A Newly Designed Modular ZnBr2 Single Cell Structure
Abstract
1. Introduction
2. Experimental
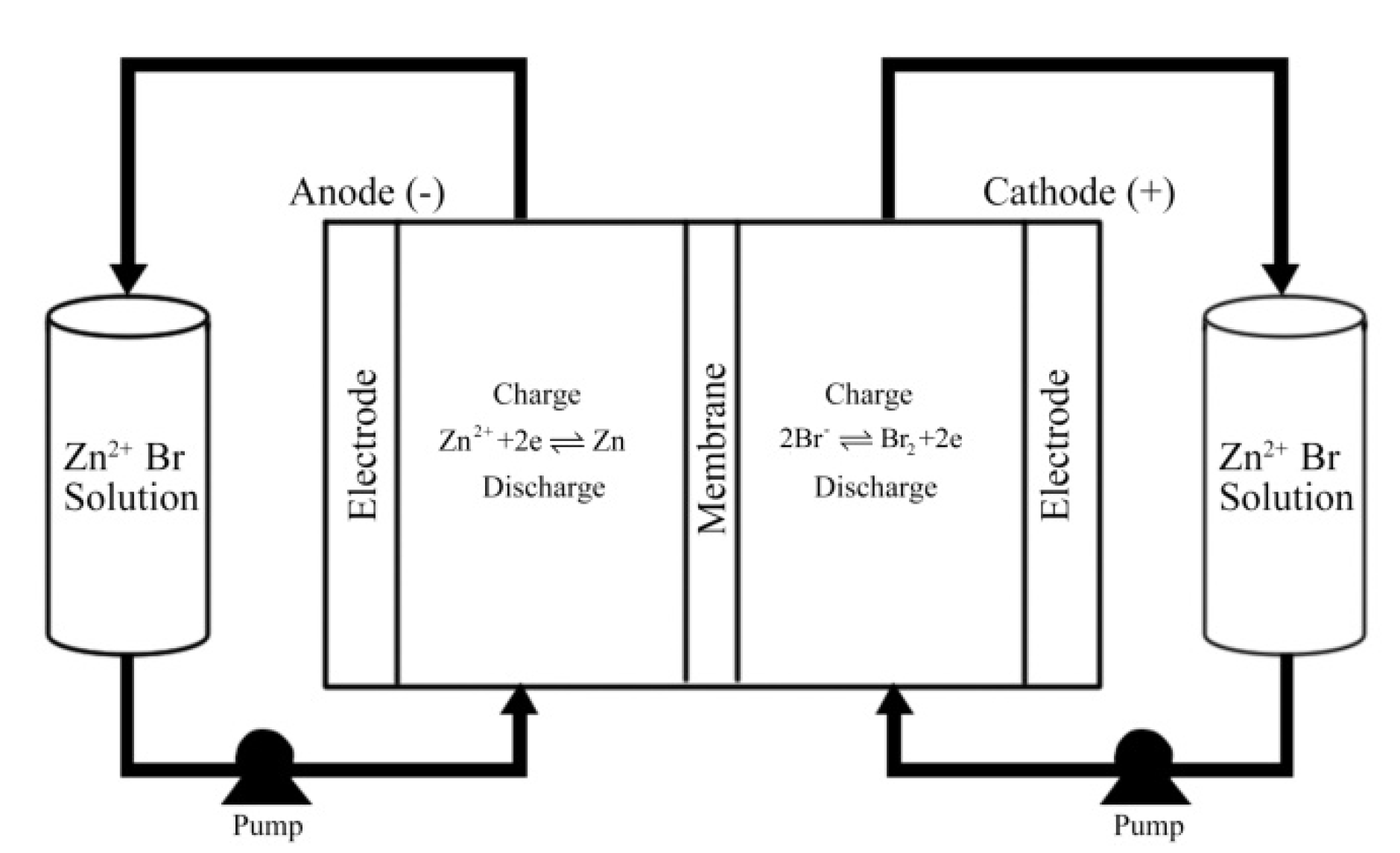
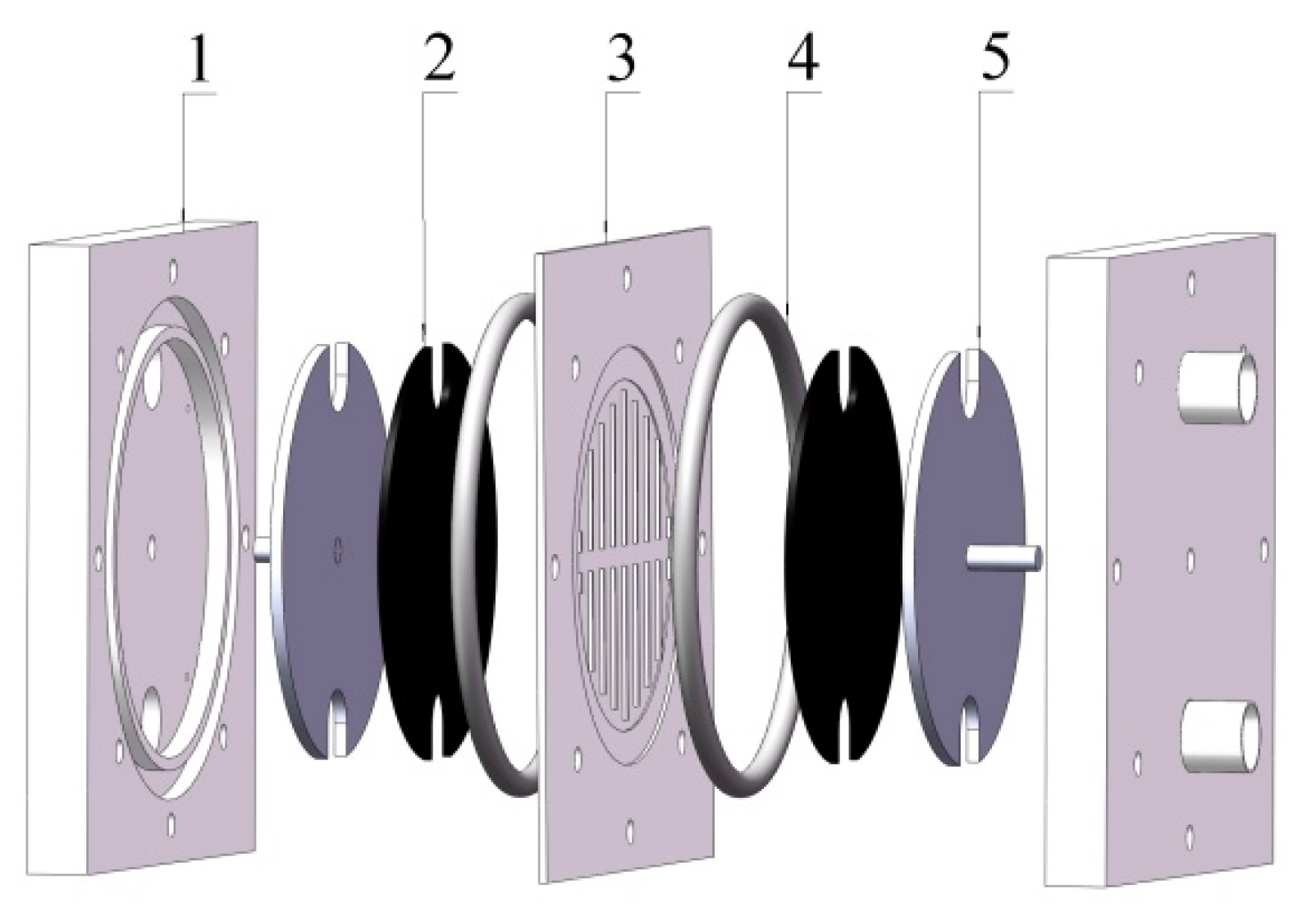
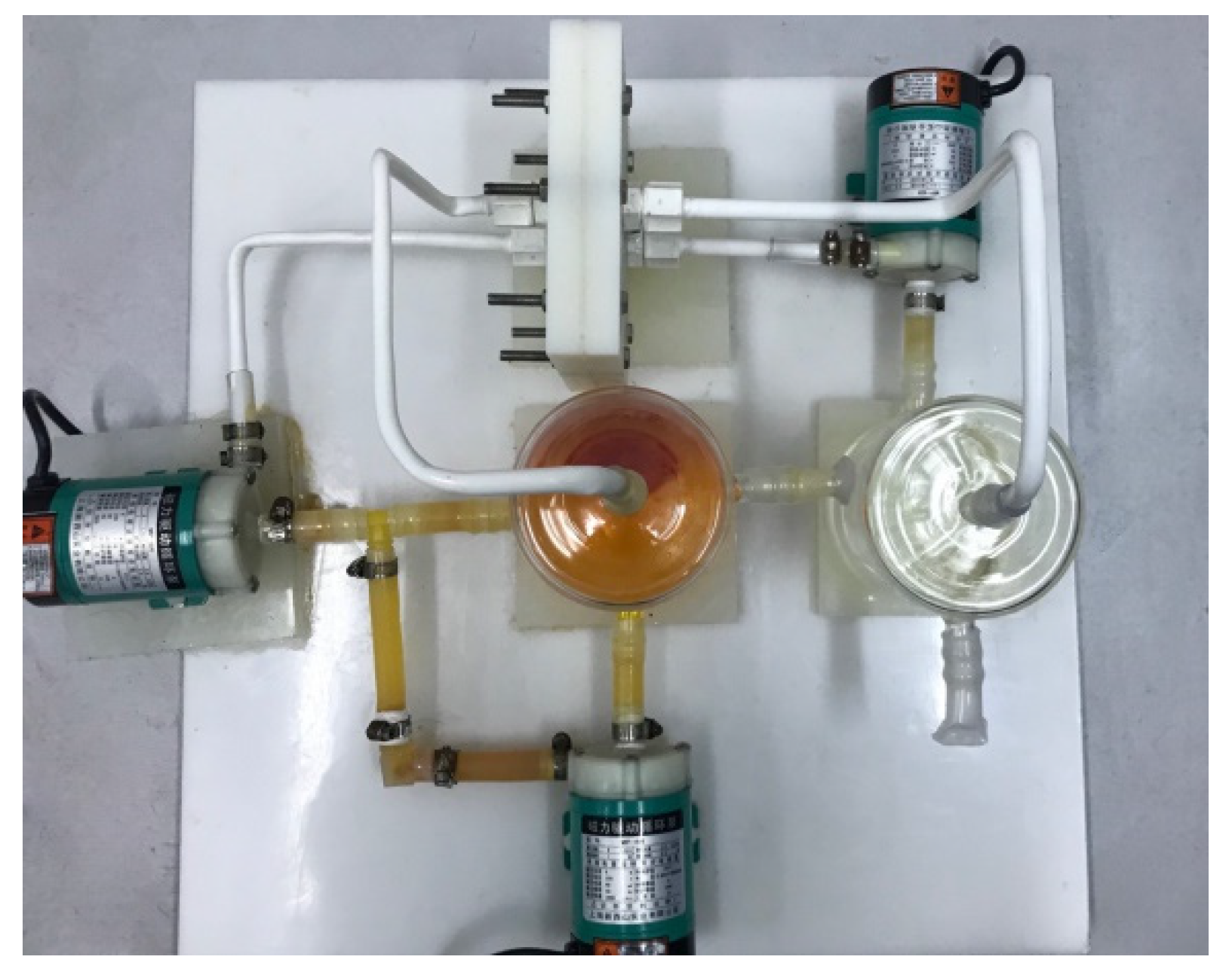
2.1. Flow Frame ZnBr2 Single Cell
2.2. Electrode and Membrane Frame
2.3. Electrolyte Configuration
3. Results and Discussion
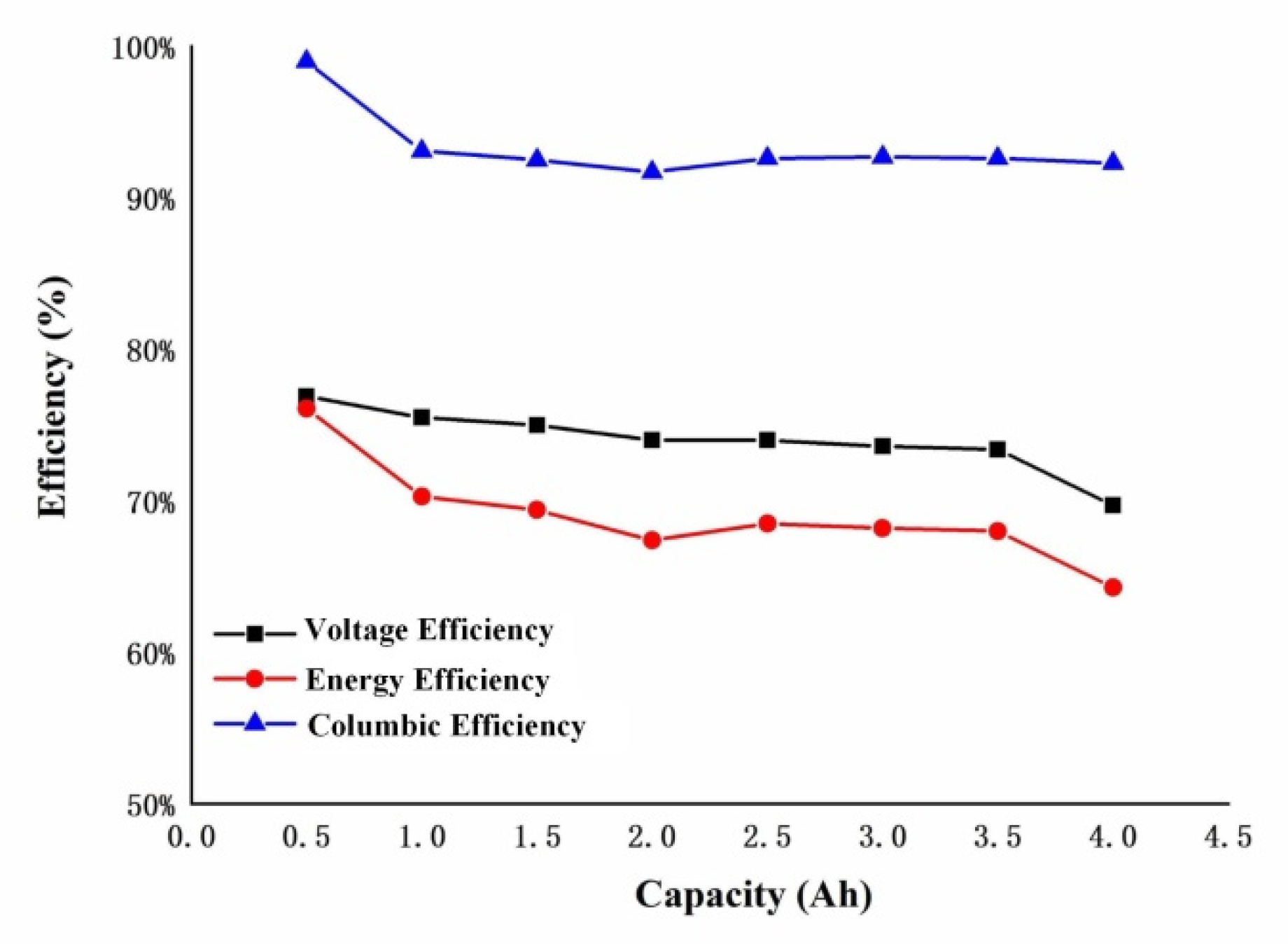
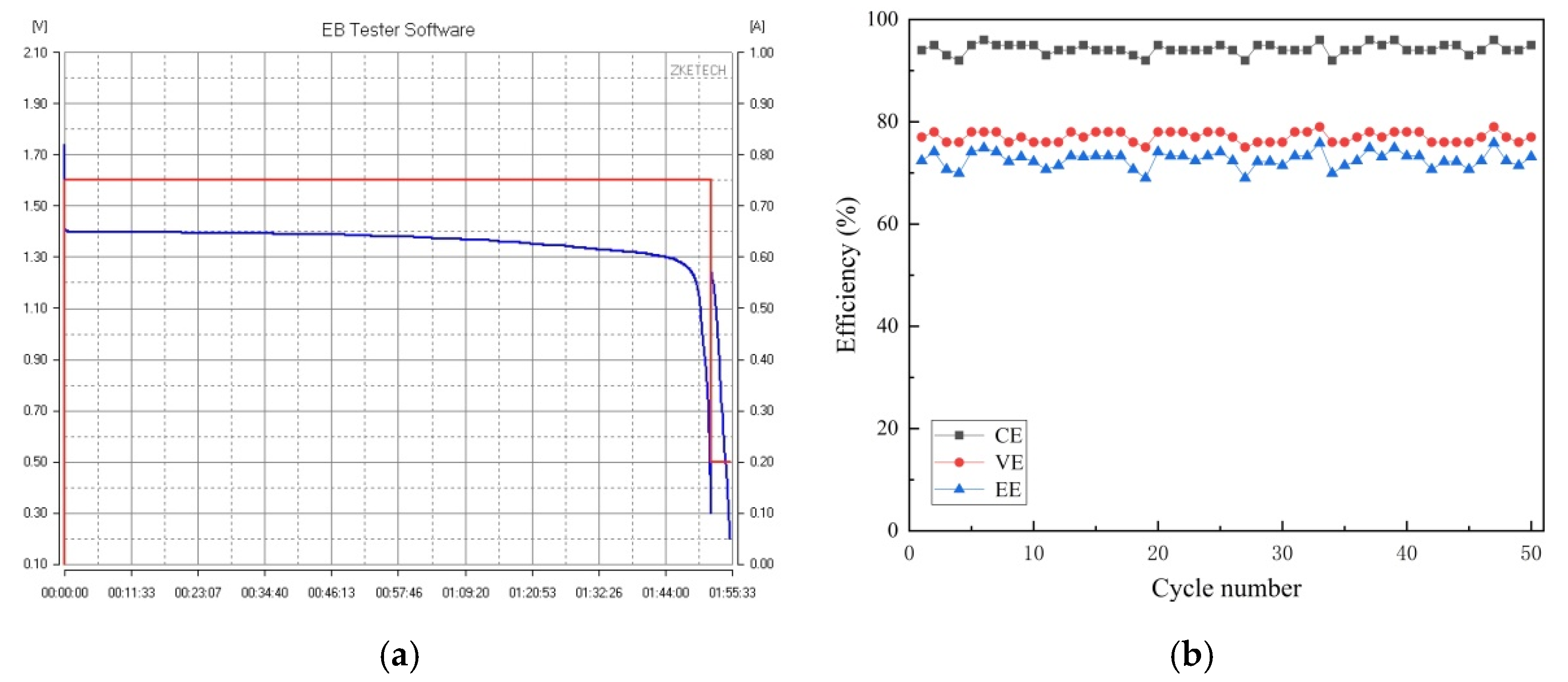
3.1. Pre-Preparation of the System Cycle
3.2. Electrolyte Configuration
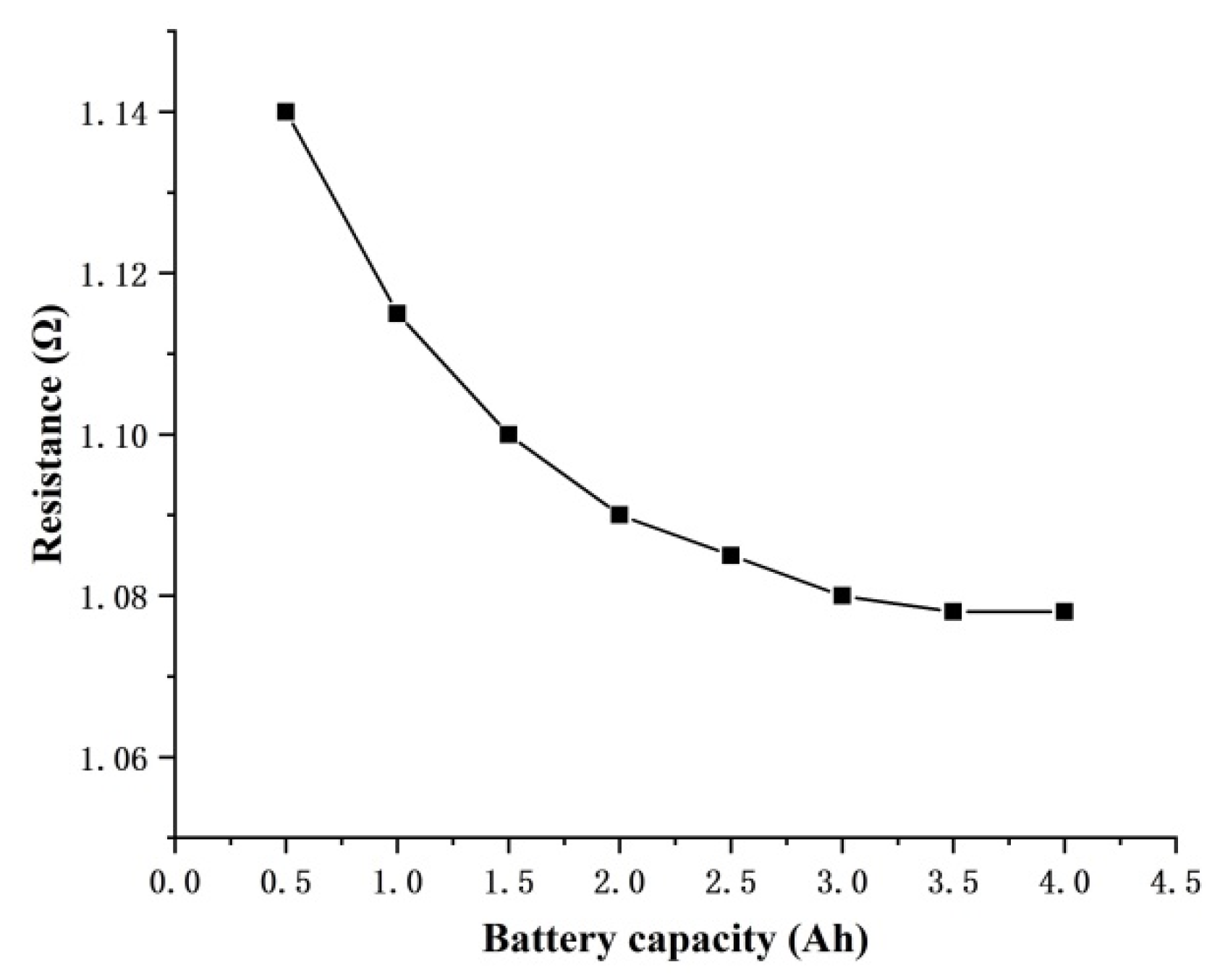
3.3. Internal Resistance of the Flow Cell
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent Advancements in All-Vanadium Redox Flow Batteries. Adv. Mater. Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Wang, C.; Lai, Q.; Xu, P.; Li, X.; Zhang, H. A non-aqueous Li/organosulfur semi-solid flow battery. Chin. Chem. Lett. 2018, 29, 716–718. [Google Scholar] [CrossRef]
- Ma, T.; Pan, Z.; Miao, L.; Chen, C.; Han, M.; Shang, Z.; Chen, J. Porphyrin-Based Symmetric Redox-Flow Batteries towards Cold-Climate Energy Storage. Angew. Chem. 2018, 130, 3212–3216. [Google Scholar] [CrossRef]
- Khor, A.; Leung, P.; Mohamed, M.R.; Flox, C.; Xu, Q.; An, L.; Wills, R.G.A.; Morante, J.R.; Shah, A.A. Review of zinc-based hybrid flow batteries: From fundamentals to applications. Mater. Today Energy 2018, 8, 80–108. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Wei, L. Critical transport issues for improving the performance of aqueous redox flow batteries. J. Power Sources 2017, 339, 1–12. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, T.; Sun, Q.; Zhao, G.; Shi, J. The Research Progress of Zinc Bromine Flow Battery. J. New Mater. Electrochem. Syst. 2018, 21, 63–70. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Xi, X.; Xu, P. RSC Advances Relationship between activity and structure of carbon materials for Br 2/Br À in zinc bromine flow. RSC Adv. 2016, 6, 40169–40174. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Jiang, H.R.; Zeng, Y.K.; Ren, Y.X. High-performance zinc bromine flow battery via improved design of electrolyte and electrode. J. Power Sources 2017, 355, 62–68. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, T.; Zhang, R.; Jiang, H.; Wei, L. A Zinc-Bromine Flow Battery with Improved Design of Cell Structure and Electrodes. Energy Technol. 2018, 6, 333–339. [Google Scholar] [CrossRef]
- Nagai, Y.; Komiyama, R.; Miyashita, H.; Lee, S. Miniaturisation of Zn/Br redox flow battery cell and investigation of electrode materials influence on its characteristics. Micro Nano Lett. 2016, 11, 577–581. [Google Scholar] [CrossRef]
- Li, B.; Gu, M.; Nie, Z.; Shao, Y.; Luo, Q.; Wei, X.; Li, X.; Xiao, J.; Wang, C.; Sprenkle, V.; et al. Bismuth nanoparticle decorating graphite felt as a high-performance electrode for an all-vanadium redox flow battery. Nano Lett. 2013, 13, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Cedzynska, K. Properties of modified electrolyte for zinc-bromine cells. Electrochim. Acta 1995, 40, 971–976. [Google Scholar] [CrossRef]
- Biswas, S.; Senju, A.; Mohr, R.; Hodson, T.; Karthikeyan, N.; Knehr, K.W.; Hsieh, A.G.; Yang, X.; Koel, B.E.; Steingart, D.A. Minimal architecture zinc-bromine battery for low cost electrochemical energy storage. Energy Environ. Sci. 2017, 10, 114–120. [Google Scholar] [CrossRef]
- Rajarathnam, G.P.; Easton, M.E.; Schneider, M.; Masters, A.F.; Maschmeyer, T.; Vassallo, A.M. The influence of ionic liquid additives on zinc half-cell electrochemical performance in zinc/bromine flow batteries. RSC Adv. 2016, 6, 27788–27797. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Li, L.; Ma, M. Photoactivity of titanium dioxide/carbon felt composites prepared with the assistance of supercritical carbon dioxide: Effects of calcination temperature and supercritical conditions. Sci. China Chem. 2011, 54, 497–505. [Google Scholar] [CrossRef]
- Leung, P.K.; Martin, T.; Shah, A.A.; Anderson, M.A.; Palma, J. Membrane-less organic–inorganic aqueous flow batteries with improved cell potential. Chem. Commun. 2016, 52, 14270–14273. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Zhang, C. Performance of a vanadium redox flow battery with and without flow fields. Electrochim. Acta 2014, 142, 61–67. [Google Scholar] [CrossRef]
- Jiang, H.R.; Wu, M.C.; Ren, Y.X.; Shyy, W.; Zhao, T.S. Towards a uniform distribution of zinc in the negative electrode for zinc bromine flow batteries. Appl. Energy 2018, 213, 366–374. [Google Scholar] [CrossRef]
- Winardi, S.; Poon, G.; Ulaganathan, M.; Parasuraman, A.; Yan, Q.; Wai, N.; Lim, T.M.; Skyllas-Kazacos, M. Effect of bromine complexing agents on the performance of cation exchange membranes in second-generation vanadium bromide battery. Chempluschem 2015, 80, 376–381. [Google Scholar] [CrossRef]
- Park, S.-K.; Shim, J.; Yang, J.H.; Jin, C.-S.; Lee, B.S.; Lee, Y.-S.; Shin, K.-H.; Jeon, J.-D. The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery. Electrochim. Acta 2014, 116, 447–452. [Google Scholar] [CrossRef]
- Yang, H.S.; Park, J.H.; Ra, H.W.; Jin, C.S.; Yang, J.H. Critical rate of electrolyte circulation for preventing zinc dendrite formation in a zinc-bromine redox flow battery. J. Power Sources 2016, 325, 446–452. [Google Scholar] [CrossRef]
- Modestov, A.D.; Konev, D.V.; Tripachev, O.V.; Antipov, A.E.; Tolmachev, Y.V.; Vorotyntsev, M.A. A Hydrogen-Bromate Flow Battery for Air-Deficient Environments. Energy Technol. 2018, 6, 242–245. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, J.; Tseng, K.J.; Skyllas-Kazacos, M.; Lim, T.M.; Zhang, Y. Thermal hydraulic behavior and efficiency analysis of an all-vanadium redox flow battery. J. Power Sources 2013, 242, 314–324. [Google Scholar] [CrossRef]
- Song, W.J.; Li, M.Q.; Su, H.; School of Energy and Power Engineering, Dalian University of Technology. Preparation and characterization of stack of zinc-bromine flow battery. Res. Rev. Electrochem. 2013, 4, 121–125. [Google Scholar]
- Yang, J.H.; Yang, H.S.; Ra, H.W.; Shim, J.; Jeon, J.-D. Effect of a surface active agent on performance of zinc/bromine redox flow batteries: Improvement in current efficiency and system stability. J. Power Sources 2015, 275, 294–297. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Zhang, R.H.; Wei, L.; Jiang, H.R. Carbonized tubular polypyrrole with a high activity for the Br2/Br− redox reaction in zinc-bromine flow batteries. Electrochim. Acta 2018, 284, 569–576. [Google Scholar] [CrossRef]
- Mohammadi, F.; Nazri, G.A.; Saif, M. A bidirectional power charging control strategy for Plug-in Hybrid Electric Vehicles. Sustainability 2019, 11, 4317. [Google Scholar] [CrossRef]
- Mohammadi, F. Design, analysis, and electrification of a solar-powered electric vehicle. J. Sol. Energy Res. 2018, 3, 293–299. [Google Scholar]
- Rajarathnam, G.P.; Vassallo, A. Half-Cell Electrochemical Performance of Hybridized Ionic Liquid Additives for Zinc/Bromine Flow Battery Applications. ECS Trans. 2016, 72, 33–55. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Z.; Gong, Y.; Yuan, M.; Li, X. A Newly Designed Modular ZnBr2 Single Cell Structure. Batteries 2020, 6, 27. https://doi.org/10.3390/batteries6020027
Pang Z, Gong Y, Yuan M, Li X. A Newly Designed Modular ZnBr2 Single Cell Structure. Batteries. 2020; 6(2):27. https://doi.org/10.3390/batteries6020027
Chicago/Turabian StylePang, Zongqiang, Yutao Gong, Ming Yuan, and Xin Li. 2020. "A Newly Designed Modular ZnBr2 Single Cell Structure" Batteries 6, no. 2: 27. https://doi.org/10.3390/batteries6020027
APA StylePang, Z., Gong, Y., Yuan, M., & Li, X. (2020). A Newly Designed Modular ZnBr2 Single Cell Structure. Batteries, 6(2), 27. https://doi.org/10.3390/batteries6020027




