Abstract
The effects of Cs2CO3 addition in a KOH-based electrolyte were investigated for applications in nickel/metal hydride batteries. Both MgNi-based and Laves phase-related body-centered cubic solid solution metal hydride alloys were tested as the anode active materials, and sintered β-Ni(OH)2 was used as the cathode active material. Certain amounts of Cs2CO3 additive in the KOH-based electrolyte improved the electrochemical performances compared with a conventional pure KOH electrolyte. For example, with Laves phase-related body-centered cubic alloys, the addition of Cs2CO3 to the electrolyte improved cycle stability (for all three alloys) and discharge capacity (for the Al-containing alloys); moreover, in the 0.33 M Cs2CO3 + 6.44 M KOH electrolyte, the discharge capacity of Mg52Ni39Co3Mn6 increased to 132%, degradation decreased to 87%, and high-rate dischargeability stayed the same compared with the conventional 6.77 M KOH electrolyte. The effects of Cs2CO3 on the physical and chemical properties of Mg52Ni39Co3Mn6 were characterized by Fourier transform infrared spectroscopy, X-ray diffraction, transmission electron microscopy, inductively coupled plasma, and electrochemical impedance spectroscopy. The results from these analyses concluded that Cs2CO3 addition changed both the alloy surface and bulk composition. A fluffy layer containing carbon was found covering the metal particle surface after cycling in the Cs2CO3-containing electrolyte, and was considered to be the main cause of the reduction in capacity degradation during cycling. Also, the Cs2CO3 additive promoted the formations of the C–O and C=O bonds on the alloy surface. The C–O and C=O bonds were believed to be active sites for proton transfer during the electrochemical process, with the C–O bond being the more effective of the two. Both bonds contributed to a higher surface catalytic ability. The addition of 0.33 M Cs2CO3 was deemed optimal in this study.
1. Introduction
Since the commercialization of nickel/metal hydride (Ni/MH) batteries in 1980s, they have been widely used as energy storage devices in hybrid electric vehicles, vacuum cleaners, electric toys, power tools, and cordless phones, to name a few uses [1,2,3]. Ni/MH batteries have many superior properties over rival battery technologies, such as high specific power, long cycle life, robust abuse tolerance, and a wide temperature operation range [2]. In the past decades, Ni/MH batteries have repeatedly attracted attention from both researchers and markets.
Basic Ni/MH battery electrochemistry is shown in the following reactions:
| Negative electrode: | M + H2O + e− ⇌ OH− + MH | (1) |
| Positive electrode: | Ni(OH)2 + OH− ⇌ NiOOH + H2O + e− | (2) |
The reaction taking place at the negative electrode (anode) is described in Equation (1). M is a metal hydride (MH) alloy capable of storing hydrogen reversibly, and MH is the corresponding hydrided metal. During charge, the added voltage splits the water molecule into a proton and a hydroxide ion. Driven by voltage and diffusion difference, protons transfer from the electrolyte to the surface of the MH alloy particles, and then into the bulk of alloy. During discharge, protons travel in a reverse route. Equation (2) represents the reaction at the positive electrode (cathode). During charge, protons are dissociated from Ni(OH)2, then move to the cathode surface, and finally recombine with the hydroxide ions in the electrolyte.
In order to promote proton transfer and improve electrochemical performance, many researchers and companies have focused on developing new anode/cathode materials, with a particular focus on anode materials. A wide set of hydrogen storage MH alloys have been studied for electrochemical applications, including AB2, AB5, A2B7, body-centered cubic (BCC) solid solution, BCC-AB2 composite, MgxNiy, TiNi and its composite, etc. [4,5,6,7,8,9]. Yu et al. reported a Ti40V30Cr15Mn15 alloy with an initial capacity of 814 mAh·g‒1 at a rate of 10 mA·g‒1 and 80 °C, which is more than twofold higher than the capacity obtained from the conventional rare earth-based AB5 MH alloy (350 mAh·g‒1) [10]. However, degradation for this alloy was very high due to the pulverization caused by hydrogen evolution inside MH alloy particles. Young and Nei reported various MgNi-based amorphous/microcrystalline MH alloys with a theoretical capacity as high as 1080 mAh·g‒1 [11]. However, most of these alloys demonstrated rapid decay during cycling. Nei reported an 80% or higher decay in capacity after 20 cycles for MgNi-based MH alloys [12]. Many methods have been attempted to improve the electrochemical performance of MH alloys. Recently, additives such as B [13], Ti [14,15], Pt [15], Pd [14], Nd [16], Cr [17], La [18], Co [19], Ni [20], Li [21], and Cu [22] were added to the bulk or surface of MH alloys to enhance the capacity, cycle stability, and high-rate dischargeability (HRD). For MgNi-based MH alloys, Ni coating [23], the addition of TiO2 [24], and substitutions of Mn [25,26] and Nb [26] have been intensively studied with the goal of improving electrochemical performances. Many studies on the alkaline electrolytes [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] and salt additives [50,51] were conducted before in NiMH and other alkaline batteries; however, focuses were on the rare earth-based AB5 and Zr-based AB2 MH alloys. Young et al. have pointed out that electrolyte modification is one of the most economic and effective methods to alter electrode performances, since it does not affect the battery gravimetric and volumetric energy densities [11]. Nei et al. reported the conductivity and corrosion behaviors of several hydroxides [16]. Later, Yan et al. published a screen test of 32 salt additives in the KOH electrolyte, of which 12 salt additives were found to efficiently decrease the corrosion of the traditional KOH electrolyte on alloy AR3 (an MgNi-based MH alloy with a nominal composition of Mg52Ni39Co3Mn6) [52]. However, no detailed investigation was done on these 12 salt additives.
This study is a continuous work from Yan’s previous report [52]. A systematic investigation of the effects of Cs2CO3 addition in a conventional KOH electrolyte on various MH alloys is performed. This additive was originally used in the KOH electrolyte for Ni/Zn batteries to extend the cycle life [53]. The total concentration of Cs2CO3 and KOH is fixed at 6.77 M. The influence of Cs2CO3 on the cell performance, electrolyte properties, and surface and bulk structure of MH alloy electrodes are examined. A possible proton transfer process for the Cs2CO3-containing electrolyte system is also discussed.
2. Experimental Setup
Both the sintered β-Ni(OH)2 and MH alloys (AR3, P31, P32, and P37) were produced in-house. The compositions and fabrication methods of the MH alloys are summarized in Table 1. AR3 is an amorphous/microcrystalline MgNi-based MH alloy made by a melt–spin method, followed by mechanical alloying [52]. The P-series MH alloys belong to a family of Laves phase-related body-centered cubic (BCC) solid solution alloys [54], which were developed during a United States (U.S.) Department of Energy-funded research program [4]. The P-series of alloys were produced by induction melting, followed by annealing under optimized conditions (900 °C for 12 h [55]). KOH and Cs2CO3 were purchased from the Sigma-Aldrich Corporation (St. Louis, MO, USA).

Table 1.
Compositions of the four metal hydride (MH) alloys used in this study. BCC: body-centered cubic.
Electrochemical charge/discharge cycling tests were performed with an Arbin BT2000 battery tester (Arbin, College Station, TX, USA) at room temperature. In the test cells, the cathode was sintered β-Ni(OH)2, the anode was made from directly dry-compacting the alloy powder onto an expanded Ni substrate without using any binder, and the separator was hydrophilic nonwoven polyolefin. Charge/discharge processes were the same as reported before by Yan et al. [52] The cell was charged at 100 mA·g‒1 for 5 h, and discharged first at 100 mA·g−1 to a cutoff voltage of 0.9 V. The initial discharge was followed by a 30 s rest for the voltage to recover, and then the cell was discharged at 24 mA·g−1 to reach a cutoff voltage of 0.9 V. The cell was put to rest for 30 s again before the final discharge at 8 mA·g−1 to 0.9 V. Testing for each alloy/electrolyte combination was repeated three times. When the total discharge capacity (sum of capacities at 100, 24, and 8 mA·g−1) of the cell decreased by 70%, it was considered to be cell failure.
Discharge capacity degradation and HRD were calculated and compared with those of a traditional 6.77 M KOH electrolyte. Degradation was determined as Yan previously reported [52]. The percent capacity loss per cycle within the initial 10 cycles is shown by the following equation:
where is the highest value of discharge capacity achieved in the initial 10 cycles, is the lowest value of discharge capacity in the initial 10 cycles, is the cycle number of the highest discharge capacity in the initial 10 cycles, and is the cycle number of the lowest discharge capacity in the initial 10 cycles. HRD is defined as the ratio of capacities measured at 100 and 8 mA·g−1.
Fourier transform infrared (FTIR) spectroscopy was performed on a Perkin Elmer Spectrum Spotlight 200™ (Perkin Elmer, Waltham, MA, USA). Powder X-ray diffraction (XRD) patterns were taken with a Rigaku RU2000 rotating anode powder diffractometer (Rigaku Americas Corporation, The Woodlands, TX, USA) equipped with Cu–Kα radiation (40 kV, 200 mA). Transmission electron microscopy (TEM) was carried out using a JEOL 2010 (JEOL, Tokyo, Japan) operated at 200 kV for microstructural and morphological studies. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was performed on a Perkin Elmer Optima TM 2100 DV ICP-OES system (Perkin Elmer, Waltham, MA, USA). Electrochemical impedance spectroscopy was measured on a Solartron S1287 potentiostat/galvanostat with a S1255 frequency response analyzer (Solartron, Hampshire, UK).
3. Results and Discussion
3.1. Electrochemical Performances for Electrolytes with Cs2CO3 Addition
Generally, KOH solutions with concentrations varying from 4.0 M to 8.5 M are used for Ni/MH batteries in the research field and for commercial applications [11,56,57,58]. In this study, the electrolyte concentration (KOH + Cs2CO3) is fixed at 6.77 M. Concentrations of KOH and Cs2CO3 in various electrolytes are shown in Table 2.

Table 2.
Normalized discharge capacities, degradations, and high-rate dischargeabilities (HRDs) of AR3 cycled in five different electrolytes.
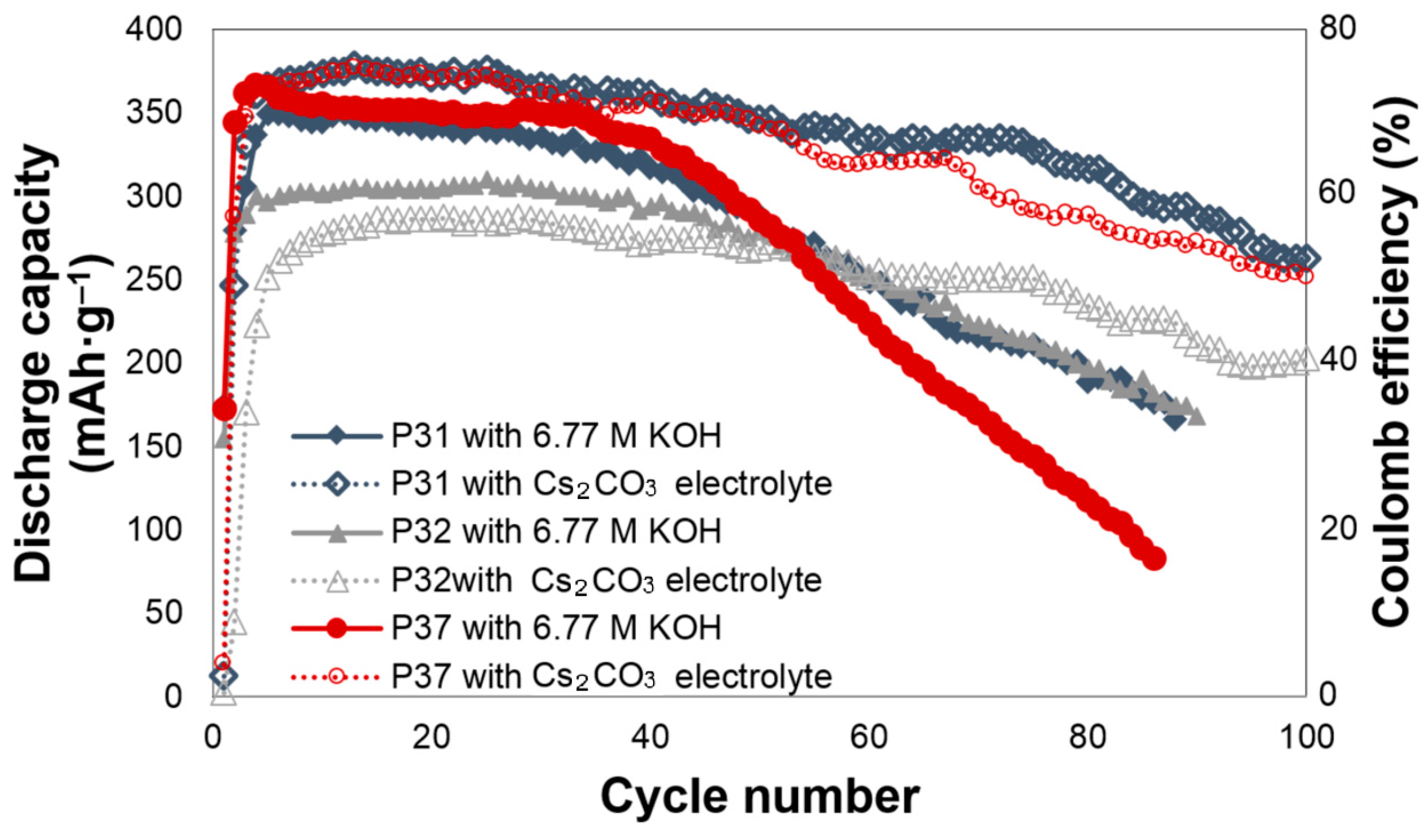
The effects of Cs2CO3 addition on the electrochemical performances of alloys P31, P32, and P37 are shown in Figure 1. All of the cells require approximately three to five cycles to be activated. For alloy P31, Cs2CO3 slightly increases the initial discharge capacity, and greatly decreases the degradation. The initial discharge capacities of alloy P31 in the 6.77 M KOH and 6.44 M KOH + 0.33 M Cs2CO3 electrolytes are 349 and 375 mAh·g‒1, respectively. In the 6.77 M KOH electrolyte, the capacity of alloy P31 begins to fade after the 20th cycle, while the capacity fade begins at the 55th cycle in the 6.44 M KOH + 0.33 M Cs2CO3 electrolyte. The same trend was observed in alloy P37, where the addition of Cs2CO3 increases the initial discharge capacity from 364 mAh·g‒1 to 375 mAh·g‒1, and changes the beginning of the capacity fade from the 30th to the 65th cycle. For alloy P32, Cs2CO3 does not increase the initial discharge capacity; however, it greatly decreases the decay, as shown in Figure 1. In comparison to alloy P32, both alloys P31 and P37 contain Al, raising the possibility that the presence of Al results in the alloy surface reacting with the CO32− ions in the electrolyte and forming Al2(CO3)3. Al2(CO3)3, which is known to be unstable in water [59]. Such phenomenon may assist in the dissolution of Al into the highly alkaline electrolyte, and therefore increase the reactive surface area.
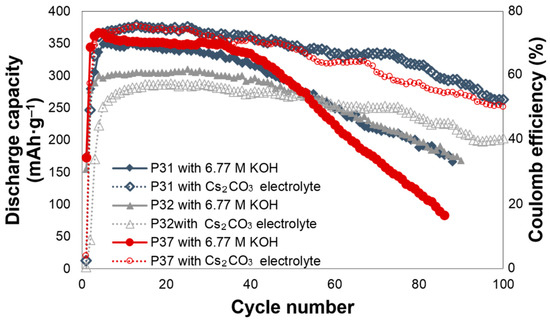
Figure 1.
Discharge capacities of alloys P31, P32, and P37 in the 6.77 M KOH and 6.44 M KOH + 0.33 M Cs2CO3 electrolytes.
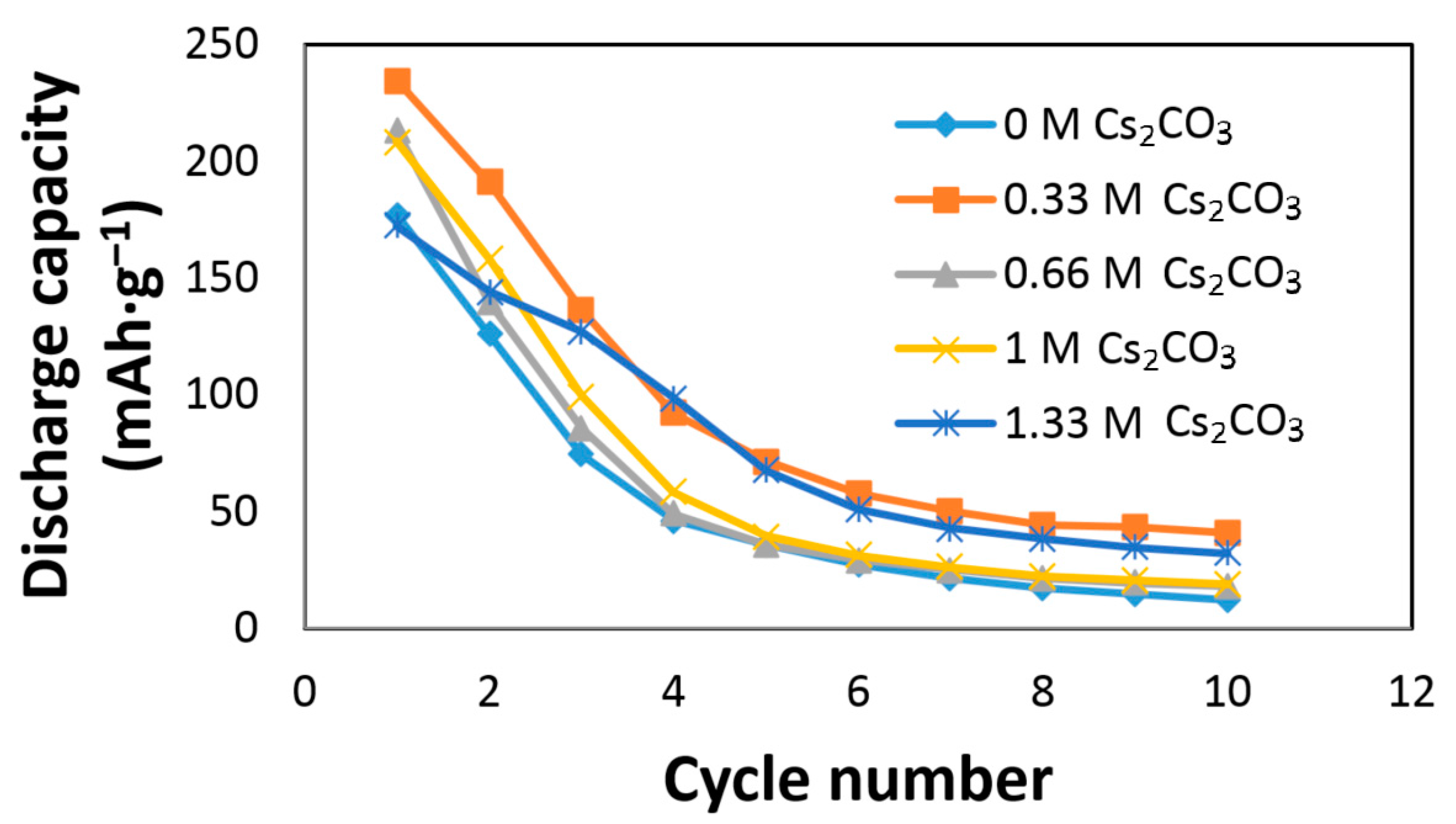
The effects of Cs2CO3 on the AR3 alloy electrode performances are shown in Figure 2. The AR3 MH alloy is very reactive to the KOH electrolyte due to the alloy’s high content of Mg and the high porosity caused by the mechanical alloying preparation [12]. A rapid decay in the KOH electrolyte was previously reported [5,6,12]. Figure 2 shows an increase in initial discharge capacity and a decrease in capacity decay with the addition of Cs2CO3. The discharge capacity, degradation, and HRD of the Cs2CO3-containing electrolytes are normalized to those of the 6.77 M KOH electrolyte and presented in Table 2. The optimized conditions were obtained at the concentration of 6.44 M KOH + 0.33 M Cs2CO3, which exhibited the highest discharge capacity and lowest degradation. Table 2 also indicates that a small addition of Cs2CO3 has an insignificant effect on HRD, while a high concentration of Cs2CO3 in the electrolyte has a negative influence on HRD.
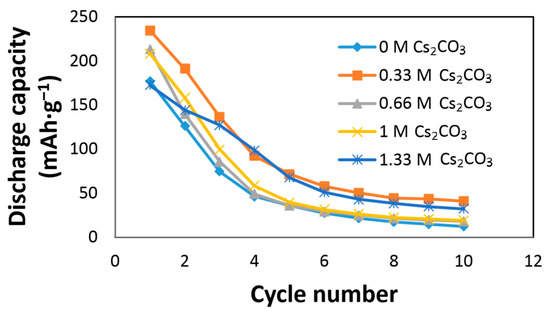
Figure 2.
Discharge capacities of AR3 in 6.77 M KOH, 6.44 M KOH + 0.33 M Cs2CO3, 6.11 M KOH + 0.66 M Cs2CO3, 5.77 M KOH + 1.00 M Cs2CO3, and 5.44 M KOH + 1.33 M Cs2CO3 electrolytes.
3.2. Effects of Cs2CO3 Addition on MgNi Alloy
Weights of the cycled AR3 alloy electrodes were measured. After 10 cycles, weights of the alloy electrodes cycled in all of the electrolytes increased due to surface metal oxidation and the deposition of some salts. Surface metal oxidation during charge leads to the formation of surface metal hydroxide, such as Mg(OH)2 [11,16]. The majority of salt depositions are carbonates and bicarbonates [52]. Weight gains of the alloy electrodes cycled in the Cs2CO3-containing electrolytes are normalized to that in the 6.77 M KOH electrolyte and presented in Table 3. As the Cs2CO3 concentration in the electrolyte increases, the weight gain decreases. The addition of Cs2CO3 changes the physical and chemical properties of the electrolyte. Cs2CO3 reacts with the MH alloy surface during electrochemical cycling, which results in a protective layer and greatly decreases the reaction rate of metal oxidation. Therefore, the weight gain due to surface oxidation (listed in Table 3) is substantially reduced by the addition of Cs2CO3 in the electrolyte.

Table 3.
Normalized weight gains of the AR3 alloy electrodes cycled in five different electrolytes.
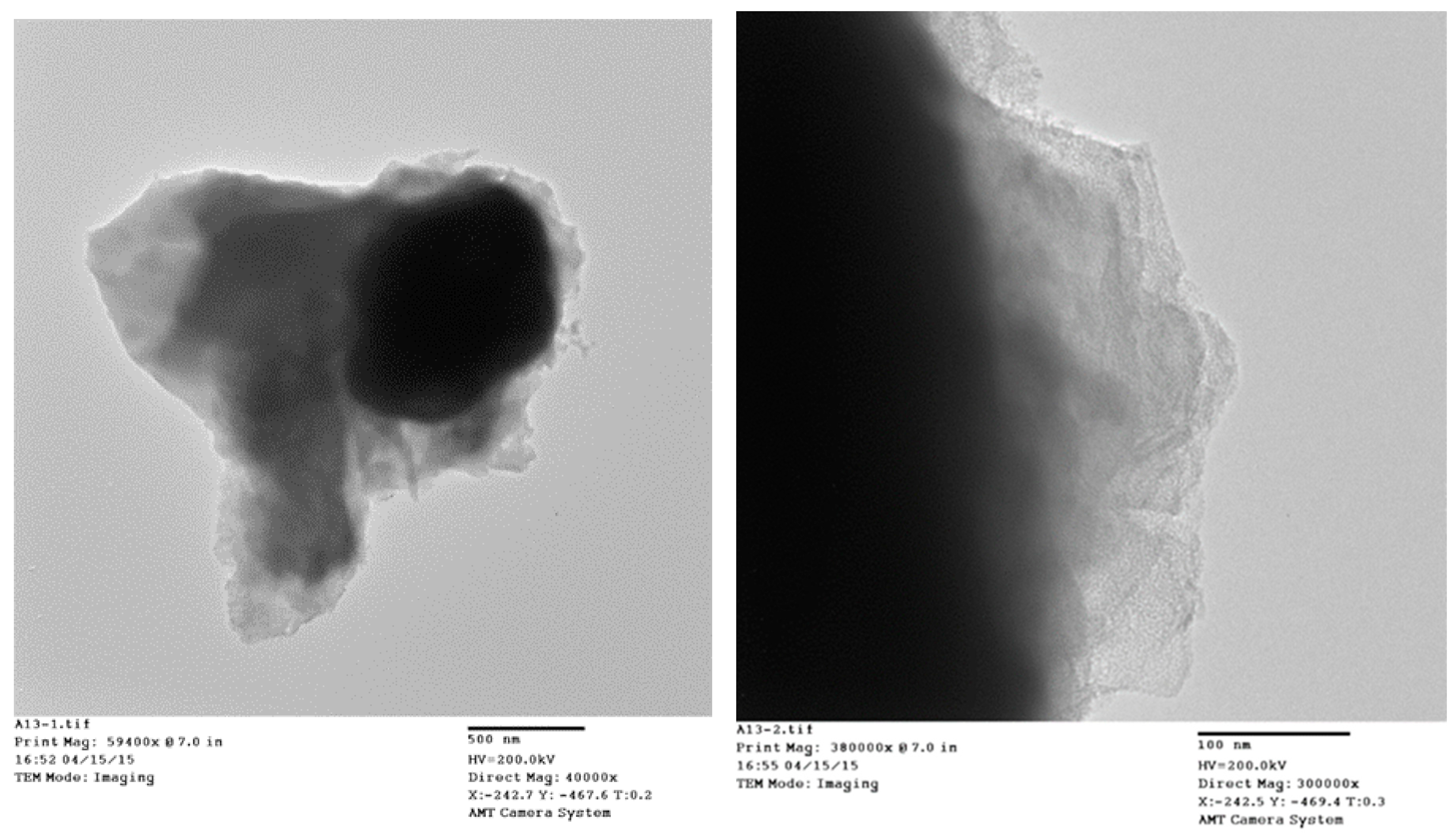
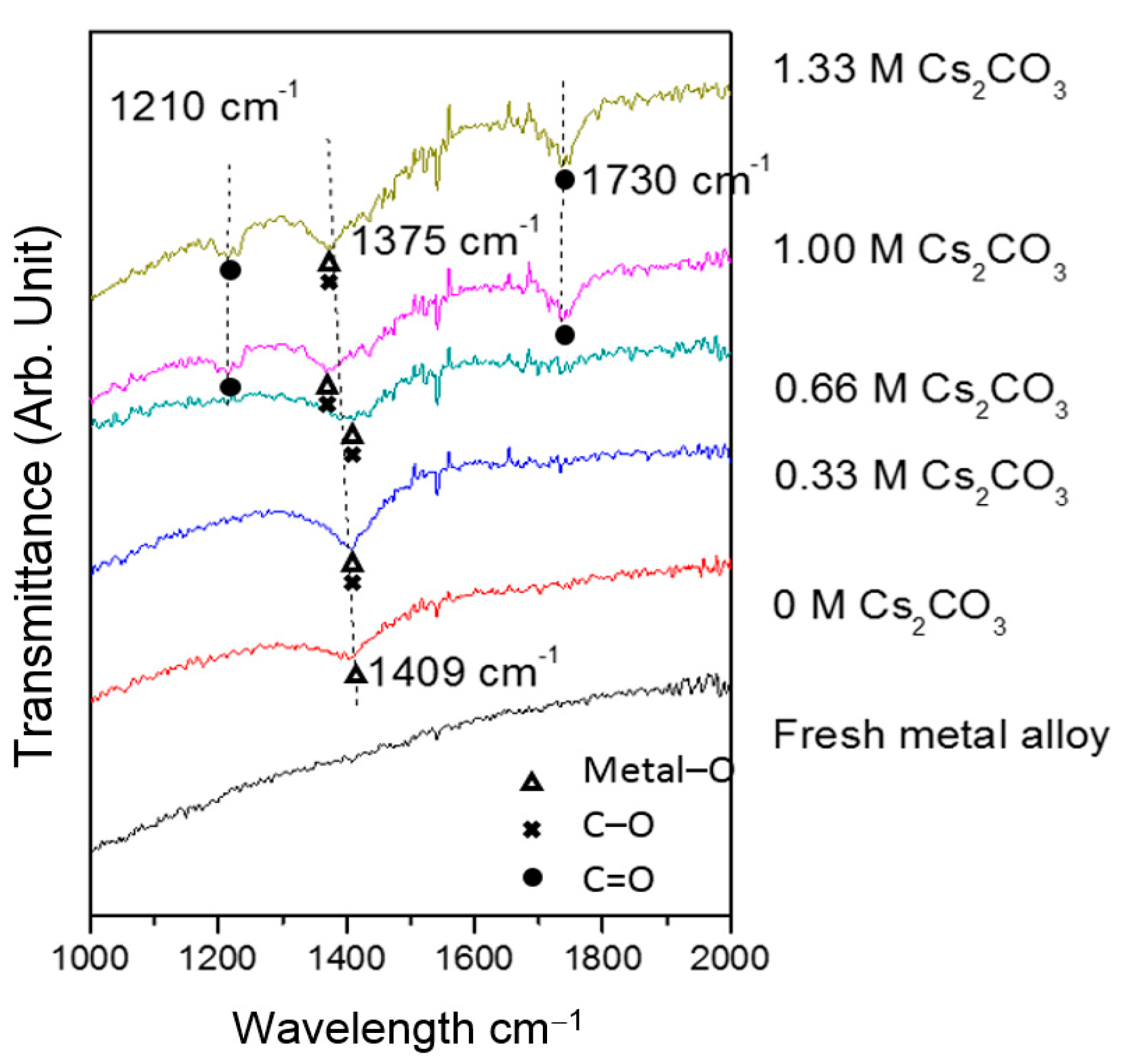
TEM micrographs of the cycled AR3 indicate that a thin layer of fluffy material covers the surface of the MH alloy (Figure 3a,b). FTIR was used to characterize the surface structure, and the results are shown in Figure 4. For the fresh MH alloy, there is no clear diffraction peak, which indicates that the alloy surface is clean. For the alloy cycled in the 6.77 M KOH electrolyte, a peak at approximately 1409 cm‒1 is observed, which is related to the vibration of surface metal–O bonds [60]. For the electrode cycled in the 6.44 M KOH + 0.33 M Cs2CO3 electrolyte, the same peak is seen, but shifted slightly to a lower wavelength, implying a decrease in bond strength. However, the peak intensity increases, which suggests the appearance of C–O bonds on the alloy surface, since the stretching vibration of the C–O bond occurs at approximately the same wavelength [61] as the metal–O bond. With further increases in concentration of Cs2CO3 in the electrolyte, the metal–O bond becomes weaker with the peak shifting even lower, to around 1375 cm‒1, and the amount of C–O bond decreases, as shown by a reduction in peak intensity compared with the electrode cycled in the 6.77 M KOH electrolyte. Moreover, peaks at approximately 1730 and 1210 cm‒1 start to appear as the content of Cs2CO3 increases, which is related to the vibration of the C=O bond in carbonate [61]. FTIR results demonstrate the changes in the surface groups on the MH alloy surface with varying Cs2CO3 concentration.
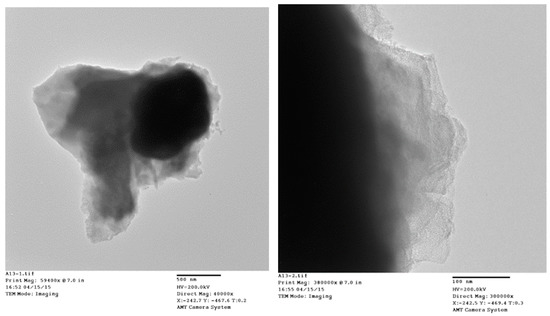
Figure 3.
Transmission electron microscopy (TEM) micrographs at (a) ×40,400 and (b) ×300,000 magnification of AR3 cycled in the 6.44 M KOH + 0.33 M Cs2CO3 electrolyte.
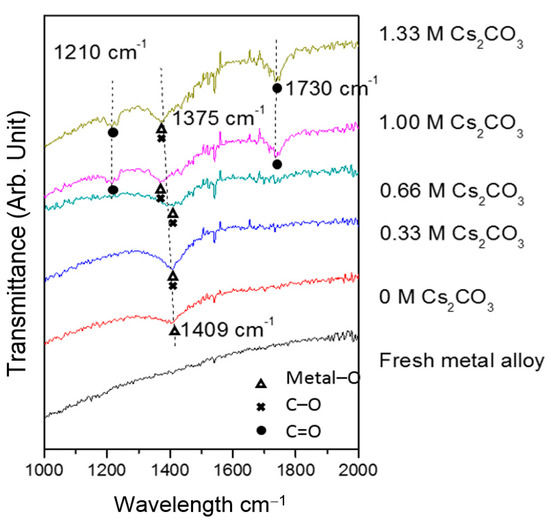
Figure 4.
Fourier transform infrared (FTIR) spectra of the fresh and cycled AR3 in the 6.77 M KOH, 6.44 M KOH + 0.33 M Cs2CO3, 6.11 M KOH + 0.66 M Cs2CO3, 5.77 M KOH + 1.00 M Cs2CO3, and 5.44 M KOH + 1.33 M Cs2CO3 electrolytes.
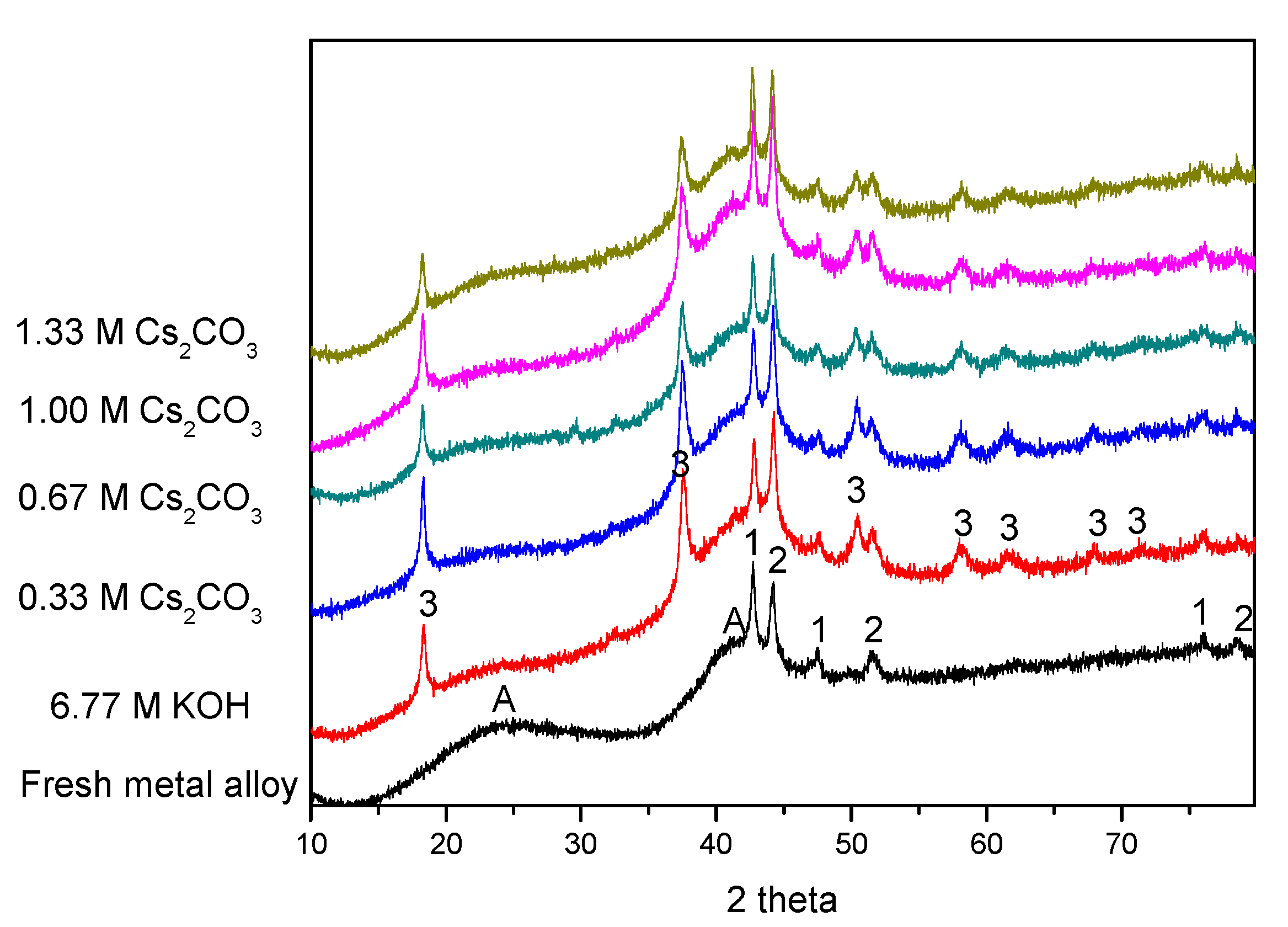
XRD patterns before and after electrochemical cycling are shown in Figure 5. As our previous study has shown [62], the two broad peaks at approximately 23° and 42° (marked as “A”) are from the broadening of the MgNi2 and Mg2Ni phases. These two broad peaks exist in all of the cycled AR3 samples, suggesting high stability for MgNi2 and Mg2Ni during cycling. Except for the peaks marked as “A”, there are some strong peaks related to metallic Mn and Co (marked as “1” and “2” in Figure 5, respectively). Mn and Co fine particles are the remnants from the mechanical alloying process, and act as catalysts for hydrogen storage to enhance the electrochemical capacity [11]. Mg(OH)2 is also found in all of the cycled AR3 (marked as “3” in Figure 5), and is the oxidation product from high-Mg AR3. The peaks at approximately 18° and 33° are the (001) and (100) diffraction peaks for the hexagonal Mg(OH)2. Results from the phase deconvolution by Jade 9.0 software (MDI, Livermore, CA, USA) are summarized in Table 4 and Table 5. While no obvious trends are found in the crystallite sizes of phases with cycling in different electrolytes, clear trends are observed in phase abundances. An increase in Cs2CO3 concentration in the exchange of KOH reduces both the amount of Mg(OH)2 (decrease in oxidation) and the Mg2Ni-to-MgNi2 ratio (last column in Table 5). The Mg2Ni phase is more oxidable compared with the MgNi2 phase, due to its higher Mg content. Upon contacting the KOH electrolyte, the Mg2Ni phase is oxidized into Mg(OH)2, which results in a reduction in the Mg2Ni-to-MgNi2 ratio from 1.50 to 0.67. Partial replacement of the corrosive KOH with Cs2CO3 increases the Mg2Ni-to-MgNi2 ratio, which is another indication of decrease in alloy oxidation.
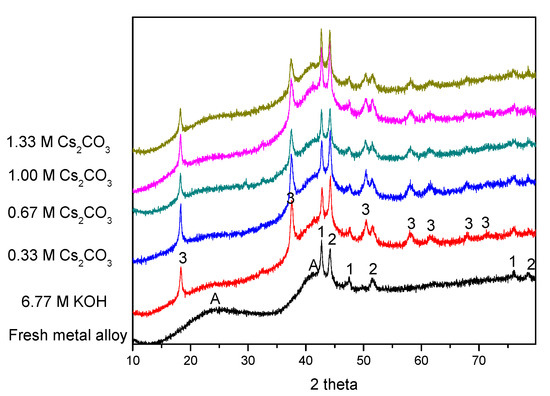
Figure 5.
X-ray diffraction (XRD) patterns of the pristine and cycled AR3 in the 6.77 M KOH, 6.44 M KOH + 0.33 M Cs2CO3, 6.11 M KOH + 0.66 M Cs2CO3, 5.77 M KOH + 1.00 M Cs2CO3, and 5.44 M KOH + 1.33 M Cs2CO3 electrolytes. Note: Peaks marked as “A” represent the microcrystalline/amorphous components of MgNi2 and Mg2Ni; peaks marked as “1” and “2” represent metallic Mn and Co, respectively, and peaks marked as “3” represent Mg(OH)2.

Table 4.
Crystallite sizes in nm of the Mg2Ni, MgNi2, Co, Mn, and Mg(OH)2 phases obtained from the XRD patterns in Figure 5.

Table 5.
Abundances in wt % of the Mg2Ni, MgNi2, Co, Mn, and Mg(OH)2 phases, and the ratio between Mg2Ni and MgNi2 obtained from the XRD patterns in Figure 5.
The chemical compositions of fresh and cycled MH alloys determined by ICP are compared in Table 6. Similar to the results in previous reports, the bulk composition changes slightly after cycling [5,6]. The loss of Mg occurs at approximately 1.5%, and results in an increase in the concentrations of other elements.

Table 6.
ICP results in at % of the fresh and cycled AR3 in five different electrolytes.
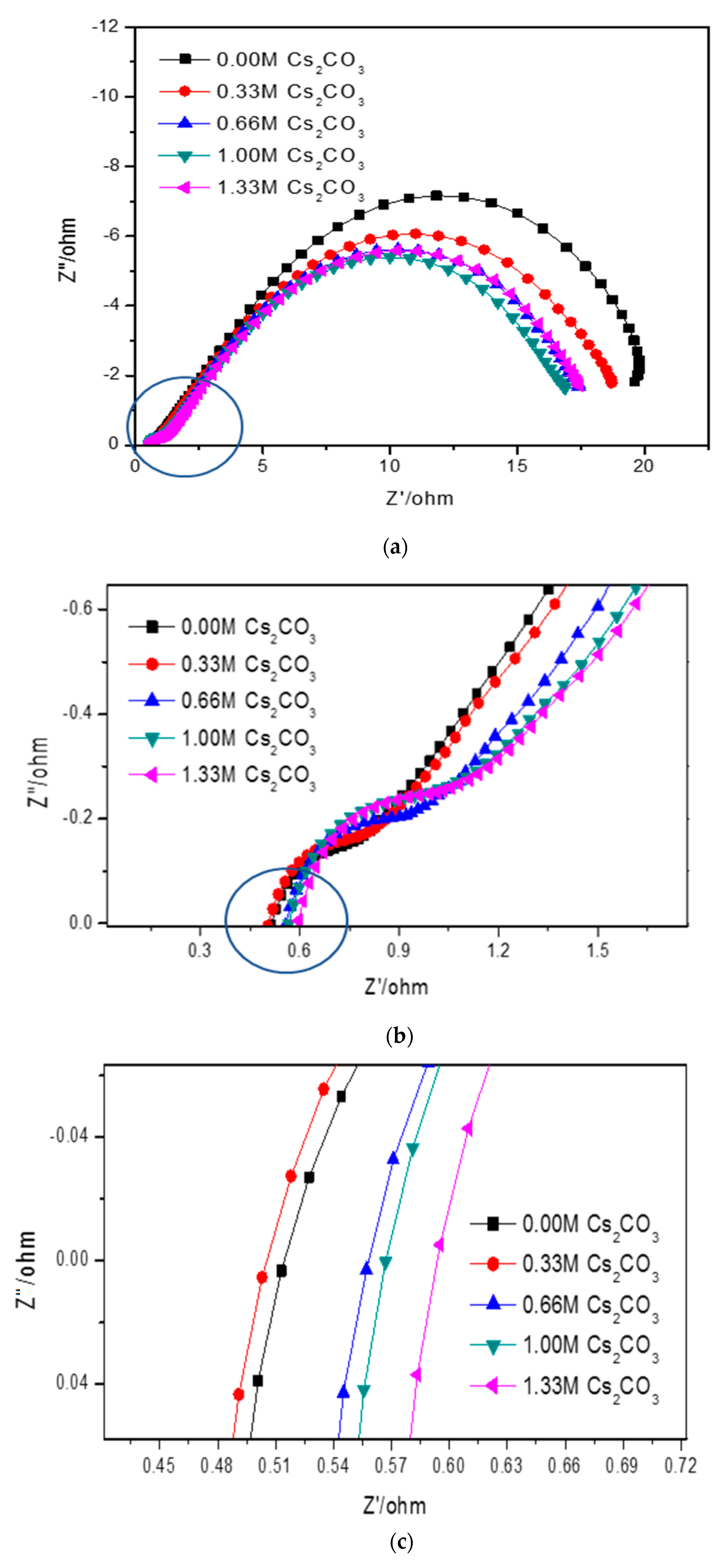
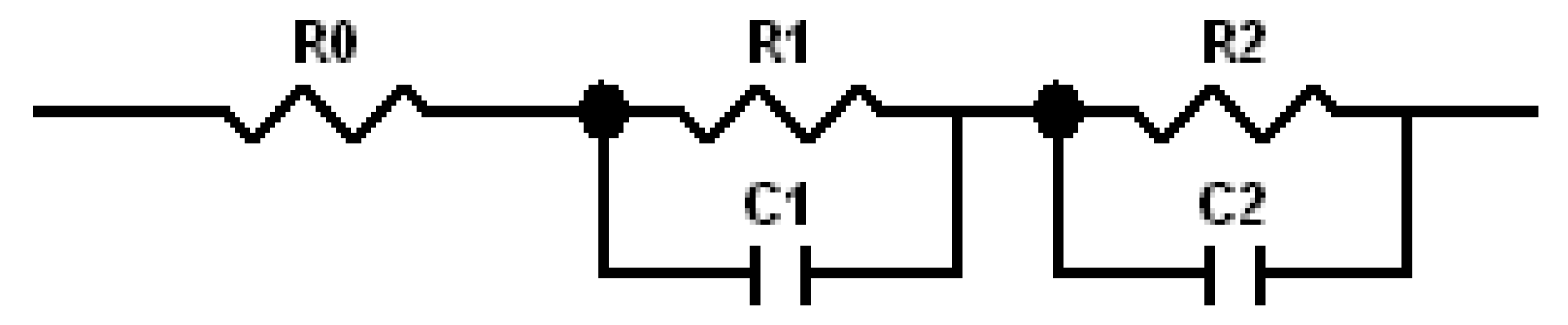
Cole–Cole plots obtained from the alternating current (AC) impedance measurements are shown in Figure 6. The reported equivalent circuit model for Ni-MH batteries using Mg-based alloy anodes is shown in Figure 7 [63,64,65,66,67]. Constant phase elements are used in this circuit model, due to the inhomogeneity properties of the electrode surface, such as porosity and roughness. Results obtained from the Cole–Cole plots are presented in Table 7. R0 represents the resistance of ions traveling through the electrolyte and separator. The electrolyte containing 6.44 M KOH + 0.33 M Cs2CO3 shows the lowest R0, which is consistent with its high discharge capacity, low degradation, and similar HRD compared with the 6.77 M KOH electrolyte. R1 is the resistance among alloy particles and increases as the Cs2CO3 concentration increases. R2 is the whole electrode resistance, and the addition of Cs2CO3 greatly decreases R2 compared with the pure KOH electrolyte. C1 represents the particle capacitance, which is closely related to the contact area among alloy particles. With increasing Cs2CO3 concentration, C1 decreases. C2 is the electrode capacitance, which is an indication of the amount of the active area in the electrode. Table 7 shows that the addition of Cs2CO3 decreases C2 as well. The product of R2 and C2 represents the electrode activity performance [68,69], and a smaller value suggests better electrochemical performances. Table 7 shows an optimized R2·C2 value at 5.77 M KOH + 1.00 M Cs2CO3.
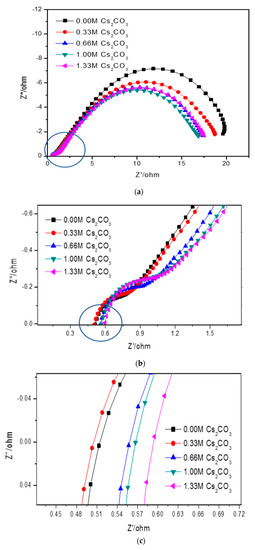
Figure 6.
(a) Full Cole–Cole plots of AR3 in the 6.77 M KOH, 6.44 M KOH + 0.33 M Cs2CO3, 6.11 M KOH + 0.66 M Cs2CO3, 5.77 M KOH + 1.00 M Cs2CO3, and 5.44 M KOH + 1.33 M Cs2CO3 electrolytes, (b) magnification of the circled section in (a), and (c) magnification of the circled section in (b).
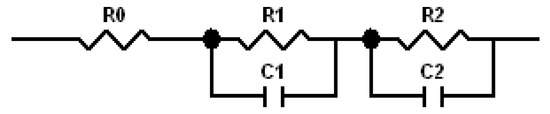
Figure 7.
Proposed equivalent circuit model for NiMH batteries using Mg-based anodes [63].

Table 7.
Data obtained by the AC impedance measurements of AR3 cycled in five different electrolytes.
3.3. Discussion
Results obtained from the current study indicate that the addition of Cs2CO3 alters the chemical and physical properties of the KOH electrolyte. As shown in Figure 6 and Table 7, an addition of 0.33 M Cs2CO3 decreases the solution resistance. Even if the electrolyte concentration was fixed at 6.77 M, the number of conductive ions increased with the addition of Cs2CO3. Therefore, the 6.44 M KOH + 0.33 M Cs2CO3 electrolyte has a decreased resistance and increased conductivity. While a low Cs2CO3 concentration promotes proton transfer in the system and improves both the discharge capacity and cycling performance, a higher Cs2CO3 concentration increases the solution resistivity due to the increased concentrations of larger cations (Cs+) and anions (CO32−) in the electrolyte, according to Stokes’ Law [12].
Cs2CO3 also changes the alloy surface structure during cycling. The FTIR results show that a small addition of Cs2CO3 decreases the strength of surface metal–O bond, but generates the C–O bond on the alloy surface. By further increasing the Cs2CO3 concentration, the C=O bond begins to appear, in relation with decreasing C–O bond. The changes in surface groups by electrolyte additive consequently change the chemical and physical properties of the alloy particles. In our previous work [52], 32 types of salt additives in KOH electrolytes were tested, and some oxyacid salts were reported to create more surface groups that promoted proton transfer. In the current study, the addition of Cs2CO3 provides C–O and C=O bonds as new active sites for proton transfer. However, protons bond to the two active sites differently; protons are covalently bound to C–O, but electrostatically bound to C=O [52,66]. With the stronger attraction to C–O, more protons can be bound and later transferred (driven by voltage). Therefore, the largest number of C–O bonds, which occur at a small addition of Cs2CO3 (6.44 M KOH + 0.33 M Cs2CO3), were demonstrated to be the most effective in improving the electrochemical performances among all of the electrolytes tested in the current study.
The addition of Cs2CO3 also changes the bulk structure of the alloy particles. The TEM images show a layer of solid covering the MH alloy particle (Figure 3), which ranges from 20 nm to 500 nm. This surface layer decreases the contact area among alloy particles (as shown by the decrease in C1) and increases the barrier for proton transfer among the particles (as shown by the increase in R1). The ICP results show that a small amount of Cs2CO3 results in this decrease, but further increases in Cs2CO3 concentration increase the loss of Mg after cycling. For the pure KOH electrolyte, the alloy particles are covered by Mg(OH)2. However, a small addition of Cs2CO3 reduces the particle size of Mg(OH)2 on the surface, as indicated by the increase in full width at half maximum of the Mg(OH)2 peaks (Figure 5). Smaller Mg(OH)2 crystals are more strongly adsorbed on the surface of the MH alloy, which is not easily removed from the electrolyte, and also protects the bulk alloy from further oxidation. As the Cs2CO3 concentration further increases in the electrolyte, more MgCO3 starts to form on the alloy surface. Since the solubility of MgCO3 in KOH solution is greater than that of Mg(OH)2, the loss of Mg occurs at a higher rate at higher Cs2CO3 concentrations. Therefore, high Cs2CO3 concentrations are not suggested for Mg–Ni alloys. On the other hand, if properly balanced with the loss of Mg, an appropriate amount of carbonate formation by adding Cs2CO3 can promote the dissolution of surface oxidation products and consequently reveal a clean metal surface exposed to the electrolyte, which can lead to a decrease in electrode resistance (as shown by the decrease in R2 as the Cs2CO3 concentration increases).
4. Conclusions
The effects of Cs2CO3 addition in a KOH-based electrolyte for Ni/MH batteries were investigated. Four different MH alloys (three Laves phase-related BCC and one MgNi-based) were used as the anode materials, and β-Ni(OH)2 was used as the cathode material. A proper amount of Cs2CO3 addition greatly improved electrochemical performances. For the Laves phase-related BCC alloys, adding Cs2CO3 into the electrolyte improved the cycle stability (for all three alloys tested) and the discharge capacity (for Al-containing alloys). For the MgNi-based alloy, the discharge capacity increased to 132%, while degradation decreased to 87% in the 6.44 M KOH + 0.33 M Cs2CO3 electrolyte (compared with those in the 6.77 M KOH electrolyte). The effects of Cs2CO3 addition on the electrolyte and alloy properties are summarized as follows:
- (1)
- A small addition of Cs2CO3 decreases the electrolyte resistance and increases the conductivity.
- (2)
- A newly-formed fluffy C-containing surface oxide by the addition of Cs2CO3 is believed to be the main cause of the decrease in capacity decay during cycling.
- (3)
- The addition of Cs2CO3 in the electrolyte changes the alloy surface structure after cycling by creating more surface groups in addition to metal–O bonds, including C–O and C=O bonds, and the C–O bond is more effective than the C=O bond during proton transfer.
- (4)
- For MgNi-based alloys, the addition of Cs2CO3 changes the alloy bulk structure after cycling. A small addition of Cs2CO3 strengthens the Mg(OH)2 layer on the alloy surface and prevents loss of Mg. However, a large addition of Cs2CO3 causes the formation of MgCO3 with higher solubility in the KOH solution, and consequently a more severe loss of Mg.
Acknowledgments
The authors would like to thank the following individuals from BASF—Ovonic for their help: Su Cronogue, Baoquan Huang, Diana F. Wong, Taihei Ouchi, Tiejun Meng, and Shiuan Chang.
Author Contributions
Shuli Yan designed and Peifeng Li performed the experiments, and analyzed the results. Jean Nei, Kwo-Hsiung Young, and Simon Ng provided guidance and helped in manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ni/MH | Nickel/metal hydride |
| M | Metal |
| MH | Metal hydride alloy |
| MH | Hydrided metal |
| HRD | High-rate dischargeability |
| BCC | Body-centered-cubic |
| Caphigh | The highest value of discharge capacity in the initial 10 cycles |
| Caplow | The lowest value of discharge capacity in the initial 10 cycles |
| nhigh | The cycle number of the highest discharge capacity in the initial 10 cycles |
| nlow | The cycle number of the lowest discharge capacity in the initial 10 cycles |
| n0,high | The cycle number of the highest discharge capacity in the initial 10 cycles for 6.77 M KOH electrolyte |
| n0,low | The cycle number of the lowest discharge capacity in the initial 10 cycles for 6.77 M KOH electrolyte |
| FTIR | Fourier transform infrared |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscopy |
| ICP-OES | Inductively coupled plasma-optical emission spectroscopy |
| AC | Alternating current |
| R0 | Resistance of ions traveling through the electrolyte and separator |
| R1 | Resistance among alloy particles |
| R2 | Whole electrode resistance |
| C1 | Particle capacitance |
| C2 | Electrode capacitance |
References
- Linden, D.; Reddy, T.B. Handbook of Batteries; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Fetcenko, M.A.; Ovshinsky, S.R.; Reichman, B.; Young, K.; Fierro, C.; Koch, J.; Zallen, A.; Mays, W.; Ouchi, T. Recent advances in NiMH battery technology. J. Power Sources 2007, 165, 544–551. [Google Scholar] [CrossRef]
- Young, K.; Cai, X.; Chang, S. Reviews on the Chinese Patents regarding nickel/metal hydride battery. Batteries 2017, 3, 24. [Google Scholar] [CrossRef]
- Young, K.; Ng, K.Y.S.; Bendersky, L.A. A Technical report of the robust affordable next generation energy storage system-BASF program. Batteries 2016, 2, 2. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.-H.; Nei, J.; Fierro, C. Reviews on the US Patents regarding nickel/metal hydride batteries. Batteries 2016, 2, 10. [Google Scholar] [CrossRef]
- Ouchi, T.; Young, K.-H.; Moghe, D. Reviews on the Japanese Patent Applications regarding nickel/metal hydride batteries. Batteries 2016, 2, 21. [Google Scholar] [CrossRef]
- Züttle, A. Materials for Hydrogen Storage. Mater. Today 2003, 24–33. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L. Recent progress in hydrogen storage alloys for nickel/metal hydride secondary batteries. Int. J. Hydrog. Energy 2009, 34, 4788–4796. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Yu, X.B.; Wu, Z.; Xia, B.J.; Xu, N.X. A Ti-V-Based BCC phase alloy for use as metal hydride electrode with high discharge capacity. J. Chem. Phys. 2004, 121, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [PubMed]
- Nei, J.; Young, K.; Rotarov, D. Studies on MgNi-based metal hydride electrode with aqueous electrolytes composed of various hytproxides. Batteries 2016, 2, 27. [Google Scholar] [CrossRef]
- Redzeb, M.; Zlatanova, Z.; Spassov, T. Influence of boron on the hydriding of nanocrystalline Mg2Ni. Intermetallics 2013, 34, 63–68. [Google Scholar] [CrossRef]
- Nikkuni, F.R.; Santos, S.F.; Ticianelli, E.A. Microstructures and electrochemical properties of Mg49Ti6Ni45−xMx (M = Pd and Pt) alloy electrodes. Int. J. Energy Res. 2013, 37, 706–712. [Google Scholar] [CrossRef]
- Zhang, X.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J.W.; Nie, A.; Chen, X.; Yassar, R.S.; Axelbaum, R.L. Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 cathode material using ALD. Adv. Energy Mater. 2013, 3, 1299–1307. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Cai, Y.; Hu, F.; Liu, Z.; Guo, S. Highly improved electrochemical hydrogen storage performances of the Nd-Cu-added Mg2Ni-type alloys by melt spinning. J. Alloy. Compd. 2014, 584, 81–86. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wan, C.B.; Wang, R.L.; Meng, X.H.; Huang, M.F.; Ju, X. Effect of Cr substitution by Ni on the cycling stability of Mg2Ni alloy using EXAFS. Int. J. Hydrog. Energy 2014, 39, 14858–14867. [Google Scholar] [CrossRef]
- Hou, X.; Hu, R.; Zhang, T.; Kou, H.; Song, W.; Li, J. Microstructure and electrochemical hydrogenation/dehydrogenation performance of melt-spun La-doped Mg2Ni alloys. Mater. Charact. 2015, 106, 163–174. [Google Scholar] [CrossRef]
- Verbovytskyy, Y.; Zhang, J.; Cuevas, F.; Paul-Boncour, V.; Zavaliy, I. Synthesis and properties of the Mg2Ni0.5Co0.5H4.4 hydride. J. Alloy. Compd. 2015, 645, S408–S411. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Yang, C.; Zhang, J.; Chen, W.; Li, L. Enhanced electrochemical hydrogen storage properties of Mg2NiH4 by coating with nano-nickel. Int. J. Hydrog. Energy 2015, 40, 13949–13956. [Google Scholar] [CrossRef]
- Shang, J.; Ouyang, Z.; Liu, K.; Xing, C.; Liu, W.; Wang, L. Effect of Li atom infiltration by the way of electro-osmosis on electrochemical properties of amorphous Mg65Ni27La8 alloy used as negative electrode materials for the nickel–metal hydride secondary batteries. J. Non-Cryst. Solids 2015, 415, 30–35. [Google Scholar] [CrossRef]
- Shao, H.; Li, X. Effect of nanostructure and partial substitution on gas absorption and electrochemical properties in Mg2Ni-based alloys. J. Alloy. Compd. 2016, 667, 191–197. [Google Scholar] [CrossRef]
- Ohara, R.; Lan, C.-H.; Hwang, C.-S. Electrochemical and structural characterization of electroless nickel coating on Mg2Ni hydrogen storage alloy. J. Alloy. Compd. 2013, 580, S368–S372. [Google Scholar] [CrossRef]
- Shahcheraghi, A.; Dehghani, F.; Raeissi, K.; Saatchi, A.; Enayati, M.H. Effects of TiO2 additive on electrochemical hydrogen storage properties of nanocrystalline/amorphous Mg2Ni intermetallic alloy. Iran. J. Mater. Sci. Eng. 2013, 10, 1–9. [Google Scholar]
- Haghighat-Shishavan, S.; Bozorg, F.K. Nano-crystalline Mg2−xMnxNi compounds synthesized by mechanical alloying: Microstructure and electrochemistry. J. Ultrafine Grained Nanostruct. Mater. 2014, 47, 43–49. [Google Scholar]
- Venkateswari, A.; Nithya, C.; Kumaran, S. Electrochemical behaviour of Mg67Ni33−xNbx (x = 0, 1, 2 and 4) alloy synthesized by high energy ball milling. Proc. Mater. Sci. 2014, 5, 679–687. [Google Scholar] [CrossRef]
- Rubin, E.J.; Baboian, R. A correlation of the solution properties and the electrochemical behavior of the nickel hydroxide electrode in binary aqueous alkali hydroxides. J. Electrochem. Soc. 1971, 118, 428–433. [Google Scholar] [CrossRef]
- Barnard, R.; Randell, C.F.; Tye, F.L. Studies concerning changes nickel hydroxide electrodes. IV. Reversible potentials in LiOH, NaOH, RbOH and CdOH. J. Appl. Electrochem. 1981, 11, 517–523. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; Guibert, A. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Leblanc, P.; Jordy, C.; Knosp, B.; Blanchard, Ph. Mechanism of alloy corrosion and consequences on sealed nickel-metal hydride battery performance. J. Electrochem. Soc. 1998, 145, 860–863. [Google Scholar] [CrossRef]
- Knosp, B.; Vallet, L.; Blamchard, P. Performance of an AB2 alloy in sealed Ni-MH batteries for electric vehicles: Qualification of corrosion rate and consequences on the battery performance. J. Alloy. Compd. 1999, 293–295, 770–774. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, H.G.; Jung, Y.H.; Ruhmann, H. Effect of LiOH, NaOH and KOH on Corrosion and Oxide Microstructure of Zr-Based Alloys. Available online: http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/30/060/30060383.pdf (accessed on 26 February 2016).
- Liu, J.; Wang, D.; Liu, S.; Feng, X. Improving high temperature performance of MH/Ni battery by orthogonal design. Battery Bimon. 2003, 33, 218–220. [Google Scholar]
- Hou, X.; Nan, J.; Han, D.; Zhao, J. Preparation and performance of high-rated A-type MN-Ni batteries. Chin. J. Appl. Chem. 2004, 21, 1169–1173. [Google Scholar]
- Lv, J.; Liu, X.; Zhang, J.; Fan, L.; Wang, L.; Zhang, Z. Studies on high-power nickel-metal hydride battery. Chin. J. Power Sources 2005, 29, 826–830. [Google Scholar]
- Li, X.; Dong, H.; Zhang, A.; Wei, Y. Electrochemical impedance and cyclic voltammetry characterization of a metal hydride electrode in alkaline electrolytes. J. Alloy. Compd. 2006, 426, 93–96. [Google Scholar] [CrossRef]
- Park, C.; Shim, J.; Jang, M.; Park, C.; Choi, J. Influences of various electrolytes on the low-temperature characteristics of Ni-MH secondary battery. Trans. Korean Hydrog. New Energy Soc. 2007, 18, 284–291. [Google Scholar]
- Chen, R.; Li, L.; Wu, F.; Qiu, X.; Chen, S. Effects of low temperature on performance of hydrogen-storage alloys and electrolyte. Min. Metall. Eng. 2007, 27, 44–46. [Google Scholar]
- Yang, D.C.; Park, C.N.; Park, C.J.; Choi, J.; Sim, J.S.; Jang, M.H. Design of additives and electrolyte for optimization of electrode characteristics of Ni-MH secondary battery at room and low temperatures. Trans. Korean Hydrog. New Energy Soc. 2007, 18, 365–373. [Google Scholar]
- Zhang, X.; Chen, Y.; Tao, M.; Wu, C. Effect of electrolyte concentration on low-temperature electrochemical properties of LaNi5 alloy electrode at 233 K. J. Rare Earths 2008, 26, 402–405. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Tao, M.; Wu, C. Effect of electrolyte on the low-temperature electrochemical properties of LaNi5 alloy electrode at 253 K. Rare Metal Mater. Eng. 2008, 37, 2012–2015. [Google Scholar]
- Pei, L.; Yi, S.; He, Y.; Chen, Q. Effect of electrolyte formula on the self-discharge properties of nickel-metal hydride batteries. J. Guangdong Univ. Technol. 2008, 25, 10–12. [Google Scholar]
- Khaldi, C.; Mathlouthi, H.; Lamloumi, J. A comparative study of 1 M and 8 M KOH electrolyte concentrations used in Ni-MH batteries. J. Alloy. Compd. 2009, 469, 464–471. [Google Scholar] [CrossRef]
- Guiose, B.; Cuevas, F.; Décamps, B.; Leroy, E.; Percheron-Guégan, A. Microstructural analysis of the aging of pseudo-binary (Ti, Zr)Ni intermetallic compounds as negative electrodes of Ni-MH batteries. Electrochim. Acta 2009, 54, 2781–2789. [Google Scholar] [CrossRef]
- Qiu, Z.; Wu, A. Study on wide temperature characteristics of Ni-MH battery. J. South China Norm. Univ. 2009, 1, 79–81. [Google Scholar]
- Song, M.; Chen, Y.; Tao, M.; Wu, C.; Zhu, D.; Yang, H. Some factors affecting the electrochemical performances of LaCrO3 as negative electrodes for Ni/MH batteries. Electrochim. Acta 2010, 55, 3103–3108. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, F.; Chen, J. Nickel-metal hydride (Ni-MH) rechargeable battery. In Electrochemical Technologies for Energy Storage and Conversion; Zhang, J., Zhang, L., Liu, H., Sun, A., Liu, R., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2011; p. 204. [Google Scholar]
- Karwowska, M.; Jaron, T.; Fijalkowski, K.J.; Leszczynski, P.J.; Rogulski, Z.; Czerwinski, A. Influence of electrolyte composition and temperature on behavior of AB5 hydrogen storage alloy used as negative electrode in Ni-MH batteries. J. Power Sources 2014, 263, 304–309. [Google Scholar] [CrossRef]
- Giza, K. Influence of electrolyte on capacity and corrosion resistance of anode material used in Ni-MH cells. Ochr. Przed Koroz. 2016, 59, 167–169. [Google Scholar] [CrossRef]
- Shangguan, E.; Li, J.; Chang, Z.; Tang, H.; Li, B.; Yuan, X.; Wang, H. Sodium tungstate as electrolyte additive to improve high-temperature performance of nickelemetal hydride batteries. Int. J. Hydrog. Energy 2013, 38, 5133–5138. [Google Scholar] [CrossRef]
- Vaidyanathan, H.; Robbins, K.; Rao, G.M. Effect of KOH concentration and anions on the performance of an Ni-H2 battery positive plate. J. Power Sources 1996, 63, 7–13. [Google Scholar] [CrossRef]
- Yan, S.; Ng, K.Y.S.; Young, K.-H. Effects of salt additives to the KOH electrolyte used in Ni/MH batteries. Batteries 2015, 1, 54–73. [Google Scholar] [CrossRef]
- Li, L. Non-Toxic Alkaline Electrolyte with Additives for Rechareable Zinc Cells. U.S. Patent 2010/0062327, 11 March 2010. [Google Scholar]
- Young, K.; Nei, J.; Wong, D.; Wang, L. Structural, hydrogen storage, and electrochemical properties of Laves-phase related body-centered-cubic solid solution metal hydride alloys. Int. J. Hydrog. Energy 2014, 39, 21489–21499. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Wang, L. Annealing effects on Laves phase-related body-centered-cubic solid solution metal hydride alloys. J. Alloy. Compd. 2016, 654, 216–225. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Martínez, P.S.; Castro, E.B.; Humana, R.; Peretti, H.A.; Visintin, A. Effect of electrolyte concentration on the electrochemical properties of an AB5-type alloy for Ni/MH batteries. Int. J. Hydrog. Energy 2013, 38, 240–245. [Google Scholar] [CrossRef]
- Martínez, P.S.; Ruiz, F.C.; Visintin, A. Influence of different electrolyte concentrations on the performance of an AB2-type alloy. J. Electrochem. Soc. 2014, 161, A326–A329. [Google Scholar] [CrossRef]
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Why is Aluminum Carbonate Unstable? Available online: https://chemistry.stackexchange.com/questions/6369/why-is-aluminium-carbonate-unstable (accessed on 16 August 2017).
- Yang, C.; Wöll, C. IR spectroscopy applied to metal oxide surfaces: Adsorbate vibrations and beyond. Adv. Phys. 2017, 2, 373–408. [Google Scholar] [CrossRef]
- Reig, F.B.; Adelantado, J.V.; Moya Moreno, M.C. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Young, K. Stoichiometry in inter-metallic compounds for hydrogen storage applications. In Stoichiometry and Materials Science—When Numbers Matter; Innocenti, A., Kamarulzaman, N., Eds.; InTech: Rijeka, Crotia, 2012. [Google Scholar]
- Zhang, W.; Kumar, M.P.S.; Srinivasan, S. AC impedance studies on metal hydride electrodes. J. Electrochem. Soc. 1995, 142, 2935–2943. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on incorporation of Mg in Zr-based AB2 metal hydride alloys. Batteries 2016, 2, 11. [Google Scholar] [CrossRef]
- Trapanese, M.; Franzitta, V.; Viola, A. Description of hysteresis of nickel metal hydride battery. In Proceedings of the 38th Annual Conference on IEEE Industrial Electronics Society, Montreal, QC, Canada, 25–28 October 2012; pp. 967–970. [Google Scholar]
- Trapanese, M.; Franzitta, V.; Viola, A. Description of hysteresis in lithium battery by classical Preisach model. Adv. Mater. Res. 2013, 622, 1099–1103. [Google Scholar]
- Trapanese, M.; Franzitta, V.; Viola, A. The Jiles Atherton model for description on hysteresis in lithium battery. In Proceedings of the Twenty-Eighth Annual IEEE Applied Power Electronics Conference and Exposition (APEC), Long Beach, CA, USA, 17–21 March 2013; pp. 2772–2775. [Google Scholar]
- Young, K.; Ouchi, T.; Nei, J.; Moghe, D. The importance of rare-earth additions in Zr-based AB2 metal hydride alloys. Batteries 2016, 2, 25. [Google Scholar] [CrossRef]
- Grabowski, J.S. What is the covalency of hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).