Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries
Abstract
1. Introduction
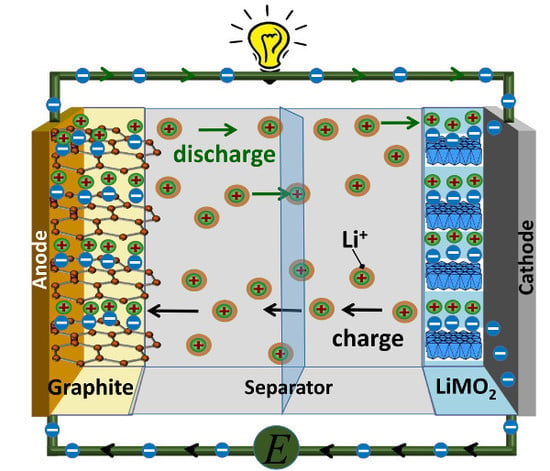
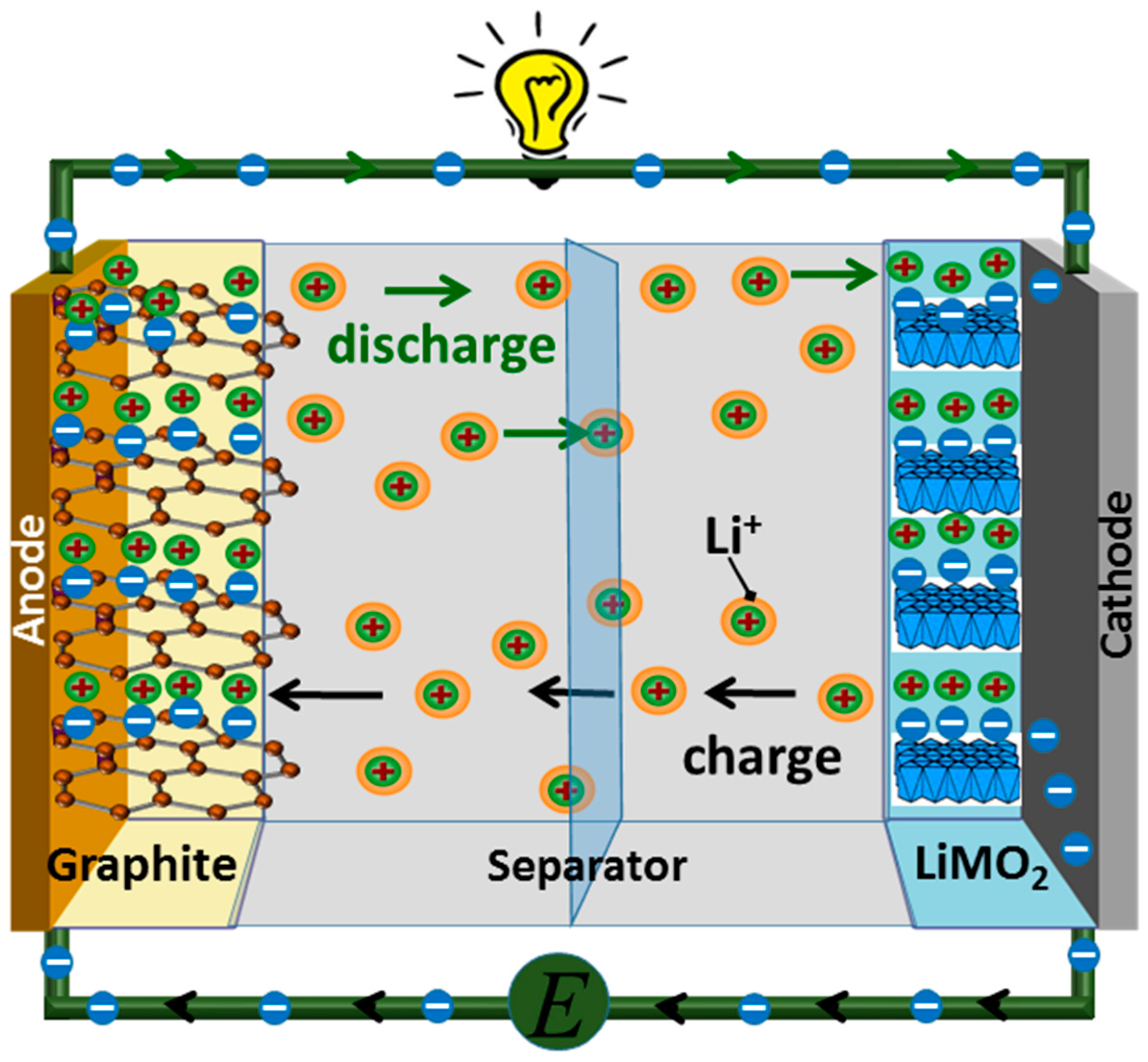
2. Lithium Ion Battery System
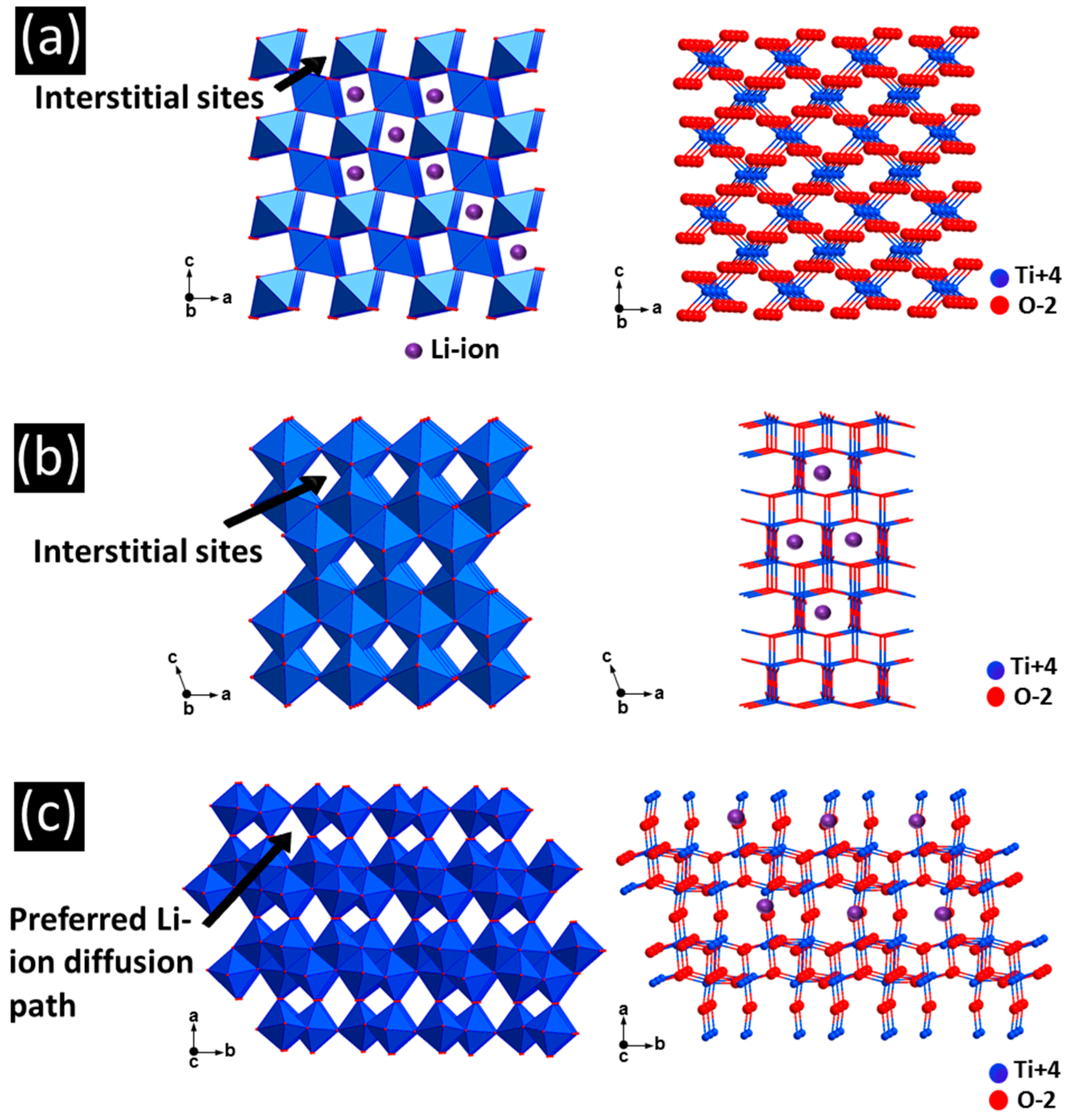
3. TiO2 Anodes
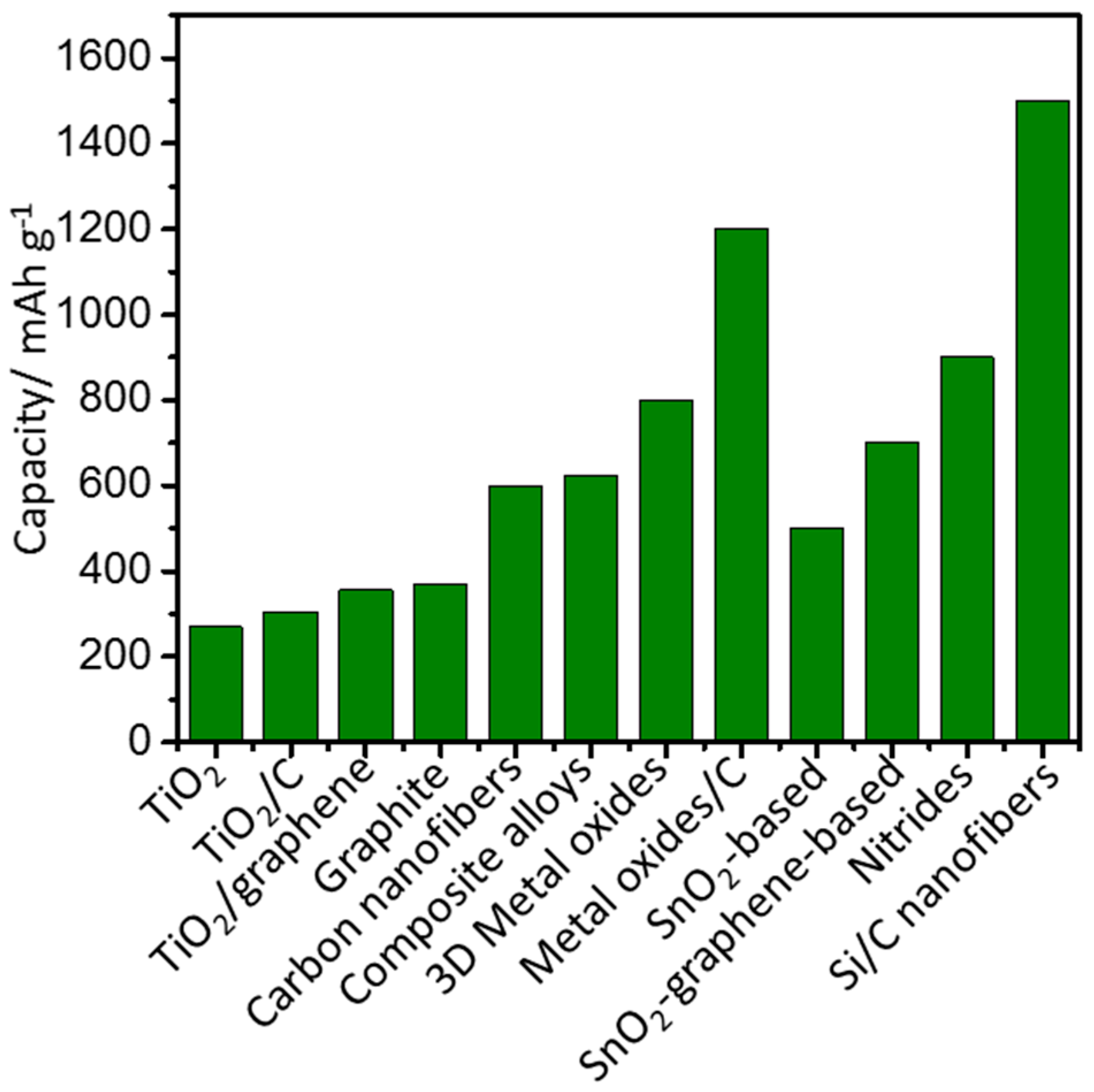
4. TiO2 Nanostructures for LIBs
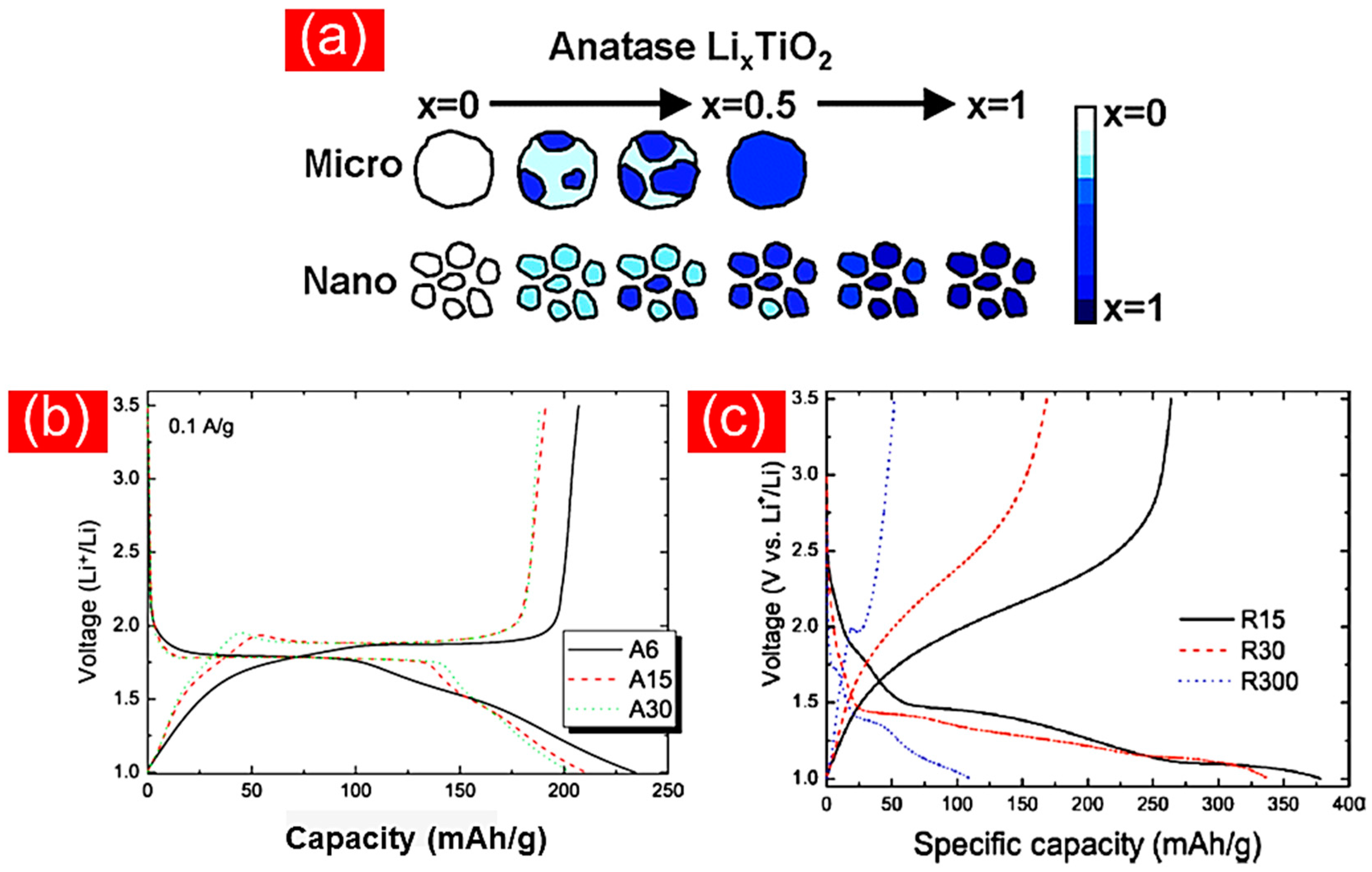
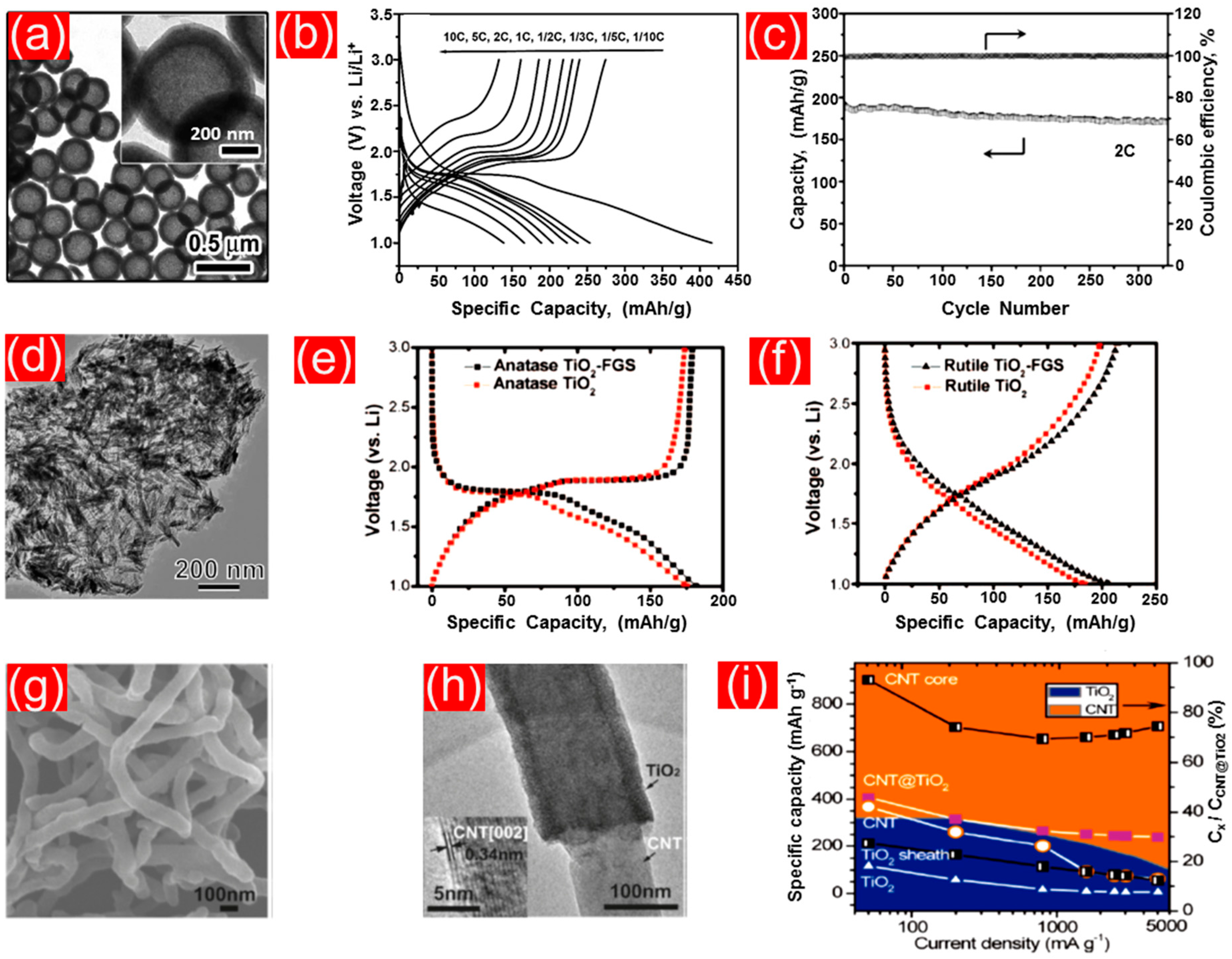
4.1. TiO2 Nanoparticles
Excursus: Nanoparticulate TiO2 Contributions in Redox-Flow Lithium Ion Batteries
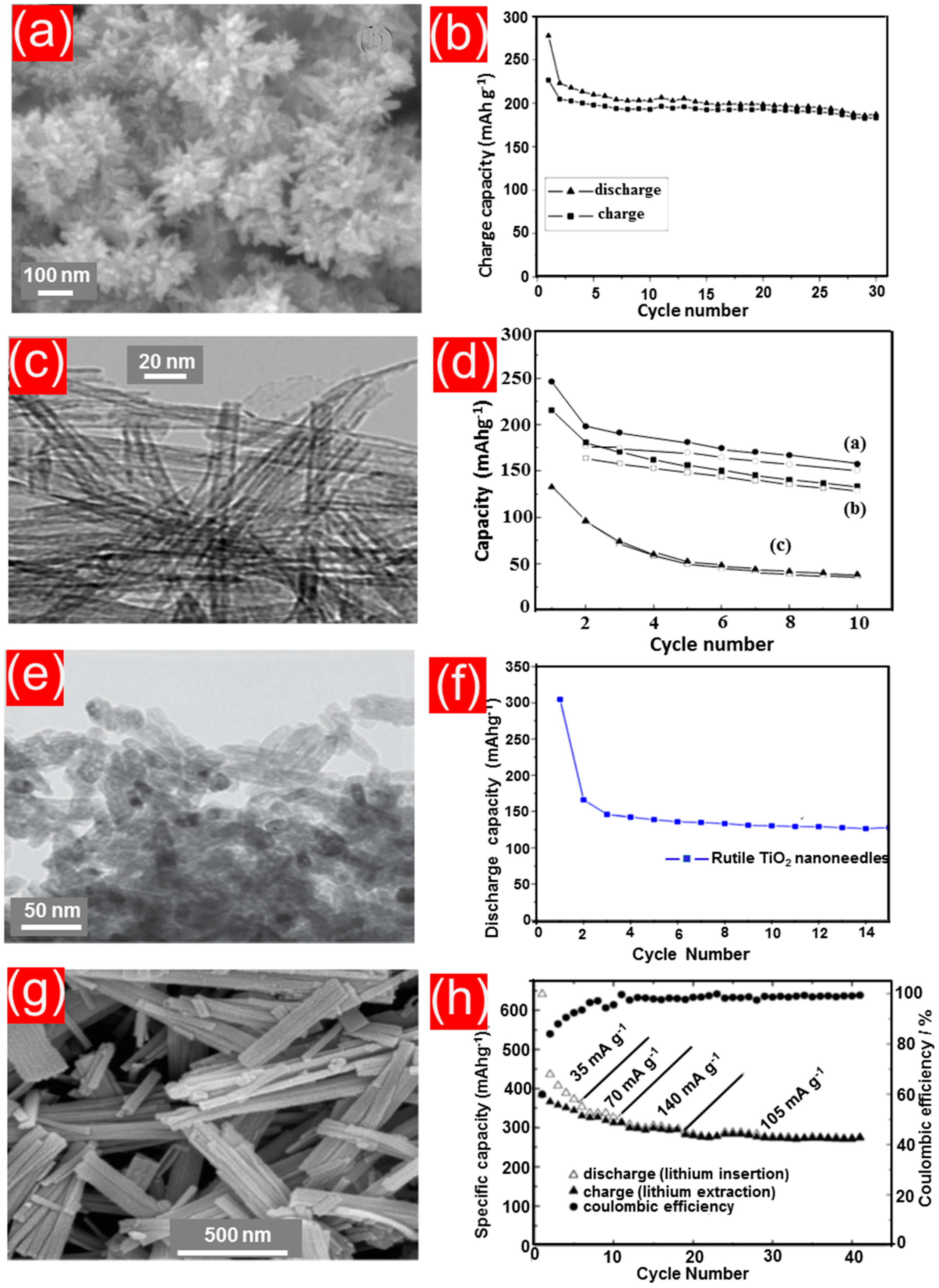
4.2. TiO2 Nanorods, Nanoneedles, Nanowires, and Nanofibers
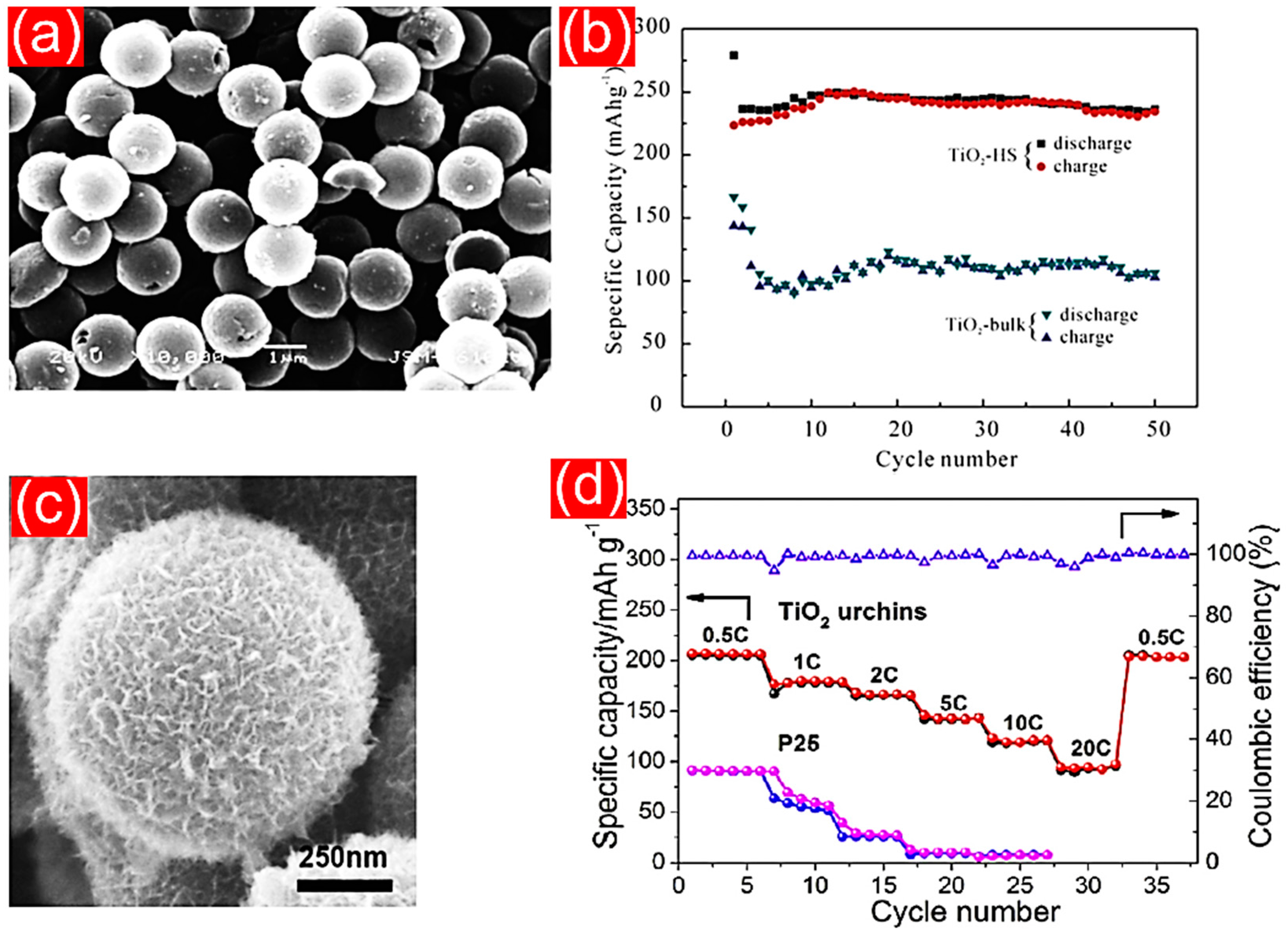
4.3. Porous TiO2 Nanostructures
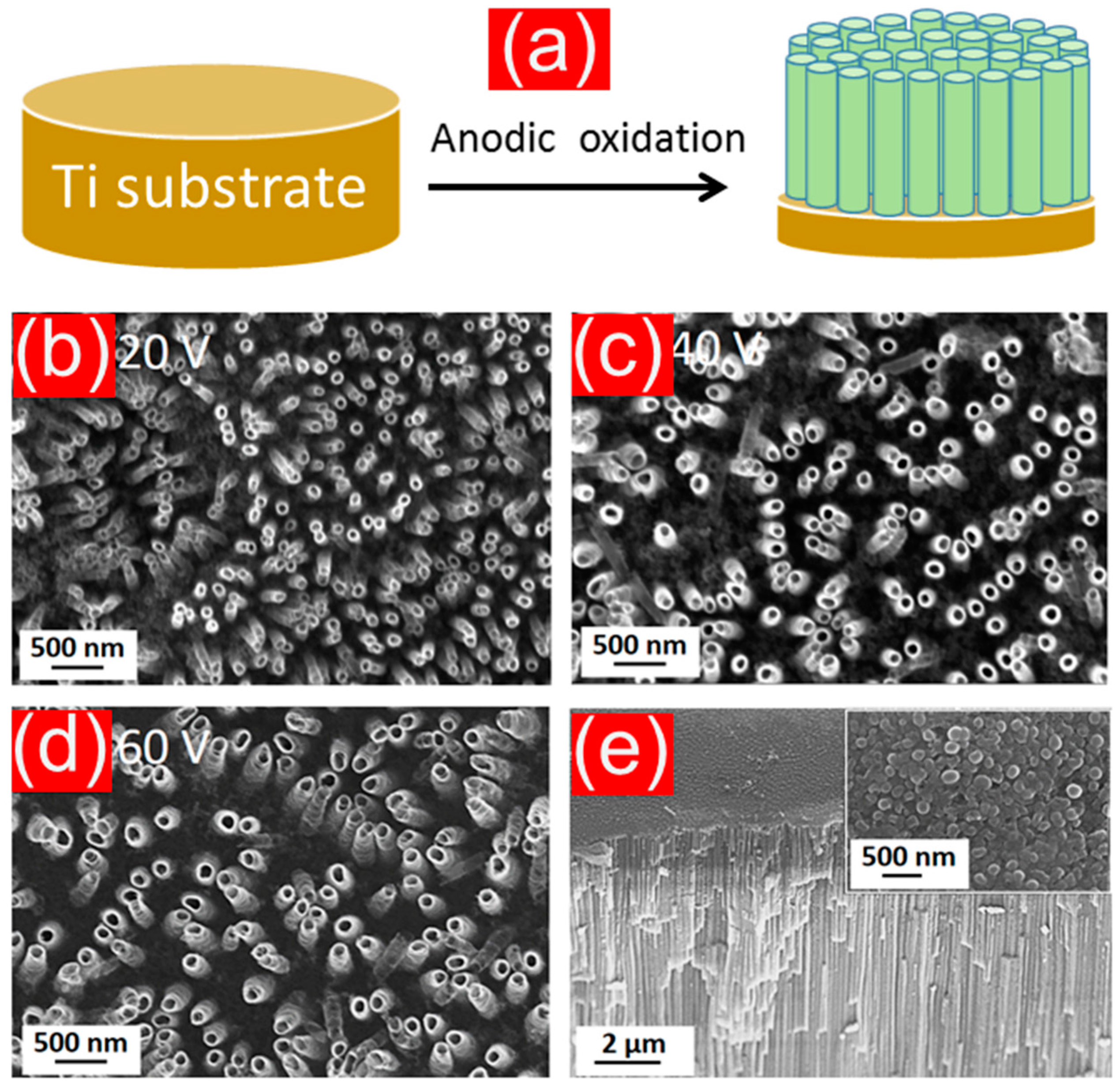
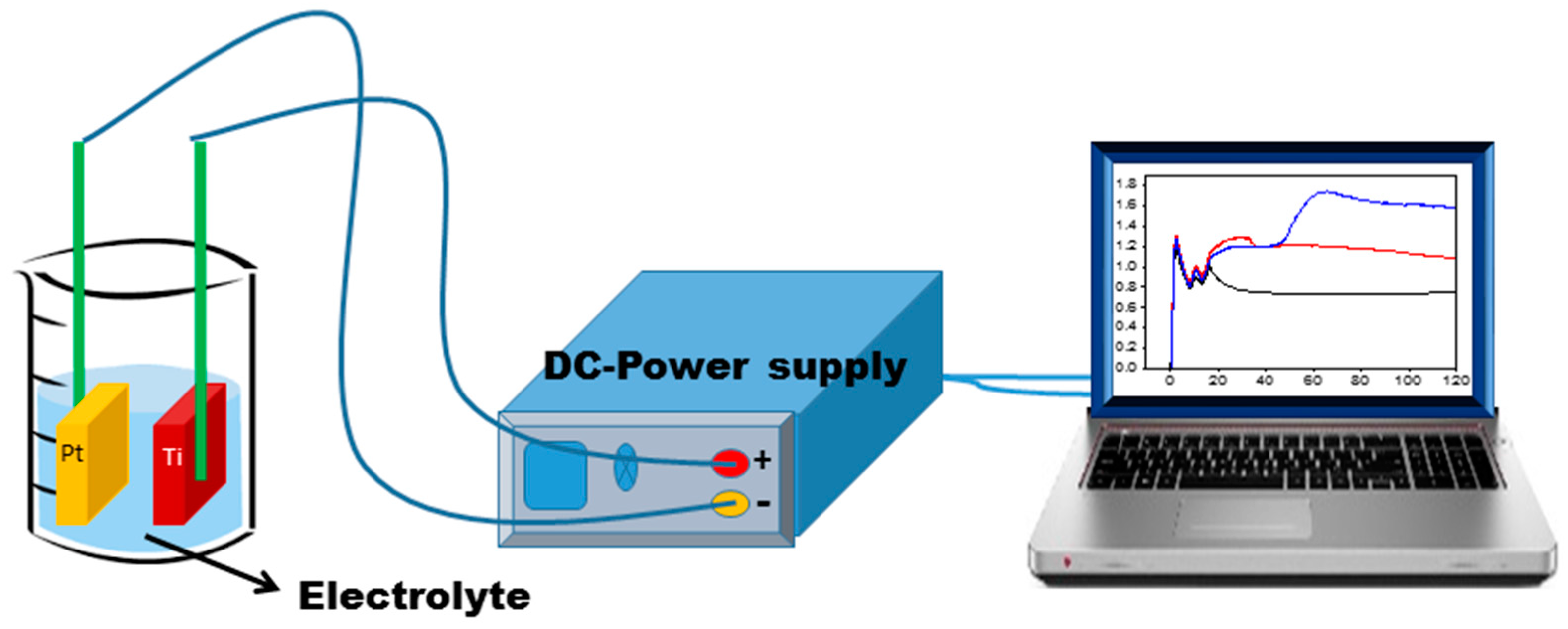
4.4. TiO2 Nanotubes Prepared by Electrochemical Anodization
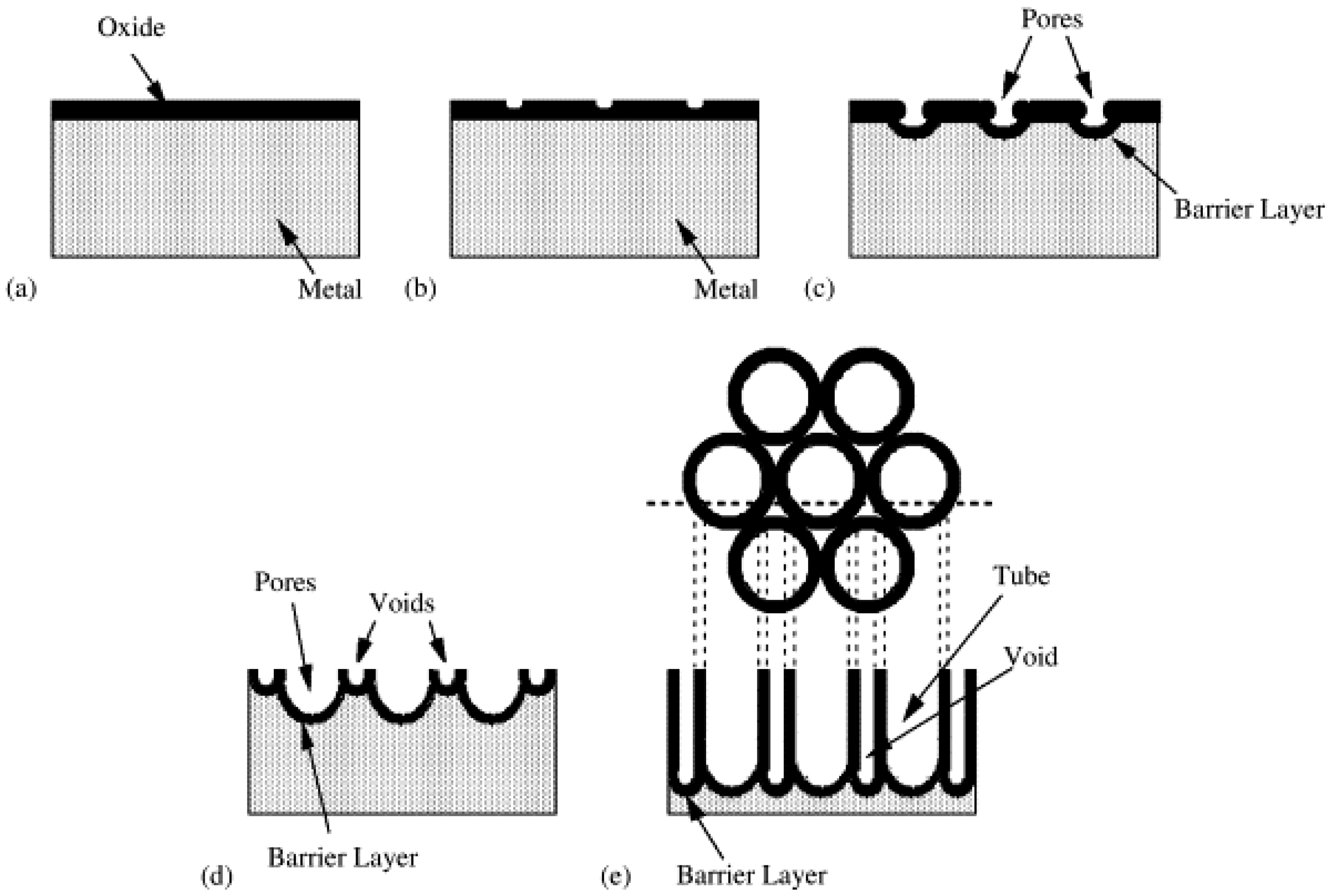
Excursus: The Mechanism of Nanotube Formation by Anodic oxidation
4.5. Anodically Fabricated TiO2 Nanotubes (NTs) as Potential Anodes in LIBs
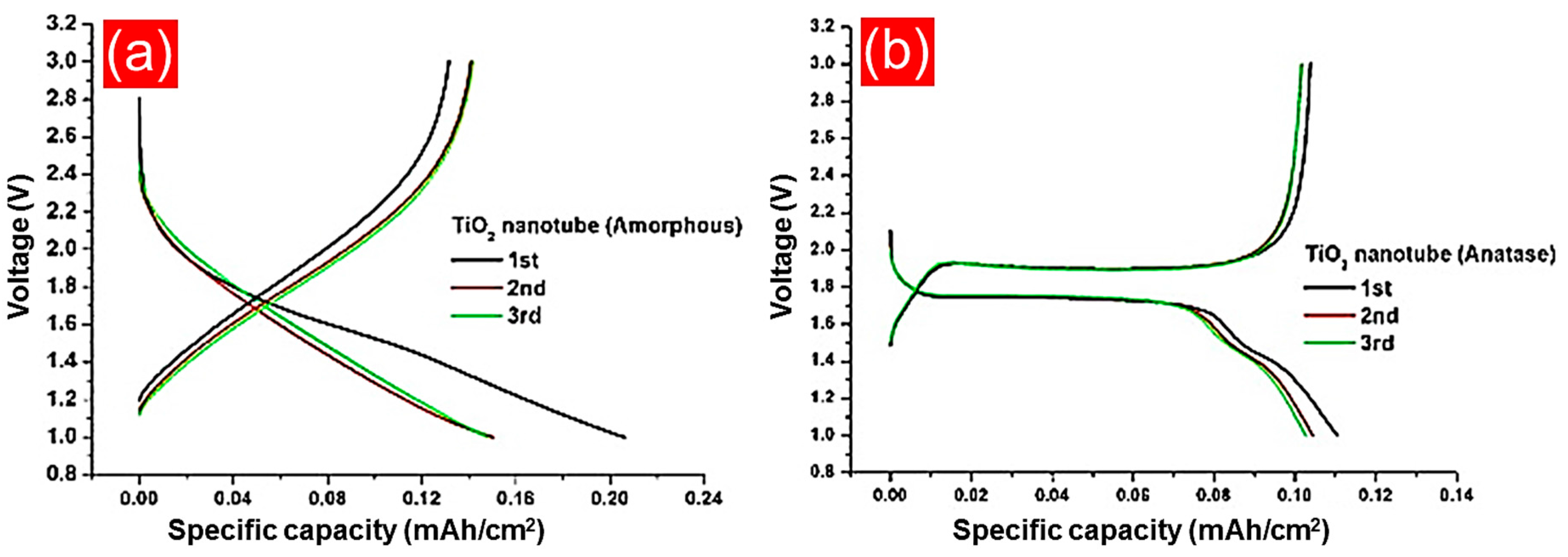
4.5.1. Amorphous and Crystalline TiO2 Anodes
4.5.2. Anodization Electrolyte
4.5.3. Influence of the Annealing Atmosphere on the Properties of TiO2 Nanotube Anodes
4.5.4. Free-Standing TiO2 Nanotube Membranes
4.5.5. TiO2 Nanotubes/Carbon Composites
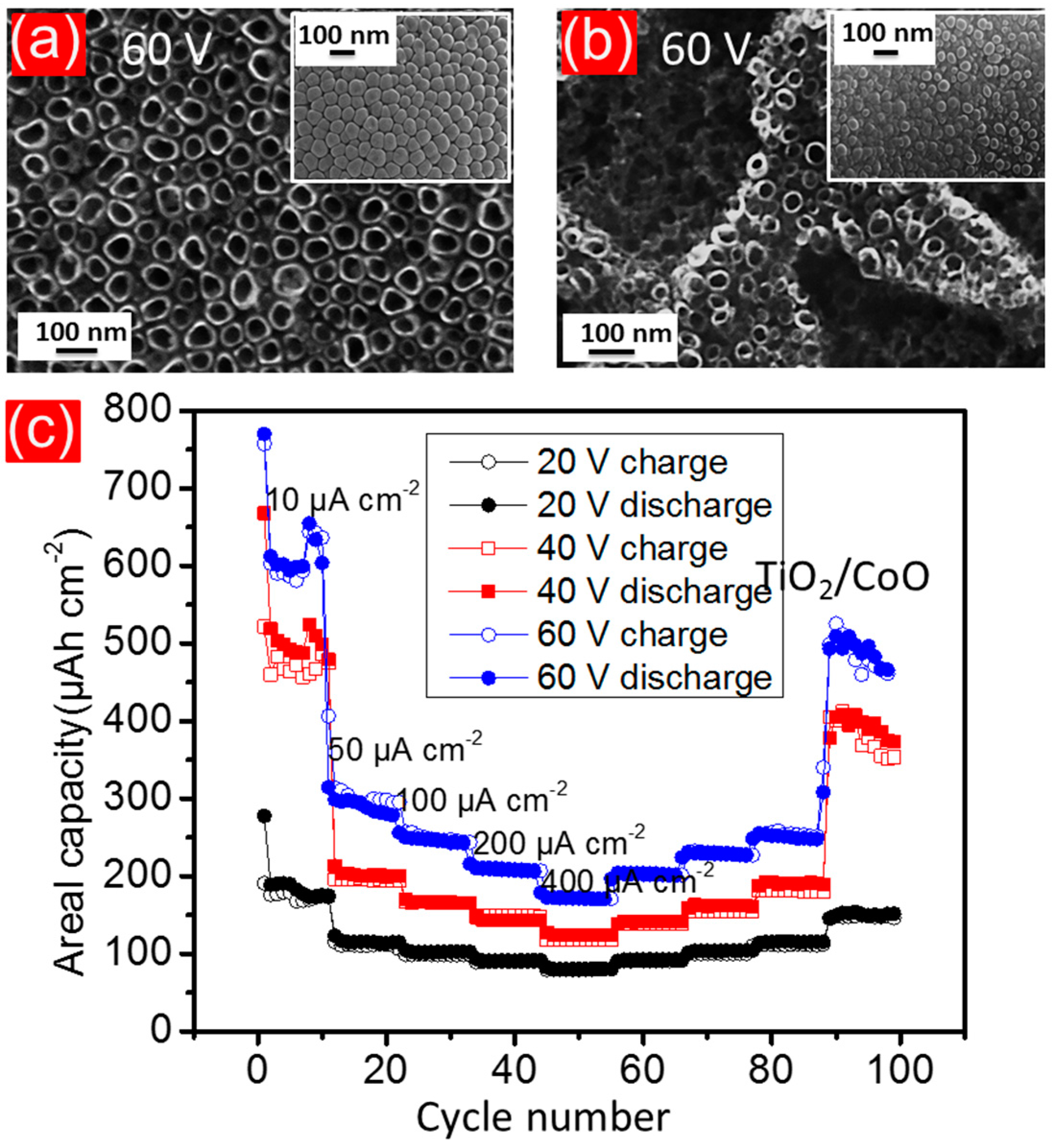
4.5.6. Mixed Oxide Nanotubes
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broussely, M.; Biensan, P.; Simon, B. Lithium insertion into host materials: The key to success for Li ion batteries. Electrochim. Acta 1999, 45, 3–22. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Aldissi, M. Multi-layered polymer electrolytes towards interfacial stability in lithium ion batteries. J. Power Sources 2001, 94, 219–224. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Yaakov, D.; Gofer, Y.; Aurbach, D.; Halalay, I.C. On the study of electrolyte solutions for Li-ion batteries that can work over a wide temperature range. J. Electrochem. Soc. 2010, 157, A1383–A1391. [Google Scholar] [CrossRef]
- Yariv, O.; Hirshberg, D.; Zinigrad, E.; Meitav, A.; Aurbach, D.; Jiang, M.; Powell, B.R. Carbon Negative Electrodes for Li-Ion Batteries: The effect of solutions and temperatures. J. Electrochem. Soc. 2014, 161, A1422–A1431. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Xu, L.; Kim, C.; Shukla, A.K.; Dong, A.; Mattox, T.M.; Milliron, D.J.; Cabana, J. Monodisperse Sn nanocrystals as a platform for the study of mechanical damage during electrochemical reactions with Li. Nano Lett. 2013, 13, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.J.; Boukamp, B.A.; Huggins, R.A.; Weppner, W. Thermodynamic and mass transport properties of “LiAl”. J. Electrochem. Soc. 1979, 126, 2258–2266. [Google Scholar] [CrossRef]
- Saint, J.; Morcrette, M.; Larcher, D.; Tarascon, J.M. Exploring the Li-Ga room temperature phase diagram and the electrochemical performances of the LixGay alloys vs. Li. Solid State Ion. 2005, 176, 189–197. [Google Scholar] [CrossRef]
- Yoon, S.; Park, C.M.; Sohn, H.J. Electrochemical characterizations of germanium and carbon-coated germanium composite anode for lithium-ion batteries. Electrochem. Solid State Lett. 2008, 11, A42–A45. [Google Scholar] [CrossRef]
- Martos, M.; Morales, J.; Sánchez, L. Lead-based systems as suitable anode materials for Li-ion batteries. Electrochim. Acta 2003, 48, 615–621. [Google Scholar] [CrossRef]
- Dailly, A.; Ghanbaja, J.; Willmann, P.; Billaud, D. Lithium insertion into new graphite-antimony composites. Electrochim. Acta 2003, 48, 977–984. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Huang, X.; Huang, X.; Chen, L.; Chen, L.; Wu, Z.; Wu, Z.; Liang, Y.; Liang, Y. A high capacity nano-Si composite anode material for lithium rechargeable batteries. Electrochem. Solid State Lett. 1999, 2, 547–549. [Google Scholar] [CrossRef]
- Cao, F.F.; Deng, J.W.; Xin, S.; Ji, H.X.; Schmidt, O.G.; Wan, L.J.; Guo, Y.G. Cu-Si nanocable arrays as high-rate anode materials for lithium-ion batteries. Adv. Mater. 2011, 23, 4415–4420. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, H.; Du, N.; Wu, P.; Xu, X.; Li, Y.; Yang, D. Vertically ordered Ni3Si2/Si nanorod arrays as anode materials for high-performance Li-ion batteries. Nanoscale 2012, 4, 5343–5347. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Recent progress of TiO2-based anodes for Li ion batteries. J. Nanomater. 2016, 2016, 8123652. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite nanofibers as advanced materials for Li-ion, Li-O2 and Li-S batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Campos, H.; Villarreal, J.; Alcoutlabi, M. A comparative study on the performance of binary SnO2/NiO/C and Sn/C composite nanofibers as alternative anode materials for lithium ion. Electrochim. Acta 2017, 224, 608–621. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Wagemaker, M.; Kearley, G.J.; van Well, A.A.; Mutka, H.; Mulder, F.M. Multiple Li positions inside oxygen octahedra in lithiated TiO2 anatase. J. Am. Chem. Soc. 2003, 125, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Nobili, F.; Tossici, R.; Wohlfahrt-Mehrens, M.; Marassi, R. High performance, environmentally friendly and low cost anodes for lithium-ion battery based on TiO2 anatase and water soluble binder carboxymethyl cellulose. J. Power Sources 2011, 196, 9665–9671. [Google Scholar] [CrossRef]
- Xie, M.; Sun, X.; Zhou, C.; Cavanagh, A.S.; Sun, H.; Hu, T.; Wang, G.; Lian, J.; George, S.M. Amorphous Ultrathin TiO2 atomic layer deposition films on carbon nanotubes as anodes for lithium ion batteries. J. Electrochem. Soc. 2015, 162, A974–A981. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J. Nanoengineering titania for high rate lithium storage: A review. J. Mater. Sci. Technol. 2013, 29, 97–122. [Google Scholar] [CrossRef]
- Nuspl, G.; Yoshizawa, K.; Yamabe, T. Lithium intercalation in TiO2 modifications. J. Mater. Chem. 1997, 7, 2529–2536. [Google Scholar] [CrossRef]
- Van de Krol, R.; Goossens, A.; Schoonman, J. Spatial extent of lithium intercalation in anatase TiO2. J. Phys. Chem. B 1999, 103, 7151–7159. [Google Scholar] [CrossRef]
- Koudriachova, M.V.; Harrison, N.M.; De Leeuw, S.W. Diffusion of Li-ions in rutile. An ab initio study. Solid State Ion. 2003, 157, 35–38. [Google Scholar] [CrossRef]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.-E. Li+ ion insertion in TiO2 (anatase). 2. Voltammetry on nanoporous films. J. Phys. Chem. B 1997, 101, 7717–7722. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Li, W.; Chen, X. Nanostructured TiO2-based anode materials for high-performance rechargeable lithium-ion batteries. ChemNanoMat 2016, 2, 764–775. [Google Scholar] [CrossRef]
- Liu, Z.; Andreev, Y.G.; Armstrong, A.R.; Brutti, S.; Ren, Y.; Bruce, P.G. Nanostructured TiO2(B): The effect of size and shape on anode properties for Li-ionbatteries. Prog. Nat. Sci. Mater. Int. 2013, 23, 235–244. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Xu, Z.R.; Gao, L.B.; Liu, Y.J.; Li, L. Recent developments in the doped LiFePO4 cathode materials for power lithium ion batteries. J. Electrochem. Soc. 2016, 163, A2600–A2610. [Google Scholar] [CrossRef]
- Qi, Z.; Koenig, G.M., Jr. High-performance LiCoO2 sub-micrometer materials from scalable microparticle template processing. Chem. Sel. 2016, 1, 3992–3999. [Google Scholar] [CrossRef]
- Eftekhari, A. LiFePO4/C nanocomposites for lithium-ion batteries. J. Power Sources 2017, 343, 395–411. [Google Scholar] [CrossRef]
- Scrosati, B. Lithium rocking chair batteries: An old concept? J. Electrochem. Soc. 1992, 139, 2776–2781. [Google Scholar] [CrossRef]
- Moseley, P.T.; Garche, J. Electrochemical Energy Storage For Renewable Sources and Grid Balancing, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444626165. [Google Scholar]
- Yang, P. The Chemistry of Nanostructured Materials; World Scientific Publishing Co.: Singapore, 2011; ISBN 9814313068. [Google Scholar]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Abraham, K.M. Prospects and limits of energy storage in batteries. J. Phys. Chem. Lett. 2015, 6, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Simons, D.; Roy, R. Pressure-temperature studies of anatase, brookite, rutile, and TiO2(II). Am. Mineral. 1968, 53, 1929–1939. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D.; Pask, J.A. Kinetics of the anatase-rutile transformation. J. Am. Ceram. Soc. 1965, 48, 391–398. [Google Scholar] [CrossRef]
- Koudriachova, M.; Harrison, N.; de Leeuw, S. Effect of diffusion on lithium intercalation in titanium dioxide. Phys. Rev. Lett. 2001, 86, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, Y.W.L.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; He, M.; Hou, Z.; Xia, T.; Liu, G.; Chen, X. TiO2 Nanomaterials as Anode Materials for Lithium-Ion Rechargeable Batteries. Energy Technol. 2015, 3, 801–814. [Google Scholar] [CrossRef]
- Wagemaker, M.; van de Krol, R.; Kentgens, A.P.M.; van Well, A.A.; Mulder, F.M. Two phase morphology limits lithium diffusion in TiO2 (anatase): A 7Li MAS NMR study. J. Am. Chem. Soc. 2001, 123, 11454–11461. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, A.; Casarin, M.; Selloni, A. Structure and stability of TiO2-B surfaces: A density functional study. J. Phys. Chem. C 2009, 113, 18973–18977. [Google Scholar] [CrossRef]
- Cromer, D.T.; Herrington, K. The structures of anatase and rutile. J. Am. Chem. Soc. 1955, 77, 4708–4709. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Zukalova, M.; Kalbac, M.; Kavan, L.; Zukalová, M.; Kalbáč, M.; Exnar, I.; Graetzel, M. Pseudocapacitive lithium storage in TiO2 (B). Chem. Mater. 2005, 17, 1248–1255. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Zhan, X.; Wu, J.; Wei, S.; Guo, Z. Advanced titania nanostructures and composites for lithium ion battery. J. Mater. Sci. 2011, 47, 2519–2534. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Mulder, F.M. Large impact of particle size on insertion reactions. A case for anatase LixTiO2. J. Am. Chem. Soc. 2007, 129, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wei, M.; Qi, Z.; Kudo, T.; Honma, I.; Zhou, H. Particle size dependence of the lithium storage capability and high rate performance of nanocrystalline anatase TiO2 electrode. J. Power Sources 2007, 166, 239–243. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; van Eck, E.R.H.; Kentgens, A.P.M.; Kearley, G.J.; Mulder, F.M. The Influence of size on phase morphology and Li-ion mobility in nanosized lithiated anatase TiO2. Chem. Eur. J. 2007, 13, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Sudant, G.; Baudrin, E.; Larcher, D.; Tarascon, J.M. Electrochemical lithium reactivity with nanotextured anatase-type TiO2. J. Mater. Chem. 2005, 15, 1263–1269. [Google Scholar] [CrossRef]
- Hu, Y.S.; Kienle, L.; Guo, Y.G.; Maier, J. High lithium electroactivity of nanometer-sized rutile TiO2. Adv. Mater. 2006, 18, 1421–1426. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbac, M.; Zukalova, M.; Exnar, I.; Lorenzen, V.; Nesper, R.; Graetzel, M. Lithium storage in nanostructured TiO2 made by hydrothermal growth. Chem. Mater. 2004, 16, 477–485. [Google Scholar] [CrossRef]
- Borghols, W.J.H.; Wagemaker, M.; Lafont, U.; Kelder, E.M.; Mulder, F.M. Impact of nanosizing on lithiated rutile TiO2. Chem. Mater. 2008, 20, 2949–2955. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Nanocrystallinity effects in lithium battery materials. Phys. Chem. Chem. Phys. 2003, 5, 5215–5220. [Google Scholar] [CrossRef]
- Jiang, C.; Honma, I.; Kudo, T.; Zhou, H. Nanocrystalline rutile TiO2 electrode for high-capacity and high-rate lithium storage. Electrochem. Solid State Lett. 2007, 10, A127–A129. [Google Scholar] [CrossRef]
- Sushko, M.L.; Rosso, K.M.; Liu, J. Size effects on Li+/electron conductivity in TiO2 nanoparticles. J. Phys. Chem. Lett. 2010, 1, 1967–1972. [Google Scholar] [CrossRef]
- Kubiak, P.; Fröschl, T.; Hüsing, N.; Hörmann, U.; Kaiser, U.; Schiller, R.; Weiss, C.K.; Landfester, K.; Wohlfahrt-Mehrens, M. TiO2 anatase nanoparticle networks: Synthesis, structure, and electrochemical performance. Small 2011, 7, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, O.; Pratsinis, S.E.; de Chambrier, E.; Crouzet, M.; Exnar, I. Electrochemical performance of granulated titania nanoparticles. J. Power Sources 2004, 134, 197–201. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Li, Z.; Wang, X.; Yu, K. Fabrication of nanostructured TiO2 using a solvothermal reaction for lithium-ion batteries. Nanomater. Nanotechnol. 2016, 6, 15. [Google Scholar] [CrossRef][Green Version]
- Kang, J.; Kim, D.H.; Mathew, V.; Lim, J.S.; Gim, J.; Kim, J. Particle size effect of anatase TiO2 nanocrystals for lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A59–A62. [Google Scholar] [CrossRef]
- Liu, L.; Fan, Q.; Sun, C.; Gu, X.; Li, H.; Gao, F.; Chen, Y.; Dong, L. Synthesis of sandwich-like TiO2@C composite hollow spheres with high rate capability and stability for lithium-ion batteries. J. Power Sources 2013, 221, 141–148. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Jiu, H.; Qiu, H.; Wang, H. Porous TiO2/C nanocomposite shells as a high-performance anode material for lithium-ion batteries. ACS Appl. Mater. Interface 2013, 5, 6478–6483. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R.; Hu, D.; Wang, C.; Saraf, L.V.; Zhang, J.; et al. Self-assembled TiO2—Graphene hybrid insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.F.; Guo, Y.G.; Zheng, S.F.; Wu, X.L.; Jiang, L.Y.; Bi, R.R.; Wan, L.J.; Maier, J. Symbiotic coaxial nanocables: Facile synthesis and an efficient and elegant morphological solution to the lithium storage problem. Chem. Mater. 2010, 22, 1908–1914. [Google Scholar] [CrossRef]
- Tao, H.C.; Fan, L.Z.; Yan, X.; Qu, X. In situ synthesis of TiO2-graphene nanosheets composites as anode materials for high-power lithium ion batteries. Electrochim. Acta 2012, 69, 328–333. [Google Scholar] [CrossRef]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-flow batteries: From metals to organic redox-active materials. Angew. Chem. Int. Ed. 2017, 56, 686–711. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Koenig, G.M. Flow battery systems with solid electroactive materials. J. Vac. Sci. Technol. B 2017, 35, 040801. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q. Redox species of redox flow batteries: A review. Molecules 2015, 20, 20499–20517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zakeeruddin, S.M.; Wang, D.; Exnar, I.; Grätzel, M. Redox targeting of insulating electrode materials: A new approach to high-energy-density batteries. Angew. Chem. Int. Ed. 2006, 45, 8197–8200. [Google Scholar] [CrossRef] [PubMed]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient vanadium redox flow cell. J. Electrochem. Soc. 1987, 134, 2950–2953. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.-M. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Huang, Q.; Li, H.; Grätzel, M.; Wang, Q. Reversible chemical delithiation/lithiation of LiFePO4: Towards a redox flow lithium-ion battery. Phys. Chem. Chem. Phys. 2013, 15, 793–1797. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Yang, J.; Huang, Q.; Wang, X.; Huang, H.; Wang, Q. Redox targeting of anatase TiO2 for redox flow lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1400567. [Google Scholar] [CrossRef]
- Jia, C.; Pan, F.; Zhu, Y.G.; Huang, Q.; Lu, L.; Wang, Q. High–energy density nonaqueous all redox flow lithium battery enabled with apolymeric membrane. Sci. Adv. 2015, 1, e1500886. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Choi, D.; Yang, Z.; Viswanathan, V.V.; Nie, Z.; Wang, C.; Song, Y.; Zhang, J.-G.; Liu, J. Synthesis and Li-ion insertion properties of highly crystalline mesoporous rutile TiO2. Chem. Mater. 2008, 20, 3435–3442. [Google Scholar] [CrossRef]
- Qiao, H.; Tao, D.; Wang, Y.; Cai, Y.; Huang, F.; Yang, X.; Wei, J.; Wei, Q. Electrochemical charge storage of flowerlike rutile TiO2 nanorods. Chem. Phys. Lett. 2010, 490, 180–183. [Google Scholar] [CrossRef]
- Lan, Y.; Gao, X.; Zhu, H.; Zheng, Z.; Yan, T.; Wu, F.; Ringer, S.P.; Song, D. Titanate nanotubes and nanorods prepared from rutile powder. Adv. Funct. Mater. 2005, 15, 1310–1318. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J. Rate Characteristics of anatase TiO2 nanotubes and nanorods for lithium battery anode materials at room temperature. J. Electrochem. Soc. 2007, 154, A542–A546. [Google Scholar] [CrossRef]
- Bao, S.J.; Bao, Q.L.; Li, C.M.; Dong, Z.L. Novel porous anatase TiO2 nanorods and their high lithium electroactivity. Electrochem. Commun. 2007, 9, 1233–1238. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, H.; Pan, G.; Ye, S.; Lan, Y.; Wu, F.; Song, D. Preparation and electrochemical characterization of anatase nanorods for lithium-inserting electrode material. J. Phys. Chem. B 2004, 108, 2868–2872. [Google Scholar] [CrossRef]
- Khomane, R.B. Microemulsion-mediated sol-gel synthesis of mesoporous rutile TiO2 nanoneedles and its performance as anode material for Li-ion batteries. J. Colloid Interface Sci. 2011, 356, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, M.; Zhang, W.F. Preparation and electrochemical characterization of TiO2 nanowires as an electrode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 7863–7868. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Wang, Z.; Guo, H.; Wu, L.; Xiong, X.; Wang, X. A novel method to synthesize anatase TiO2 nanowires as an anode material for lithium-ion batteries. J. Alloy. Compd. 2011, 509, 3711–3715. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2-B nanowires. Angew. Chem. Int. Ed. 2004, 116, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Tammawat, P.; Meethong, N. Synthesis and characterization of stable and binder-free electrodes of TiO2 nanofibers for Li-ion batteries. J. Nanomater. 2013, 2013, 413692. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; Prato, M.; Scarpellini, A.; Marras, S.; Monaco, S.; Toma, A.; Messina, G.C.; Alabastri, A.; de Angelis, F.; et al. Direct Synthesis of carbon-doped TiO2-bronze nanowires as anode materials for high performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 25139–25146. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, X.; Li, H.; Yuan, C.; Cao, G. Design and tailoring of a three-dimensional TiO2-graphene-carbon nanotube nanocomposite for fast lithium storage. J. Phys. Chem. Lett. 2011, 2, 3096–3101. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Xiong, B.; Zhao, Y.; Huang, X.; Shao, Z. Fast lithium-ion insertion of TiO2 nanotube and graphene composites. Electrochim. Acta 2013, 88, 847–857. [Google Scholar] [CrossRef]
- He, L.; Ma, R.; Du, N.; Ren, J.; Wong, T.; Li, Y.; Lee, S.T. Growth of TiO2 nanorod arrays on reduced graphene oxide with enhanced lithium-ion storage. J. Mater. Chem. 2012, 22, 19061–19066. [Google Scholar] [CrossRef]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO2/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloy. Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Thirugunanama, L.; Kaveric, S.; Etacherib, V.; Ramaprabhua, S.; Duttad, M.; Polb, V.G. Electrospun nanoporous TiO2 nanofibers wrapped with reduced graphene oxide for enhanced and rapid lithium-ion storage. Mater. Charact. 2017, 131, 64–71. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Luan, D.; Yin, F.; Boey, C.; Madhavib, S.; Lou, X.W.D. Graphene-supported anatase TiO2 nanosheets for fast lithium storage. Chem. Commun. 2011, 47, 5780–5782. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhou, X.; Wu, J.; Yao, X.; Liu, Z. Scalable synthesis of TiO2/graphene nanostructured composite with high-rate performance for lithium ion batteries. ACS Nano 2012, 6, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Wang, D.; Viswanathan, V.V.; Bae, I.; Wang, W.; Nie, Z.; Zhang, J.; Graff, G.L.; Liu, J.; Yang, Z.; et al. Li-ion batteries from LiFePO4 cathode and anatase/graphene composite anode for stationary energy storage. Electrochem. Commun. 2010, 12, 378–381. [Google Scholar] [CrossRef]
- Wang, H.E.; Cheng, H.; Liu, C.; Chen, X.; Jiang, Q.; Lu, Z.; Li, Y.Y.; Chung, C.Y.; Zhang, W.; Zapien, J.A.; et al. Facile synthesis and electrochemical characterization of porous and dense TiO2 nanospheres for lithium-ion battery applications. J. Power Sources 2011, 196, 6394–6399. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, M.; Mei, D.; Guo, Y.; Yao, L.; Qu, D.; Deng, B. Preparation and electrochemical lithium storage features of TiO2 hollow spheres. J. Power Sources 2013, 238, 197–202. [Google Scholar] [CrossRef]
- Saravanan, K.; Ananthanarayanan, K.; Balaya, P. Mesoporous TiO2 with high packing density for superior lithium storage. Energy Environ. Sci. 2010, 3, 939–948. [Google Scholar] [CrossRef]
- Wang, H.E.; Jin, J.; Cai, Y.; Xu, J.M.; Chen, D.S.; Zheng, X.F.; Deng, Z.; Li, Y.; Bello, I.; Su, B.L. Facile and fast synthesis of porous TiO2 spheres for use in lithium ion batteries. J. Colloid Interface Sci. 2014, 417, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhang, L.D.; Li, W.; Yu, A.S.; Wu, P.Y. Carbon-coated mesoporous TiO2 nanocrystals grown on graphene for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 10395–10400. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Wang, K.X.; Guo, X.X.; Wei, X.; Wang, J.F.; Chen, J.S. Mesoporous titania rods as an anode material for high performance lithium-ion batteries. J. Power Sources 2012, 214, 298–302. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Binetti, E.; Krueger, S.; Striccoli, M.; Winter, M.; Passerini, S. Percolating networks of TiO2 nanorods and carbon for high power lithium insertion electrodes. J. Power Sources 2012, 206, 301–309. [Google Scholar] [CrossRef]
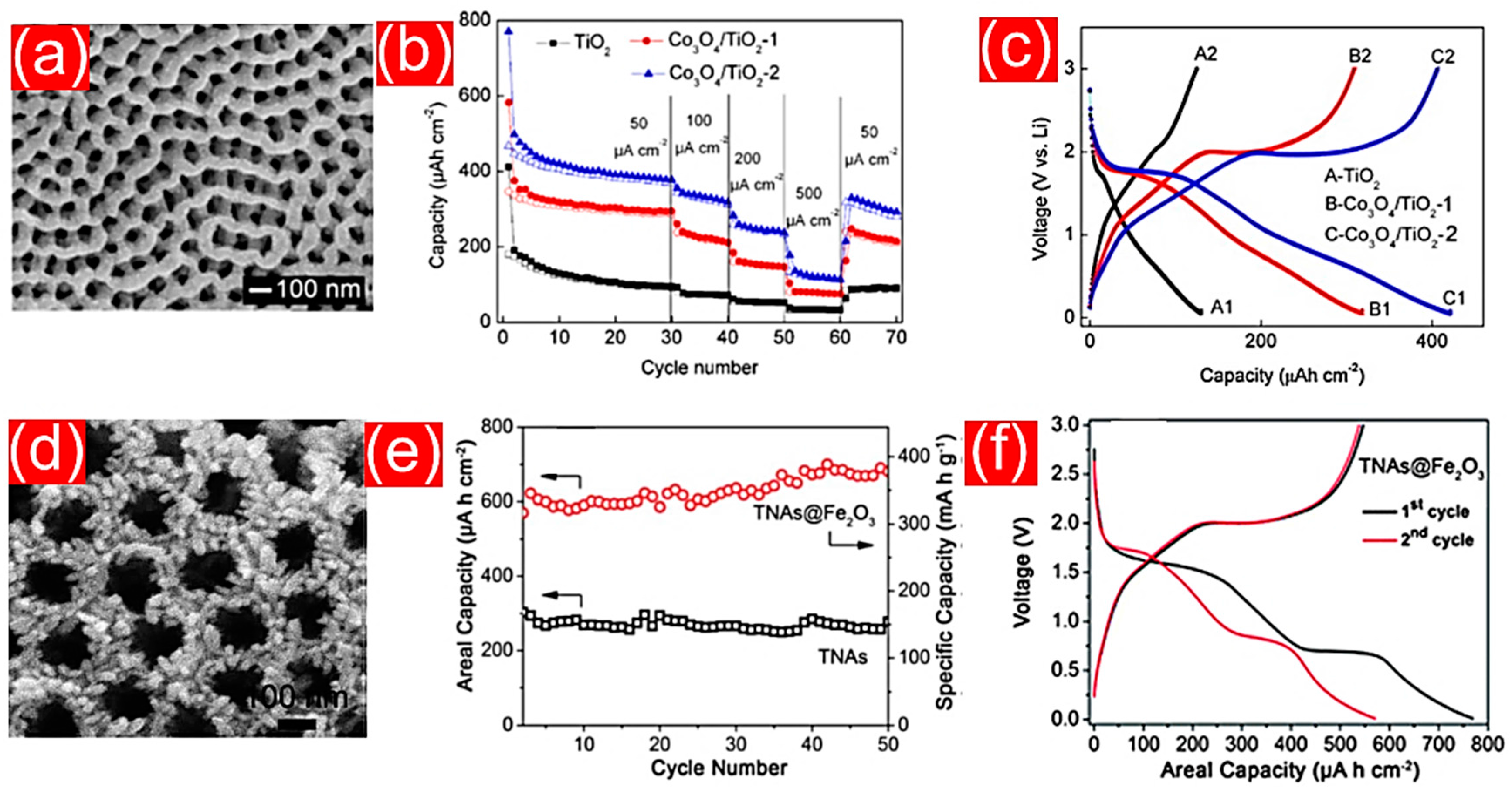
- Zhang, X.; Chen, H.; Xie, Y.; Guo, J. Ultralong life lithium-ion battery anode with superior high-rate capability and excellent cyclic stability from mesoporous Fe2O3@TiO2 core–shell nanorods. J. Mater. Chem. A 2014, 2, 3912–3918. [Google Scholar] [CrossRef]
- Wang, H.-G.; Li, Y.-H.; Liu, W.-Q.; Wan, Y.-C.; Li, Y.-W.; Duan, Q. One-step facile synthesis of TiO2/Fe2O3 fiber-in-tube hierarchical heterostructures with improved lithium-ion battery performance. RSC Adv. 2014, 4, 23125–23130. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, H.E.; Huang, S.Z.; Yuen, M.F.; Cai, H.H.; Wang, C.; Yu, Y.; Li, Y.; Zhang, W.J.; Su, B.L. Porous TiO2 urchins for high performance Li-ion battery electrode: Facile synthesis, characterization and structural evolution. Electrochim. Acta 2016, 210, 206–214. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Liu, F.; Liang, H.; You, X.; Wang, K.; Lou, X.; Shuang, W.; Xiao, L.; Cai, B.; et al. The template-free synthesis of hierarchically porous anatase TiO2 via acid-etching for enhancing the cycling stability and reversible capacity of lithium ion batteries. RSC Adv. 2016, 6, 48985–48994. [Google Scholar] [CrossRef]
- Maier, J. Mass storage in space charge regions of nano-sized systems, Nano-ionics. Part V. Faraday Discuss. Chem. Soc. 2007, 134, 51–66. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Samuelis, D.; Maier, J. Sustained lithium-storage performance of hierarchical, nanoporous anatase TiO2 at high rates: Emphasis on interfacial storage phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Jin, J.; Huang, S.Z.; Li, Y.; Tian, H.; Wang, H.E.; Yu, Y.; Chen, L.H.; Hasan, T.; Su, B.L. Hierarchical nanosheet-constructed yolk-shell TiO2 porous microspheres for lithium batteries with high capacity, superior rate and long cycle capability. Nanoscale 2015, 7, 12979–12989. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.; Li, G.; Wang, X.; Jiang, G.; Lin, E.; Fowler, M.; Yu, A.; Chen, Z. Flexible, three-dimensional ordered macroporous TiO2 electrode with enhanced electrode-electrolyte interaction in high-power li-ion batteries. Nano Energy 2016, 24, 72–77. [Google Scholar] [CrossRef]
- Ye, J.; Baumgaertel, A.C.; Wang, Y.M.; Biener, J.; Biener, M.M. Structural optimization of 3D porous electrodes for high-rate performance lithium ion batteries. Nano Lett. 2015, 9, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Aldeyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Kelly, J.J. The influence of fluoride ions on the passive dissolution of titanium. Electrochim. Acta 1979, 24, 1273–1282. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Madian, M.; Giebeler, L.; Klose, M.; Jaumann, T.; Uhlemann, M.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J. Self-Organized TiO2/CoO Nanotubes as Potential Anode Materials for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2015, 3, 909–919. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of tapered, conical-shaped titania nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Ruan, C.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Fabrication of highly ordered TiO2 nanotube arrays using an organic electrolyte. J. Phys. Chem. B 2005, 109, 15754–15759. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 µm long anodic TiO2 nanotubes with hexagonal self-ordering. Phys. Status Solidi Rapid Res. Lett. 2007, 1, R65–R67. [Google Scholar] [CrossRef]
- Quan, X.; Yang, S.; Ruan, X.; Zhao, H. Preparation of titania nanotubes and their environmental applications as electrode. Environ. Sci. Technol. 2005, 39, 3770–3775. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim. Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Balaur, E.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-organized TiO2 nanotubes prepared in ammonium fluoride containing acetic acid electrolytes. Electrochem. Commun. 2005, 7, 576–580. [Google Scholar] [CrossRef]
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. Appl. Phys. Lett. 1998, 72, 1173–1175. [Google Scholar] [CrossRef]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-organized porous titanium oxide prepared in H2SO4/HF electrolytes. Electrochem. Solid State Lett. 2003, 6, B12–B14. [Google Scholar] [CrossRef]
- Grimes, C.A. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 2007, 17, 1451. [Google Scholar] [CrossRef]
- Parkhutik, V.P.; Shershulsky, V.I. Theoretical modelling of porous oxide growth on aluminium. J. Phys. D Appl. Phys. 1992, 25, 1258–1263. [Google Scholar] [CrossRef]
- Macdonald, D.D. On the formation of voids in anodic oxide films on aluminum. J. Electrochem. Soc. 1993, 140, L27–L30. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.R.; Stevens, R.; Allsopp, D. Macro, micro and nanostructure of TiO2 anodised films prepared in a fluorine-containing electrolyte. J. Mater. Sci. 2007, 42, 6729–6734. [Google Scholar] [CrossRef]
- Siejka, J. An O18 study of field-assisted pore formation in compact anodic oxide films on aluminum. J. Electrochem. Soc. 1977, 124, 883–891. [Google Scholar] [CrossRef]
- Lohrengel, M.M. Thin anodic oxide layers on aluminium and other valve metals: High field regime. Mater. Sci. Eng. Rep. 1993, 11, 243–294. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Kirchgeorg, R.; Kallert, M.; Liu, N.; Hahn, R.; Killian, M.S.; Schmuki, P. Key factors for an improved lithium ion storage capacity of anodic TiO2 nanotubes. Electrochim. Acta 2016, 198, 56–65. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Lee, E.K.; Devadoss, A.; Jeon, Y.; Ha, J.; Chung, Y.C.; Choi, Y.M.; Jung, Y.G.; Paik, U. Dominant factors governing the rate capability of a TiO2 nanotube anode for high power lithium ion batteries. ACS Nano 2012, 6, 8308–8315. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, Z.; Jiang, R.; Bian, C.; Huang, T.; Yu, A. TiO2 nanotube array film prepared by anodization as anode material for lithium ion batteries. J. Solid State Electrochem. 2009, 14, 1045–1050. [Google Scholar] [CrossRef]
- Shannon, R.D. Phase transformation studies in TiO2 supporting different defect mechanisms in vacuum-reduced and hydrogen-reduced rutile. J. Appl. Phys. 1964, 35, 3414–3416. [Google Scholar] [CrossRef]
- Roman, I.; Trusca, R.D.; Soare, M.L.; Fratila, C.; Krasicka-Cydzik, E.; Stan, M.S.; Dinischiotu, A. Titanium dioxide nanotube films: Preparation, characterization and electrochemical biosensitivity towards alkaline phosphatase. Mater. Sci. Eng. C 2014, 37, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Prakasam, H.E.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Vertically oriented Ti-Fe-O nanotube array films: Toward a useful material architecture for solar spectrum water photoelectrolysis. Nano Lett. 2007, 7, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Cheng, L.; Wulfmeier, H.; Albrecht, D.; Fritze, H.; Bund, A. Electrochemical behavior of anodically obtained titania nanotubes in organic carbonate and ionic liquid based Li ion containing electrolytes. Electrochim. Acta 2013, 104, 228–235. [Google Scholar] [CrossRef]
- Li, H.; Martha, S.K.; Unocic, R.R.; Luo, H.; Dai, S.; Qu, J. High cyclability of ionic liquid-produced TiO2 nanotube arrays as an anode material for lithium-ion batteries. J. Power Sources 2012, 218, 88–92. [Google Scholar] [CrossRef]
- Ryu, W.H.; Nam, D.H.; Ko, Y.S.; Kim, R.H.; Kwon, H.-S. Electrochemical performance of a smooth and highly ordered TiO2 nanotube electrode for Li-ion batteries. Electrochim. Acta 2012, 61, 19–24. [Google Scholar] [CrossRef]
- Fang, H.T.; Liu, M.; Wang, D.W.; Sun, T.; Guan, D.S.; Li, F.; Zhou, J.; Sham, T.K.; Cheng, H.M. Comparison of the rate capability of nanostructured amorphous and anatase TiO2 for lithium insertion using anodic TiO2 nanotube arrays. Nanotechnology 2009, 20, 225701. [Google Scholar] [CrossRef] [PubMed]
- Tighineanu, A.; Ruff, T.; Albu, S.; Hahn, R.; Schmuki, P. Conductivity of TiO2 nanotubes: Influence of annealing time and temperature. Chem. Phys. Lett. 2010, 494, 260–263. [Google Scholar] [CrossRef]
- Guan, D.; Cai, C.; Wang, Y. Amorphous and Crystalline TiO2 Nanotube Arrays for Enhanced Li-ion intercalation properties. J. Nanosci. Nanotechnol. 2011, 11, 3641–3650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Huang, K.; Fang, D.; Zhuang, S. Electrochemical properties of freestanding TiO2 nanotube membranes annealed in Ar for lithium anode material. J. Solid State Electrochem. 2012, 16, 723–729. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, P.; Zhang, Y.; Garcia, B.B.; Zhang, Q.; Guo, Q.; Champion, R.; Cao, G. TiO2 nanotube arrays annealed in N2 for efficient lithium-ion intercalation. J. Phys. Chem. C 2008, 112, 11175–11180. [Google Scholar] [CrossRef]
- Lu, Z.; Yip, C.T.; Wang, L.; Huang, H.; Zhou, L. Hydrogenated TiO2 nanotube arrays as high-rate anodes for lithium-ion microbatteries. ChemPlusChem 2012, 77, 991–1000. [Google Scholar] [CrossRef]
- Wei, W.; Oltean, G.; Tai, C.W.; Edström, K.; Björefors, F.; Nyholm, L. High energy and power density TiO2 nanotube electrodes for 3D Li-ion microbatteries. J. Mater. Chem. A 2013, 1, 8160–8169. [Google Scholar] [CrossRef]
- Liu, G.; Hoivik, N.; Wang, K. Small diameter TiO2 nanotubes with enhanced photoresponsivity. Electrochem. Commun. 2013, 28, 107–110. [Google Scholar] [CrossRef]
- Kilinç, N.; Şennik, E.; Işik, M.; Ahsen, A.Ş.; Öztürk, O.; Öztürk, Z.Z. Fabrication and gas sensing properties of C-doped and un-doped TiO2 nanotubes. Ceram. Int. 2014, 40, 109–115. [Google Scholar] [CrossRef]
- Mole, F.; Wang, J.; Clayton, D.A.; Xu, C.; Pan, S. Highly conductive nanostructured C-TiO2 electrodes with enhanced electrochemical stability and double layer charge storage capacitance. Langmuir 2012, 28, 10610–10619. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Y.; Luo, S.; Liu, C.; Li, Y. TiO2 nanotube arrays co-loaded with Au nanoparticles and reduced graphene oxide: Facile synthesis and promising photocatalytic application. J. Alloy. Compd. 2013, 578, 242–248. [Google Scholar] [CrossRef]
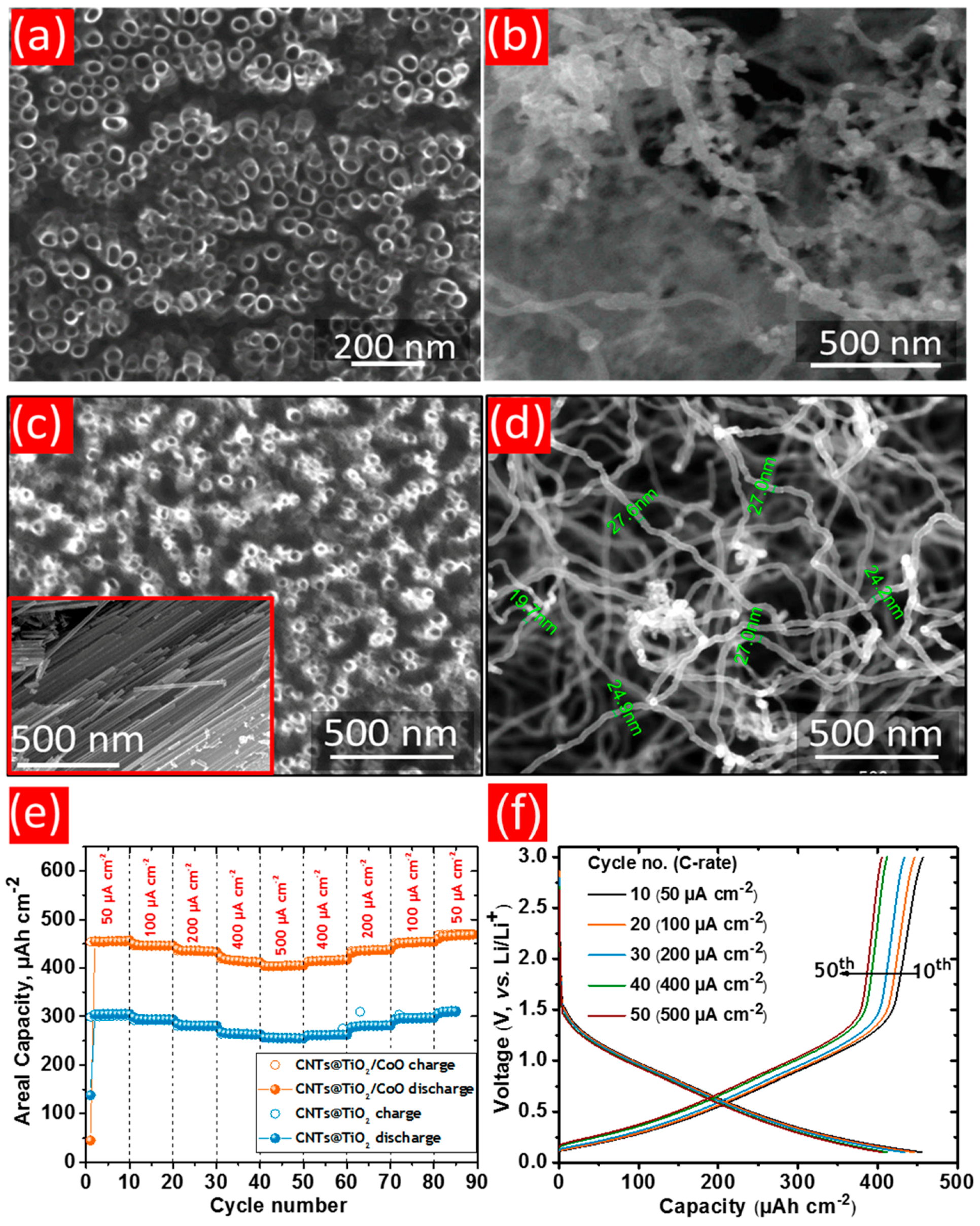
- Madian, M.; Ummethala, R.; Naga, A.O.A.E.; Ismail, N.; Rümmeli, M.H.; Eychmüller, A.; Giebeler, L. Ternary CNTs@TiO2/CoO nanotube composites: Improved anode materials for high performance lithium ion batteries. Materials 2017, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, N.; Zhang, L.; Shao, H.; Wang, J.; Zhang, J.; Cao, C. Co3O4-coated TiO2 nanotube composites synthesized through photo-deposition strategy with enhanced performance for lithium-ion batteries. Electrochim. Acta 2013, 94, 285–293. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.G.; Shen, W.D.; Wang, S.X.; Liu, H.; Wang, J.L. Cobalt oxide modified highly ordered TiO2 nanotube arrays: Enhanced visible light photoelectrochemical properties. Sci. Adv. Mater. 2013, 5, 1256–1263. [Google Scholar] [CrossRef]
- Macak, J.M.; Gong, B.G.; Hueppe, M.; Schmuki, P. Filling of TiO2 nanotubes by self-doping and electrodeposition. Adv. Mater. 2007, 19, 3027–3031. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Banerjee, S.; Misra, M. Synthesis of Fe2O3/TiO2 nanorod-nanotube arrays by filling TiO2 nanotubes with Fe. Nanotechnology 2008, 19, 315601. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Z.; Zhang, L.; Wu, H.B.; Lou, X.W.D. TiO2 nanotube arrays grafted with Fe2O3 hollow nanorods as integrated electrodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 122–127. [Google Scholar] [CrossRef]
- Herrera, J.E.; Isimjan, T.T.; Abdullahi, I.; Ray, A.; Rohani, S. A novel nanoengineered VOx catalyst supported on highly ordered TiO2 nanotube arrays for partial oxidation reactions. Appl. Catal. A Gen. 2012, 417, 13–18. [Google Scholar] [CrossRef]
- Guan, D.; Li, J.; Gao, X.; Yuan, C. Controllable synthesis of MoO3-deposited TiO2 nanotubes with enhanced lithium-ion intercalation performance. J. Power Sources 2014, 246, 305–312. [Google Scholar] [CrossRef]
- Gobal, F.; Faraji, M. Fabrication of nanoporous nickel oxide by de-zincification of Zn–Ni/(TiO2-nanotubes) for use in electrochemical supercapacitors. Electrochim. Acta 2013, 100, 133–139. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Hanzu, I.; Lavela, P.; Knauth, P.; Tirado, J.L.; Djenizian, T. Nanoarchitectured TiO2/SnO2: A future negative electrode for high power density Li-ion microbatteries. Chem. Mater. 2010, 22, 1926–1932. [Google Scholar] [CrossRef]
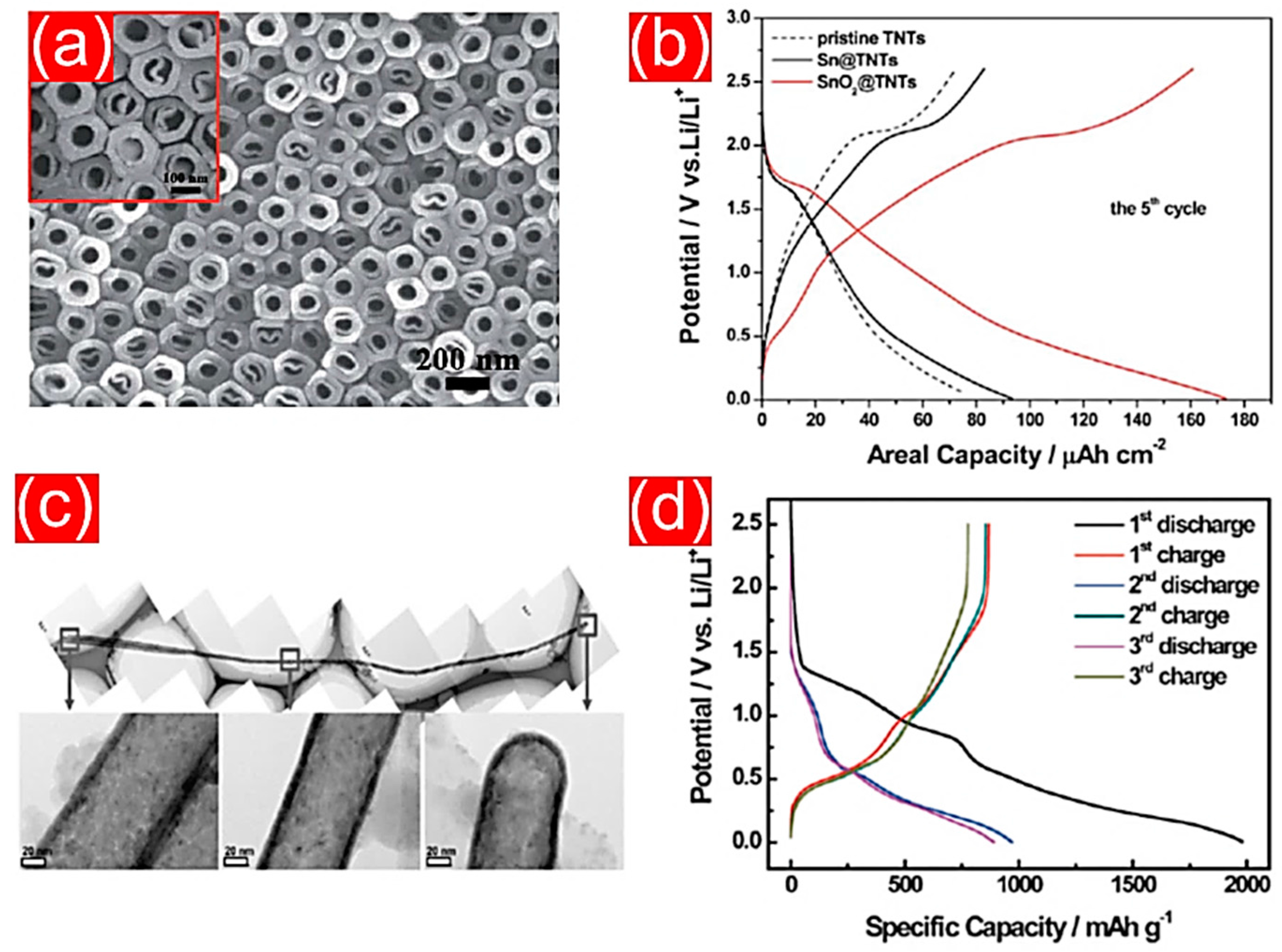
- Wu, X.; Zhang, S.; Wang, L.; Du, Z.; Fang, H.; Ling, Y.; Huang, Z. Coaxial SnO2@TiO2 nanotube hybrids: From robust assembly strategies to potential application in Li+ storage. J. Mater. Chem. 2012, 22, 11151–11158. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Lee, S.; Seo, S.; Bae, C.; Shin, H. Nanotubular heterostructure of tin dioxide/titanium dioxide as a binder-free anode in lithium-ion batteries. ChemSusChem 2015, 8, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; Ortiz, G.F.; González, J.R.; Alcántara, R.; Tirado, J.L. Improving the Performance of Titania Nanotube Battery Materials by Surface Modification with Lithium Phosphate. ACS Appl. Mater. Interfaces 2014, 6, 5669–5678. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.; Hahn, R.; Schmuki, P. Ultrafast oxide nanotube formation on TiNb, TiZr and TiTa alloys by rapid breakdown anodization. Electrochim. Acta 2010, 55, 8883–8887. [Google Scholar] [CrossRef]
- Allam, N.K.; Poncheri, A.J.; El-Sayed, M.A. Vertically oriented Ti-Pd mixed oxynitride nanotube arrays for enhanced photoelectrochemical water splitting. ACS Nano 2011, 5, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, K.-H.; Shin, J.; Park, S.H.; Kang, J.S.; Han, K.S.; Sung, M.M.; Pinna, N.; Sung, Y.-E. Highly ordered and vertically oriented TiO2/Al2O3 nanotube electrodes for application in dye-sensitized solar cells. Nanotechnology 2014, 25, 504003. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.T.C.; Verdério, J.F.; Bolfarini, C. Obtaining self-organized nanotubes on biomedical Ti–Mo alloys. Electrochem. Commun. 2013, 35, 139–141. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, D.; Yang, M.; Schmuki, P. Vertically aligned mixed V2O5-TiO2 nanotube arrays for supercapacitor applications. Chem. Commun. 2011, 47, 7746–7748. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, I.; Nah, Y.C.; Das, C.; Shrestha, N.K.; Schmuki, P. WO3/TiO2 nanotubes with strongly enhanced photocatalytic activity. Chem. Eur. J. 2010, 16, 8993–8997. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.S.; Misra, M. Ordered Arrays of Ti-Mn Oxide Nanotubes for High Capacity Li-ion Battery. ECS Trans. 2011, 33, 31–44. [Google Scholar] [CrossRef]
- Kim, J.H.; Zhu, K.; Yan, Y.; Perkins, C.L.; Frank, A.J. Microstructure and pseudocapacitive properties of electrodes constructed of oriented NiO-TiO2 nanotube arrays. Nano Lett. 2010, 10, 4099–4104. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Deyab, N.M.; Ghany, N.A. Ternary Ti-Mo-Ni mixed oxide nanotube arrays as photoanode materials for efficient solar hydrogen production. Phys. Chem. Chem. Phys. 2013, 15, 12274–12282. [Google Scholar] [CrossRef] [PubMed]
- Filova, E.; Fojt, J.; Kryslova, M.; Moravec, H.; Joska, L.; Bacakova, L. The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells. Int. J. Nanomed. 2015, 10, 7145–7163. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, G.; Spiecker, E.; Lee, K.; Schmuki, P. Ordered “superlattice” TiO2/Nb2O5 nanotube arrays with improved ion insertion stability. Chem. Commun. 2013, 49, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Madian, M.; Klose, M.; Jaumann, T.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J.; Giebeler, L. Anodically fabricated TiO2–SnO2 nanotubes and their application in lithium ion batteries. J. Mater. Chem. A 2016, 2, 5542–5552. [Google Scholar] [CrossRef]

| Rechargeable Battery | Specific Energy, Wh kg−1 | Energy Density, Wh L−1 |
|---|---|---|
| Pb–acid | 30 | 80 |
| Ni–Cd | 40 | 90 |
| Ni–MH | 55 | 165 |
| Ni–Zn | 70 | 145 |
| Ag–Zn | 75 | 200 |
| Li ion | 265 | 690 |
| Composition | Synthesis | Capacity after 20th Cycle | Current Rate | Number of Reversible Cycles | Reference |
|---|---|---|---|---|---|
| Rutile nanoparticles | Commercially obtained | 207 mAh g−1 | 0.05 A g−1 | 20 | [65] |
| Anatase nanoparticles | solvothermal | 196 mAh g−1 | 0.2 C | 100 | [69] |
| TiO2@C hollow spheres | sol-gel hydrothermal | 170 mAh g−1 | 2 C | 300 | [72] |
| TiO2 nanoparticles@graphene | in-situ hydrolysis | 70 mAh g−1 | 5 A g−1 | 400 | [75] |
| Core-shell CNTs/TiO2 | hydrolysis | 240 mAh g−1 | 5 A g−1 | 100 | [74] |
| Anatase nanorods | hydrolysis | 206 mAh g−1 | 50 mA g−1 | 30 | [90] |
| Anatase nanowires | hydrolysis | 280 mAh g−1 | 140 mA g−1 | 40 | [92] |
| TiO2 nanofibers | electrospinning | 170 mAh g−1 | 0.3 C | 50 | [95] |
| C-doped TiO2 nanowires | hydrothermal | 306 mAh g−1 | 0.1 C | 1000 | [96] |
| TiO2 NT@graphene | hydrothermal | 357 mAh g−1 | 10 mA g−1 | 50 | [98] |
| TiO2/C hollow nanofibers | force spinning | 229 mAh g−1 | 100 mA g−1 | 100 | [100] |
| Graphene-wrapped TiO2 nanofibers | electrospinning | 200 mAh g−1 | 0.1 C | 35 | [101] |
| Anatase/graphene nanosheets | solvothermal | 161 mAh g−1 | 1 C | 120 | [102] |
| TiO2/graphene | hydrolysis | 230 mAh g−1 | 0.1 C | 100 | [103] |
| TiO2 nanoporous hollow spheres | template-assisted | 230 mAh g−1 | 33.5 mA g−1 | 50 | [106] |
| Mesoporous TiO2 nanoparticles | template-assisted | 268 mAh g−1 | 0.2 C | 30 | [107] |
| C-coated mesoporous TiO2@graphene sheets | solvothermal | 111 mAh g−1 | 0.2 A g−1 | 100 | [109] |
| Fe2O3 nanorods-TiO2 | thermal treatments | 860 mAh g−1 | 1 A g−1 | 1000 | [112] |
| Hierarchical TiO2/Fe2O3 fiber-in-tube | electrospinning | 987 mAh g−1 | 100 mA g−1 | 240 | [113] |
| Hierarchically ordered porous anatase | chemical etching | 191 mAh g−1 | 1 A g−1 | 60 | [115] |
| Hierarchical nanosheets | solvothermal alcoholysis | 225 mAh g−1 | 1 C | 700 | [118] |
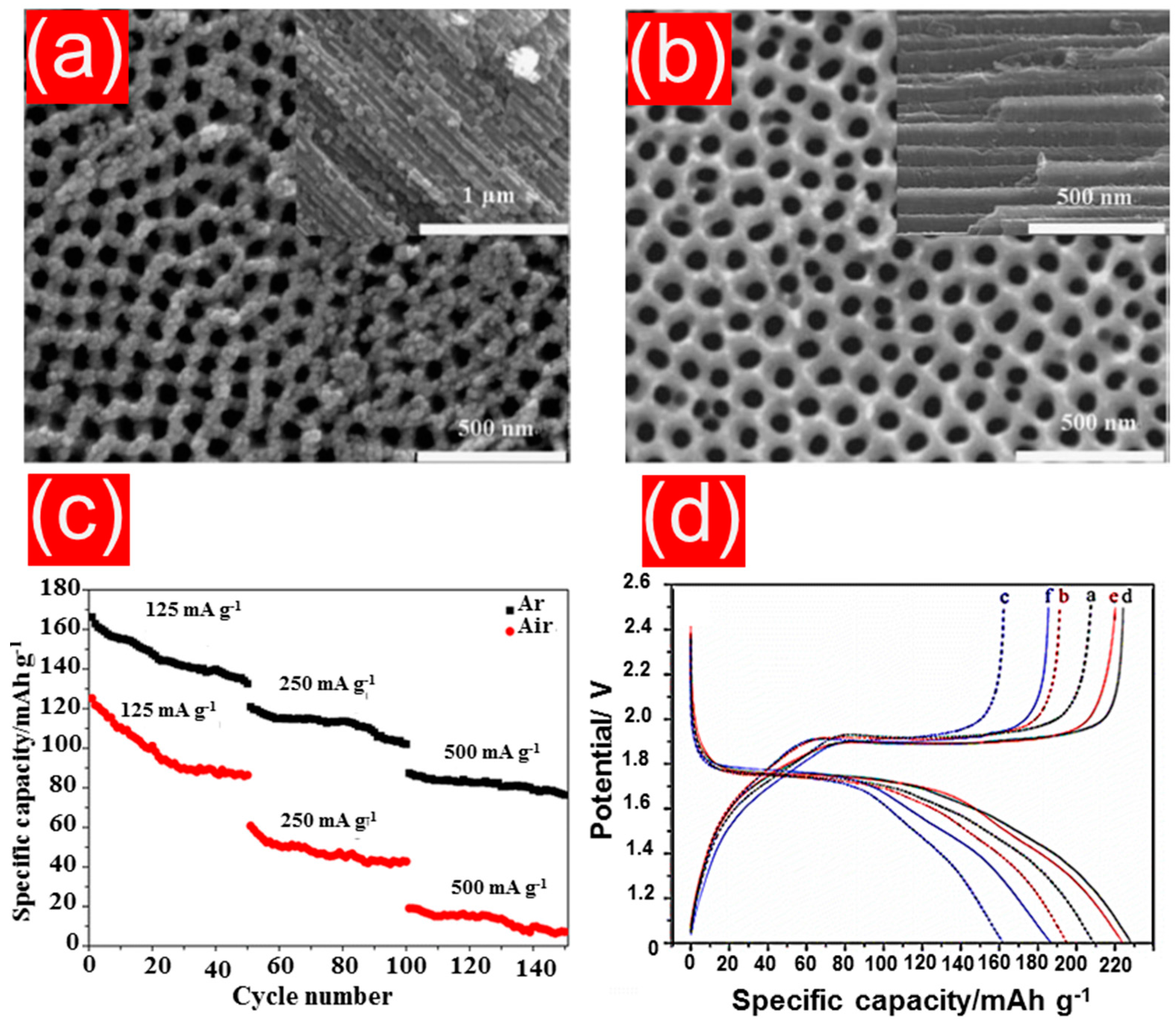
| TiO2 NTs | anodization | 140 mAh g−1 | 0.1 C | 1000 | [145] |
| 3D free-standing TiO2 NTs | anodization | 184 mAh g−1 | 0.05 mA cm−2 | 500 | [157] |
| SnO2/TiO2 NTs | atomic layer deposition | 250 mAh g−1 | 0.1 C | 50 | [173] |
| Ti-Mn-O NTs | anodization | 474 mAh g−1 | 175 mA g−1 | 30 | [181] |
| CoO/TiO2 NTs | anodization | 600 μAh cm−2 | 10 µA cm−2 | 100 | [125] |
| TiO2-SnO2 NTs | anodization | 405 μAh cm−2 | 504 µA cm−2 | 400 | [186] |
| CoO/TiO2NTs | anodization—chemical vapor deposition | 450 μAh cm−2 | 50 µA cm−2 | 90 | [156] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. https://doi.org/10.3390/batteries4010007
Madian M, Eychmüller A, Giebeler L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries. 2018; 4(1):7. https://doi.org/10.3390/batteries4010007
Chicago/Turabian StyleMadian, Mahmoud, Alexander Eychmüller, and Lars Giebeler. 2018. "Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries" Batteries 4, no. 1: 7. https://doi.org/10.3390/batteries4010007
APA StyleMadian, M., Eychmüller, A., & Giebeler, L. (2018). Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries, 4(1), 7. https://doi.org/10.3390/batteries4010007