Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries †
Abstract
1. Introduction
2. Methods
3. Materials
4. Catalysis, Catalysts and Electrode Materials
- cheap and abundantly available
- easily manufactured into particular shapes and forms
- chemically stable against practically all reactant solutions encountered in RFB
- mechanically stable during operation
- impermeable for electrolyte solution
- show reasonable electrocatalytic activities for many redox reactions in their pristine state
4.1. Modified Carbons and Graphites
- Structural modification of carbon (e.g., by carbon fiber or graphene deposition on graphite)
- Mechanical or chemical surface treatment (e.g., by nitrogen doping of graphite felt)
- Foreign metal deposits
- Metal oxide deposits
4.1.1. Structural Modification of Carbon
4.1.2. Mechanical, Thermal or Chemical Surface Treatment
4.1.3. Foreign Metal Deposits
4.1.4. Metal Oxide Deposits
4.2. Composite Materials
4.3. Non-Carbon Materials and Miscellaneous Concepts
4.4. Catalysis and Surface Enlargement Effects—Concluding Remarks
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Kangro, W. Verfahren zur Speicherung von Elektrischer Energie. German Patent DE914264C, 28 June 1954. [Google Scholar]
- Kangro, W.; Pieper, H. Zur Frage der Speicherung von Elektrischer Energie in Flüssigkeiten. Electrochim. Acta 1962, 7, 435–448. [Google Scholar] [CrossRef]
- Thaller, L.H. Electrically rechargeable redox flow cells. In Proceedings of the 9th Intersociety Energy Conversion Engineering Conference, San Francisco, CA, USA, 26–30 August 1974; pp. 924–928. [Google Scholar]
- Hagedorn, N.H.; Thaller, L.H. Redox storage systems for solar applications. Power Sources 1981, 8, 227–243. [Google Scholar]
- Skyllas-Kazacos, M.; Robins, R. All-Vanadium Redox Battery. U.S. Patent 4,786,567, 11 February 1986. [Google Scholar]
- Bartolozzi, M. Development of redox flow batteries. A historical bibliography. J. Power Sources 1989, 27, 219–234. [Google Scholar] [CrossRef]
- Giner, J.; Jalan, V.; Swette, L. Redox Storage Batteries. In DECHEMA-Monographie; Vielstich, W., Ed.; Verlag Chemie: Weinheim, Germany, 1982; Volume 92, pp. 381–393. [Google Scholar]
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55–R79. [Google Scholar] [CrossRef]
- Tomazic, G.; Skyllas-Kazacos, M. Redox Flow Batteries. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 309–336. [Google Scholar]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Guarnieri, M.; Mattavelli, P.; Petrone, G.; Spagnuolo, G. Vanadium Redox Flow Batteries. IEEE Ind. Electron. Mag. 2016, 10, 20–31. [Google Scholar] [CrossRef]
- Ravikumar, M.K.; Rathod, S.; Jaiswal, N.; Patil, S.; Shukla, A. The renaissance in redox flow batteries. J. Solid State Electrochem. 2017, 21, 2467–2488. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Shigematsu, T. Redox flow battery for energy storage. SEI Tech. Rev. 2011, 73, 5–13. [Google Scholar]
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Recent advances with UNSW vanadium-based redox flow batteries. Int. J. Energy Res. 2010, 34, 182–189. [Google Scholar] [CrossRef]
- Fabjan, C.; Garche, J.; Harrer, B.; Jörissen, L.; Kolbeck, C.; Philippi, F.; Tomazic, G.; Wagner, F. The vanadium redox-battery: An efficient storage unit for photovoltaic systems. Electrochim. Acta 2007, 47, 825–831. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Cunha, A.; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium redox flow batteries: A technology review. Int. J. Energy Res. 2015, 39, 889–918. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Deng, Q.; Mo, J.; Xie, H.; Liu, Z.; Xiong, Y.; Wu, X.; Wu, Y. Aqueous Rechargeable Batteries for Large-scale Energy Storage. Israel J. Chem. 2015, 55, 521–536. [Google Scholar] [CrossRef]
- Walsh, F.C.; Ponce de Leon, C.; Berlouis, L.; Nikiforidis, G.; Arenas-Martinez, L.F.; Hodgson, D.; Hall, D. The Development of Zn-Ce Hybrid Redox Flow Batteries for Energy Storage and Their Continuing Challenges. ChemPlusChem 2015, 80, 288–311. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Xiang, X.; Zhang, J.; Hu, J.; Wu, Y. Electrolytes for vanadium redox flow batteries. Pure Appl. Chem. 2014, 86, 661–669. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Li, X.; Ponce De León, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium Electrolyte Studies for the Vanadium Redox Battery A Review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, H.; Li, X.; Liu, T.; Xing, F. Vanadium Flow Battery for Energy Storage: Prospects and Challenges. J. Phys. Chem. Lett. 2013, 4, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.-G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials Science and Materials Chemistry for Large Scale Electrochemical Energy Storage: From Transportation to Electrical Grid. Adv. Funct. Mater. 2013, 23, 929–946. [Google Scholar] [CrossRef]
- Parasuraman, A.; Mariana Lim, T.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M.; Mustafa, I. Redox Flow Battery for Energy Storage. Arab. J. Sci. Eng. 2013, 38, 723–739. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. 2015, A3, 16913–16933. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Mariana Lim, T. Recent Advancements in All-Vanadium Redox Flow Batteries. Adv. Mater. Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Wei, G.-J.; Fan, X.-Z.; Liu, J.-G.; Yan, C.-W. A review of the electrochemical activity of carbon materials in vanadium redox flow batteries. Xinxing Tan Cailao/New Carbon Mater. 2014, 29, 272–279, reprinted in Carbon 2015, 81, 850. [Google Scholar] [CrossRef]
- Zhang, H. Liquid Redox Rechargeable Batteries. In Electrochemical Technologies for Energy Storage and Conversion; Liu, R.S., Zhang, L., Sun, X., Liu, H., Zhang, J., Eds.; WILEY-VCH: Weinheim, Germany, 2012; pp. 279–316. [Google Scholar]
- Park, M.; Ryu, J.; Wang, W.; Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2017, 2, 16080. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhao, T.S.; An, L.; Zhou, X.L.; Wei, L. A comparative study of all-vanadium and iron-chromium redox flow batteries for large-scale energy storage. J. Power Sources 2015, 300, 438–443. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Liu, J.; Zhou, Q.; Zhou, W.; Li, H.; Wu, Y. Ion exchange membranes for vanadium redox flow batteries. Pure Appl. Chem. 2014, 86, 633–649. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, H.M.; Mai, Z.S.; Zhang, H.Z.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Wei, X.; Li, B.; Wang, W. Porous Polymeric Composite Separators for Redox Flow Batteries. Polym. Rev. 2015, 55, 247–272. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; et al. Materials and Systems for Organic Redox Flow Batteries: Status and Challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- Rosenberg, D.; Pansegrau, S.; Wachholz, M.; Rehling, A.; Busker, M.; Jansen, W. Redox Flow Batteries—Organic Batteries with Future Prospects. CHEMKON 2017, 24, 325–340. [Google Scholar] [CrossRef]
- Leung, P.; Shah, A.A.; Sanz, L.; Flox, C.; Morante, J.R.; Xu, Q.; Mohamed, M.R.; Ponce de Leon, C.; Walsh, F.C. Recent developments in organic redox flow batteries: A critical review. J. Power Sources 2017, 360, 243–283. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Dryfe, R.A.W.; Roberts, E.P.L. Evaluation of electrolytes for redox flow battery applications. Electrochim. Acta 2007, 52, 2189–2195. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ould Amrouche, S.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Minke, C.; Turek, T. Materials, system designs and modelling approaches in techno-economic assessment of all-vanadium redox flow batteries—A review. J. Power Sources 2018, 376, 66–81. [Google Scholar] [CrossRef]
- Arbabzadeh, M.; Johnson, J.X.; Kleine, R.De; Keoleian, G.A. Vanadium redox flow batteries to reach greenhouse gas emissions targets in an off-grid configuration. Appl. Energy 2015, 146, 397–408. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, H.; Zhou, H.; Chen, J.; Gao, S.; Yi, B. Characteristics and performance of 10 kW class all-vanadium redox-flow battery stack. J. Power Sources 2006, 162, 1416–1420. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Li, B. Research on the characteristics of the vanadium redox-flow battery in power systems applications. J. Power Sources 2013, 241, 396–399. [Google Scholar]
- Arbabzadeh, M.; Johnson, J.X.; Keoleian, G.A.; Rasmussen, P.G.; Thompson, L.T. Twelve Principles for Green Energy Storage in Grid Applications. Environ. Sci. Technol. 2016, 50, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vizcaino, R.; Mena, E.; Millan, M.; Rodrigo, M.A.; Lobato, J. Performance of a vanadium redox flow battery for the storage of electricity produced in photovoltaic solar panels. Renew. Energy 2017, 114, 1123–1133. [Google Scholar] [CrossRef]
- Zhou, Z.; Benbouzid, M.E.H.; Charpentier, J.F.; Scuiller, F. Hybrid Diesel/MCT/Battery Electricity Power Supply System for Power Management in Small Islanded Sites: Case Study for the Ouessant French Island. In Smart Energy Grid Design for Island Countries: Challenges and Opportunities; Rabiul Islam, F.M., Al Mamun, K., Oo Amanullah, M.T., Eds.; Springer: New York, NY, USA, 2017; pp. 415–445. [Google Scholar]
- Lawder, M.T.; Suthar, B.; Northrop, P.W.C.; De, S.; Hoff, C.M.; Leitermann, O.; Crow, M.L.; Santhanagopalan, S.; Subramanian, V.R. Battery Energy Storage System (BESS) and Battery Management System (BMS) for Grid-Scale Applications. Proc. IEEE 2014, 102, 1014–1030. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F.; Stella, A. Large scale energy storage with redox flow batteries. COMPEL 2013, 32, 1459–1470. [Google Scholar] [CrossRef]
- Zhou, Z.; Scuiller, F.; Charpentier, J.F.; Benbouzid, M.; Tang, T. Application of Flow Battery in Marine Current Turbine System for Daily Power Management. In Proceedings of the 2014 International Conference on Freen Energy, Sfax, Tunisia, 25–27 March 2014; pp. 8–13. [Google Scholar]
- Baumann, M.; Peters, J.F.; Weil, M.; Grunwald, A. CO2 Footprint and Life-Cycle Costs of Electrochemical Energy Storage for Stationary Grid Applications. Energy Technol. 2017, 5, 1071–1083. [Google Scholar] [CrossRef]
- Lei, J.; Gong, Q. Operating strategy and optimal allocation of large-scale VRB energy storage system in active distribution networks for solar/wind power applications. IET Gener. Transm. Distrib. 2017, 11, 2403–2411. [Google Scholar] [CrossRef]
- Ontiveros, L.J.; Suvire, G.O.; Mercado, P.E. Power conditioning system coupled with a flow battery for wind energy applications: Modelling and control design. IET Renew. Power Gener. 2017, 11, 987–995. [Google Scholar] [CrossRef]
- Shibata, A.; Sato, K. Development of vanadium redox flow battery for electricity storage. Power Eng. J. 1999, 13, 130–135. [Google Scholar] [CrossRef]
- Vanýsek, P.; Novák, V. Redox flow batteries as the means for energy storage. J. Energy Storage 2017, 13, 435–441. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Battery Technologies for Large-Scale Stationary Energy Storage. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Zhao, W.; Chen, X.; Tao, W.-Q. Economic analysis of a new class of vanadium redox-flow battery for medium- and large-scale energy storage in commercial applications with renewable energy. Appl. Therm. Energy 2017, 114, 802–814. [Google Scholar] [CrossRef]
- Holze, R. Beiträge der Elektrochemie zu einer sich wandelnden Energielandschaft; Sitzungsberichte der Sächsischen Akademie der Wissenschaften zu Leipzig Mathematisch-Naturwissenschaftliche Klasse; S. Hirzel: Stuttgart/Leipzig, Germany, 2018; Volume 133. [Google Scholar]
- Vanysek, P.; Novak, V. Availability of Suitable Raw Materials Determining the Prospect for Energy Storage Systems Based on Redox Flow Batteries. Acta Montan. Slov. 2018, 23, 90–99. [Google Scholar]
- Wu, X.; Yuan, X.; Wang, Z.; Liu, J.; Hu, Y.; Deng, Q.; Yin, X.; Zhou, Q.; Zhou, W.; Wu, Y. Electrochemical performance of 5 kW all-vanadium redox flow battery stack with a flow frame of multi-distribution channels. J. Solid State Electrochem. 2017, 21, 429–435. [Google Scholar] [CrossRef]
- Mena, E.; Lopez-Vizcaino, R.; Millan, M.; Canizares, P.; Lobato, J.; Rodrigo, M.A. Vanadium redox flow batteries for the storage of electricity produced in wind turbines. Int. J. Energy Res. 2018, 42, 720–730. [Google Scholar] [CrossRef]
- Aaron, D.S.; Liu, Q.; Tang, Z.; Grim, G.M.; Papandrew, A.B.; Turhan, A.; Zawodzinski, T.A.; Mench, M.M. Dramatic performance gains in vanadium redox flow batteries through modified cell architecture. J. Power Sources 2012, 206, 450–453. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Habisch, A.; Kjeang, E. In Situ Enhancement of Flow-through Porous Electrodes with Carbon Nanotubes via Flowing Deposition. Electrochim. Acta 2016, 206, 36–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, P.; Skyllas-Kazacos, M.; Xiong, B.; Badrinarayanan, R. A comprehensive equivalent circuit model of all-vanadium redox flow battery for power system analysis. J. Power Sources 2015, 290, 14–24. [Google Scholar] [CrossRef]
- Houser, J.; Clement, J.; Pezeshki, A.; Mench, M.M. Influence of architecture and material properties on vanadium redox flow battery performance. J. Power Sources 2016, 302, 369–377. [Google Scholar] [CrossRef]
- Luo, S.-W.; Lee, S.; Fuh, Y.-K. Micro-pillars enhanced all vanadium redox flow batteries and the effect of assembly torque on the electrochemical performance. Microsyst. Technol. 2017, 23, 2065–2074. [Google Scholar] [CrossRef]
- Gencten, M.; Gursu, H.; Sahin, Y. Effect of alpha- and gamma-alumina on the precipitation of positive electrolyte in vanadium redox battery. Int. J. Hydrogen Energy 2017, 42, 25598–25607. [Google Scholar] [CrossRef]
- Gencten, M.; Gursu, H.; Sahin, Y. Anti-precipitation effects of TiO2 and TiOSO4 on positive electrolyte of vanadium redox battery. Int. J. Hydrogen Energy 2017, 42, 25608–25618. [Google Scholar] [CrossRef]
- Wang, G.; Ciobotaru, M.; Agelidis, V.G. Power Management for Improved Dispatch of Utility-Scale PV Plants. IEEE Trans. Power Syst. 2016, 31, 2297–2306. [Google Scholar] [CrossRef]
- Viswanathan, V.; Crawford, A.; Stephenson, D.; Kim, S.; Wang, W.; Li, B.; Coffey, G.; Thomsen, E.; Graff, G.; Balducci, P.; et al. Cost and performance model for redox flow batteries. J. Power Sources 2014, 247, 1040–1051. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Wie, L. Critical transport issues for improving the performance of aqueous redox flow batteries. J. Power Sources 2017, 339, 1–12. [Google Scholar] [CrossRef]
- Monteiro, R.; Ler¢s, J.; Boaventura, M.; Mendes, A. Insights into all-vanadium redox flow battery: A case study on components and operational conditions. Electrochim. Acta 2018, 267, 80–93. [Google Scholar] [CrossRef]
- Pan, J.; Huang, M.; Li, X.; Wang, S.; Li, W.; Ma, T.; Xie, X.; Ramani, V. The performance of all vanadium redox flow batteries at below-ambient temperatures. Energy 2016, 107, 784–790. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, X.; Cheng, Y.; Ning, G.; Xing, F.; Zhang, H. Development and perspective in vanadium flow battery modeling. Appl. Energy 2014, 132, 254–266. [Google Scholar] [CrossRef]
- Qiu, G.; Joshi, A.S.; Dennison, C.R.; Knehr, K.W.; Kumbur, E.C.; Sun, Y. 3-D pore-scale resolved model for coupled species/charge/fluid transport in a vanadium redox flow battery. Electrochim. Acta 2012, 64, 46–64. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new All-Vanadium Redox Flow Battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Crawford, A.; Viswanathan, V.; Stephenson, D.; Wang, W.; Thomsen, E.; Reed, D.; Li, B.; Balducci, P.; Kintner-Meyer, M.; Sprenkle, V. Comparative analysis for various redox flow batteries chemistries using a cost performance model. J. Power Sources 2015, 293, 388–399. [Google Scholar] [CrossRef]
- Fares, R.L.; Webber, M.E. What are the tradeoffs between battery energy storage cycle life and calendar life in the energy arbitrage application? J. Energy Storage 2018, 16, 37–45. [Google Scholar] [CrossRef]
- Hiksas, M.M.; Aninditio, M.L. Redox Flow Batteries for Small Scale Energy Storage. In Proceedings of the 2016 IEEE Conference on Technologies for Sustainability (SUSTECH), Phoenix, AZ, USA, 9–11 October 2016. [Google Scholar]
- Hosseina, M.; Bathaee, S.M.T. Optimal scheduling for distribution network with redox flow battery storage. Energy Convers. Manag. 2016, 121, 145–151. [Google Scholar] [CrossRef]
- He, G.; Chen, Q.; Kang, C.; Xia, Q. Optimal operating strategy and revenue estimates for the arbitrage of a vanadium redox flow battery considering dynamic efficiencies and capacity loss. IET Gener. Transm. Distrib. 2016, 10, 1278–1285. [Google Scholar] [CrossRef]
- Yamamura, T.; Wu, X.W.; Ohta, S.; Shirasaki, K.; Sakuraba, H.; Satoh, I.; Shikama, T. Vanadium solid-salt battery: Solid state with two redox couples. J. Power Sources 2011, 196, 4003–4011. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Wu, X.; Ye, H.; Zhou, W.; Wu, Y.; Holze, R. Amembrane based on sulfonated polystyrene for a vanadium solid-salt battery. J. Solid State Electrochem. 2016, 20, 943–948. [Google Scholar] [CrossRef]
- Shinkle, A.A.; Sleightholme, A.E.S.; Thompson, L.T.; Monroe, C.W. Electrode kinetics in non-aqueous vanadium acetylacetonate redox flow batteries. J. Appl. Electrochem. 2011, 41, 1191–1199. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Kwon, K.W.; Park, M.-S.; Hwang, J.-H.; Kim, K.J. 3D Graphene-Ni Foam as an Advanced Electrode for High-Performance Nonaqueous Redox Flow Batteries. ACS Appl. Mater. Interfaces 2017, 9, 22502–22508. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.; Bamgbopa, M.O.; Alraeesi, E.; Shao-Horn, Y.; Sun, H.; Almheiri, S. Insights on the Electrochemical Activity of Porous Carbonaceous Electrodes in Non-Aqueous Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2017, 164, A3673–A3683. [Google Scholar] [CrossRef]
- Lee, J.; Park, M.-S.; Kim, K.J. Highly enhanced electrochemical activity of Ni foam electrodes decorated with nitrogen-doped carbon nanotubes for non-aqueous redox flow batteries. J. Power Sources 2017, 341, 212–218. [Google Scholar] [CrossRef]
- Sun, C.-N.; Delnick, F.M.; Aaron, D.S.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. Probing Electrode Losses in All-Vanadium Redox Flow Batteries with Impedance Spectroscopy. ECS Electrochem. Lett. 2013, 2, A43–A45. [Google Scholar] [CrossRef]
- Sun, C.-N.; Delnick, F.M.; Aaron, D.S.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A., Jr. Resolving Losses at the Negative Electrode in All-Vanadium Redox Flow Batteries Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2014, 161, A981–A988. [Google Scholar] [CrossRef]
- Holze, R. Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, New Series, Group IV: Physical Chemistry, Volume 9: Electrochemistry, Subvolume A: Electrochemical Thermodynamics and Kinetics; Martienssen, W., Lechner, M.D., Eds.; Springer: Berlin, Germany, 2007. [Google Scholar]
- The acronym ASA for active surface area seems to apply only on surface area determined by dioxygen chemisorption [117], it should not be confused with EASA.
- Trasatti, S.; Petrii, O.A. Real Surface-Area Measurements in Electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.E. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Watt-Smith, M.J.; Friedrich, J.M.; Rigby, S.P.; Ralph, T.R.; Walsh, F.C. Determination of the electrochemically active surface area of Pt/C PEM fuel cell electrodes using different adsorbates. J. Phys. D Appl. Phys. 2008, 41, 74004. [Google Scholar] [CrossRef]
- Binninger, T.; Fabbri, E.; Koetz, R.; Schmidt, T.J. Determination of the Electrochemically Active Surface Area of Metal-Oxide Supported Platinum Catalyst. J. Electrochem. Soc. 2014, 161, H121–H128. [Google Scholar] [CrossRef]
- Ganassin, A.; Maljusch, A.; Colic, V.; Spanier, L.; Brandl, K.; Schuhmann, W.; Bandarenka, A. Benchmarking the Performance of Thin-Film Oxide Electrocatalysts for Gas Evolution Reactions at High Current Densities. ACS Catal. 2016, 6, 3017–3024. [Google Scholar] [CrossRef]
- Maksimov, Y.M.; Podlovchenko, B.I. Use of silver adatoms for the determination of the electrochemically active surface area of polycrystalline gold. Mendeleev Commun. 2017, 27, 64–66. [Google Scholar] [CrossRef]
- Watzele, S.; Bandarenka, A.S. Quick Determination of Electroactive Surface Area of Some Oxide Electrode Materials. Electroanalysis 2016, 28, 2394–2399. [Google Scholar] [CrossRef]
- Wiberg, G.K.H.; Mayrhofer, K.J.J.; Arenz, M. Investigation of the Oxygen Reduction Activity on Silver—A Rotating Disc Electrode Study. Fuel Cells 2010, 10, 575–581. [Google Scholar] [CrossRef]
- Xie, X.; Holze, R. Electrode kinetic data: Geometric vs. real surface area. 2018; in progress. [Google Scholar]
- Ahn, J.; Holze, R. Bifunctional Electrodes for an Integrated Water-Electrolysis and Hydrogen-Oxygen Fuel Cell with a Solid Polymer Electrolyte. J. Appl. Electrochem. 1992, 22, 1167–1174. [Google Scholar] [CrossRef]
- Friedl, J.; Stimming, U. Determining Electron Transfer Kinetics at Porous Electrodes. Electrochim. Acta 2017, 227, 235–245. [Google Scholar] [CrossRef]
- Friedl, J.; Bauer, C.M.; Rinaldi, A.; Stimming, U. Electron transfer kinetics of the Reaction on multi-walled carbon nanotubes. Carbon 2013, 63, 228–239. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Jagiello, J.; Ania, C.; Parra, J.B.; Cook, C. Dual gas analysis of microporous carbons using 2D-NL heterogeneous surface model and combined adsorption data of N2 and CO2. Carbon 2015, 91, 330–337. [Google Scholar] [CrossRef]
- Jagiello, J.; Ania, C.O.; Parra, J.B.; Jagiello, L.; Pis, J.J. Using DFT analysis of adsorption data of multiple gases including H2 for the comprehensive characterization of microporous carbons. Carbon 2007, 45, 1066–1071. [Google Scholar] [CrossRef]
- Holze, R.; Vielstich, W. Double-Layer Capacity Measurements as a Method to Characterize Porous Fuel Cell Electrodes. Electrochim. Acta 1984, 29, 607–610. [Google Scholar] [CrossRef]
- Kneten Cline, K.; McDermott, M.T.; McCreery, R.L. Anomalously Slow-Electron Transfer at Ordered Graphite-Electrodes—Influence of Electronic Factors and Reactive Sites. J. Phys. Chem. 1994, 98, 5314–5319. [Google Scholar] [CrossRef]
- McCreery, R.L.; Kneten Cline, K.; McDermott, C.A.; McDermott, M.T. Control of reactivity at carbon electrode surfaces. Colloid. Surf. 1994, A93, 211–219. [Google Scholar] [CrossRef]
- Wei, G.; Su, W.; Wei, Z.; Fan, X.; Liu, J.; Yan, C. Electrocatalytic effect of the edge planes sites at graphite electrode on the vanadium redox couples. Electrochim. Acta 2016, 204, 263–269. [Google Scholar] [CrossRef]
- Rabbow, T.J.; Whitehead, A.H. Deconvolution of electrochemical double layer capacitance between fractions of active and total surface area of graphite felts. Carbon 2017, 111, 782–788. [Google Scholar] [CrossRef]
- Pour, N.; Kwabi, D.G.; Carney, T.; Darling, R.M.; Perry, M.L.; Shao-Horn, Y. Influence of Edge- and Basal-Plane Sites on the Vanadium Redox Kinetics for Flow Batteries. J. Phys. Chem. 2015, C119, 5311–5318. [Google Scholar] [CrossRef]
- Jow, J.-J.; Hsieh, L.-Y.; Cho, H.-P.; Chen, H.-R.; Kuo, C.-W. Determination of surface area of carbon-black by simple cyclic-voltammetry measurements in aqueous H2SO4. J. Ind. Eng. Chem. 2013, 19, 1730–1734. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Holze, R. Electrode impedance measurements: A versatile tool for electrochemists. Bull. Electrochem. 1994, 10, 56–67. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy; WILEY-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Fink, H.; Friedl, J.; Stimming, U. Composition of the Electrode Determines Which Half-Cell’s Rate Constant is Higher in a Vanadium Flow Battery. J. Phys. Chem. 2016, C120, 15893–15901. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Sacci, R.L.; Delnick, F.M.; Aaron, D.S.; Mench, M.M. Elucidating effects of cell architecture, electrode material, and solution composition on overpotentials in redox flow batteries. Electrochim. Acta 2017, 229, 261–270. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. Half-Cell Steady-State Flow-Battery Experiments. ECS Trans. 2013, 53, 31–38. [Google Scholar] [CrossRef]
- Aaron, D.S.; Tang, Z.; Lawton, J.S.; Papandrew, A.B.; Zawodzinski, T.A., Jr. In-situ single electrode studies of an all-vanadium redox flow battery. ECS Trans. 2012, 41, 43–51. [Google Scholar]
- Schweiss, R.; Meiser, C.; Goh, F.W.T. Steady-State Measurements of Vanadium Redox-Flow Batteries to Study Particular Influences of Carbon Felt Properties. ChemElectroChem 2017, 4, 1969–1974. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Wu, Y. On the difference between cell and electrode impedances. Electrochem. Energy Technol. 2018. submitted. [Google Scholar]
- Park, J.H.; Park, J.J.; Park, O.O.; Jin, C.-S.; Yang, J.H. Highly accurate apparatus for electrochemical characterization of the felt electrodes used in redox flow batteries. J. Power Sources 2016, 310, 137–144. [Google Scholar] [CrossRef]
- Diener, F.; Roscher, J.; Holze, R. A miniature flow cell for electrochemical studies of graphite electrodes. 2018; in progress. [Google Scholar]
- Wang, Q.; Qu, Z.G.; Jiang, Z.Y.; Yang, W.W. Experimental study on the performance of a vanadium redox flow battery with non-uniformly compressed carbon felt electrode. Appl. Energy 2018, 213, 293–305. [Google Scholar] [CrossRef]
- Park, S.-K.; Shim, J.; Yang, J.H.; Jin, C.-S.; Lee, B.S.; Lee, Y.-S.; Shin, K.-H.; Jeon, J.-D. The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery. Electrochim. Acta 2014, 116, 447–452. [Google Scholar] [CrossRef]
- Becker, M.; Bredemeyer, N.; Tenhumberg, N.; Turek, T. Polarization curve measurements combined with potential probe sensing for determining current density distribution in vanadium redox-flow batteries. J. Power Sources 2016, 307, 826–833. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Eikerling, M.; Kjeang, E. Direct measurement of electrochemical reaction kinetics in flow-through porous electrodes. Electrochem. Commun. 2015, 57, 14–17. [Google Scholar] [CrossRef]
- Li, L.; Nikiforidis, G.; Leung, M.K.H.; Daoud, W.A. Vanadium microfluidic fuel cell with novel multi-layer flow-through porous electrodes: Model, simulations and experiments. Appl. Energy 2016, 177, 729–739. [Google Scholar] [CrossRef]
- Lee, J.W.; Hong, J.K.; Kjeang, E. Electrochemical characteristics of vanadium redox reactions on porous carbon electrodes for microfluidic fuel cell applications. Electrochim. Acta 2012, 83, 430–438. [Google Scholar] [CrossRef]
- Sato, Y.; Narita, A.; Kaneko, Y.; Negishi, A.; Nozaki, K.; Kato, T. Characterization of Carbon Materials for Redox Flow Battery Electrodes by Voltage-Step Coulometry. ECS Trans. 2017, 75, 37–47. [Google Scholar] [CrossRef]
- Jing, M.; Qin, Y.; Shi, Q.; Liu, J.; Yan, C. A new insight into electrode processes of vanadium redox flow battery by thermo-electro-chemistry method. J. Energy Storage 2017, 14, 163–167. [Google Scholar] [CrossRef]
- Ashraf Gandomi, Y.; Aaron, D.S.; Houser, J.R.; Daugherty, M.C.; Clement, J.T.; Pezeshki, A.M.; Ertugrul, T.Y.; Moseley, D.P.; Mench, M.M. Critical Review—Experimental Diagnostics and Material Characterization Techniques Used on Redox Flow Batteries. J. Electrochem. Soc. 2018, 165, A970–A1010. [Google Scholar] [CrossRef]
- Tang, Z. Characterization Techniques and Electrolyte Separator Performance Investigation for All Vanadium Redox Flow Battery. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2013. [Google Scholar]
- Wu, L.; Wang, J.; Shen, Y.; Liu, L.; Xi, J. Electrochemical evaluation methods of vanadium flow battery electrodes. Phys. Chem. Chem. Phys. 2017, 19, 14708–14717. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Bredemeyer, N.; Tenhumberg, N.; Turek, T. Kinetic studies at carbon felt electrodes for vanadium redox-flow batteries under controlled transfer current density conditions. Electrochim. Acta 2017, 252, 12–24. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Liang, J.; Tao, Z.L.; Chen, J. Functional Materials for Rechargeable Batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.H.; Brandon, N.P.; Hajimolana, S.A.; Tariq, E.; Yufit, V.; Hashim, M.A.; Hussain, M.A.; Low, C.T.J.; Aravind, P.V. Application of carbon materials in redox flow batteries. J. Power Sources 2014, 253, 150–166. [Google Scholar] [CrossRef]
- Satola, B.; Komsiyska, L.; Wittstock, G. Corrosion of Graphite-Polypropylene Current Collectors during Overcharging in Negative and Positive Vanadium Redox Flow Battery Half-Cell Electrolytes. J. Electrochem. Soc. 2018, 165, A963–A969. [Google Scholar] [CrossRef]
- Satola, B.; Komsiyska, L.; Wittstock, G. Bulk Aging of Graphite-Polypropylene Current Collectors Induced by Electrochemical Cycling in the Positive Electrolyte of Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2017, 164, A2566–A2572. [Google Scholar] [CrossRef]
- Satola, B.; Nunes Kirchner, C.; Komsiyska, L.; Wittstock, G. Chemical Stability of Graphite-Polypropylene Bipolar Plates for the Vanadium Redox Flow Battery at Resting State. J. Electrochem. Soc. 2016, 163, A2318–A2325. [Google Scholar] [CrossRef]
- Mohammadi, F.; Timbrell, P.; Zhong, S.; Padeste, C.; Skyllas-Kazacos, M. Overcharge in the Vanadium Redox Battery and Changes in Electrical Resistivity and Surface Functionality of Graphite Felt Electrodes. J. Power Sources 1994, 52, 61–68. [Google Scholar] [CrossRef]
- Haddadi-Asl, V.; Rabbani, M.S. Effect of electrochemical cell overcharge on electrical and electrochemical properties of polymer composite electrodes (II): Mechanism of electrode deterioration. Iran. Polym. J. 1998, 7, 185–194. [Google Scholar]
- Lee, N.J.; Lee, S.-W.; Kim, K.J.; Kim, J.-H.; Park, M.-S.; Jeong, G.; Kim, Y.-J.; Byun, D. Development of Carbon Composite Bipolar Plates for Vanadium Redox Flow Batteries. Bull. Korean Chem. Soc. 2012, 33, 3589–3592. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, B.G.; Lee, D.G. Development of carbon composite bipolar plate (BP) for vanadium redox flow battery (VRFB). Compos. Struct. 2014, 109, 253–259. [Google Scholar] [CrossRef]
- Nam, S.; Lee, D.; Lee, D.G.; Kim, J. Nano carbon/fluoroelastomer composite bipolar plate for a vanadium redox flow battery (VRFB). Compos. Struct. 2017, 159, 220–227. [Google Scholar] [CrossRef]
- Li, W.; Jing, S.; Wang, S.; Wang, C.; Xie, X. Experimental investigation of expanded graphite/phenolic resin composite bipolar plate. Int. J. Hydrogen Energy 2016, 41, 16240–16246. [Google Scholar] [CrossRef]
- Choe, J.; Kim, K.H.; Lee, D.G. Corrugated carbon/epoxy composite bipolar plate for vanadium redox flow batteries. Compos. Struct. 2015, 119, 534–542. [Google Scholar] [CrossRef]
- Park, M.; Jung, Y.-J.; Ryu, J.; Cho, J. Material selection and optimization for highly stable composite bipolar plates in vanadium redox flow batteries. J. Mater. Chem. 2014, 2, 15808–15815. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Yan, C. On-line mass spectrometry study of electrochemical corrosion of the graphite electrode for vanadium redox flow battery. Electrochem. Commun. 2013, 28, 58–62. [Google Scholar] [CrossRef]
- Hagg, C.M.; Skyllas-Kazacos, M. Novel bipolar electrodes for battery applications. J. Appl. Electrochem. 2002, 32, 1063–1069. [Google Scholar] [CrossRef]
- Caglar, B.; Fischer, P.; Kauranen, P.; Karttunen, M.; Eisner, P. Development of carbon nanotube and graphite filled polyphenylene sulfide based bipolar plates for all-vanadium redox flow batteries. J. Power Sources 2014, 256, 88–95. [Google Scholar] [CrossRef]
- Derr, I.; Przyrembel, D.; Schweer, J.; Fetyan, A.; Langner, J.; Melke, J.; Weinelt, M.; Roth, C. Electroless chemical aging of carbon felt electrodes for the all-vanadium redox flow battery (VRFB) investigated by Electrochemical Impedance and X-ray Photoelectron Spectroscopy. Electrochim. Acta 2017, 246, 783–793. [Google Scholar] [CrossRef]
- Derr, I.; Bruns, M.; Langner, J.; Fetyan, A.; Melke, J.; Roth, C. Degradation of all-vanadium redox flow batteries (VRFB) investigated by electrochemical impedance and X-ray photoelectron spectroscopy: Part 2 electrochemical degradation. J. Power Sources 2016, 325, 351–359. [Google Scholar] [CrossRef]
- Derr, I.; Fetyan, A.; Schutjajew, K.; Roth, C. Electrochemical analysis of the performance loss in all vanadium flow batteries using different cut-off voltages. Electrochim. Acta 2017, 224, 9–16. [Google Scholar] [CrossRef]
- Nibel, O.; Taylor, S.M.; Patru, A.; Fabbri, E.; Gubler, L.; Schmidt, T.J. Performance of Different Carbon Electrode Materials: Insights into Stability and Degradation under Real Vanadium Redox Flow Battery Operating Conditions. J. Electrochem. Soc. 2017, 164, A1608–A1615. [Google Scholar] [CrossRef]
- Mayer, P.; Holze, R. Electrocatalysis of redox reactions by metal nanoparticles on graphite electrodes. J. Solid State Electrochem. 2001, 5, 402–411. [Google Scholar] [CrossRef]
- Climent, M.A.; Garces, P.; Aldaz, A. Cyclic voltammetric study of Fe3+/Fe2+ electrodic reaction for use in a Fe/Cr battery. Bull. Electrochem. 1988, 4, 845–848. [Google Scholar]
- Kaneko, H.; Nozaki, K.; Wada, Y.; Aoki, T.; Negishi, A.; Kamimoto, M. Vanadium Redox Reactions and Carbon Electrodes for Vanadium Redox Flow Battery. Electrochim. Acta 1991, 36, 1191–1196. [Google Scholar] [CrossRef]
- Kim, H.S. Electrochemical Properties of Graphite-based Electrodes for Redox Flow Batteries. Bull. Korean Chem. Soc. 2011, 32, 571–575. [Google Scholar] [CrossRef]
- Cnobloch, H.; Nischik, H.; Pantel, K.; Ledjeff, K.; Heinzel, A. Application of carbon as a construction material in redox-flow-storage-batteries. In Proceedings of the 4th International Carbon Conference (Carbon’86), Baden-Baden, Germany, 30 June–4 July 1986; pp. 367–369. [Google Scholar]
- Holze, R. Underpotential deposit electrocatalysis of fast redox reactions for electrochemical energy storage systems. J. Solid State Electrochem. 1998, 2, 73–77. [Google Scholar] [CrossRef]
- Yang, C.Y. Catalytic electrodes for the Redox Flow Cell energy storage device. J. Appl. Electrochem. 1982, 12, 425–434. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; Zeng, Y.K.; An, L.; Wie, L. A highly permeable and enhanced surface area carbon-cloth electrode for vanadium redox flow batteries. J. Power Sources 2016, 329, 247–254. [Google Scholar] [CrossRef]
- Aaron, D.; Sun, C.-N.; Bright, M.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. In Situ Kinetics Studies in All-Vanadium Redox Flow Batteries. ECS Electrochem. Lett. 2013, 2, A29–A31. [Google Scholar] [CrossRef]
- Maruyama, J.; Shinagawa, T.; Hayashida, A.; Matsuo, Y.; Nishihara, H.; Kyotani, T. Vanadium-Ion Redox Reactions in a Three-Dimensional Network of Reduced Graphite Oxide. ChemElectroChem 2016, 3, 650–657. [Google Scholar] [CrossRef]
- Park, S.; Kim, H. Fabrication of nitrogen-doped graphite felts as positive electrodes using polypyrrole as a coating agent in vanadium redox flow batteries. J. Mater. Chem. 2015, 3, 12276–12283. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.-S.; Kim, J.-H.; Hwang, U.; Lee, N.J.; Jeong, G.; Kim, Y.-J. Novel catalytic effects of Mn3O4 for all vanadium redox flow batteries. Chem. Commun. 2012, 48, 5455–5457. [Google Scholar] [CrossRef] [PubMed]
- Moghim, M.H.; Eqra, R.; Babaiee, M.; Zarei-Jelyani, M.; Mohsen Loghavi, M. Role of reduced graphene oxide as nano-electrocatalyst in carbon felt electrode of vanadium redox flow battery. J. Electroanal. Chem. 2017, 789, 67–75. [Google Scholar] [CrossRef]
- Sun, B.; Sykllas-Kazacos, M. Modification of Graphite Electrode Materials for Vanadium Redox Flow Battery Application—1. Thermal-Treatment. Electrochim. Acta 1992, 37, 1253–1260. [Google Scholar] [CrossRef]
- Flox, C.; Rubio-Garcia, J.; Skoumal, M.; Andreu, T.; Ramon Morante, J. Thermo-chemical treatments based on NH3/O2 for improved graphite-based fiber electrodes in vanadium redox flow batteries. Carbon 2013, 60, 280–288. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, J.; Li, Z.; Zhou, H.; Liu, L.; Wu, Z.; Qiu, X. Electrochemical activation of graphite felt electrode for redox couple application. Electrochim. Acta 2013, 89, 429–435. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Flox, C.; Blanco, C.; Granda, M.; Morante, J.R.; Menendez, R.; Santamaria, R. Outstanding electrochemical performance of a graphene-modified graphite felt for vanadium redox flow battery application. J. Power Sources 2017, 338, 155–162. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Sanchez, A.; Blanco, C.; Granda, M.; Menendez, R.; Santamaria, R. Enhanced performance of a Bi-modified graphite felt as the positive electrode of a vanadium redox flow battery. Electrochem. Commun. 2011, 13, 1379–1382. [Google Scholar] [CrossRef]
- Zhong, S.; Padeste, C.; Kazacos, M.; Skyllas-Kazacos, M. Comparison of the Physical, Chemical and Electrochemical Properties of Rayon-Based and Polyacrylonitrile-Based Graphite Felt Electrodes. J. Power Sources 1993, 45, 29–41. [Google Scholar] [CrossRef]
- Oriji, G.; Katayama, Y.; Miura, T. Investigation on V(IV)/V(V) species in a vanadium redox flow battery. Electrochim. Acta 2004, 49, 3091–3095. [Google Scholar] [CrossRef]
- Wen, Y.H.; Zhang, H.M.; Qian, P.; Zhao, P.; Zhou, H.T.; Yi, B.L. Investigations on the electrode process of concentrated V(IV)/V(V) species in a vanadium redox flow battery. Act. Phys.-Chim. Sin. 2006, 22, 403–408. [Google Scholar] [CrossRef]
- Wang, Q.; Daoud, W.A. Temperature influence on the reaction kinetics of V(IV)/V(V) in methanesulfonic acid for all-vanadium redox flow battery. Electrochim. Acta 2016, 214, 11–18. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar]
- Noack, J.; Tübke, J. A Comparison of Materials and Treatment of Materials for Vanadium Redox Flow Battery. ECS Trans. 2010, 25, 235–245. [Google Scholar]
- Gang, W.; Jinwei, C.; Shifu, Z.; Jie, Z.; Xiaojiang, L.; Ruilin, W. Activation of Carbon Electrodes for All-Vanadium Redox Flow Battery. Prog. Chem. 2015, 27, 1343–1355. [Google Scholar]
- Goulet, M.-A.; Skyllas-Kazacos, M.; Kjeang, E. The importance of wetting in carbon paper electrodes for vanadium redox reactions. Carbon 2016, 101, 390–398. [Google Scholar] [CrossRef]
- Certainly any combination of two materials can be called a composite; a generally accepted definition of this term is absent. According to common usage a composite can be a combination of two distinctly different materials yielding a new one—A composite—With properties distinctly different from those of the constituents. Accordingly the combination of CNF and MnO2 may be called a composite whereas graphene platelets deposited on carbon fiber hardly qualify. The latter can be called modified carbon fiber—And this classification is observed in the present text.
- Rychcik, M.; Skyllas-Kazacos, M. Evaluation of electrode materials for vanadium redox cell. J. Power Sources 1987, 19, 45–54. [Google Scholar] [CrossRef]
- Jiang, Z.; Klyukin, K.; Alexandrov, V. First-principles study of adsorption-desorption kinetics of aqueous V2+/V3+ redox species on graphite in a vanadium redox flow battery. Phys. Chem. Chem. Phys. 2017, 19, 14897–14901. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zeng, X.-X.; Zhou, C.; Deng, Q.; Wang, P.-F.; Zuo, T.-T.; Zhang, X.-D.; Yin, Y.-X.; Wu, X.; Chai, L.-Y.; et al. Designing High-Performance Composite Electrodes for Vanadium Redox Flow Batteries: Experimental and Computational Investigation. ACS Appl. Mater. Interface 2018, 10, 22381–22388. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Shyy, W.; Zeng, L.; Zhang, R.H.; Zhao, T.S. Highly efficient and ultra-stable boron-doped graphite felt electrodes for vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 13244–13253. [Google Scholar] [CrossRef]
- Holze, R. Kinetics of Fast Redox Systems for Energy Storage. In Springer Handbook of Electrochemical Energy; Breitkopf, C., Swider-Lyons, K., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 591–610. [Google Scholar]
- Schweiss, R.; Pritzl, A.; Meiser, C. Parasitic Hydrogen Evolution at Different Carbon Fiber Electrodes in Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2016, 163, A2089–A2094. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Chen, H.; Yan, C. Study on hydrogen evolution reaction at a graphite electrode in the all-vanadium redox flow battery. Int. J. Electrochem. Sci. 2012, 7, 3750–3764. [Google Scholar]
- Gahn, R.F.; Hagedorn, N.H.; Johnson, J.A. Cycling performance of the iron-chromium redox energy storage system. In Proceedings of the 20th Intersociety Energy Conversion Engineering Conference 1985, Miami Beach, FL, USA, 18–23 August 1985; Volume 2, pp. 91–97. [Google Scholar]
- Skyllas-Kazakos, M. All-Vanadium Redox Battery and Additives. WO Patent Affliation 89/05526, 15 June 1989. [Google Scholar]
- Cnobloch, H.; Nischik, H.; Pantel, K.; Ledjeff, K.; Heinzel, A.; Reiner, A. Eisen-Chrom-Redoxionen-Speicher. In DECHEMA-Monographie; VCH Verlagsgesellschaft: Weinheim, Germany, 1987; Volume 109, pp. 427–445. [Google Scholar]
- Liu, C.C.; Galasco, R.T.; Savinell, F. Operating Performance of an Fe-Ti Stationary Redox Battery in the Presence of Lead. J. Electrochem. Soc. 1982, 129, 2502–2505. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Xiang, Z.-P.; Deng, H.-Q.; Wan, K.; Liu, Q.-B.; Piao, J.-H.; Zheng, Y.-Y.; Liang, Z.-X. Electrochemical Behavior of Vanadium Redox Couples on Carbon Electrode. J. Electrochem. Soc. 2016, 163, H937–H942. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Oriji, G.; Katayama, Y.; Miura, T. Investigations on V(IV)/V(V) and V(II)/V(III) redox reactions by various electrochemical methods. J. Power Sources 2005, 139, 321–324. [Google Scholar] [CrossRef]
- Liu, W.; Luo, D.; Zeng, F.; Meng, X.; Li, D. Investigations on the V(III) Reduction Process of All-Vanadium Redox Flow Battery. Int. J. Electrochem. Sci. 2016, 11, 3492–3501. [Google Scholar] [CrossRef]
- Roznyatovskaya, N.; Noack, J.; Fühl, M.; Pinkwart, K.; Tübke, J. Towards an all-vanadium redox-flow battery electrolyte: Electrooxidation of V(III) in V(IV)/V(III) redox couple. Electrochim. Acta 2016, 211, 926–932. [Google Scholar] [CrossRef]
- Gattrell, M.; Qian, J.; Stewar, C.; Graham, P.; MacDougall, B. The electrochemical reduction of VO2. Electrochim. Acta 2005, 51, 395–407. [Google Scholar] [CrossRef]
- Taylor, S.M.; Patru, A.; Streich, D.; El Kazzi, M.; Fabbri, E.; Schmidt, T.J. Vanadium (V) reduction reaction on modified glassy carbon electrodes—Role of oxygen functionalities and microstructure. Carbon 2016, 109, 472–478. [Google Scholar] [CrossRef]
- Melke, J.; Jakes, P.; Langner, J.; Riekehr, L.; Kunz, U.; Zhao-Karger, Z.; Nefedov, A.; Sezen, H.; Woell, C.; Ehrenberg, H.; et al. Carbon materials for the positive electrode in all-vanadium redox flow batteries. Carbon 2014, 78, 220–230. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Y.; Li, J.; Xue, B. Studies on properties of rayon- and polyacrylonitrile-based graphite felt electrodes affecting Fe/Cr redox flow battery performance. Electrochim. Acta 2017, 248, 603–613. [Google Scholar] [CrossRef]
- Langner, J.; Bruns, M.; Dixon, D.; Nefedov, A.; Woell, Ch.; Scheiba, F.; Ehrenberg, H.; Roth, C.; Melke, J. Surface properties and graphitization of polyacrylonitrile based fiber electrodes affecting the negative half-cell reaction in vanadium redox flow batteries. J. Power Sources 2016, 321, 210–218. [Google Scholar] [CrossRef]
- Ling, W.; Wang, Z.; Deng, Q.; Wang, H.; Zeng, X.; Hu, Y.; He, S.; Wu, X.; Chen, G.; Wu, Y.; et al. A three-dimensional conducting network from rGO-in-graphite-felt as electrode for vanadium redox flow batteries. Electrochem. Energy Technol. 2018. submitted. [Google Scholar]
- Sabou, D.-M.; Dorneanu, S.-A.; Ilea, P. Spectral graphite as electrode material for the all-vanadium redox flow battery. Stud. Univ. Babes-Bolyai Chem. 2015, 60, 193–203. [Google Scholar]
- Yang, H.-Y.; Hsueh, K.-L.; Hsieh, C.-L.; Hung, J.-S. Study of the Kinetics of Vanadium (II)/(III) Redox Reaction. ECS Trans. 2013, 50, 87–92. [Google Scholar] [CrossRef]
- Wang, W.; Fan, X.; Liu, J.; Yan, C.; Zeng, C. Unusual phenomena in the reduction process of vanadium(V) on a graphite electrode at high overpotentials. Phys. Chem. Chem. Phys. 2014, 16, 19848–19851. [Google Scholar] [CrossRef] [PubMed]
- Gattrell, M.; Park, J.; MacDougall, B.; Apte, J.; McCarthy, S.; Wu, C.W. Study of the mechanism of the vanadium 4+/5+ redox reaction in acidic solutions. J. Electrochem. Soc. 2004, 151, A123–A130. [Google Scholar] [CrossRef]
- Wang, W.; Fan, X.; Liu, J.; Yan, C.; Zeng, C. A novel mechanism for the oxidation reaction of VO2+ on a graphite electrode in acidic solutions. J. Power Sources 2014, 261, 212–220. [Google Scholar] [CrossRef]
- Yamamura, T.; Watanabe, N.; Yano, T.; Shiokawa, Y. Electron-transfer kinetics of Np3+/Np4+, , V2+/V3+, and at carbon electrodes. J. Electrochem. Soc. 2005, 152, A830–A836. [Google Scholar] [CrossRef]
- Narita, A.; Kaneko, Y.; Sato, Y.; Negishi, A.; Nozaki, K.; Kato, T. Characterization of Carbon Fiber Electrode for Vanadium-Based Redox Flow Batteries. ECS Trans. 2015, 68, 89–95. [Google Scholar] [CrossRef]
- Maruyama, J.; Hasegawa, T.; Iwasaki, S.; Fukuhara, T.; Nogami, M. Mechanism of Dioxovanadium Ion Reduction on Oxygen-Enriched Carbon Surface. J. Electrochem. Soc. 2013, 160, A1293–A1298. [Google Scholar] [CrossRef]
- Wu, X.W.; Yamamura, T.; Ohta, S.; Zhang, Q.X.; Lv, F.C.; Liu, C.M.; Shirasaki, K.; Satoh, I.; Shikama, T.; Lu, D.; et al. Acceleration of the redox kinetics of and V3+/V2+ couples on carbon paper. J. Appl. Electrochem. 2011, 41, 1183–1190. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zeng, Y.K.; Zhu, X.B.; Wei, L.; Zhao, T.S. A high-performance dual-scale porous electrode for vanadium redox flow batteries. J. Power Sources 2016, 325, 329–336. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, J.; Zhou, H.; Qiu, X. KOH etched graphite felt with improved wettability and activity for vanadium flow batteries. Electrochim. Acta 2016, 218, 15–23. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, Z.; Guo, B.; Qiu, Y.; Fan, H.; Sun, S.; Wu, T.; Jin, L.; Fan, L. Preparation of activated carbon paper by modified Hummer’s method and application as vanadium redox battery. Ionics 2015, 21, 283–287. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Shen, Y.; Xu, P.; Lu, L.; Fu, J.; Zhao, H. Treatment of graphite felt by modified Hummers method for the positive electrode of vanadium redox flow battery. Electrochim. Acta 2014, 138, 264–269. [Google Scholar] [CrossRef]
- Gencten, M.; Gursu, H.; Sahin, Y. Electrochemical investigation of the effects of V(V) and sulfuric acid concentrations on positive electrolyte for vanadium redox flow battery. Int. J. Hydrogen Energy 2016, 41, 9868–9875. [Google Scholar] [CrossRef]
- Yang, H.; Fan, C.; Zhu, Q. Activated Charcoal Modified Graphite Felts Using for Positive Electrodes of Vanadium Redox Flow Battery. J. Electrochem. Energy Convers. Storage 2017, 14, 041004. [Google Scholar] [CrossRef]
- Lee, M.E.; Lee, S.; Jin, H.-J.; Yun, Y.S. Standalone macroporous graphitic nanowebs for vanadium redox flow batteries. J. Ind. Eng. Chem. 2018, 60, 85–90. [Google Scholar] [CrossRef]
- Schmidt, C.N.; Cao, G. Properties of mesoporous carbon modified carbon felt for anode of all-vanadium redox flow battery. Sci. China Mater. 2016, 59, 1037–1050. [Google Scholar] [CrossRef]
- Jeong, S.; An, S.; Jeong, J.; Lee, J.; Kwon, Y. Effect of mesocelluar carbon foam electrode material on performance of vanadium redox flow battery. J. Power Sources 2015, 278, 245–254. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Kwon, Y. Performance enhancement in vanadium redox flow battery using platinum-based electrocatalyst synthesized by polyol process. Electrochim. Acta 2013, 114, 439–447. [Google Scholar] [CrossRef]
- Peng, L.; Fang, Z.; Zhu, Y.; Yan, C.; Yu, G. Holey 2D Nanomaterials for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702179. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y.; Yu, L.; Liu, L.; Liang, F.; Qiu, X.; Xi, J. Holey-engineered electrodes for advanced vanadium flow batteries. Nano Energy 2018, 43, 55–62. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-Based Nanocomposites for Energy Storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Duan, W.; Wang, W.; Lin, Y.; Wang, Y.; Li, J. Nanostructured Electrocatalysts for PEM Fuel Cells and Redox Flow Batteries: A Selected Review. ACS Catal. 2015, 5, 7288–7298. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Cho, J. Nanostructured Electrocatalysts for All-Vanadium Redox Flow Batteries. Chem. Asian J. 2015, 10, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, X.; Li, C.; Zhu, Y.; Fu, L.; Wu, Y.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Shen, Y.; Wu, L.; Yu, L.; Liang, F.; Xi, J. Carbon dots promoted vanadium flow batteries for all-climate energy storage. Chem. Commun. 2017, 53, 7565–7568. [Google Scholar] [CrossRef] [PubMed]
- Robarts, L.; Santhanam, K.S.V. Interfacial Electron Transfer Involving Vanadium and Graphene Quantum Dots for Redox Flow Battery. MRS Adv. 2018, 3, 1221–1228. [Google Scholar] [CrossRef]
- Sankar, A.; Michos, I.; Dutta, I.; Dong, J.; Angelopoulos, A.P. Enhanced vanadium redox flow battery performance using graphene nanoplatelets to decorate carbon electrodes. J. Power Sources 2018, 387, 91–100. [Google Scholar] [CrossRef]
- Park, M.; Jeon, I.-Y.; Ryu, J.; Jang, H.; Back, J.-B.; Cho, J. Edge-halogenated graphene nanoplatelets with F, Cl, or Br as electrocatalysts for all-vanadium redox flow batteries. Nano Energy 2016, 26, 233–240. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, C.-S.; Zhang, W. Vertically Aligned Graphene Nanosheet Arrays: Synthesis, Properties and Applications in Electrochemical Energy Conversion and Storage. Adv. Energy Mater. 2017, 7, 1700678. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Botas, C.; Alvarez, P.; Roldan, S.; Blanco, C.; Santamaria, R.; Granda, M.; Menendez, R. Thermally reduced graphite oxide as positive electrode in Vanadium Redox Flow Batteries. Carbon 2012, 50, 828–834. [Google Scholar] [CrossRef]
- Shi, L.; Liu, S.; He, Z.J. Shen Nitrogen-Doped Graphene: Effects of nitrogen species on the properties of the vanadium redox flow battery. Electrochim. Acta 2014, 138, 93–100. [Google Scholar] [CrossRef]
- Han, P.; Wang, H.; Liu, Z.; Chen, X.; Ma, W.; Yao, J.; Zhu, Y.; Cui, G. Graphene oxide nanoplatelets as excellent electrochemical active materials for and V2+/V3+ redox couples for a vanadium redox flow battery. Carbon 2011, 49, 693–700. [Google Scholar] [CrossRef]
- Sun, B.T.; Skyllas-Kazacos, M. Chemical modification of graphite electrode materials for vanadium redox flow battery application—Part II. Acid treatments. Electrochim. Acta 1992, 37, 2459–2465. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Nir, D.; Yufit, V.; Tariq, F.; Rubio-Garcia, J.; Maher, R.; Kucernak, A.; Aravind, P.V.; Brandon, N. Performance Enhancement of Reduced Graphene Oxide-Modified Carbon Electrodes for Vanadium Redox-Flow Systems. ChemElectroChem 2017, 4, 194–200. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Reduced graphene oxide with tunable C/O ratio and its activity towards vanadium redox pairs for an all vanadium redox flow battery. Carbon 2013, 55, 313–320. [Google Scholar] [CrossRef]
- Deng, Q.; Huang, P.; Zhou, W.-X.; Ma, Q.; Zhou, N.; Xie, H.; Ling, W.; Zhou, C.-J.; Yin, Y.-X.; Wu, X.-W.; et al. A High-Performance Composite Electrode for Vanadium Redox Flow Batteries. Adv. Energy Mater. 2017, 7, 1700461. [Google Scholar] [CrossRef]
- Reversibility is used as a term with several meanings frequently confusing. Reversibility in its thermodynamic sense meaning under reversible conditions certainly does not apply here, in its mechanistic sense meaning a reaction going forward and backward in the same direction with opposite orientations may be correct here, but is presumably not intended (The authors statement regarding reaction mechanism are not supported in the article), and the electrochemical meaning of a fast reaction—Which obviously applies here.
- Di Blasi, O.; Briguglio, N.; Busacca, C.; Ferraro, M.; Antonucci, V.; Di Blasi, A. Electrochemical investigation of thermically treated graphene oxides as electrode materials for vanadium redox flow battery. Appl. Energy 2015, 147, 74–81. [Google Scholar] [CrossRef]
- Etesami, M.; Abouzari-Lotf, E.; Ripin, A.; Nasef, M.M.; Ting, T.M.; Saharkhiz, A.; Ahmad, A. Phosphonated graphene oxide with high electrocatalytic performance for vanadium redox flow battery. Int. J. Hydrogen Energy 2018, 43, 189–197. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Botas, C.; Blanco, C.; Santamaria, R.; Granda, M.; Alvarez, P.; Menendez, R. Thermally reduced graphite and graphene oxides in VRFBs. Nano Energy 2013, 2, 1322–1328. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Botas, C.; Blanco, C.; Santamaria, R.; Granda, M.; Alvarez, P.; Menendez, R. Graphite oxide-based graphene materials as positive electrodes in vanadium redox flow batteries. J. Power Sources 2013, 241, 349–354. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Choi, K.; Kim, J.-Y.; Kim, I.; Jeong, D.S.; Choi, H.-J.; Mizuseki, H.; Lee, W.-S. Onion-like carbon as dopant/modification-free electrocatalyst for redox reaction: Performance-control mechanism. Carbon 2018, 127, 31–40. [Google Scholar] [CrossRef]
- Dai, L.; Jiang, Y.; Meng, W.; Zhou, H.; Wang, L.; He, Z. Improving the electrocatalytic performance of carbon nanotubes for redox reaction by KOH activation. Appl. Surf. Sci. 2017, 401, 106–113. [Google Scholar] [CrossRef]
- He, Z.; Liu, L.; Gao, C.; Zhou, Z.; Liang, X.; Lei, Y.; He, Z.; Liu, S. Carbon nanofibers grown on the surface of graphite felt by chemical vapour deposition for vanadium redox flow batteries. RSC Adv. 2013, 3, 19774–19777. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, J.; Lv, Y.; Cheng, Y.; Jiang, S.P.; Xiang, Y.; Lu, S. The electrocatalytic characterization and mechanism of carbon nanotubes with different numbers of walls for the redox couple. Phys. Chem. Chem. Phys. 2018, 20, 7791–7797. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Cochell, T.; Manthiram, A. Nitrogen-Doped Carbon Nanotube/Graphite Felts as Advanced Electrode Materials for Vanadium Redox Flow Batteries. J. Phys. Chem. Lett. 2012, 3, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-S.; Lee, J.Y.; Jo, S.-W.; Yoon, S.J.; Kim, T.-H.; Hong, Y.T. Electrocatalytic activity of nitrogen-doped CNT graphite felt hybrid for all-vanadium redox flow batteries. Int. J. Hydrogen Energy 2018, 43, 1516–1522. [Google Scholar] [CrossRef]
- Steimecke, M.; Ruemmler, S.; Schuhmacher, N.-F.; Lindenberg, T.; Hartmann, M.; Bron, M. A Comparative Study of Functionalized High-Purity Carbon Nanotubes towards the V(IV)/V(V) Redox Reaction Using Cyclic Voltammetry and Scanning Electrochemical Microscopy. Electroanalysis 2017, 29, 1056–1061. [Google Scholar] [CrossRef]
- Steimecke, M.; Ruemmler, S.; Kuehhirt, M.; Bron, M. A Linear Sweep Voltammetric Procedure Applied to Scanning Electrochemical Microscopy for the Characterization of Carbon Materials towards the Vanadium(IV)/(V) Redox System. ChemElectroChem 2016, 3, 318–322. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Lin, X.; Yang, L.; Yu, A. Study on Nitrogen-Doped Carbon Nanotubes for Vanadium Redox Flow Battery Application. Int. J. Electrochem. Sci. 2016, 11, 665–674. [Google Scholar]
- Chang, Y.-C.; Shih, Y.-C.; Chen, J.-Y.; Lin, G.-Y.; Hsu, N.-Y.; Chou, Y.-S.; Wang, C.-H. High efficiency of bamboo-like carbon nanotubes on functionalized graphite felt as electrode in vanadium redox flow battery. RSC Adv. 2016, 6, 102068–102075. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Shih, Y.-C.; Chen, J.-Y.; Lin, G.-Y.; Hsu, N.-Y.; Chou, Y.-S.; Wang, C.-H. High efficiency of bamboo-like carbon nanotubes on functionalized graphite felt as electrode in vanadium redox flow battery. RSC Adv. 2016, 6, 107294. [Google Scholar] [CrossRef]
- Mazurenko, I.; Etienne, M.; Francius, G.; Vakulko, I.; Walcarius, A. Macroporous carbon nanotube-carbon composite electrodes. Carbon 2016, 109, 106–116. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Engelhard, M.H.; Du, D.; Lin, Y. Three-dimensional Nitrogen-Doped Reduced Graphene Oxide/Carbon Nanotube Composite Catalysts for Vanadium Flow Batteries. Electroanalysis 2017, 29, 1469–1473. [Google Scholar] [CrossRef]
- Yang, H.; Fan, C.; Zhu, Q. Sucrose pyrolysis assembling carbon nanotubes on graphite felt using for vanadium redox flow battery positive electrode. J. Energy Chem. 2018, 27, 451–454. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Multi-walled carbon nanotubes used as an electrode reaction catalyst for catalyst for for a vanadium redox flow battery. Carbon 2011, 49, 3463–3470. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. The electrochemical catalytic activity of single-walled carbon nanotubes towards and V3+/V2+ redox pairs for an all vanadium redox flow battery. Electrochim. Acta 2012, 79, 102–108. [Google Scholar] [CrossRef]
- Wei, G.; Jia, C.; Liu, J.; Yan, C. Carbon felt supported carbon nanotubes catalysts composite electrode for vanadium redox flow battery application. J. Power Sources 2012, 220, 185–192. [Google Scholar] [CrossRef]
- Huang, K.-L.; Chen, R.-Y.; Liu, S.-Q.; Shi, X.-H.; Zhang, Q.-H. Characteristics of Carbon Nanotube-graphite Composite Electrodes for Vanadium Redox Flow Battery. J. Inorg. Mater. 2010, 25, 659–663. [Google Scholar] [CrossRef]
- Chu, Y.Q.; Li, D.D.; Li, W.W.; Ma, C.A. Electrocatalytic Activity of Multi-walled Carbon Nanotubes for of a Vanadium Redox Flow Battery. In Proceedings of the 2013 International Conference on Materials for Renewable Energy and Environment (ICMREE), Chengdu, China, 19–21 August 2014; Volume 1–3, pp. 537–540. [Google Scholar]
- Mustafa, I.; Lopez, I.; Younes, H.; Susantyoko, R.A.; Abu Al-Rub, R.; Almheiri, S. Fabrication of Freestanding Sheets of Multiwalled Carbon Nanotubes (Buckypapers) for Vanadium Redox Flow Batteries and Effects of Fabrication Variables on Electrochemical Performance. Electrochim. Acta 2017, 230, 222–235. [Google Scholar] [CrossRef]
- Mustafa, I.; Al Shehhi, A.; Al Hammadi, A.; Susantyoko, R.; Palmisano, G.; Almheiri, S. Effects of carbonaceous impurities on the electrochemical activity of multiwalled carbon nanotube electrodes for vanadium redox flow batteries. Carbon 2018, 131, 47–59. [Google Scholar] [CrossRef]
- Tenny, K.M.; Lakhanpal, V.S.; Dowd, R.P., Jr.; Yarlagadda, V.; Van Nguyen, T. Impact of Multi-Walled Carbon Nanotube Fabrication on Carbon Cloth Electrodes for Hydrogen-Vanadium Reversible Fuel Cells. J. Electrochem. Soc. 2017, 164, A2534–A2538. [Google Scholar] [CrossRef]
- Zarei Jelyani, M.; Rashid-Nadimi, S.; Asghari, S. Treated carbon felt as electrode material in vanadium redox flow batteries: A study of the use of carbon nanotubes as electrocatalyst. J. Solid State Electrochem. 2017, 21, 69–79. [Google Scholar] [CrossRef]
- González, Z.; Álvarez, P.; Blanco, C.; Vega-Diaz, S.; Tristaán-Lopez, F.; Pulickal Rajukumar, L.; Cruz-Silva, R.; Laura Elias, A.; Terrones, M.; Menéndez, R. The influence of carbon nanotubes characteristics in their performance as positive electrodes in vanadium redox flow batteries. Sustain. Energy Technol. Assess. 2015, 9, 105–110. [Google Scholar] [CrossRef]
- Han, P.; Yue, Y.; Liu, Z.; Xu, W.; Zhang, L.; Xu, H.; Dong, S.; Cui, G. Graphene oxide nanosheets/multi-walled carbon nanotubes hybrid as an excellent electrocatalytic material towards redox couples for vanadium redox flow batteries. Energy Environ. Sci. 2011, 4, 4710–4717. [Google Scholar] [CrossRef]
- Rui, X.; Ohnmar Oo, M.; Sim, D.H.; Raghu, S.C.; Yan, Q.; Lim, T.M.; Skyllas-Kazacos, M. Graphene oxide nanosheets/polymer binders as superior electrocatalytic materials for vanadium bromide redox flow batteries. Electrochim. Acta 2012, 85, 175–181. [Google Scholar] [CrossRef]
- Park, M.; Jung, Y.-J.; Kim, J.; Lee, H.I.; Cho, J. Synergistic Effect of Carbon Nanofiber/Nanotube Composite Catalyst on Carbon Felt Electrode for High-Performance All-Vanadium Redox Flow Battery. NANO Lett. 2013, 13, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Q.; Zhang, Y.M.; Yue, L.; Li, W.S.; Li, G.L.; Shu, D.; Chen, H.Y. Graphite-carbon nanotube composite electrodes for all vanadium redox flow battery. J. Power Sources 2008, 184, 637–640. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Modified multiwalled carbon nanotubes as an electrode reaction catalyst for an all vanadium redox flow battery. J. Solid State Electrochem. 2013, 17, 1369–1376. [Google Scholar] [CrossRef]
- Yang, H.; Hung, C.-H.; Wang, S.-P.; Chiang, I.-L. Graphite felt with vapor grown carbon fibers as electrodes for vanadium redox flow batteries. Rare Met. 2011, 30, 1–4. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Tang, Y.; Bian, H.; Ng, T.-W.; Zhang, W.; Lee, C.-S. Graphene-Nanowall-Decorated Carbon Felt with Excellent Electrochemical Activity Toward Couple for All Vanadium Redox Flow Battery. Adv. Sci. 2016, 3, 1500276. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Z.; Vizireanu, S.; Dinescu, G.; Blanco, C.; Santamaria, R. Carbon nanowalls thin films as nanostructured electrode materials in vanadium redox flow batteries. Nano Energy 2012, 1, 833–839. [Google Scholar] [CrossRef]
- Park, M.; Jeon, I.-Y.; Ryu, J.; Baek, J.-B.; Cho, J. Exploration of the Effective Location of Surface Oxygen Defects in Graphene-Based Electrocatalysts for All-Vanadium Redox-Flow Batteries. Adv. Energy Mater. 2015, 5, 1401550. [Google Scholar] [CrossRef]
- Park, M.; Jeon, I.-Y.; Ryu, J.; Baek, J.-B.; Cho, J. Exploration of the effective location of surface oxygen defects in graphene-based electrocatalysts for all-vanadium redox flow batteries. In Proceedings of the 249th National ACS Meeting, Denver, CO, USA, 22–26 March 2015. [Google Scholar]
- He, Z.; Cheng, G.; Jiang, Y.; Wang, L.; Dai, L. Sulfonated Carbon Nanotubes as Superior Catalysts towards V3+/V2+ Redox Reaction for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2018, 165, A932–A938. [Google Scholar] [CrossRef]
- Noh, C.; Moon, S.; Chung, Y.; Kwon, Y. Chelating functional group attached to carbon nanotubes prepared for performance enhancement of vanadium redox flow battery. J. Mater. Chem. 2017, 5, 21334–21342. [Google Scholar] [CrossRef]
- Wu, L.; Shen, Y.; Yu, L.; Xi, J.; Qiu, X. Boosting vanadium flow battery performance by Nitrogen-doped carbon nanospheres electrocatalyst. Nano Energy 2016, 28, 19–28. [Google Scholar] [CrossRef]
- He, Z.; Jiang, Y.; Wei, Y.; Zhao, C.; Jiang, F.; Li, L.; Zhou, H.; Meng, W.; Wang, L.; Dai, L. N,P co-doped carbon microsphere as superior electrocatalyst for redox reaction. Electrochim. Acta 2018, 259, 122–130. [Google Scholar] [CrossRef]
- Han, M.; Kim, H. Development of Boron Doped Carbon Using CO2 Reduction with NaBH4 for Vanadium Redox Flow Battery. J. Korean Electrochem. Soc. 2018, 21, 1–5. [Google Scholar]
- Gursu, H.; Gencten, M.; Sahin, Y. Preparation of Sulphur-Doped Graphene-Based Electrodes by Cyclic Voltammetry: A Potential Application for Vanadium Redox Flow Battery. Int. J. Electrochem. Sci. 2018, 13, 875–885. [Google Scholar] [CrossRef]
- Jiang, H.R.; Shyy, W.; Wu, M.C.; Wei, L.; Zhao, T.S. Highly active, bi-functional and metal-free B4C-nanoparticle-modified graphite felt electrodes for vanadium redox flow batteries. J. Power Sources 2017, 365, 34–42. [Google Scholar] [CrossRef]
- Abbas, S.; Lee, H.; Hwang, J.; Mehmood, A.; Shin, H.-J.; Mehboob, S.; Lee, J.-Y.; Ha, H.Y. A novel approach for forming carbon nanorods on the surface of carbon felt electrode by catalytic etching for high-performance vanadium redox flow battery. Carbon 2018, 128, 31–37. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Graphite–graphite oxide composite electrode for vanadium redox flow battery. Electrochim. Acta 2011, 56, 5290–5294. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.A.; Wu, X.W.; Yuan, X.H.; Hu, J.P.; Zhou, Q.M.; Liu, Z.H.; Wu, Y.P. Porous carbon derived from disposable shaddock peel as an excellent catalyst toward couple for vanadium redox battery. J. Power Sources 2015, 299, 301–308. [Google Scholar] [CrossRef]
- Maharjan, M.; Bhattarai, A.; Ulaganathan, M.; Wai, N.; Oo, M.O.; Wang, J.-Y.; Lim, T.M. High surface area bio-waste based carbon as a superior electrode for vanadium redox flow battery. J. Power Sources 2017, 362, 50–56. [Google Scholar] [CrossRef]
- Ryu, J.; Park, M.; Cho, J. Catalytic Effects of B/N-co-Doped Porous Carbon Incorporated with Ketjenblack Nanoparticles for All-Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2016, 163, A5144–A5149. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Jain, A.; Aravindan, V.; Jayaraman, S.; Ling, W.C.; Lim, T.M.; Srinivasan, M.P.; Yan, Q.; Madhavi, S. Bio-mass derived mesoporous carbon as superior electrode in all vanadium redox flow battery with multicouple reactions. J. Power Sources 2015, 274, 846–850. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Kim, Y.; Cho, J. Corn protein-derived nitrogen-doped carbon materials with oxygen-rich functional groups: A highly efficient electrocatalyst for all-vanadium redox flow batteries. Energy Environ. Sci. 2014, 7, 3727–3735. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Zhao, G.; An, L.; Zeng, L. A high-performance carbon nanoparticle-decorated graphite felt electrode for vanadium redox flow batteries. Appl. Energy 2016, 176, 74–79. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhao, T.S.; Bai, B.F.; Zeng, L.; Wie, L. A highly active biomass-derived electrode for all vanadium redox flow batteries. Electrochim. Acta 2017, 248, 197–205. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H. Development of nitrogen-doped carbons using the hydrothermal method as electrode materials for vanadium redox flow batteries. J. Appl. Electrochem. 2013, 43, 553–557. [Google Scholar] [CrossRef]
- Schnucklake, M.; Kuecken, S.; Fetyan, A.; Schmidt, J.; Thomas, A.; Roth, C. Salt-templated porous carbon-carbon composite electrodes for application in vanadium redox flow batteries. J. Mater. Chem. 2017, 5, 25193–25199. [Google Scholar] [CrossRef]
- Mazur, P.; Mrlik, J.; Benes, J.; Pocedic, J.; Vrana, J.; Dundalek, J.; Kosek, J. Performance evaluation of thermally treated graphite felt electrodes for vanadium redox flow battery and their four-point single cell characterization. J. Power Sources 2018, 380, 105–114. [Google Scholar] [CrossRef]
- Noh, T.H.; Kim, M.Y.; Kim, D.H.; Yang, S.H.; Lee, J.H.; Park, H.S.; Noh, H.S.; Lee, M.S.; Kim, H.S. Electrochemical Studies of Carbon Felt Electrode Modified Under Airless Conditions for Redox Flow Batteries. J. Electrochem. Sci. Technol. 2017, 8, 155–161. [Google Scholar]
- Liu, S.-Q.; Shi, X.-H.; Huang, K.-L.; Li, X.-G. Characteristics of Carbon Paper as Electrode for Vanadium Redox Flow Battery. J. Inorg. Mater. 2009, 24, 798–802. [Google Scholar] [CrossRef]
- Cho, Y.I.; Park, S.J.; Hwang, H.J.; Lee, J.G.; Jeon, Y.K.; Chu, Y.H.; Shul, Y.-G. Effects of Microwave Treatment on Carbon Electrode for Vanadium Redox Flow Battery. ChemElectroChem 2015, 2, 872–876. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Xu, P.; Shen, Y.; Lu, L.; Shi, J.; Fu, J.; Zhao, H. Microwave-treated graphite felt as the positive electrode for all-vanadium redox flow battery. J. Power Sources 2014, 263, 104–109. [Google Scholar] [CrossRef]
- Boehm, H.-P. Funktionelle Gruppen an Festkörper-Oberflächen. Angew. Chem. 1966, 78, 617–628. [Google Scholar] [CrossRef]
- Di Blasi, A.; Di Blasi, O.; Briguglio, N.; Arico, A.S.; Sebastian, D.; Lazaro, M.J.; Monforte, G.; Antonucci, V. Investigation of several graphite-based electrodes for vanadium redox flow cell. J. Power Sources 2013, 227, 15–23. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, Y.-J.; Kim, J.-H.; Park, M.-S. The effects of surface modification on carbon felt electrodes for use in vanadium redox flow batteries. Mater. Chem. Phys. 2011, 131, 547–553. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.; Wie, L. Revealing the Performance Enhancement of Oxygenated Carbonaceous Materials for Vanadium Redox Flow Batteries: Functional Groups or Specific Surface Area? Adv. Sustain. Syst. 2018, 2, 1700148. [Google Scholar] [CrossRef]
- Maruyama, J.; Maruyama, S.; Fukuhara, T.; Hanafusa, K. Efficient Edge Plane Exposure on Graphitic Carbon Fiber for Enhanced Flow-Battery Reactions. J. Phys. Chem. 2017, C121, 24425–24433. [Google Scholar] [CrossRef]
- Mayrhuber, I.; Dennison, C.R.; Kalra, V.; Kumbur, E.C. Laser-perforated carbon paper electrodes for improved mass-transport in high power density vanadium redox flow batteries. J. Power Sources 2014, 260, 251–258. [Google Scholar] [CrossRef]
- He, Z.; Jiang, Y.; Li, Y.; Zhu, J.; Zhou, H.; Meng, W.; Wang, L.; Dai, L. Carbon layer-exfoliated, wettability-enhanced, SO3H-functionalized carbon paper: A superior positive electrode for vanadium redox flow battery. Carbon 2018, 127, 297–304. [Google Scholar] [CrossRef]
- Kötz, R.; Barbero, C.; Schnyder, B.; Haas, O. Spectroscopic Ellipsometry of Carbon Electrodes during Electrochemical Activation. Thin Solid Films 1993, 233, 69–73. [Google Scholar] [CrossRef]
- Sullivan, M.G.; Schnyder, B.; Bartsch, M.; Alliata, D.; Barbero, C.; Imhof, R.; Kötz, R. Electrochemically modified glassy carbon for capacitor electrodes characterization of thick anodic layers by cyclic voltammetry, differential electrochemical mass spectrometry, spectroscopic ellipsometry, X-ray photoelectron spectroscopy, FTIR, and AFM. J. Electrochem. Soc. 2000, 147, 2636–2643. [Google Scholar] [CrossRef]
- Kabir, H.; Gyan, I.O.; Cheng, I.F. Electrochemical modification of a pyrolytic graphite sheet for improved negative electrode performance in the vanadium redox flow battery. J. Power Sources 2017, 342, 31–37. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Z.; Su, W.; Fan, X.; Liu, J.; Yan, C.; Zeng, C. Kinetic investigation of vanadium (V)/(IV) redox couple on electrochemically oxidized graphite electrodes. Electrochim. Acta 2016, 205, 102–112. [Google Scholar] [CrossRef]
- Cao, L.; Skyllas-Kazacos, M.; Wang, D.-W. Effects of Surface Pretreatment of Glassy Carbon on the Electrochemical Behavior of V(IV)/V(V) Redox Reaction. J. Electrochem. Soc. 2016, 163, A1164–A1174. [Google Scholar] [CrossRef]
- Bourke, A.; Miller, M.A.; Lynch, R.P.; Gao, X.; Landon, J.; Wainright, J.S.; Savinell, R.F.; Buckley, D.N. Electrode Kinetics of Vanadium Flow Batteries: Contrasting Responses of V-II-V-III and V-IV-V-V to Electrochemical Pretreatment of Carbon. J. Electrochem. Soc. 2016, 163, A5097–A5105. [Google Scholar] [CrossRef]
- Miller, M.A.; Bourke, A.; Quill, N.; Wainright, J.S.; Lynch, R.P.; Buckley, D.N.; Savinell, R.F. Kinetic Study of Electrochemical Treatment of Carbon Fiber Microelectrodes Leading to In Situ Enhancement of Vanadium Flow Battery Efficiency. J. Electrochem. Soc. 2016, 163, A2095–A2102. [Google Scholar] [CrossRef]
- Bourke, A.; Miller, M.A.; Lynch, R.P.; Wainright, J.S.; Savinell, R.F.; Buckley, D.N. Effect of Cathodic and Anodic Treatments of Carbon on the Electrode Kinetics of V-IV/V-V Oxidation-Reduction. J. Electrochem. Soc. 2015, 162, A1547–A1555. [Google Scholar] [CrossRef]
- Bourke, A.; Lynch, R.P.; Buckley, D.N. Effect of Electrode Pretreatment on the Cyclic Voltammetry of at a Glassy Carbon Electrode. ECS Trans. 2013, 53, 59–67. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Xu, Q.; Yan, C. An electrochemically activated graphite electrode with excellent kinetics for electrode processes of V(II)/V(III) and V(IV)/V(V) couples in a vanadium redox flow battery. RSC Adv. 2014, 4, 55666–55670. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, K.-L.; Tan, N.; Liu, S.-Q.; Chen, L.-Q. Electrochemical modification of graphite felt electrode for vanadium redox flow battery. J. Inorg. Mater. 2006, 21, 1114–1120. [Google Scholar] [CrossRef]
- Tan, N.; Huang, K.L.; Liu, S.Q.; Li, X.G.; Chang, Z.F. Activation mechanism study of electrochemical treated graphite felt for vanadium redox cell by electrochemical impedance spectrum. Acta Chim. Sin. 2006, 64, 584–588. [Google Scholar]
- Xi, J.; Zhang, W.; Li, Z.; Zhou, H.; Liu, H.; Wu, Z.; Qiu, X. Effect of Electro-Oxidation Current Density on Performance of Graphite Felt Electrode for Vanadium Redox Flow Battery. Int. J. Electrochem. Sci. 2013, 8, 4700–4711. [Google Scholar]
- Men, Y.; Sun, T. Carbon felts electrode treated in different weak acid solutions through electrochemical oxidation method for all vanadium redox flow battery. Int. J. Electrochem. Sci. 2012, 7, 3482–3488. [Google Scholar]
- He, Z.; Jiang, Y.; Zhou, H.; Cheng, G.; Meng, W.; Wang, L.; Dai, L. Graphite felt electrode modified by square wave potential pulse for vanadium redox flow battery. Int. J. Energy Res. 2017, 41, 439–447. [Google Scholar] [CrossRef]
- Buckley, D.N.; Bourke, A.; Lynch, R.P.; Quill, N.; Miller, M.A.; Wainright, J.S.; Savinell, R.F. Influence of Pretreatment on Kinetics at Carbon Electrodes and Consequences for Flow Battery Performance. MRS Adv. 2017, 2, 1131–1142. [Google Scholar] [CrossRef]
- Raj, S.C.; Skyllas-Kazacos, M. Electrochemical studies on wettability of sintered TiB2 electrodes in aluminum electrolysis. Electrochim. Acta 1992, 37, 1395–1401. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, K.-L.; Liu, S.-Q.; Chen, L.-Q. Electrochemical behavior of diverse vanadium ions at modified graphite felt electrode in sulphuric solution. J. Cent. South Univ. Technol. 2007, 14, 51–56. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Shi, X.-H.; Huang, K.-L.; Li, X.-G.; Li, Y.-J.; Wu, X.-W. Characteristics of carbon paper as electrode for vanadium redox flow battery. Chin. J. Inorg. Chem. 2008, 24, 1079–1083. [Google Scholar]
- Yue, L.; Li, W.; Sun, F.; Zhao, L.; Xing, L. Highly hydroxylated carbon fibres as electrode materials of all-vanadium redox flow battery. Carbon 2010, 48, 3079–3090. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, H.S.; Kim, J.; Park, M.-S.; Kim, J.H.; Kim, Y.-J.; Skyllas-Kazacos, M. Superior Electrocatalytic Activity of a Robust Carbon-Felt Electrode with Oxygen-Rich Phosphate Groups for All-Vanadium Redox Flow Batteries. ChemSusChem 2016, 9, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Kil, D.; Lee, H.J.; Park, S.; Kim, S.; Kim, H. Synthesis of Activated Graphite Felts Using Short-Term Ozone/Heat Treatment for Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2017, 164, A3011–A3017. [Google Scholar] [CrossRef]
- Dixon, D.; Babu, D.J.; Langner, J.; Bruns, M.; Pfaffmann, L.; Bhaskar, A.; Schneider, J.J.; Scheiba, F.; Ehrenberg, H. Effect of oxygen plasma treatment on the electrochemical performance of the rayon and polyacrylonitrile based carbon felt for the vanadium redox flow battery application. J. Power Sources 2016, 332, 240–248. [Google Scholar] [CrossRef]
- Estevez, L.; Reed, D.; Nie, Z.; Schwarz, A.M.; Nandasiri, M.I.; Kizewski, J.P.; Wang, W.; Thomsen, E.; Liu, J.; Zhang, J.-G.; et al. Tunable Oxygen Functional Groups as Electrocatalysts on Graphite Felt Surfaces for All-Vanadium Flow Batteries. ChemSusChem 2016, 9, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, S.-W.; Yim, T.; Kim, J.-G.; Choi, J.W.; Kim, J.H.; Park, M.-S.; Kim, Y.-J. A new strategy for integrating abundant oxygen functional groups into carbon felt electrode for vanadium redox flow batteries. Sci. Rep. 2014, 4, 6906. [Google Scholar] [CrossRef] [PubMed]
- Su, A.-Q.; Wang, N.-F.; Liu, S.-Q.; Wu, T.; Peng, S. Modification of Carbon Paper Electrode via Hydrothermal Oxidation Applied in the Vanadium Redox Battery. Act. Phys.-Chim. Sin. 2012, 28, 1387–1392. [Google Scholar]
- Taylor, S.M.; Patru, A.; Fabbri, E.; Schmidt, T.J. Influence of surface oxygen groups on V(II) oxidation reaction kinetics. Electrochem. Commun. 2017, 75, 13–16. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Liao, W.-Y.; Hsieh, W.-Y.; Hsu, C.-C.; Chen, Y.-S. All-vanadium redox flow batteries with graphite felt electrodes treated by atmospheric pressure plasma jets. J. Power Sources 2015, 274, 894–898. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, J.-Y.; Kabtamu, D.M.; Lin, G.-Y.; Hsu, N.-Y.; Chou, Y.-S.; Wei, H.-J.; Wang, C.-H. High efficiency of CO2-activated graphite felt as electrode for vanadium redox flow battery. J. Power Sources 2017, 364, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, J.; He, J.; He, Z. Enhancement of nitrogen and sulfur co-doping on the electrocatalytic properties of carbon nanotubes for (+) redox reaction. RSC Adv. 2017, 7, 13184–13190. [Google Scholar] [CrossRef]
- Gursu, H.; Gencten, M.; Sahin, Y. Novel chlorine doped graphene electrodes for positive electrodes of a vanadium redox flow battery. Int. J. Energy Res. 2018, 42, 3303–3314. [Google Scholar] [CrossRef]
- He, Z.; Jiang, Y.; Meng, W.; Jiang, F.; Zhou, H.; Li, Y.; Zhu, J.; Wang, L.; Dai, L. HF/H2O2 treated graphite felt as the positive electrode for vanadium redox flow battery. Appl. Surf. Sci. 2017, 423, 111–118. [Google Scholar] [CrossRef]
- Park, J.J.; Park, J.H.; Park, O.O.; Yang, J.H. Highly porous graphenated graphite felt electrodes with catalytic defects for high-performance vanadium redox flow batteries produced via NiO/Ni redox reactions. Carbon 2016, 110, 17–26. [Google Scholar] [CrossRef]
- Aoki, K.; Kaneko, H.; Nozaki, K. Estimation of charge transfer kinetic parameters from irreversible cyclic voltammograms at carbon fibre. J. Electroanal. Chem. 1988, 247, 29–36. [Google Scholar] [CrossRef]
- Warburg, O.; Brefeld, W. Über die Aktivierung stickstoffhaltiger Kohlen durch Eisen. Biochem. Z. 1924, 145, 461–480. [Google Scholar]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- He, Z.-X.; Liu, J.-L.; He, Z.; Liu, S.-Q. Nitrogen-doped Carbon Paper as Electrodes for Vanadium Redox Flow Batteries. J. Inorg. Mater. 2015, 30, 779–784. [Google Scholar]
- Shao, Y.Y.; Wang, X.D.; Engelhard, M.; Wang, C.M.; Dai, S.; Liu, J.; Yang, Z.G.; Lin, Y.H. Nitrogen-doped mesoporous carbon for energy storage in vanadium redox flow batteries. J. Power Sources 2010, 195, 4375–4379. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Xiang, Z.-P.; Piao, J.-H.; Shi, J.-Y.; Liang, Z.-X. Electrochemistry of vanadium redox couples on nitrogen-doped carbon. Electrochim. Acta 2018, 259, 687–693. [Google Scholar] [CrossRef]
- Kim, D.S.; Chung, D.J.; Park, H.-I.; Ansari, M.Z.; Song, T.; Kim, H. Direct Nitradated Graphite Felt as an Electrode Material for the Vanadium Redox Flow Battery. Bull. Korean Chem. Soc. 2018, 39, 281–286. [Google Scholar] [CrossRef]
- Lee, H.J.; Kil, D.; Kim, H. Synthesis of Activated Graphite Felt Using Consecutive Post-Treatments for Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2016, 163, A2586–A2591. [Google Scholar] [CrossRef]
- He, Z.; Shi, L.; Shen, J.; He, Z.; Liu, S. Effects of nitrogen doping on the electrochemical performance of graphite felts for vanadium redox flow batteries. Int. J. Energy Res. 2015, 39, 709–716. [Google Scholar] [CrossRef]
- Kim, J.; Lim, H.; Jyoung, J.-Y.; Lee, E.-S.; Yi, J.S.; Lee, D. Effects of Doping Methods and Kinetic Relevance of N and O Atomic Co-Functionalization on Carbon Electrode for V(IV)/V(V) Redox Reactions in Vanadium Redox Flow Battery. Electrochim. Acta 2017, 245, 724–733. [Google Scholar] [CrossRef]
- Wu, T.; Huang, K.; Liu, S.; Zhuang, S.; Fang, D.; Li, S.; Lu, D.; Su, A. Hydrothermal ammoniated treatment of PAN-graphite felt for vanadium redox flow battery. J. Solid State Electrochem. 2012, 16, 579–585. [Google Scholar] [CrossRef]
- He, Z.; Chen, Z.; Meng, W.; Jiang, Y.; Cheng, G.; Dai, L.; Wang, L. Modified carbon cloth as positive electrode with high electrochemical performance for vanadium redox flow batteries. J. Energy Chem. 2016, 25, 720–725. [Google Scholar] [CrossRef]
- He, Z.; Su, A.; Gao, C.; Zhou, Z.; Pan, C.; Liu, S. Carbon paper modified by hydrothermal ammoniated treatment for vanadium redox battery. Ionis 2013, 19, 1021–1026. [Google Scholar] [CrossRef]
- Jin, J.; Fu, X.; Liu, Q.; Liu, Y.; Wei, Z.; Niu, K.; Zhang, J. Identifying the Active Site in Nitrogen-Doped Graphene for the Redox Reaction. ACS Nano 2013, 7, 4764–4773. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, Q.; Wu, X.; Wang, S. N, O Co-doped carbon felt for high-performance all-vanadium redox flow battery. Int. J. Hydrogen Energy 2017, 42, 7177–7185. [Google Scholar] [CrossRef]
- Kim, J.; Lim, H.; Jyoung, J.-Y.; Lee, E.-S.; Yi, J.S.; Lee, D. High electrocatalytic performance of N and O atomic co-functionalized carbon electrodes for vanadium redox flow battery. Carbon 2017, 111, 592–601. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H. Graphite Felt Coated with Dopamine-Derived Nitrogen-Doped Carbon as a Positive Electrode for a Vanadium Redox Flow Battery. J. Electrochem. Soc. 2015, 162, A1675–A1681. [Google Scholar] [CrossRef]
- Flox, C.; Skoumal, M.; Rubio-Garcia, J.; Andreu, T.; Morante, J.R. Strategies for enhancing electrochemical activity of carbon-based electrodes for all-vanadium redox flow batteries. Appl. Energy 2013, 109, 344–351. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Chen, J.-Y.; Chang, Y.-C.; Wang, C.-H. Water-activated graphite felt as a high-performance electrode for vanadium redox flow batteries. J. Power Sources 2017, 341, 270–279. [Google Scholar] [CrossRef]
- Mrha, J. Katalysatoren für die Elektroden der Brennstoffelemente II. Aktivkohle als Katalysator der Elektroreduktion des Sauerstoffes. Collect. Czechoslov. Chem. Commun. 1966, 31, 715–733. [Google Scholar] [CrossRef]
- Mrha, J. Study of catalysts for fuel cell electrodes. IV. Active carbon electrodes for oxygen in alkaline electrolyte. Collect. Czechoslov. Chem. Commun. 1967, 32, 708–719. [Google Scholar] [CrossRef]
- Hollax, E.; Cheng, D.S. The Influence of Oxidative Pretreatment of Graphite-Electrodes on the Catalysis of the Cr3+/Cr2+ and Fe3+/Fe2+ Redox Reactions. Carbon 1985, 23, 655–664. [Google Scholar] [CrossRef]
- Lee, M.E.; Jin, H.-J.; Yun, Y.S. Synergistic catalytic effects of oxygen and nitrogen functional groups on active carbon electrodes for all-vanadium redox flow batteries. RSC Adv. 2017, 7, 43227–43232. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s Reagent Revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Brillas, E.; Sires, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, N.F.; Peng, S.; Lin, S.Q.; Lei, Y.; Liang, X.X.; Zeng, S.S.; Zi, H.F. Influence of Fenton’s reagent treatment on electrochemical properties of graphite felt for all vanadium redox flow battery. Electrochim. Acta 2013, 88, 193–202. [Google Scholar] [CrossRef]
- He, Z.; Jiang, Y.; Li, Y.; Wang, L.; Dai, L. Boosting the electrocatalytic performance of carbon nanotubes toward V(V)/V(IV) reaction by sulfonation treatment. Int. J. Energy Res. 2018, 42, 1625–1634. [Google Scholar] [CrossRef]
- Huang, P.; Ling, W.; Sheng, H.; Zhou, Y.; Wu, X.; Zeng, X.-X.; Wu, X.; Guo, Y.-G. Heteroatom-doped electrodes for all-vanadium redox flow batteries with ultralong lifespan. J. Mater. Chem. 2018, A6, 41–44. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, L.; He, Z.; Li, Y.; Jiang, Y.; Meng, W.; Dai, L. Electrocatalytic activity of cobalt phosphide-modified graphite felt toward redox reaction. Appl. Surf. Sci. 2018, 436, 1030–1037. [Google Scholar] [CrossRef]
- Maruyama, J.; Hasegawa, T.; Iwasaki, S.; Fukuhara, T.; Orikasa, Y.; Uchimoto, Y. Catalysis of Vanadium Ion Redox Reactions on Carbonaceous Material with Metal-N-4 Sites. ChemCatChem 2015, 7, 2305–2308. [Google Scholar] [CrossRef]
- Maruyama, J.; Hasegawa, T.; Iwasaki, S.; Fukuhara, T.; Orikasa, Y.; Uchimoto, Y. Carbonaceous thin film coating with Fe-N-4 site for enhancement of dioxovanadium ion reduction. J. Power Sources 2016, 324, 521–527. [Google Scholar] [CrossRef]
- Maruyama, J.; Shinagawa, T. Catalyst Layer Structures for Enhancement of Redox Reactions of V(IV/V) Ions. Electrochim. Acta 2016, 210, 854–861. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Jeng, K.-T.; Yen, S.-C. Characterization and electrochemical properties of graphite felt-based electrode modified using an ionomer impregnation approach for vanadium redox flow battery. Electrochim. Acta 2017, 251, 109–118. [Google Scholar] [CrossRef]
- Pupkevich, V.; Glibin, V.; Karamanev, D. The effect of activation on the electrochemical behaviour of graphite felt towards the Fe3+/Fe2+ redox electrode reaction. Electrochem. Commun. 2007, 9, 1924–1930. [Google Scholar] [CrossRef]
- Kokkinidis, G. Underpotential deposition and electrocatalysis. J. Electroanal. Chem. 1986, 201, 217–236. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis of Hydrogen Evolution: Progress in Cathode Activation. In Advances in Electrochemical Science and Engineering; Gerischer, H., Tobias, C.W., Eds.; VCH: Weinheim, Germany, 1990; Volume 2, pp. 1–85. [Google Scholar]
- Ross, P.N. The Science of Catalysis on Bimetallic Surfaces. In Electrocatalysis; Lipkowski, J., Ross, P.N., Eds.; Wiley-VCH: New York, NY, USA, 1998; pp. 43–74. [Google Scholar]
- Dapperheld, S. Elektrokatalytisches Verfahren zur Aufbereitung von Mutterlaugen aus der Chloressigsäure-Produktion. In DECHEMA-Monographie; VCH Verlagsgesellschaft: Weinheim, Germany, 1988; Volume 112, pp. 317–324. [Google Scholar]
- Holze, R.; Fette, U. The Electrocatalysis of Dichloroacetic Acid Reduction at Lead-Modified Carbon Electrodes. J. Electroanal. Chem. 1992, 339, 247–253. [Google Scholar] [CrossRef]
- Holze, R.; Fette, U. Zur Elektrokatalyse der Dichloressigsäurereduktion. In DECHEMA-Monographie; VCH Verlagsgesellschaft: Weinheim, Germany, 1992; Volume 125, pp. 769–775. [Google Scholar]
- Wang, W.; Wang, X. Study of the electrochemical properties of a transition metallic ions modified electrode in acidic VOSO4 solution. Rare Met. 2007, 26, 131–135. [Google Scholar] [CrossRef]
- Sun, B.T.; Skyllas-Kazacos, M. Chemical modification and electrochemical behaviour of graphite fibre in acidic vanadium solution. Electrochim. Acta 1991, 36, 513–517. [Google Scholar] [CrossRef]
- Mehboob, S.; Mehmood, A.; Lee, J.-Y.; Shin, H.-J.; Hwang, J.; Abbas, S.; Ha, H.Y. Excellent electrocatalytic effects of tin through in situ electrodeposition on the performance of all-vanadium redox flow batteries. J. Mater. Chem. 2017, A5, 17388–17400. [Google Scholar] [CrossRef]
- Xiang, Y.; Daoud, W.A. Electrochemical Enhancement of Carbon Paper by Indium Modification for the Positive Side of Vanadium Redox Flow Battery. J. Electrochem. Soc. 2017, 164, A2256–A2261. [Google Scholar] [CrossRef]
- Suarez, D.J.; Gonzalez, Z.; Blanco, C.; Granda, M.; Menendez, R.; Santamaria, R. Graphite Felt Modified with Bismuth Nanoparticles as Negative Electrode in a Vanadium Redox Flow Battery. ChemSusChem 2014, 7, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Nie, H.; Xu, C.; Zhang, H. Investigation on the effect of catalyst on the electrochemical performance of carbon felt and graphite felt for vanadium flow batteries. J. Power Sources 2015, 286, 73–81. [Google Scholar] [CrossRef]
- Li, B.; Gu, M.; Nie, Z.; Shao, Y.; Luo, Q.; Wei, X.; Li, X.; Xiao, J.; Wang, C.; Sprenkle, V.; et al. Bismuth Nanoparticle Decorating Graphite Felt as a High-Performance Electrode for an All-Vanadium Redox Flow Battery. Nano Lett. 2013, 13, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, T.; Xu, C.; Zhang, H.; Li, X.; Zhang, H. The catalytic effect of bismuth for and V3+/V2+ redox couples in vanadium flow batteries. J. Energy Chem. 2017, 26, 1–7. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Lv, Z.; Wu, C.; Liu, Y.; Wang, H.; Lu, S.; Xiang, Y. Enhanced electrochemical activity of carbon felt for V3+/V2+ redox reaction via combining KOH-etched pretreatment with uniform deposition of Bi nanoparticles. Electrochim. Acta 2017, 253, 78–84. [Google Scholar] [CrossRef]
- Shen, J.; Liu, S.; He, Z.; Shi, L. Influence of antimony ions in negative electrolyte on the electrochemical performance of vanadium redox flow batteries. Electrochim. Acta 2015, 151, 297–305. [Google Scholar] [CrossRef]
- Flox, C.; Rubio-Garcia, J.; Nafria, R.; Zamani, R.; Skoumal, M.; Andreu, T.; Arbiol, J.; Cabot, A.; Morante, J.R. Active nano-CuPt3 electrocatalyst supported on graphene for enhancing reactions at the cathode in all-Vanadium redox flow batteries. Carbon 2012, 50, 2372–2374. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Zeng, L.; Zhou, X.L.; Zeng, Y.K. Copper nanoparticle-deposited graphite felt electrodes for all vanadium redox flow batteries. Appl. Energy 2016, 180, 386–391. [Google Scholar] [CrossRef]
- Han, P.; Wang, X.; Zhang, L.; Wang, T.; Yao, J.; Huang, C.; Gu, L.; Cui, G. RuSe/reduced graphene oxide: An efficient electrocatalyst for redox couples in vanadium redox flow batteries. RSC Adv. 2014, 4, 20379–20381. [Google Scholar] [CrossRef]
- Huang, R.-H.; Sun, C.-H.; Tseng, T.-M.; Chao, W.-K.; Hsueh, K.-L.; Shieu, F.-S. Investigation of Active Electrodes Modified with Platinum/Multiwalled Carbon Nanotube for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2012, 159, A1579–A1586. [Google Scholar] [CrossRef]
- Tseng, T.-M.; Huang, R.-H.; Huang, C.-Y.; Hsueh, K.-L.; Shieu, F.-S. A Kinetic Study of the Platinum/Carbon Anode Catalyst for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2013, 160, A690–A696. [Google Scholar] [CrossRef]
- Tsai, H.-M.; Yang, S.-J.; Ma, C.-C.M.; Xie, X. Preparation and electrochemical activities of iridium-decorated graphene as the electrode for all-vanadium redox flow batteries. Electrochim. Acta 2012, 77, 232–236. [Google Scholar] [CrossRef]
- Chang, C.H.; Yuen, T.S.; Nagao, Y.; Yugami, H. Electrocatalytic activity of iridium oxide nanoparticles coated on carbon for oxygen reduction as cathode catalyst in polymer electrolyte fuel cell. J. Power Sources 2010, 195, 5938–5941. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, X.D. Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electrochim. Acta 2007, 52, 6755–6762. [Google Scholar] [CrossRef]
- Fuh, Y.-K.; Chang, T.-C.; Zhang, J.-P. Electroless Plating on Porous Carbon Felts in Redox Flow Batteries and Thickness Effect on the Electrical and Mechanical Properties. Int. J. Electrochem. Sci. 2013, 8, 8989–8999. [Google Scholar]
- Ma, Q.; Deng, Q.; Sheng, H.; Ling, W.; Wang, H.R.; Jiao, H.W.; Wu, X.W.; Zhou, W.X.; Zeng, X.X.; Yin, Y.X.; et al. High electro-catalytic graphite felt/MnO2 composite electrodes for vanadium redox flow batteries. Sci. China Chem. 2018, 48, 1–7. [Google Scholar] [CrossRef]
- He, Z.; Dai, L.; Liu, S.; Wang, L.; Li, C. Mn3O4 anchored on carbon nanotubes as an electrode reaction catalyst of V(IV)/V(V) couple for vanadium redox flow batteries. Electrochim. Acta 2015, 176, 1434–1440. [Google Scholar] [CrossRef]
- Ejigu, A.; Edwards, M.; Walsh, D.A. Synergistic Catalyst-Support Interactions in a Graphene-Mn3O4 Electrocatalyst for Vanadium Redox Flow Batteries. ACS Catal. 2015, 5, 7122–7130. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Lu, L.; Zhao, H.; Fu, J.; Shen, Y.; Xu, P.; Dong, Y. PbO2-modified graphite felt as the positive electrode for an all-vanadium redox flow battery. J. Power Sources 2014, 250, 274–278. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, T.; Li, X.; Liu, Z. Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J. Power Sources 2012, 218, 455–461. [Google Scholar] [CrossRef]
- Faraji, M.; Hassanzadeh, A.; Mohseni, M. Interlaced WO3-carbon nanotube nanocomposite electrodeposited on graphite as a positive electrode in vanadium redox flow battery. Thin Solid Films 2017, 642, 188–194. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, H.; Xu, P.; Wu, X.; Dong, Y.; Lu, L. Electrochemical catalytic activity of tungsten trioxide- modified graphite felt toward redox reaction. Electrochim. Acta 2014, 132, 37–41. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Xi, J.; Qiu, X.; Chen, L. ZrO2-Nanoparticle-Modified Graphite Felt: Bifunctional Effects on Vanadium Flow Batteries. ACS Appl. Mater. Interf. 2016, 8, 15369–15378. [Google Scholar] [CrossRef] [PubMed]
- Kabtamu, D.M.; Chen, J.-Y.; Chang, Y.-C.; Wang, C.-H. Electrocatalytic activity of Nb-doped hexagonal WO3 nanowire-modified graphite felt as a positive electrode for vanadium redox flow batteries. J. Mater. Chem. 2016, A4, 11472–11480. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mousavihashemi, S.; Murcia-Lopez, S.; Flox, C.; Andreu, T.; Morante, J.R. High-power positive electrode based on synergistic effect of N- and WO3-decorated carbon felt for vanadium redox flow batteries. Carbon 2018, 136, 444–453. [Google Scholar] [CrossRef]
- Cao, L.; Skyllas-Kazacos, M.; Wang, D.-W. Modification Based on MoO3 as Electrocatalysts for High Power Density Vanadium Redox Flow Batteries. ChemElectroChem 2017, 4, 1836–1839. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Jo, C.; Lee, J.; Kwon, Y. MoO2 nanocrystals interconnected on mesocellular carbon foam as a powerful catalyst for vanadium redox flow battery. RSC Adv. 2016, 6, 17574–17582. [Google Scholar] [CrossRef]
- Vazquez-Galvan, J.; Flox, C.; Fàbrega, C.; Ventosa, E.; Parra, A.; Andreu, T.; Morante, J.R. Hydrogen-Treated Rutile TiO2 Shell in Graphite-Core Structure as a Negative Electrode for High-Performance Vanadium Redox Flow Batteries. ChemSusChem 2017, 10, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-M.; Huang, R.-H.; Huang, C.-Y.; Hsueh, K.-L.; Shieu, F.-S. Improvement of Titanium Dioxide Addition on Carbon Black Composite for Negative Electrode in Vanadium Redox Flow Battery. J. Electrochem. Soc. 2013, 160, A1269–A1275. [Google Scholar] [CrossRef]
- Tseng, T.-M.; Huang, R.-H.; Huang, C.-Y.; Liu, C.-C.; Hsueh, K.-L.; Shieu, F.-S. Carbon Felt Coated with Titanium Dioxide/Carbon Black Composite as Negative Electrode for Vanadium Redox Flow Battery. J. Electrochem. Soc. 2014, 161, A1132–A1138. [Google Scholar] [CrossRef]
- He, Z.; Li, M.; Li, Y.; Zhu, J.; Jiang, Y.; Meng, W.; Zhou, H.; Wang, L.; Dai, L. Flexible electrospun carbon nanofiber embedded with TiO2 as excellent negative electrode for vanadium redox flow battery. Electrochim. Acta 2018, 281, 601–610. [Google Scholar] [CrossRef]
- Bayeh, A.W.; Kabtamu, D.M.; Chang, Y.-C.; Chen, G.-C.; Chen, H.-Y.; Lin, G.-Y.; Liu, T.-R.; Wondimu, T.H.; Wang, K.-C.; Wang, C.-H. Ta2O5-Nanoparticle-Modified Graphite Felt As a High-Performance Electrode for a Vanadium Redox Flow Battery. ACS Sustain. Chem. Eng. 2018, 6, 3019–3028. [Google Scholar] [CrossRef]
- Fetyan, A.; El-Nagar, G.A.; Derr, I.; Kubella, P.; Dau, H.; Roth, C. A neodymium oxide nanoparticle-doped carbon felt as promising electrode for vanadium redox flow batteries. Electrochim. Acta 2018, 268, 59–65. [Google Scholar] [CrossRef]
- Li, B.; Gu, M.; Nie, Z.; Wie, X.; Wang, C.; Sprenkle, V.; Wang, W. Nanorod Niobium Oxide as Powerful Catalysts for an All Vanadium Redox Flow Battery. Nano Lett. 2014, 14, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Rui, X.; Chen, G.; Xu, W.; Zou, G.; Luo, H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar] [CrossRef] [PubMed]
- Gobal, F.; Faraji, M. RuO2/MWCNT/stainless steel mesh as a novel positive electrode in vanadium redox flow batteries. RSC Adv. 2015, 5, 68378–68384. [Google Scholar] [CrossRef]
- Zhou, H.; Xi, J.; Li, Z.; Zhang, Z.; Yu, L.; Liu, L.; Qiu, X.; Chen, L. CeO2 decorated graphite felt as a high-performance electrode for vanadium redox flow batteries. RSC Adv. 2014, 4, 61912–61918. [Google Scholar] [CrossRef]
- Holze, R. Carbon as Electrocatalyst for Applications in Electrochemical Energy Conversion—An Overview. In Proceedings of the 4th International Carbon Conference (Carbon’86), Baden-Baden, Germany, 30 June–4 July 1986; pp. 361–363. [Google Scholar]
- Krüger, A. Neue Kohlenstoffmaterialien; Teubner-Verlag: Wiesbaden, Germany, 2007. [Google Scholar]
- Haddadi-Asl, V.; Kazacos, M.; Skyllas-Kazacos, M. Carbon-Polymer Composite Electrodes for Redox Cells. J. Appl. Polym. Sci. 1995, 57, 1455–1463. [Google Scholar] [CrossRef]
- Haddadi-Asl, V.; Kazacos, M.; Skyllas-Kazacos, M. Conductive carbon-polypropylene composite electrodes for vanadium redox battery. J. Appl. Electrochem. 1995, 25, 29–33. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Huang, Q.-M.; Li, W.-S.; Peng, H.-Y.; Hu, S.-J. Graphite-acetylene black composite electrodes for all vanadium redox flow battery. J. Inorg. Mater. 2007, 22, 1051–1055. [Google Scholar]
- Tsai, H.-M.; Yang, S.-Y.; Ma, C.-C.M.; Xie, X. Preparation and Electrochemical Properties of Graphene-Modified Electrodes for All-Vanadium Redox Flow Batteries. Electroanalysis 2011, 23, 2139–2143. [Google Scholar] [CrossRef]
- Ji, Y.; Li, J.L.; Li, S.F.Y. Synergistic effect of the bifunctional polydopamine-Mn3O4 composite electrocatalyst for vanadium redox flow batteries. J. Mater. Chem. 2017, A5, 15154–15166. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Chang, Y.-C.; Lin, G.-Y.; Bayeh, A.W.; Chen, J.-Y.; Wondimu, T.H.; Wang, C.-H. Three-dimensional annealed WO3 nanowire/graphene foam as an electrocatalytic material for all vanadium redox flow batteries. Sustain. Energy Fuels 2017, 1, 2091–2100. [Google Scholar] [CrossRef]
- Bayeh, A.W.; Kabtamu, D.M.; Chang, Y.-C.; Chen, G.-C.; Chen, H.-Y.; Lin, G.-Y.; Liu, T.-R.; Wondimu, T.H.; Wang, K.-C.; Wang, C.-H. Synergistic effects of a TiNb2O7-reduced graphene oxide nanocomposite electrocatalyst for highperformance all-vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 13908–13917. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Fetyan, A.; Derr, I.; Kayarkatte, M.K.; Langner, J.; Bernsmeier, D.; Kraehnert, R.; Roth, C. Electrospun Carbon Nanofibers as Alternative Electrode Materials for Vanadium Redox Flow Batteries. ChemElectroChem 2015, 2, 2055–2060. [Google Scholar] [CrossRef]
- Flox, C.; Fabrega, C.; Andreu, T.; Morata, A.; Skoumal, M.; Rubio-Garcia, J.; Morante, J.R. Highly electrocatalytic flexible nanofiber for improved vanadium-based redox flow battery cathode electrodes. RSC Adv. 2013, 3, 12056–12059. [Google Scholar] [CrossRef]
- Wei, G.; Su, W.; Wei, Z.; Jing, M.; Fan, X.; Liu, J.; Yan, C. Effect of the graphitization degree for electrospun carbon nanofibers on their electrochemical activity towards redox couple. Electrochim. Acta 2016, 199, 147–153. [Google Scholar] [CrossRef]
- Etesami, M.; Abouzari-Lotf, E.; Sha’rani, S.S.; Miyake, M.; Nia, P.M.; Ripin, A.; Ahmad, A. Self-assembled heteropolyacid on nitrogen-enriched carbon nanofiber for vanadium flow batteries. Nanoscale 2018, 10, 13212–13222. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Zhang, X.; Fan, X.; Zhao, L.; Liu, J.; Yan, C. CeO2 embedded electrospun carbon nanofibers as the advanced electrode with high effective surface area for vanadium flow battery. Electrochim. Acta 2016, 215, 57–65. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X.; Li, X.; Liu, T.; Zhang, H. Ultrathin free-standing electrospun carbon nanofibers web as the electrode of the vanadium flow batteries. J. Energy Chem. 2017, 26, 730–737. [Google Scholar] [CrossRef]
- Wei, G.; Liu, J.; Zhao, H.; Yan, C. Electrospun carbon nanofibres as electrode materials toward redox couple for vanadium flow battery. J. Power Sources 2013, 241, 709–717. [Google Scholar] [CrossRef]
- Wei, G.; Gao, Z.; Wei, Z.; Fan, X.; Liu, J.; Yan, C. Coupling effect between the structure and surface characteristics of electrospun carbon nanofibres on the electrochemical activity towards the redox couple. Phys. Chem. Chem. Phys. 2015, 17, 20368–20375. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Fan, X.; Liu, J.; Yan, C. Investigation of the electrospun carbon web as the catalyst layer for vanadium redox flow battery. J. Power Sources 2014, 270, 634–645. [Google Scholar] [CrossRef]
- Shi, L.; Liu, S.; He, Z.; Yuan, H.; Shen, J. Synthesis of boron and nitrogen co-doped carbon nanofiber as efficient metal-free electrocatalyst for the Redox Reaction. Electrochim. Acta 2015, 178, 748–757. [Google Scholar] [CrossRef]
- Busacca, C.; Di Blasi, O.; Briguglio, N.; Ferraro, M.; Antonucci, V.; Di Blasi, A. Electrochemical performance investigation of electrospun urchin-like V2O3-CNF composite nanostructure for vanadium redox flow battery. Electrochim. Acta 2017, 230, 174–180. [Google Scholar] [CrossRef]
- Di Blasi, A.; Busaccaa, C.; Di Blasia, O.; Briguglioa, N.; Squadritoa, G.; Antonuccia, V. Synthesis of flexible electrodes based on electrospun carbon nanofibers with Mn3O4 nanoparticles for vanadium redox flow battery application. Appl. Energy 2017, 190, 165–171. [Google Scholar] [CrossRef]
- Wei, G.; Fan, X.; Liu, J.; Yan, C. Electrospun carbon nanofibers/electrocatalyst hybrids as asymmetric electrodes for vanadium redox flow battery. J. Power Sources 2015, 281, 1–6. [Google Scholar] [CrossRef]
- Han, J.; Yoo, H.; Kim, M.; Lee, G.; Choi, J. High-performance bipolar plate of thin IrOx-coated TiO2 nanotubes in vanadium redox flow batteries. Catal. Today 2017, 295, 132–139. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Zeng, L.; Zeng, Y.K.; Jiang, H.R. Highly catalytic and stabilized titanium nitride nanowire array-decorated graphite felt electrodes for all vanadium redox flow batteries. J. Power Sources 2017, 341, 318–326. [Google Scholar] [CrossRef]
- Zhao, F.-M.; Wen, G.; Kong, L.-Y.; Chu, Y.-Q.; Ma, C.-A. Structure Characteristic of Titanium Nitride Nanowires and Its Electrode Processes for V(II)/V(III) Redox Couple. Acta Phys. Chim. Sin. 2017, 33, 1181–1188. [Google Scholar]
- Zhao, F.-M.; Wen, G.; Kong, L.-Y.; Chu, C.-Q.; Ma, C.-A. Electrochemical Performance of Titanium Nitride Nanotubes as Negative Electrode in a Static Vanadium Battery Towards V(II)/V(III) Redox Couple. Chin. J. Inorg. Chem. 2017, 33, 501–508. [Google Scholar]
- Wei, L.; Zhao, T.; Zeng, L.; Zhou, X.; Zeng, Y. Titanium Carbide Nanoparticle-Decorated Electrode Enables Significant Enhancement in Performance of All-Vanadium Redox Flow Batteries. Energy Technol. 2016, 4, 990–996. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Lu, S.; Wu, C.; Liu, Y.; Tan, Q.; Liang, D.; Xiang, Y. Titanium nitride as an electrocatalyst for V(II)/V(III) redox couples in all-vanadium redox flow batteries. Electrochim. Acta 2015, 182, 834–840. [Google Scholar] [CrossRef]
- Huang, Y.; Huo, J.; Dou, S.; Hu, K.; Wang, S. Graphitic C3N4 as a powerful catalyst for all-vanadium redox flow batteries. RSC Adv. 2016, 6, 66368–66372. [Google Scholar] [CrossRef]
- Su, J.; Zhao, Y.; Xi, J. Phosphorus-doped carbon nitride as powerful electrocatalyst for highpower vanadium flow battery. Electrochim. Acta 2018, 286, 22–28. [Google Scholar] [CrossRef]
- Liu, H.; Cai, T.; Song, Q.; Yang, L.; Xu, Q.; Yan, C. Electrochemical Behavior of the Titanium Plate with Carbon Films in a Vanadium Sulfate Solution. Int. J. Electrochem. Sci. 2013, 8, 2515–2523. [Google Scholar]
- Chandrabose Raghu, S.; Ulaganathan, M.; Lim, T.M.; Skyllas-Kazacos, M. Electrochemical behaviour of titanium/iridium(IV) oxide: Tantalum pentoxide and graphite for application in vanadium redox flow battery. J. Power Sources 2013, 238, 103–108. [Google Scholar] [CrossRef]
- Lee, W.; Jo, C.; Youk, S.; Shin, H.Y.; Lee, J.; Chung, Y.; Kwon, Y. Mesoporous tungsten oxynitride as electrocatalyst for promoting redox reactions of vanadium redox couple and performance of vanadium redox flow battery. Appl. Surf. Sci. 2018, 429, 187–195. [Google Scholar] [CrossRef]
- Flox, C.; Murcia-Lopez, S.; Carretero, N.M.; Ros, C.; Morante, J.R.; Andreu, T. Role of Bismuth in the Electrokinetics of Silicon Photocathodes for Solar Rechargeable Vanadium Redox Flow Batteries. ChemSusChem 2018, 11, 125–129. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, L.; He, Y.; Chen, C.; Jiang, Y.; He, Z.; Liu, S. Effect of In3+ ions on the electrochemical performance of the positive electrolyte for vanadium redox flow batteries. Ionics 2013, 19, 1915–1920. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Luo, C.-H.; Wang, G.X.; Yan, K.P.; Luo, D.M. Influence of Cr3+ concentration on the electrochemical behavior of the anolyte for vanadium redox flow batteries. Chin. Sci. Bull. 2012, 57, 4237–4243. [Google Scholar] [CrossRef]
- Huang, F.; Wang, G.-X.; Yan, K.-P.; Luo, D.-M. Influence of Mn2+ Concentration on the Electrochemical Behavior of the Anolyte for Vanadium Redox Flow Batteries. Chin. J. Inorg. Chem. 2012, 28, 898–904. [Google Scholar]
- Kim, M.; Yoo, H.; Lee, G.; Choi, J. Enhanced VRB electrochemical performance using tungsten as an electrolyte additive. Electrochim. Acta 2017, 246, 190–196. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.; Lu, S.; Fang, D.; Wu, X.; Lu, D.; Wu, T. Effect of organic additives on positive electrolyte for vanadium redox battery. Electrochim. Acta 2011, 56, 5483–5487. [Google Scholar] [CrossRef]
- Kazacos, M.; Skyllas-Kazacos, M. High Energy Density Vanadium Electrolyte Solutions. WO Patent 96/35239, 7 November 1996. [Google Scholar]
- Skyllas-Kazacos, M.; Kazacos, M. Stabilised Electrolyte Solutions, Methods of Preparation Thereof and Redox Cells and Batteries Containing Stabilised Electrolyte Solutions. U.S. Patent 6,143,443, 7 November 2000. [Google Scholar]
- Samad, M.; Kazakos, M.; Skyllas-Kazakos, M. Stabilised Electrolyte Solutions. WO Patent 95/12219, 4 May 1992. [Google Scholar]
- Hwang, J.; Kim, B.-M.; Moon, J.; Mehmood, A.; Ha, H.Y. A highly efficient and stable organic additive for the positive electrolyte in vanadium redox flow batteries: Taurine biomolecules containing -NH2 and -SO3H functional groups. J. Mater. Chem. 2018, A6, 4695–4705. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Liu, T.; Huang, J. Improved properties of positive electrolyte for a vanadium redox flow battery by adding taurine. Res. Chem. Intermed. 2018, 44, 769–786. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, B.; Song, S.; Chen, X. Effect of Polyhydroxy-Alcohol on the Electrochemical Behavior of the Positive Electrolyte for Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2012, 159, A843–A847. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Wang, N.; Peng, S.; He, Z. Influence of organic additives on electrochemical properties of the positive electrolyte for all-vanadium redox flow battery. Electrochim. Acta 2012, 78, 475–482. [Google Scholar] [CrossRef]
- Han, H.; He, Z.; Liu, J.; Chen, Y.; Liu, S. Effects of pyridine carboxylic acid on the positive electrolyte for vanadium redox flow battery. Ionics 2015, 21, 167–174. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Xu, Y.; Yan, B.; Wang, X.; Zhu, X.; Zhang, Y.; Liu, X.; Wang, R. Several ionic organic compounds as positive electrolyte additives for a vanadium redox flow battery. RSC Adv. 2014, 4, 63025–63035. [Google Scholar] [CrossRef]
- Wu, X.-W.; Liu, S.-Q.; Huang, R.-L. Characteristics of CTAB as Electrolyte Additive for Vanadium Redox Flow Battery. J. Inorg. Mater. 2010, 25, 641–646. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Zhang, J.; Chen, J.; Zhu, S.; Liu, X.; Wang, R. Effect of different additives with -NH2 or functional groups on V(V) electrolytes for a vanadium redox flow battery. J. Electroanal. Chem. 2016, 768, 62–71. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Wang, X.; Tian, J.; Kang, H.; Zhu, X.; Zhang, Y.; Liu, X.; Wang, R. Influence of several additives on stability and electrochemical behavior of V(V) electrolyte for vanadium redox flow battery. J. Electroanal. Chem. 2013, 709, 31–38. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; He, Z.; Han, H.; Chen, Y. Effects of organic additives with oxygen- and nitrogen-containing functional groups on the negative electrolyte of vanadium redox flow battery. Electrochim. Acta 2014, 130, 314–321. [Google Scholar] [CrossRef]
- Liang, X.; Peng, S.; Lei, Y.; Gao, C.; Wang, N.; Liu, S.; Fang, D. Effect of l-glutamic acid on the positive electrolyte for all-vanadium redox flow battery. Electrochim. Acta 2013, 95, 80–86. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Han, H.; Chen, Y.; Zhou, Z.; Zheng, S.; Lu, W.; Liu, S.; He, Z. Effects of organic additives containing -NH2 and -SO3H on electrochemical properties of vanadium redox flow battery. Electrochim. Acta 2013, 106, 556–562. [Google Scholar] [CrossRef]
- Chang, F.; Hu, C.; Liu, X.; Liu, L.; Zhang, J. Coulter dispersant as positive electrolyte additive for the vanadium redox flow battery. Electrochim. Acta 2012, 60, 334–338. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Whitehead, A.; Scherer, G.G.; Wai, N.; Oo, M.O.; Bhattarai, A.; Chandra, G.P.; Xu, Z.J. The oxidation of organic additives in the positive vanadium electrolyte and its effect on the performance of vanadium redox flow battery. J. Power Sources 2016, 334, 94–103. [Google Scholar] [CrossRef]
- Li, L.Y.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.M.; Chen, B.W.; Zhang, J.L.; Xia, G.G.; Hu, J.Z.; Graff, G.; et al. A Stable Vanadium Redox-Flow Battery with High Energy Density for Energy Storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A High Energy Density Vanadium Redox Flow Battery with 3 M Vanadium Electrolyte. J. Electrochem. Soc. 2016, 163, A5023–A5028. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Peng, C.; Cheng, M. Evaluation of precipitation inhibitors for supersaturated vanadyl electrolytes for the vanadium redox battery. Electrochem. Solid State Lett. 1999, 2, 121–122. [Google Scholar] [CrossRef]
- Mousa, A.; Skyllas-Kazacos, M. Effect of Additives on the Low-Temperature Stability of Vanadium Redox Flow Battery Negative Half-Cell Electrolyte. ChemElectroChem 2015, 2, 1742–1751. [Google Scholar] [CrossRef]
- Kausar, N.; Mousa, A.; Skyllas-Kazacos, M. The Effect of Additives on the High-Temperature Stability of the Vanadium Redox Flow Battery Positive Electrolytes. ChemElectroChem 2016, 3, 276–282. [Google Scholar] [CrossRef]
- Rahman, F.; Skyllas-Kazacos, M. Evaluation of additive formulations to inhibit precipitation of positive electrolyte in vanadium battery. J. Power Sources 2017, 340, 139–149. [Google Scholar] [CrossRef]
- Park, S.-K.; Shim, J.; Yang, J.H.; Jin, C.-S.; Lee, B.S.; Lee, Y.-S.; Shin, K.-H.; Jeon, J.-D. Effect of inorganic additive sodium pyrophosphate tetrabasic on positive electrolytes for a vanadium redox flow battery. Electrochim. Acta 2014, 121, 321–327. [Google Scholar] [CrossRef]
- Ding, C.; Ni, X.; Li, X.; Xi, X.; Han, X.; Bao, X.; Zhang, H. Effects of phosphate additives on the stability of positive electrolytes for vanadium flow batteries. Electrochim. Acta 2015, 164, 307–314. [Google Scholar] [CrossRef]
- Peng, S.; Wang, N.; Gao, C.; Lei, Y.; Liang, X.; Liu, S.; Liu, Y. Influence of trishydroxymethyl aminomethane as a positive electrolyte additive on performance of vanadium redox flow battery. Int. J. Electrochem. Sci. 2012, 7, 4314–4321. [Google Scholar]
- Peng, S.; Wang, N.; Gao, C.; Lei, Y.; Liang, X.; Liu, S.; Liu, Y. Influence of trishydroxymethyl aminomethane as a positive electrolyte additive on performance of vanadium redox flow battery. Int. J. Electrochem. Sci. 2012, 7, 2440–2447. [Google Scholar]
- Kim, D.; Jeon, J. An electrolyte with high thermal stability for the vanadium redox flow battery. In Advanced Materials, Mechanical and Structural Engineering; Hong, S.H., Seo, J., Moon, K., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 371–375. [Google Scholar]
- Flox, C.; Rubio-Garcia, J.; Skoumal, M.; Vazquez-Galvan, J.; Ventosa, E.; Ramon, J. Morante Thermally Stable Positive Electrolytes with a Superior Performance in All-Vanadium Redox Flow Batteries. ChemPlusChem 2015, 80, 354–358. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, S.-Q.; Gao, C.; Liang, X.-X.; He, Z.-X.; Deng, Y.-H.; He, Z. Effect of Amino Acid Additives on the Positive Electrolyte of Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2013, 160, A722–A727. [Google Scholar] [CrossRef]
- Lim, J.W.; Lee, D.G. Carbon fiber/polyethylene bipolar plate-carbon felt electrode assembly for vanadium redox flow batteries (VRFB). Compos. Struct. 2015, 134, 483–492. [Google Scholar] [CrossRef]
- Qian, P.; Zhang, H.; Chen, J.; Wen, Y.; Luo, Q.; Liu, Z.; You, D.; Yi, B. A novel electrode-bipolar plate assembly for vanadium redox flow battery applications. J. Power Sources 2008, 175, 613–620. [Google Scholar] [CrossRef]
- Zhong, S.; Skyllas-Kazacos, M. Electrochemical behaviour of vanadium(V)/vanadium(IV) redox couple at graphite electrodes. J. Power Sources 1992, 39, 1–9. [Google Scholar] [CrossRef]
- Herrmann, J. Entwicklung und Anwendung einer elektrochemischen Methode zur Untersuchung schneller zwischengelagerter Reaktionen an Ringelektroden in turbulenter Rohrströmung. Ph.D. Thesis, Bonn University, Bonn, Germany, 1983. [Google Scholar]
- Herrmann, J.; Schmidt, H.; Vielstich, W. Electrochemical Investigations of a Fast Chemical Step between two Charge Transfer Reactions. Z. Phys. Chem. NF 1984, 139, 83–96. [Google Scholar] [CrossRef]
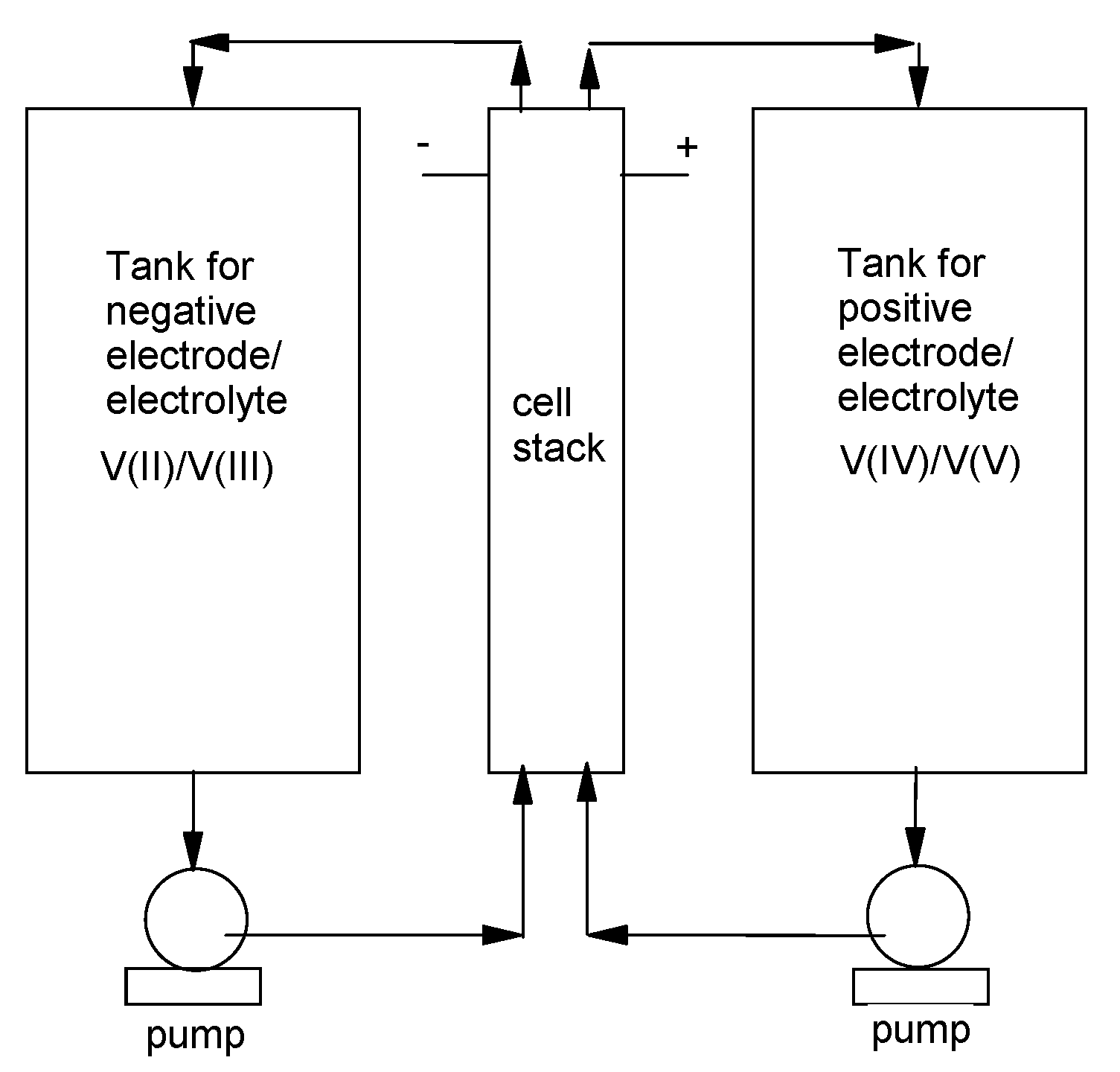

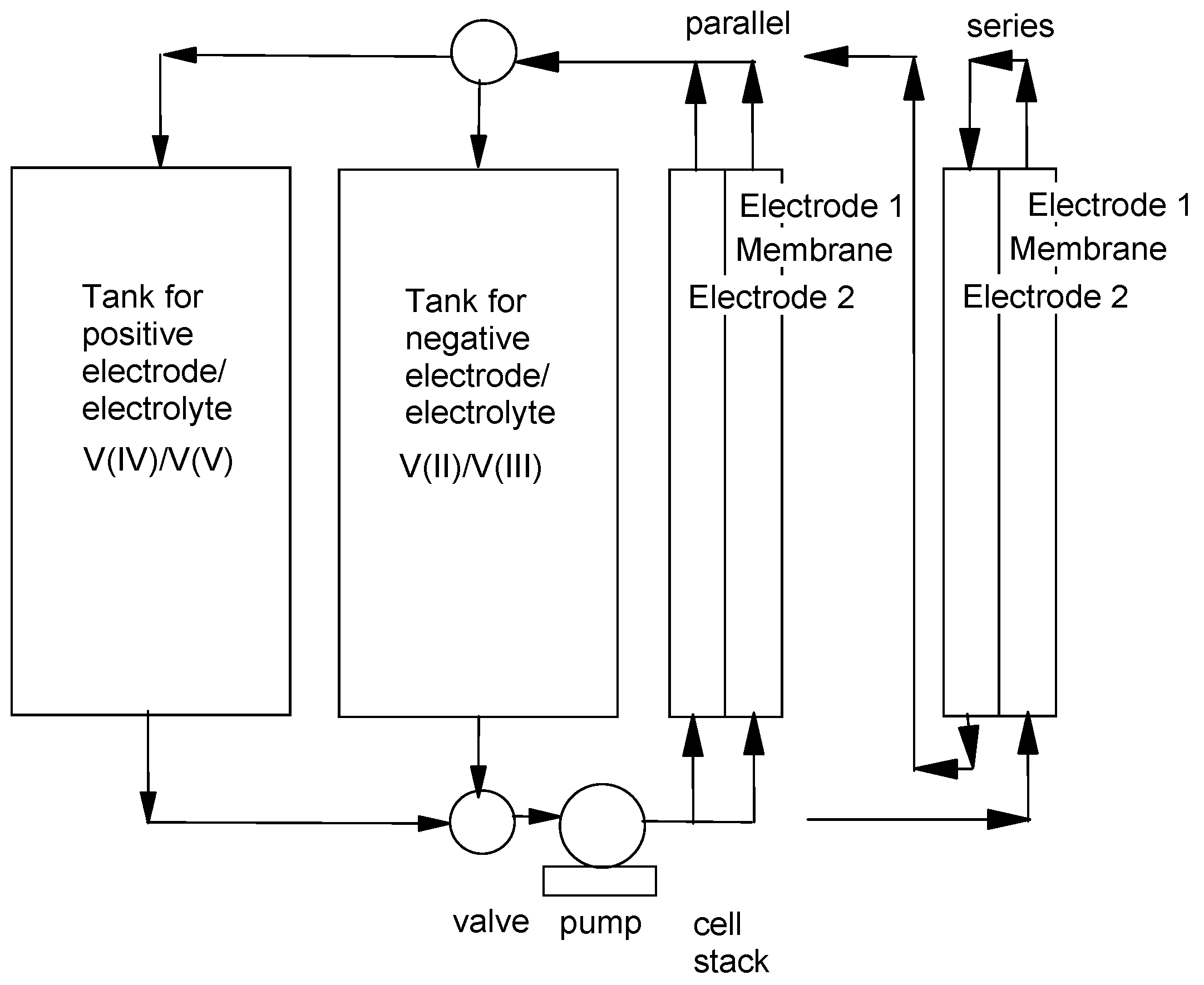
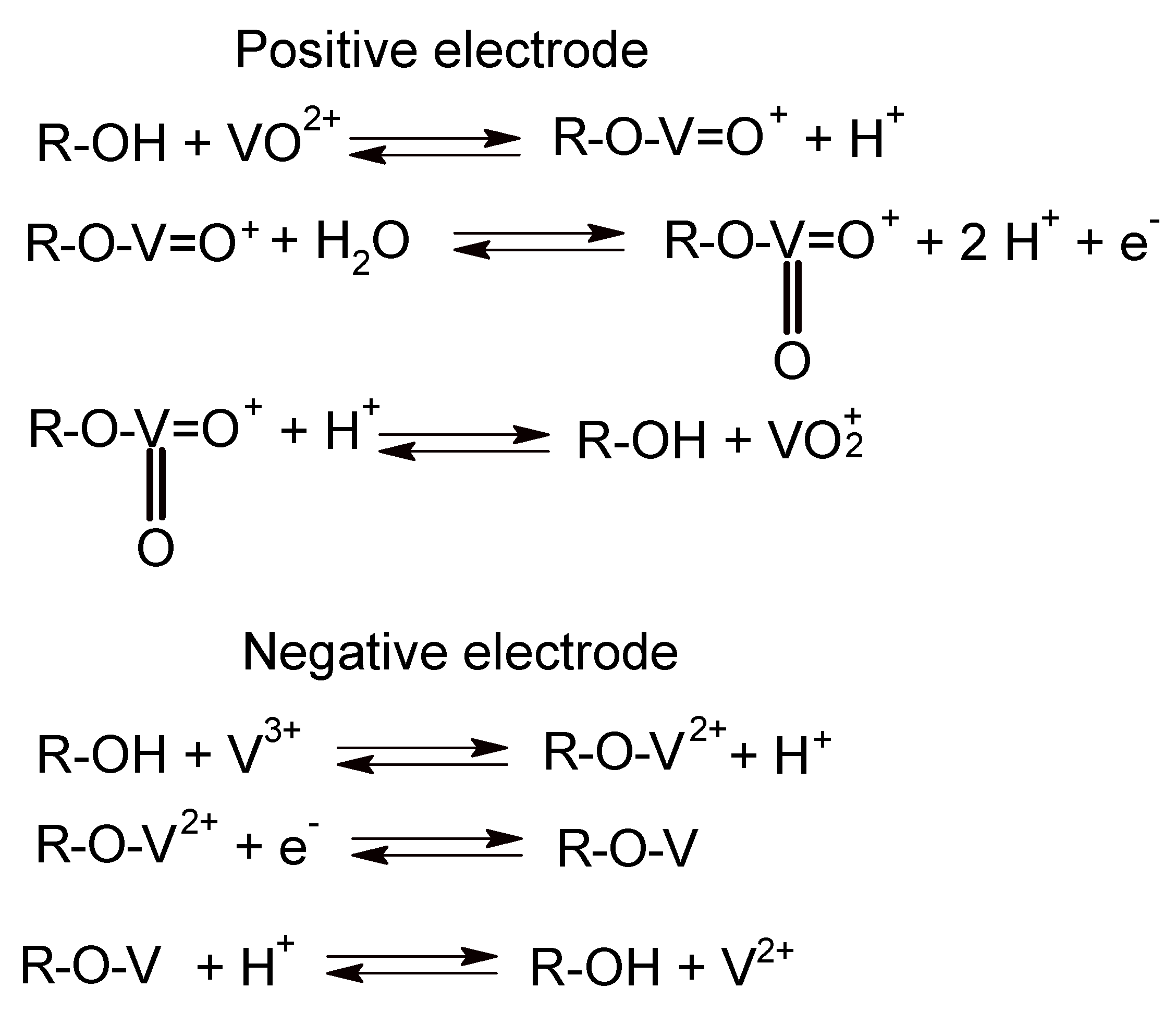
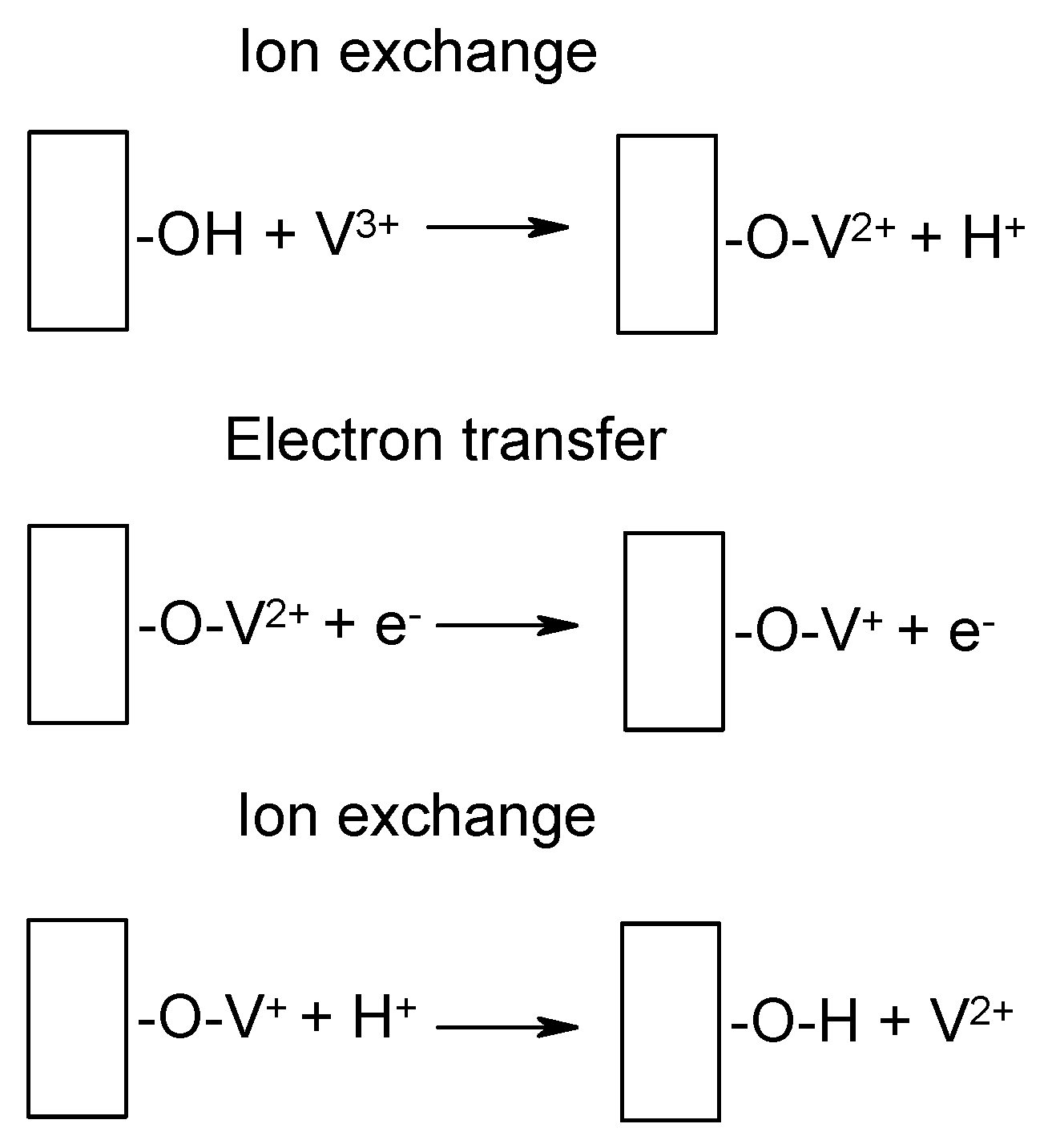
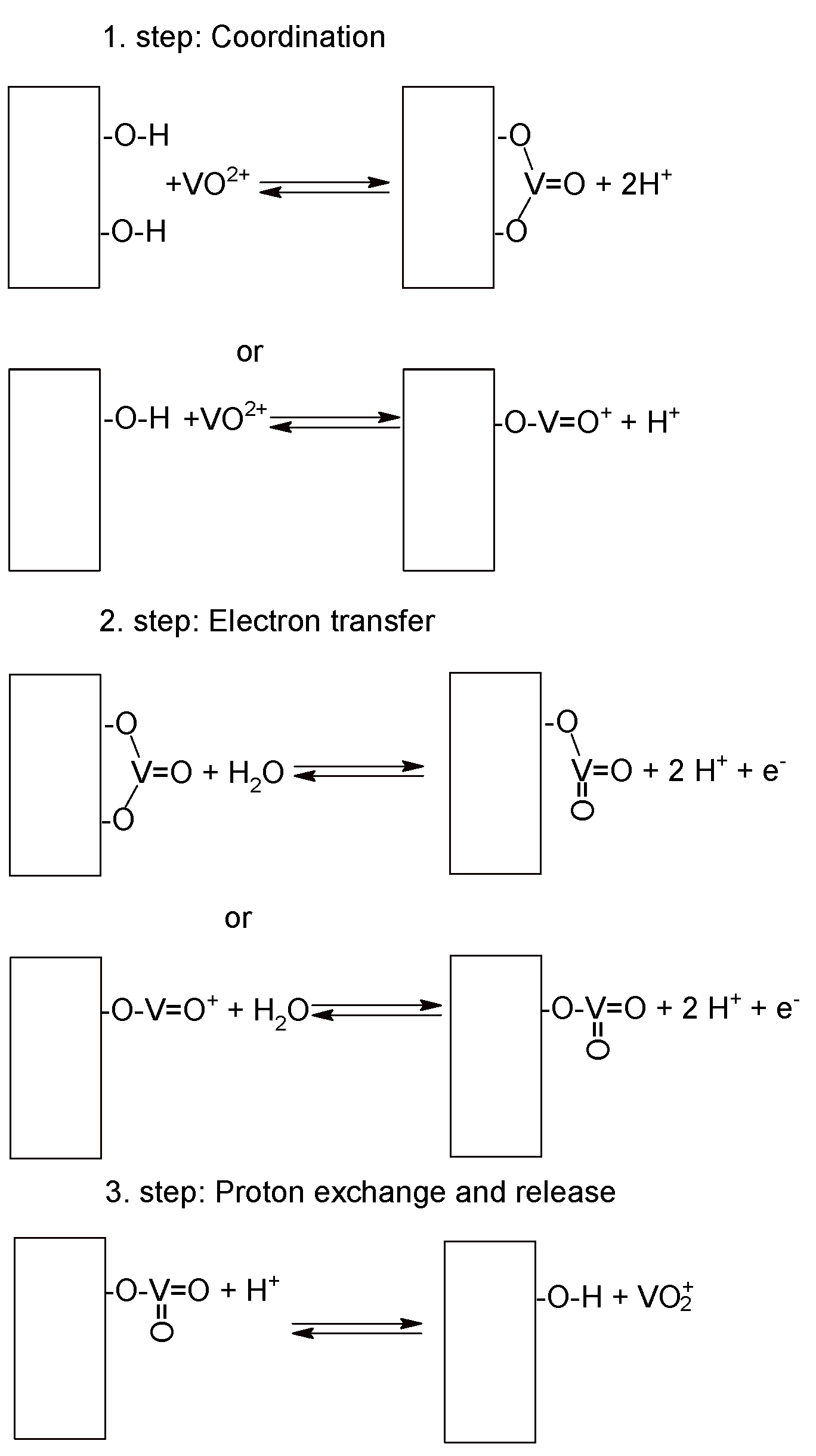
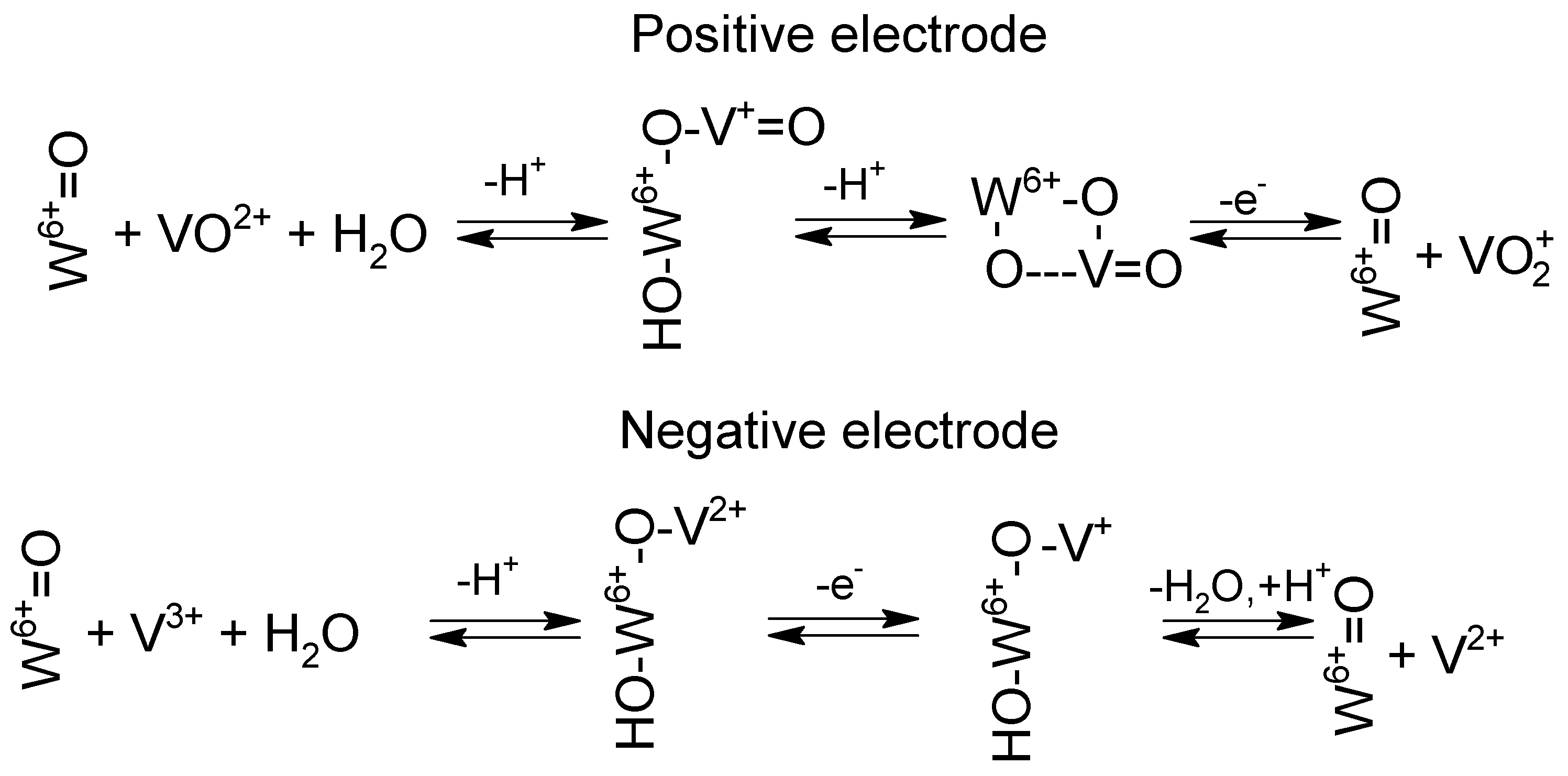
| Trade Name | Manufacturer | Generic Designation * | Properties | Studied Redox Reactions | Ref. |
|---|---|---|---|---|---|
| ATJ | Le Carbone-Lorraine | porous bulk graphite | Fe2+/3+ | [164] | |
| BW-309 | Toyobo Co., Ltd. | carbon fiber | V4+/5+, V3+/4+ | [165] | |
| GRC | - | graphite reinforcement carbon | V4+/5+, V3+/4+ | [165] | |
| GF-20 | Nippon Carbon Co., Ltd. | carbon fiber | V4+/5+, V3+/4+ | [165] | |
| JP845 | Le Carbone-Lorraine | non-porous bulk graphite | Fe2+/3+ | [164] | |
| NG | natural graphite | V4+/5+ | [166] | ||
| PG60 | Le Carbone-Lorraine | porous bulk graphite | Fe2+/3+ | [164] | |
| RVC-45PPI | Electrosynthesis Corp. | reticulated vitreous carbon | Fe2+/3+ | [164] | |
| RVC-60PPI | Electrosynthesis Corp. | reticulated vitreous carbon | Fe2+/3+ | [164] | |
| SGF | Stackpole | graphite felt | Fe2+/3+ | [164] | |
| - | - | plastic-bonded natural graphite flake | Cr2+/3+ | [164] | |
| - | - | Electrographite | Cr2+/3+, Fe2+/3+ | [167] | |
| - | - | natural graphite | Cr2+/3+, Fe2+/3+ | [166] | |
| Electrode graphite EH | Sigri | - | Fe2+/3+ | [163,168] | |
| OPG | Ringsdorff | ordinary pyrolytic graphite | Fe2+/3+ | [163,169] | |
| GC | Sigri, Metrohm | glassy carbon | electrochemically activated | Fe2+/3+ | [163,169] |
| Diabon N | Sigri | graphitized, polymer-bonded material | Fe2+/3+ | [163,169] | |
| Ridurid V1017 | Ringsdorff | non-porous material bonded with phenolic resin | Fe2+/3+ | [163,169] | |
| WCA cloth | Union Carbide | porous graphite | Cr2+/3+ | [169] | |
| ELAT carbon cloth | Fuel Cells Etc | V2+/3+, V4+/5+ | [170] | ||
| SGL 10AA | SGL Technologies | carbon paper | untreated | V2+/3+, V4+/5+ | [171] |
| TORAY carbon paper | Toray | carbon paper | V2+/3+, V4+/5+ | [126,162] | |
| Donacarbo paper, S-251 | Osaka Gas Chemicals | carbon paper | V4+/5+ | [172] | |
| Felt | Union Carbide | porous graphite | Cr2+/3+ | [169] | |
| GF-20-3 graphite felt | Nippon carbon | V4+/5+ | [173] | ||
| PAN GF-3F carbon felt | Nippon carbon | V2+/3+, V4+/5+ | [174] | ||
| GFA6 carbon felt | SGL Carbon, Germany | V2+/3+, V4+/5+ | [159] | ||
| Carbon felt + | SGL Carbon | V2+/3+, V4+/5+ | [127] | ||
| Carbon felt | Baofeng Jinshi New Materials Co. | V4+/5+ | [175] | ||
| Fiber graphite felt | Material Inc. | V2+/3+, V4+/5+ | [176] | ||
| Graphite felt | Beijing Great Wall | PAN ?-based | V4+/5+ | [177] | |
| Graphite felt | Shanghai Qijie Limited Co. | PAN-based | V4+/5+ | [178] | |
| Graphite felt | SGL Carbon | V2+/3+, V4+/5+ | [127] | ||
| Graphite felt RVG-2000 | Société Carbon-Lorraine | Rayon #-based | V4+/5+ | [179] | |
| Graphite felt | Carbone Lorraine | Rayon-based | V4+/5+ | [180] | |
| G 2225 cloth | Hitco | porous graphite | Cr2+/3+ | [169] | |
| FMI | Fibre Materials Inc. | Rayon-based | V2+/3+, V4+/5+ | [181] | |
| GFD 2 | Sigri | PAN-based | V2+/3+, V4+/5+ | [181] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Holze, R. Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries. Batteries 2018, 4, 47. https://doi.org/10.3390/batteries4030047
Wu Y, Holze R. Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries. Batteries. 2018; 4(3):47. https://doi.org/10.3390/batteries4030047
Chicago/Turabian StyleWu, Yuping, and Rudolf Holze. 2018. "Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries" Batteries 4, no. 3: 47. https://doi.org/10.3390/batteries4030047
APA StyleWu, Y., & Holze, R. (2018). Electrocatalysis at Electrodes for Vanadium Redox Flow Batteries. Batteries, 4(3), 47. https://doi.org/10.3390/batteries4030047






