Low Reversible Capacity of Nitridated Titanium Electrical Terminals
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Cells
2.2. Electrical Terminals
2.3. Nitridation of Ti Electrode Terminals
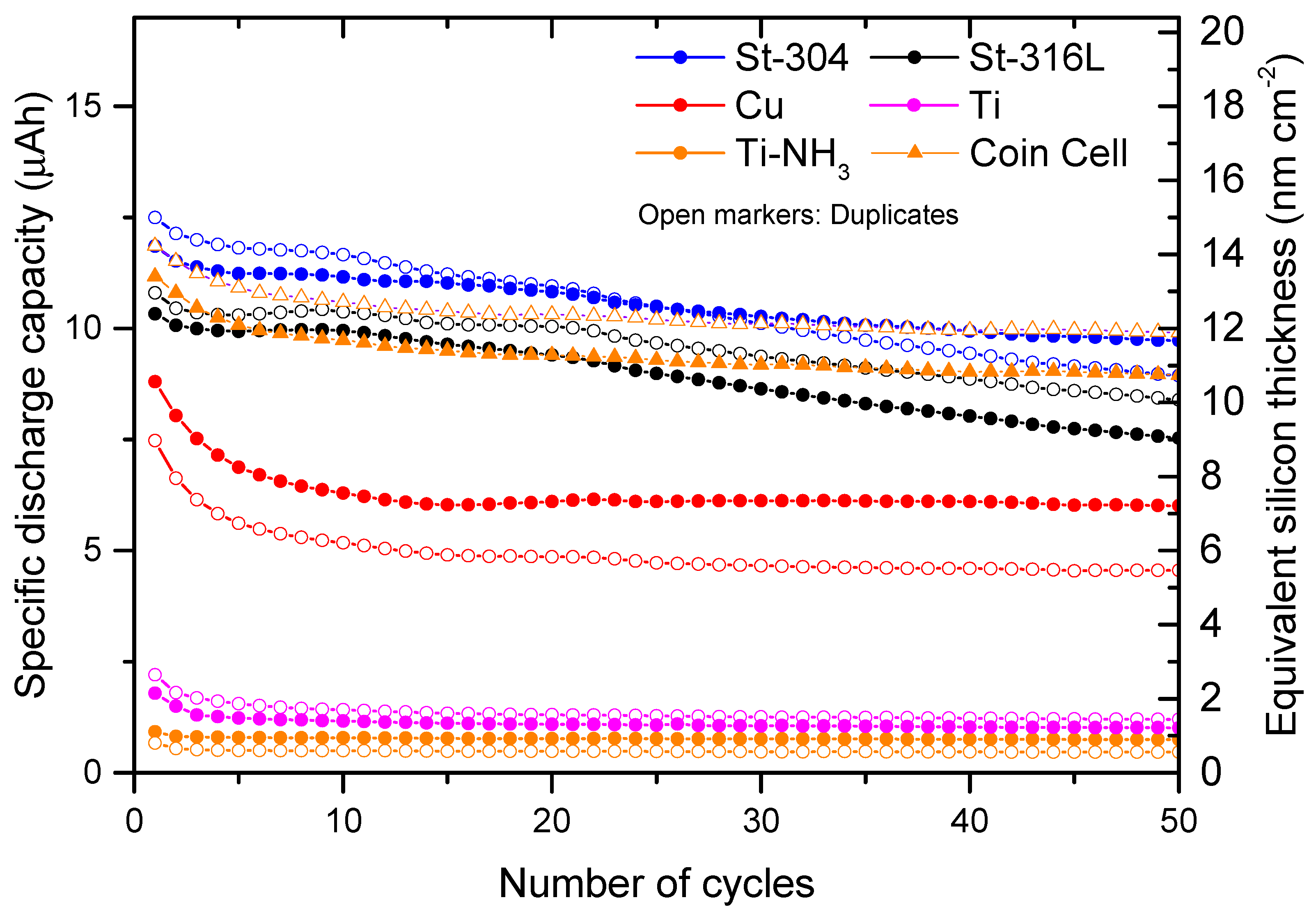
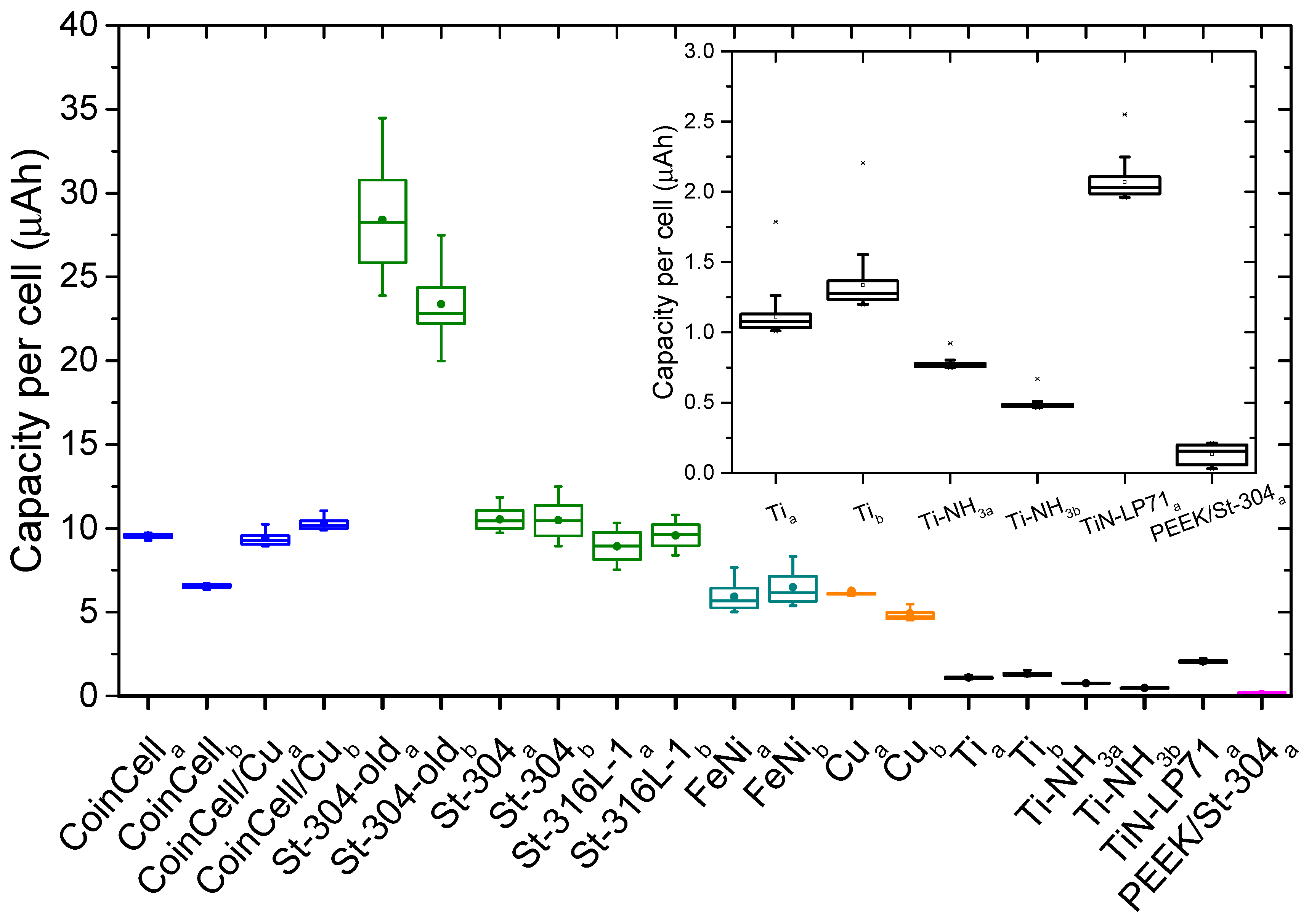
2.4. Determination of Reversible Capacities
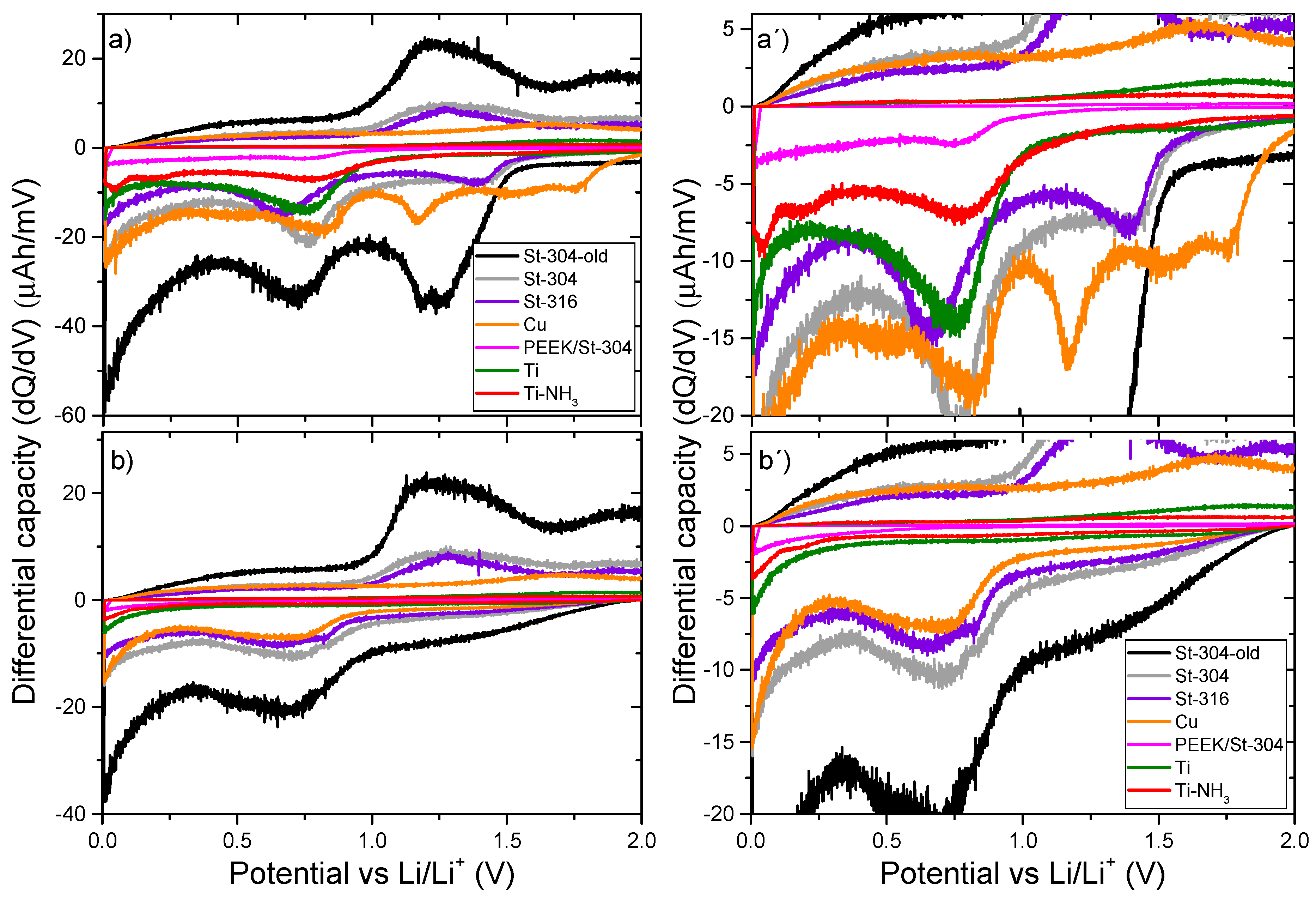
2.5. Differential Capacity Characterization (dQ/dV)
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Erickson, E.M.; Markevich, E.; Salitra, G.; Sharon, D.; Hirshberg, D.; de la Llave, E.; Shterenberg, I.; Rozenman, A.; Frimer, A.; Aurbach, D. Review—Development of advanced rechargeable batteries: a continuous challenge in the choice of suitable electrolyte solutions. J. Electrochem. Soc. 2015, 162, A2424–A2438. [Google Scholar] [CrossRef]
- Kohanoff, J.; Galli, G.; Parrinello, M. Theoretical study of LiC6. J. Phys. IV 1991, 1, C5-351–C5-356. [Google Scholar] [CrossRef]
- Tozawa, T.N.K. Lithium ion rechargeable battery. Prog. Batter. Solar. Cells 1990, 9, 209. [Google Scholar]
- Su, D.S.; Schlögl, R. Nanostructured carbon and carbon nanocomposites for electrochemical energy storage applications. ChemSusChem 2010, 3, 136–168. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Dogan, F.; Ilavsky, J.; Maroni, V.A.; Gosztola, D.J.; Lu, W. Mechanisms for lithium nucleation and dendrite growth in selected carbon allotropes. Chem. Mater. 2017, 29, 6205–6213. [Google Scholar] [CrossRef]
- Lu, W.; López, C.M.; Liu, N.; Vaughey, J.T.; Jansen, A.; Dees, D.W. Overcharge effect on morphology and structure of carbon electrodes for lithium-ion batteries. J. Electrochem. Soc. 2012, 159, A566. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Aravindan, V.; Lee, Y.S.; Madhavi, S. Research progress on negative electrodes for practical Li-ion batteries: beyond carbonaceous anodes. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.X.; Wan, L.J.; Guo, Y.G. Silicon-based nanomaterials for lithium-ion batteries. Chin. Sci. Bull. 2012, 57, 4104–4110. [Google Scholar] [CrossRef]
- Hatchard, T.D.; Dahn, J.R. In situ XRD and electrochemical study of the reaction of lithium with amorphous silicon. J. Electrochem. Soc. 2004, 151, A838–A842. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L.; Le, D.B.; Dahn, J.R. Alloy design for lithium-ion battery anodes. J. Electrochem. Soc. 2007, 154, A849. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based anode materials for li-ion batteries: A. mini review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef]
- Hu, Y.S.; Demir-Cakan, R.; Titirici, M.M.; Müller, J.O.; Schlögl, R.; Antonietti, M.; Maier, J. Superior storage performance of a Si@SiOx/C nanocomposite as anode material for lithium-ion batteries. Angew. Chemie. 2008, 47, 1645–1649. [Google Scholar] [CrossRef]
- Abraham, K.M. Prospects and limits of energy storage in batteries. J. Phys. Chem. Lett. 2015, 6, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Z.W.; Qin, Q.Z. Microstructure and Li alloy formation of nano-structured amorphous Si and Si/TiN composite thin film electrodes. Electrochem. Commun. 2004, 6, 484–491. [Google Scholar] [CrossRef]
- Bourderau, S.; Brousse, T.; Schleich, D. Amorphous silicon as a possible anode material for Li-ion batteries. J. Power Sources 1999, 81–82, 233–236. [Google Scholar] [CrossRef]
- Demirkan, M.T.; Trahey, L.; Karabacak, T. Low-density silicon thin films for lithium-ion battery anodes. Thin Solid Films 2016, 600, 126–130. [Google Scholar] [CrossRef]
- Ohara, S.; Suzuki, J.; Sekine, K.; Takamura, T. A thin film silicon anode for Li-ion batteries having a very large specific capacity and long cycle life. J. Power Sources 2004, 136, 303–306. [Google Scholar] [CrossRef]
- Mukanova, A.; Jetybayeva, A.; Myung, S.T.; Kim, S.S.; Bakenov, Z. A mini-review on the development of Si-based thin film anodes for Li-ion batteries. Mater. Today Energy 2018, 9, 49–66. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D integrated all-solid-state rechargeable batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Baggetto, L.; Niessen, R.A.H.; Roozehoom, F.; Notten, P.H.L. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Adv. Funct. Mater. 2008, 18, 1057–1066. [Google Scholar] [CrossRef]
- Reyes Jiménez, A.; Klöpsch, R.; Wagner, R.; Rodehorst, U.C.; Kolek, M.; Nölle, R.; Winter, M.; Placke, T. A Step toward high-energy silicon-based thin film lithium ion batteries. ACS Nano 2017, 11, 4731–4744. [Google Scholar] [CrossRef]
- Kermani, G.; Sahraei, E. Review: Characterization and modeling of the mechanical properties of lithium-ion batteries. Energies 2017, 10, 1730. [Google Scholar] [CrossRef]
- Behrou, R.; Maute, K. Numerical modeling of damage evolution phenomenon in solid-state lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A2573–A2589. [Google Scholar] [CrossRef]
- Behrou, R.; Maute, K. Multiscale modeling of non-local damage evolution in lithium-ion batteries. ECS Trans. 2017, 77, 1163–1177. [Google Scholar] [CrossRef]
- Franco, A. A multiscale modelling and numerical simulation of rechargeable lithium ion batteries: concepts, methods and challenges. Rsc Adv. 2013, 3, 13027–13058. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, Y.; Wang, E. First-principles approaches to simulate lithiation in silicon electrodes. Model. Simul. Mater. Sci. Eng. 2013, 21, 074001. [Google Scholar] [CrossRef]
- Myung, S.T.; Sasaki, Y.; Saito, T.; Sun, Y.K.; Yashiro, H. Passivation behavior of Type 304 stainless steel in a non-aqueous alkyl carbonate solution containing LiPF6 salt. Electrochim. Acta 2009, 54, 5804–5812. [Google Scholar] [CrossRef]
- Myung, S.T.; Sasaki, Y.; Sakurada, S.; Sun, Y.K.; Yashiro, H. Electrochemical behavior of current collectors for lithium batteries in non-aqueous alkyl carbonate solution and surface analysis by ToF-SIMS. Electrochim. Acta 2009, 55, 288–297. [Google Scholar] [CrossRef]
- Myung, S.-T.; Hitoshi, Y.; Sun, Y.-K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891. [Google Scholar] [CrossRef]
- Janski, R.; Forster, M.F.M.; Dunst, M.S.A. Lithium barrier materials for on-chip Si-based microbatteries. J. Mater. Sci. 2017, 28, 14605–14614. [Google Scholar] [CrossRef]
- Grigorov, K.G.; Grigorov, G.I.; Stoyanova, M.; Vignes, J.L.; Langeron, J.P.; Denjean, P.; Perriere, J. Diffusion of silicon in titanium nitride films. Efficiency of TiN barrier layers. Appl. Phys. A Solids Surfaces 1992, 55, 502–504. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Baggetto, L.; Langereis, E.; van de Sanden, M.C.M.; Klootwijk, J.H.; Roozeboom, F.; Niessen, R.A.H.; Notten, P.H.L.; Kessels, W.M.M. Deposition of TiN and TaN by remote plasma ALD for Cu and Li diffusion barrier applications. J. Electrochem. Soc. 2008, 155, G287. [Google Scholar] [CrossRef]
- Freixas, J.; Eustache, E.; Roussel, P.; Brillard, C.; Deresmes, D.; Nuns, N.; Rolland, N.; Brousse, T.; Lethien, C. Sputtered titanium nitride: A bifunctional material for li-Ion microbatteries. J. Electrochem. Soc. 2015, 162. [Google Scholar] [CrossRef]
- Pohrelyuk, I.; Fedirko, V. Chemico-thermal treatment of titanium alloys—Nitriding. In Titanium Alloys—Towards Achieving Enhanced Properties For Diversified Applications; IntechOpen: London, UK, 2012; pp. 141–174. [Google Scholar]
- Ajikumar, P.K.; Kamruddin, M.; Nithya, R.; Shankar, P.; Dash, S.; Tyagi, A.K.; Raj, B. Surface nitridation of Ti and Cr in ammonia atmosphere. Scr. Mater. 2004, 51, 361–366. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Kamruddin, M.; Shankar, P.; Gouda, R.; Balamurugan, A.K.; Nithya, R.; Tyagi, A.K.; Jayaram, V.; Biswas, S.K.; Raj, B. Internal nitride formation during gas-phase thermal nitridation of titanium. Scr. Mater. 2009, 61, 403–406. [Google Scholar] [CrossRef]
- Velasco-Velez, J.J.; Davaasuren, B.; Scherzer, M.; Cap, S.; Willinger, M.; Guo, J.H.; Schlögl, R.; Knop-Gericke, A. Exploring the incorporation of nitrogen in titanium and its influence on the electrochemical corrosion resistance in acidic media. Surf. Sci. 2016, 650, 272–278. [Google Scholar] [CrossRef]
- Nulman, J.; Alto, P.; Materials, A.; Clara, S. Gas in an Integrated Processing. U.S. Patent 5,236,868, 17 August 1993. [Google Scholar]
- Kurtz, S.R.; Gordon, R.G. Chemical vapor deposition of titanium nitride at low temperatures. Thin Solid Films 1986, 140, 277–290. [Google Scholar] [CrossRef]
- Rebenne, H.E.; Bhat, D.G. Review of CVD TiN coatings for wear-resistant applications: deposition processes, properties and performance. Surf. Coatings Technol. 1994, 63, 1–13. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Zhang, X.; Kostecki, R.; Richardson, T.J.; Pugh, J.K.; Ross, P.N. Electrochemical and infrared studies of the reduction of organic carbonates. J. Electrochem. Soc. 2001, 148, A1341. [Google Scholar] [CrossRef]
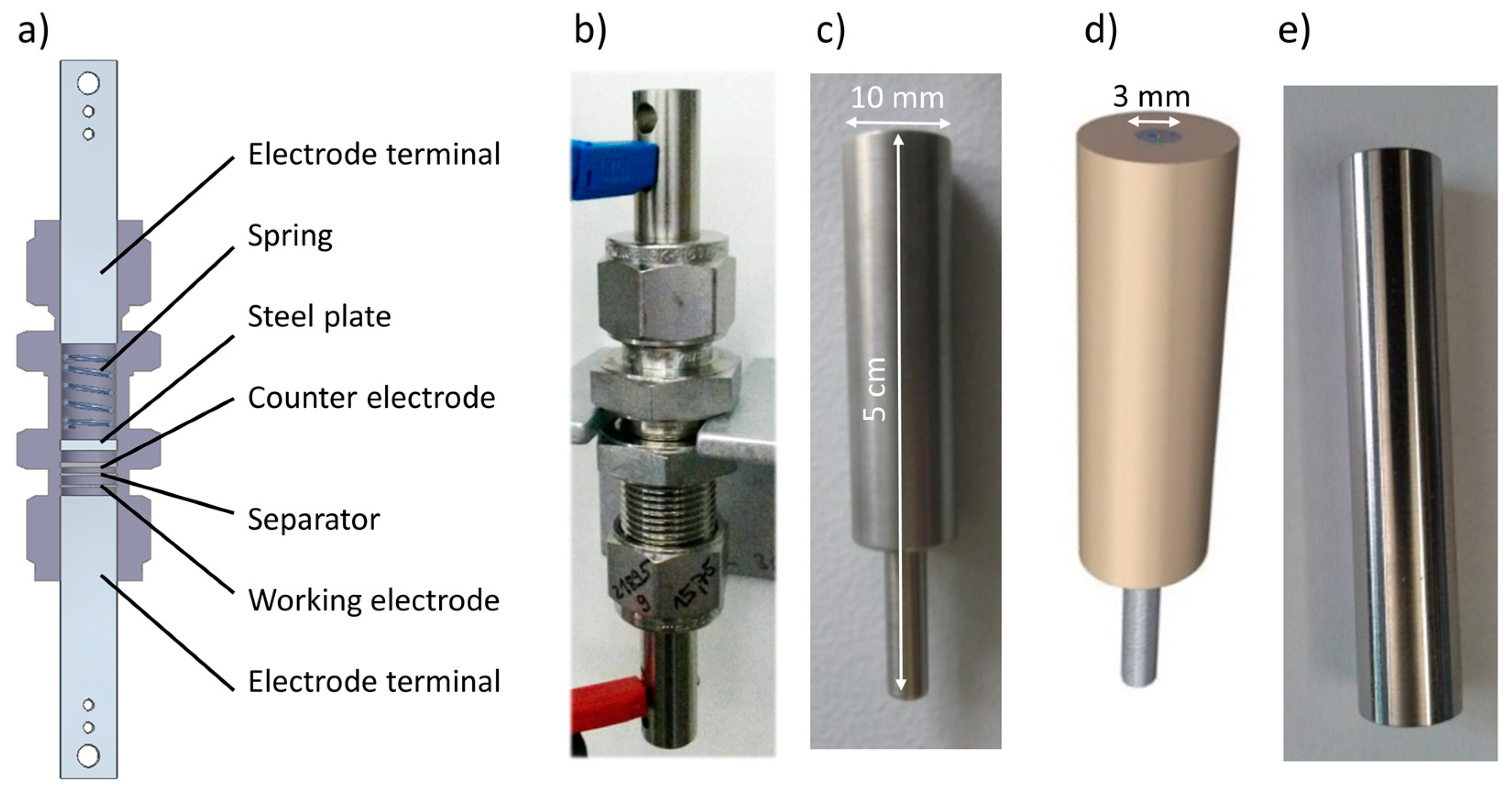
| Electrical Terminal | Ra (μm) | Rz (μm) | Rq (μm) |
|---|---|---|---|
| Pristine Cu | 0.20 ± 0.044 | 1.37 ± 0.044 | 0.26 ± 0.050 |
| Pristine St-304 | 0.26 ± 0.088 | 1.77 ± 0.79 | 0.30 ± 0.11 |
| Pristine TiN | 0.18 ± 0.025 | 1.35 ± 0.26 | 0.24 ± 0.035 |
| Pristine St-316L | 0.19 ± 0.017 | 1.25 ± 0.10 | 0.24 ± 0.021 |
| Pristine PEEK | 0.13 ± 0.04 | 0.93 ± 0.14 | 0.18 ± 0.052 |
| St-304-old | 1.36 ± 0.74 | 6.83 ± 2.97 | 1.69 ± 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, D.; Xu, Y.; Schlögl, R.; Cap, S. Low Reversible Capacity of Nitridated Titanium Electrical Terminals. Batteries 2019, 5, 17. https://doi.org/10.3390/batteries5010017
Klein D, Xu Y, Schlögl R, Cap S. Low Reversible Capacity of Nitridated Titanium Electrical Terminals. Batteries. 2019; 5(1):17. https://doi.org/10.3390/batteries5010017
Chicago/Turabian StyleKlein, David, Yaolin Xu, Robert Schlögl, and Sébastien Cap. 2019. "Low Reversible Capacity of Nitridated Titanium Electrical Terminals" Batteries 5, no. 1: 17. https://doi.org/10.3390/batteries5010017
APA StyleKlein, D., Xu, Y., Schlögl, R., & Cap, S. (2019). Low Reversible Capacity of Nitridated Titanium Electrical Terminals. Batteries, 5(1), 17. https://doi.org/10.3390/batteries5010017





