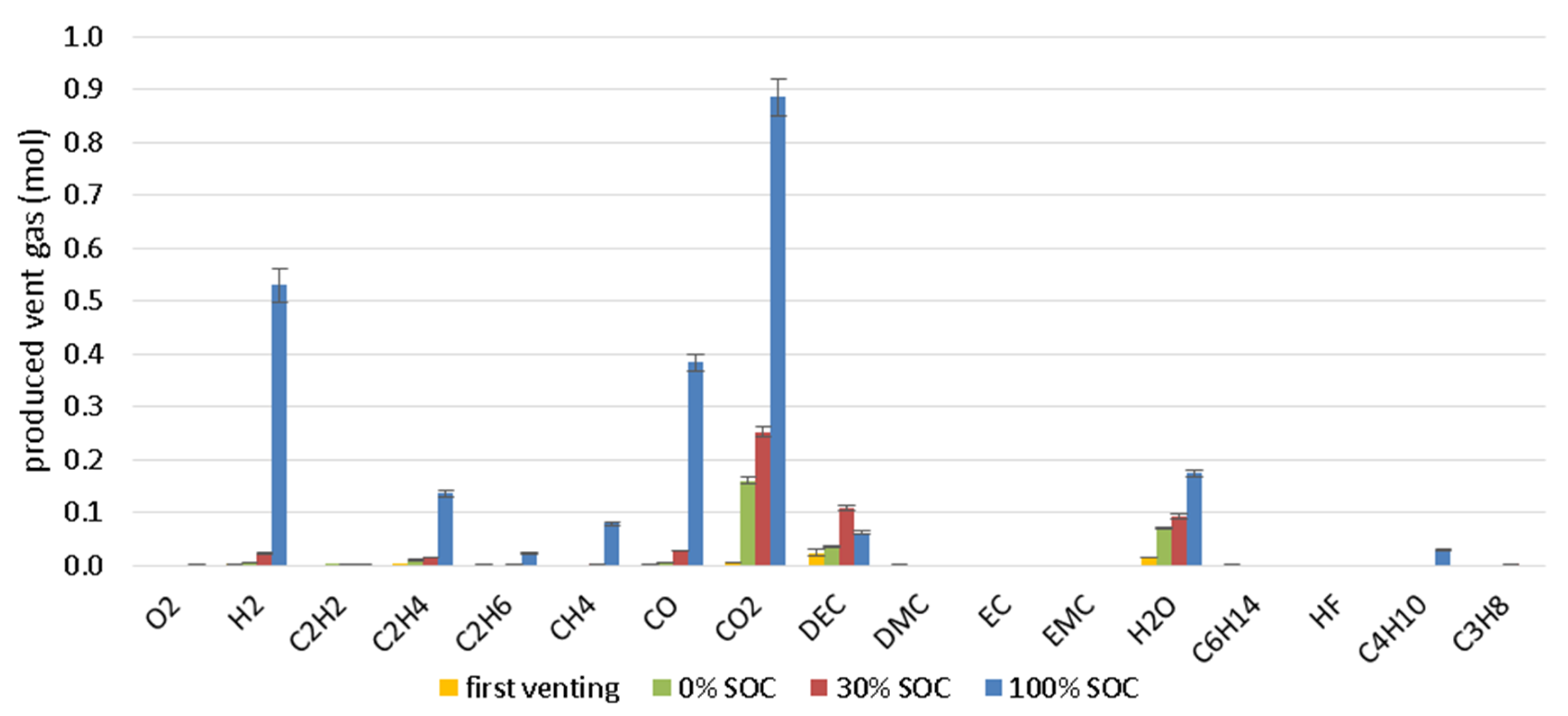
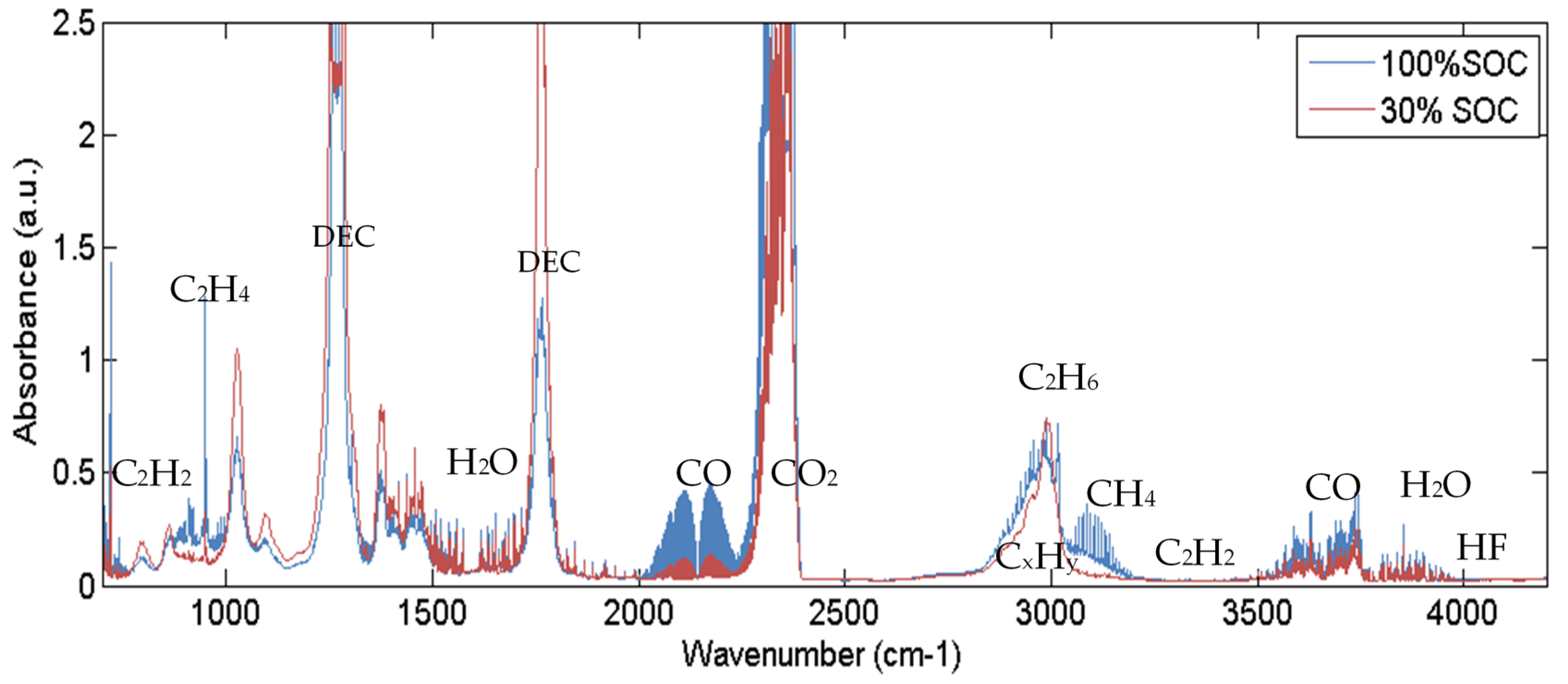
4.3. Vent Gas Composition
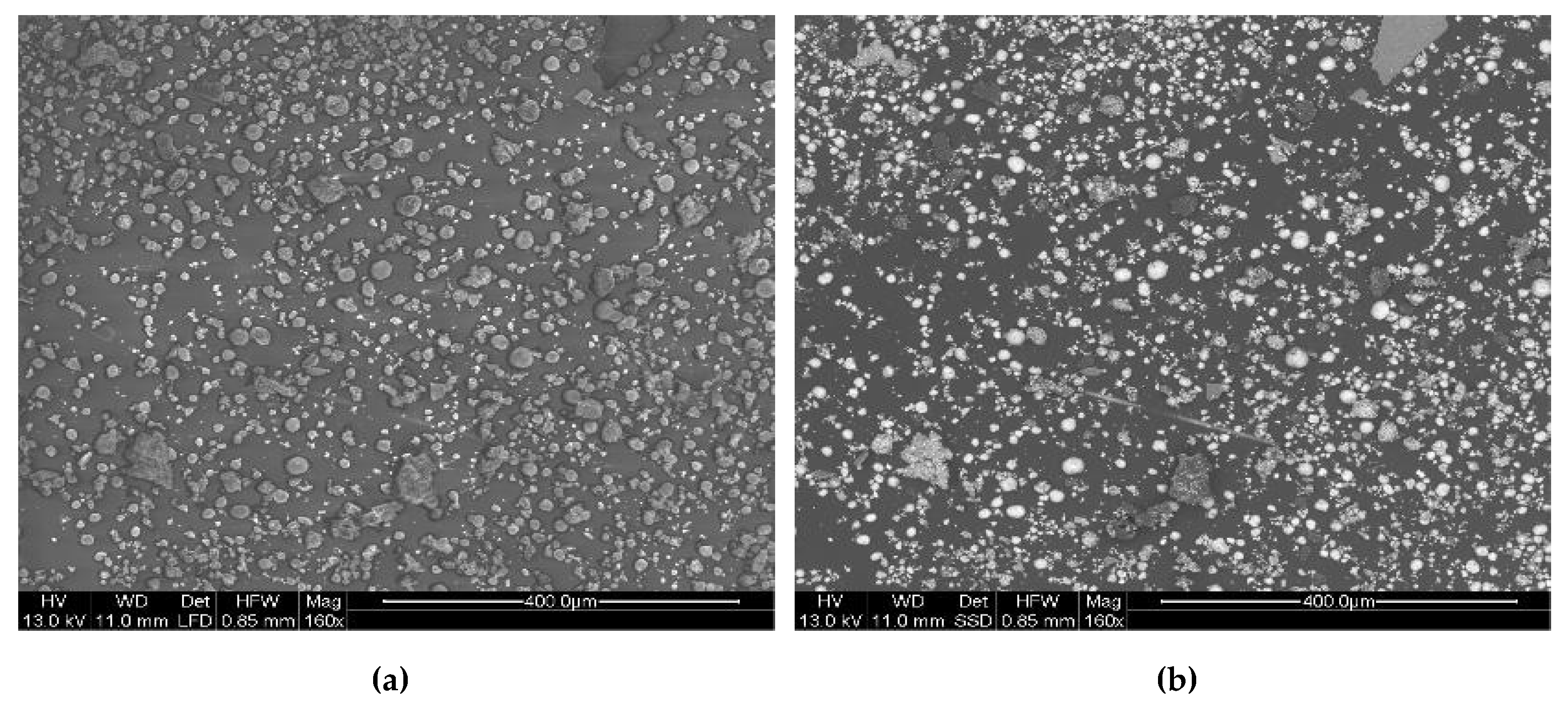
4.4. Particle Emission
- Particles mainly consisting of Al and O. Their assumed chemical formula is Al2O3 (Figure 13).
- Particles with huge amounts of nickel (Ni), manganese (Mn), O and smaller amounts of cobalt (Co). The assumed chemical formula is (Li + NMC)3O4.
- Particles mainly consisting of Mn and O. The average elemental composition has the estimated chemical formula Mn2O3 or its decomposition products.
- Particles with a high content of C. Very small EDX peaks of O, fluorine (F) and phosphorus (P) were measured.
- The fifth particle class describes agglomerates with several different material composites which do not fit into one of the listed classes.
4.5. Mass Reduction
4.6. Optical Observation of the Cell after TR
5. Discussion
5.1. Hazard Analysis of Failing Automotive Pouch Cells
5.1.1. Heat Generation/Temperature Increase
5.1.2. Gas Emission
5.1.3. Gas Composition below SOCcrit—30% and 0% SOC
5.1.4. Gas Composition—100% SOC
5.1.5. Particle Emission
5.1.6. Mass Reduction
5.2. Forecast for Failing Behavior of Future Cells
5.3. Forecast for Failing Behavior of Aged Cells
5.4. Recommended Failure Detection
6. Conclusions
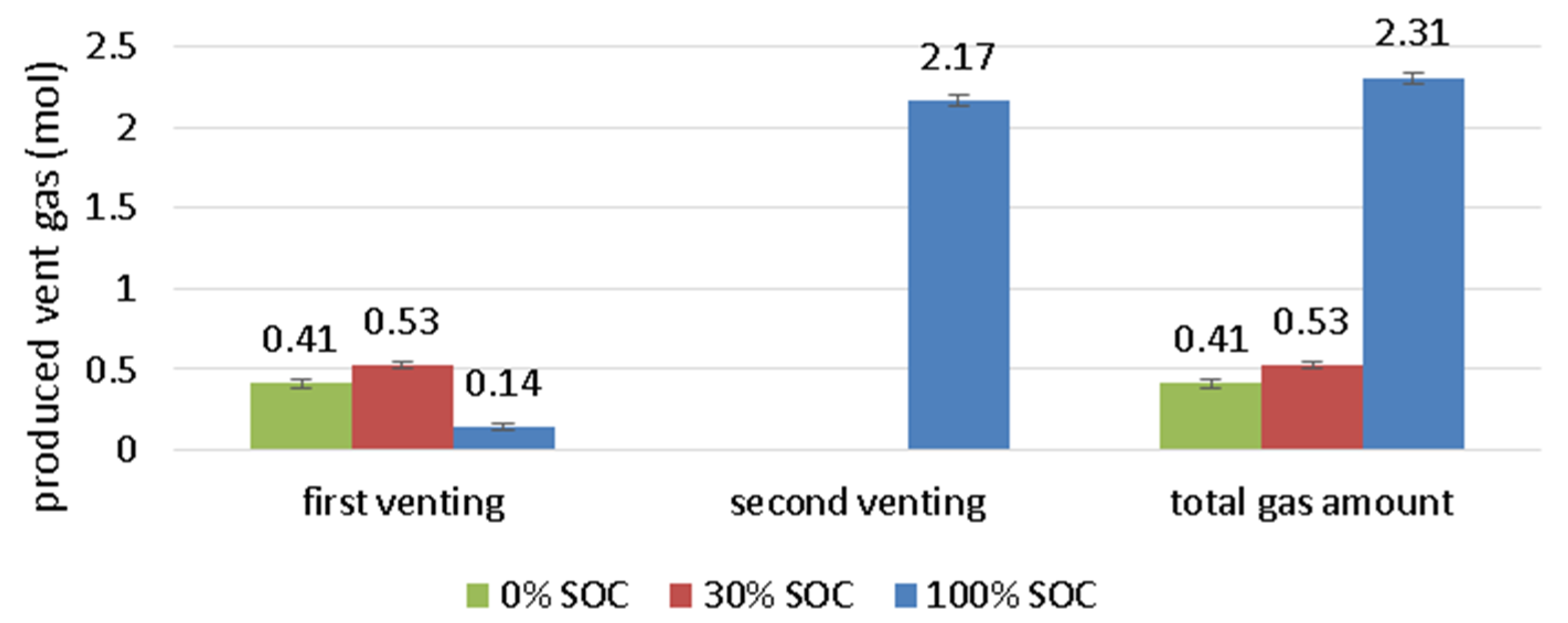
- The first venting is measured at 120–130 °C cell surface temperature independent of the SOC.
- At the 30% and 0% SOC cell:
- ○
- The main gas components are after the first venting and constant gas production until the heating is stopped in descending order CO2, DEC, H2O, CO, H2, C2H4, CH4, C3H8.
- ○
- One presented hazard is electrolyte vaporization. Commonly used electrolyte components such as EC, DEC, DMC, EMC in an unsealed cell are critical due to the consequential irritant, toxic, cancerogenic and flammable atmosphere. At this cell EC (irritant, PAC-1: 30 mg/m3) and DEC (flammable, PAC-1: 2 mg/m3) are the main electrolyte components. It is important to address this hazard especially in large traction battery EV applications, where significant amounts of electrolyte may vaporize inside a closed system (pack, garage, tunnel).
- At the fully charged (100% SOC) pouch cell two venting stages were observed: A first venting and a second venting (TR). The second venting starts above average cell temperature of 212 °C. The TR has the following hazards and consequences, which end up as safety and health risks:
- ○
- Enormous heat is generated by the cell, the cell surface temperatures increased above 700 °C. The main exothermic reaction developed to a rapid TR when the hottest measured part of the cell reached 231 °C. Within 4.28 s the TR propagated through the cell. This high surface temperature can lead to TR propagation to neighboring cells and irreversible damage of the battery pack.
- ○
- Overall, 2.31 mol (57 L, 1.3 L/Ah) of gas is produced. The cell released 0.14 mol before the TR. During the TR, the cell released in 4 s additional 2.17 mol with a characteristic rate of 0.8 mol/s (18.7 L/s). 50% of the gas is produced in 1.4 s. The abrupt pressure increase at the TR is a serious risk inside a closed volume.
- ○
- The cell mass reduces by 43% of the initial mass. This mass reduction can be explained as the sum of released gas and ejected particles at TR.
- ○
- The main gas components are: 38% CO2, 23% H2, 17% CO, 8% H2O, 6% C2H4, 4% CH4 and 3% electrolyte vapor (DEC). The measured gas components are about 20% of the lost cell mass during TR and 9% of the initial cell mass. Toxic (CO) and flammable (H2, CH4, DEC, etc.) gas components are dangerous when entering the passenger compartment.
- ○
- A large number of ejected particles are smaller than 10 μm2. Novel nondestructive sampling and analysis methods were used to evaluate the particle parameters: The smallest analyzed particles have an area of 0.1 µm2, thus a circle equivalent diameter of roughly 6 nm. A total of twelve elements were detected in the particles, including elements like Al, Ni or F. These ejected hot particles (~35% of the initial cell mass) may ignite the vent gas, are carcinogenic and respirable for humans.
- The NMC/LMO cell is comparable to results of failing NMC cells concerning heat generation (max. reached temperature), gas emission and main gas components. Although, the exact gas composition is highly sensitive to the electrolyte mixture.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LIB | Lithium-ion battery | EV | Electric vehicle |
| TR | Thermal runaway | SOC | State-of-charge |
| BEV | Battery electric vehicle | HEV | Hybrid electric vehicles |
| EC | Ethylene carbonate | DEC | Diethylene carbonate |
| DMC | Dimethyl carbonate | EMC | Ethyl methyl carbonate |
| SEM | Scanning electron microscope | EDX | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier-transform infrared (spectroscopy) | BSE | Backscattered electron |
| GC | Gas chromatograph | SE | Secondary electrons |
| ppm | Parts per million | PSD | Particle size distribution |
| SOH | State-of-health | IC | Ion chromatography |
| SEI | Solid electrolyte interface | LOD | Limit of detection |
| ICTM | Institute of Chemistry and Technology of Materials, Graz University of Technology | FELMI | Institute of Electron Microscopy and Nanoanalysis, Graz University of Technology |
References
- Wagner, I. 2017—Statista-Statistic_id264754_Worldwide-Vehicle-Sales-by-Propulsion-Technology-2017-2030 (1).pdf. Statista. 2017. Available online: https://www.statista.com/statistics/264754/worldwide-vehicle-sales-by-propulsion-technology-2025/ (accessed on 2 March 2020).
- Ahlswede, A. 2020—Statista-Statistic_id681259_Absatz-von-Elektroautos-in-Ausgewaehlten-Maerkten-Weltweit-Bis-2019.pdf. Statista. 2020. Available online: https://de.statista.com/statistik/daten/studie/681259/umfrage/absatz-von-elektroautos-in-ausgewaehlten-maerkten-weltweit/ (accessed on 2 March 2020).
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles; Springer: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Pfrang, A.; Kriston, A.; Ruiz, V.; Lebedeva, N.; di Persio, F. Safety of Rechargeable Energy Storage Systems with a Focus on Li-Ion Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal Behavior of Lithiated Graphite with Electrolyte in Lithium-Ion Batteries. J. Electrochem. Soc. 2006, 153, A329. [Google Scholar] [CrossRef]
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef]
- Jhu, C.Y.; Wang, Y.W.; Wen, C.Y.; Shu, C.M. Thermal runaway potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 batteries determined with adiabatic calorimetry methodology. Appl. Energy 2012, 100, 127–131. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Lamb, J.; Steele, L.A.M.; Spangler, S.W.; Langendorf, J. Quantification of Lithium-Ion Cell Thermal Runaway Energetics; Sandia Report; Sandia National Laboratories (SNL-NM): Albuquerque, NM, USA, 2016; p. 0486.
- Huang, P.; Wang, Q.; Li, K.; Ping, P.; Sun, J. The combustion behavior of large scale lithium titanate battery. Sci. Rep. 2015, 5, 7788. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Di Michiel, M.; Offer, G.J.; Hinds, G.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef]
- Harris, S.J.; Timmons, A.; Pitz, W.J. A combustion chemistry analysis of carbonate solvents used in Li-ion batteries. J. Power Sources 2009, 193, 855–858. [Google Scholar] [CrossRef]
- Mikolajczak, C.; Michael Kahn, P.; White, K.; Thomas Long, R. Lithium-Ion Batteries Hazard and Use Assessment Final Report; Fire Protection Research Foundation: Quincy, MA, USA, 2011. [Google Scholar]
- Roth, E.P.; Doughty, D.H.; Franklin, J. DSC investigation of exothermic reactions occurring at elevated temperatures in lithium-ion anodes containing PVDF-based binders. J. Power Sources 2004. [Google Scholar] [CrossRef]
- Ren, D.; Hsu, H.; Li, R.; Feng, X.; Guo, D.; Han, X.; Lu, L.; He, X.; Gao, S.; Hou, J.; et al. A comparative investigation of aging effects on thermal runaway behavior of lithium-ion batteries. eTransportation 2019, 2, 100034. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Planteu, R.; Krohn, P.; Rasch, B.; Brunnsteiner, B.; Thaler, A.; Hacker, V. Thermal runaway of large automotive Li-ion batteries. RSC Adv. 2018, 8, 40172–40186. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Gachot, G.; Ribière, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.B.; Pilard, S.; Laruelle, S. Gas chromatography/mass spectrometry as a suitable tool for the li-ion battery electrolyte degradation mechanisms study. Anal. Chem. 2011, 83, 478–485. [Google Scholar] [CrossRef]
- Dahn, J.R.; Fuller, E.W.; Obrovac, M.; von Sacken, U. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Solid State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Kumai, K.; Miyashiro, H.; Kobayashi, Y.; Takei, K.; Ishikawa, R. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell. J. Power Sources 1999, 81–82, 715–719. [Google Scholar] [CrossRef]
- Kenichiroh, K. Journey to a New Regulatory Option. OICA Submission to IWG for GTR 20, Phase 2 2019(IWG#19). pp. 1–31. Available online: https://wiki.unece.org/download/attachments/86311372/EVS19-E1TP-0300 %5BOICA%5DJourney to a New Regulatory Option.pdf?api=v2 (accessed on 23 February 2020).
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Lebedeva, N.P.; Boon-Brett, L. Considerations on the Chemical Toxicity of Contemporary Li-Ion Battery Electrolytes and Their Components. J. Electrochem. Soc. 2016, 163, A821–A830. [Google Scholar] [CrossRef]
- Roth, E.P.; Orendorff, C.J. How electrolytes influence battery safety. Electrochem. Soc. Interface 2012, 21, 45–49. [Google Scholar] [CrossRef]
- Larsson, F. Assessment of Safety Characteristics for Li-Ion Battery Cells by Abuse Testing; Chalmers University of Technology: Göteborg, Sweden, 2014. [Google Scholar]
- Hollmotz, L.; Hackmann, M. Lithium Ion Batteries for Hybrid and Electric Vehicles—Risks, Requirements and Solutions out of the Crash Safety Point of View; 11–0269; International Technical Conference on the Enhanced Safety of Vehicles (ESV): Washington, DC, USA, 2011.
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Mellander, B.E. Lithium-Ion Battery Aspects on Fires in Electrified Vehicles on the Basis of Experimental Abuse Tests. Batteries 2016, 2, 9. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; de Persis, S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Somandepalli, V.; Marr, K.; Horn, Q. Quantification of Combustion Hazards of Thermal Runaway Failures in Lithium-Ion Batteries. SAE Int. J. Altern. Powertrains 2014, 3, 98–104. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Wang, Q. Thermal runaway hazards investigation on 18650 lithium-ion battery using extended volume accelerating rate calorimeter. J. Energy Storage 2020, 28, 101232. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Planteu, R.; Rasch, B.; Fuchs, A. Transport of Li-Ion Batteries: Early Failure Detection by Gas Composition Measurements. In Proceedings of the 7th Transport Research Arena (TRA), Vienna, Austria, 16–19 April 2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Size distribution and elemental composition of vent particles from abused prismatic Ni-rich automotive lithium-ion batteries. J. Energy Storage 2019, 26, 100991. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Liu, X.; Li, M.; Ouyang, M. Key characteristics for thermal runaway of Li-ion batteries. Energy Procedia 2019, 158. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Nagasubramanian, G.; Fenton, K. Reducing Li-ion safety hazards through use of non-flammable solvents and recent work at Sandia National Laboratories. Electrochim. Acta 2013, 101, 3–10. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes—Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Perea, A.; Paolella, A.; Dubé, J.; Champagne, D.; Mauger, A.; Zaghib, K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J. Power Sources 2018, 399, 392–397. [Google Scholar] [CrossRef]
- Waldmann, T.; Wohlfahrt-Mehrens, M. Effects of rest time after Li plating on safety behavior—ARC tests with commercial high-energy 18650 Li-ion cells. Electrochim. Acta 2017. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C. Quantitative identification of emissions from abused prismatic Ni-rich lithium-ion batteries. eTransportation 2019, 2, 100031. [Google Scholar] [CrossRef]
- Gao, S.; Feng, X.; Lu, L.; Ouyang, M.; Kamyab, N.; White, R.E.; Coman, P. Thermal Runaway Propagation Assessment of Different Battery Pack Designs Using the TF5 Draft as Framework. J. Electrochem. Soc. 2019, 166, A1653–A1659. [Google Scholar] [CrossRef]
- Kovachev, G.; Schröttner, H.; Gstrein, G.; Aiello, L.; Hanzu, I. Analytical Dissection of an Automotive Li-Ion Pouch Cell. Batteries 2019, 5, 67. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Planteu, R.; Rasch, B.; Essl, C.; Thaler, A.; Hacker, V. Thermal runaway and battery fire: Comparison of Li-ion, Ni-MH and sealed lead-acid batteries. In Proceedings of the 7th Transport Research Arena (TRA), Vienna, Austria, 16–19 April 2018; Volume 43. [Google Scholar] [CrossRef]
- Gasser, E. Characterization of Gas and Particle Released during Thermal Runaway of Li-Ion Batteries. Master’s Thesis, Graz University of Technology, Graz, Austria, 2019; pp. 2–92. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Self, J.; Aiken, C.P.; Petibon, R.; Dahn, J.R. Survey of Gas Expansion in Li-Ion NMC Pouch Cells. J. Electrochem. Soc. 2015, 162, 796–802. [Google Scholar] [CrossRef]
- Onuki, M.; Kinoshita, S.; Sakata, Y.; Yanagidate, M.; Otake, Y.; Ue, M.; Deguchi, M. Identification of the Source of Evolved Gas in Li-Ion Batteries Using [sup 13]C-labeled Solvents. J. Electrochem. Soc. 2008, 155, A794. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Liu, Y.; Zhang, Y.; Wang, C.; Xu, J.; Ning, F.; Wang, D. Investigation on gas generation of Li4Ti5O 12/LiNi1/3Co1/3Mn1/3O2 cells at elevated temperature. J. Power Sources 2013, 237, 285–290. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Jimenez-Gordon, I.; Eshetu, G.G.; Boyanov, S.; Lecocq, A.; Marlair, G.; Pilard, S.; Laruelle, S. Gas chromatography/Fourier transform infrared/mass spectrometry coupling: A tool for Li-ion battery safety field investigation. Anal. Methods 2014, 6120–6124. [Google Scholar] [CrossRef]
- Sun, W.; Yang, B.; Hansen, N.; Westbrook, C.K.; Zhang, F.; Wang, G.; Moshammer, K.; Law, C.K. An experimental and kinetic modeling study on dimethyl carbonate (DMC) pyrolysis and combustion. Combust. Flame 2016, 164, 224–238. [Google Scholar] [CrossRef]
- Spangl, W.; Kaiser, A.; Schneider, J. Herkunftsanalyse der PM10-Belastung in Österreich—Ferntransport und Regionale Beiträge; Umweltbundesamt: Wien, Austria, 2006.
- Schmidt, R.; Fitzek, H.; Nachtnebel, M.; Mayrhofer, C.; Schroettner, H.; Zankel, A. The Combination of Electron Microscopy, Raman Microscopy and Energy Dispersive X-Ray Spectroscopy for the Investigation of Polymeric Materials. Macromol. Symp. 2019, 384, 1–10. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Nominal capacity | 41 Ah |
| Cathode material | NMC/LMO |
| Anode material | graphite |
| Electrolyte | 48% EC, 48% DEC, 4% DMC, 1 mol/L LiPF6 |
| Nominal voltage | ~3.8 V |
| Initial mass | 865 g |
| Volume | 0.459 L |
| Aging state | fresh, unused |
| SOC | 0%, 30% and 100% |
| Gas | FTIR | GC | ||||
|---|---|---|---|---|---|---|
| Optimized Concentration/% | Accuracy/% rel. | LOD/ ppm | Calibrated Concentration/% | Accuracy/% rel. | LOD/ ppm | |
| O2 | - | - | - | 0–20 | ±5 | 14 |
| N2 | - | - | - | 22–100 | ±3 | 220,000 |
| H2 | - | - | - | 0.1–35 | ±6 | 22 |
| C2H2 | 0–10 | ±4 | 81 | 0.1–5 | ±4 | 200 |
| C2H4 | 2–10 | ±5 | 14 | 0.1–5 | ±4 | 195 |
| C2H6 | 0–10 | ±6 | 33 | 0.1–2 | ±5 | 184 |
| CH4 | 0–10 | ±4 | 114 | 0.1–5 | ±5 | 272 |
| CO | 0–30 | ±4 | 65 | 0.1–55 | ±6 | 534 |
| CO2 | 0–35 | ±4 | 121 | 0.1–28 | ±4 | 189 |
| DEC | - | ±4 | 20 | - | - | - |
| DMC | - | ±4 | 28 | - | - | - |
| EC | - | ±4 | 2 | - | - | - |
| EMC | - | ±4 | 25 | - | - | - |
| H2O | 0–3 | ±4 | 120 | - | - | - |
| C6H14 | - | ±4 | 16 | - | - | - |
| HF | 0–30 | ±4(min 5 ppm) | 4 | - | - | - |
| C4H10 | - | ±4 | 15 | - | - | - |
| C3H8 | - | 30 | - | - | - | |
| Gas | 100% SOC | 30% SOC | ||||
|---|---|---|---|---|---|---|
| Measured Gas | Vent Gas (without N2) | Vent Gas (without N2) | Measured Gas | Vent Gas (without N2) | Vent Gas (without N2) | |
| cm/%vol | cv/% vol | cv/mol | cm/%vol | cv/% vol | cv/mol | |
| O2 | 0.01 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| N2 | 69.21 | 89.01 | ||||
| H2 | 7.06 | 22.93 | 0.53 | 0.41 | 4.47 | 0.02 |
| C2H2 | 0.02 | 0.05 | 0.00 | 0.01 | 0.12 | 0.00 |
| C2H4 | 1.81 | 5.88 | 0.14 | 0.27 | 2.93 | 0.02 |
| C2H6 | 0.30 | 0.99 | 0.02 | 0.03 | 0.36 | 0.00 |
| CH4 | 1.06 | 3.46 | 0.08 | 0.05 | 0.52 | 0.00 |
| CO | 5.11 | 16.59 | 0.38 | 0.47 | 5.15 | 0.03 |
| CO2 | 11.80 | 38.33 | 0.89 | 4.39 | 47.73 | 0.25 |
| DEC | 0.83 | 2.69 | 0.06 | 1.91 | 20.72 | 0.11 |
| DMC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| EC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| EMC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H2O | 2.32 | 7.55 | 0.17 | 1.61 | 17.50 | 0.09 |
| C6H14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| HF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C4H10 | 0.39 | 1.26 | 0.03 | 0.00 | 0.00 | 0.00 |
| C3H8 | 0.00 | 0.00 | 0.00 | 0.05 | 0.50 | 0.00 |
| Gas amount | 2.31 mol | 0.53 mol | ||||
| Area of Particles/µm2 | Average Number of Particles/% |
|---|---|
| 1 < a ≤ 2 | 21.8 ± 7.6 |
| 2 < a ≤ 3 | 11.6 ± 2.2 |
| 3 < a ≤ 5 | 12.2 ± 2.7 |
| 5 < a ≤ 10 | 15.8 ± 0.6 |
| 10 < a ≤ 50 | 26.2 ± 5.5 |
| 50 < a ≤ 100 | 6.6 ± 3.4 |
| 100 < a | 5.9 ± 5.5 |
| Safety Relevant Parameter | 100% SOC | 30% SOC | 0% SOC |
|---|---|---|---|
| First venting (electrolyte vapor) | yes | yes | yes |
| Thermal runaway | yes | no | no |
| Start voltage (V) | 4.18 | 3.67 | 3.11 |
| Heat ramp (°C/min) | 0.39 | 0.36 | 0.33 |
| (°C) | 130 | 127 | 120 |
| (°C) | 203 | 190 | 190 |
| (°C) | 231 | - | - |
| (°C) | 715 (self-heating) | 309 (external heating) | 242 (external heating) |
| Duration of TR (s) | 4 | - | - |
| Amount of vent gas nv (mol) | 2.31 (57 l) | 0.53 (13 l) | 0.41 (10 l) |
| Characteristic venting rate ch (mol/s) | 0.8 (18.7 L/s) | - | - |
| Main gas compounds | CO2, CO, H2 | DEC, CO2, H2O | DEC, CO2, H2O |
| CH2 (vol%) | 23 | 4 | 1 |
| CCO (vol%) | 17 | 5 | 1 |
| Particle release (g) | ~300 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries 2020, 6, 30. https://doi.org/10.3390/batteries6020030
Essl C, Golubkov AW, Gasser E, Nachtnebel M, Zankel A, Ewert E, Fuchs A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries. 2020; 6(2):30. https://doi.org/10.3390/batteries6020030
Chicago/Turabian StyleEssl, Christiane, Andrey W. Golubkov, Eva Gasser, Manfred Nachtnebel, Armin Zankel, Eduard Ewert, and Anton Fuchs. 2020. "Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments" Batteries 6, no. 2: 30. https://doi.org/10.3390/batteries6020030
APA StyleEssl, C., Golubkov, A. W., Gasser, E., Nachtnebel, M., Zankel, A., Ewert, E., & Fuchs, A. (2020). Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries, 6(2), 30. https://doi.org/10.3390/batteries6020030