Lithium-Ion Capacitor Safety Testing for Commercial Application
Abstract
1. Introduction
2. Experimental Method
3. Electrochemical–Thermal Reaction Mechanisms Governing Equation
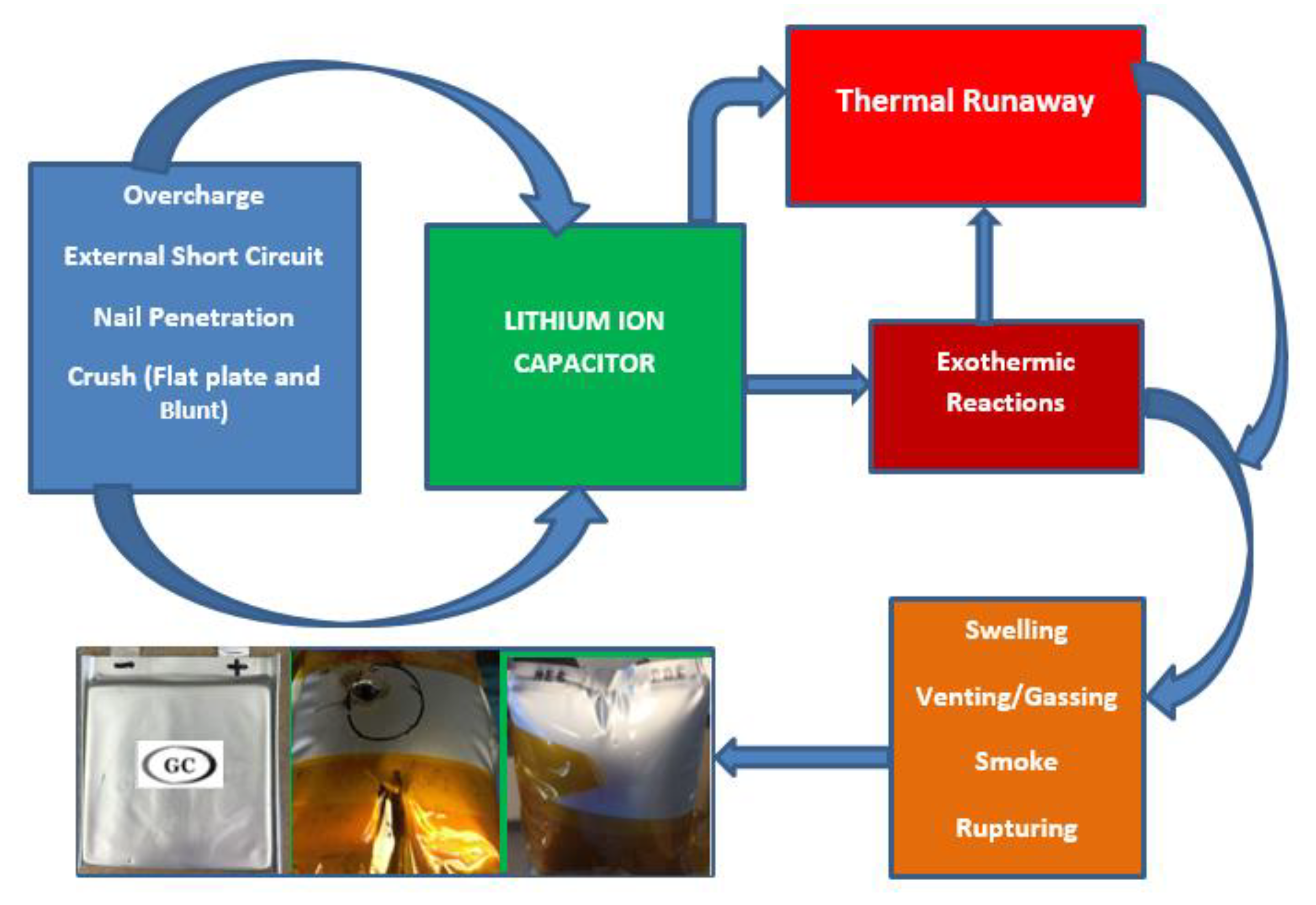
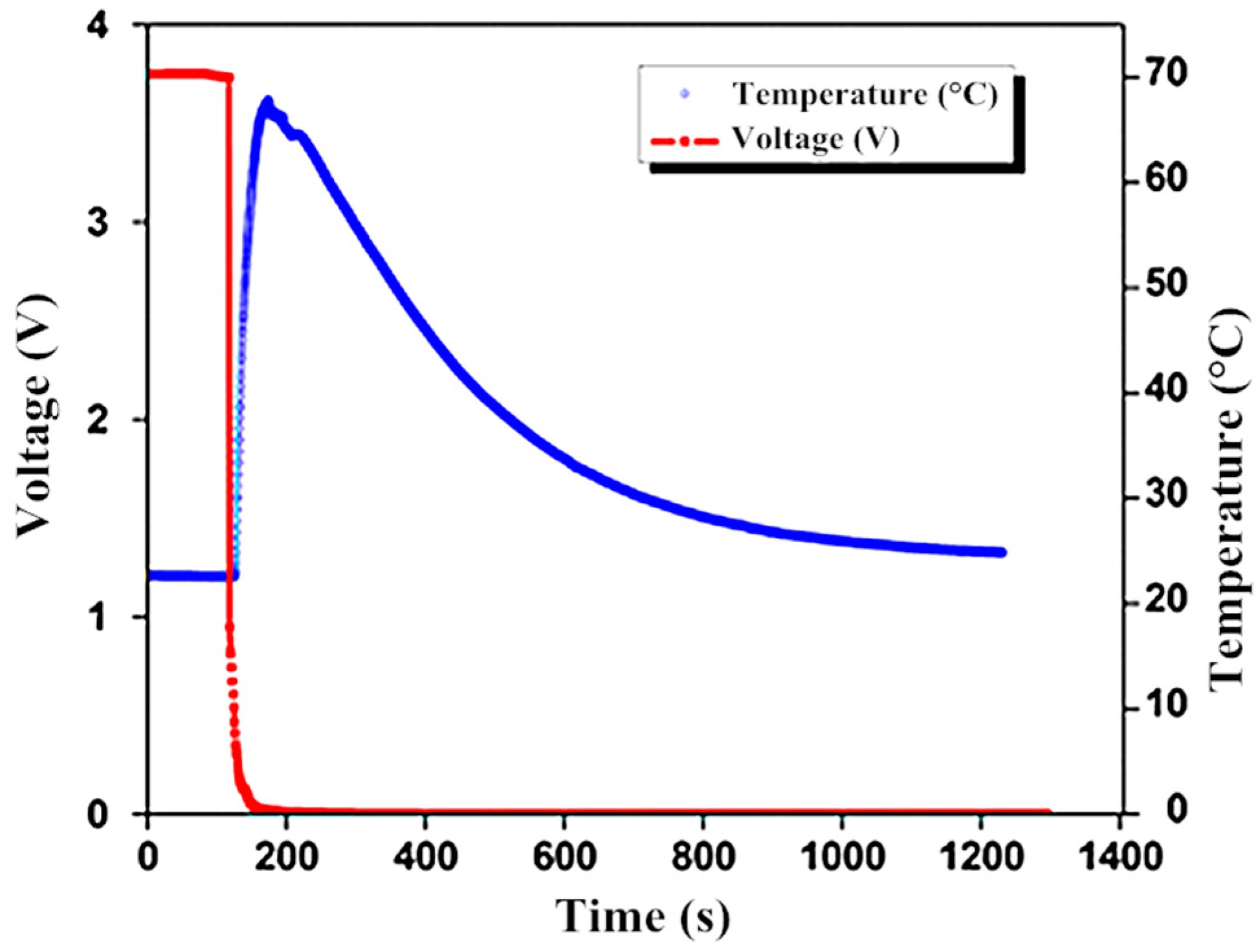
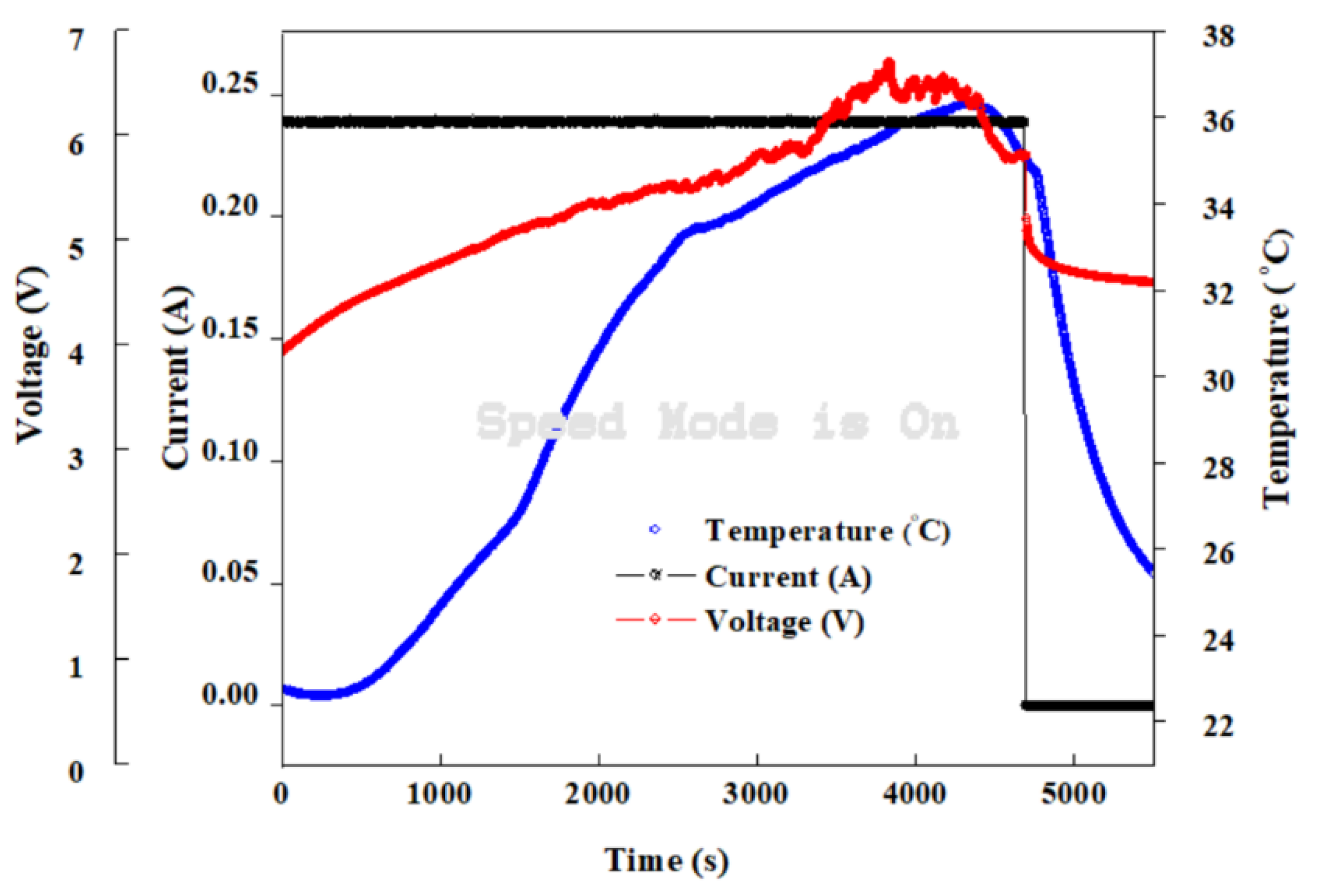
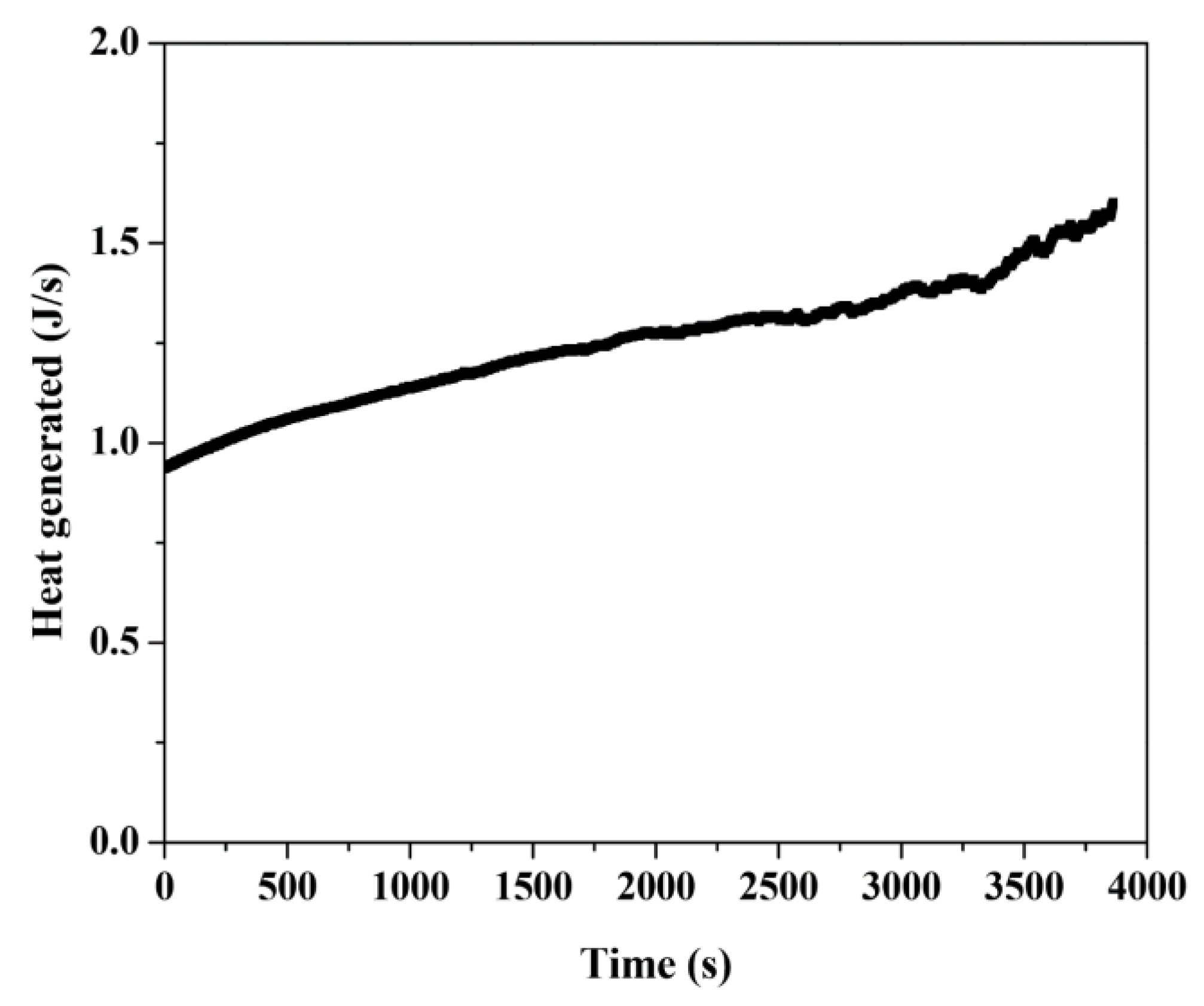
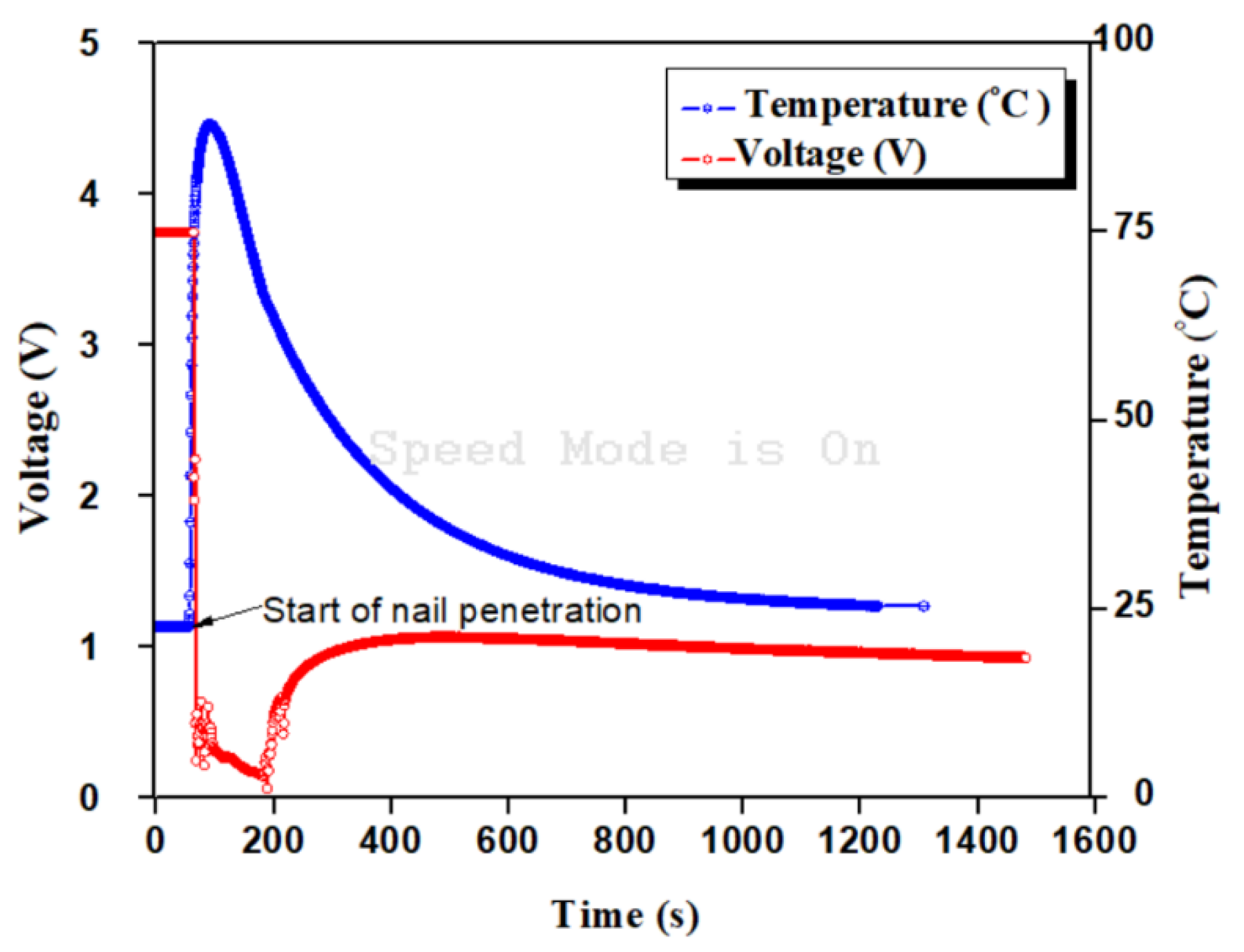
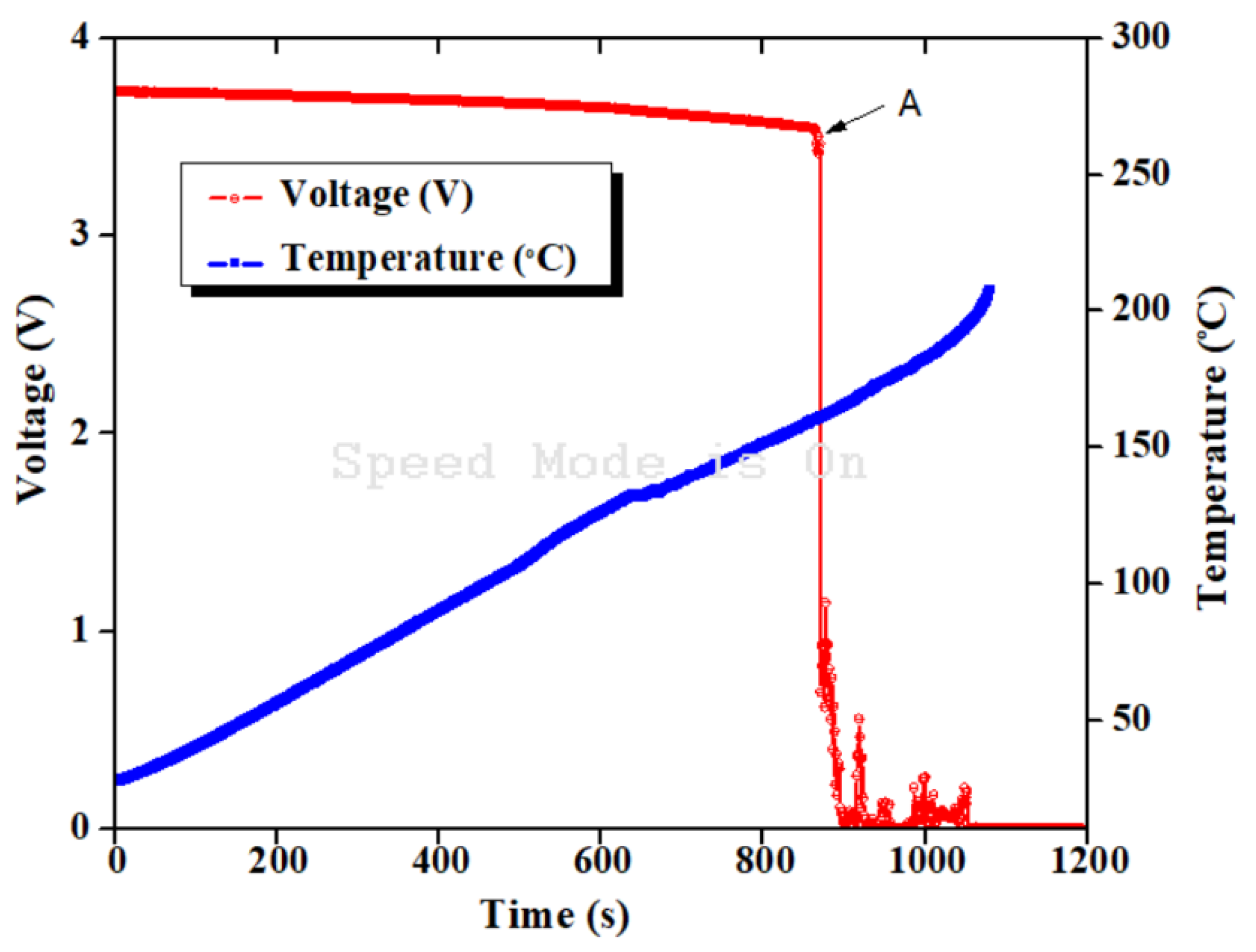
4. Result and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, W.J.; Zheng, J.P. The effect of cathode and anode potentials on the cycling performance of Li-Ion capacitors. J. Electrochem. Soc. 2013, 160, A1572–A1576. [Google Scholar] [CrossRef]
- Zheng, J.P. The limitations of energy density of battery/double-layer capacitor asymmetric cells. J. Electrochem. Soc. 2003, 150, A484–A492. [Google Scholar] [CrossRef]
- Zheng, J.P. Theoretical energy density for electrochemical capacitors with intercalation electrodes. J. Electrochem. Soc. 2005, 152, A1864–A1869. [Google Scholar] [CrossRef]
- Zheng, J.P. High energy density electrochemical capacitors without consumption of electrolyte. J. Electrochem. Soc. 2009, 156, A500–A505. [Google Scholar] [CrossRef]
- Cao, W.J.; Zheng, J.P. Li-ion capacitors with carbon cathode and hard carbon/stabilized lithium metal powder anode electrodes. J. Power Sour. 2012, 213, 180–185. [Google Scholar] [CrossRef]
- Gao, X.; Zhan, C.; Yu, X.; Liang, Q.; Lv, R.; Gai, G.; Shen, W.; Kang, F.; Huang, Z.H. A high performance lithium-ion capacitor with both electrodes prepared from Sri Lanka graphite ore. Materials 2017, 10, 414. [Google Scholar] [CrossRef]
- Adelowo, E.; Baboukani, A.; Chen, C.; Wang, C. Electrostatically sprayed reduced graphene oxide-carbon nanotubes electrodes for lithium-ion capacitors. J. Carbon Res. 2018, 4, 31. [Google Scholar] [CrossRef]
- Belov, D.; Yang, M.H. Failure mechanism of Li-ion battery at overcharge conditions. J. Sol. State Electrochem. 2008, 12, 885–894. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, U.S.; Sim, C.B. Modelling of the thermal behaviour of an ultracapacior for a 42-V automotive electrical system. J. Power Sour. 2008, 175, 664–668. [Google Scholar] [CrossRef]
- Gualous, H.; Louahlia-Gualous, H.; Gallay, R.; Miraoui, A. Supercapacitor thermal modelling and characterization in transient state for industrial applications. IEEE Trans. Ind. Appl. 2009, 45, 1035–1044. [Google Scholar] [CrossRef]
- Monzer Al, S.; Gualous, H.; Van Mierlo, J.; Culcu, H. Thermal modeling and heat management of supercapacitor modules for vehicle applications. J. Power Sour. 2009, 194, 581–587. [Google Scholar]
- Zubieta, L.; Bonert, R. Characterization of double-layer capacitors for power electronics applications. IEEE Trans. Ind. Appl. 2000, 36, 199–205. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.B.; Zhang, R.; Bin, Y.Z.; Qiu, J.S. Polymer casting of ultralight graphene aerogels for the production of conductive nanocomposites with low filling Content. J. Mater. Chem. A 2014, 2, 3756–3760. [Google Scholar] [CrossRef]
- Song, Z.; Sun, K. Adaptive backstepping sliding mode control with fuzzy monitoring strategy for a kind of mechanical system. ISA Trans. 2014, 53, 125–133. [Google Scholar] [CrossRef]
- Guo, G.; Bo, L. Three-dimensional thermal finite element modeling of lithium-ion battery in thermal abuse application. J. Power Sour. 2010, 195, 2393–2398. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Zhang, T.; Liu, Z. Nitrogen-doped graphene for supercapacitor with long-term electrochemical stability. Energy 2014, 70, 612–617. [Google Scholar] [CrossRef]
- Finegan, D.P.; Tjaden, B.; Heenan, T.M.; Jervis, R.; Di Michiel, M.; Rack, A.; Hinds, G.; Brett, D.J.; Shearing, P.R. Tracking internal temperature and structural dynamics during nail penetration of lithium-ion cells. J. Electrochem. Soc. 2017, 164, A3285–A3291. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Yin, H.; Zhang, T.; Wan, W. Thermal modelling analysis of spiral wound supercapacitor under constant-current cycling. PLoS ONE 2015, 10, e0138672. [Google Scholar] [CrossRef]
- Al-zubaidi, A.; Ji, X.; Yu, J. Thermal charging of supercapacitors: A perspective. Sustain. Energy Fuels 2017, 1, 1457–1474. [Google Scholar] [CrossRef]
- Kermani, G.; Sahraei, E. Characterization and modeling of the mechanical properties of lithium-ion batteries. Energies 2017, 10, 1730. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B. Abuse by external heating, overcharge and short circuiting of commercial lithium-ion battery cells. J. Electrochem. Soc. 2014, 161, A1611–A1617. [Google Scholar] [CrossRef]
- Lee, C.W.; Venkatachalapathy, R.; Prakash, J. A Novel Flame-Retardant Additive for Lithium Batteries. Electrochem. Sol. State Lett. 2000, 3, 63–65. [Google Scholar] [CrossRef]
- Cappetto, A.; Cao, W.J.; Luo, J.F.; Hagen, M.; Adams, D.; Shelikeri, A.; Xu, K.; Zheng, J.P. Performance of wide temperature range electrolytes for Li-Ion capacitor pouch cells. J. Power Sour. 2017, 359, 205–214. [Google Scholar] [CrossRef]
- Arora, A.; Medora, N.K.; Livernois, T.; Swart, J. Safety of Lithium-Ion Batteries for Hybrid Electric Vehicles. In Electric and Hybrid Vehicle; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Spotnitz, R.; Franklin, J. Abuse Behavior of High-Power, Lithium-Ion Cells. J. Power Sour. 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Tobishima, S.; Yamaki, J. A consideration of Lithium Cell Safety. J. Power Sour. 1999, 81–82, 882–886. [Google Scholar] [CrossRef]
- Biensan, P.; Simon, B.; Peres, J.P. On Safety of Lithium-Ion Cells. J. Power Sour. 1999, 81–82, 906–912. [Google Scholar] [CrossRef]
- Saito, Y.; Takano, K.; Negishi, A. Thermal behaviors of lithium-ion cells during overcharge. J. Power Sour. 2001, 97–98, 693–696. [Google Scholar] [CrossRef]
- Leising, R.A.; Palazzo, M.J.; Takeuchi, E.S.; Takeuchi, K.J. Abuse Testing of Lithium-Ion Batteries: Characterization of the Overcharge Reaction of LiCoO2/Graphite Cells. J. Electrochem. Soc. 2001, 148, A838. [Google Scholar] [CrossRef]
- Leising, R.A.; Palazzo, M.J.; Takeuchi, E.S.; Takeuchi, K.J. A study of the overcharge reaction of lithium-ion batteries. J. Power Sour. 2001, 97–98, 681–683. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety Focused Modeling of Lithium-Ion Batteries: A Review. J. Power Sour. 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Sahraei, E.; Meier, J.; Wierzbicki, T. Characterizing and modeling mechanical properties and onset of short circuit for three types of lithium-ion pouch cells. J. Power Sour. 2014, 247, 503–516. [Google Scholar] [CrossRef]
- Zavalis, T.G.; Behm, M.; Lindbergh, G. Investigation of Short-Circuit Scenarios in a Lithium-Ion Battery Cell. J. Electrochem. Soc. 2012, 159, A848. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A review on the thermal hazards of the lithium-ion battery and the corresponding countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef]
- Yamauchi, T.; Mizushima, K.; Satoh, Y.; Yamada, S. Development of a simulator for both property and safety of a lithium secondary battery. J. Power Sour. 2004, 136, 99–107. [Google Scholar] [CrossRef]
- Melcher, A.; Ziebert, C.; Lei, B.; Zhao, W.; Luo, J.; Rohde, M.; Seifert, H.J. Modeling and Simulation of Thermal Runaway in Cylindrical 18650 Lithium-Ion Batteries. In Meeting Abstracts the Electrochemical Society; The Electrochemical Society: Pennington, NJ, USA, 2016; p. 425. [Google Scholar]
- Matula, R.A. Electrical resistivity of copper, gold, palladium and silver. J. Phys. Chem. 1979, 8, 1147. [Google Scholar] [CrossRef]
- Benger, R.; Wenzl, H.; Beck, H.; Jiang, M.; Ohms, D.; Schaedlich, G. 2009 Electrochemical thermal modeling of lithium-ion cells for use in HEV or EV application. World Electr. Veh. J. 2009, 3, 342. [Google Scholar] [CrossRef]
- Jeon, D.H.; Baek, S.M. Thermal modeling of cylindrical lithium ion battery during discharge cycle. Energy Convers. Manag. 2011, 52, 2973–2981. [Google Scholar] [CrossRef]
- Wang, F.; Li, M. Thermal performance analysis of the Lithium-ion Batteries. In Proceedings of the 2010 International Conference on Parallel and Distributed Computing, Applications and Technologies (PDCAT), Wuhan, China, 8–11 December 2010; pp. 483–486. [Google Scholar]
- Soltani, M.; Ronsmans, J.; Kakihara, S.; Jaguemont, J.; Van den Bossche, P.; van Mierlo, J.; Omar, N. Hybrid battery/lithium-ion capacitor energy storage system for a pure electric bus for an urban transportation application. Appl. Sci. 2018, 8, 1176. [Google Scholar] [CrossRef]
- Onda, K.; Ohshima, T.; Nakayama, M.; Fukuda, K.; Araki, T. Thermal behavior of small lithium-ion battery during rapid charge and discharge cycles. J. Power Sour. 2006, 158, 535–542. [Google Scholar] [CrossRef]
- Ismail, N.H.F.; Toha, S.F.; Azubir, N.A.M.; Ishak, N.H.M.; Hassan, M.K.; Ibrahim, B.S.K. Simplified heat generation model for lithium ion battery used in electric vehicle. IOP Conf. Ser. Mater. Sci. Eng. 2013, 53, 012014. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanism of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Sol. State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Dahn, J.R.; Fuller, E.W.; Obrovac, M.; Von Sacken, U. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Sol. State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.W. Thermal analysis of lithium-ion batteries. J. Electrochem. Soc. 1996, 143, 2708–2712. [Google Scholar] [CrossRef]
- Kriston, A.; Pfrang, A.; Döring, H.; Fritsch, B.; Ruiz, V.; Adanouj, I.; Kosmidou, T.; Ungeheuer, J.; Boon-Brett, L. External short circuit performance of Graphite-LiNi1/3Co1/3Mn1/3O2 and Graphite-LiNi0.8Co0.15Al0.05O2 cells at different external. J. Power Sour. 2017, 361, 170–181. [Google Scholar] [CrossRef]
- Orendorff, C.J. The Role of Separators in Lithium-Ion Cell Safety. Electrochem. Soc. Interface 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Jiang, J.R.D.J.; Dahn, J.R. ARC studies of the thermal stability of three different cathode materials: LiCoO2; Li [Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC electrolytes. Electrochem. Commun. 2004, 6, 39–43. [Google Scholar] [CrossRef]




| Parameters | Specification |
|---|---|
| Dimension | Thickness = 4.5 mm, Heights = 58 mm, Width = 48 mm |
| Weight | 16 g |
| Specific Power | 6 kW/kg |
| Specific Energy | 14 Wh/g |
| Voltage Range | 2.2–3.8 V |
| Maximum Voltage | 4.0 V |
| Capacitance | 200 F |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolufawi, O.; Shellikeri, A.; Zheng, J.P. Lithium-Ion Capacitor Safety Testing for Commercial Application. Batteries 2019, 5, 74. https://doi.org/10.3390/batteries5040074
Bolufawi O, Shellikeri A, Zheng JP. Lithium-Ion Capacitor Safety Testing for Commercial Application. Batteries. 2019; 5(4):74. https://doi.org/10.3390/batteries5040074
Chicago/Turabian StyleBolufawi, Omonayo, Annadanesh Shellikeri, and Jim P. Zheng. 2019. "Lithium-Ion Capacitor Safety Testing for Commercial Application" Batteries 5, no. 4: 74. https://doi.org/10.3390/batteries5040074
APA StyleBolufawi, O., Shellikeri, A., & Zheng, J. P. (2019). Lithium-Ion Capacitor Safety Testing for Commercial Application. Batteries, 5(4), 74. https://doi.org/10.3390/batteries5040074





