Degradation and Aging Routes of Ni-Rich Cathode Based Li-Ion Batteries
Abstract
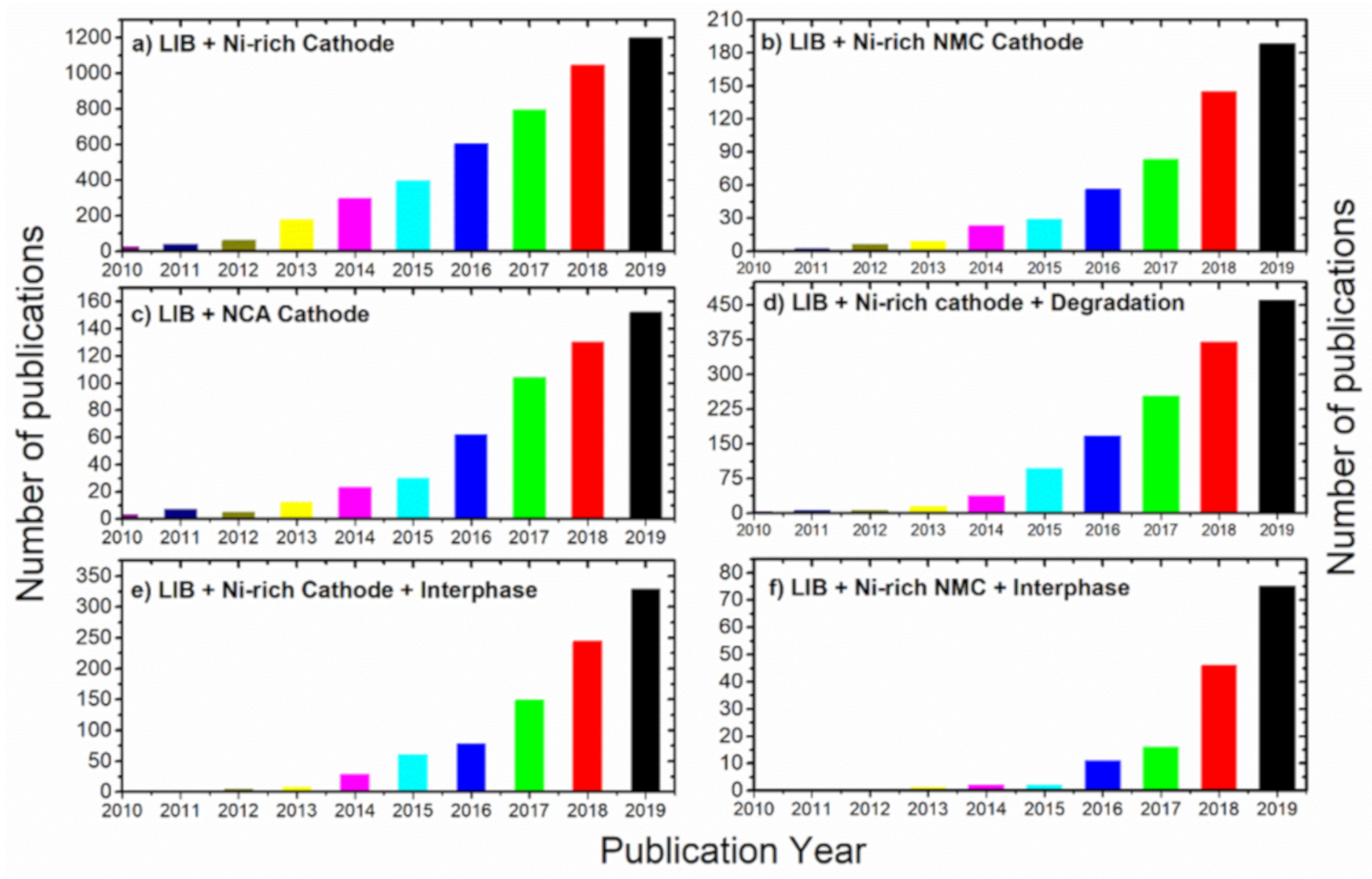
1. Introduction
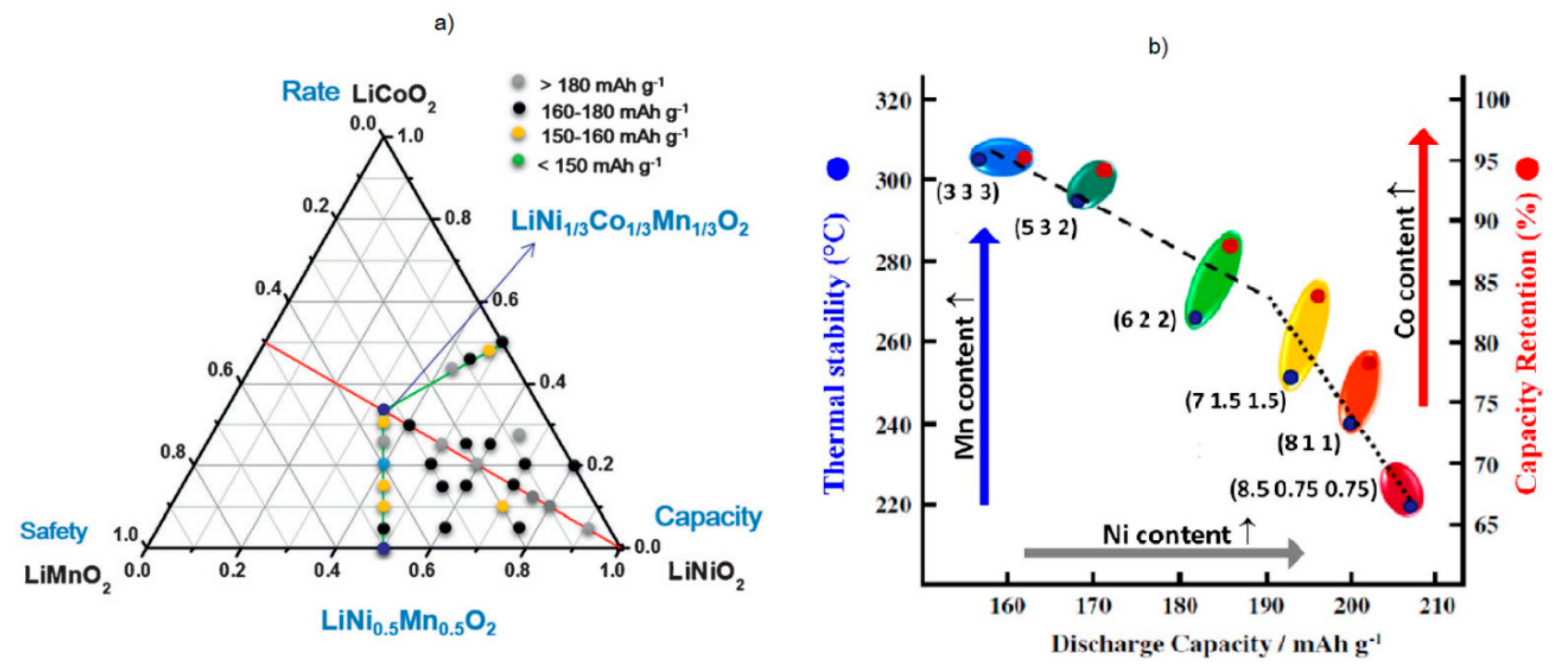
2. Chemistry of Ni-Rich Cathode Materials
3. Challenges and Origins Associated with Ni-Rich Cathode Materials
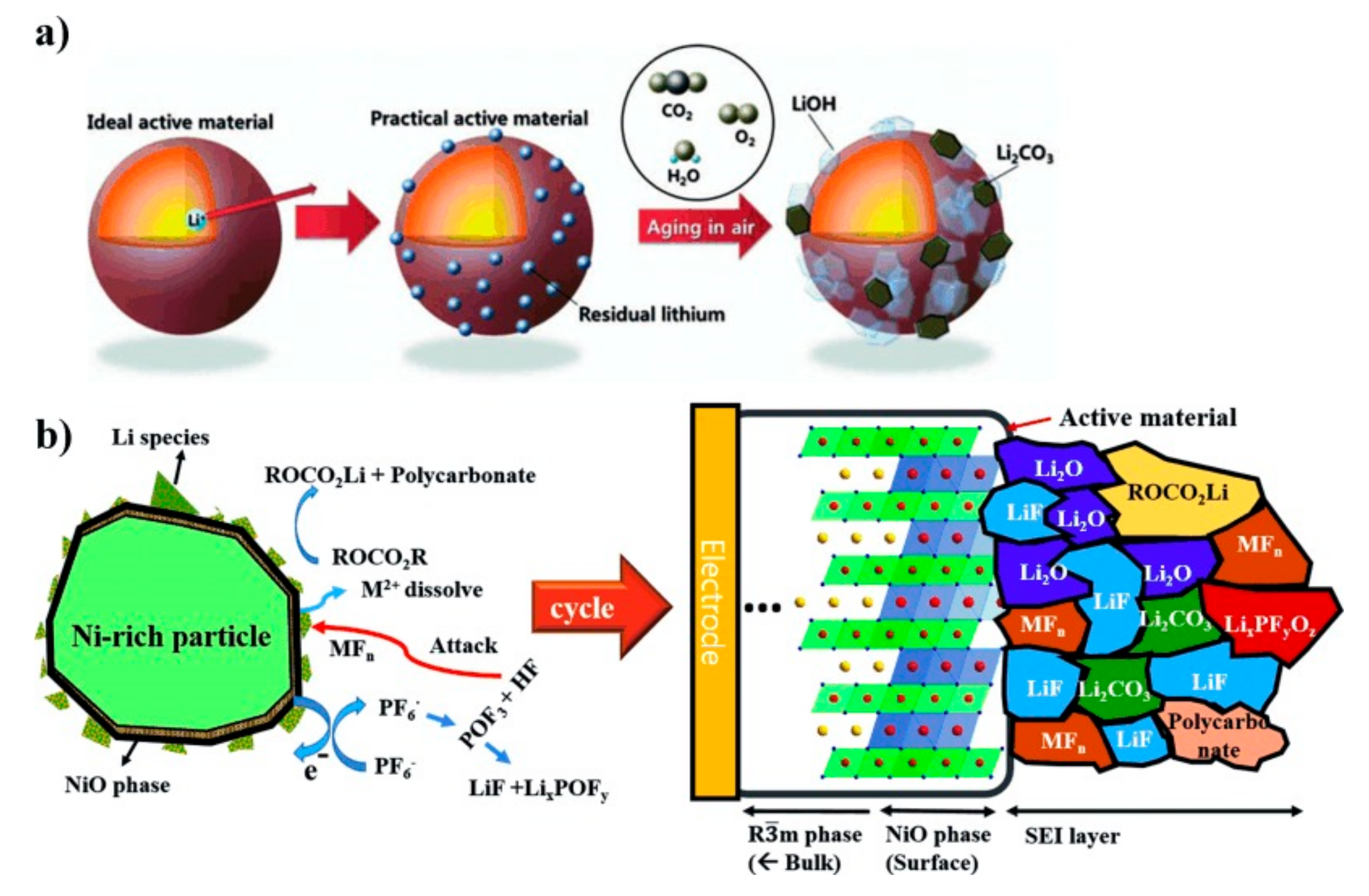
3.1. Lithium Residual Compounds from Synthesis
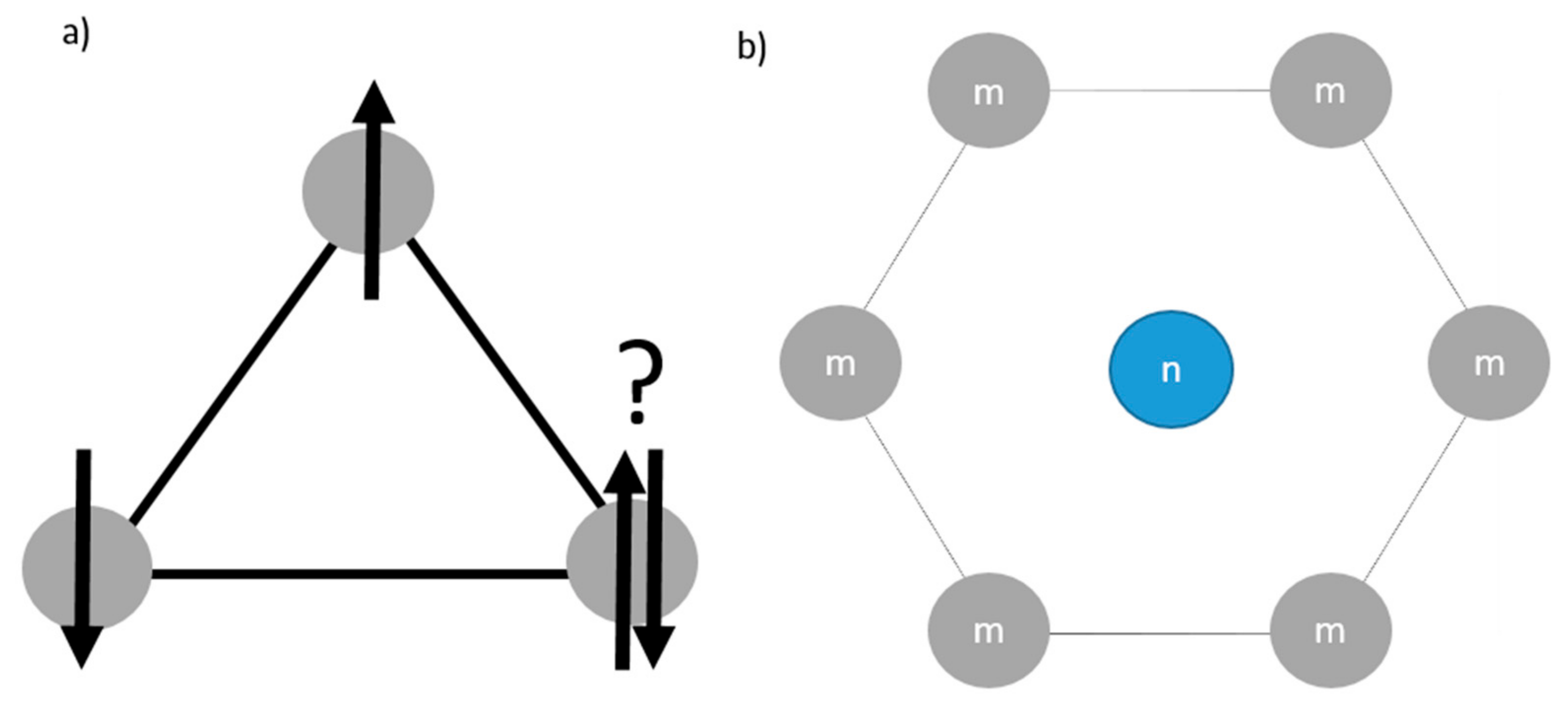
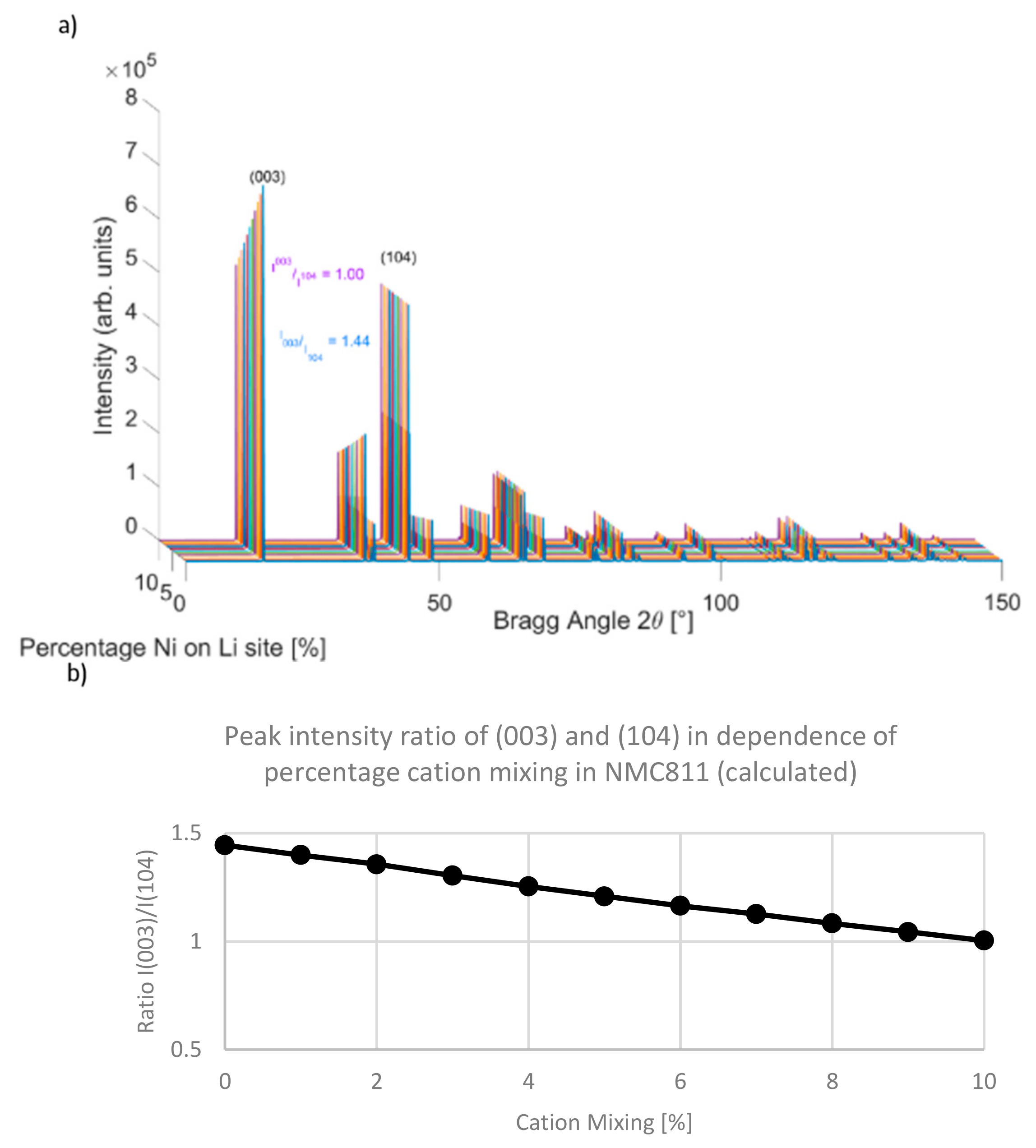
3.2. M/Li Cationic Mixing
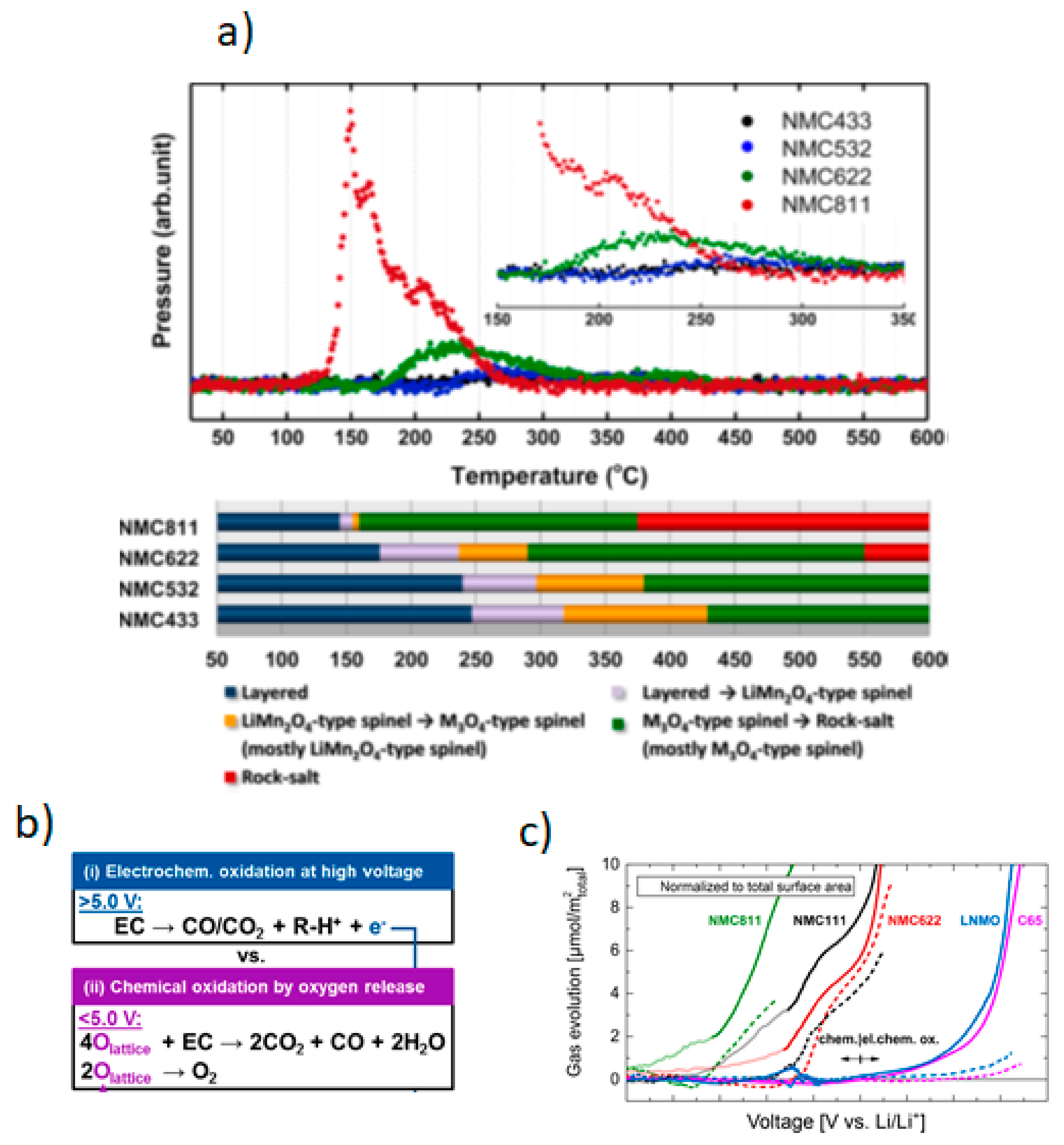
3.3. Safety of Ni-Rich Cathodes
3.4. Effect of Electrode Manufacturing on Ni-Rich Cathode Ageing
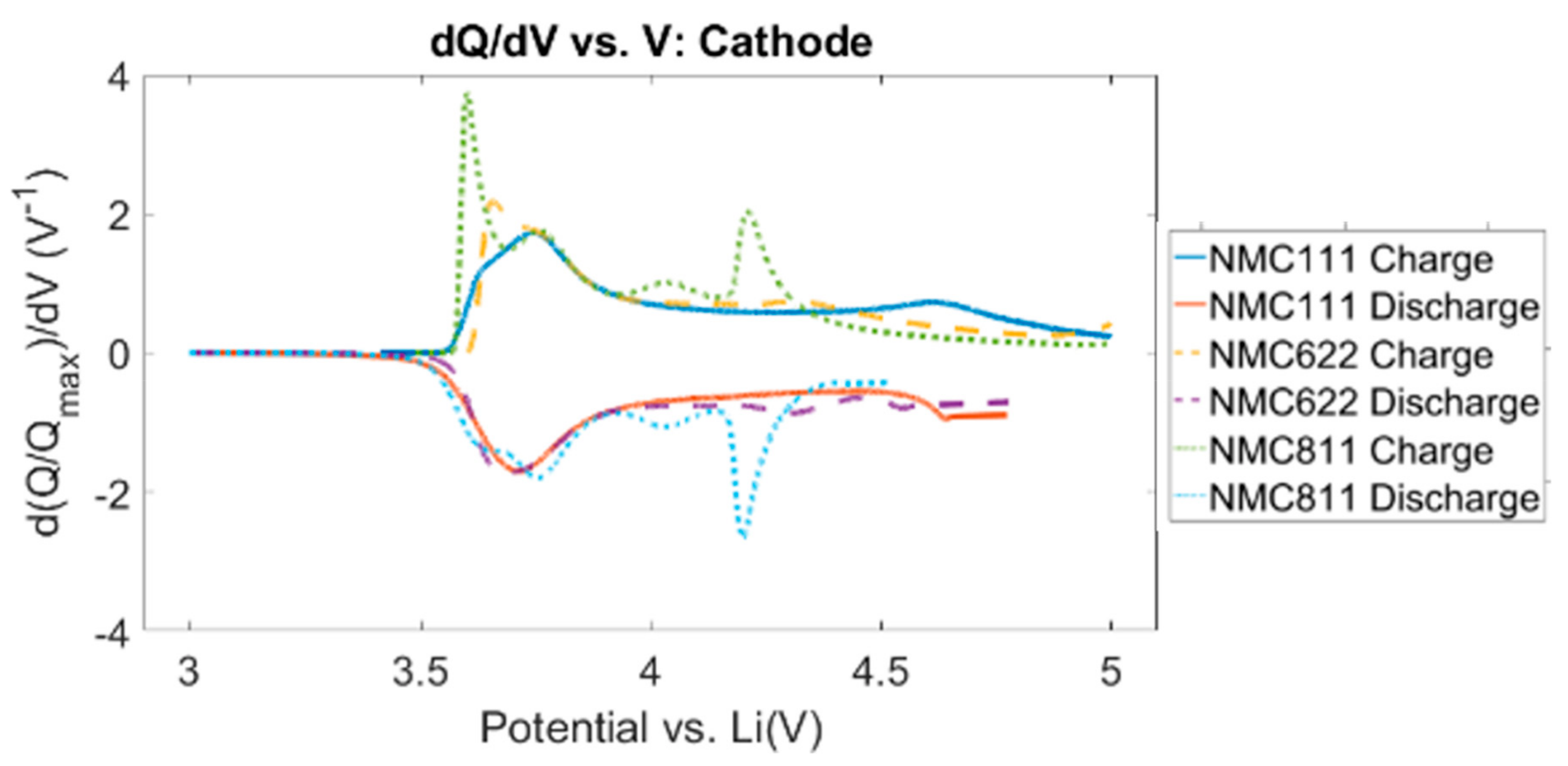
3.5. Ni-Rich Cathode Ageing
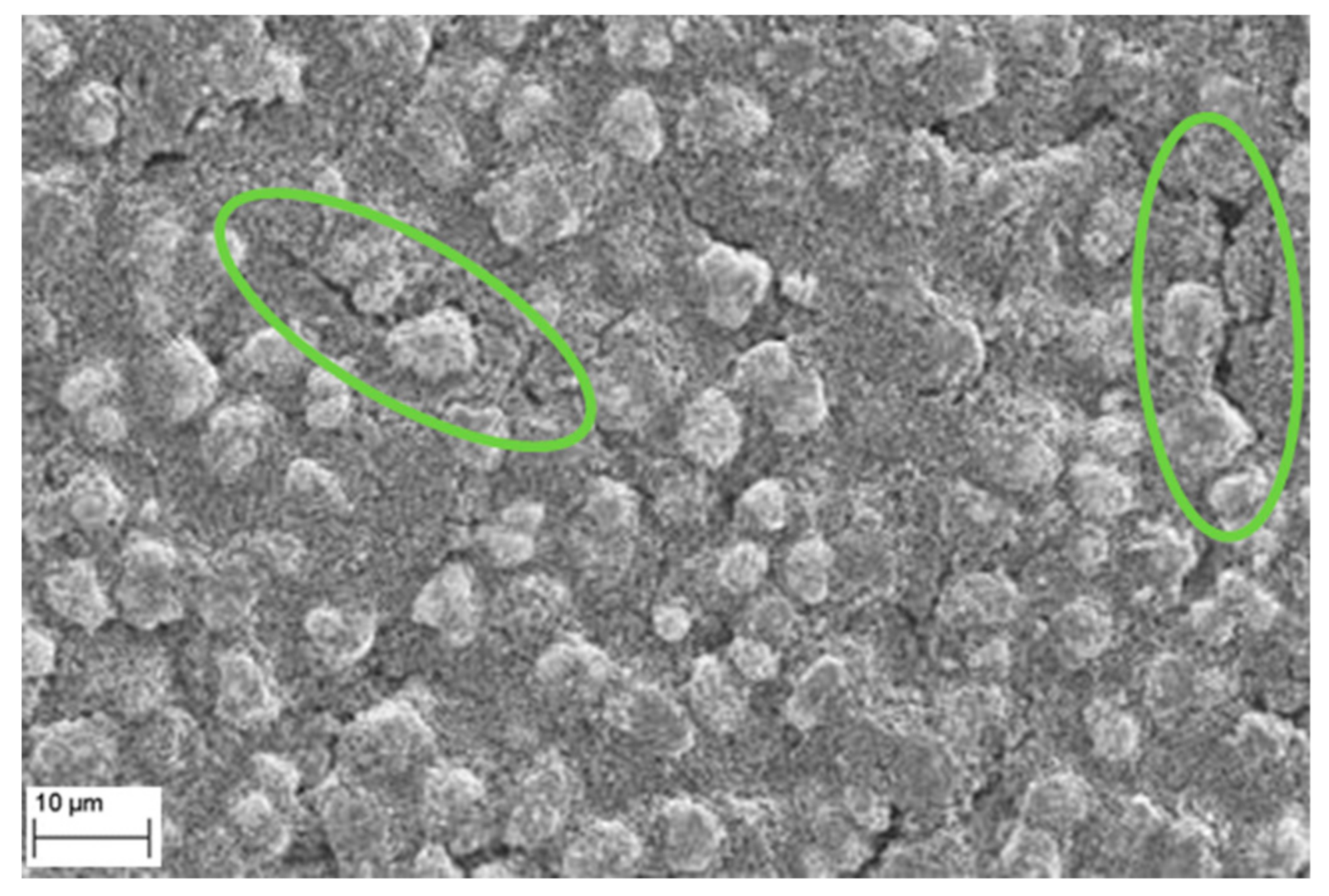
3.5.1. Micro Cracking of Secondary Particle Structure
3.5.2. Electrolyte Degradation and Interphasial Reactions
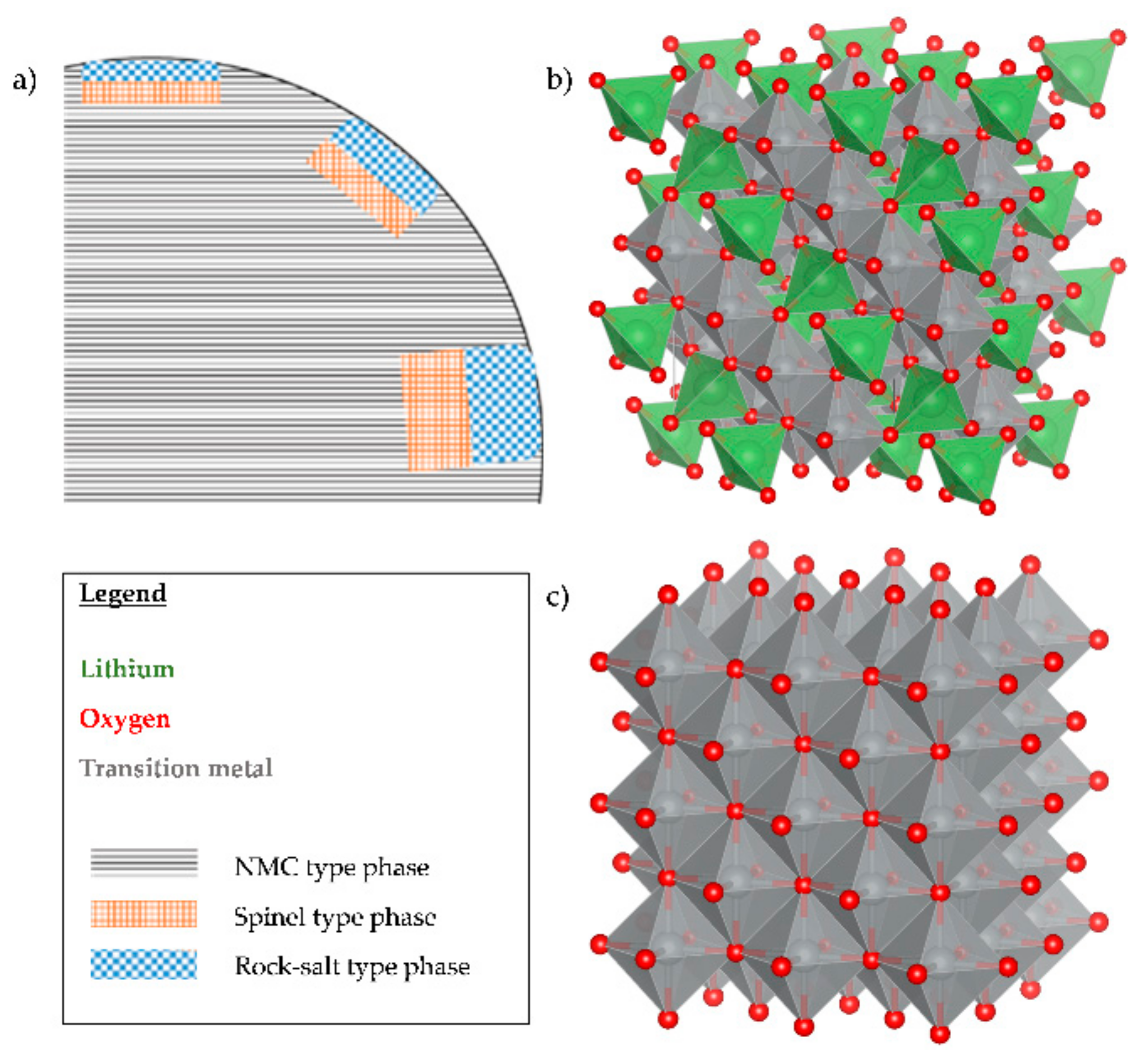
3.5.3. Layered-Spinel to Rock Salt Phase Transition
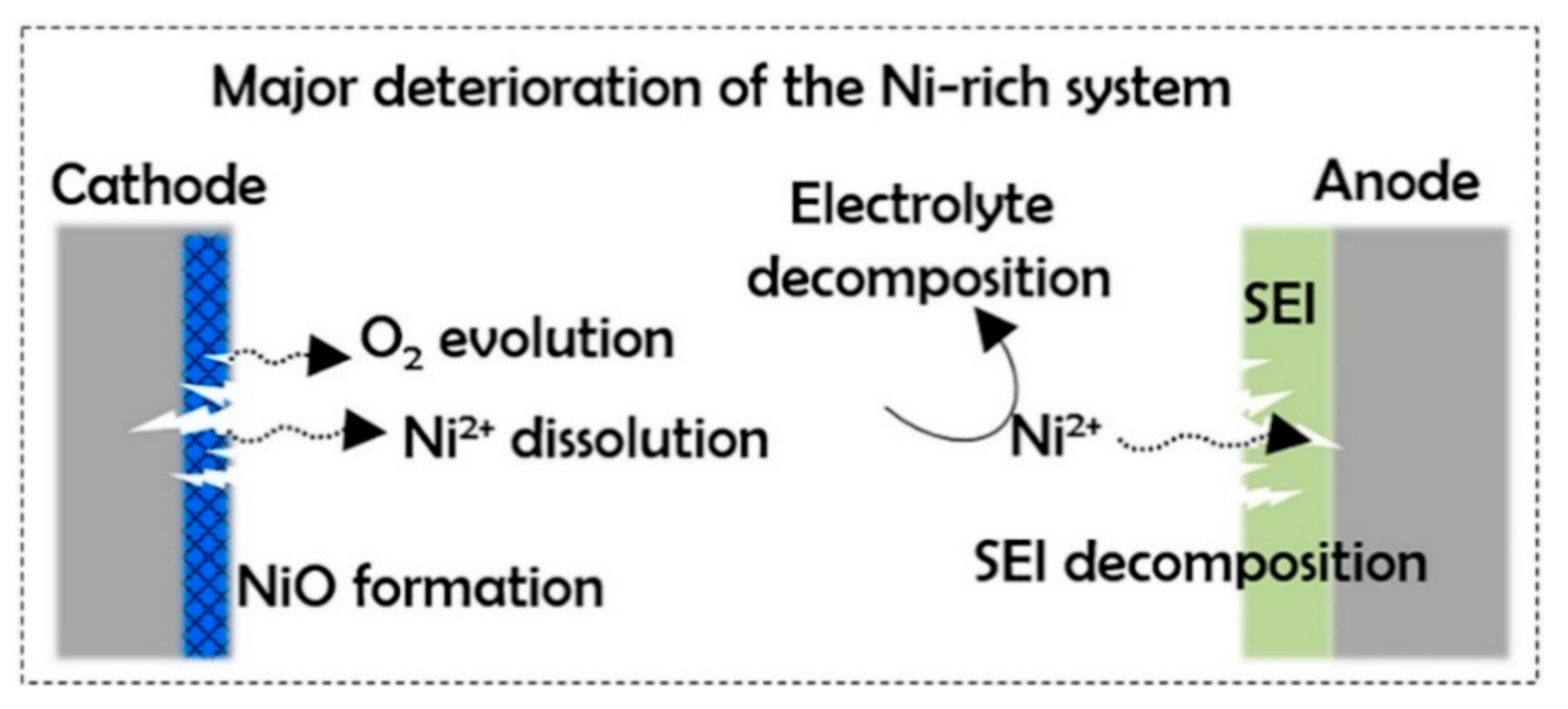
3.5.4. Transition Metal-Ion Dissolution and Anode-Cathode Dialogue
4. Mitigating Strategies
4.1. Tailoring Morphology of Ni-Rich Cathode Particles
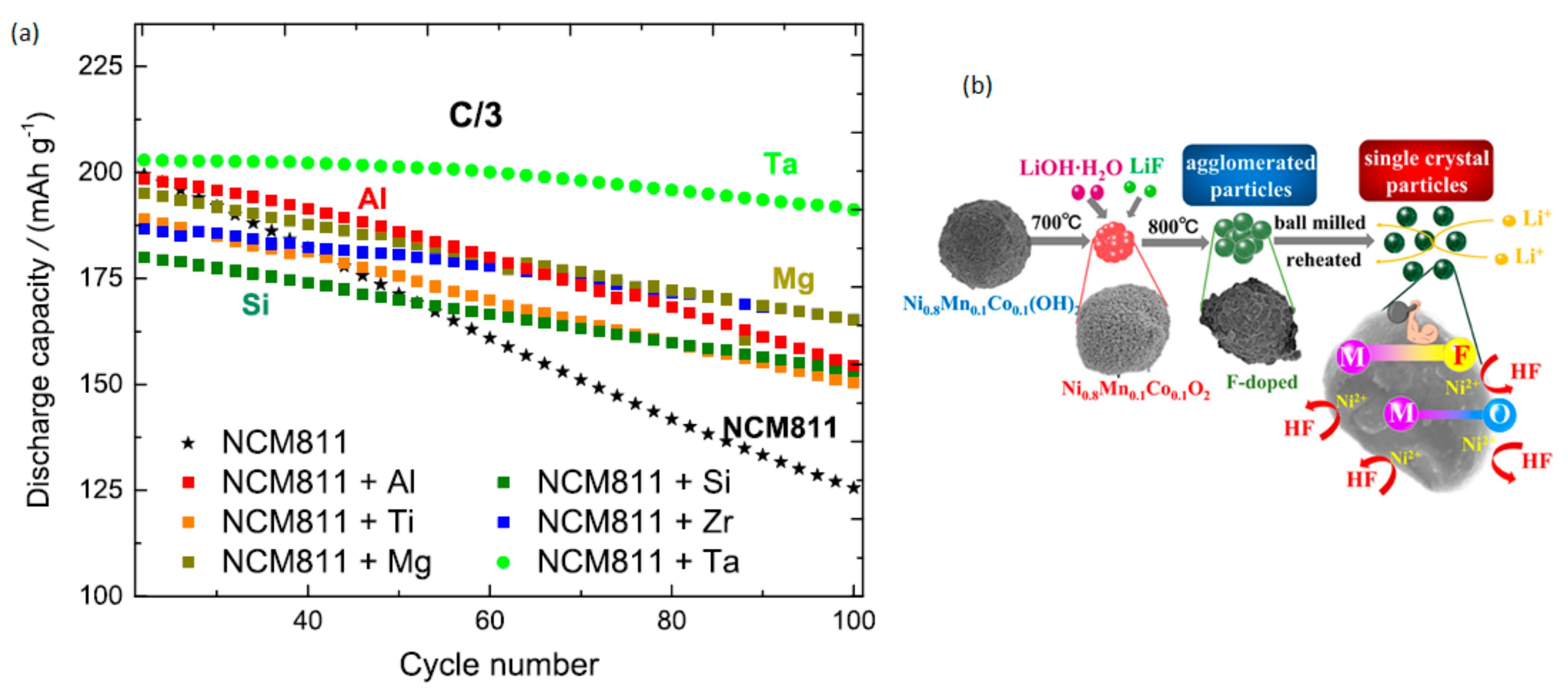
4.2. Foreign-Ion Doping
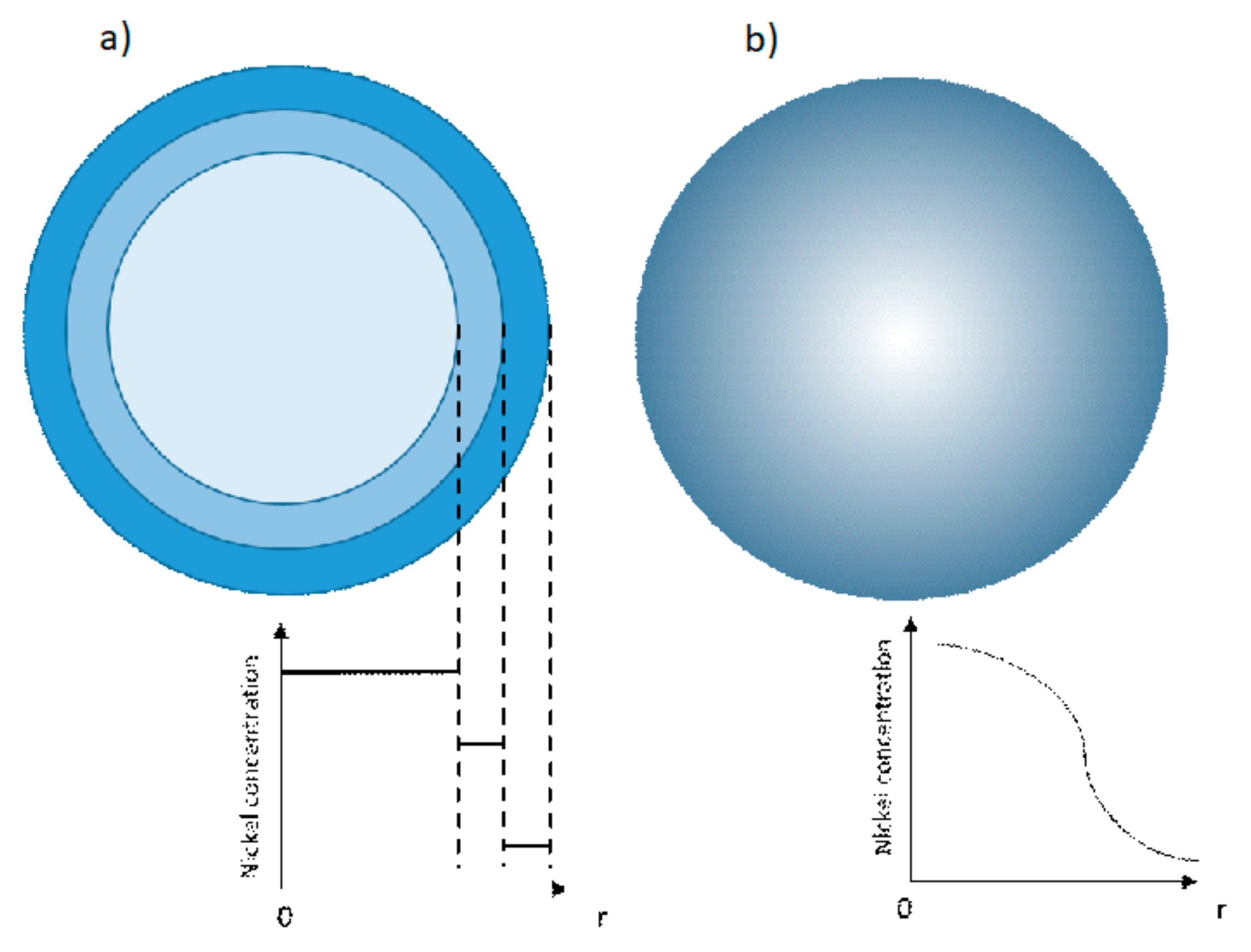
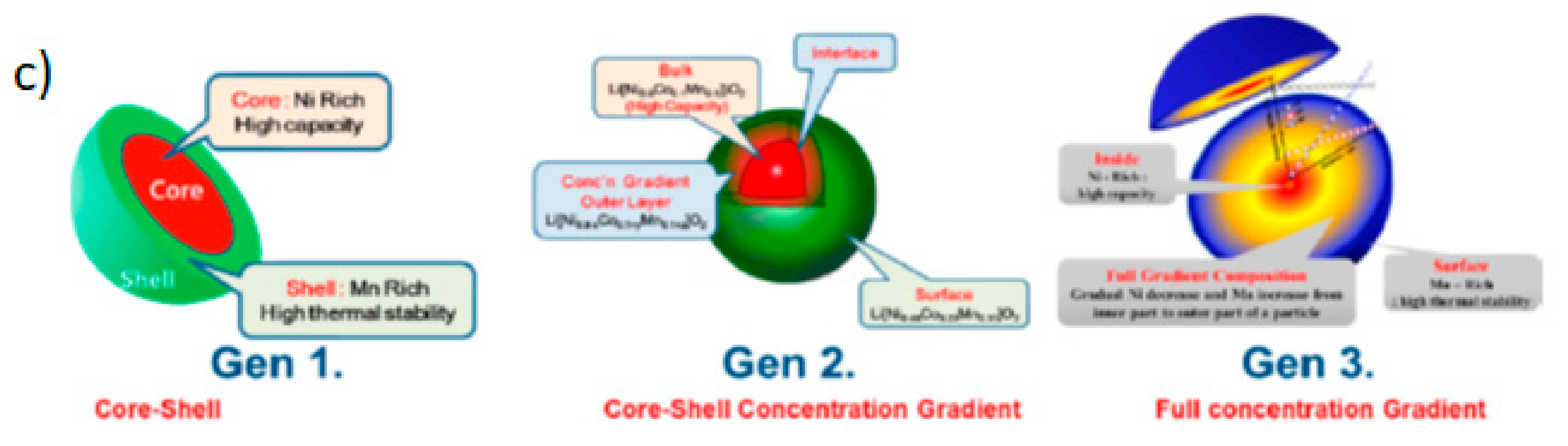
4.3. Core Shell and Gradient: Full Concentration Gradient
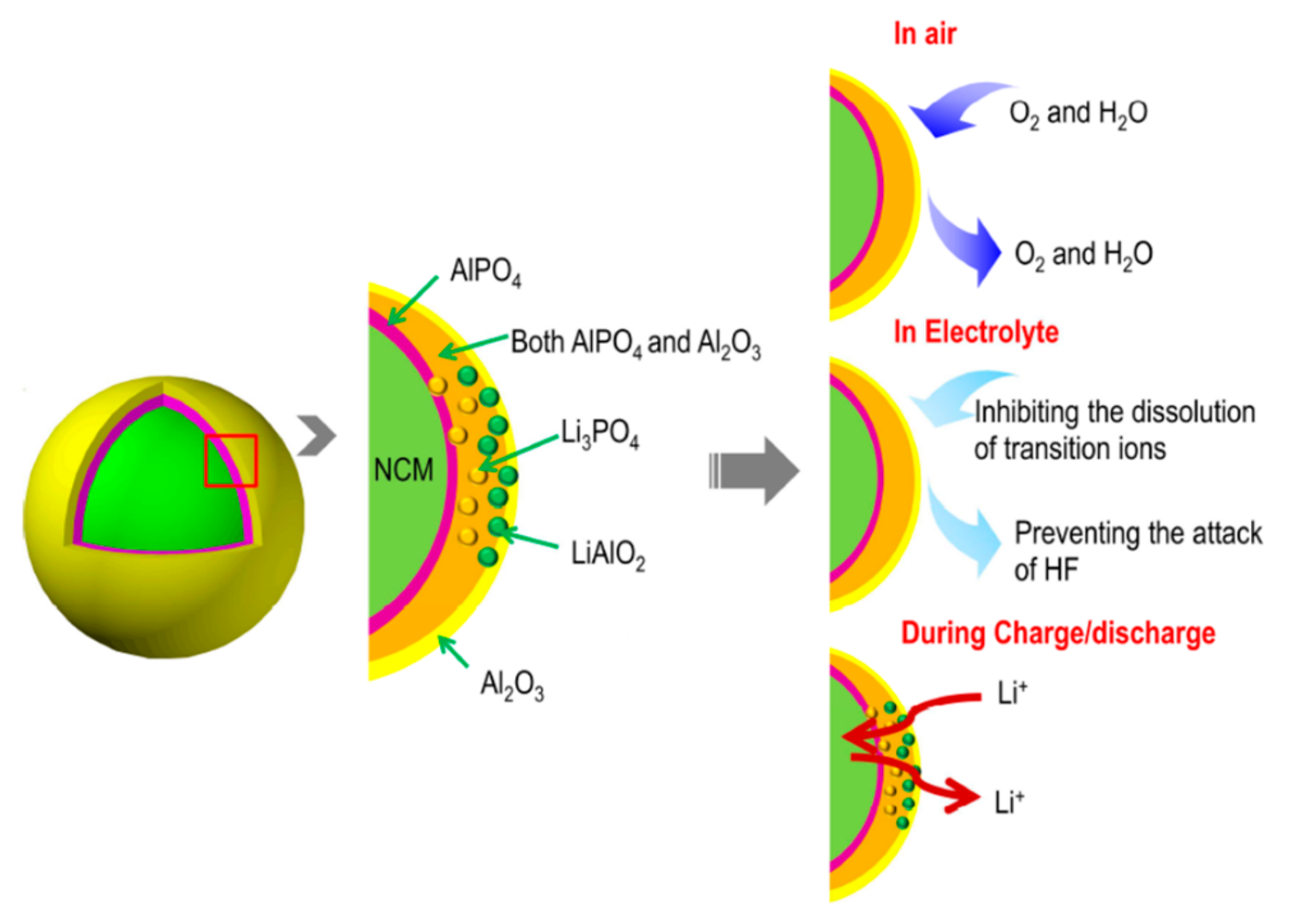
4.4. Surface Modification
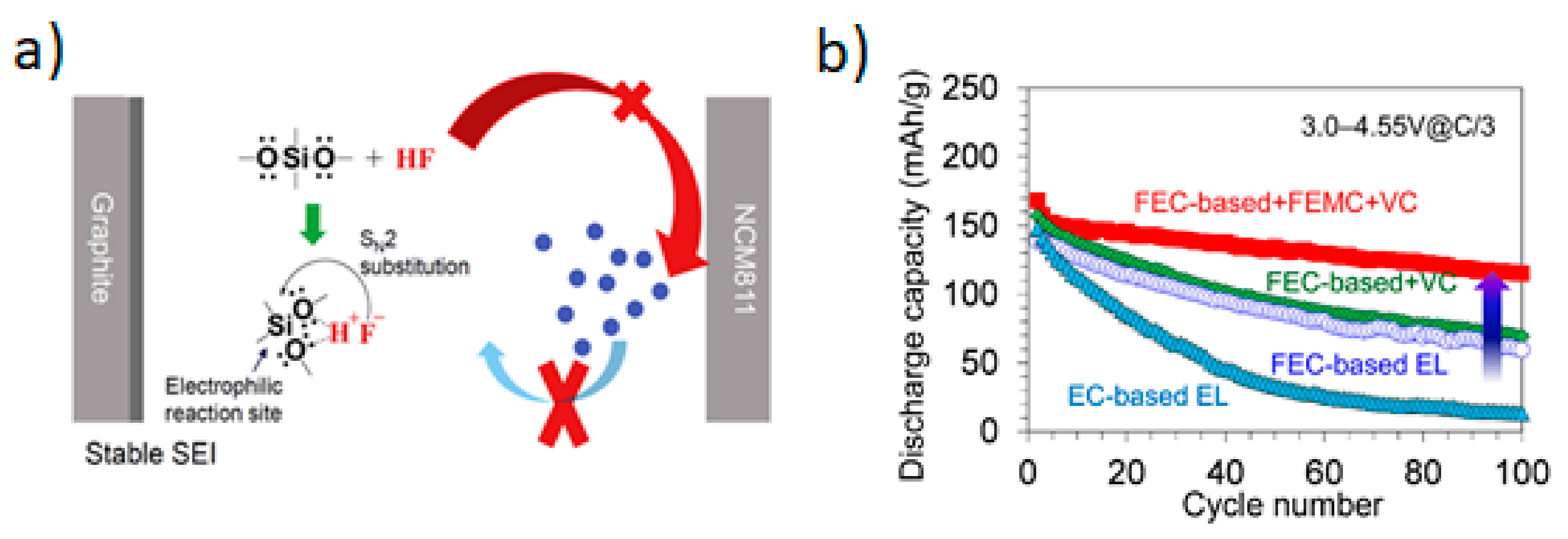
4.5. Functional Electrolyte Additives
4.6. Designer Polymeric Binders
5. Conclusions
- (i)
- Novel electrolyte design: The development of designer electrolyte salt anions and tailored electrolyte additives presents as one of the emerging avenues for the realization of high-voltage nickel rich cathode materials. Salt anions stable at high voltage and those do not result in the formation of reactive species are needed. On the additive side, molecules or ionic salts of multi-functionality and/or single functionality with synergistic effect are desirable.
- (ii)
- Degradation phenomenon: The chemistry, major routes, and ultimate effects of the different degradation mechanisms require an exhaustive investigation and understanding. Of particular interest, the cathode/electrolyte interface and its dialogue with the anode and/or anode-electrolyte interface is of supreme importance and it deserves a deep characterization and, thus, comprehension while using innovative experimental and theoretical calculations. Moreover, the quantification of the different aging behaviors and understanding of the correlations among the various degradation mechanisms of Ni-rich cathode materials will be highly appreciated.
- (iii)
- Safety appraisal: The full-scale safety profiling of high voltage and Ni-rich cathode materials is one of the stern requirement for practical operations and it needs an in-depth investigation enlisting both thermal and chemical threats making use of various arsenal characterization tools.
- (iv)
- Advanced characterization: The use of nondestructive advanced characterization tools is required to better understand and profile the degradation and aging routes.
Author Contributions
Funding
Conflicts of Interest
References
- Eshetu, G.G.; Armand, M.; Ohno, H.; Scrosati, B.; Passerini, S. Ionic Liquids as Tailored Media for the Synthesis and Processing of Energy Conversion Materials. Energy Environ. Sci. 2016, 9, 49–61. [Google Scholar] [CrossRef]
- Zhang, H.; Eshetu, G.G.; Judez, X.; Li, C.; Rodriguez-Martínez, L.M.; Armand, M. Electrolyte Additives for Lithium Metal Anodes and Rechargeable Lithium Metal Batteries: Progress and Perspectives. Angew. Chem. 2018, 57. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Mecerreyes, D.; Forsyth, M.; Zhang, H.; Armand, M. Polymeric Ionic Liquids for Lithium-Based Rechargeable Batteries. Mol. Syst. Des. Eng. 2019, 4, 294–309. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, D.R.; Aishova, A.; Yoon, C.S.; Sun, Y.K. Li[Ni0.9.Co0.09W0.01]O2: A New Type of Layered Oxide Cathode with High Cycling Stability. Adv. Energy Mater. 2019, 9, 1902698. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Figgemeier, E. Confronting the Challenges of Next-Generation Silicon Anode-Based Lithium-Ion Batteries: Role of Designer Electrolyte Additives and Polymeric Binders. ChemSusChem 2019, 12, 2515–2539. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Spherical Carbon Particles are Used for Efficient and Stable “Metal Lithium Storage Room”. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Armand, M. Nature Lithium Battery. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding Electrochemical Potentials of Cathode Materials in Rechargeable Batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Jin, B.S.; Kim, H.S. Optimized Electrochemical Performance of Ni Rich LiNi0.91Co0.06Mn0.03O2 Cathodes for High-Energy Lithium Ion Batteries. Sci. Rep. 2019, 9, 8901. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Song, B.; Li, W. A Perspective on Nickel-Rich Layered Oxide Cathodes for Lithium-Ion Batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x <1): A new cathode material for batteries of high energy density. Mater. Res. Bulletin 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Schmidt, T.; Buchert, M.; Schebek, L. Investigation of the Primary Production Routes of Nickel and Cobalt Products Used for Li-Ion Batteries. Resour. Conserv. Recycl. 2016, 112, 107–122. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent Progress in Cathode Materials Research for Advanced Lithium Ion Batteries. Mater. Sci. Eng. R Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Zhang, W.J. Structure and Performance of LiFePO4 Cathode Materials: A Review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Winter, M. Thorough Look by Consideration of the Li+ Extraction Ratio. ACS Appl. Energy Mater. 2019, 2, 7733–7737. [Google Scholar] [CrossRef]
- Hou, P.; Yin, J.; Ding, M.; Huang, J.; Xu, X. Surface/Interfacial Structure and Chemistry of High-Energy Nickel-Rich Layered Oxide Cathodes: Advances and Perspectives. Small 2017, 13, 1–29. [Google Scholar] [CrossRef]
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.J.; Sun, Y.K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Rozier, P.; Tarascon, J.M. Review-Li-Rich Layered Oxide Cathodes for next-Generation Li-Ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-Art and Energy Management System of Lithium-Ion Batteries in Electric Vehicle Applications: Issues and Recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; Von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 1900161, 1–25. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267. [Google Scholar] [CrossRef]
- Wangda, L.; Erickson, E.M.; Arumugam, M. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Ajayi, B.P.; Thapa, A.K.; Cvelbar, U.; Jasinski, J.B.; Sunkara, M.K. Atmospheric Plasma Spray Pyrolysis of Lithiated Nickel-Manganese-Cobalt Oxides for Cathodes in Lithium Ion Batteries. Chem. Eng. Sci. 2017, 174, 302–310. [Google Scholar] [CrossRef]
- Križan, G.; Križan, J.; Dominko, R.; Gaberšček, M. Pulse Combustion Reactor as a Fast and Scalable Synthetic Method for Preparation of Li-Ion Cathode Materials. J. Power Sources 2017, 363, 218–226. [Google Scholar] [CrossRef]
- Kim, D.; Shim, H.C.; Yun, T.G.; Hyun, S.; Han, S.M. High Throughput Combinatorial Analysis of Mechanical and Electrochemical Properties of Li[NixCoyMnz]O2 Cathode. Extrem. Mech. Lett. 2016, 9, 439–448. [Google Scholar] [CrossRef]
- Sun, Y.K.; Chen, Z.; Noh, H.J.; Lee, D.J.; Jung, H.G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.T.; Amine, K. Nanostructured High-Energy Cathode Materials for Advanced Lithium Batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Shen, Y.; Yang, Y.; Shen, L.; Levin, B.D.A.; Yu, Y.; Muller, D.A.; Abruña, H.D. Systematic Optimization of Battery Materials: Key Parameter Optimization for the Scalable Synthesis of Uniform, High-Energy, and High Stability LiNi0.6Mn0.2Co0.2O2 Cathode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 35811–35819. [Google Scholar] [CrossRef] [PubMed]
- Peralta, D.; Salomon, J.; Colin, J.F.; Boulineau, A.; Fabre, F.; Bourbon, C.; Amestoy, B.; Gutel, E.; Bloch, D.; Patoux, S. Submicronic LiNi1/3Mn1/3Co1/3O2 Synthesized by Co-Precipitation for Lithium Ion Batteries—Tailoring a Classic Process for Enhanced Energy and Power Density. J. Power Sources 2018, 396, 527–532. [Google Scholar] [CrossRef]
- Tian, C.; Xu, Y.; Nordlund, D.; Lin, F.; Liu, J.; Sun, Z.; Liu, Y.; Doeff, M. Charge Heterogeneity and Surface Chemistry in Polycrystalline Cathode Materials. Joule 2018, 2, 464–477. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Ohtsuka, H.; Ichimura, M.; Yamaki, J. Characterization and Cathode Performance of Li1 − XNi1 + XO2 Prepared with the Excess Lithium Method. Solid State Ion. 1995, 80, 261–269. [Google Scholar] [CrossRef]
- Oh, P.; Song, B.; Li, W.; Manthiram, A. Overcoming the Chemical Instability on Exposure to Air of Ni-Rich Layered Oxide Cathodes by Coating with Spinel LiMn1.9Al0.1O4. J. Mater. Chem. A 2016, 4, 5839–5841. [Google Scholar] [CrossRef]
- Cho, D.H.; Jo, C.H.; Cho, W.; Kim, Y.J.; Yashiro, H.; Sun, Y.K.; Myung, S.T. Effect of Residual Lithium Compounds on Layer Ni-Rich Li[Ni0.7Mn0.3]O2. J. Electrochem. Soc. 2014, 161, 920–926. [Google Scholar] [CrossRef]
- Zhang, S.S. Insight into the Gassing Problem of Li-Ion Battery. Front. Energy Res. 2014, 2, 2–5. [Google Scholar] [CrossRef]
- Dahn, J.R.; Fuller, E.W.; Obrovac, M.; von Sacken, U. Thermal Stability of LixCoO2, LixNiO2 and λ-MnO2 and Consequences for the Safety of Li-Ion Cells. Solid State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Hatsukade, T.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Origin of Carbon Dioxide Evolved during Cycling of Nickel-Rich Layered NCM Cathodes. ACS Appl. Mater. Interfaces 2018, 10, 38892–38899. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Dahn, J.R. The Reactivity of Delithiated Li(Ni1/3Co1/3Mn1/3)O2, Li(Ni0.8Co0.15Al0.05)O2 or LiCoO2 with Non-Aqueous Electrolyte. Electrochem. Commun. 2007, 9, 2534–2540. [Google Scholar] [CrossRef]
- Jo, C.H.; Cho, D.H.; Noh, H.J.; Yashiro, H.; Sun, Y.K.; Myung, S.T. An Effective Method to Reduce Residual Lithium Compounds on Ni-Rich Li[Ni0.6Co0.2Mn0.2]O2 Active Material Using a Phosphoric Acid Derived Li3PO4 Nanolayer. Nano Res. 2015, 8, 1464–1479. [Google Scholar] [CrossRef]
- Kim, U.H.; Jun, D.W.; Park, K.J.; Zhang, Q.; Kaghazchi, P.; Aurbach, D.; Major, D.T.; Goobes, G.; Dixit, M.; Leifer, N.; et al. Pushing the Limit of Layered Transition Metal Oxide Cathodes for High-Energy Density Rechargeable Li Ion Batteries. Energy Environ. Sci. 2018, 11, 1271–1279. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.Y.; Chesneau, F.F.; Aurbach, D. Review-Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes I. Nickel-Rich, LiNixCoyMnzO2. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries; Springer: Singapore, 2019; Volume 2018. [Google Scholar] [CrossRef]
- Tang, M.; Yang, J.; Chen, N.; Zhu, S.; Wang, X.; Wang, T.; Zhang, C.; Xia, Y. Overall Structural Modification of a Layered Ni-Rich Cathode for Enhanced Cycling Stability and Rate Capability at High Voltage. J. Mater. Chem. A 2019, 7, 6080–6089. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, C.Y.; Cho, S.W.; Missiul, A.; Kim, J.K.; Ahn, Y.J.; Lee, S. Effects of Heat-Treatment Atmosphere on Electrochemical Performances of Ni-Rich Mixed-Metal Oxide (LiNi0.8Co0.15Mn0.05O2) as a Cathode Material for Lithium Ion Battery. Electrochim. Acta 2014, 138, 15–21. [Google Scholar] [CrossRef]
- Lim, J.M.; Hwang, T.; Kim, D.; Park, M.S.; Cho, K.; Cho, M. Intrinsic Origins of Crack Generation in Ni-Rich LiNi0.8Co0.1Mn0.1O2 Layered Oxide Cathode Material. Sci. Rep. 2017, 7, 39669. [Google Scholar] [CrossRef]
- Tian, C.; Nordlund, D.; Xin, H.L.; Xu, Y.; Liu, Y.; Sokaras, D.; Lin, F.; Doeff, M.M. Depth-Dependent Redox Behavior of LiNi0.6Mn0.2Co0.2O2. J. Electrochem. Soc. 2018, 165, A696–A704. [Google Scholar] [CrossRef]
- Koyama, Y.; Yabuuchi, N.; Tanaka, I.; Adachi, H.; Ohzuku, T. Solid-State Chemistry and Electrochemistry of LiCo1/3Ni1/3Mn1/3O2 for Advanced Lithium-Ion Batteries I. First-Principles Calculation on the Crystal and Electronic Structures. J. Electrochem. Soc. 2004, 151, A1545–A1551. [Google Scholar] [CrossRef]
- Bak, S.M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.J.; Kim, K.B.; Chung, K.Y.; Yang, X.Q.; Nam, K.W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Chemical versus Electrochemical Electrolyte Oxidation on NMC111, NMC622, NMC811, LNMO, and Conductive Carbon. J. Phys. Chem. Lett. 2017, 8, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative Binders for Sustainable Electrochemical Energy Storage—The Transition to Aqueous Electrode Processing and Bio-Derived Polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Doberdò, I.; Löffler, N.; Laszczynski, N.; Cericola, D.; Penazzi, N.; Bodoardo, S.; Kim, G.-T.; Passerini, S. Enabling Aqueous Binders for Lithium Battery Cathodes—Carbon Coating of Aluminum Current Collector. J. Power Sources 2014, 248, 1000–1006. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.P.; Daniel, C.; Wood, D.L. Enabling Aqueous Processing for Crack-Free Thick Electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef]
- Ahmadi, L.; Young, S.B.; Fowler, M.; Fraser, R.A.; Achachlouei, M.A. A Cascaded Life Cycle: Reuse of Electric Vehicle Lithium-Ion Battery Packs in Energy Storage Systems. Int. J. Life Cycl. Assess. 2017, 22, 111–124. [Google Scholar] [CrossRef]
- Pavoni, F.H.; Sita, L.E.; dos Santos, C.S.; da Silva, S.P.; da Silva, P.R.C.; Scarminio, J. LiCoO2 Particle Size Distribution as a Function of the State of Health of Discarded Cell Phone Batteries. Powder Technol. 2018, 326, 78–83. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium Recycling and Cathode Material Regeneration from Acid Leach Liquor of Spent Lithium-Ion Battery via Facile Co-Extraction and Co-Precipitation Processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal Treatment Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent Lithium-Ion Battery Recycling—Reductive Ammonia Leaching of Metals from Cathode Scrap by Sodium Sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Mohtat, P.; Nezampasandarbabi, F.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; James, A.; Uddin, K.; Chouchelamane, G.H.; Suttman, A.; Gong, X.; et al. Aging Phenomena for Lithium-Ion Batteries. J. Power Sources 2017, 147, 98–103. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Wu, Y.; Saxena, S.; Xing, Y.; Wang, Y.; Li, C.; Yung, W.K.C.; Pecht, M. Analysis of Manufacturing-Induced Defects and Structural Deformations in Lithium-Ion Batteries Using Computed Tomography. Energies 2018, 11, 925. [Google Scholar] [CrossRef]
- Li, J.; Du, Z.; Ruther, R.E.; An, S.J.; David, L.A.; Hays, K.; Wood, M.; Phillip, N.D.; Sheng, Y.; Mao, C.; et al. Toward Low-Cost, High-Energy Density, and High-Power Density Lithium-Ion Batteries. JOM 2017, 69, 1484–1496. [Google Scholar] [CrossRef]
- Mohanty, D.; Hockaday, E.; Li, J.; Hensley, D.K.; Daniel, C.; Wood, D.L. Effect of Electrode Manufacturing Defects on Electrochemical Performance of Lithium-Ion Batteries: Cognizance of the Battery Failure Sources. J. Power Sources 2016, 312, 70–79. [Google Scholar] [CrossRef]
- Mohanty, D.; Li, J.; Born, R.; Maxey, L.C.; Dinwiddie, R.B.; Daniel, C.; Wood, D.L. Non-Destructive Evaluation of Slot-Die-Coated Lithium Secondary Battery Electrodes by in-Line Laser Caliper and IR Thermography Methods. Anal. Methods 2014, 6, 674–683. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Dahn, H.M.; Smith, A.J.; Burns, J.C.; Stevens, D.A.; Dahn, J.R. User-Friendly Differential Voltage Analysis Freeware for the Analysis of Degradation Mechanisms in Li-Ion Batteries. J. Electrochem. Soc. 2012, 159, A1405–A1409. [Google Scholar] [CrossRef]
- Gabrisch, H.; Yi, T.; Yazami, R. Transmission Electron Microscope Studies of LiNi1/3Mn1/3Co1/3O2 before and after Long-Term Aging at 70 °C. Electrochem. Solid-State Lett. 2008, 11, 7–12. [Google Scholar] [CrossRef]
- Mu, L.; Lin, R.; Xu, R.; Han, L.; Xia, S.; Sokaras, D.; Steiner, J.D.; Weng, T.C.; Nordlund, D.; Doeff, M.M.; et al. Oxygen Release Induced Chemomechanical Breakdown of Layered Cathode Materials. Nano Lett. 2018, 18, 3241–3249. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.Y.; Seo, D.H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Downie, L.E.; Ma, L.; Qiu, W.; Dahn, J.R. Study of the Failure Mechanisms of LiNi0.8Mn0.1Co0.1O2 Cathode Material for Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1401–A1408. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2(NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Nam, K.W.; Yoon, W.S.; Yang, X.Q. Structural Changes and Thermal Stability of Charged LiNi1/3Co1/3Mn1/3O2 Cathode Material for Li-Ion Batteries Studied by Time-Resolved XRD. J. Power Sources 2009, 189, 515–518. [Google Scholar] [CrossRef]
- Young, D.; Park, I.; Shin, Y.; Seo, D.; Kang, Y.; Doo, S.; Koh, M. Ni-Stabilizing Additives for Completion of Ni-Rich Layered Cathode Systems in Lithium-Ion Batteries: An Ab Initio Study. J. Power Sources 2019, 418, 74–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Katayama, Y.; Tatara, R.; Giordano, L.; Yu, Y.; Fraggedakis, D.; Sun, J.; Maglia, F.; Jung, R.; Bazant, M.Z.; et al. Revealing Electrolyte Oxidation via Carbonate Dehydrogenation on Ni-Based Oxides in Li-Ion Batteries by in Situ Fourier Transform Infrared Spectroscopy. Energy Environ. Sci. 2019. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface Reconstruction and Chemical Evolution of Stoichiometric Layered Cathode Materials for Lithium-Ion Batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Kim, J.M.; Chung, H.T. Role of Transition Metals in Layered Li[Ni,Co,Mn]O2 under Electrochemical Operation. Electrochim. Acta 2004, 49, 3573–3580. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic Lattice Strain and Mechanical Degradation of High- and Low-Nickel NCM Cathode Materials for Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Guo, S.; Xu, Z.; Zhu, H.; Zhang, X.; Yuan, Y.; He, P.; Ishida, M.; Zhou, H. Direct Visualization of the Reversible O2−/O− Redox Process in Li-Rich Cathode Materials. Adv. Mater. 2018, 30, 2–7. [Google Scholar] [CrossRef]
- Nation, L.; Li, J.; James, C.; Qi, Y.; Dudney, N.; Sheldon, B.W. In Situ Stress Measurements during Electrochemical Cycling of Lithium-Rich Cathodes. J. Power Sources 2017, 364, 383–391. [Google Scholar] [CrossRef]
- Ko, D.S.; Park, J.H.; Park, S.; Ham, Y.N.; Ahn, S.J.; Park, J.H.; Han, H.N.; Lee, E.; Jeon, W.S.; Jung, C. Microstructural Visualization of Compositional Changes Induced by Transition Metal Dissolution in Ni-Rich Layered Cathode Materials by High-Resolution Particle Analysis. Nano Energy 2019, 56, 434–442. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Zhang, Y.; Zhao, K. Recent Advance in Understanding the Electro-Chemo-Mechanical Behavior of Lithium-Ion Batteries by Electron Microscopy. Mater. Today Nano 2019, 7, 100040. [Google Scholar] [CrossRef]
- Ruan, Y.; Song, X.; Fu, Y.; Song, C.; Battaglia, V. Structural Evolution and Capacity Degradation Mechanism of LiNi0.6Mn0.2Co0.2O2 Cathode Materials. J. Power Sources 2018, 400, 539–548. [Google Scholar] [CrossRef]
- Besli, M.M.; Xia, S.; Kuppan, S.; Huang, Y.; Metzger, M.; Shukla, A.K.; Schneider, G.; Hellstrom, S.; Christensen, J.; Doeff, M.M.; et al. Mesoscale Chemomechanical Interplay of the LiNi0.8Co0.15Al0.05O2 Cathode in Solid-State Polymer Batteries. Chem. Mater. 2019, 31, 491–501. [Google Scholar] [CrossRef]
- Nara, H.; Morita, K.; Mukoyama, D.; Yokoshima, T.; Momma, T.; Osaka, T. Impedance Analysis of LiNi1/3Mn1/3Co1/3O2 Cathodes with Different Secondary-Particle Size Distribution in Lithium-Ion Battery. Electrochim. Acta 2017, 241, 323–330. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of Performance Degradation of LiNi0.80Co0.10Mn0.10O2 Cathode Material Operating at High Potentials. J. Energy Chem. 2020, 41, 135–141. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. The Role of Cations on the Performance of Lithium Ion Batteries: A Quantitative Analytical Approach. Acc. Chem. Res. 2018, 51, 265–272. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, S.Y.; Chung, K.Y.; Stach, E.A.; Kim, S.M.; Chang, W. Determination of the Mechanism and Extent of Surface Degradation in Ni-Based Cathode Materials after Repeated Electrochemical Cycling. APL Mater. 2016, 4. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, J.; Zou, L.; Jia, H.; Liu, B.; Wang, H.; Engelhard, M.H.; Wang, C.; Xu, W.; Yang, Y.; et al. High Voltage Operation of Ni-Rich NMC Cathodes Enabled by Stable Electrode/Electrolyte Interphases. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Xiong, D.J.; Ellis, L.D.; Li, J.; Li, H.; Hynes, T.; Allen, J.P.; Xia, J.; Hall, D.S.; Hill, I.G.; Dahn, J.R. Measuring Oxygen Release from Delithiated LiNixMnyCo1-x-yO2 and Its Effects on the Performance of High Voltage Li-Ion Cells. J. Electrochem. Soc. 2017, 164, A3025–A3037. [Google Scholar] [CrossRef]
- Hu, E.; Wang, X.; Yu, X.; Yang, X.Q. Probing the Complexities of Structural Changes in Layered Oxide Cathode Materials for Li-Ion Batteries during Fast Charge-Discharge Cycling and Heating. Acc. Chem. Res. 2018, 51, 290–298. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon N. Y. 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Kropf, A.J.; Marin, T.W.; Li, Y.; Poluektov, O.G.; Niklas, J.; Abraham, D.P. Manganese in Graphite Anode and Capacity Fade in Li Ion Batteries. J. Phys. Chem. C 2014, 118, 24335–24348. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Shkrob, I.A.; Abraham, D.P. Transition Metal Dissolution, Ion Migration, Electrocatalytic Reduction and Capacity Loss in Lithium-Ion Full Cells. J. Electrochem. Soc. 2017, 164, A389–A399. [Google Scholar] [CrossRef]
- Yeon, N.; Yim, T.; Ho, J.; Yu, J.; Lee, Z. Microstructural Study on Degradation Mechanism of Layered Electron Microscopy. J. Power Sources 2016, 307, 641–648. [Google Scholar] [CrossRef]
- Guéguen, A.; Streich, D.; He, M.; Mendez, M.; Chesneau, F.F.; Novák, P.; Berg, E.J. Decomposition of LiPF6 in High Energy Lithium-Ion Batteries Studied with Online Electrochemical Mass Spectrometry. J. Electrochem. Soc. 2016, 163, A1095–A1100. [Google Scholar] [CrossRef]
- Freiberg, A.T.S.; Roos, M.K.; Wandt, J.; De Vivie-Riedle, R.; Gasteiger, H.A. Singlet Oxygen Reactivity with Carbonate Solvents Used for Li-Ion Battery Electrolytes. J. Phys. Chem. A 2018, 122, 8828–8839. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental Investigation of the Lithium-Ion Battery Impedance Characteristic at Various Conditions and Aging States and Its Influence on the Application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Mu, L.; Yuan, Q.; Tian, C.; Wei, C.; Zhang, K.; Liu, J.; Pianetta, P.; Doeff, M.M.; Liu, Y.; Lin, F. Propagation Topography of Redox Phase Transformations in Heterogeneous Layered Oxide Cathode Materials. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Radin, M.D.; Hy, S.; Sina, M.; Fang, C.; Liu, H.; Vinckeviciute, J.; Zhang, M.; Whittingham, M.S.; Meng, Y.S.; Van der Ven, A. Narrowing the Gap between Theoretical and Practical Capacities in Li-Ion Layered Oxide Cathode Materials. Adv. Energy Mater. 2017, 7, 1–33. [Google Scholar] [CrossRef]
- Solchenbach, S.; Metzger, M.; Egawa, M.; Beyer, H.; Gasteiger, H.A. Quantification of PF5 and POF3 from Side Reactions of LiPF6 in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3022–A3028. [Google Scholar] [CrossRef]
- Pierre-Etienne, C.; David, P.; Mikael, C.; Pascal, M. Impact of Morphological Changes of LiNi1/3Mn1/3Co1/3O2 on Lithium-Ion Cathode Performances. J. Power Sources 2017, 346, 13–23. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Chao, D.; Baikie, T.; Bai, L.; Chen, S.; Zhao, Y.; Sum, T.C.; Lin, J.; Shen, Z. Hierarchical Porous LiNi1/3Co1/3Mn1/3O2 Nano-/Micro Spherical Cathode Material: Minimized Cation Mixing and Improved Li+ Mobility for Enhanced Electrochemical Performance. Sci. Rep. 2016, 6, 25771. [Google Scholar] [CrossRef]
- Wang, Y.; Roller, J.; Maric, R. Morphology-Controlled One-Step Synthesis of Nanostructured LiNi1/3Mn1/3Co1/3O2 Electrodes for Li-Ion Batteries. ACS Omega 2018, 3, 3966–3973. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Stone, W.; Weber, R.; Hy, S.; Dahn, J.R. Synthesis of Single Crystal LiNi0.5Mn0.3Co0.2O2 for Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A3529–A3537. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, X.; Dahn, J.R. Synthesis of Single Crystal LiNi0.6Mn0.2Co0.2O2 with Enhanced Electrochemical Performance for Lithium Ion Batteries. J. Electrochem. Soc. 2018, 165, A1038–A1045. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Stone, W.; Glazier, S.; Dahn, J.R. Development of Electrolytes for Single Crystal NMC532/Artificial Graphite Cells with Long Lifetime. J. Electrochem. Soc. 2018, 165, A626–A635. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Chen, G.; Croy, J.; Balasubramanian, M.; Shahbazian-Yassar, R. Aberration-Corrected Scanning Transmission Electron Microscopy of Single Crystals and Chemically-Gradient NMC Cathodes. Microsc. Microanal. 2018, 24, 1536–1537. [Google Scholar] [CrossRef]
- Xie, H.; Du, K.; Hu, G.; Peng, Z.; Cao, Y. The Role of Sodium in LiNi0.8Co0.15Al0.05O2 Cathode Material and Its Electrochemical Behaviors. J. Phys. Chem. C 2016, 120, 3235–3241. [Google Scholar] [CrossRef]
- Pouillerie, C.; Croguennec, L.; Delmas, C. Modifications Observed Upon Cycling. Solid State Ion. 2000, 132, 15–29. [Google Scholar] [CrossRef]
- Jang, Y.I.; Huang, B.; Wang, H.; Maskaly, G.R.; Ceder, G.; Sadoway, D.R.; Chiang, Y.M.; Liu, H.; Tamura, H. Synthesis and Characterization of LiAlyCO1-yO2 and LiAlyNi1-yO2. J. Power Sources 1999, 81, 589–593. [Google Scholar] [CrossRef]
- Croguennec, L.; Suard, E.; Willmann, P.; Delmas, C. Structural and Electrochemical Characterization of the LiNi1-yTiyO2 Electrode Materials Obtained by Direct Solid-State Reactions. Chem. Mater. 2002, 14, 2149–2157. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Gendron, F.; Julien, C.M. Minimization of the Cation Mixing in Li1+x(NMC)1-xO2 as Cathode Material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on Challenges and Recent Advances in the Electrochemical Performance of High Capacity Li- and Mn-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Wang, R.; Qian, G.; Liu, T.; Li, M.; Liu, J.; Zhang, B.; Zhu, W.; Li, S.; Zhao, W.; Yang, W.; et al. Tuning Li-Enrichment in High-Ni Layered Oxide Cathodes to Optimize Electrochemical Performance for Li-Ion Battery. Nano Energy 2019, 62, 709–717. [Google Scholar] [CrossRef]
- Tian, C.; Lin, F.; Doeff, M.M. Electrochemical Characteristics of Layered Transition Metal Oxide Cathode Materials for Lithium Ion Batteries: Surface, Bulk Behavior, and Thermal Properties. Acc. Chem. Res. 2018, 51, 89–96. [Google Scholar] [CrossRef]
- Eilers-Rethwisch, M.; Winter, M.; Schappacher, F.M. Synthesis, Electrochemical Investigation and Structural Analysis of Doped Li[Ni0.6Mn0.2Co0.2-xMx]O2 (x = 0, 0.05; M = Al, Fe, Sn) Cathode Materials. J. Power Sources 2018, 387, 101–107. [Google Scholar] [CrossRef]
- Mi, C.; Han, E.; Li, L.; Zhu, L.; Cheng, F.; Dai, X. Effect of Iron Doping on LiNi0.35Co0.30Mn0.35O2. Solid State Ion. 2018, 325, 24–29. [Google Scholar] [CrossRef]
- Weigel, T.; Schipper, F.; Erickson, E.M.; Susai, F.A.; Markovsky, B.; Aurbach, D. Structural and Electrochemical Aspects of LiNi0.8Co0.1Mn0.1O2 Cathode Materials Doped by Various Cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, B.; Wang, M.; Yang, X.; Gu, Y. Facile Synthesis of Fluorine Doped Single Crystal Ni-Rich Cathode Material for Lithium-Ion Batteries. Solid State Ion. 2019, 342, 115065. [Google Scholar] [CrossRef]
- Konarov, A.; Myung, S.T.; Sun, Y.K. Cathode Materials for Future Electric Vehicles and Energy Storage Systems. ACS Energy Lett. 2017, 2, 703–708. [Google Scholar] [CrossRef]
- Lin, F.; Nordlund, D.; Li, Y.; Quan, M.K.; Cheng, L.; Weng, T.C.; Liu, Y.; Xin, H.L.; Doeff, M.M. Metal Segregation in Hierarchically Structured Cathode Materials for High-Energy Lithium Batteries. Nat. Energy 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, H.; Zi, Z.; Zhang, L.; Xu, X. Core-Shell and Concentration-Gradient Cathodes Prepared via Co-Precipitation Reaction for Advanced Lithium-Ion Batteries. J. Mater. Chem. A 2017, 5, 4254–4279. [Google Scholar] [CrossRef]
- Sun, Y.K.; Myung, S.T.; Park, B.C.; Amine, K. Synthesis of Spherical Nano- To Microscale Core-Shell Particles Li[(Ni0.8Co0.1Mn0.1)1-x(Ni0.5Mn0.5)x]O2 and Their Applications to Lithium Batteries. Chem. Mater. 2006, 18, 5159–5163. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Mo, Y.; Jia, X.; Jia, J.; Yao, C.; Chen, D.; Chen, Y. Cathode Materials with Cross-Stack Structures for Suppressing Intergranular Cracking and High-Performance Lithium-Ion Batteries. Electrochim. Acta 2018, 261, 513–520. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhang, Y.; Huang, X.; Duan, J.; Wang, D.; Nayaka, G.P.; Li, X.; Dong, P. Beneficial Effect of Incorporating Ni-Rich Oxide and Layered over-Lithiated Oxide into High-Energy-Density Cathode Materials for Lithium-Ion Batteries. J. Power Sources 2018, 400, 341–349. [Google Scholar] [CrossRef]
- Li, G.; Qi, L.; Xiao, P.; Yu, Y.; Chen, X.; Yang, W. Effect of Precursor Structures on the Electrochemical Performance of Ni-Rich LiNi0.88Co0.12O2 Cathode Materials. Electrochim. Acta 2018, 270, 319–329. [Google Scholar] [CrossRef]
- Hou, P.; Li, F.; Sun, Y.; Pan, M.; Wang, X.; Shao, M.; Xu, X. Improving Li+ Kinetics and Structural Stability of Nickel-Rich Layered Cathodes by Heterogeneous Inactive-Al3+ Doping. ACS Sustain. Chem. Eng. 2018, 6, 5653–5661. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.K. Role of Surface Coating on Cathode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-Rich LiNi0.5Co0.2Mn0.3O2 Cathode Properties at High Operating Voltage by Double Coating Layer of Al2O3 and AlPO4. J. Alloys Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Co, L.; Battery, M.O.; Lee, Y.; Nam, K.; Hwang, E.; Kwon, Y.; Kang, D.; Kim, S.; Song, S. Interfacial Origin of Performance Improvement and Fade for 4.6 V. J. Phys. Chem. C 2014, 118, 10631–10639. [Google Scholar]
- Gao, H.; Maglia, F.; Lamp, P.; Amine, K.; Chen, Z. Mechanistic Study of Electrolyte Additives to Stabilize High-Voltage Cathode-Electrolyte Interface in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 44542–44549. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Su, C.C.; Peebles, C.; Feng, Z.; Connell, J.G.; Liao, C.; Wang, Y.; Shkrob, I.A.; Zhang, Z. Mechanistic Insight in the Function of Phosphite Additives for Protection of LiNi0.5Co0.2Mn0.3O2 Cathode in High Voltage Li-Ion Cells. ACS Appl. Mater. Interfaces 2016, 8, 11450–11458. [Google Scholar] [CrossRef]
- Nie, M.; Madec, L.; Xia, J.; Hall, D.S.; Dahn, J.R. Some Lewis Acid-Base Adducts Involving Boron Trifluoride as Electrolyte Additives for Lithium Ion Cells. J. Power Sources 2016, 328, 433–442. [Google Scholar] [CrossRef]
- Liu, Z.; Chai, J.; Xu, G.; Wang, Q.; Cui, G. Functional Lithium Borate Salts and Their Potential Application in High Performance Lithium Batteries. Coord. Chem. Rev. 2015, 292, 56–73. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kang, J.; Nam, K.M.; Paik, Y.; Song, S.W. Understanding Interfacial Chemistry and Stability for Performance Improvement and Fade of High-Energy Li-Ion Battery of LiNi0.5Co0.2Mn0.3O2//Silicon-Graphite. J. Power Sources 2016, 303, 150–158. [Google Scholar] [CrossRef]
- Jang, S.H.; Jung, K.; Yim, T. Silyl-Group Functionalized Organic Additive for High Voltage Ni-Rich Cathode Material. Curr. Appl. Phys. 2018, 18, 1345–1351. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teichert, P.; Eshetu, G.G.; Jahnke, H.; Figgemeier, E. Degradation and Aging Routes of Ni-Rich Cathode Based Li-Ion Batteries. Batteries 2020, 6, 8. https://doi.org/10.3390/batteries6010008
Teichert P, Eshetu GG, Jahnke H, Figgemeier E. Degradation and Aging Routes of Ni-Rich Cathode Based Li-Ion Batteries. Batteries. 2020; 6(1):8. https://doi.org/10.3390/batteries6010008
Chicago/Turabian StyleTeichert, Philipp, Gebrekidan Gebresilassie Eshetu, Hannes Jahnke, and Egbert Figgemeier. 2020. "Degradation and Aging Routes of Ni-Rich Cathode Based Li-Ion Batteries" Batteries 6, no. 1: 8. https://doi.org/10.3390/batteries6010008
APA StyleTeichert, P., Eshetu, G. G., Jahnke, H., & Figgemeier, E. (2020). Degradation and Aging Routes of Ni-Rich Cathode Based Li-Ion Batteries. Batteries, 6(1), 8. https://doi.org/10.3390/batteries6010008





