Comparison among Constituent Phases in Superlattice Metal Hydride Alloys for Battery Applications
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussion
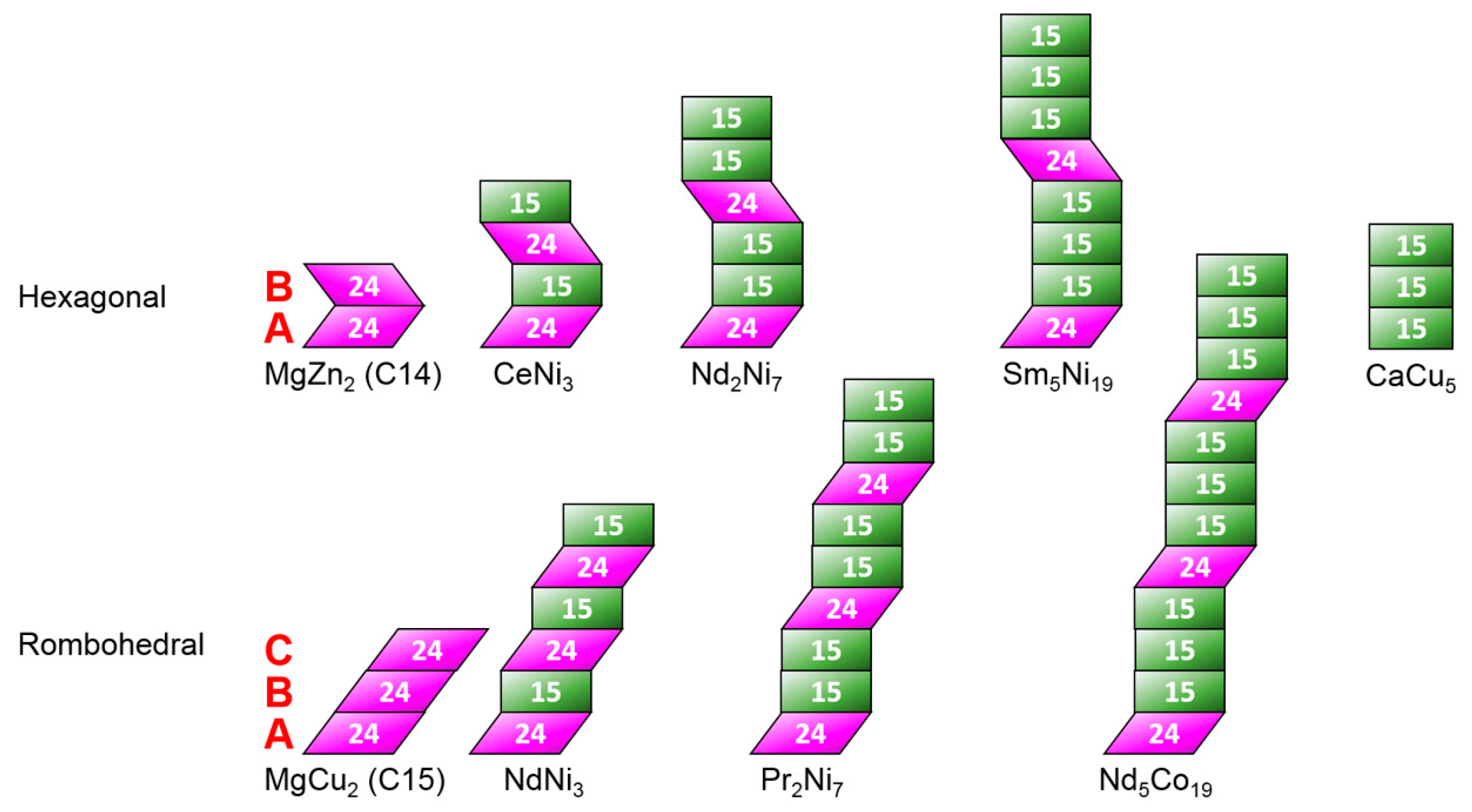
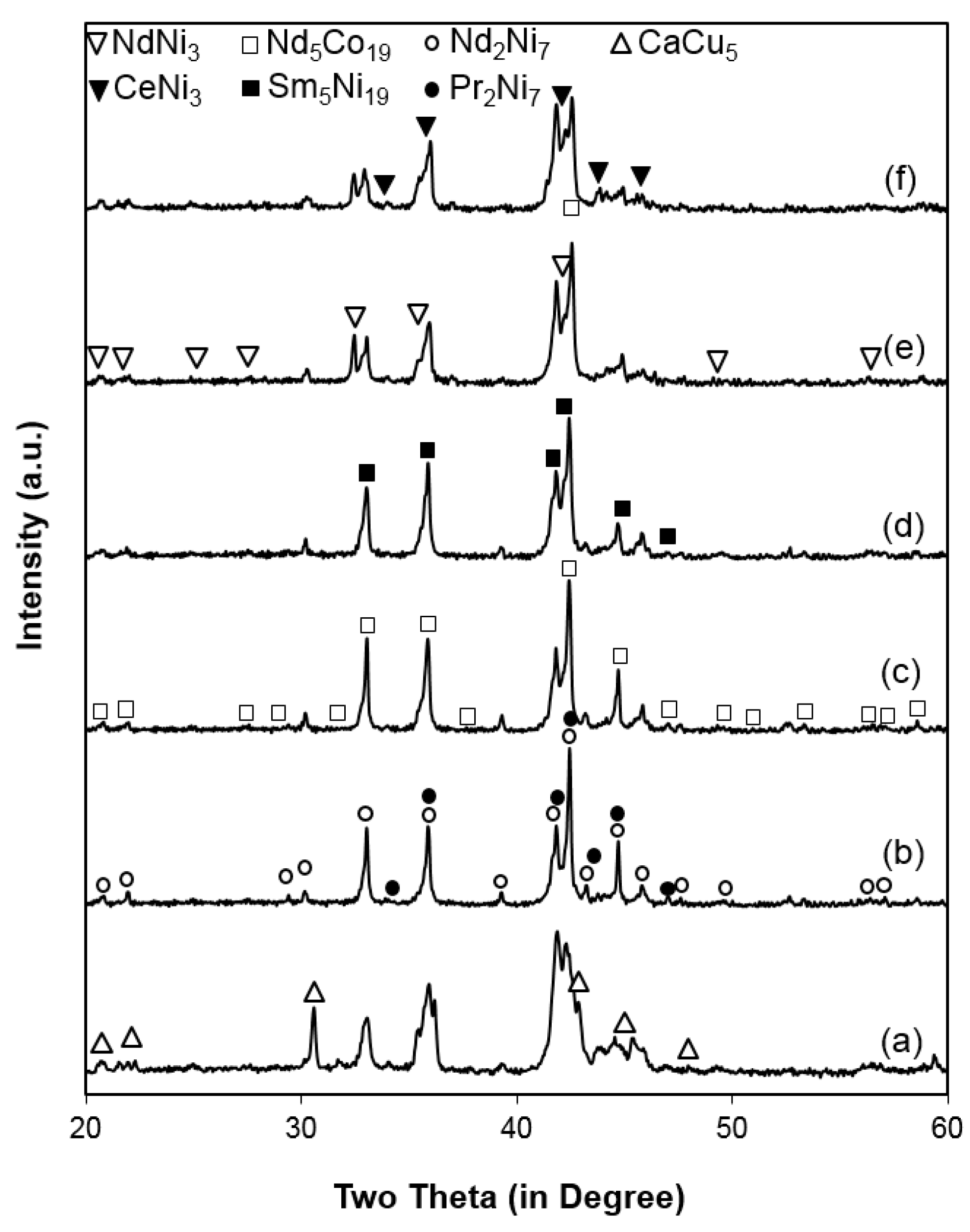
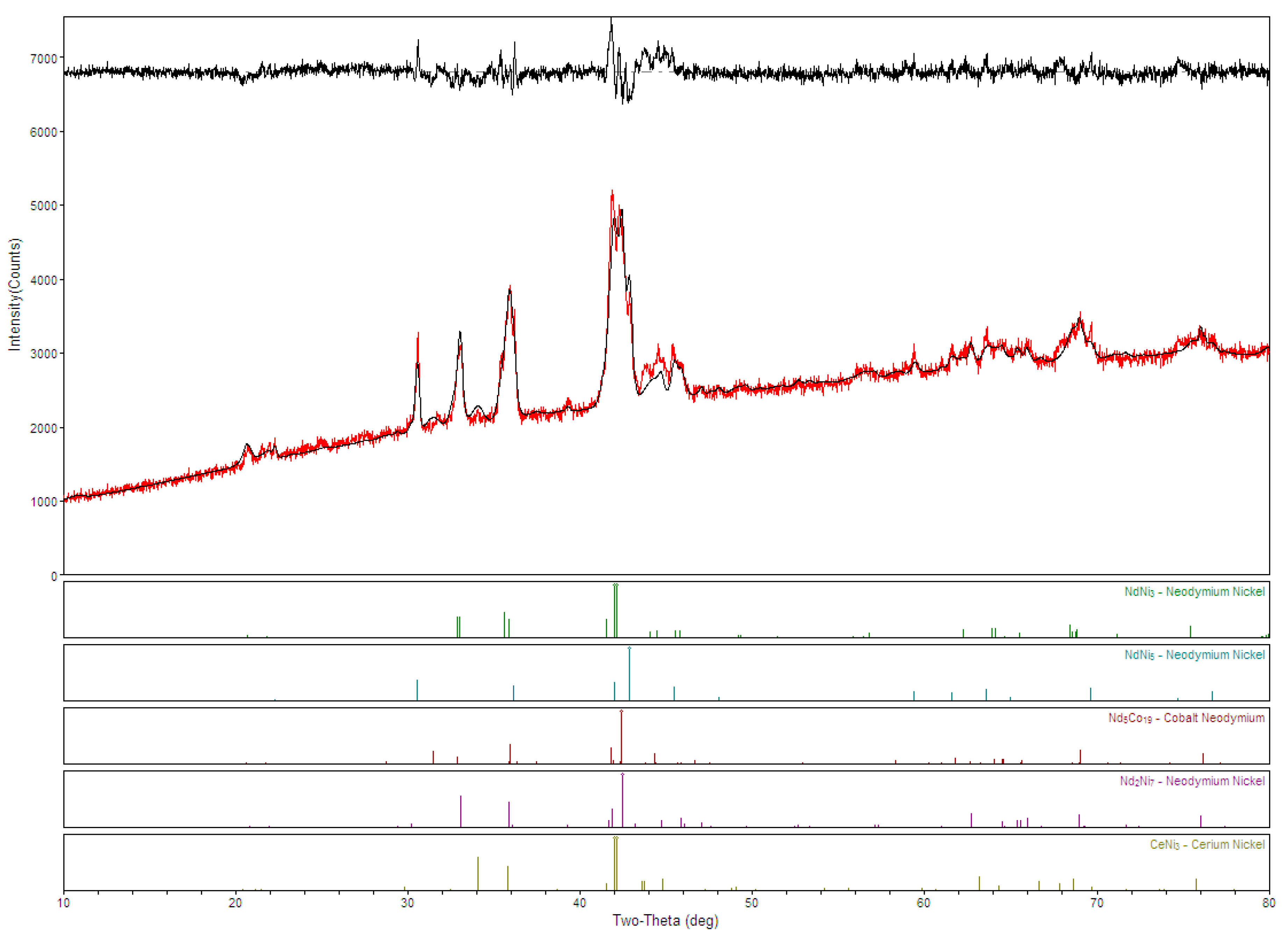
3.1. Microsctructure Analysis
3.2. Gaseous Phase Hydrogen Storage
3.3. Electrochemical and Magnetic Susceptibility Measurements
3.4. Sealed Cell Performance
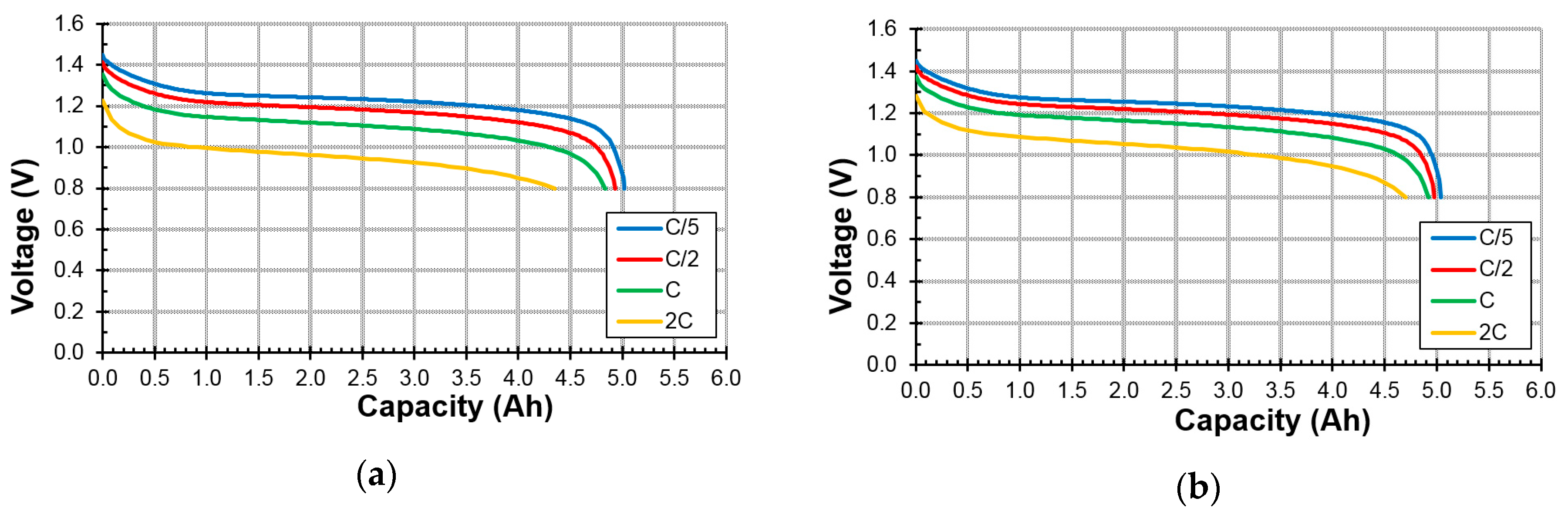
3.4.1. High-Rate Performance
3.4.2. Low-Temperature Performance
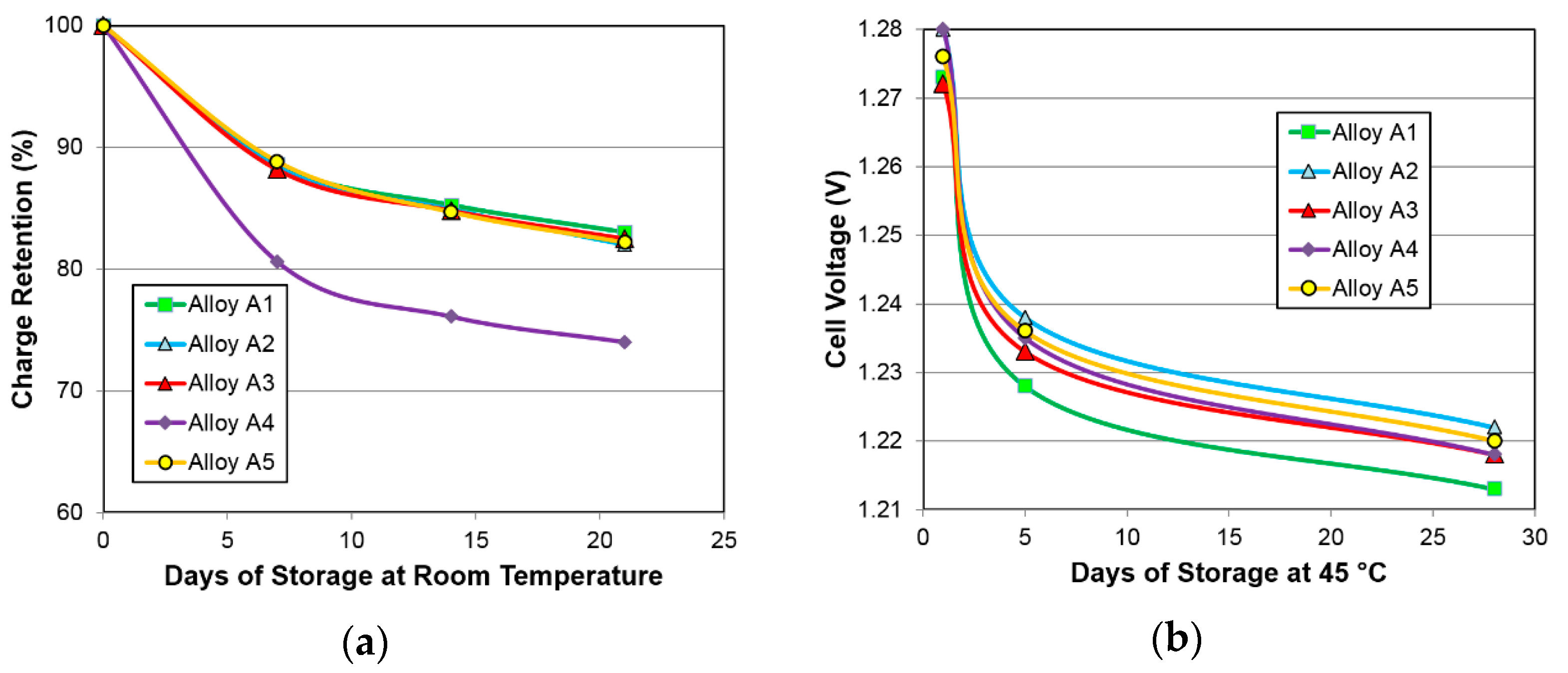
3.4.3. Charge Retention
3.4.4. Peak Power
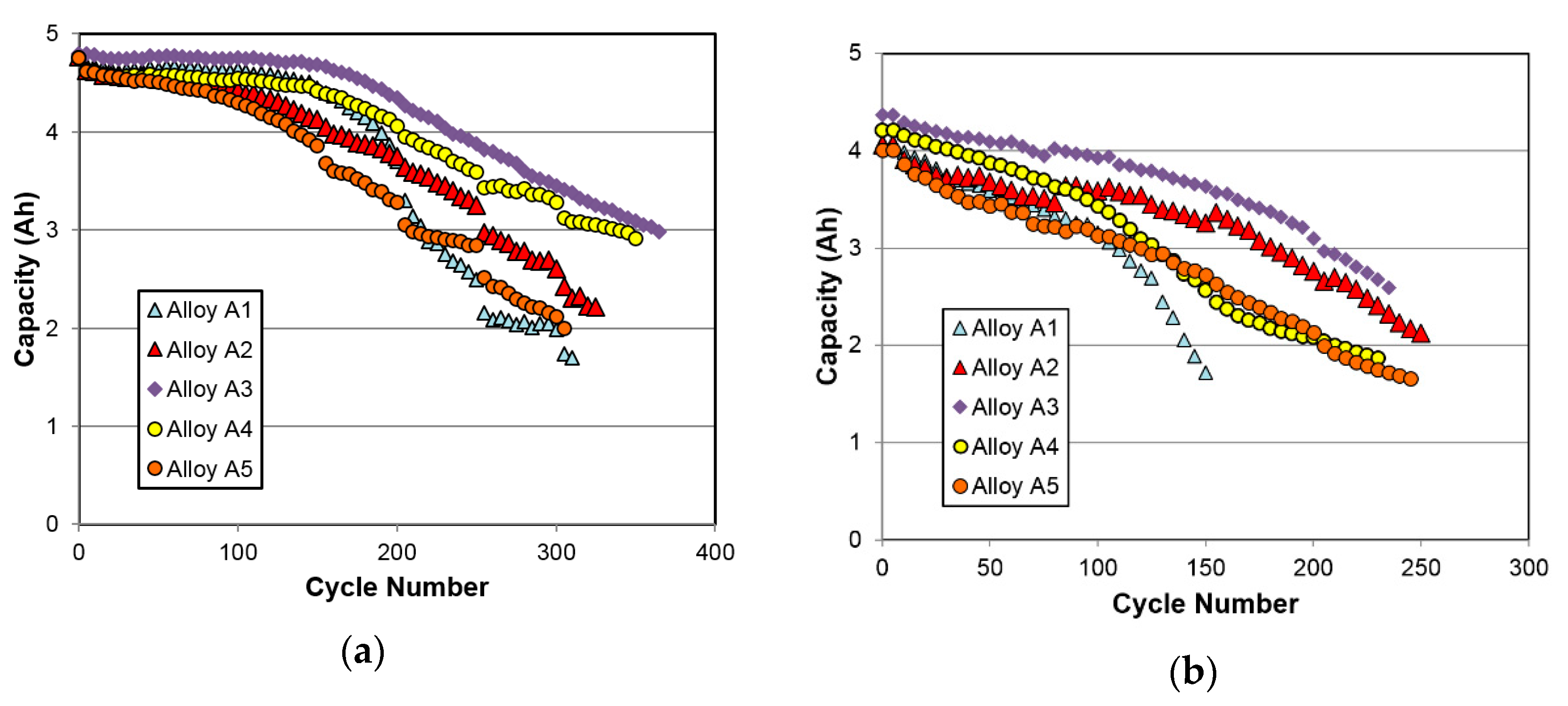
3.4.5. Cycle Life
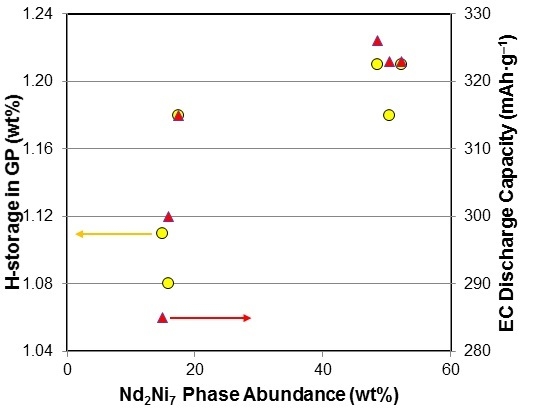
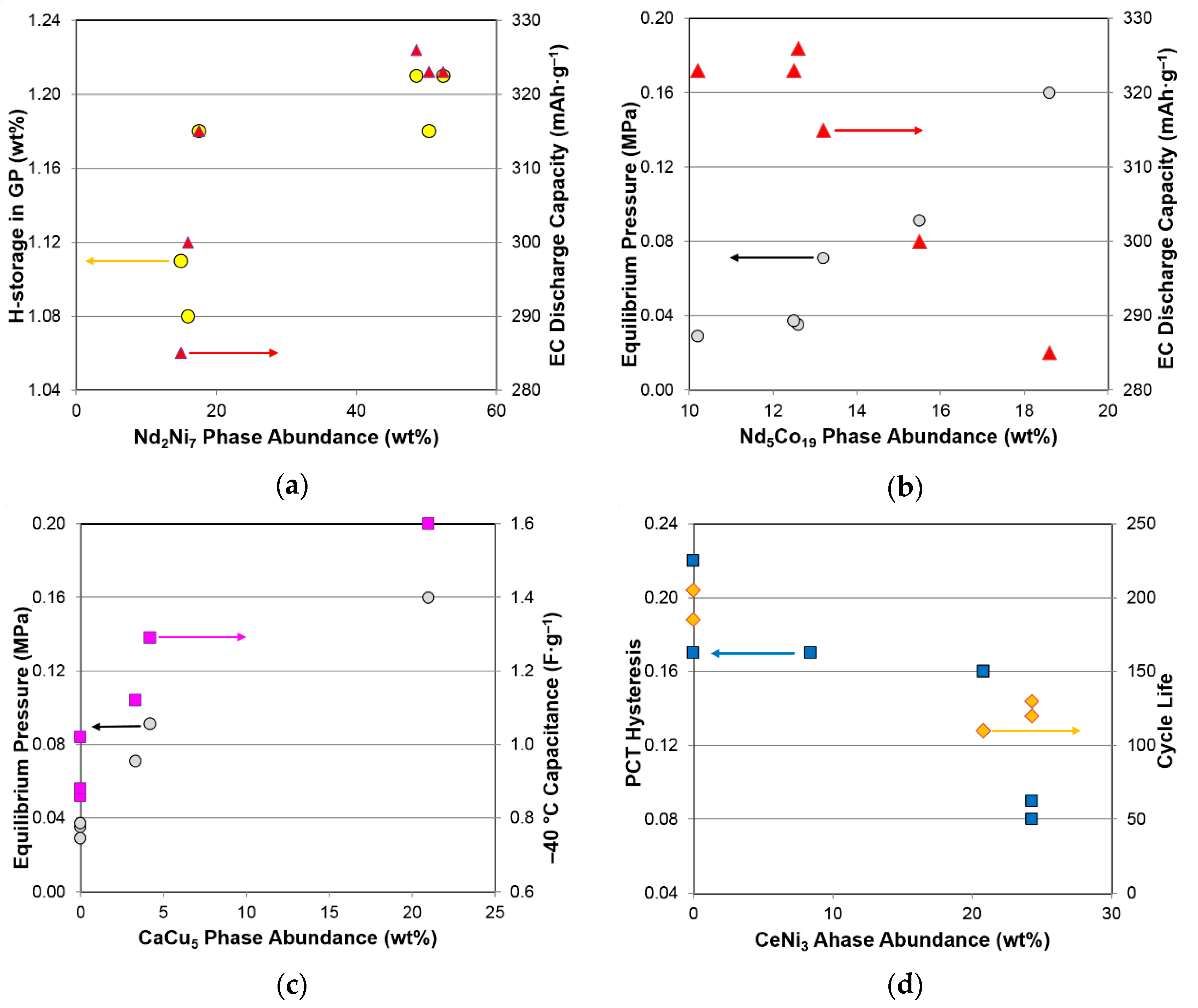
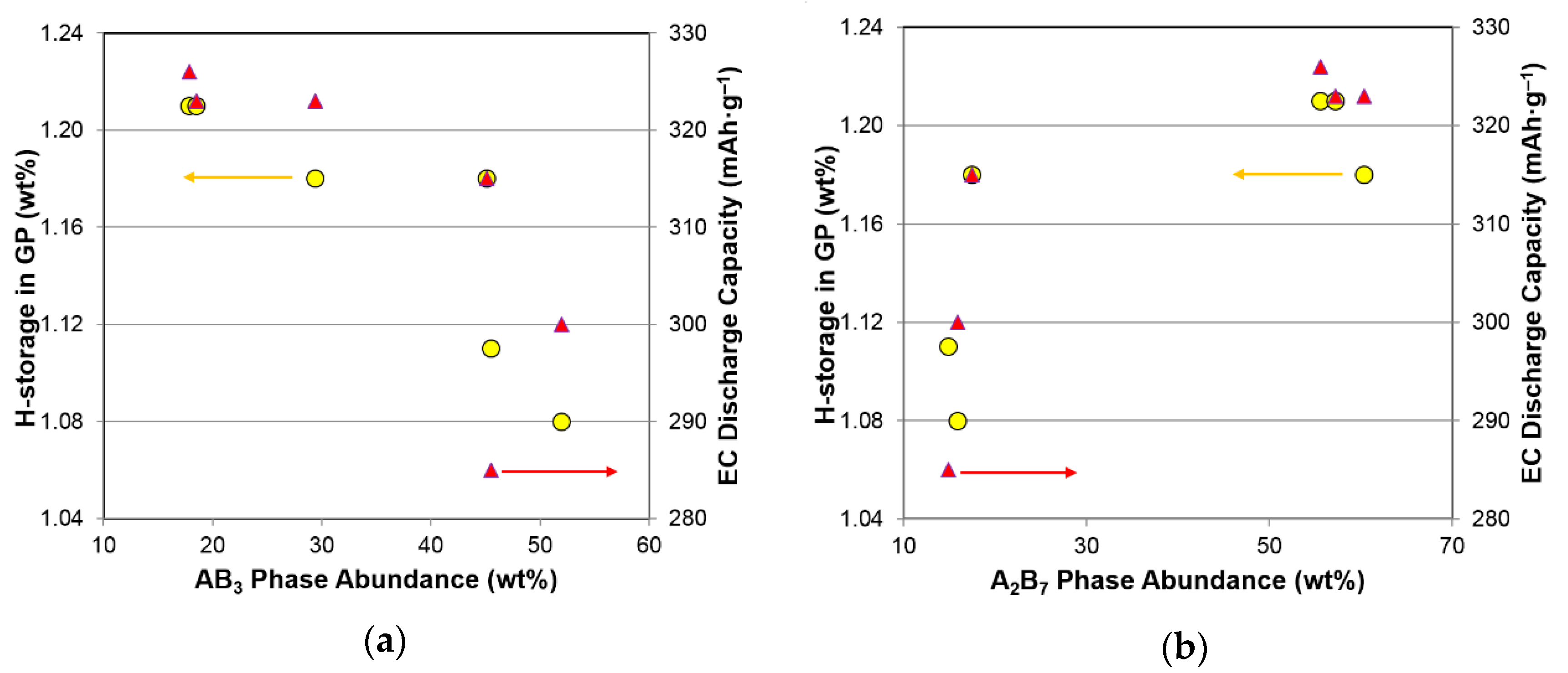
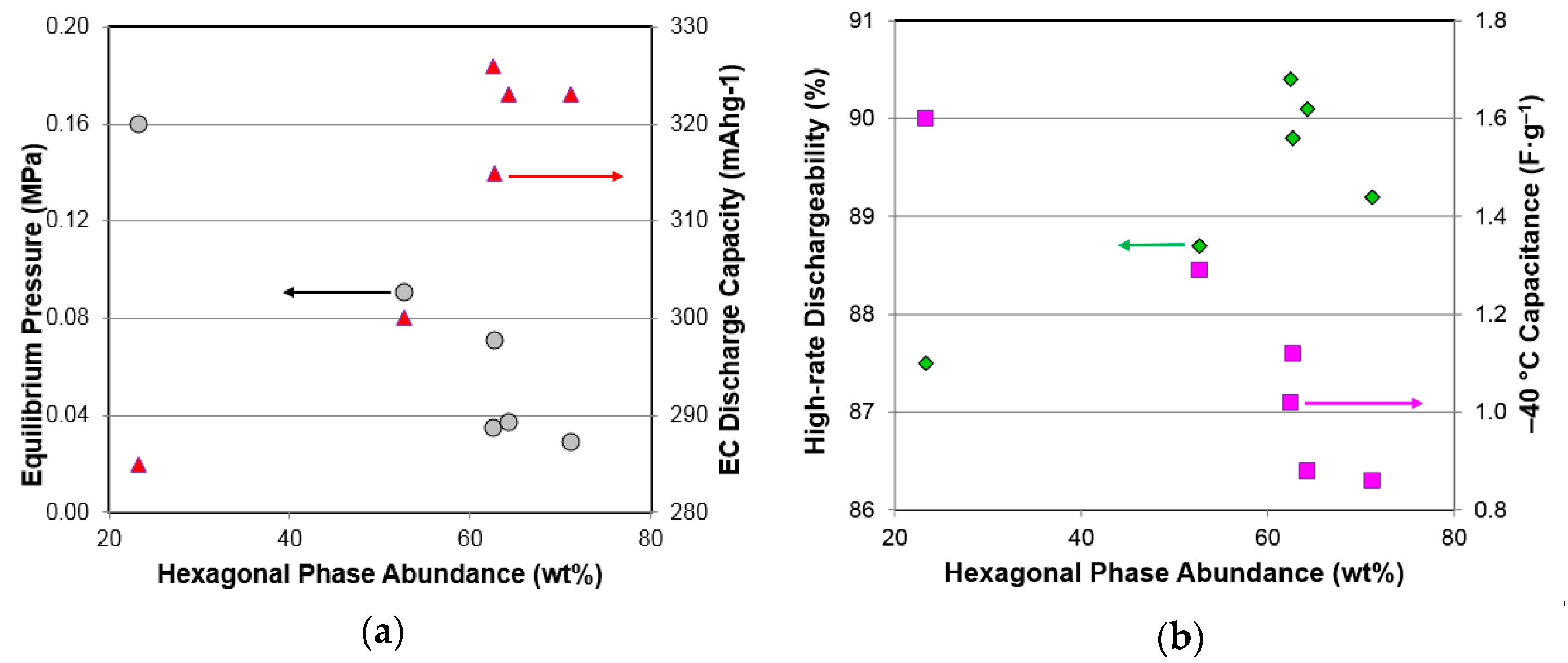
3.5. Performance Correlation with Individual Phase
3.6. Performance Correlation with Phase Stoichiometry
3.7. Performance Correlation with Phase Structure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Mm | Misch metal |
| MH | Metal hydride alloy |
| Ni/MH | Nickel/metal hydride |
| H-storage | Hydrogen-storage |
| HRD | High-rate dischargeability |
| GP | Gaseous phase |
| RE | Rare earth |
| EC | Electrochemical |
| bcc | Body-centered-cubic |
| ICP-OES | Inductively coupled plasma-optical emission spectrometer |
| XRD | X-ray diffractometer |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectroscopy |
| PCT | Pressure-concentration-temperature |
| BEI | Backscattering electron image |
| ΔH | Change in enthalpy or heat of hydride formation |
| ΔS | Change in entropy |
| P | Desorption pressure |
| T | Temperature |
| R | Ideal gas constant |
| D | Bulk hydrogen diffusion coefficient |
| Io | Surface exchange current |
| R | Surface charge-transfer resistance |
| C | Surface double-layer capacitance |
| MS | Saturated magnetic susceptibility |
| H1/2 | Applied field strength corresponding to half of MS |
| RT | Room temperature |
| R2 | Correlation factor |
References
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Low Self-Discharge Nickel-Metal Hydride Battery. Available online: http://www.scribd.com/doc/9704685/Teraoka-Article-En (accessed on 9 April 2016).
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The effect of nickel-metal hydride battery’s characteristics with structure of the alloy. In Proceedings of the 54th Battery Symposium in Japan, Osaka, Japan, 7–9 October 2013; p. 210. [Google Scholar]
- Takasaki, T.; Nishimura, K.; Saito, M.; Fukunaga, H.; Iwaki, T.; Sakai, T. Cobalt-free nickel-metal hydride battery for industrial applications. J. Alloys Compd. 2013, 580, S378–S381. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Ni-MH EThSS with Lifetime and Performance Estimation Technology. Presented at the 34th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 20–23 March 2017. [Google Scholar]
- Teraoka, H. Ni-MH Stationary Energy Storage: Extreme Temperature & Long Life Developments. Presented at the 33th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 21–24 March 2016. [Google Scholar]
- Teraoka, H. Development of Highly Durable and Long Life Ni-MH Batteries for Energy Storage Systems. Presented at the 32th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 9–12 March 2015. [Google Scholar]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese Patent Applications regarding nickel/metal hydride batteries. Batteries 2016, 2, 21. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Synthesis and structure determination of a new series of hydrogen storage alloys; RMg2Ni9 (R = La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers. J. Alloys Compd. 1997, 257, 115–121. [Google Scholar] [CrossRef]
- Kadir, K.; Kuriyama, N.; Sakai, T.; Uehara, I.; Eriksson, L. Structural investigation and hydrogen capacity of CaMg2Ni9: A new phase in the AB2C9 system isostructural with LaMg2Ni9. J. Alloys Compd. 1999, 284, 145–154. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Structural investigation and hydrogen storage capacity of LaMg2Ni9 and (La0.65Ca0.35)(Mg1.32Ca0.68)Ni9 of the AB2C9 type structure. J. Alloys Compd. 2000, 302, 112–117. [Google Scholar]
- Hayakawa, H.; Akiba, E.; Gotho, M.; Kohno, T. Crystal structure of hydrogen storage alloys, La-Mg-Nix (x = 3–4) system. Jpn. Inst. Met. 2005, 69, 170–178. (In Japanese) [Google Scholar] [CrossRef]
- Kohno, T.; Yoshida, H.; Kawashima, F.; Inaba, T.; Sakai, I.; Yamamoto, M.; Kanda, M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14. J. Alloys Compd. 2000, 311, L5–L7. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamamoto, M.; Sakai, I.; Inaba, T.; Takabayashi, J.; Irie, S.; Suzuki, H.; Takeno, K. Hydrogen Storage Alloy, Alkali Secondary Battery, Hybrid Car and Electric Vehicle. Jpn. Patent 069554, 2002. [Google Scholar]
- Inaba, T.; Sakai, I.; Yoshida, H.; Takabayashi, J.; Yamamoto, M.; Suzuki, H.; Irie, S.; Takeno, K. Nickel Hydrogen Secondary Battery, Hybrid Car and Electric Vehicle. Jpn. Patent 083593, 2002. [Google Scholar]
- Kawashima, F.; Sakamoto, T.; Arai, T. Hydrogen Storage Alloy and Nickel-Hydrogen Secondary Battery Using the Same. Jpn. Patent 105563, 2002. [Google Scholar]
- Liao, B.; Lei, Y.; Chen, L.; Lu, G.; Pan, H.; Wang, Q. A study on the structure and electrochemical properties of La2Mg(Ni0.95M0.05)9 (M = Co, Mn, Fe, Al, Cu, Sn) hydrogen storage electrode alloys. J. Alloys Compd. 2004, 376, 186–195. [Google Scholar] [CrossRef]
- Pan, H.; Liu, Y.; Gao, M.; Lei, Y.; Wang, Q. Electrochemical properties of the La0.7Mg0.3Ni2.65–xMn0.1Co0.75Alx (x = 0–5) hydrogen storage alloy electrode. J. Electrochem. Soc. 2005, 152, A326–A332. [Google Scholar] [CrossRef]
- Liao, B.; Lei, Y.; Chen, L.; Lu, G.; Pan, H.; Wang, Q. The effect of Al substitution for Ni on the structure and electrochemical properties of AB3-type La2Mg(Ni1–xAlx)9 (x = 0–0.05) alloys. J. Alloys Compd. 2005, 404–406, 665–668. [Google Scholar] [CrossRef]
- Qiu, S.; Chu, H.; Zhang, Y.; Qi, Y.; Sun, L.; Xu, F. Investigation on the structure and electrochemical properties of AB3-type La-Mg-Ni-Co-based hydrogen storage composites. J. Alloys Compd. 2008, 462, 392–397. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, L.; Shen, X.; Wang, L.; Wu, Y.; Wang, L. Cooperative effect of Co and Al on the microstructure and electrochemical properties of AB3-type hydrogen storage electrode alloys for advanced MH/Ni secondary battery. J. Alloys Compd. 2011, 36, 893–900. [Google Scholar] [CrossRef]
- Belgacem, Y.B.; Khaldi, C.; Lamloumi, J. The effect of the discharge rate on the electrochemical properties of AB3-type hydrogen storage alloy as anode in nickel-metal hydride batteries. Int. J. Hydrogen Energy 2017, 42, 12797–12807. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, X.; Wang, N.; Chai, Y.; Hou, D. Cyclic stability and high rate discharge performance of (La,Mg)5Ni19 multiphase alloy. Int. J. Hydrogen Energy 2011, 36, 4370–4374. [Google Scholar] [CrossRef]
- Guo, X.; Luo, Y.; Gao, Z.; Zhang, G.; Kang, L. The effect of Mg on the microstructure and electrochemical properties of La0.8–xGd0.2MgxNi3.3Co0.3Al0.1 (x = 0–0.4) hydrogen storage alloys. Funct. Mater. 2012, 43, 2450–2455. (In Chinese) [Google Scholar]
- Zhao, Y.; Han, S.; Li, Y.; Liu, J.; Zhang, L.; Yang, S.; Ke, D. Characterization and improvement of electrochemical properties of Pr5Co19-type single-phase La0.84Mg0.16Ni3.80 alloy. Electrochim. Acta 2015, 152, 265–273. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Ren, H.; Liu, Z.; Sun, H. Mechanism of distinct high rate dischargeability of La4MgNi19 electrode alloys prepared by casting and rapid quenching followed by annealing treatment. Int. J. Hydrogen Energy 2016, 41, 18571–18581. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, L.; Fan, Y.; Fan, G.; Liu, B.; Han, S. Phase transformation and electrochemical hydrogen storage performance of La3RMgNi19 (R = La, Pr, Nd, Sm, Gd, and Y) alloys. Int. J. Hydrogen Energy 2017, 42, 6051–6064. [Google Scholar] [CrossRef]
- Liu, J.; Han, S.; Li, Y.; Zhang, L.; Zhao, Y.; Yang, S.; Liu, B. Phase structures and electrochemical properties of La–Mg–Ni-based hydrogen storage alloys with superlattice structure. Int. J. Hydrogen Energy 2016, 41, 20261–20275. [Google Scholar] [CrossRef]
- Yan, H.; Xiong, W.; Wang, L.; Li, B.; Li, J.; Zhao, X. Investigations on AB3-, A2B7- and A5B19-type La-Y-Ni system hydrogen storage alloys. Int. J. Hydrogen Energy 2017, 42, 2257–2264. [Google Scholar] [CrossRef]
- Young, K.; Yasuoka, S. Past, present, and future of metal hydride alloys in nickel-metal hydride batteries. In Proceedings of the 14th International Symposium on Metal-Hydrogen Systems, Manchester, UK, 21–25 July 2014. [Google Scholar]
- Young, K.; Chang, S.; Lin, X. C14 Laves phase metal hydride alloys for Ni/MH batteries applications. Batteries 2017, 3, 27. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Power Sources 2015, 277, 426–432. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; Ouchi, T.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 1 structural, hydrogen storage and electrochemical properties. J. Alloys Compd. 2016, 660, 407–415. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 2 Ni/MH battery performance and failure mechanism. J. Power Sources 2015, 277, 433–442. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; English, N.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 2 battery performance and failure mechanism. J. Alloys Compd. 2016, 664, 417–427. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ishida, J.; Kai, T.; Kajiwara, T.; Doi, S.; Yamazaki, T.; Kishida, K.; Inui, H. Function of aluminum in crystal structure of rare earth-Mg-Ni hydrogen-absorbing alloy and deterioration mechanism of Nd0.9Mg0.1Ni3.4 and Nd0.9Mg0.1Ni3.3Al0.2 alloys. Int. J. Hydrogen Energy 2017, 42, 11574–11583. [Google Scholar] [CrossRef]
- Yasuoka, S.; Ishida, J.; Kishida, K.; Inui, H. Effects of cerium on the hydrogen absorption-desorption properties of rare earth-Mg-Ni hydrogen-absorbing alloys. J. Power Sources 2017, 346, 56–62. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Wang, C.S.; Wang, F.S.; Wang, Q.D. Structure of the secondary phase and its effects on hydrogen-storage properties in a Ti0.7Zr0.2V0.1Ni alloy. J. Power Sources 1998, 75, 288–291. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jankowska, E.; Makowiecka, M.; Wieczorek, I. Electrode characteristics of nanocrystalline TiFe-type alloys. J. Alloys Compd. 2003, 354, L1–L4. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Yang, X.G.; Ren, K.; Wang, Q.D. Annealing treatment of AB2-type hydrogen storage alloys: I. crystal structures. J. Alloys Compd. 1999, 292, 236–240. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Yang, X.G.; Du, Y.L.; Wang, Q.D. Effects of annealing treatment on phase structures, hydrogen absorption–desorption characteristics and electrochemical properties of a V3TiNi0.56Hf0.24Mn0.15Cr0.1 alloy. J. Alloys Compd. 2000, 305, 125–129. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, Q.A.; Shu, K.Y.; Du, Y.L.; Lei, Y.Q.; Wang, Q.D.; Zhang, W.K. The effect of annealing on the electrochemical; properties of Zr0.5Ti0.5Mn0.5V0.3Co0.2Ni1.1 alloy electrode. J. Power Sources 2000, 90, 170–175. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Chao, B.; Fetcenko, M.A.; Bendersky, L.A.; Wang, K.; Chiu, C. The correlation of C14/C15 phase abundance and electrochemical properties in the AB2 alloys. J. Alloys Compd. 2010, 506, 841–848. [Google Scholar] [CrossRef]
- Pan, H.; Liu, Y.; Gao, M.; Zhu, Y.; Lei, Y.; Wang, Q. A study on the effect of annealing treatment on the electrochemical properties of La0.67Mg0.33Ni2.5Co0.5 alloy electrode. Int. J. Hydrogen Energy 2003, 28, 113–117. [Google Scholar] [CrossRef]
- Yang, Z.P.; Li, Q.; Zhao, X.J. Influence of magnetic annealing on electrochemical performance of La0.67Mg0.33Ni3.0 hydride electrode. J. Alloys Compd. 2013, 558, 99–104. [Google Scholar] [CrossRef]
- Hu, W.; Denys, R.V.; Nwakwuo, C.C.; Holm, T.; Maehlen, J.P.; Solberg, J.K.; Yartys, V.A. Annealing effect on phase composition and electrochemical properties of the Co-free La2MgNi9 anode for Ni-metal hydride batteries. Electrochim. Acta 2013, 96, 27–133. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Zhai, F.; Ma, G.; Xu, L.; Qu, X. Effect of annealing treatment on the anti-pulverization and anti-corrosion properties of La0.67Mg0.33Ni2.5Co0.5 hydrogen storage alloy. J. Rare Earths 2015, 33, 417–424. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B. Effects of annealing and stoichiometry to (Nd, Mg)(Ni, Al)3.5 metal hydride alloys. J. Power Sources 2012, 215, 152–159. [Google Scholar] [CrossRef]
- Balcerzak, M.; Nowak, M.; Jurczyk, M. Hydrogenation and electrochemical studies of La-Mg-Ni alloy. Int. J. Hydrogen Energy 2017, 42, 1436–1443. [Google Scholar] [CrossRef]
- Xiong, W.; Uan, H.; Wang, L.; Zhao, X.; Li, J.; Li, B.; Wang, Y. Effects of annealing temperature on the structure and properties of the LaY2Ni10Mn0.5 hydrogen storage alloy. Int. J. Hydrogen Energy 2017, 42, 15319–15327. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.; Zhai, T.; Yuan, Z.; Zhang, G.; Guo, S. Effects of stoichiometric ratio La/Mg on structures and electrochemical performances of as-cast and annealed La-Mg-Ni-based A2B7-type electrode alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1968–1977. [Google Scholar] [CrossRef]
- Hayakawa, H.; Enoki, H.; Akiba, E. Annealing conditions with Mg vapor-pressure control and hydrogen storage characteristic of La4MgNi19 hydrogen storage alloy. Jpn. Inst. Met. 2006, 70, 158–161. (In Japanese) [Google Scholar] [CrossRef]
- Hu, W.K.; Kim, D.M.; Jeon, S.W.; Lee, J.Y. Effect of annealing treatment on electrochemical properties of Mm-based hydrogen storage alloys for Ni/MH batteries. J. Alloys Compd. 1998, 270, 255–264. [Google Scholar] [CrossRef]
- Ma, Z.; Qiu, J.; Chen, L.; Lei, Y. Effects of annealing on microstructure and electrochemical properties of the low Co-containing alloy MI(NiCoMnAlFe)5 for Ni/MH battery electrode. J. Power Sources 2004, 125, 267–272. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, Y.; Cui, S.; Huang, C.; Qian, W.; Lin, C.; Zhang, Y.; Lin, Y. Effect of annealing treatment on structure and electrochemical performance of quenched MmNi4.2Co0.3Mn0.4Al0.3Mg0.03 hydrogen storage alloy. J. Alloys Compd. 2010, 501, 47–53. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La and Hf): Structural characteristics. J. Alloys Compd. 2012, 516, 144–152. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; NG, K.Y.S. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La and Hf): Hydrogen storage and electrochemical properties. Int. J. Hydrogen Energy 2012, 37, 8418–8427. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Wang, L. Annealing effects on Laves phase-related body-centered-cubic solid solution metal hydride alloys. J. Alloys Compd. 2016, 654, 216–225. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Zhang, J.; Latroche, M. Structural stability of ABy phases in the (La,Mg)-Ni system obtained by density functional theory calculations. J. Phys. Chem. 2011, 115, 25470–25478. [Google Scholar]
- Wong, D.F.; Young, K. Phase stability of superlattice metal hydride alloy estimated by first principle calculation. Unpublished work. 2017. [Google Scholar]
- Zhang, L.; Zhang, J.; Han, S.; Li, Y.; Yang, S.; Liu, J. Phase transformation and electrochemical properties of La0.70Mg0.30Ni3.3 super-stacking metal hydride alloy. Intermetallics 2015, 58, 65–70. [Google Scholar]
- Li, F.; Young, K.; Ouchi, T.; Fetcenko, M.A. Annealing effects on structural and electrochemical properties of (LaPrNdZr)0.83Mg0.17(NiCoAlMn)3.3 alloy. J. Alloys Compd. 2009, 471, 371–377. [Google Scholar] [CrossRef]
- Young, K.; Wang, L.; Yan, S.; Liao, X.; Meng, T.; Shen, H.; May, W.C. Fabrications of high-capacity alpha-Ni(OH)2. Batteries 2017, 3, 6. [Google Scholar] [CrossRef]
- Young, K.; Wu, A.; Qiu, Z.; Tan, J.; Mays, W. Effects of H2O2 addition to the cell balance and self-discharge of Ni/MH batteries with AB5 and A2B7 alloys. Int. J. Hydrogen Energy 2012, 37, 9882–9891. [Google Scholar] [CrossRef]
- Young, K.; Koch, J.M.; Wan, C.; Denys, R.V.; Yartys, V.A. Cell performance comparison between C14- and C15-predominated AB2 metal hydride alloys. Batteries 2017, 3, 29. [Google Scholar] [CrossRef]
- Charbonnier, V.; Monnier, J.; Zhang, J.; Paul-Boucour, V.; Joiret, S.; Puga, B.; Goubault, L.; Bernard, P.; Latroche, M. Relationship between H2 sorption properties and aqueous corrosion mechanisms in A2B7 hydride forming alloys (A = Y, Gd or Sm). J. Power Sources 2016, 326, 146–155. [Google Scholar] [CrossRef]
- Tang, R.; Wei, X.; Liu, Y.; Zhu, C.; Zhu, J.; Yu, G. Effect of the Sm content on the structure and electrochemical properties of La1.3–xSmxCaMg0.7Ni9 (x = 0–0.3) hydrogen storage alloys. J. Power Sources 2006, 155, 456–460. [Google Scholar]
- Ping, L.; Hou, Z.; Yang, T.; Shang, H.; Qu, X.; Zhang, Y. Structure and electrochemical hydrogen storage characteristics of the as-cast and annealed La0.8–xSmxMg0.2Ni3.15Co0.2Al0.1Si0.05 (x = 0–0.4) alloys. J. Rare Earths 2012, 30, 696–704. [Google Scholar]
- Zhang, Y.; Hou, Z.; Li, B.; Ren, H.; Zhang, G.; Zhao, D. An investigation on electrochemical hydrogen storage performances of the as-cast and -annealed La0.8–xSmxMg0.2Ni3.35Al0.1Si0.05 (x = 0–0.4) alloys. J. Alloys Compd. 2012, 537, 175–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Yang, T.; Zhai, T.; Yuan, Z.; Guo, S. Effects of substituting La with M (M = Sm, Nd, Pr) on electrochemical hydrogen storage characteristics of A2B7-type electrode alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 4012–4022. [Google Scholar] [CrossRef]
- Liu, J.; Han, S.; Li, Y.; Zhao, X.; Yang, S.; Zhao, Y. Cooperative effects of Sm and Mg on electrochemical performance of La–Mg–Ni-based alloys with A2B7- and A5B19-type super-stacking structure. Int. J. Hydrogen Energy 2015, 40, 1116–1127. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Wang, L.; Wong, D.F. The effects of Al substitution on the phase abundance, structure and electrochemical performance of La0.7Mg0.3Ni2.8Co0.5–xAlx (x = 0, 0.1, 0.2) alloys. J. Power Sources 2015, 279, 172–179. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Zhao, Y.; Du, W.; Li, Y.; Yang, S.; Han, S. Phase structure and cycling stability of A2B7 superlattice La0.60Sm0.15Mg0.25Ni3.4 metal hydride alloy. Int. J. Hydrogen Energy 2016, 41, 1791–1800. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. The role of Mn in C14 Laves phase multi-component alloys for NiMH battery application. J. Alloys Compd. 2009, 477, 749–758. [Google Scholar] [CrossRef]
- Osumi, Y. Suiso Kyuzou Goukin; Agune Technology Center: Tokyo, Japan, 1999; p. 218. (In Japanese) [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Ouchi, T.; Nei, J.; Moghe, D. The importance of rare-earth additions in Zr-based AB2 metal hydride alloys. Batteries 2016, 2, 25. [Google Scholar] [CrossRef]
- Stucki, F.; Schlapbach, L. Magnetic properties of LaNi5, FeTi, Mg2Ni and their hydrides. J. Less-Comm. Met. 1980, 74, 143–151. [Google Scholar] [CrossRef]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Pawlik, D.; Shen, H.T. Transmission electron microscope studies in the surface oxide on the La-containing AB2 metal hydride alloy. J. Alloys Compd. 2016, 672, 356–365. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Hu, C.; Reichman, B. Effects of alkaline pre-etching to metal hydride alloys. Batteries 2017, 3, 30. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Wan, C.; Denys, R.V.; Yartys, V.A. Comparison of C15- and C15-predominated AB2 metal hydride alloys for electrochemical applications. Batteries 2017, 3, 22. [Google Scholar] [CrossRef]
| Alloy | Annealing Temperature | Source | La | Sm | Mg | Ni | Al | B/A |
|---|---|---|---|---|---|---|---|---|
| - | - | Design | 5.7 | 14.0 | 4.0 | 73.0 | 3.3 | 3.2 |
| A0 | - | ICP | 5.7 | 14.0 | 3.9 | 73.0 | 3.4 | 3.2 |
| A1 | 880 °C | ICP | 6.0 | 14.5 | 4.2 | 72.1 | 3.2 | 3.0 |
| A2 | 900 °C | ICP | 5.8 | 13.9 | 4.0 | 72.9 | 3.4 | 3.2 |
| A3 | 920 °C | ICP | 5.8 | 13.9 | 4.0 | 73.0 | 3.3 | 3.2 |
| A4 | 940 °C | ICP | 5.8 | 14.3 | 4.0 | 72.7 | 3.2 | 3.1 |
| A5 | 960 °C | ICP | 5.8 | 14.5 | 4.0 | 72.5 | 3.2 | 3.1 |
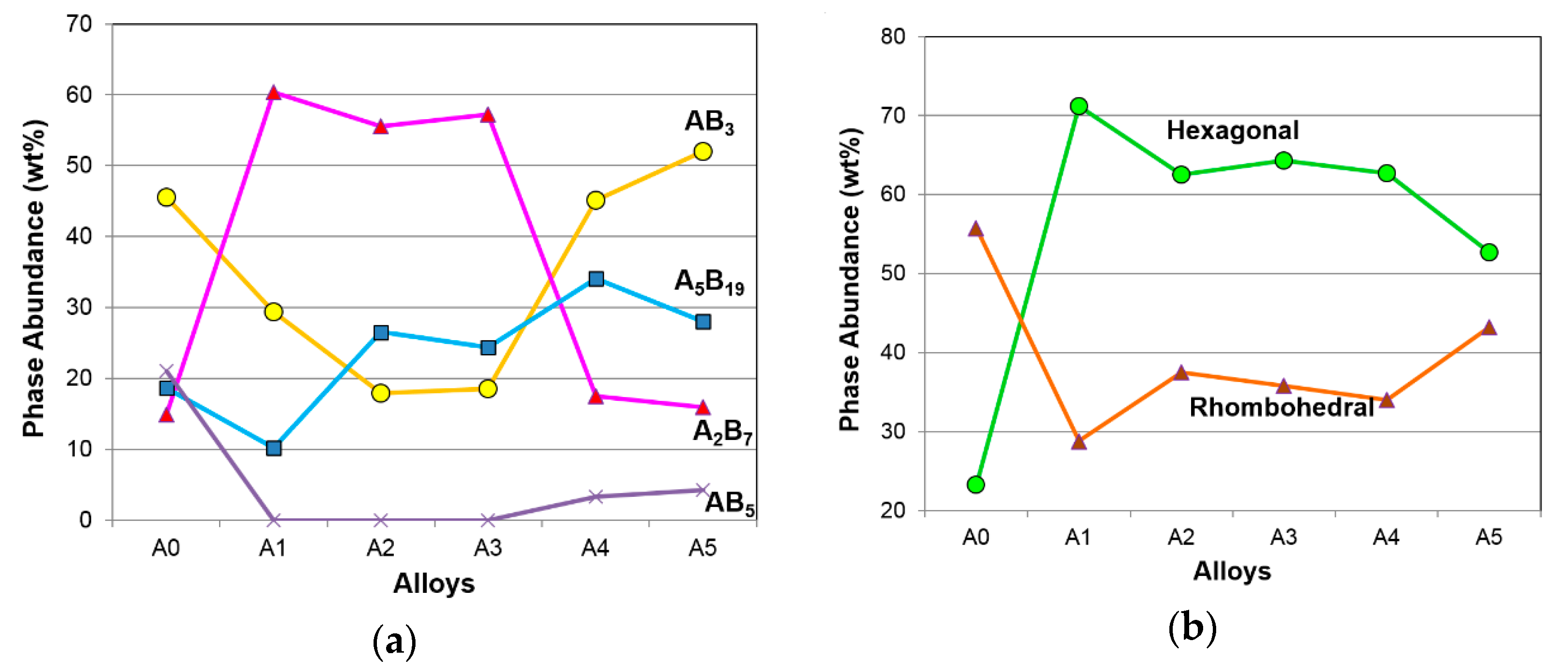
| Alloy | CeNi3 (H) | NdNi3 (R) | Nd2Ni7 (H) | Pr2Ni7 (R) | Sm5Ni19 (H) | Nd5Co19 (R) | CaCu5 (H) |
|---|---|---|---|---|---|---|---|
| A0 | 8.4 | 37.1 | 14.9 | - | - | 18.6 | 21.0 |
| A1 | 20.8 | 8.6 | 50.4 | 10.0 | - | 10.2 | - |
| A2 | - | 17.9 | 48.6 | 7.0 | 13.9 | 12.6 | - |
| A3 | - | 18.5 | 52.4 | 4.8 | 11.9 | 12.5 | - |
| A4 | 24.3 | 20.8 | 17.5 | - | 20.9 | 13.2 | 3.3 |
| A5 | 24.3 | 27.7 | 15.9 | - | 12.5 | 15.5 | 4.2 |
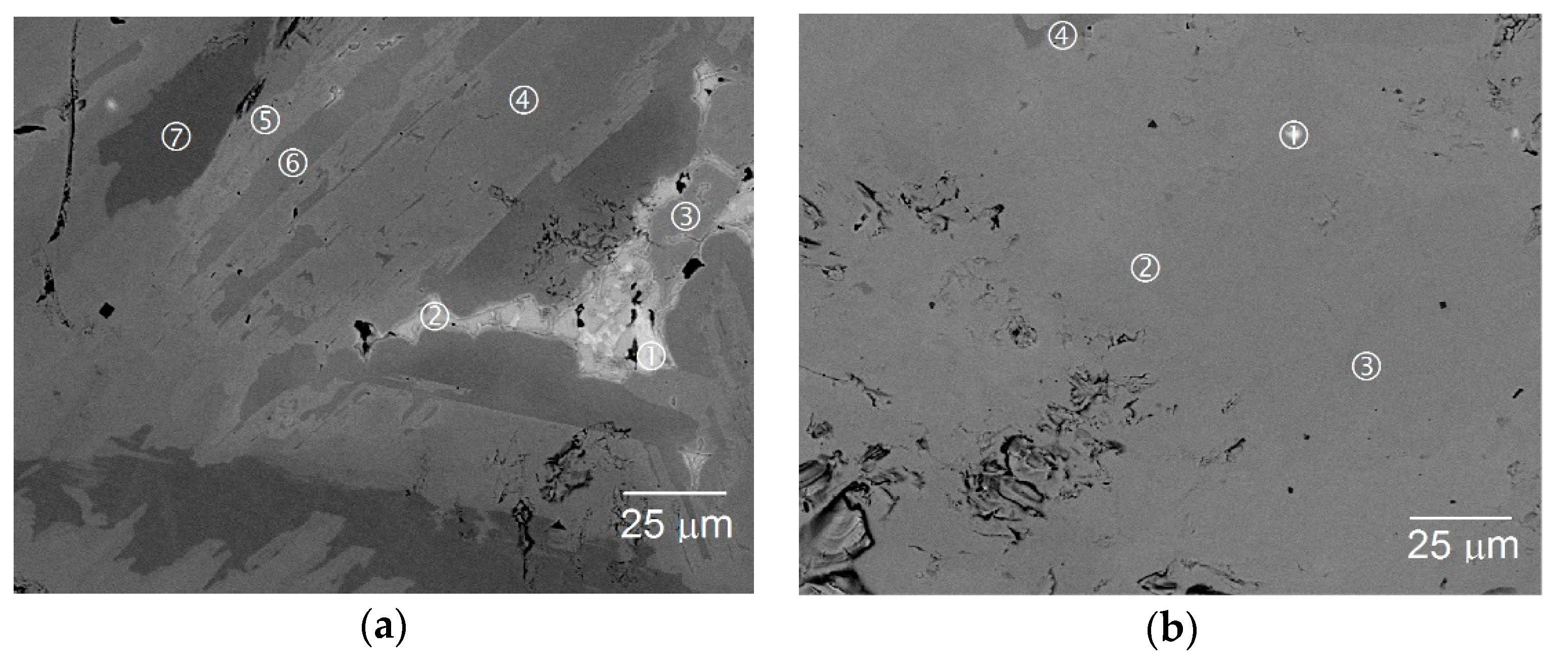
| Sample | Location | La | Sm | Mg | Ni | Al | B/A | Phase |
|---|---|---|---|---|---|---|---|---|
| A0 | 3a-1 | 90.8 | 5.1 | 1.5 | 1.9 | 0.7 | 0.03 | La |
| 3a-2 | 2.5 | 42.9 | 7.3 | 47.0 | 0.3 | 0.90 | AB | |
| 3a-3 | 9.3 | 13.6 | 5.2 | 65.7 | 6.2 | 2.56 | AB2/AB3 | |
| 3a-4 | 4.1 | 15.3 | 5.4 | 71.6 | 3.6 | 3.03 | AB3 | |
| 3a-5 | 3.4 | 15.0 | 4.4 | 73.6 | 3.6 | 3.39 | AB3/A2B7/A5B19 | |
| 3a-6 | 7.2 | 14.3 | 13.5 | 63.7 | 1.2 | 1.85 | AB2 | |
| 3a-7 | 3.5 | 14.0 | 0.6 | 78.3 | 3.6 | 4.52 | AB5 | |
| A3 | 3b-1 | 27.9 | 61.0 | 2.7 | 7.3 | 1.1 | 0.09 | La/Sm |
| 3b-2 | 5.1 | 14.4 | 5.1 | 71.1 | 4.3 | 3.07 | AB3/A2B7 | |
| 3b-3 | 3.1 | 13.9 | 4.0 | 75.2 | 3.8 | 3.76 | A2B7/A5B19 | |
| 3b-4 | 4.8 | 14.5 | 15.6 | 64.0 | 1.0 | 1.86 | AB2 |
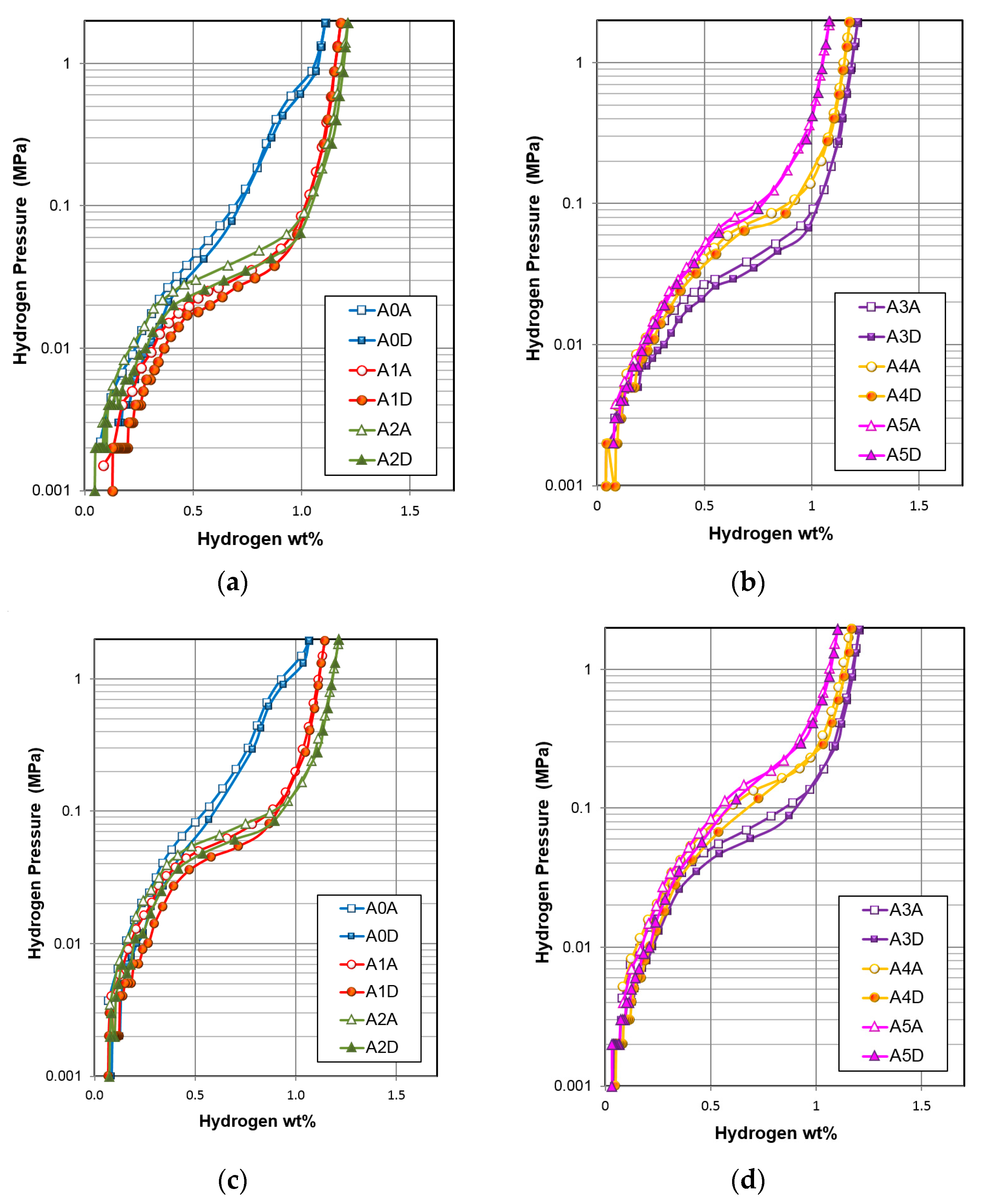
| Gaseous Phase Properties | Unit | A0 | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|---|---|
| Capacity at 2 MPa | wt % | 1.11 | 1.18 | 1.21 | 1.21 | 1.18 | 1.08 |
| Reversible capacity | wt % | 0.95 | 0.98 | 1.17 | 1.17 | 1.13 | 1.00 |
| Desorption pressure | MPa | 0.16 | 0.029 | 0.035 | 0.037 | 0.071 | 0.091 |
| Slope factor | % | 69 | 78 | 76 | 72 | 77 | 79 |
| Hysteresis | 0.17 | 0.16 | 0.22 | 0.17 | 0.08 | 0.09 | |
| –∆H | kJ·mol H2−1 | 25.8 | 38.8 | 35.2 | 33.6 | 31.8 | 39.3 |
| –∆S | J·mol H2−1·K−1 | 89 | 118 | 107 | 102 | 102 | 129 |
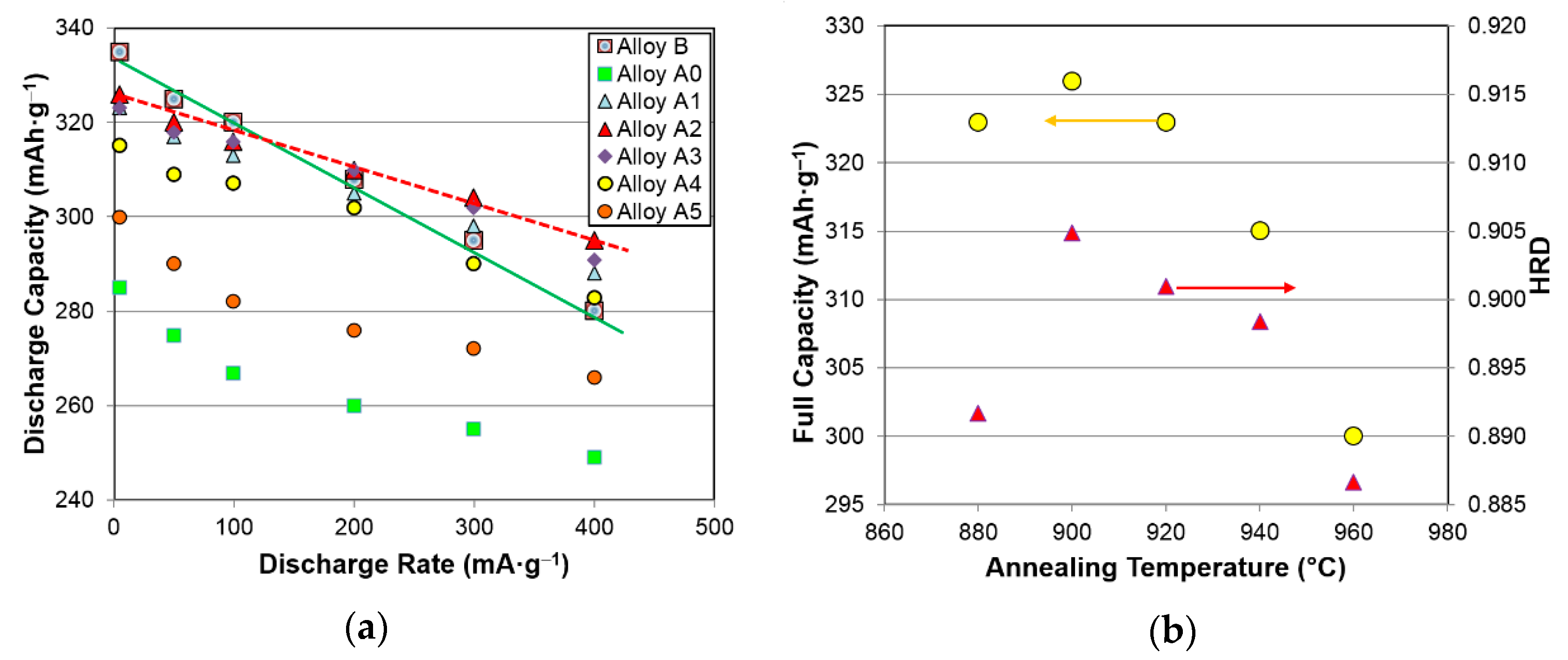
| Electrochemical and Magnetics Properties | Unit | A0 | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|---|---|
| High-rate discharge capacity | mAh·g−1 | 249 | 288 | 295 | 291 | 283 | 266 |
| Full discharge capacity | mAh·g−1 | 285 | 323 | 326 | 323 | 315 | 300 |
| Half-cell HRD | % | 87.5 | 89.2 | 90.4 | 90.1 | 89.8 | 88.7 |
| Diffusion coefficient, D | 10−10 cm2·s−1 | 4.0 | 4.0 | 4.2 | 4.4 | 4.2 | 4.1 |
| Surface reaction current, Io | mA·g−1 | 24.2 | 24.0 | 33.1 | 23.8 | 21.9 | 17.6 |
| Charge-transfer resistance at −40 °C, R | Ω·g | 4.9 | 8.8 | 4.0 | 3.7 | 3.4 | 5.3 |
| Double-layer capacitance at −40 °C, C | F·g−1 | 1.6 | 0.86 | 1.02 | 0.88 | 1.12 | 1.29 |
| RC product at −40 °C | s | 7.7 | 7.6 | 4.1 | 3.3 | 3.8 | 6.8 |
| Total saturated magnetic susceptibility, MS | emu·g−1 | 1.45 | 1.36 | 0.96 | 0.60 | 1.05 | 1.12 |
| Applied field where M.S. = ½ MS, H1/2 | kOe | 0.11 | 0.10 | 0.11 | 0.12 | 0.10 | 0.10 |
| C-Cell Results | Unit | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|---|
| 2C at RT capacity/0.2C at RT capacity | % | 87 | 90 | 93 | 90 | 87 |
| 1C at −10 °C capacity/0.2C at RT capacity | % | 92 | 94 | 95 | 94 | 91 |
| 14-day charge retention | % | 85.3 | 84.9 | 84.8 | 76.1 | 85.3 |
| 28-day 45 °C voltage stand | V | 1.213 | 1.222 | 1.218 | 1.218 | 1.220 |
| Peak power at RT (20th cycle) | W·kg−1 | 183 | 198 | 206 | 200 | 194 |
| 0.5C/0.5C cycle life (before reaching 3 Ah) | Number of cycles | 220 | 255 | 365 | 340 | 210 |
| C/C cycle life (before reaching 3 Ah) | Number of cycles | 110 | 185 | 205 | 130 | 120 |
| Properties | CeNi3 | NdNi3 | Nd2Ni7 | Pr2Ni7 | Sm5Ni19 | Nd5Co19 | CaCu5 |
|---|---|---|---|---|---|---|---|
| GP maximum capacity | 0.26− | 0.51− | 0.63+ | 0.42+ | 0.07+ | 0.55− | 0.38− |
| GP reversible capacity | 0.24− | 0.14− | 0.21+ | 0.03+ | 0.57+ | 0.20− | 0.37− |
| Equilibrium pressure | 0.02+ | 0.89+ | 0.68− | 0.58− | 0.09− | 0.92+ | 0.92+ |
| PCT slope factor | 0.42+ | 0.31− | 0.00 | 0.05+ | 0.14+ | 0.31− | 0.46− |
| PCT hysteresis | 0.73− | 0.03− | 0.46+ | 0.39+ | 0.17− | 0.02− | 0.00 |
| ∆H | 0.12− | 0.43+ | 0.16− | 0.24− | 0.02− | 0.42+ | 0.61+ |
| ∆S | 0.28− | 0.16+ | 0.01− | 0.05− | 0.01− | 0.15+ | 0.33+ |
| EC high-rate capacity | 0.06− | 0.79− | 0.65+ | 0.49+ | 0.17+ | 0.84− | 0.84− |
| EC full capacity | 0.05− | 0.84− | 0.69+ | 0.56+ | 0.13+ | 0.89− | 0.84− |
| HRD | 0.09− | 0.52− | 0.43+ | 0.22+ | 0.42+ | 0.59− | 0.76− |
| Diffusion constant, D | 0.25− | 0.05− | 0.16+ | 0.00 | 0.42+ | 0.09− | 0.27− |
| Exchange Current, Io | 0.52− | 0.08− | 0.33+ | 0.31+ | 0.00 | 0.07− | 0.02− |
| −40 °C resistivity, R | 0.15+ | 0.17− | 0.06+ | 0.32+ | 0.53− | 0.11− | 0.01− |
| −40 °C capacitance, C | 0.02+ | 0.91+ | 0.71− | 0.59− | 0.05+ | 0.94+ | 0.85+ |
| RC product | 0.17+ | 0.08+ | 0.11− | 0.00 | 0.65− | 0.13+ | 0.29+ |
| High rate | 0.54− | 0.00 | 0.14+ | 0.00 | 0.20+ | 0.00 | 0.15− |
| Low temperature | 0.50− | 0.02− | 0.18+ | 0.01+ | 0.19+ | 0.07− | 0.23− |
| Charge retention | 0.17− | 0.03− | 0.31+ | 0.30+ | 0.48− | 0.01− | 0.21− |
| Peak power | 0.29− | 0.25+ | 0.00 | 0.23− | 0.56+ | 0.17+ | 0.00 |
| Cycle life | 0.90− | 0.00 | 0.31+ | 0.02+ | 0.07+ | 0.00 | 0.30− |
| Properties | AB3 | A2B7 | A5B19 | Hexagonal | Rhombohedral |
|---|---|---|---|---|---|
| GP maximum capacity | 0.75− | 0.60+ | 0.00 | 0.44+ | 0.46− |
| GP reversible capacity | 0.40− | 0.17+ | 0.36+ | 0.29+ | 0.21− |
| Equilibrium pressure | 0.52+ | 0.68− | 0.00 | 0.93− | 0.85+ |
| PCT slope factor | 0.02+ | 0.01+ | 0.03+ | 0.45+ | 0.41− |
| PCT hysteresis | 0.61− | 0.46+ | 0.21− | 0.00 | 0.00 |
| ∆H | 0.03+ | 0.17− | 0.01+ | 0.54− | 0.43+ |
| ∆S | 0.02− | 0.01− | 0.00 | 0.26− | 0.18+ |
| EC high-rate capacity | 0.60− | 0.63+ | 0.01+ | 0.84+ | 0.78− |
| EC full capacity | 0.61− | 0.68+ | 0.00 | 0.86+ | 0.81− |
| HRD | 0.49− | 0.40+ | 0.14+ | 0.70+ | 0.59− |
| Diffusion constant, D | 0.29− | 0.11+ | 0.29+ | 0.18+ | 0.11− |
| Exchange Current, Io | 0.56− | 0.34+ | 0.01− | 0.02+ | 0.01− |
| −40 °C resistivity, R | 0.00 | 0.10+ | 0.72− | 0.04+ | 0.08− |
| −40 °C capacitance, C | 0.53+ | 0.71− | 0.01+ | 0.90− | 0.88+ |
| RC product | 0.26+ | 0.08− | 0.46− | 0.23− | 0.16+ |
| High rate | 0.34− | 0.09+ | 0.13+ | 0.02+ | 0.00 |
| Low temperature | 0.42− | 0.13+ | 0.10+ | 0.11+ | 0.09− |
| Charge retention | 0.17− | 0.32+ | 0.38− | 0.00 | 0.04+ |
| Peak power | 0.05− | 0.02− | 0.53+ | 0.11− | 0.16+ |
| Cycle life | 0.58− | 0.24+ | 0.05+ | 0.00 | 0.03+ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-H.; Ouchi, T.; Nei, J.; Koch, J.M.; Lien, Y.-L. Comparison among Constituent Phases in Superlattice Metal Hydride Alloys for Battery Applications. Batteries 2017, 3, 34. https://doi.org/10.3390/batteries3040034
Young K-H, Ouchi T, Nei J, Koch JM, Lien Y-L. Comparison among Constituent Phases in Superlattice Metal Hydride Alloys for Battery Applications. Batteries. 2017; 3(4):34. https://doi.org/10.3390/batteries3040034
Chicago/Turabian StyleYoung, Kwo-Hsiung, Taihei Ouchi, Jean Nei, John M. Koch, and Yu-Ling Lien. 2017. "Comparison among Constituent Phases in Superlattice Metal Hydride Alloys for Battery Applications" Batteries 3, no. 4: 34. https://doi.org/10.3390/batteries3040034
APA StyleYoung, K.-H., Ouchi, T., Nei, J., Koch, J. M., & Lien, Y.-L. (2017). Comparison among Constituent Phases in Superlattice Metal Hydride Alloys for Battery Applications. Batteries, 3(4), 34. https://doi.org/10.3390/batteries3040034