Olivine Positive Electrodes for Li-Ion Batteries: Status and Perspectives
Abstract
:1. Introduction
2. LiFePO4
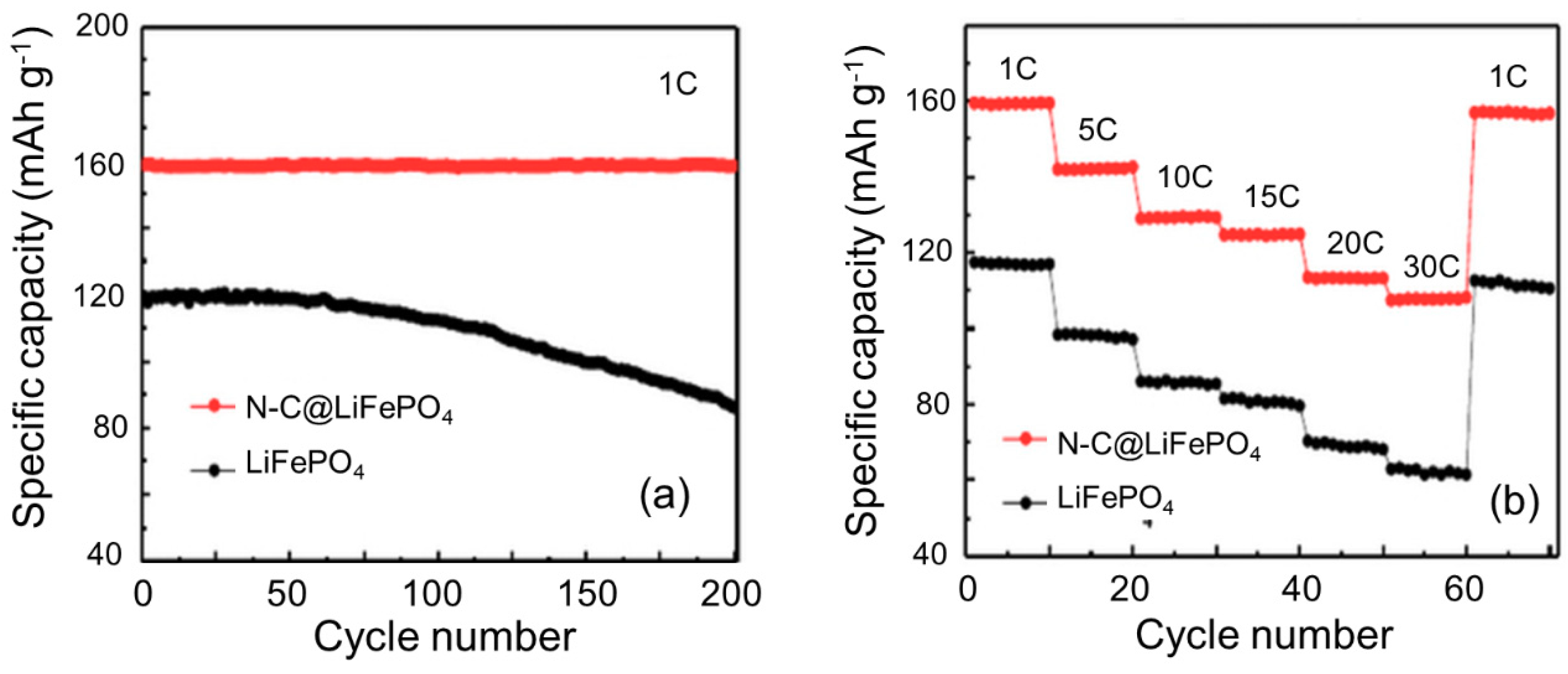
2.1. Morphology
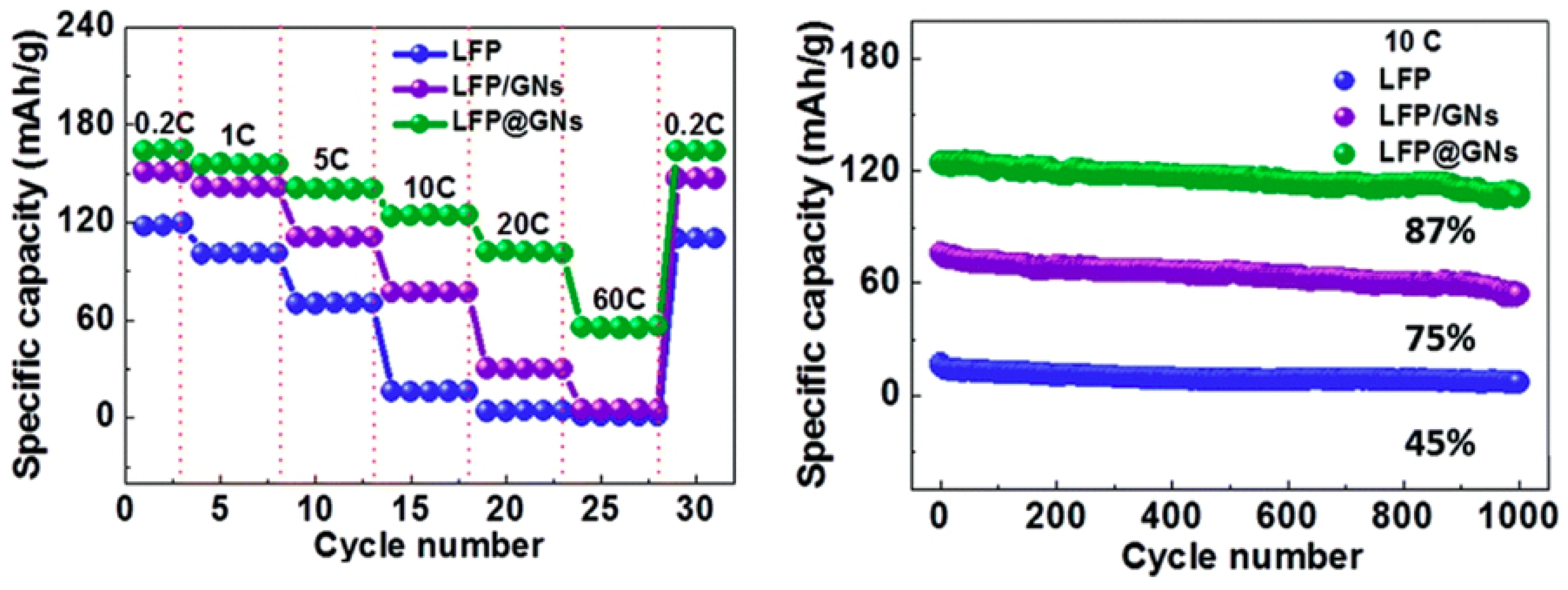
2.2. LFP/Graphene Composites
2.3. Tap Density
2.4. Doping
2.5. Impurities and Defects
2.6. Structural Changes during Cycling
2.7. Characteristic Properties of Commercial LFP
3. LiMnPO4
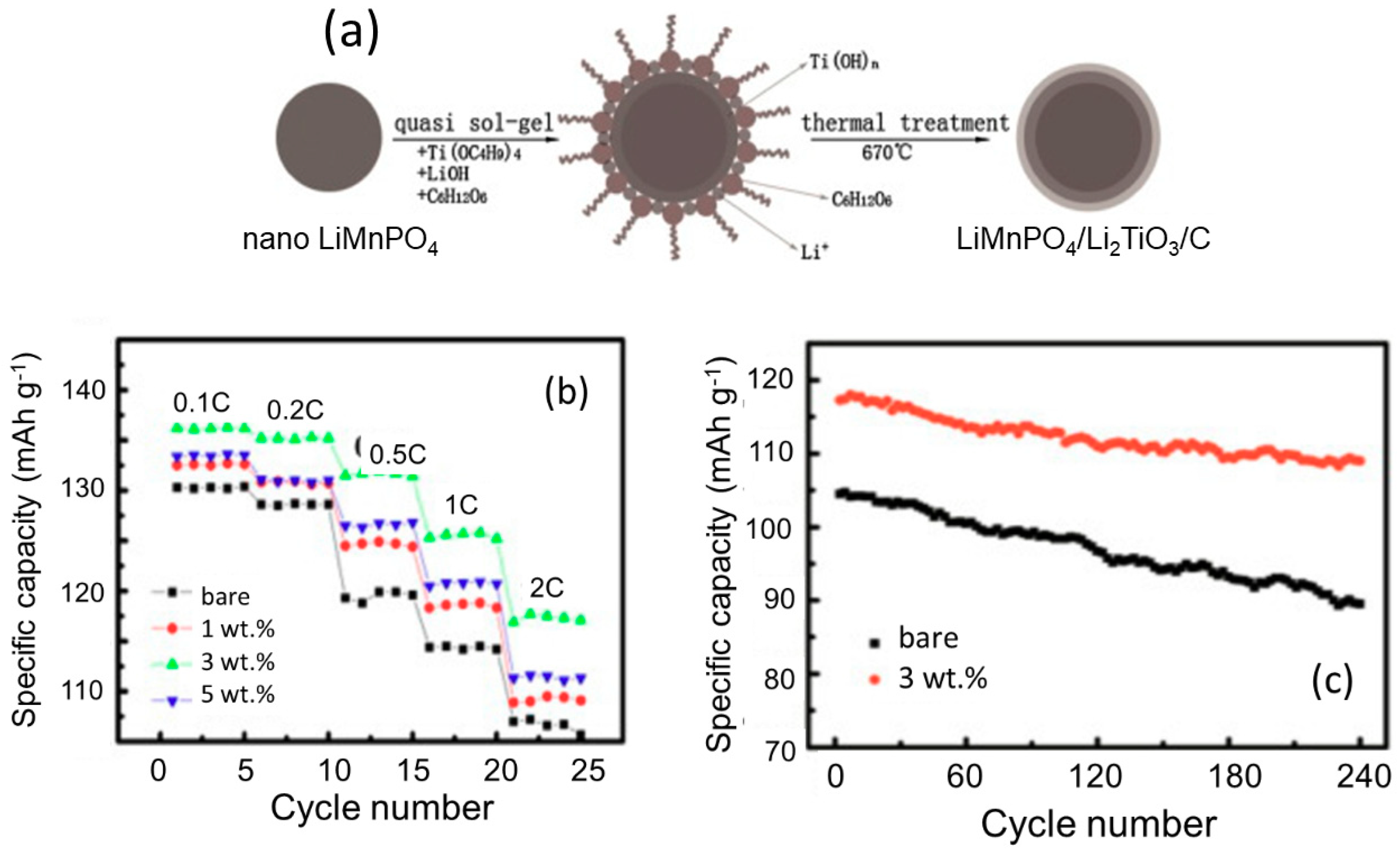
3.1. Carbon Coating
3.2. Particle Size
3.3. Cycle Life
3.4. Substitution and Doping
3.5. Graphene-LMP Composites
4. LiMn1−xFexPO4
4.1. Carbon Coating
4.2. Doping
4.3. Core-Shell Composites
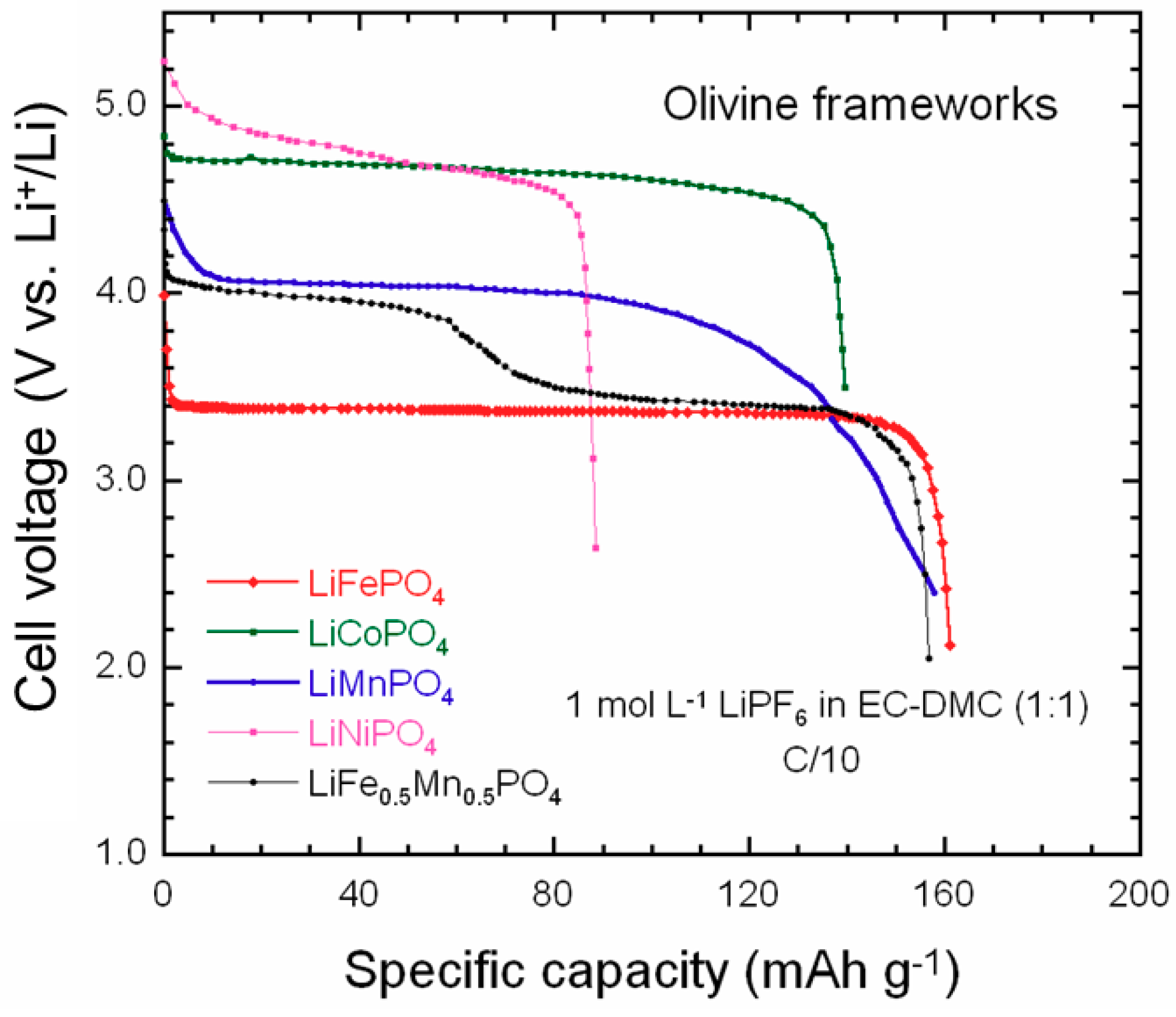
5. LiCoPO4
6. LiNiPO4
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Zaghib, K.; Mauger, A.; Julien, C.M. Olivine-based cathode materials. In Green Energy, Technology: Rechargeable Batteries Materials, Technologies and New Trends; Zhang, Z., Zhang, S.S., Eds.; Springer Science: Hoboken, NJ, USA, 2015; Chapter 3; pp. 25–65. [Google Scholar]
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.M. Critical review on lithium-ion batteries: Are they safe? Sustainable? Ionics 2017, 23, 1933–1947. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid-State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Kim, C.-S.; Zaghib, K.; Mauger, A.; Julien, C.M. Advances in lithium-sulfur batteries. Mater. Sci. Eng. R Rep. 2017, 121, 1–29. [Google Scholar]
- Mauger, A.; Julien, C.M.; Paollela, A.; Armand, M.; Zaghib, K. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R Rep. 2018; in press. [Google Scholar]
- Zaghib, K.; Mauger, A.; Julien, C.M. Rechargeable lithium batteries for energy storage in smart grids. In Rechargeable Lithium Batteries: From Fundamentals to Applications; Franco, A.A., Ed.; Woodhead Publ. Ltd.: Cambridge, UK, 2015; Chapter 12; pp. 319–351. [Google Scholar]
- Manthiram, A.; Goodenough, J.B. Lithium insertion into Fe2(SO4)3 frameworks. J. Power Sources 1989, 26, 403–408. [Google Scholar] [CrossRef]
- Muraliganth, T.; Manthiram, A. Understanding the shifts in the redox potentials of olivine LiM1−yMyPO4 (M = Fe, Mn, Co, and Mg) solid solution cathodes. J. Phys. Chem. C 2010, 114, 15530–15540. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Gong, J. A wavelet transform-adaptive unscented Kalman filter approach for state of charge estimation of LiFePO4 battery. Int. J. Energy Res. 2018, 42, 587–600. [Google Scholar] [CrossRef]
- Shen, Y. Supervised chaos genetic algorithm-based state of charge determination for LiFePO4 batteries in electric vehicles. AIP Conf. Proc. 2018, 1955, 040050. [Google Scholar]
- Trudeau, M.L.; Laul, D.; Veillette, R.; Serventi, A.M.; Zaghib, K.; Mauger, A.; Julien, C.M. In-situ High-resolution transmission electron microscopy synthesis observation of nanostructured LiFePO4. J. Power Sources 2011, 196, 7383–7394. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, K.; Zeng, H. The effect of phenol–formaldehyde resin on the electrochemical properties of carbon-coated LiFePO4 materials in pilot scale. Ionics 2015, 21, 1525–1531. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, L.; Xu, G.; Huang, J.; Fang, X.; Wang, Y.; Jin, Y.; Tang, X. Preparation of high tap density LiFePO4/C through carbothermal reduction process using beta-cyclodextrin as carbon Source. Int. J. Electrochem. Sci. 2018, 13, 2958–2968. [Google Scholar] [CrossRef]
- Bhuvaneswari, D.; Kalaiselvi, N. In situ carbon coated LiFePO4/C microrods with improved lithium intercalation behavior. Phys. Chem. Chem. Phys. 2014, 16, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, Z.; Feng, H.; Li, J. Sucrose-assisted loading of LiFePO4 nanoparticles on graphene for high-performance lithium-ion battery cathodes. Chem. Eur. J. 2013, 19, 5631–5636. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xue, Z.; Wen, S.; Ye, Y.; Xie, X. Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J. Power Sources 2016, 318, 93–112. [Google Scholar] [CrossRef]
- Bazzi, K.; Mandal, B.P.; Nazri, M.; Naik, V.M.; Garg, V.K.; Oliveira, A.C.; Vaishnava, P.P.; Nazri, G.A.; Naik, R.; Li, Q. Effect of surfactants on the electrochemical behavior of LiFePO4 cathode material for lithium ion batteries. J. Power Sources 2014, 265, 67–74. [Google Scholar] [CrossRef]
- Zheng, F.; Huang, Y.; Zhang, X.; Wu, Q.; Fu, D.; Zhang, J.; Yin, J.; Wang, H. Surfactants assisted synthesis of nano-LiFePO4/C composite as cathode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 2025–2035. [Google Scholar]
- Gong, G.; Deng, F.; Tsui, C.P.; Xue, Z.; Ye, Y.S.; Tang, C.Y.; Zhou, X.; Xie, X. PANI–PEG copolymer modified LiFePO4 as a cathode material for high-performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 19315–19323. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, J.; Zhang, J.; Yu, F.; Wang, J.; Nie, N.; Li, W. LiFePO4 nanoparticles growth with preferential (010) face modulated by Tween-80. RSC Adv. 2015, 5, 9745–9751. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Lou, J.J.; Teng, X.J.; Lu, X.X.; Liu, S.Y.; Chi, H.Z.; Ji, Z.G. Controllable synthesis of N-C@LiFePO4 nanospheres as advanced cathode of lithium ion batteries. J. Alloy. Compd. 2018, 743, 377–382. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, K.; Li, J.; Wang, X. Morphological and orientational diversity of LiFePO4 crystallites: Remarkable reaction path dependence in hydrothermal/solvothermal syntheses. CrystEngComm 2014, 16, 10112–10122. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, S.; Lu, A.; Yao, J. Immobilization of nanosized LiFePO4 spheres by 3D coralloid carbon structure with large pore volume and thin walls for high power lithium-ion batteries. J. Power Sources 2013, 229, 249–257. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, S.; Liu, Z.; Xiao, G.; Liu, Q.; Song, H. High performance LiFePO4 microsphere composed with nanofibers with an alcohol-thermal approach. J. Mater. Chem. A 2013, 1, 4546–4551. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, H.-Y.; Uchaker, E.; Zhang, M.; Liu, S.Q.; Liu, Y.-N.; Cao, G. Additive-free solvothermal synthesis and Li-ion intercalation properties of dumbbell-shaped LiFePO4/C mesocrystals. J. Power Sources 2013, 239, 103–110. [Google Scholar] [CrossRef]
- Ghafarian-Zahmatkesh, H.; Javanbakht, M.; Ghaemi, M. Ethylene-Glycol assisted hydrothermal synthesis and characterization of bow-tie-like lithium iron phosphate nanocrystals for lithium-ion batteries. J. Power Sources 2015, 284, 339–348. [Google Scholar] [CrossRef]
- Zheng, Z.; Pang, W.K.; Tang, X.; Jia, D.; Huang, Y.; Guo, Z. Solvothermal synthesis and electrochemical performance of hollow LiFePO4 nanoparticles. J. Alloy. Compd. 2015, 640, 95–100. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Shao, G.; Shi, M.; Ma, Z.; Wang, G.; Song, W.; Liu, S.; Wang, C. Controllable synthesis, morphology evolution and electrochemical properties of LiFePO4 cathode materials for Li-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 7728–7733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, B.; Wang, Y.; Wang, F. Solvothermal synthesis of LiFePO4 nanorods as high-performance cathode materials for lithium ion batteries. Ceram. Int. 2016, 42, 10297–10303. [Google Scholar] [CrossRef]
- Tian, R.; Liu, G.; Liu, H.; Zhang, L.; Gu, X.; Guo, Y.; Wang, H.; Sun, L.; Chu, W. Very high power and superior rate capability LiFePO4 nanorods hydrothermally synthesized using tetraglycol as surfactant. RSC Adv. 2015, 5, 1859–1866. [Google Scholar] [CrossRef]
- Fisher, M.G.; Hua, X.; Wilts, B.D.; Castillo-Martinez, E.; Steiner, U. Polymer-templated LiFePO4/C nanonetworks as high-performance cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Geng, Y.; Li, N.; Liu, X.; Zuo, X. Nonstoichiometric LiFePO4/C nanofibers by electrospinning as cathode materials for lithium-ion battery. Mater. Chem. Phys. 2014, 144, 226–229. [Google Scholar] [CrossRef]
- Shao, D.; Wang, J.; Dong, X.; Yu, W.; Liu, G.; Zhang, F.; Wang, L. Electrospinning fabrication and electrochemical properties of LiFePO4/C composite nanofibers. J. Mater. Sci. Mater. Electron. 2013, 24, 4263–4269. [Google Scholar] [CrossRef]
- Lin, H.Y.; Yeh, S.M.; Chen, J.S. Physical and electrochemical properties of LiFePO4/C nanofibers synthesized by electrospinning. Int. J. Electrochem. Sci. 2014, 9, 6936–6948. [Google Scholar]
- Toprakci, O.; Ji, L.; Lin, Z.; Toprakci, H.A.K.; Zhang, X. Fabrication and electrochemical characteristics of electrospun LiFePO4/carbon composite fibers for lithium-ion batteries. J. Power Sources 2011, 196, 7692–7699. [Google Scholar] [CrossRef]
- Shao, D.; Wang, J.; Dong, X.; Yu, W.; Liu, G.; Zhang, F.; Wang, L. Preparation and electrochemical performances of LiFePO4/C composite nanobelts via facile electrospinning. J. Mater. Sci. Mater. Electron. 2014, 25, 1040–1046. [Google Scholar] [CrossRef]
- Chen, L.L.; Shen, X.Q.; Jing, M.X.; Zhu, S.W.; Pi, Z.C.; Li, J.Q.; Zhai, H.A.; Xiao, K.S. Electrospun LiFePO4/C composite fiber membrane as a binder-free, self-standing cathode for power lithium-ion battery. J. Nanosci. Nano Technol. 2018, 18, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Xu, G.; Sun, X.; Zeng, H.; Zhao, R.; Yang, X.; Shen, G.; Han, G.; Zhou, S. Mono-dispersed LiFePO4@C core-shell [001] nanorods for a high power Li-ion battery cathode. J. Alloy. Compd. 2017, 708, 685–693. [Google Scholar] [CrossRef]
- Xu, G.; Li, F.; Tao, Z.H.; Wei, X.; Liu, Y.; Li, X.; Ren, Z.H.; Shen, G.; Han, G.R. Monodispersed LiFePO4@C core-shell nanostructures for a high power Li-ion battery cathode. J. Power Sources 2014, 246, 696–702. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.Y.; Wu, Q.; Shu, H.B.; Yang, X.K. Effects of Ni and Mn doping on physicochemical and electrochemical performances of LiFePO4/C. J. Alloy. Compd. 2016, 675, 187–194. [Google Scholar] [CrossRef]
- Saravanan, K.; Vittal, J.J.; Reddy, M.V.; Chowdari, B.V.R.; Balaya, P. Storage performance of Li1−xMnxPO4 nanoplates (x = 0, 0.5, and 1). J. Solid State Electrochem. 2010, 23, 1933–1947. [Google Scholar]
- Saravanan, K.; Reddy, M.V.; Balaya, P.; Gong, H.; Chowdari, B.V.R.; Vittal, J.J. Storage performance of LiFePO4 nanoplates. J. Mater. Chem. 2009, 19, 605–610. [Google Scholar] [CrossRef]
- Saravanan, K.; Balaya, P.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Morphology controlled synthesis of LiFePO4/C nanoplates for Li-ion batteries. Energy Environ. Sci. 2010, 3, 457–464. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Benedek, P.; Wenzler, N.; Yarema, M.; Wood, V.C. Low temperature hydrothermal synthesis of battery grade lithium iron phosphate. RSC Adv. 2017, 7, 17763. [Google Scholar] [CrossRef]
- Yang, J.L.; Wang, J.J.; Tang, Y.J.; Wang, D.N.; Li, X.F.; Hu, Y.H.; Li, R.Y.; Liang, G.X.; Sham, T.K.; Sun, X.L. LiFePO4-graphene as a superior cathode material for rechargeable lithium batteries: Impact of stacked graphene and unfolded graphene. Energy Environ. Sci. 2013, 6, 1521–1528. [Google Scholar] [CrossRef]
- Jegal, J.P.; Kim, K.C.; Kim, M.S.; Kim, K.B. A lithium iron phosphate/nitrogen-doped reduced graphene oxide nanocomposite as a cathode material for high-power lithium ion batteries. J. Mater. Chem. A 2014, 2, 9594–9599. [Google Scholar] [CrossRef]
- Wu, H.X.; Liu, Q.J.; Guo, S.W. Composites of graphene and LiFePO4 as cathode materials for lithium-ion battery: A mini-review. Nano-Micro Lett. 2014, 6, 316–326. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Guo, H.-F.; Yan, P. A rapid microwave heating route to synthesize graphene modified LiFePO4/C nanocomposite for rechargeable lithium-ion batteries. Ceram. Int. 2014, 40, 15801–15806. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhu, Y.; Liu, Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 3353–3358. [Google Scholar] [CrossRef]
- Shang, W.L.; Kong, L.Y.; Ji, X.W. Synthesis, characterization and electrochemical performances of LiFePO4/graphene cathode material for high power lithium-ion batteries. Solid State Sci. 2014, 38, 79–84. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Mo, R.W.; Lei, Z.Y.; Rooney, D.; Sun, K.N. Facile synthesis of nanocrystalline LiFePO4/graphene composite as cathode material for high power lithium ion batteries. Electrochim. Acta 2014, 130, 594–599. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Liu, P.; Deng, J.; Xu, B.H.; Liu, T.F.; Wang, D.L.; Zhao, X.S. Growth of LiFePO4 nanoplatelets with orientated (010) facets on graphene for fast lithium storage. Mater. Lett. 2014, 118, 137–141. [Google Scholar] [CrossRef]
- Long, Y.; Shu, Y.; Ma, X.; Ye, M. In-situ synthesizing superior high-rate LiFePO4/C nanorods embedded in graphene matrix. Electrochim. Acta 2014, 117, 105–112. [Google Scholar] [CrossRef]
- Xu, H.; Chang, J.; Sun, J.; Gao, L. Graphene-encapsulated LiFePO4 nanoparticles with high electrochemical performance for lithium ion batteries. Mater. Lett. 2012, 83, 27–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.C.; Li, P.H.; Fu, Y.B.; Ma, X.H. A simple solvothermal route to synthesize graphene-modified LiFePO4 cathode for high power lithium ion batteries. J. Power Sources 2012, 210, 47–53. [Google Scholar] [CrossRef]
- Fathollahi, F.; Javanbakht, M.; Omidvar, H.; Ghaemi, M. Improved electrochemical properties of LiFePO4/graphene cathode nanocomposite prepared by one-step hydrothermal method. J. Alloy. Compd. 2015, 627, 146–152. [Google Scholar] [CrossRef]
- Tang, Y.F.; Huang, F.Q.; Bi, H.; Liu, Z.Q.; Wan, D.-Y. Highly conductive three-dimensional graphene for enhancing the rate performance of LiFePO4 cathode. J. Power Sources 2012, 203, 130–134. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.-S.; Chen, J.-J.; Zhang, C. Synthesis and electrochemical performance of LiFePO4/graphene composites by solid-state reaction. Mater. Lett. 2012, 71, 54–56. [Google Scholar] [CrossRef]
- Wu, K.; Hu, G.; Du, K.; Peng, Z.; Cao, Y. Improved electrochemical properties of LiFePO4/graphene/carbon composite synthesized from FePO4·2H2O/graphene oxide. Ceram. Int. 2015, 41, 13867–13871. [Google Scholar] [CrossRef]
- Wang, B.; Liu, A.; Abdulla, W.A.; Wang, D.; Zhao, X.S. Desired crystal oriented LiFePO4 nanoplatelets in situ anchored on a graphene cross-linked conductive network for fast lithium storage. Nanoscale 2015, 7, 8819–8828. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon 2018, 127, 149–157. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, Y.; Tu, X.; Zhang, Z.; Du, G. Well-dispersed LiFePO4 nanoparticles anchored on a three-dimensional graphene aerogel as high-performance positive electrode materials for lithium-ion batteries. J. Power Sources 2017, 340, 40–50. [Google Scholar] [CrossRef]
- Yang, J.L.; Wang, J.J.; Wang, D.N.; Li, X.F.; Geng, D.S.; Liang, G.X.; Gauthier, M.; Li, R.Y.; Sun, X.L. 3D porous LiFePO4/graphene hybrid cathodes with enhanced performance for Li-ion batteries. J. Power Sources 2012, 208, 340–344. [Google Scholar] [CrossRef]
- Li, X.L.; Li, T.T.; Zhang, Y.L.; Zhang, X.L.; Li, H.Y.; Huang, J.M. Graphene nanoribbon-wrapping LiFePO4 by electrostatic absorbing with improved electrochemical performance for rechargeable lithium batteries. Electrochim. Acta 2014, 139, 69–75. [Google Scholar] [CrossRef]
- Wu, G.; Ran, R.; Zhao, B.T.; Sha, Y.J.; Su, C.; Zhou, Y.K.; Shao, Z.P. 3D amorphous carbon and graphene co-modified LiFePO4 composite derived from polyol process as electrode for high power lithium-ion batteries. J. Energy Chem. 2014, 23, 363–375. [Google Scholar] [CrossRef]
- Wei, X.; Guan, Y.; Zheng, X.; Zhu, Q.; Shen, J.; Qiao, N.; Zhou, S.; Xu, B. Improvement on high rate performance of LiFePO4 cathodes using graphene as a conductive agent. Appl. Surf. Sci. 2018, 440, 748–754. [Google Scholar] [CrossRef]
- Eftekhari, A. LiFePO4/C nanocomposites for lithium-ion batteries. J. Power Sources 2017, 343, 395–411. [Google Scholar] [CrossRef]
- Mei, R.; Yang, Y.; Song, X.; An, Z.; Zhang, J. Triple carbon coated LiFePO4 composite with hierarchical conductive architecture as high-performance cathode for Li-ion batteries. Electrochim. Acta 2015, 153, 523–550. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhang, H.Y.; Chen, Y.M.; Qin, G.; Lei, X.L.; Liu, L.Y. Graphene-carbon nanotubes-modified LiFePO4 cathode materials for high-performance lithium-ion batteries. Mater. Sci. Forum 2018, 913, 818–830. [Google Scholar] [CrossRef]
- Wu, R.; Xia, G.; Shen, S.; Zhu, F.; Jiang, F.; Zhang, J. In-situ growth of LiFePO4 nanocrystals on interconnected carbon nanotubes/mesoporous carbon nanosheets for high-performance lithium ion batteries. Electrochim. Acta 2015, 153, 334–342. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, M.; Cao, Y.; Ai, X.; Yang, H. Template-free hydrothermal synthesis of nanoembossed mesoporous LiFePO4 microspheres for high-performance lithium-ion batteries. J. Phys. Chem. C 2010, 114, 3477–3482. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-Y.; Kim, K.-B.; Lee, J.-W.; Kim, H.; Kim, H.; Kang, K.; Roh, K.C. Defect-free solvothermally assisted synthesis of microspherical mesoporous LiFePO4/C. RSC Adv. 2013, 3, 3421–3427. [Google Scholar] [CrossRef]
- Ni, L.; Zheng, J.; Qin, C.; Lu, Y.; Liu, P.; Wu, T.; Tang, Y.; Chen, Y. Fabrication and characteristics of spherical hierarchical LiFePO4/C cathode material by a facile method. Electrochim. Acta 2014, 147, 330–336. [Google Scholar] [CrossRef]
- Kulka, A.; Braun, A.; Huang, T.W.; Wolska, A.; Klepka, M.T.; Szewczyk, A.; Baster, D.; Zajac, W.; Swierczek, K.; Molenda, J. Evidence for Al doping in lithium sublattice of LiFePO4. Solid State Ion. 2015, 270, 33–38. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Goodenough, J.B.; Gendron, F.; Julien, C.M. Electronic, optical, and magnetic properties of LiFePO4: small magnetic polaron effects. Chem. Mater. 2007, 19, 3740–3747. [Google Scholar] [CrossRef]
- Islam, M.S.; Driscoll, D.J.; Fisher, C.A.J.; Slater, P.R. Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem. Mater. 2005, 17, 5085–5092. [Google Scholar] [CrossRef]
- Wagemaker, M.; Ellis, B.L.; Lutzenkirchen-Hecht, D.; Mulder, F.M.; Nazar, L.F. Proof of supervalent doping in olivine LiFePO4. Chem. Mater. 2008, 20, 6313–6315. [Google Scholar] [CrossRef]
- Omenya, F.; Chernova, N.A.; Upreti, S.; Zavalij, P.Y.; Nam, K.-W.; Yang, X.-Q.; Whittingham, M.S. Can vanadium be substituted into LiFePO4? Chem. Mater. 2011, 23, 4733–4740. [Google Scholar] [CrossRef]
- Harrison, K.; Bridges, C.A.; Paranthaman, M.P.; Segre, C.U.; Katsoudas, J.; Maroni, V.A.; Idrobo, J.C.; Goodenough, J.B.; Manthiram, A. Temperature dependence of aliovalent-vanadium doping in LiFePO4 cathodes. Chem. Mater. 2013, 25, 768–781. [Google Scholar] [CrossRef]
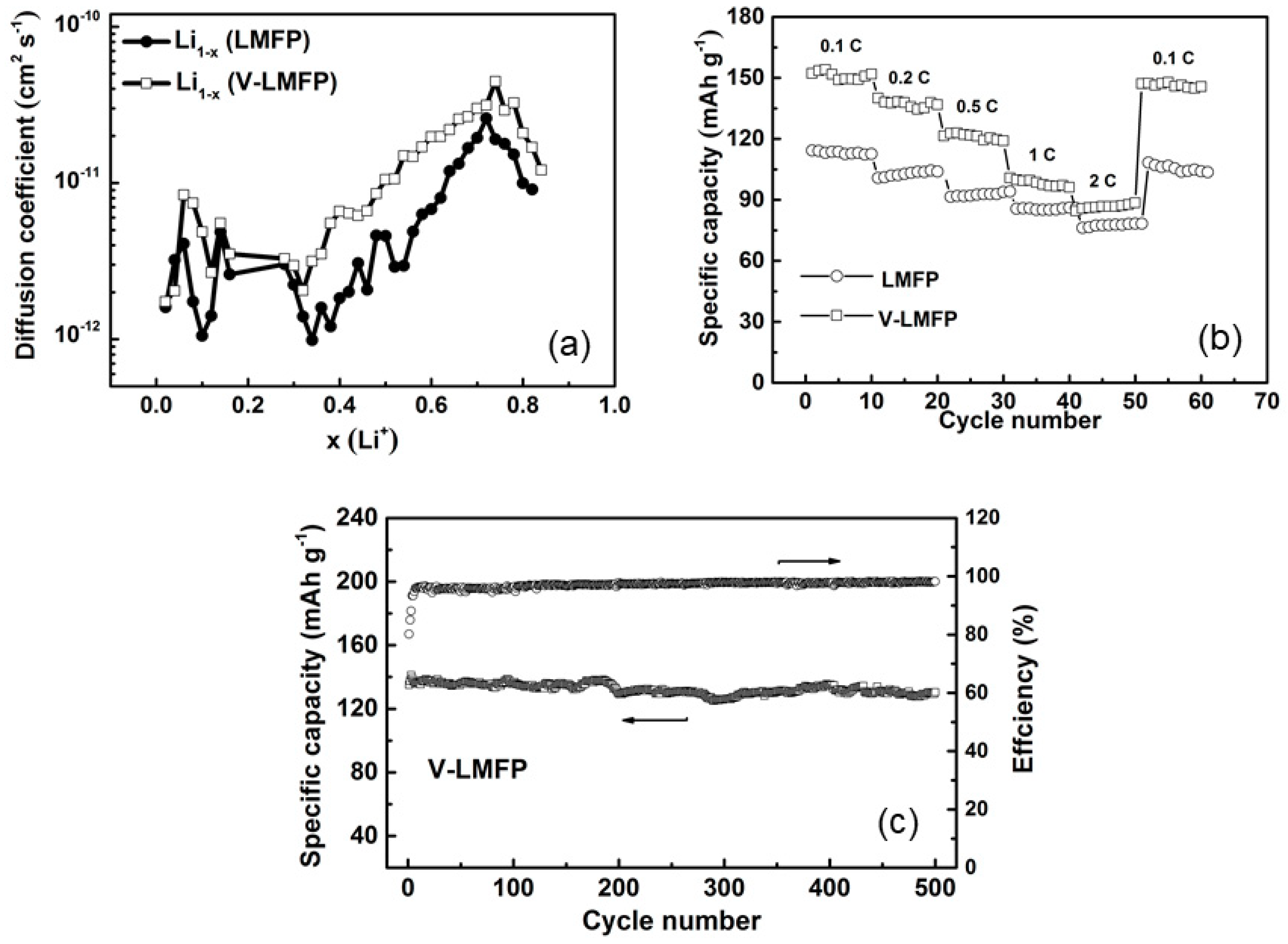
- Wu, T.; Liu, J.; Sun, L.; Cong, L.; Xie, H.; Abdel-Ghany, A.; Mauger, A.; Julien, C.M. V-insertion in Li(Fe,Mn)FePO4. J. Power Sources 2018, 383, 133–143. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Su, H.C.; Wu, P.J.; Liu, H.J.; Hu, C.W.; Sharma, N.; Peterson, V.K.; Hsieh, H.W.; Lin, Y.F.; Chou, W.C.; et al. Vanadium substitution of LiFePO4 cathode materials to enhance the capacity of LiFePO4-based lithium-ion batteries. J. Phys. Chem. C 2012, 116, 24424–24429. [Google Scholar] [CrossRef]
- Axmann, P.; Stinner, C.; Wohlfahrt-Mehrens, M.; Mauger, A.; Gendron, F.; Julien, C.M. Non-stoichiometric LiFePO4: Defects and related properties. Chem. Mater. 2009, 21, 1636–1644. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Goodenough, J.B.; Gendron, F.; Julien, C.M. Design and properties of LiFePO4 electrode materials for Li-ion batteries. In Advanced Materials and Methods for Lithium batteries; Zhang, S.S., Ed.; Research Signpost: Trivandrum, India, 2007; Chapter 6; pp. 115–149. [Google Scholar]
- Zaghib, K.; Mauger, A.; Goodenough, J.B.; Gendron, F.; Julien, C.M. Positive electrode: Lithium iron phosphate. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 264–296. [Google Scholar]
- Zhang, L.; Tang, Y.; Liu, Z.; Huang, H.; Fang, Y.; Huang, F. Synthesis of Fe2P coated LiFePO4 nanorods with enhanced Li-storage performance. J. Alloy. Compd. 2015, 627, 132–135. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Surface modifications of electrode materials for lithium-ion batteries: Status and trends. Ionics 2014, 20, 751–787. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Julien, C.M. Overview of olivines in lithium batteries for green transportation and energy storage. J. Solid State Electrochem. 2012, 16, 835–845. [Google Scholar] [CrossRef]
- Halankar, K.K.; Mandal, B.P.; Jangid, M.; Mukhopadhyay, A.; Meena, S.S.; Acharya, R.; Tyagi, A.K. Optimization of lithium content in LiFePO4 for superior electrochemical performance: The role of impurities. RSC Adv. 2018, 8, 1140–1147. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Zhou, T.; Zhao, G.; Huang, Y.; Huang, Z. Enhanced electrochemical performances of LiFePO4/C by surface modification with Sn nanoparticles. J. Power Sources 2013, 226, 20–26. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Trottier, J.; Hamel-Paquet, J.; Gariepy, V.; Galoutov, K.; Hovington, P.; Mauger, A.; Groult, H.; et al. An improved high-power battery with increased thermal operating range: C-LiFePO4//C-Li4Ti5O12. J. Power Sources 2012, 216, 192–200. [Google Scholar] [CrossRef]
- Yamada, A.; Koizumi, H.; Sonoyama, N.; Kanno, R. Phase change in LixFePO4. Electrochem. Solid-State Lett. 2005, 8, A409–A413. [Google Scholar] [CrossRef]
- Ramana, C.V.; Mauger, A.; Gendron, F.; Julien, C.M.; Zaghib, K. Study of the Li-insertion/extraction process in LiFePO4/FePO4. J. Power Sources 2009, 187, 555–564. [Google Scholar] [CrossRef]
- Bai, P.; Cogswell, D.A.; Bazant, M.Z. Suppression of phase separation in LiFePO4 nanoparticles during battery discharge. Nano Lett. 2011, 11, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Strobridge, F.C.; Borkiewicz, O.J.; Wiaderek, K.M.; Chapman, K.W.; Chupas, P.J.; Grey, C.P. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes. Science 2014, 344, 1252817. [Google Scholar] [CrossRef] [PubMed]
- Kuss, C.; Lepage, D.; Liang, G.; Schougaard, S.B. Ultrafast charging of LiFePO4 with gaseous oxidants under ambient conditions. Chem. Sci. 2013, 4, 4423–4427. [Google Scholar] [CrossRef]
- Kuss, C.; Trinh, N.D.; Andjelic, S.; Saulnier, M.; Dufresne, E.M.; Liang, G.; Schougaard, S.B. Structural transformation of LiFePO4 during ultrafast delithiation. Phys. Chem. Lett. 2017, 8, 6160–6164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Yang, F.; He, H.; Xiao, X.; Ren, Y.; Lu, W.; Stach, E.; Xie, J. Capacity fading mechanism of the commercial 18650 LiFePO4-based lithium-ion batteries: An in situ time-resolved high-energy synchrotron XRD study. ACS Appl. Mater. Interfaces 2018, 10, 4622–4629. [Google Scholar] [CrossRef] [PubMed]
- Panasonic Batteries. Available online: http://www.meircell.co.il/files/NCR18650A%20datasheet.pdf (accessed on 7 July 2018).
- Zhao, J.; Gao, Y.; Guo, J.; Chu, L.; Burke, A.F. Cycle life testing of lithium batteries: The effect of load-leveling. Int. J. Electrochem. Sci. 2018, 13, 1773–1786. [Google Scholar] [CrossRef]
- Rissouli, K.; Benkhouja, K.; Ramos-Barrado, J.R.; Julien, C.M. Electrical conductivity in lithium orthophosphates. Mater. Sci. Eng. B 2003, 98, 185–189. [Google Scholar] [CrossRef]
- Bramnik, N.N.; Ehrenberg, H. Precursor-based synthesis and electrochemical performance of LiMnPO4. J. Alloy. Compd. 2008, 464, 259–264. [Google Scholar] [CrossRef]
- Di Lecce, D.; Hu, T.; Hassoun, J. Electrochemical features of LiMnPO4 olivine prepared by sol-gel pathway. J. Alloy. Compd. 2017, 693, 730–737. [Google Scholar] [CrossRef]
- Choi, D.; Wan, D.; Bae, L.T.; Xiao, J.; Nie, Z.; Wang, W.; Viswanathan, V.V.; Lee, Y.J.; Zhang, J.G.; Graff, G.L.; et al. LiMnPO4 nanoplate grown via solid-state reaction in molten hydrocarbon for Li-ion battery cathode. Nano Lett. 2010, 10, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Oh, S.W.; Yoon, C.S.; Scrosati, B.; Amine, K.; Sun, Y.K. High-performance carbon-LiMnPO4 nanocomposite cathode for lithium batteries. Adv. Funct. Mater. 2010, 20, 3260–3265. [Google Scholar] [CrossRef]
- Fujimoto, D.; Lei, Y.; Huang, Z.-H.; Kang, F.; Kawamura, J. Synthesis and electrochemical performance of LiMnPO4 by hydrothermal method. Int. J. Electrochem. 2014, 2014, 768912. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, R.R.; Xie, Y.C.; Lv, X.Y.; Su, J.; Long, Y.F.; Wen, Y.X. Deep eutectic solvent synthesis of LiMnPO4/C nanorods as a cathode material for lithium Ion batteries. Materials 2017, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Long, Y.F.; Lv, X.Y.; Su, J.; Wen, Y.X. Microwave heating synthesis of spindle-like LiMnPO4/C in a deep eutectic solvent. Ceram. Int. 2017, 43, 6089–6095. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Wang, M.; Fang, H. Cycling stability of LiMnPO4/C composite obtained by different processing routes. Int. J. Electrochem. Sci. 2017, 12, 2909–2916. [Google Scholar] [CrossRef]
- Kwon, N.H.; Yin, H.; Vavrova, T.; Lim, J.H.-W.; Steiner, U.; Grobéty, B.; Fromm, K.M. Nanoparticle shapes of LiMnPO4, Li-diffusion orientation and diffusion coefficients for high volumetric energy Li-ion cathodes. J. Power Sources 2017, 342, 231–240. [Google Scholar] [CrossRef]
- Wang, D.; Buqa, H.; Crouzet, M.; Deghenghi, G.; Drezen, T.; Exnar, I.; Kwon, N.H.; Miners, J.; Poletto, L.; Gratzel, M. High-performance nano-structured LiMnPO4 synthesized via a polyol method. J. Power Sources 2009, 189, 624–628. [Google Scholar] [CrossRef]
- Martha, S.K.; Markovsky, B.; Grinblat, J.; Gofer, Y.; Haik, O.; Zinigrad, E.; Aurbach, D.; Drezen, T.; Wang, D.; Deghenghi, G.; et al. LiMnPO4 as an advanced cathode material for rechargeable lithium batteries. J. Electrochem. Soc. 2009, 156, A541–A552. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Z.; Wu, Z.; Su, J.; Lv, X.; Wen, Y. Microwave-assisted polyol synthesis of LiMnPO4/C and its use as a cathode material in lithium-ion batteries. Particuology 2017, 33, 42–49. [Google Scholar] [CrossRef]
- Kumar, P.R.; Venkateswarlu, M.; Misra, M.; Mohanty, A.K.; Satyanarayana, N. Carbon coated LiMnPO4 nanorods for lithium batteries. J. Electrochem. Soc. 2011, 158, A227–A230. [Google Scholar] [CrossRef]
- Pivko, M.; Bele, M.; Tchernychova, E.; Logar, Z.; Dominko, R.; Gaberscek, M. Synthesis of nanometric LiMnPO4 via a two-step technique. Chem. Mater. 2012, 24, 1041–1047. [Google Scholar] [CrossRef]
- Guo, H.; Wu, C.; Xie, J.; Zhang, S.; Cao, G.; Zhao, X. Controllable synthesis of high-performance LiMnPO4 nanocrystals by a facile one-spot solvothermal process. J. Mater. Chem. A 2014, 2, 10581–10588. [Google Scholar] [CrossRef]
- Julien, C.M.; Zaghib, K.; Mauger, A.; Massot, M.; Ait-Salah, A.; Selmane, M.; Gendron, F. Characterization of the carbon-coating of LiFePO4 by transmission electron microscopy and Raman spectroscopy. J. Appl. Phys. 2006, 100, 063511. [Google Scholar] [CrossRef]
- Ravet, N.; Gauthier, M.; Zaghib, K.; Mauger, A.; Goodenough, J.B.; Gendron, F.; Julien, C.M. Mechanism of the Fe2+ reduction at low temperature, for LiFePO4 synthesis from a polymer additive. Chem. Mater. 2007, 19, 2595–2602. [Google Scholar] [CrossRef]
- Mizuno, Y.; Kotobuki, M.; Munakata, H.; Kanamura, K. Effect of carbon source on electrochemical performance of carbon coated LiMnPO4 cathode. J. Ceram. Soc. Jpn. 2009, 117, 1225–1228. [Google Scholar] [CrossRef]
- Li, L.E.; Liu, J.; Chen, L.; Xu, H.; Yang, J.; Qian, Y. Effect of different carbon sources on the electrochemical properties of rod-like LiMnPO4/C nanocomposites. RSC Adv. 2013, 3, 6847–6852. [Google Scholar] [CrossRef]
- Fan, J.; Yu, Y.; Wang, Y.; Wu, Q.H.; Zheng, M.; Dong, Q. Nonaqueous synthesis of nano-sized LiMnPO4@C as a cathode material for high performance lithium ion batteries. Electrochim. Acta 2016, 194, 52–58. [Google Scholar] [CrossRef]
- Moon, S.; Muralidharan, P.; Kim, D.K. Carbon coating by high-energy milling and electrochemical properties of LiMnPO4 obtained in polyol process. Ceram. Int. 2012, 38, S471–S475. [Google Scholar] [CrossRef]
- Liu, J.L.; Hu, D.G.; Huang, T.; Yu, A.S. Synthesis of flower-like LiMnPO4/C with precipitated NH4MnPO4·H2O as precursor. J. Alloy. Compd. 2012, 518, 58–62. [Google Scholar] [CrossRef]
- Aono, S.; Urita, K.; Yamada, H.; Moriguchi, I. Rapid synthesis and charge‒discharge properties of LiMnPO4 nanocrystallite-embedded porous carbons. Chem. Lett. 2012, 41, 162–164. [Google Scholar] [CrossRef]
- Doan, N.L.; Taniguchi, I. Cathode performance of LiMnPO4/C nanocomposites prepared by a combination of spray pyrolysis and wet ball-milling followed by heat treatment. J. Power Sources 2011, 196, 1399–1408. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Gao, P.F.; Li, Y.N.; Wang, J.L. Morphology regulation and carbon coating of LiMnPO4 cathode material for enhanced electrochemical performance. J. Power Sources 2011, 196, 10258–10262. [Google Scholar] [CrossRef]
- Zhao, M.; Fu, Y.; Xu, N.; Li, G.; Wu, M.; Gao, X. High performance LiMnPO4/C prepared by a crystallite size control method. J. Mater. Chem. A 2014, 2, 15070–15077. [Google Scholar] [CrossRef]
- Koleva, A.; Zhecheva, E.; Stoyanova, R. A new phosphate-formate precursor method for the preparation of carbon coated nano-crystalline LiFePO4. J. Alloy. Compd. 2009, 476, 950–957. [Google Scholar] [CrossRef]
- Koleva, V.; Stoyanova, R.; Zhecheva, E. Nano-crystalline LiMnPO4 prepared by a new phosphate-formate precursor method. Mater. Chem. Phys. 2010, 121, 370–377. [Google Scholar] [CrossRef]
- Su, K.; Liu, F.; Chen, J.T. Preparation of high performance carbon-coated LiMnPO4 nanocomposite by an acetate-assisted antisolvent precipitation method. J. Power Sources 2013, 232, 234–239. [Google Scholar] [CrossRef]
- Dinh, H.C.; Mho, S.I.; Kang, Y.K.; Yeo, I.H. Large discharge capacities at high current rates for carbon-coated LiMnPO4 nanocrystalline cathodes. J. Power Sources 2013, 244, 189–195. [Google Scholar] [CrossRef]
- Tang, Y.H.; Tang, Z.; Wang, S.; Quan, W.; Zhang, Z. High-performance LiMnPO4 nanorods synthesized via a facile EG-assisted solvothermal approach. J. Mater. Chem. 2015, 3, 10267–10274. [Google Scholar]
- Zheng, X.; Huang, B.; Fan, X.; Song, G.; Lu, M. Enhanced rate performance of nano–micro structured LiFePO4/C by improved process for high-power Li-ion batteries. Electrochim. Acta 2011, 56, 4865–4868. [Google Scholar]
- Wang, Y.; He, P.; Zhou, H. LiFePO4: Development and future. Energy Environ. Sci. 2011, 4, 805–817. [Google Scholar] [CrossRef]
- Duan, J.; Cao, Y.; Jiang, J.; Du, K.; Peng, Z.; Hu, G. Novel efficient synthesis of nanosized carbon coated LiMnPO4 composite for lithium ion batteries and its electrochemical performance. J. Power Sources 2014, 268, 146–152. [Google Scholar] [CrossRef]
- Fan, C.F.; Barai, P.; Mukherjee, P.P. Diffusion induced damage and impedance response in lithium-ion battery electrodes. J. Electrochem. Soc. 2014, 161, A2138–A2152. [Google Scholar]
- Norberg, N.S.; Kostecki, R. The degradation mechanism of a composite LiMnPO4 cathode. J. Electrochem. Soc. 2012, 159, A1431–A1434. [Google Scholar] [CrossRef]
- Moskon, J.; Pivko, M.; Jerman, I.; Tchernychova, E.; Zabukovec-Logar, M.; Zorko, N.; Selih, V.S.; Dominko, R.; Gaberscek, M. Cycling stability and degradation mechanism of LiMnPO4 based electrodes. J. Power Sources 2016, 303, 97–108. [Google Scholar] [CrossRef]
- Syzdek, J.; Marcinek, M.; Kostecki, R. Electrochemical activity of carbon blacks in LiPF6-based organic electrolytes. J. Power Sources 2014, 245, 739–744. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, G.; Cao, Y.; Duan, J.; Du, K.; Peng, Z. Enhanced electrochemical performance of nano LiMnPO4 with multifunctional surface co-coating of Li2TiO3 and carbon. Solid State Ion. 2015, 283, 115–122. [Google Scholar] [CrossRef]
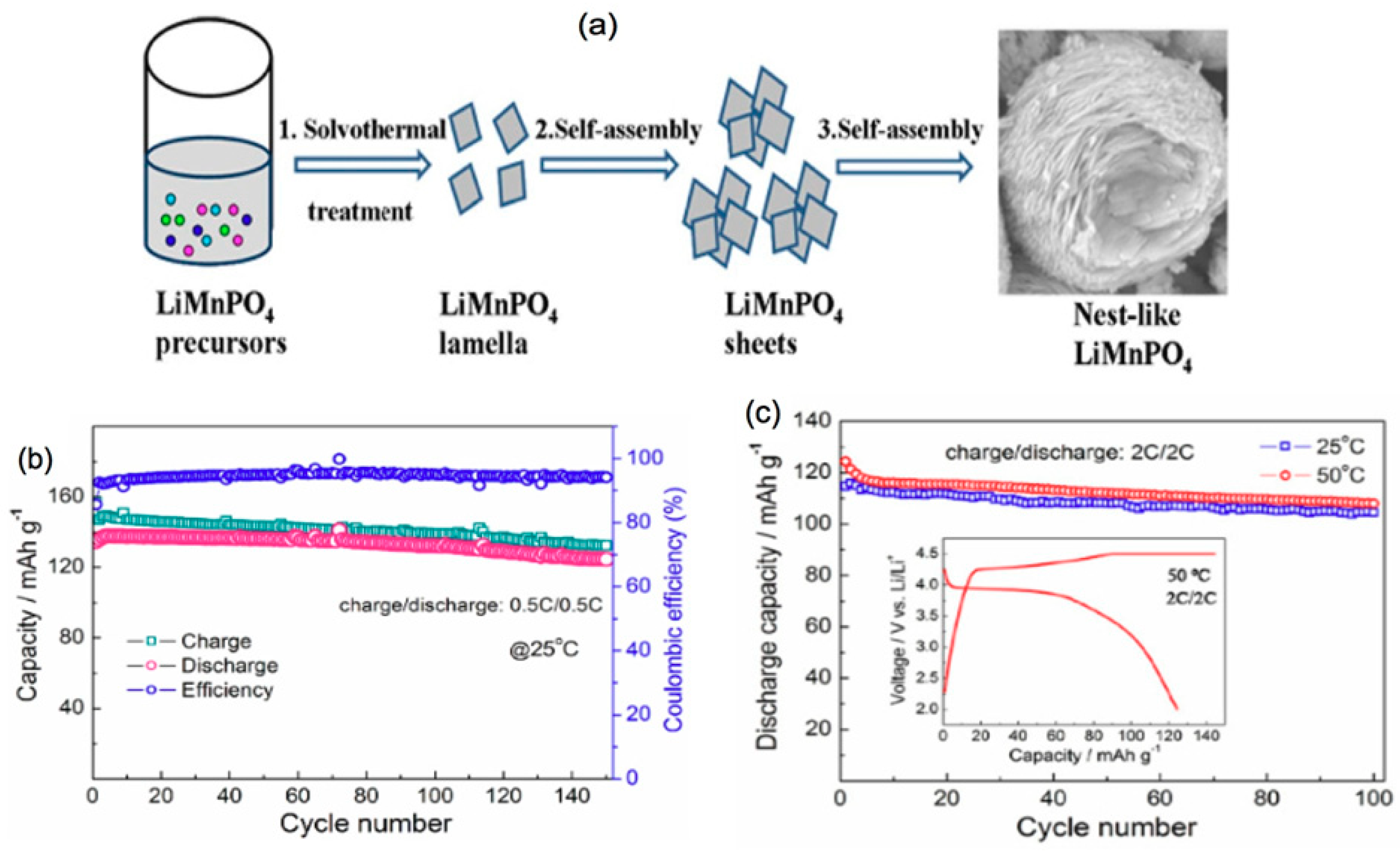
- Wang, Y.; Wang, F.; Feng, X. Porous nest-like LiMnPO4 microstructures assembled by nanosheets for lithium ion battery cathodes. J. Mater. Sci. Mater. Electron. 2018, 29, 1426–1434. [Google Scholar] [CrossRef]
- Ramar, V.; Saravanan, K.; Gajjela, S.R.; Harihran, S.; Balaya, P. The effect of synthesis parameters on the lithium storage performance of LiMnPO4/C. Electrochim. Acta 2013, 105, 496–505. [Google Scholar] [CrossRef]
- Hu, X.; Sun, X.; Yang, M.; Ji, H.; Li, X.; Cai, S.; Guo, R.; Hou, F.; Zheng, C.; Hu, W. Sandwich nanostructured LiMnPO4/C as enhanced cathode materials for lithium-ion batteries. J. Mater. Sci. 2017, 52, 3597–3612. [Google Scholar] [CrossRef]
- Fang, H.; Yi, H.; Hu, C.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Effect of Zn doping on the performance of LiMnPO4 cathode for lithium ion batteries. Electrochim. Acta 2012, 71, 266–269. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chen, Y.F.; Cheng, S.Q.; He, L.N. Improving electrochemical performance of LiMnPO4 by Zn doping using a facile solid state method. Korean J. Chem. Eng. 2011, 28, 964–968. [Google Scholar] [CrossRef]
- Shiratsuchi, T.; Okada, S.; Doi, T.; Yamaki, J.I. Cathodic performance of LiMn1−xMxPO4 (M = Ti, Mg and Zr) annealed in an inert atmosphere. Electrochim. Acta 2009, 54, 3145–3151. [Google Scholar] [CrossRef]
- Ni, J.; Gao, L. Effect of copper doping on LiMnPO4 prepared via hydrothermal route. J. Power Sources 2011, 196, 6498–6501. [Google Scholar] [CrossRef]
- Kou, L.Q.; Chen, F.J.; Tao, F.; Dong, Y.; Chen, L. High rate capability and cycle performance of Ce-doped LiMnPO4/C via an efficient solvothermal synthesis in water/diethylene glycol system. Electrochim. Acta 2015, 173, 721–727. [Google Scholar] [CrossRef]
- Verma, S.; Bamzai, K.K. Preparation of cerium orthophosphate nanosphere by coprecipitation route and its structural, thermal, optical, and electrical characterization. J. Nanopart. 2014, 2014, 125360. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, C.; Liu, J.; Bian, P.; Hao, H.; Yu, A. Enhancing the performance of LiMnPO4/C composites through Cr doping. J. Alloy. Compd. 2015, 620, 350–357. [Google Scholar] [CrossRef]
- Kellerman, D.G.; Chukalkin, Y.G.; Mukhina, N.A.; Gorshkov, V.S.; Semenova, A.S.; Teplykh, A.E. Some aspects of antiferromagnetic ordering in LiMnP0.85V0.15O4: Neutron diffraction and DC-magnetization studies. J. Magn. Magn. Mater. 2012, 324, 3181–3188. [Google Scholar] [CrossRef]
- Kellerman, D.G.; Chukalkin, Y.G.; Medvedeva, N.I.; Kuznetsov, M.V.; Mukhina, N.A.; Semenova, A.S.; Gorshkov, V.S. Hydrogen reduction of vanadium in vanadium-doped LiMnPO4. Mater. Chem. Phys. 2015, 149–150, 209–215. [Google Scholar] [CrossRef]
- Mauger, A.; Ferré, J.; Beauvillain, P. Scaling of the non-linear magnetic susceptibility in the spin glass CdMnTe. Phys. Rev. B 1989, 40, 862. [Google Scholar] [CrossRef]
- Clemens, O.; Bauer, M.; Haberkorn, R.; Springborg, M.; Philipp, H. Synthesis and characterization of vanadium-doped LiMnPO4-compounds (LiMnPO4)x(VO4)1−x (0.8 < x < 1). Chem. Mater. 2012, 24, 4717–4724. [Google Scholar]
- Hong, J.; Wang, C.S.; Chen, X.; Upretim, S.; Whittingham, M.S. Vanadium modified LiFePO4 cathode for Li-ion batteries and energy storage. Electrochem. Solid-State Lett. 2009, 12, A33–A38. [Google Scholar] [CrossRef]
- Dai, E.R.; Fang, H.S.; Yang, B.; Ma, W.H.; Dai, Y.N. Synthesis of vanadium doped LiMnPO4 by an improved solid-state method. Ceram. Int. 2015, 41, 8171–8176. [Google Scholar] [CrossRef]
- Chen, L.B.; Zhang, M.; Wei, W.F. Graphene-based composites as cathode materials for lithium-ion batteries. J. Nanomater. 2013, 2013, 940389. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.Z.; Xu, W.W.; Jiao, Z.; Wu, M.H.; Chu, Y.L.; Su, L.; Cao, H.; Hou, M.; Zhao, B. A novel graphene modified LiMnPO4 as a performance-improved cathode material for lithium-ion batteries. J. Mater. Res. 2013, 28, 2584–2589. [Google Scholar] [CrossRef]
- Zong, J.; Liu, X.J. Graphene nanoplates structured LiMnPO4/C composite for lithium-ion battery. Electrochim. Acta 2014, 116, 9–18. [Google Scholar] [CrossRef]
- Fu, X.; Chang, K.; Li, B.; Tang, H.; Shangguan, E.; Chang, Z. Low-temperature synthesis of LiMnPO4/RGO cathode material with excellent voltage platform and cycle performance. Electrochim. Acta 2017, 225, 272–282. [Google Scholar] [CrossRef]
- Kopek, M.; Yamada, A.; Kobayashi, G.; Nishimura, S.; Kanno, R.; Mauger, A.; Gendron, F.; Julien, C.M. Structural and magnetic properties of Lix(MnyFe1−y)PO4 electrode materials for Li-ion batteries. J. Power Sources 2009, 189, 1154–1163. [Google Scholar] [CrossRef]
- Oh, S.M.; Jung, H.G.; Yoon, C.S.; Myung, S.T.; Chen, Z.H.; Amine, K.; Sun, Y.K. Enhanced electrochemical performance of carbon-LiMn1−xFexPO4 nanocomposite cathode for lithium-ion batteries. J. Power Sources 2011, 196, 6924–6928. [Google Scholar] [CrossRef]
- Tan, Z.; Gao, P.; Cheng, F.; Luo, H.; Chen, J.; Zhou, H.; Tan, S. High power performance of multicomponent olivine cathode material for lithium-ion batteries. Funct. Mater. Lett. 2011, 4, 293–299. [Google Scholar] [CrossRef]
- Wang, H.L.; Yang, Y.; Liang, Y.Y.; Cui, L.F.; Casalongue, H.S.; Li, Y.G.; Hong, G.S.; Cui, Y.; Dai, H.J. LiMn1−xFexPO4 nanorods grown on graphene sheets for ultrahigh-rate-performance lithium ion batteries. Angew. Chem. Int. Ed. 2011, 50, 7364–7368. [Google Scholar] [CrossRef] [PubMed]
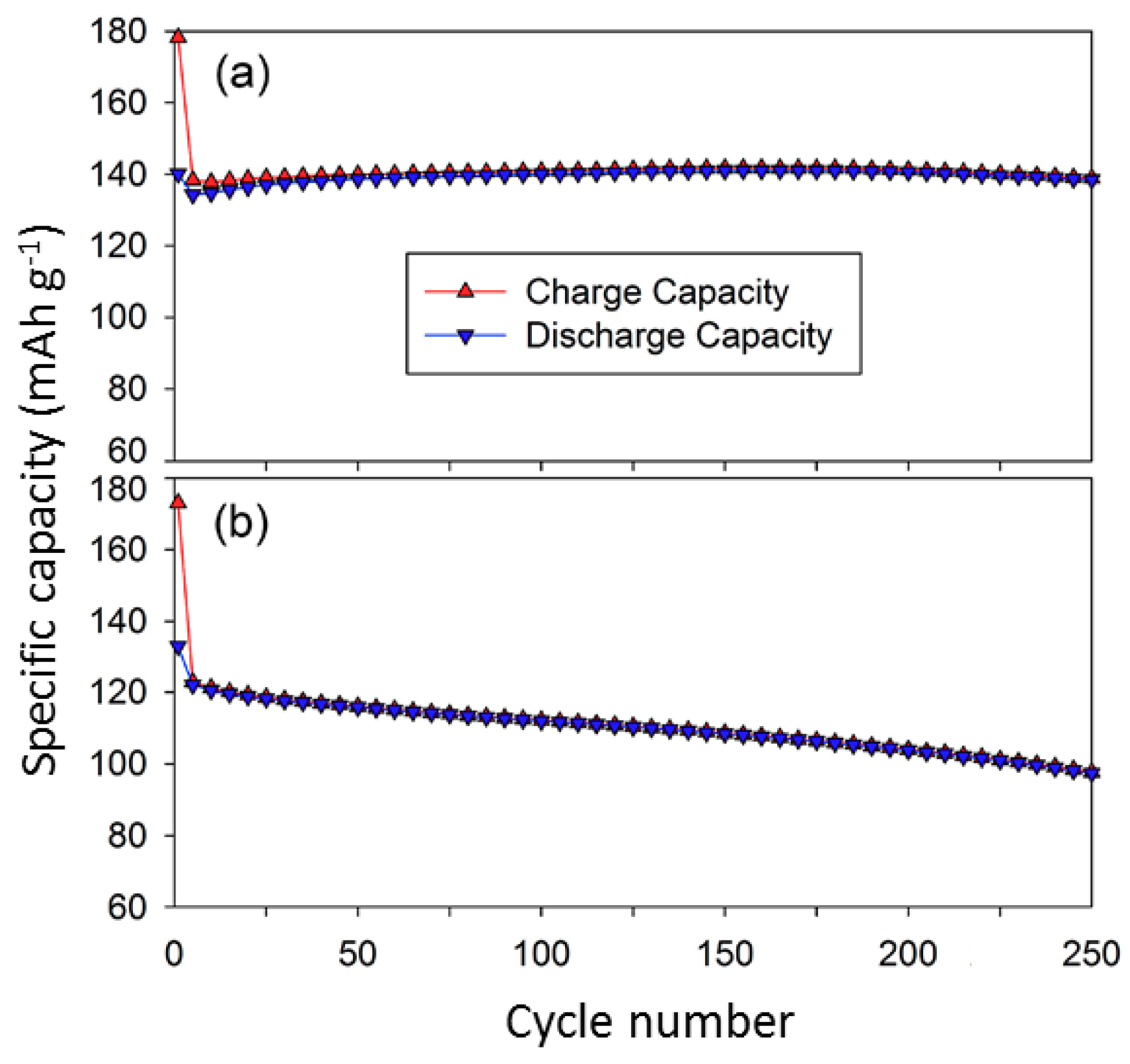
- Saravanan, K.; Ramar, V.; Balaya, P.; Vittal, J.J. Li(MnxFe1−x)PO4/C (x = 0.5, 0.75 and 1) nanoplates for lithium storage application. J. Mater. Chem. 2011, 21, 14925–14935. [Google Scholar] [CrossRef]
- Von Hagen, R.; Lorrmann, H.; Moller, K.C.; Mathur, S. Electrospun LiFe1−yMnyPO4/C nanofiber composites as self-supporting cathodes in Li-ion batteries. Adv. Energy Mater. 2012, 2, 553–559. [Google Scholar] [CrossRef]
- Hong, Y.; Tang, Z.; Hong, Z.; Zhang, Z. LiMn1−xFexPO4 (x = 0, 0.1, 0.2) nanorods synthesized by a facile solvothermal approach as high performance cathode materials for lithium-ion batteries. J. Power Sources 2014, 248, 655–659. [Google Scholar] [CrossRef]
- Hu, L.; Qiu, B.; Xia, Y.G.; Qin, Z.H.; Qin, L.F.; Zhou, X.F.; Liu, Z.P. Solvothermal synthesis of Fe-doping LiMnPO4 nanomaterials for Li-ion batteries. J. Power Sources 2014, 248, 246–252. [Google Scholar] [CrossRef]
- Seo, I.; Senthilkumar, B.; Kim, K.H.; Kim, J.K.; Kim, Y.; Ahn, J.H. Atomic structural and electrochemical impact of Fe substitution on nano porous LiMnPO4. J. Power Sources 2016, 320, 59–67. [Google Scholar] [CrossRef]
- Jo, M.; Yoo, H.; Jung, Y.S.; Cho, J. Carbon-coated nanoclustered LiMn0.71Fe0.29PO4 cathode for lithium-ion batteries. J. Power Sources 2012, 216, 162–168. [Google Scholar] [CrossRef]
- Wang, K.; Hou, M.Y.; Yuan, S.Y.; Yu, H.C.; Wang, Y.G.; Wang, C.X.; Xia, Y.Y. An additional discharge plateau of Mn3+ in LiFe0.5Mn0.5PO4 at high current rates. Electrochem. Commun. 2015, 55, 6–9. [Google Scholar] [CrossRef]
- Yan, S.Y.; Wang, C.Y.; Gu, R.M.; Sun, S.; Li, M.W. Synergetic Fe substitution and carbon connection in LiMn1−xFexPO4/C cathode materials for enhanced electrochemical performances. J. Alloy. Compd. 2015, 628, 471–479. [Google Scholar] [CrossRef]
- Yan, S.Y.; Wang, C.Y.; Gu, R.M.; Li, M.W. Enhanced kinetic behaviors of LiFe0.5Mn0.5PO4/C cathode material by Fe substitution and carbon coating. J. Solid State Electrochem. 2015, 19, 2943–2950. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, E.H.; Chen, M.Z.; Shen, H.H.; Chou, S.L.; Chen, H.; Guo, X.D.; Zhong, B.H.; Wang, X.L. Hierarchical structured LiFe0.5Mn0.5PO4 spheres synthesized by template-engaged reaction as cathodes for high power Li-ion batteries. Electrochim. Acta 2015, 178, 353–360. [Google Scholar] [CrossRef]
- Liu, W.; Gao, P.; Mi, Y.; Chen, J.; Zhou, H.; Zhang, X. Fabrication of high tap density LiFe0.6Mn0.4PO4/C microspheres by a double carbon coating-spray drying method for high rate lithium ion batteries. J. Mater. Chem. A 2013, 1, 2411–2417. [Google Scholar] [CrossRef]
- Nie, P.; Shen, L.F.; Zhang, F.; Chen, L.; Deng, H.F.; Zhang, X.G. Flower-like LiMnPO4 hierarchical microstructures assembled from single-crystalline nanosheets for lithium-ion batteries. CrystEngComm 2012, 14, 4284–4288. [Google Scholar] [CrossRef]
- Pan, X.L.; Xu, C.Y.; Zhen, L. Synthesis of LiMnPO4 microspheres assembled by plates, wedges and prisms with different crystallographic orientations and their electrochemical performance. CrystEngComm 2012, 14, 6412–6418. [Google Scholar] [CrossRef]
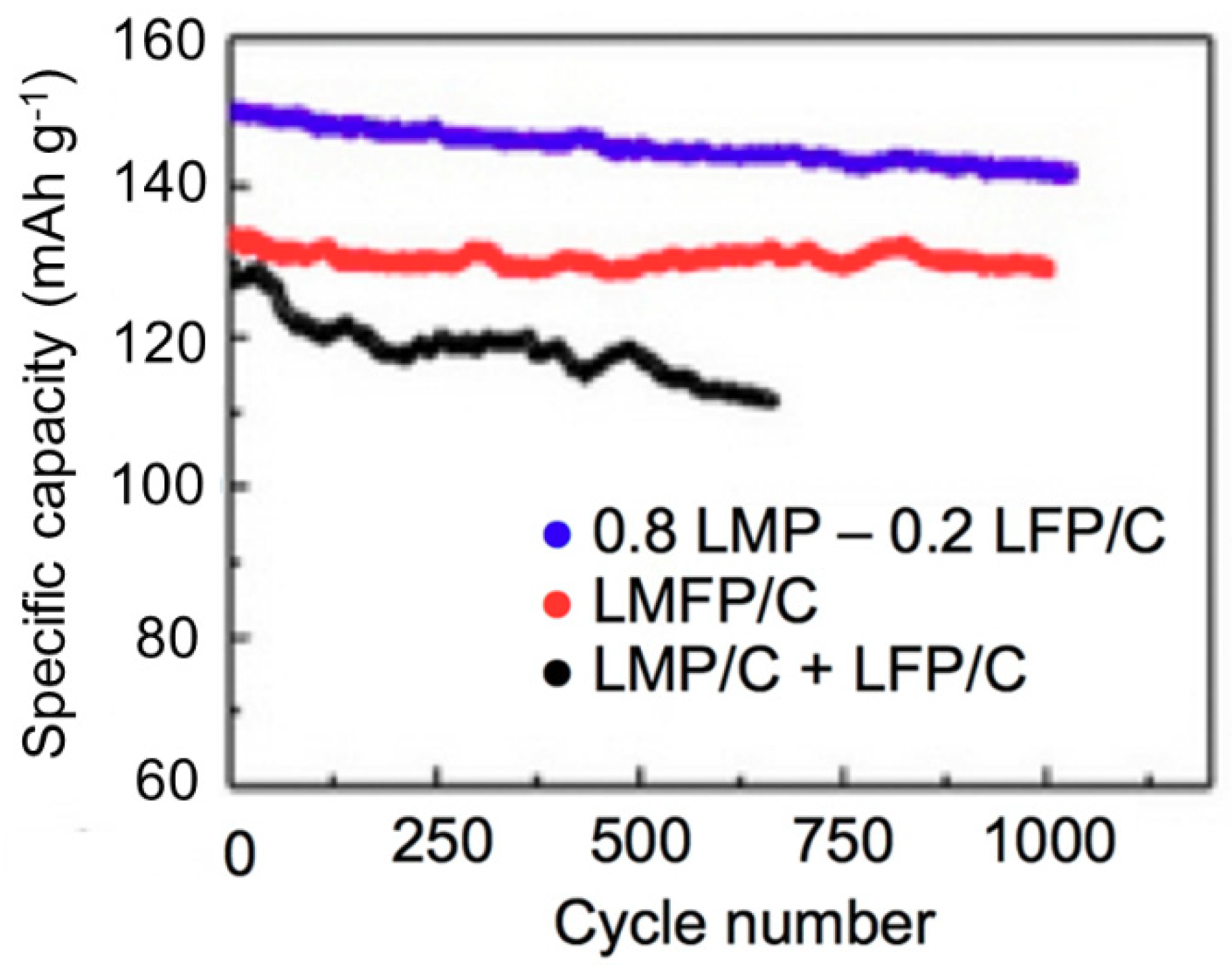
- Oh, S.M.; Myung, S.T.; Park, J.B.; Scrosati, B.; Amine, K.; Sun, Y.K. Double-structured LiMn0.85Fe0.15PO4 coordinated with LiFePO4 for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2012, 51, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, G.; Du, B.; Cui, Y.; Ke, X.; Liu, J.; Guo, Z.; Shi, Z.; Zhang, H.; Chou, S. Nano-sized cathode material LiMn0.6Fe0.4PO4/C synthesized via improved sol-gel routine and its magnetic and electrochemical properties. Electrochim. Acta 2017, 255, 205–211. [Google Scholar] [CrossRef]
- Yang, L.; Xia, Y.; Fan, X.; Qin, L.; Qiu, B.; Liu, Z. Constructing durable carbon layer on LiMn0.8Fe0.2PO4 with superior long-term cycling performance for lithium-ion battery. Electrochim. Acta 2016, 191, 200–206. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, Y.; Deng, Y.; Qin, X.; Chen, G. The enhanced rate performance of LiMn0.5Fe0.5PO4/C cathode material via synergistic strategies of surfactant-assisted solid state method and carbon coating. J. Mater. Chem. A 2015, 3, 996–1004. [Google Scholar] [CrossRef]
- Mi, Y.; Gao, P.; Liu, W.; Zhang, W.; Zhou, H. Carbon nanotube-loaded mesoporous LiFe0.6Mn0.4PO4/C microspheres as high performance cathodes for lithium-ion batteries. J. Power Sources 2014, 267, 459–468. [Google Scholar] [CrossRef]
- Duan, J.; Hu, G.; Cao, Y.; Du, K.; Peng, Z. Synthesis of high-performance Fe-Mg co-doped LiMnPO4/C via a mechano-chemical liquid-phase activation technique. Ionics 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Liu, S.; Fang, H.; Dai, E.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Effect of carbon content on properties of LiMn0.8Fe0.19Mg0.01PO4/C composite cathode for lithium ion batteries. Electrochim. Acta 2014, 116, 97–102. [Google Scholar] [CrossRef]
- Liu, S.; Fang, H.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Improving rate performance of LiMnPO4 based material by forming electron-conducting iron phosphides. J. Power Sources 2013, 230, 267–270. [Google Scholar] [CrossRef]
- Kisu, K.; Iwama, E.; Onishi, W.; Nakashima, S.; Naoi, W.; Naoi, K. Ultrafast nano-spherical single-crystalline LiMn0.792Fe0.198Mg0.010PO4 solid-solution confined among unbundled interstices of SGCNTs. J. Mater. Chem. A 2014, 2, 20789–20798. [Google Scholar] [CrossRef]
- Ramar, V.; Balaya, P. Enhancing the electrochemical kinetics of high voltage olivine LiMnPO4 by isovalent co-doping. Phys. Chem. Chem. Phys. 2013, 15, 17240–17249. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Palanisamy, K.; Yoon, J.; Kim, Y.; Yoon, W.S. Crystal and local structure studies of LiFe0.48Mn0.48Mg0.04PO4 cathode material for lithium rechargeable batteries. J. Power Sources 2013, 244, 581–585. [Google Scholar] [CrossRef]
- Yi, H.; Hu, C.; Fang, H.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Electrochemical performance of LiMn0.9Fe0.09Mg0.01PO4/C synthesized under vacuum condition. Int. J. Electrochem. Sci. 2012, 7, 663–670. [Google Scholar]
- Fang, H.; Dai, E.; Yang, B.; Yao, Y.; Ma, W. LiMn0.8Fe0.19Mg0.01PO4/C as a high performance cathode material for lithium ion batteries. J. Power Sources 2012, 204, 193–196. [Google Scholar] [CrossRef]
- Yi, H.; Hu, C.; Fang, H.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Optimized electrochemical performance of LiMn0.9Fe0.1−xMgxPO4/C for lithium ion batteries. Electrochim. Acta 2011, 56, 4052–4057. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.U.; Seo, D.H.; Kim, J.; Kim, S.W.; Kang, K. Mg and Fe Co-doped Mn based olivine cathode material for high power capability. J. Electrochem. Soc. 2011, 158, A250–A254. [Google Scholar] [CrossRef]
- Hu, C.; Yi, H.; Fang, H.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Improving the electrochemical activity of LiMnPO4 via Mn-site co-substitution with Fe and Mg. Electrochem. Commun. 2010, 12, 1784–1787. [Google Scholar] [CrossRef]
- Sronsri, C.; Noisong, P.; Danvirutai, C. Synthesis, characterization and vibrational spectroscopic study of Co, Mg co-doped LiMnPO4. Spectrochim. Acta A 2016, 153, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhong, Y.; Tang, Y.; Shen, H.; Wang, E.; Liu, H.; Zhong, B.; Guo, X. Improving the electrochemical kinetics of lithium manganese phosphate via co-substitution with iron and cobalt. J. Alloy. Compd. 2015, 635, 180–187. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Wu, Z.; Su, J.; Long, Y.F.; Lv, X.Y.; Wen, Y.X. Synthesis and electrochemical performance of Ti-Fe co-doped LiMnPO4/C as cathode material for lithium-ion batteries. Ceram. Int. 2016, 42, 11348–11354. [Google Scholar] [CrossRef]
- Jung, Y.H.; Park, W.B.; Pyo, M.; Sohn, K.S.; Ahn, D. A multi-element doping design for a high performance LiMnPO4 cathode via metaheuristic computation. J. Mater. Chem A 2017, 5, 8939–8945. [Google Scholar] [CrossRef]
- Qin, L.; Xia, Y.; Cao, H.; Yang, L.; Liu, Z. Synthesis and electrochemical performance of LiMnxFey(V□)1−x−yPO4 cathode materials for lithium-ion batteries. Electrochim. Acta 2016, 222, 1660–1667. [Google Scholar] [CrossRef]
- Di Lecce, D.; Hassoun, J. Lithium transport properties in LiMn1−xFexPO4 olivine cathodes. J. Phys. Chem. C 2015, 119, 20855–20863. [Google Scholar] [CrossRef]
- Zaghib, K.; Trudeau, M.; Guerfi, A.; Trottier, J.; Mauger, A.; Veillette, R.; Julien, C.M. New advanced cathode material: LiMnPO4 encapsulated with LiFePO4. J. Power Sources 2012, 204, 177–181. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Bi, Y.; Liu, M.; Yang, W.; Peng, Z.; Wang, D. Improvement of electrochemical activity of LiMnPO4-based cathode by surface iron enrichment. J. Power Sources 2017, 341, 175–182. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Fu, Y.Q. Density functional theory study of lithium diffusion at the interface between olivine-type LiFePO4 and LiMnPO4. J. Phys. D Appl. Phys. 2016, 49, 505601. [Google Scholar] [CrossRef]
- Guorong, H.; Yanbing, C.; Zhongdong, P.; Ke, D.; Zhijian, Z.; Min, L. Method for modifying nanometer LiMnPO4/C cathode material coated with lithium ion conductor. Chinese Patent CN 201510845237, 26 November 2015. [Google Scholar]
- Zhang, B.; Wang, X.W.; Zhang, J.F. Novel synthesis of LiMnPO4@Li3V2(PO4)3/C composite cathode material. RSC Adv. 2014, 4, 49123–49127. [Google Scholar] [CrossRef]
- Strobridge, F.C.; Clément, R.J.; Leskes, M.; Middlemiss, D.S.; Borkiewicz, O.J.; Wiaderek, K.M.; Chapman, K.W.; Chupas, P.J.; Grey, C.P. Identifying the structure of the intermediate, Li2/3CoPO4, formed during electrochemical cycling of LiCoPO4. Chem. Mater. 2014, 26, 6193–6205. [Google Scholar] [CrossRef] [PubMed]
- Mauger, A.; Julien, C.M.; Armand, M.; Goodenough, J.B.; Zaghib, K. Li(Ni,Co)PO4 as cathode materials for lithium batteries: Will the dream come true? Curr. Opin. Electrochem. 2017, 6, 63–69. [Google Scholar] [CrossRef]
- Markevich, E.; Sharabi, R.; Gottlieb, H.; Borgel, V.; Fridman, K.; Salitra, G.; Aurbach, D.; Semrau, G.; Schmidt, M.A.; Schall, N.; et al. Reasons for capacity fading of LiCoPO4 cathodes in LiPF6 containing electrolyte solutions. Electrochem. Commun. 2012, 15, 22–25. [Google Scholar] [CrossRef]
- Wu, B.R.; Xu, H.L.; Mu, D.B.; Shi, L.L.; Jiang, B.; Gai, L.; Wang, L.; Liu, Q.; Ben, L.B.; Wu, F. Controlled solvothermal synthesis and electrochemical performance of LiCoPO4 submicron single crystals as a cathode material for lithium ion batteries. J. Power Sources 2016, 304, 181–188. [Google Scholar] [CrossRef]
- Truong, Q.D.; Devaraju, M.K.; Ganbe, Y.; Tomai, T.; Honma, I. Controlling the shape of LiCoPO4 nanocrystals by supercritical fluid process for enhanced energy storage properties. Sci. Rep. 2014, 4, 3975–3983. [Google Scholar] [CrossRef] [PubMed]
- Tussupbayev, R.; Taniguchi, I. Physical and electrochemical properties of LiCoPO4/C nanocomposites prepared by a combination of emulsion drip combustion and wet ball-milling followed by heat treatment. J. Power Sources 2013, 236, 276–284. [Google Scholar] [CrossRef]
- Ludwig, J.; Nordlund, D.; Doeff, M.M.; Nilges, T. Synthesis and characterization of metastable, 20 nm-sized Pna21-LiCoPO4 nanospheres. J. Solid State Chem. 2017, 248, 9–17. [Google Scholar] [CrossRef]
- Maeyoshi, Y.; Miyamoto, S.; Noda, Y.; Munakata, H.; Kanamura, K. Effect of organic additives on characteristics of carbon-coated LiCoPO4 synthesized by hydrothermal method. J. Power Sources 2017, 337, 92–99. [Google Scholar] [CrossRef]
- Ornek, A. A new and effective approach to 4.8 V cathode synthesis with superior electrochemical qualities for lithium-ion applications. J. Alloy. Compd. 2017, 710, 809–818. [Google Scholar] [CrossRef]
- Laszczynski, N.; Birrozzi, A.; Maranski, K.; Copley, M.; Schuster, M.E.; Passerini, S. Effect of coatings on the green electrode processing and cycling behaviour of LiCoPO4. J. Mater. Chem. A 2016, 4, 17121–17128. [Google Scholar] [CrossRef]
- Ni, J.F.; Gao, L.J.; Lu, L. Carbon coated lithium cobalt phosphate for Li-ion batteries: Comparison of three coating techniques. J. Power Sources 2013, 221, 35–41. [Google Scholar] [CrossRef]
- Prabu, M.; Selvasekarapandian, S.; Reddy, M.V.; Chowdari, B.V.R. Impedance studies on the 5-V cathode material, LiCoPO4. J. Solid State Electrochem. 2012, 16, 1833–1839. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Li, Y.N.; Wang, J. Novel hedgehog-like 5 V LiCoPO4 positive electrode material for rechargeable lithium battery. J. Power Sources 2011, 196, 4806–4810. [Google Scholar] [CrossRef]
- Yamada, Y.; Noda, Y.; Munakata, H.; Yoshida, S.; Shibata, D.; Kanamura, K. Investigation of carbon-coating effect on the electrochemical performance of LiCoPO4 single particle. Electrochemistry 2018, 86, 145–151. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Yu, Z.; Ming, H.; Li, M.; Zhang, S.; Yang, Y. AlF3-modified LiCoPO4 for an advanced cathode towards high energy lithium-ion battery. Ceram. Int. 2018, 44, 1312–1320. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, Y.; Wang, G.; Chen, Y.; Liu, X. Synthesis of nanosphere-like LiCoPO4 with excellent electrochemical performance via micro reactor assisted co-precipitation method. Funct. Mater. Lett. 2018. [Google Scholar] [CrossRef]
- Kreder, K.J.; Manthiram, A. Vanadium-substituted LiCoPO4 core with a monolithic LiFePO4 shell for high-voltage lithium-ion batteries. ACS Energy Lett. 2017, 2, 64–69. [Google Scholar] [CrossRef]
- Jang, I.C.; Son, C.G.; Yang, S.M.G.; Lee, J.W.; Cho, A.R.; Aravindan, V.; Park, G.J.; Kang, K.S.; Kim, W.S.; Cho, W.I.; et al. LiFePO4 modified Li1.02(Co0.9Fe0.1)0.98PO4 cathodes with improved lithium storage properties. J. Mater. Chem. 2011, 21, 6510–6515. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podgornova, O.A.; Devyatkina, E.T.; Podugolnikov, V.R.; Petrov, S.A. Effect of Fe2+ substitution on the structure and electrochemistry of LiCoPO4 prepared by mechanochemically assisted carbothermal reduction. J. Mater. Chem. A 2014, 2, 20697–20705. [Google Scholar] [CrossRef]
- Allen, J.L.; Thompson, T.; Delp, S.A.; Wolfenstine, J.; Jow, T.R. Cr and Si substituted-LiCo0.9Fe0.1PO4: Structure, full and half Li-ion cell performance. J. Power Sources 2016, 327, 229–234. [Google Scholar] [CrossRef]
- Hanafusa, R.; Oka, Y.; Nakamura, T. Electrochemical and magnetic studies of Li-deficient Li1−xCo1−xFexPO4 olivine cathode compounds. J. Electrochem. Soc. 2014, 162, A3045–A3051. [Google Scholar] [CrossRef]
- Kang, Y.M.; Kim, Y.I.; Oh, M.W.; Yin, R.Z.; Lee, Y.; Han, D.W.; Kwon, H.S.; Kim, J.H.; Ramanath, G. Structurally stabilized olivine lithium phosphate cathodes with enhanced electrochemical properties through Fe doping. Energy Environ. Sci. 2011, 4, 4978–4983. [Google Scholar] [CrossRef]
- Brutti, S.; Manzi, J.; Meggiolaro, D.; Vitucci, F.M.; Trequattrini, F.; Paolone, A.; Palumbo, O. Interplay between local structure and transport properties in iron-doped LiCoPO4 olivines. J. Mater. Chem. A 2017, 5, 14020–14030. [Google Scholar] [CrossRef]
- Ni, J.F.; Han, Y.; Liu, J.; Wang, H.; Gao, L. Improving electrochemical properties of LiCoPO4 by Mn substitution: A case research on LiCo0.5Mn0.5PO4. ECS Electrochem. Lett. 2012, 2, A3–A5. [Google Scholar] [CrossRef]
- Allen, J.L.; Thompson, T.; Sakamoto, J.; Becker, C.R.; Jow, T.R.; Wolfenstine, J. Transport properties of LiCoPO4 and Fe-substituted LiCoPO4. J. Power Sources 2014, 254, 204–208. [Google Scholar] [CrossRef]
- Dimesso, L.; Spanheimer, C.; Jaegermann, W. Influence of isovalent ions (Ca and Mg) on the properties of LiCo0.9M0.1PO4 powders. J. Power Sources 2013, 243, 668–675. [Google Scholar] [CrossRef]
- Dimesso, L.; Spanheimer, C.; Jaegermann, W. Investigation of the LiCo1−xMgxPO4 (0 ≤ x ≤ 0.1) system. J. Alloy. Compd. 2014, 582, 69–74. [Google Scholar] [CrossRef]
- Dimesso, L.; Spanheimer, C.; Mueller, M.M.; Kleebe, H.J.; Jaegermann, W. Properties of Ca-containing LiCoPO4-graphitic carbon foam composites. Ionics 2015, 21, 2101–2107. [Google Scholar] [CrossRef]
- Kreder, K.J.; Assat, G.; Manthiram, A. Aliovalent substitution of V3+ for Co2+ in LiCoPO4 by a low-temperature microwave-assisted solvothermal process. Chem. Mater. 2016, 28, 1847–1853. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Yang, X.; Liu, L.; Chen, L.; Wei, J. Improved electrochemical performance of 5 V LiCoPO4 cathode materials via yttrium doping. Solid State Ion. 2014, 255, 84–88. [Google Scholar] [CrossRef]
- Wolfenstine, J. Electrical conductivity of doped LiCoPO4. J. Power Sources 2006, 158, 1431–1435. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Qiu, J.; Yu, Z.; Ming, H.; Li, M.; Zhang, S.; Yang, Y. Cr-substituted LiCoPO4 core with a conductive carbon layer towards high-voltage lithium-ion batteries. J. Solid State Chem. 2018, 258, 32–41. [Google Scholar] [CrossRef]
- Amin, R.; Lin, C.; Peng, J.; Weichert, K.; Acartürk, T.; Starke, U.; Maier, J. Silicon-doped LiFePO4 single crystals: Growth, conductivity behavior, and diffusivity. Adv. Funct. Mater. 2009, 19, 1697–1704. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Kim, C.; Cho, M.; Guerfi, A.; Delp, S.A.; Allen, J.L.; Jow, T.R.; Zaghib, K. High-energy lithium-ion battery using substituted LiCoPO4: From coin type to 1 Ah cell. J. Power Sources 2018, 388, 52–56. [Google Scholar] [CrossRef]
- Cherkashinin, G.; Eilhardt, R.; Lebedev, M.V.; Nappini, S.; Magnano, E.; Jaegermann, W. Olivine-LiNiPO4 thin films: Chemical compatibility with liquid electrolyte and interface stability at high potential. J. Electrochem. Soc. 2018, 165, H3143–H3147. [Google Scholar] [CrossRef]
- Örnek, A. Positive effects of a particular type of microwave-assisted methodology on the electrochemical properties of olivine LiMPO4 (M = Fe, Co and Ni) cathode materials. Chem. Eng. J. 2018, 331, 501–509. [Google Scholar] [CrossRef]
- Devi, L.S.; Babu, K.V.; Madhavilatha, B.; Reddi, M.S.; Samatha, K.; Veeraiah, V. Structural and electrochemical characterizations of nanostructured olivine LiNi1−xCoxPO4 (x = 0 and 0.5) cathode materials for lithium-ion batteries. S. Afr. J. Chem. Eng. 2018, 25, 42–47. [Google Scholar]
- Karthikprabhu, S.; Karuppasamy, K.; Vikraman, D.; Prasanna, K.; Maiyalagan, T.; Nichelson, A.; Kathalingam, A.; Kim, H.-S. Electrochemical performances of LiNi1−xMnxPO4 (x = 0.05–0.2) olivine cathode materials for high voltage rechargeable lithium ion batteries. Appl. Surf. Sci. 2018, 449, 435–444. [Google Scholar] [CrossRef]
| Cathode | Density (g cm−3) | Specific Capacity (mAh g−1) | Specific Energy (Wh g−1) | Energy Density (kWh L−1) |
|---|---|---|---|---|
| LiFePO4 | 3.60 | 169 | 0.59 | 2.10 |
| LiFePO4 + 5%C | 3.48 | 159 | 0.56 | 1.95 |
| LiMn2O4 | 4.3 | 148 | 0.56 | 2.40 |
| LiNi0.8Co0.15Al0.05O2 | 4.85 | 274 | 0.98 | 4.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, A.; Julien, C.M. Olivine Positive Electrodes for Li-Ion Batteries: Status and Perspectives. Batteries 2018, 4, 39. https://doi.org/10.3390/batteries4030039
Mauger A, Julien CM. Olivine Positive Electrodes for Li-Ion Batteries: Status and Perspectives. Batteries. 2018; 4(3):39. https://doi.org/10.3390/batteries4030039
Chicago/Turabian StyleMauger, Alain, and Christian M. Julien. 2018. "Olivine Positive Electrodes for Li-Ion Batteries: Status and Perspectives" Batteries 4, no. 3: 39. https://doi.org/10.3390/batteries4030039
APA StyleMauger, A., & Julien, C. M. (2018). Olivine Positive Electrodes for Li-Ion Batteries: Status and Perspectives. Batteries, 4(3), 39. https://doi.org/10.3390/batteries4030039






