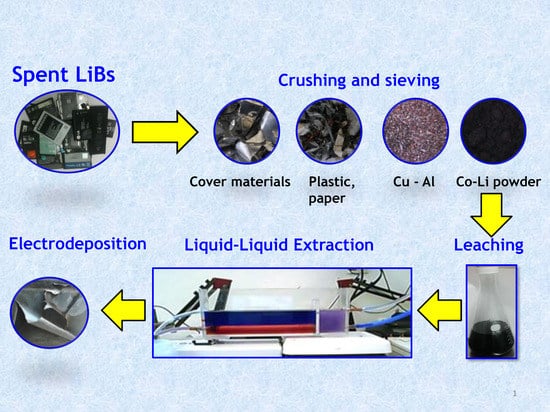
Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
3. Results
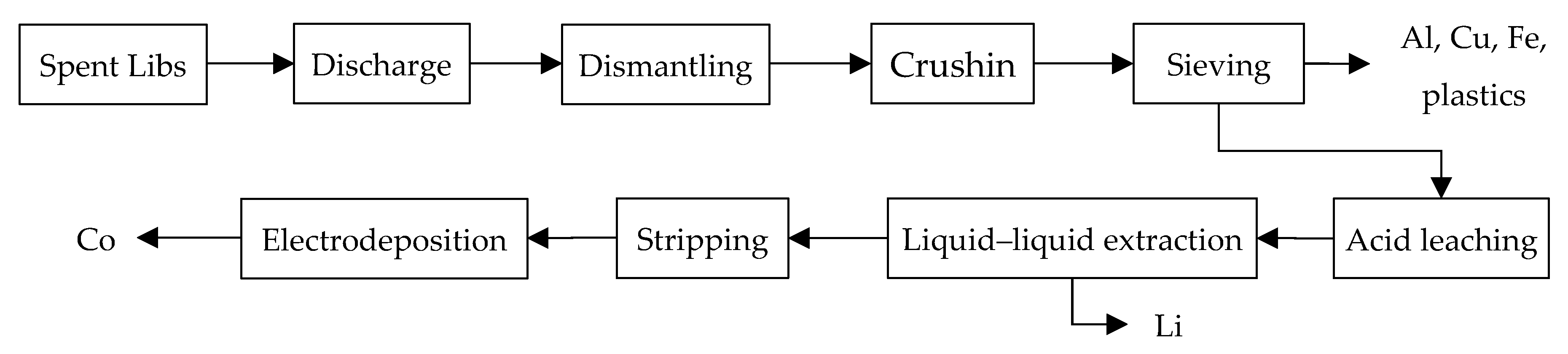
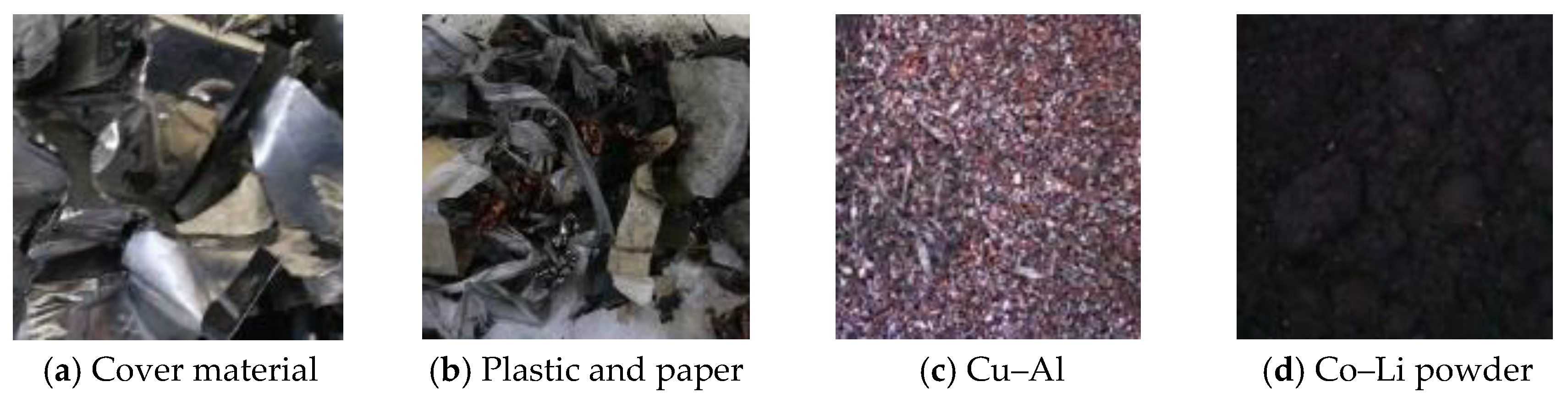
3.1. Collection, Discharge, Dismantling, Drushing, and Sieving
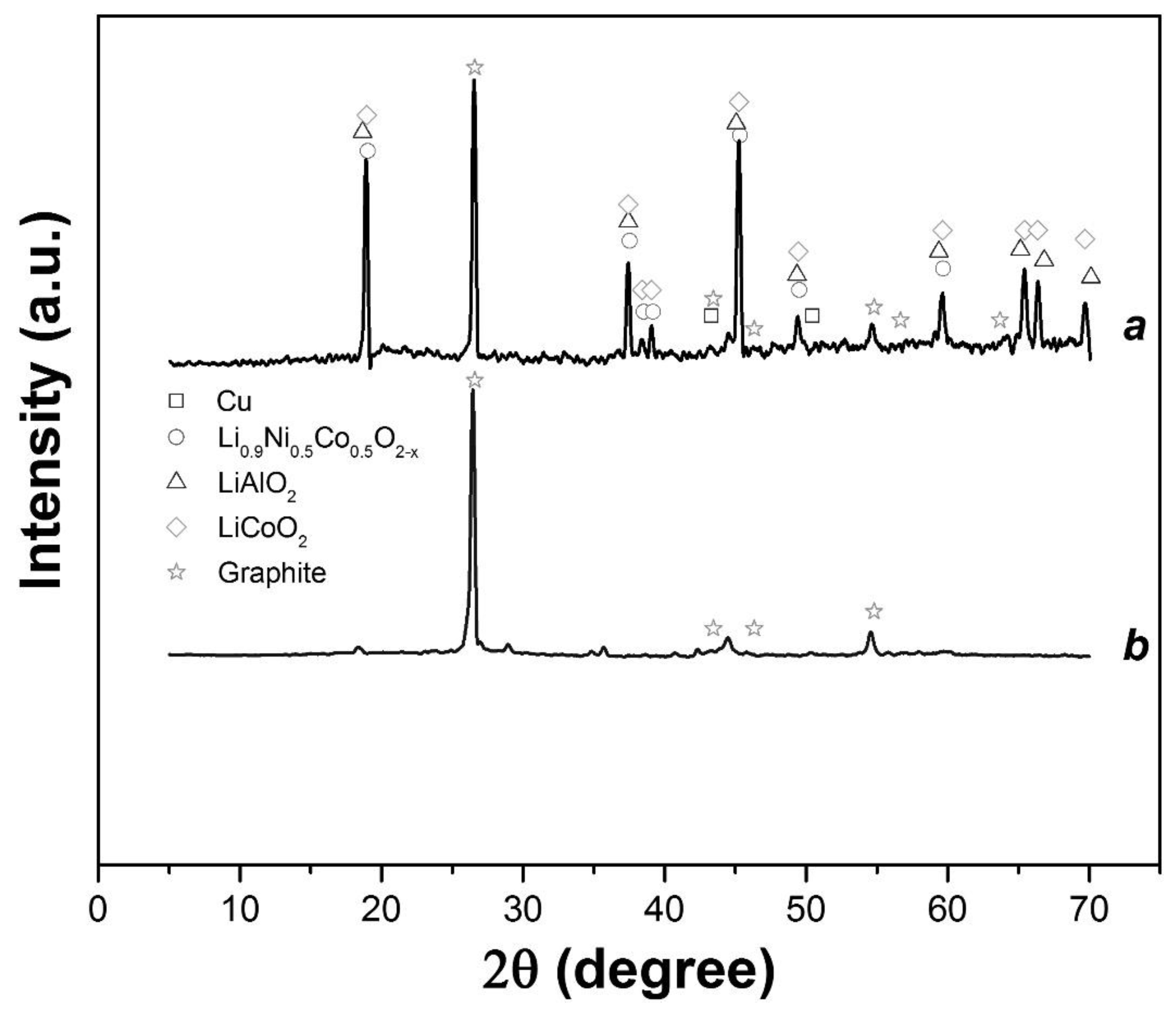
3.2. Leaching
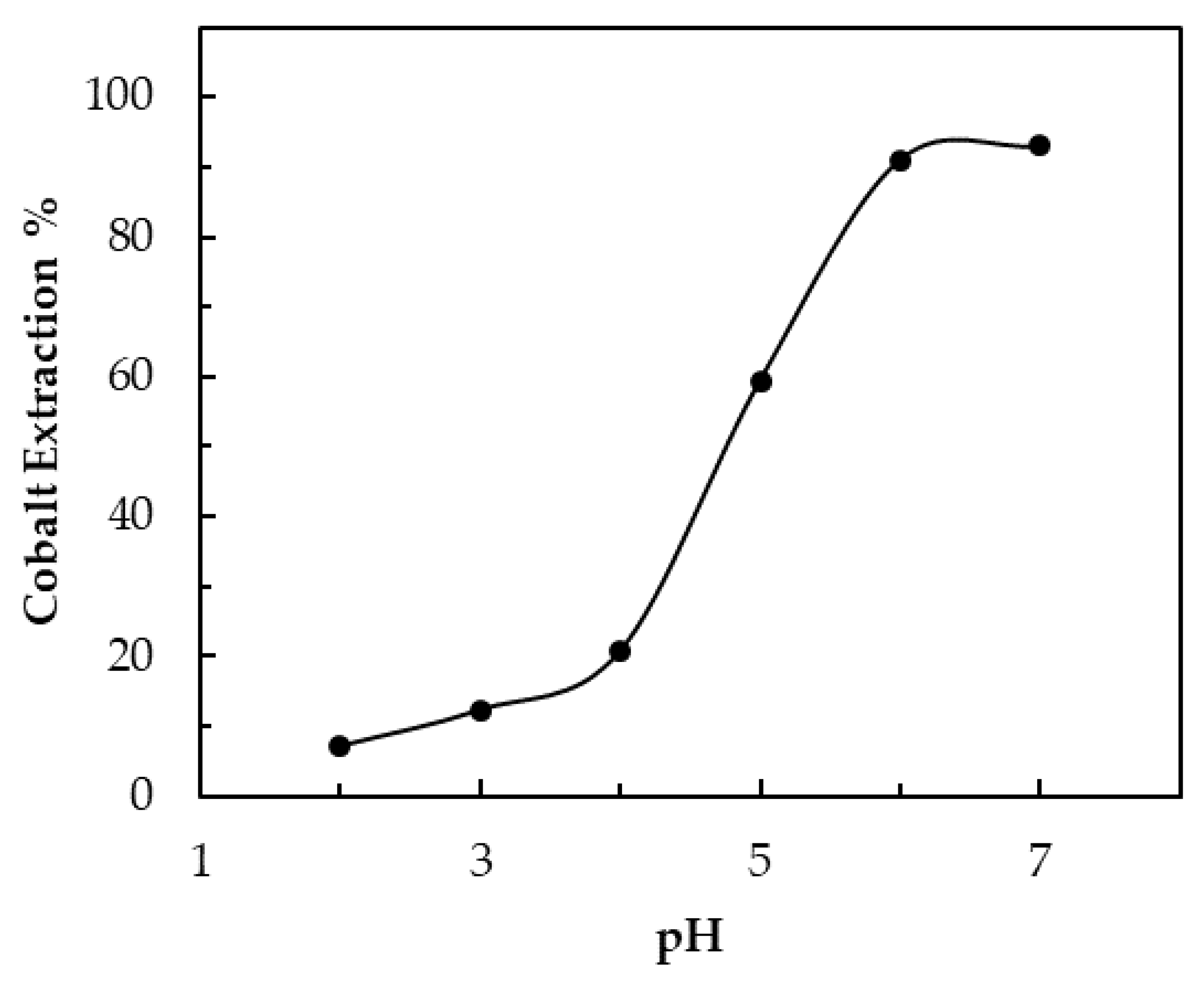
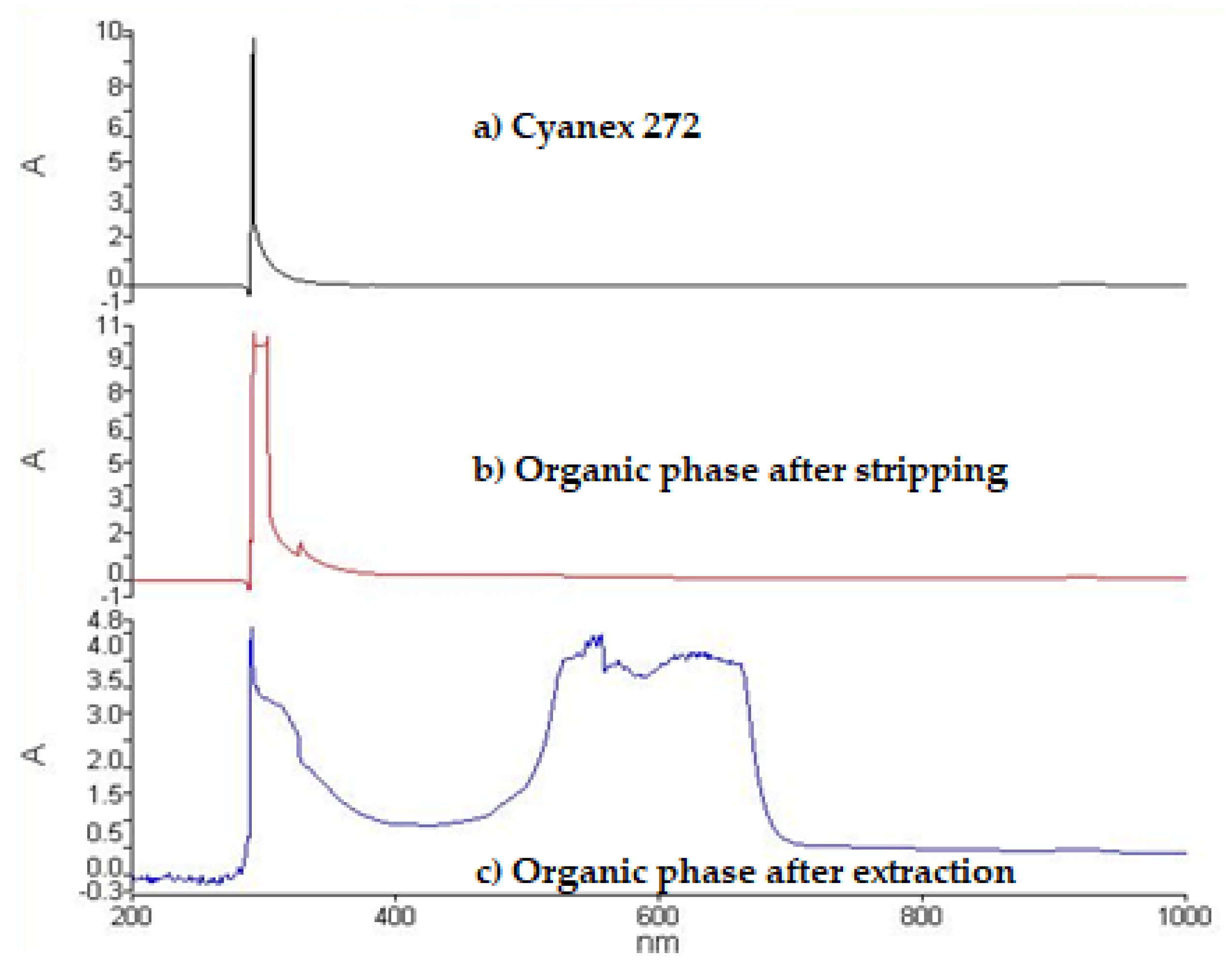
3.3. Effect of pH on Extraction
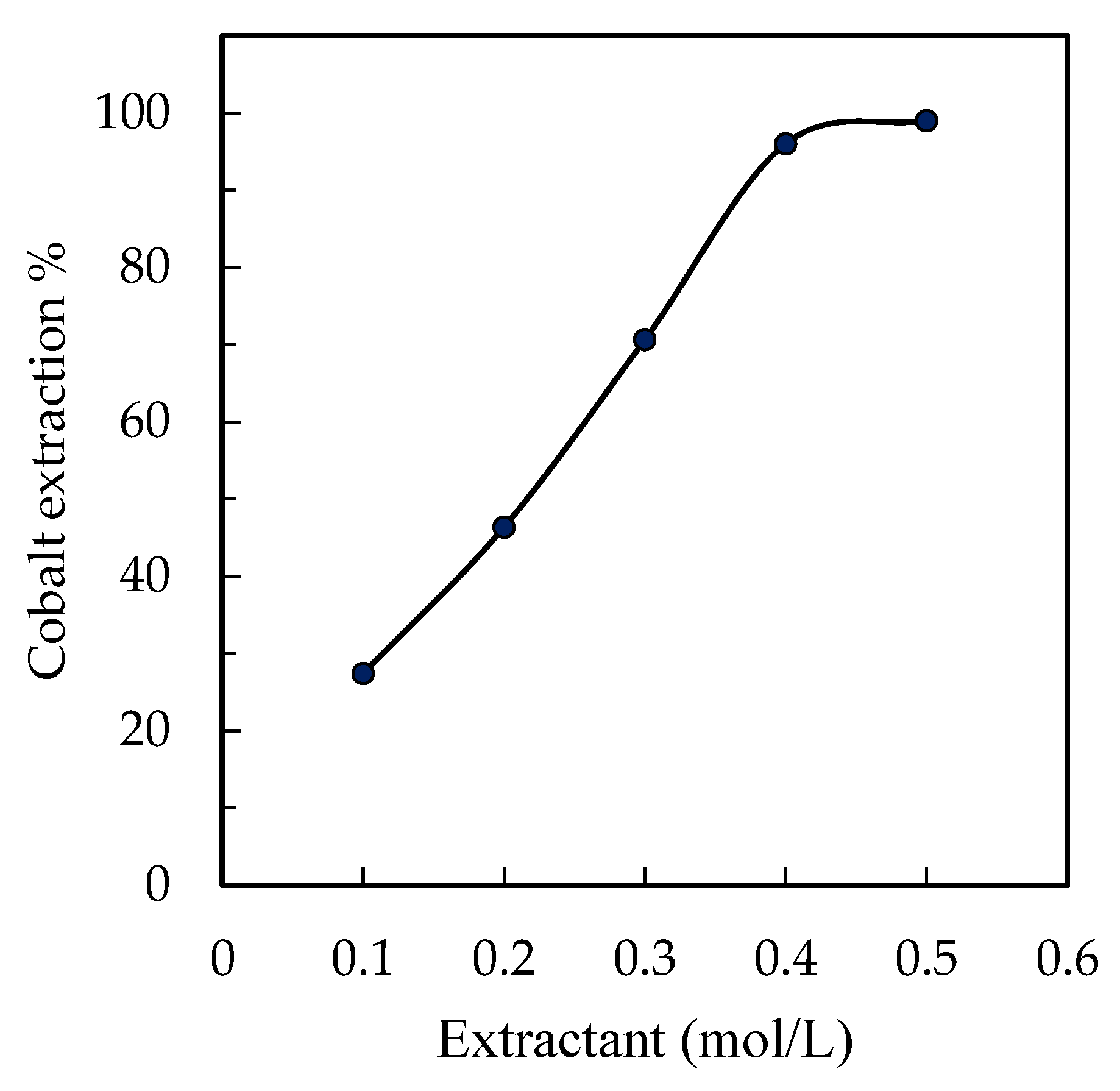
3.4. Effect of Extractant Concentration
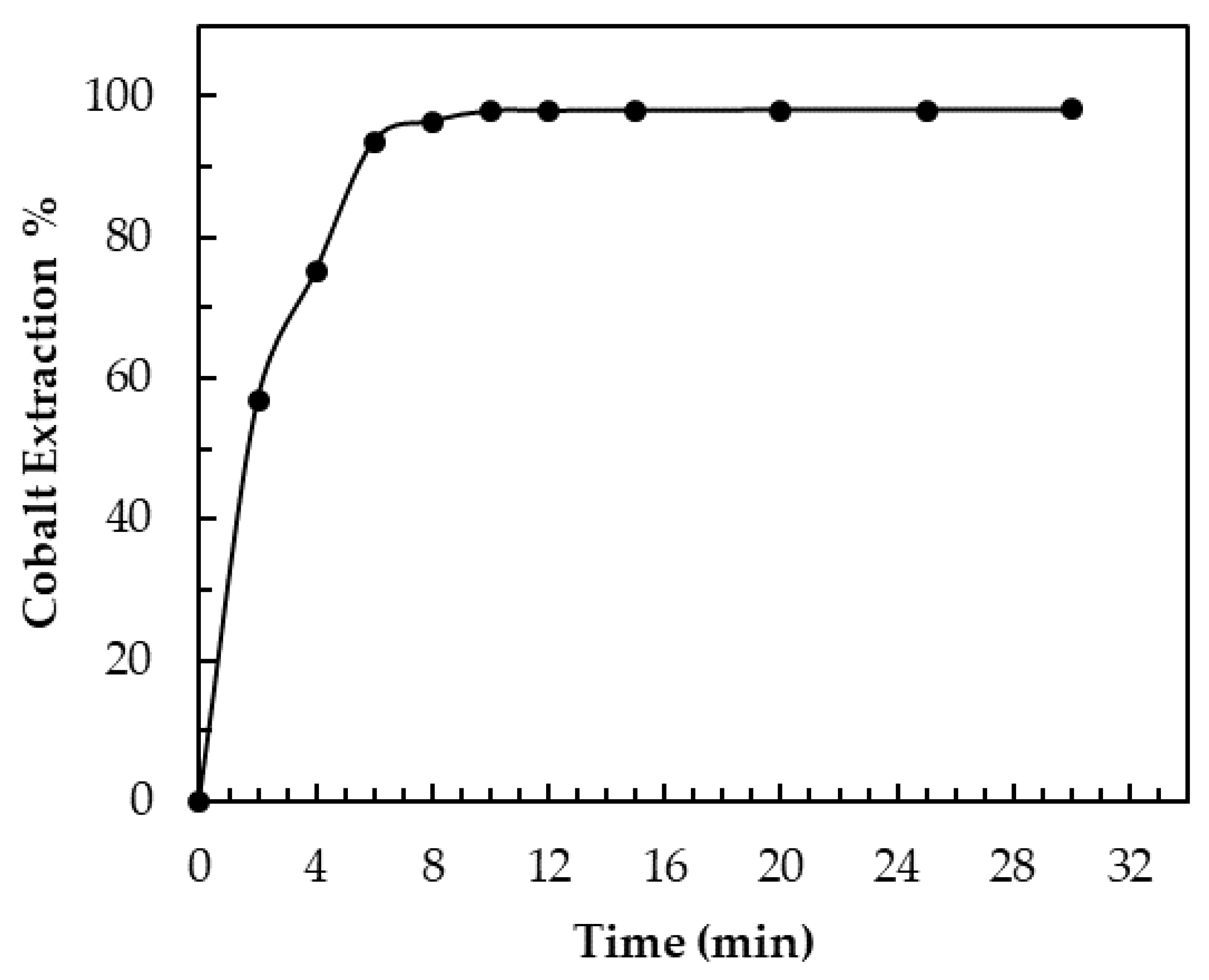
3.5. Extraction Kinetics
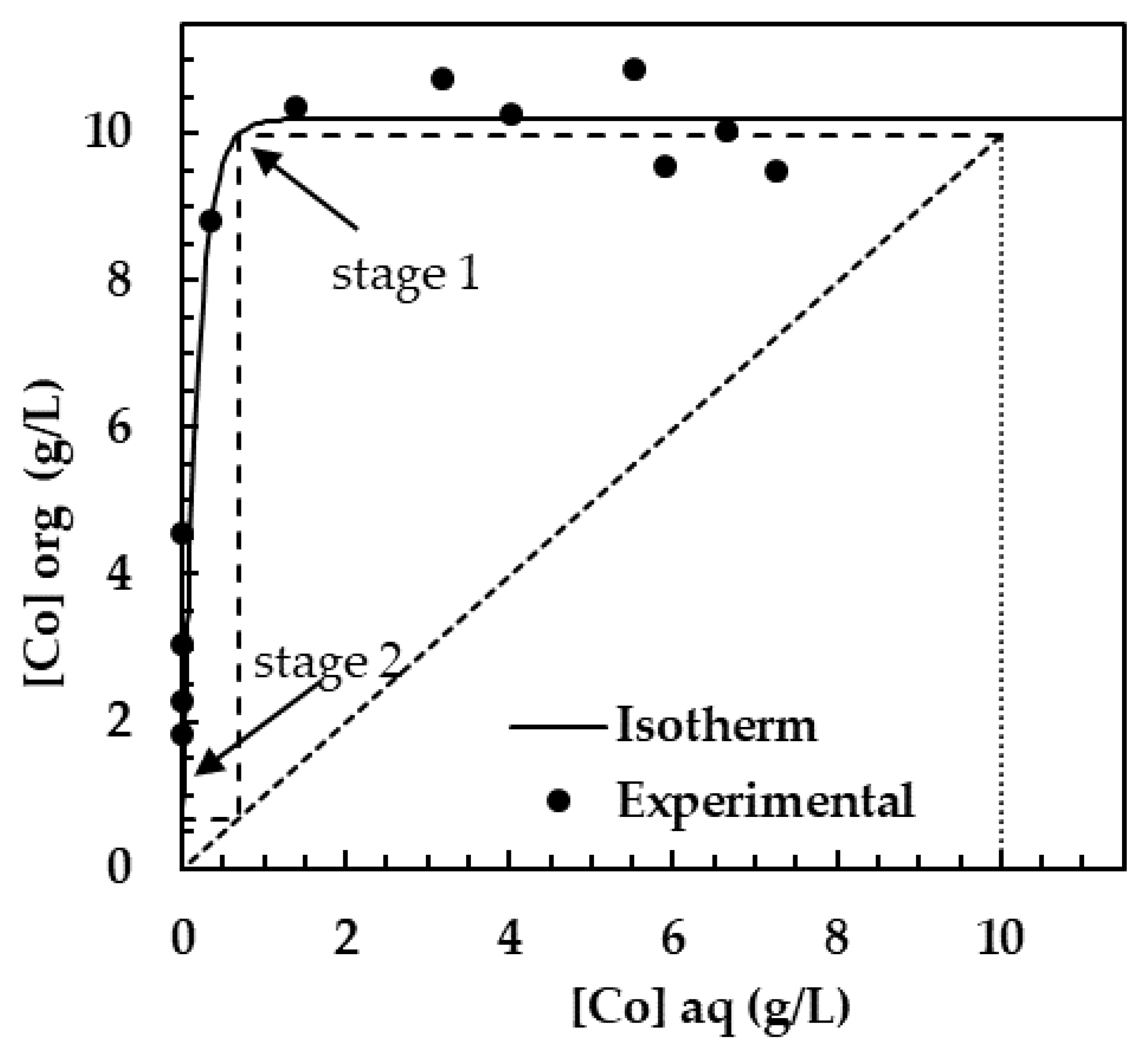
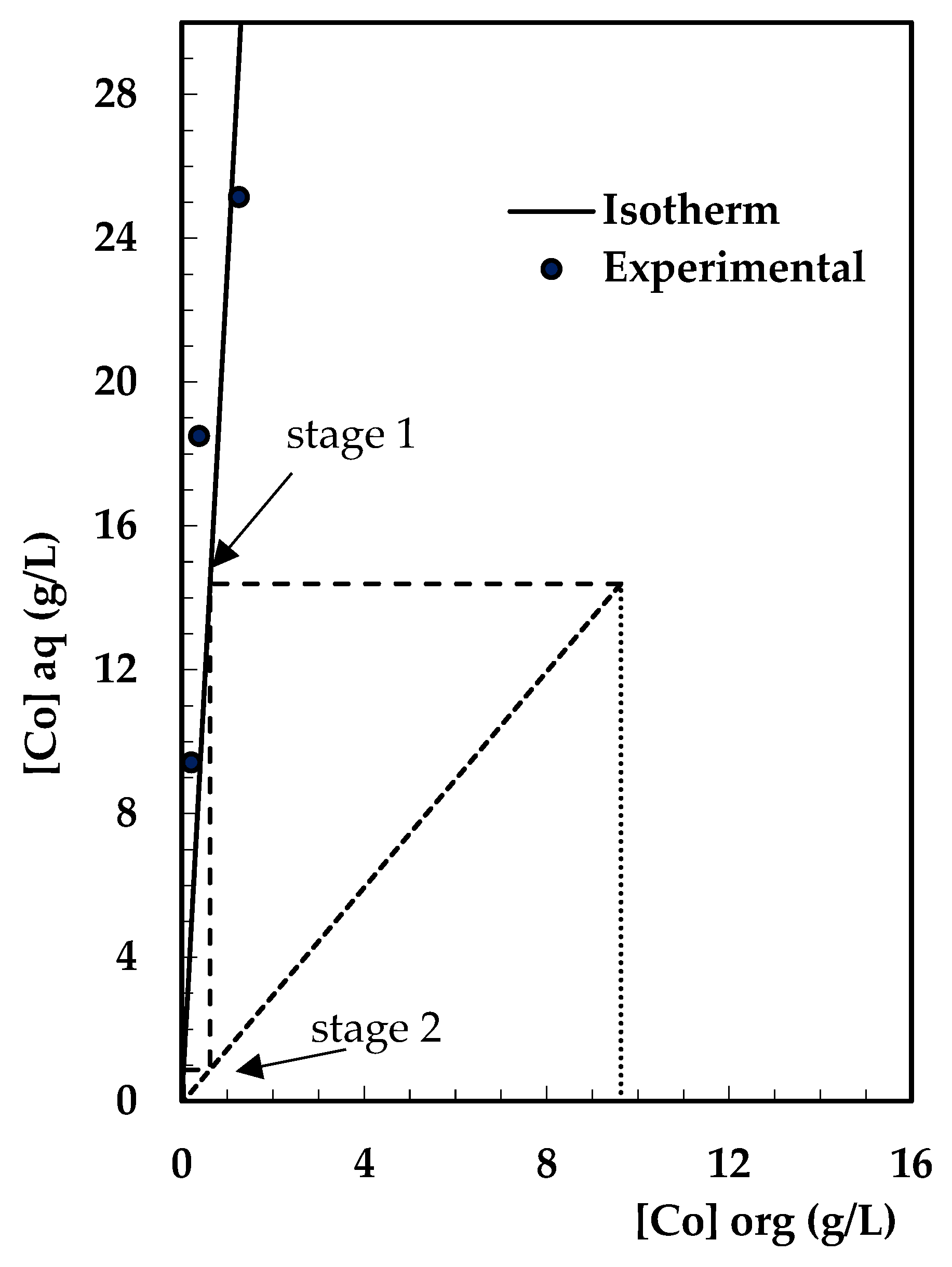
3.6. Extraction Isotherm and Stages
Calculation of Theoretical Stages
3.7. Electroplating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor 2017; United Nations University (UNU), International Telecommunication Union (ITU) & International Solid Waste Association (ISWA): Bonn, Germany; Geneva, Switzerland; Viena, Austria, 2017. [Google Scholar]
- Song, Q.; Liang, Y.; Li, J.; Liu, L.; Tan, Q.; Dong, Q. Potential recycling availability and capacity assessment on typical metals in waste mobile phones: A current research study in China. J. Clean. Prod. 2017, 148, 509–517. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Ren, Y. Prediction of various discarded lithium batteries in China. IEEE Int. Symp. Sustain. Syst. Technol. 2012, 1–4. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus Niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Aaltonen, M.; Peng, C.; Wilson, B.; Lundström, M. Leaching of Metals from Spent Lithium-Ion Batteries. Recycling 2017, 2, 20. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Jian, G.; Guo, J.; Wang, X.; Sun, C.; Zhou, Z.; Yu, L.; Kong, F.; Qiu, J. Study on Separation of Cobalt and Lithium Salts from Waste Mobile-phone Batteries. Procedia Environ. Sci. 2012, 16, 495–499. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhang, W.; Chen, Y.; Wang, C. Efficient and economical recovery of lithium, cobalt, nickel, manganese from cathode scrap of spent lithium-ion batteries. J. Clean. Prod. 2018, 204, 437–446. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J. Hazard. Mater. 2017, 338, 124–131. [Google Scholar] [CrossRef] [PubMed]
- El-Nadi, Y.A. Solvent Extraction and Its Applications on Ore Processing and Recovery of Metals: Classical Approach. Sep. Purif. Rev. 2017, 46, 195–215. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Wang, F.; He, F.; Zhao, J.; Sui, N.; Xu, L.; Liu, H. Extraction and separation of cobalt(II), copper(II) and manganese(II) by Cyanex272, PC-88A and their mixtures. Sep. Purif. Technol. 2012, 93, 8–14. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Yang, M.; Cui, M.; Hou, X. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 2006, 84, 75–80. [Google Scholar] [CrossRef]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef]
- Sharma, I.G.; Alex, P.; Bidaye, A.C.; Suri, A.K. Electrowinning of cobalt from sulphate solutions. Hydrometallurgy 2005, 80, 132–138. [Google Scholar] [CrossRef]
- Mishra, K.G.; Singh, P.; Muir, D.M. Electrowinning of cobalt from sulphate solutions contaminated with organic impurities. Hydrometallurgy 2002, 65, 97–102. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Liu, J.; Wei, R.; Weng, J.; Wang, J. Investigation of a commercial lithium-ion battery under overcharge/over-discharge failure conditions. RSC Adv. 2018, 58, 33414–33424. [Google Scholar] [CrossRef]
- Rodriguez Godinez, M.A. Estudio de lixiviación para la recuperación de cobalto, litio, cobre, aluminio y niquel de las baterias de teléfonos móviles (Spanish). Bachelor’s Thesis, Universidad de Guanajuato, Guanajuato, Mexico, 2017. [Google Scholar]
- Evans, H.A.; Bahri, P.A.; Rumball, J.A.; Barnard, K.R. Modelling cobalt extraction with Cyanex 272. In Proceedings of the International Solvent Extraction Conference 2008—Volume I, Tucson, AZ, USA, 15–19 September 2008; pp. 459–465. [Google Scholar]





| Anode | Cathode | Plastic | Electrolyte | Case | Loss |
|---|---|---|---|---|---|
| 18 | 35 | 6 | 11 | 26 | 4 |
| Battery Group (g) | Average Weight (g) | Cover (% w/w) | Plastic, Paper (% w/w) | Cu Al (% w/w) | Co Li Powder (% w/w) |
|---|---|---|---|---|---|
| 15–30 | 21.20 | 24.52 | 24.16 | 8.84 | 41.90 |
| 30–40 | 34.23 | 21.73 | 4.83 | 15.97 | 57.40 |
| Current (A) | Potential (V) | Current Density (A/m2) | Mass (g) | Current Efficiency (%) |
|---|---|---|---|---|
| 0.25 | 2.71 | 50.1 | 1.1947 | 66.37 |
| 0.50 | 3.07 | 100.2 | 1.5484 | 68.82 |
| 0.75 | 4.02 | 150.3 | 1.3698 | 76.10 |
| 1.00 | 4.19 | 200.4 | 1.3194 | 73.30 |
| 1.50 | 5.51 | 300.6 | 1.4410 | 80.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Almanza, D.; Gamiño-Arroyo, Z.; Sánchez-Cadena, L.E.; Gómez-Castro, F.I.; Uribe-Ramírez, A.R.; Aguilera-Alvarado, A.F.; Ocampo Carmona, L.M. Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction. Batteries 2019, 5, 44. https://doi.org/10.3390/batteries5020044
Quintero-Almanza D, Gamiño-Arroyo Z, Sánchez-Cadena LE, Gómez-Castro FI, Uribe-Ramírez AR, Aguilera-Alvarado AF, Ocampo Carmona LM. Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction. Batteries. 2019; 5(2):44. https://doi.org/10.3390/batteries5020044
Chicago/Turabian StyleQuintero-Almanza, Daniel, Zeferino Gamiño-Arroyo, Lorena Eugenia Sánchez-Cadena, Fernando Israel Gómez-Castro, Agustín Ramón Uribe-Ramírez, Alberto Florentino Aguilera-Alvarado, and Luz Marina Ocampo Carmona. 2019. "Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction" Batteries 5, no. 2: 44. https://doi.org/10.3390/batteries5020044
APA StyleQuintero-Almanza, D., Gamiño-Arroyo, Z., Sánchez-Cadena, L. E., Gómez-Castro, F. I., Uribe-Ramírez, A. R., Aguilera-Alvarado, A. F., & Ocampo Carmona, L. M. (2019). Recovery of Cobalt from Spent Lithium-Ion Mobile Phone Batteries Using Liquid–Liquid Extraction. Batteries, 5(2), 44. https://doi.org/10.3390/batteries5020044