Studies on MgNi-Based Metal Hydride Electrode with Aqueous Electrolytes Composed of Various Hydroxides
Abstract
:1. Introduction
2. Experimental Setup
3. Results
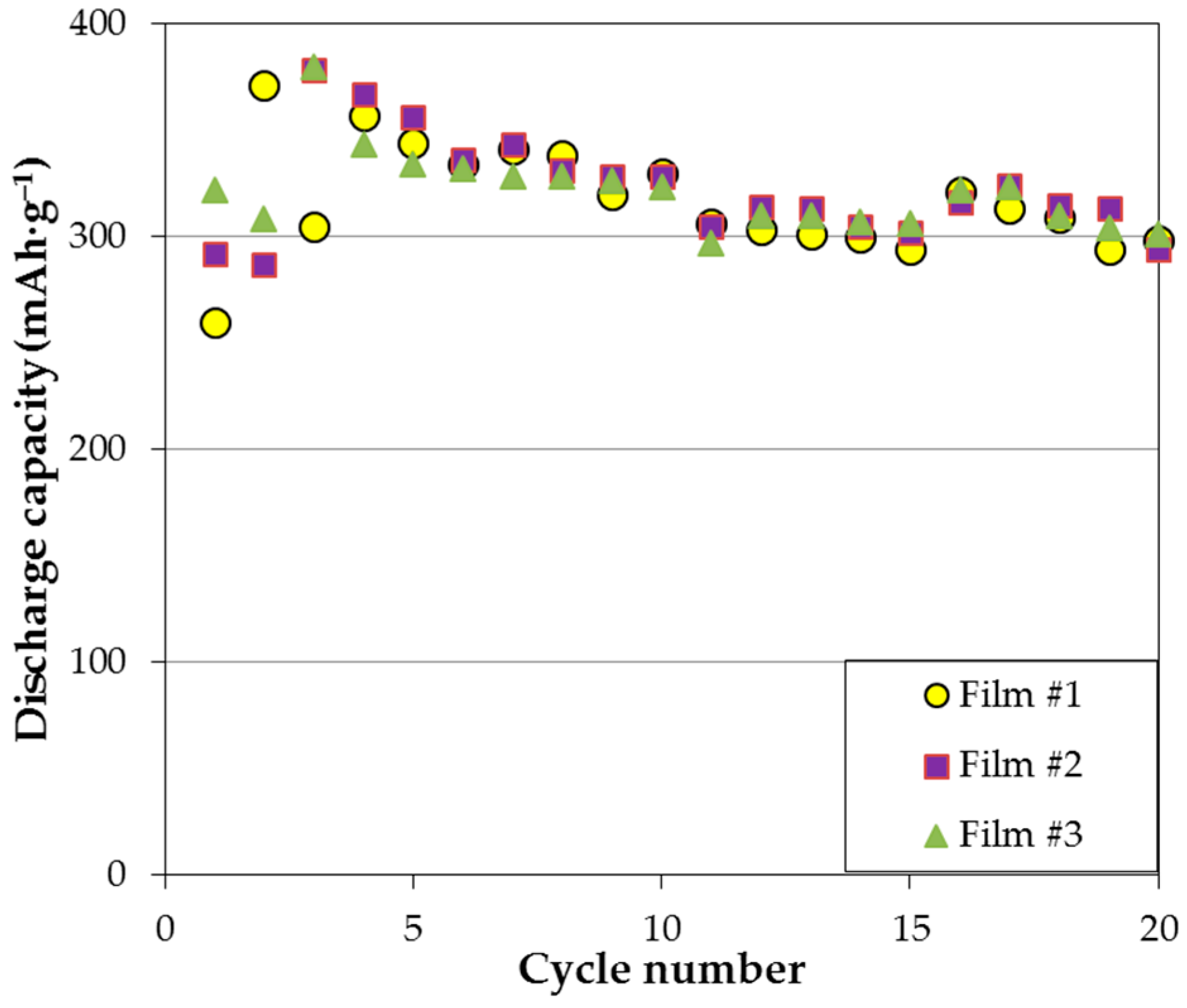
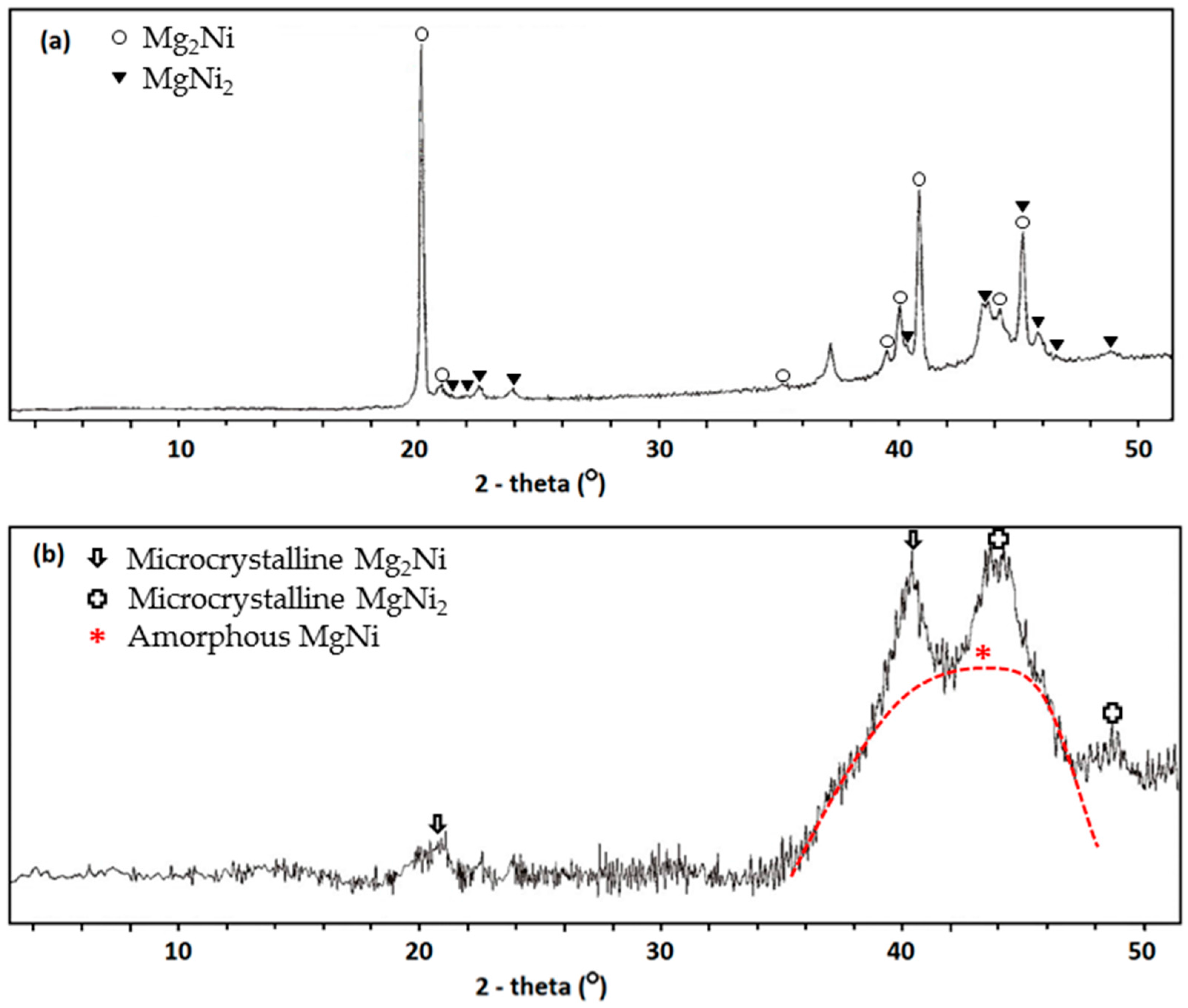
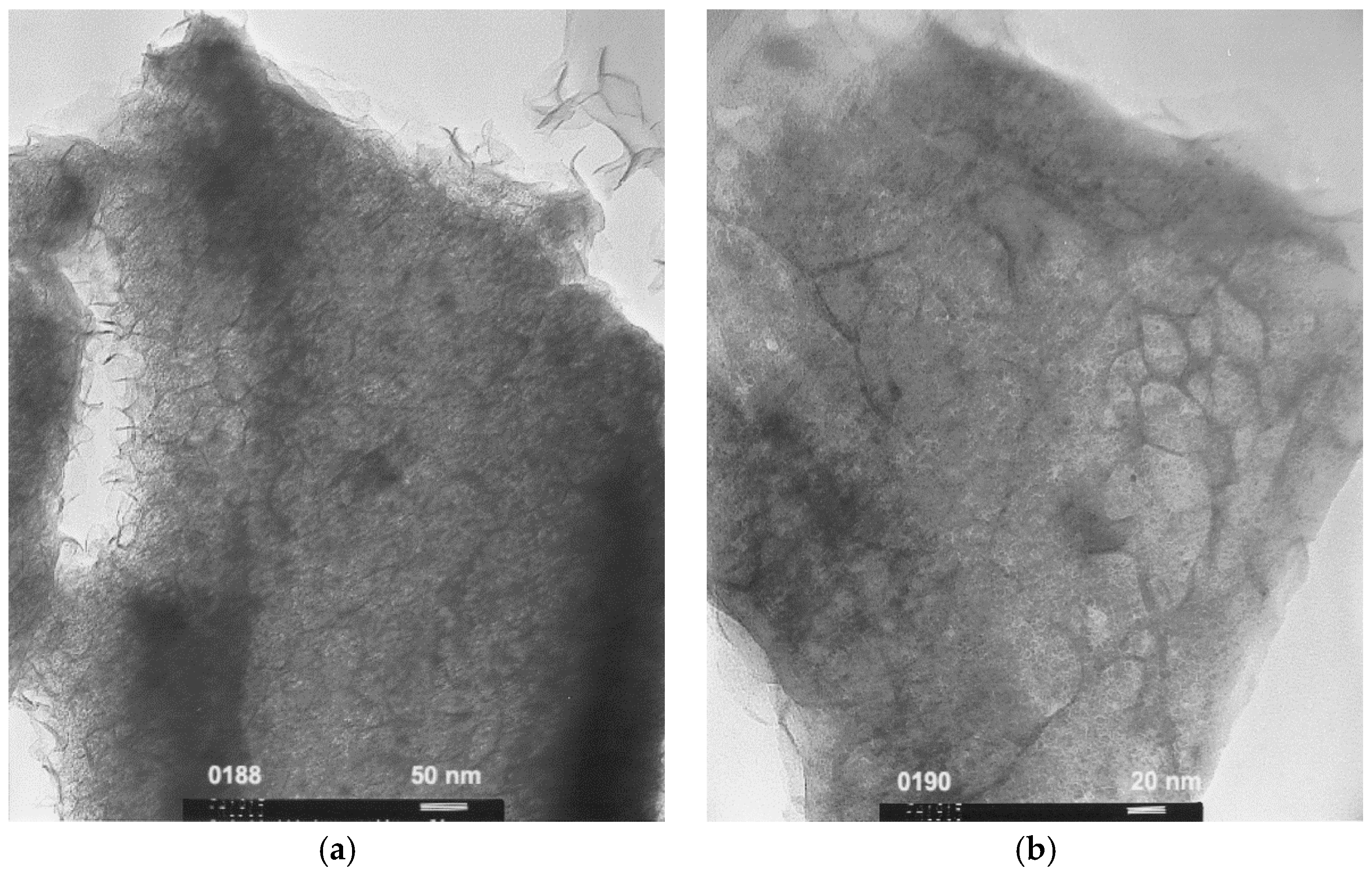
3.1. MgNi-Based Thin Film Prepared by Radio Frequency Sputtering
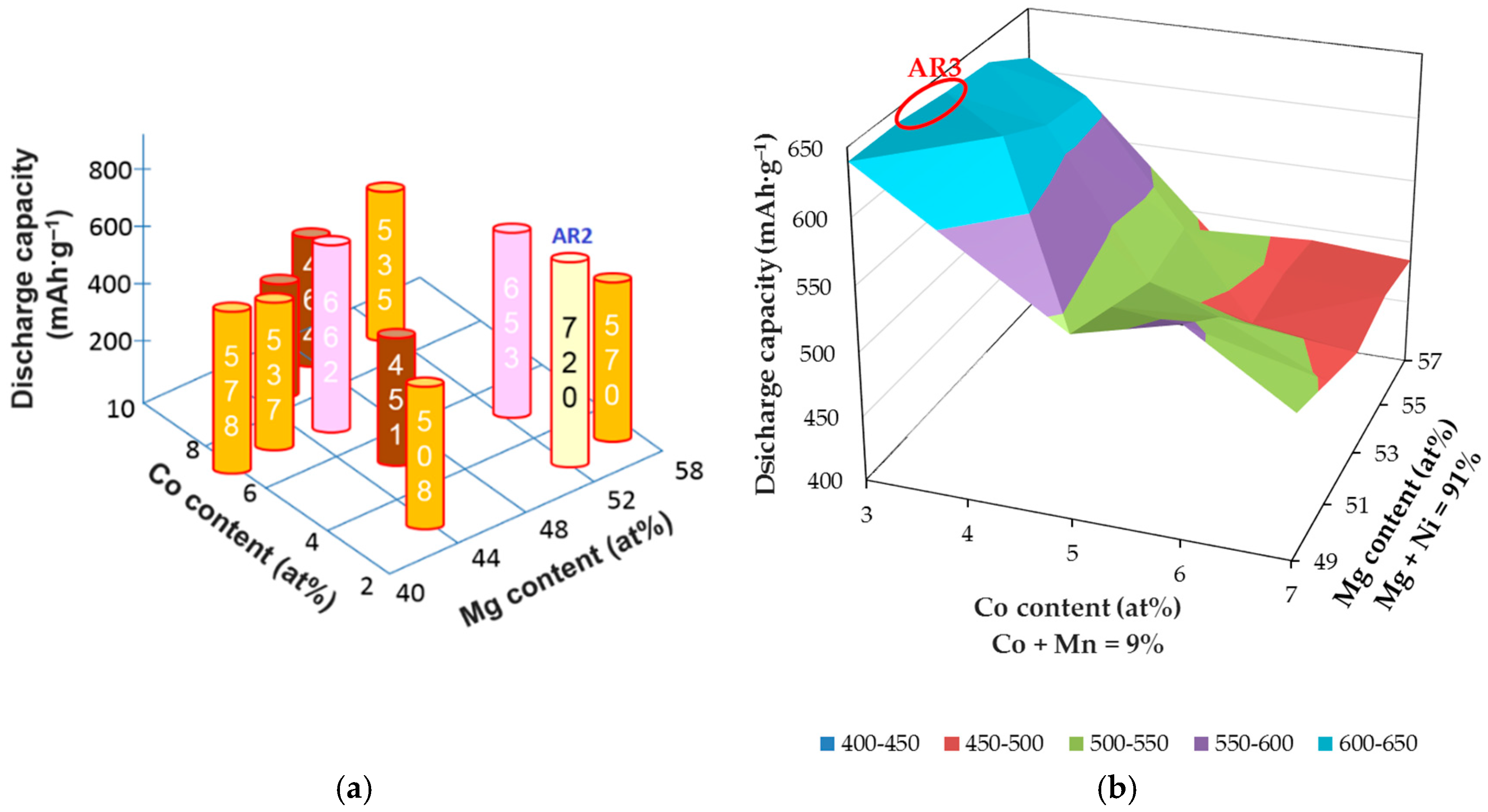
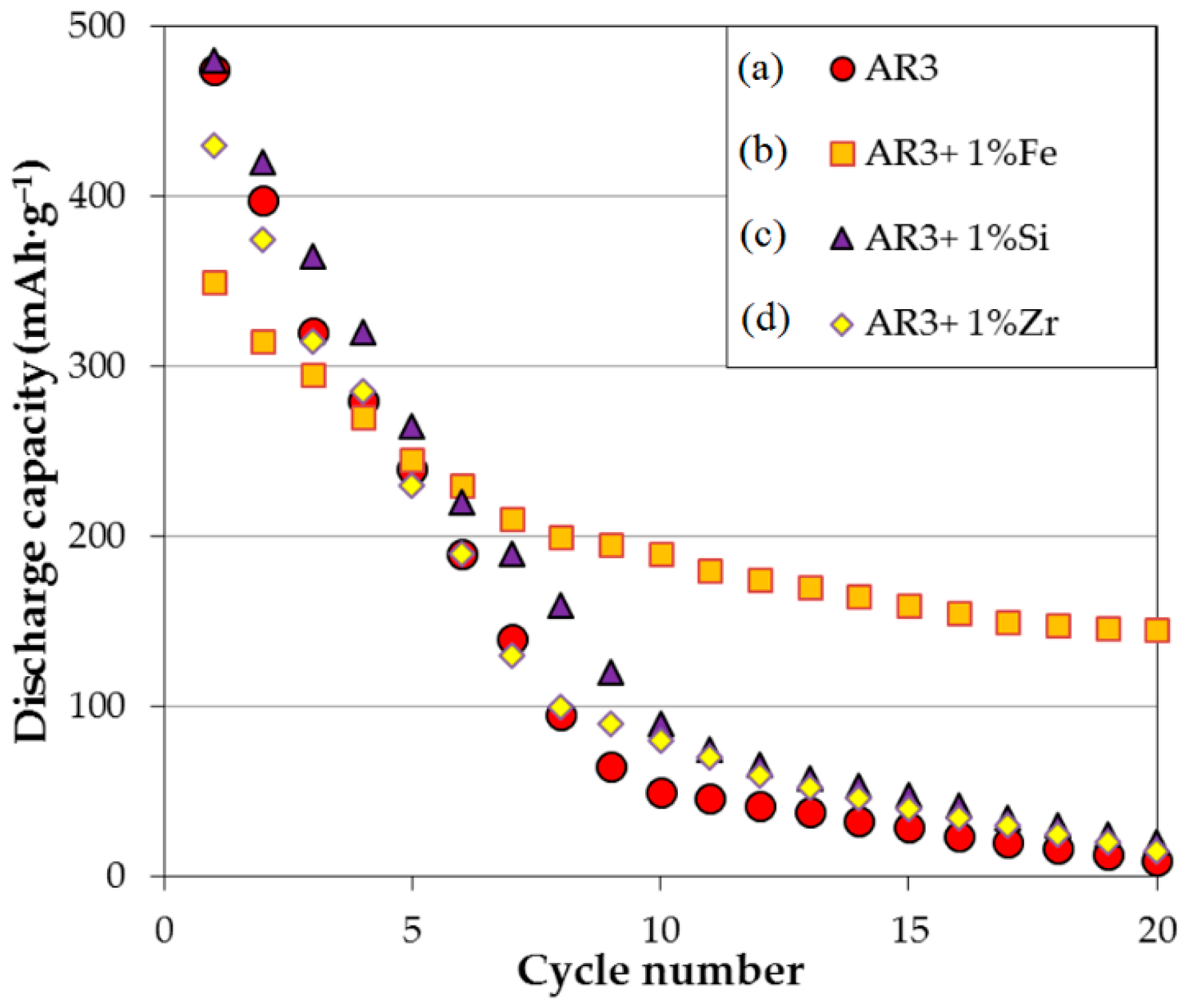
3.2. MgNi-Based Alloy Powder Prepared by Melt Spinning + Mechanical Alloying
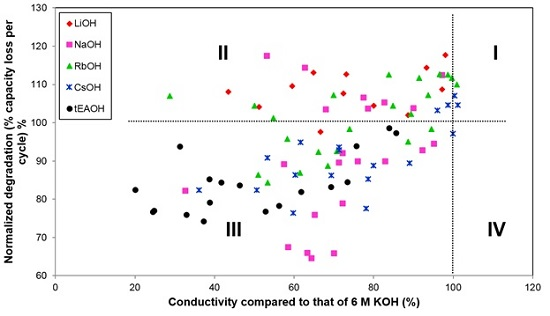
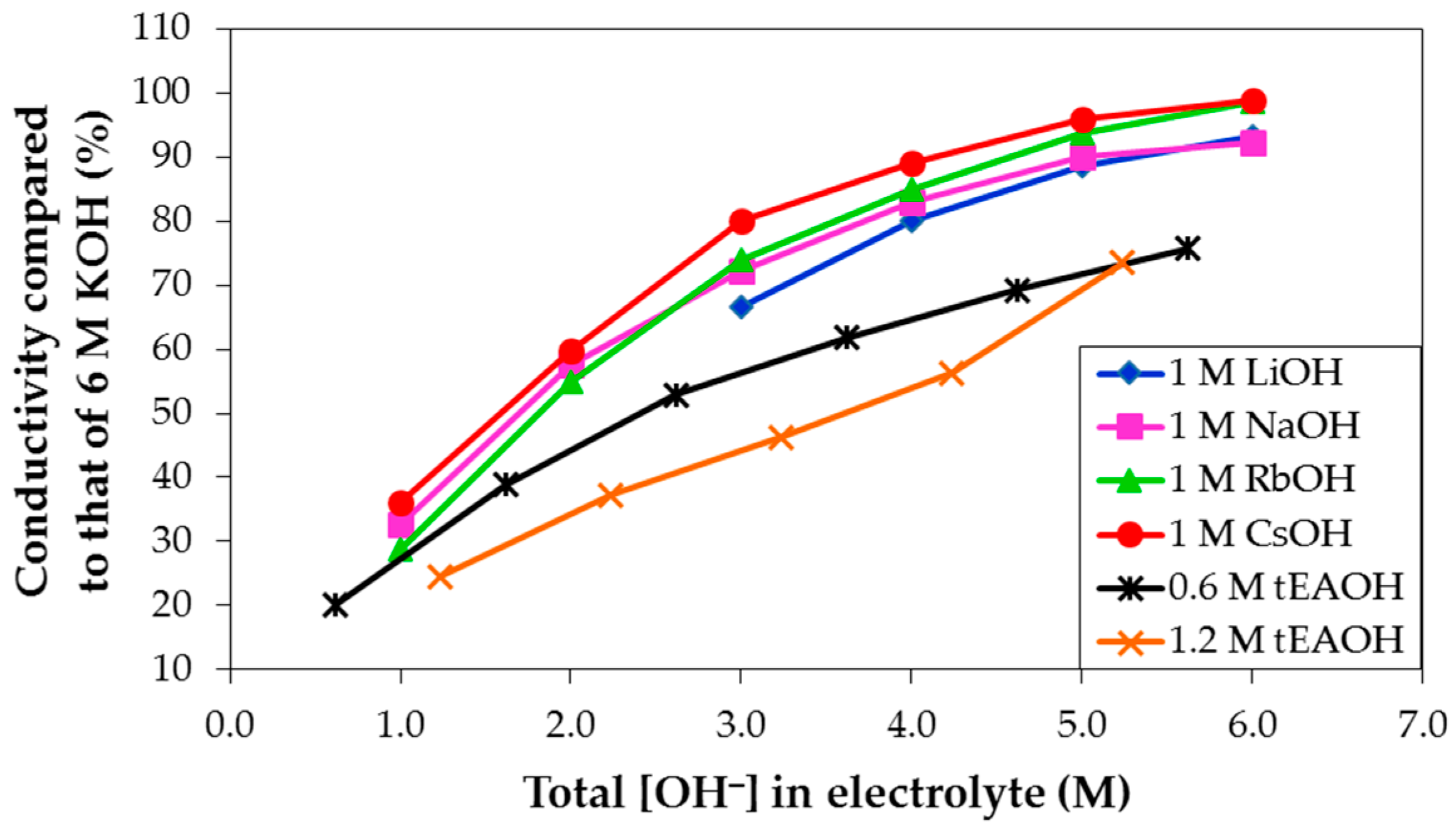
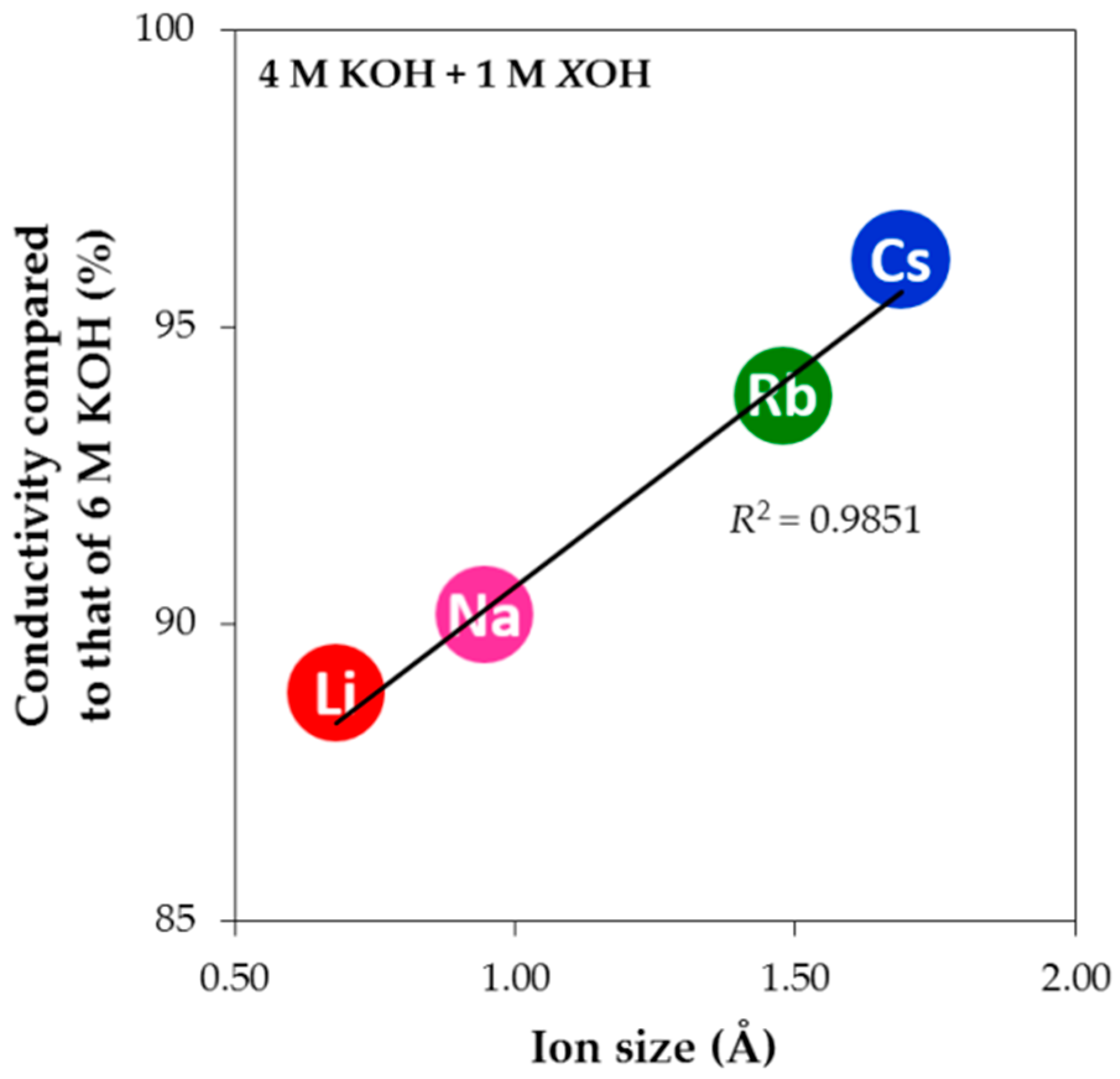
3.3. Conductivities of Various Hydroxide Electrolytes
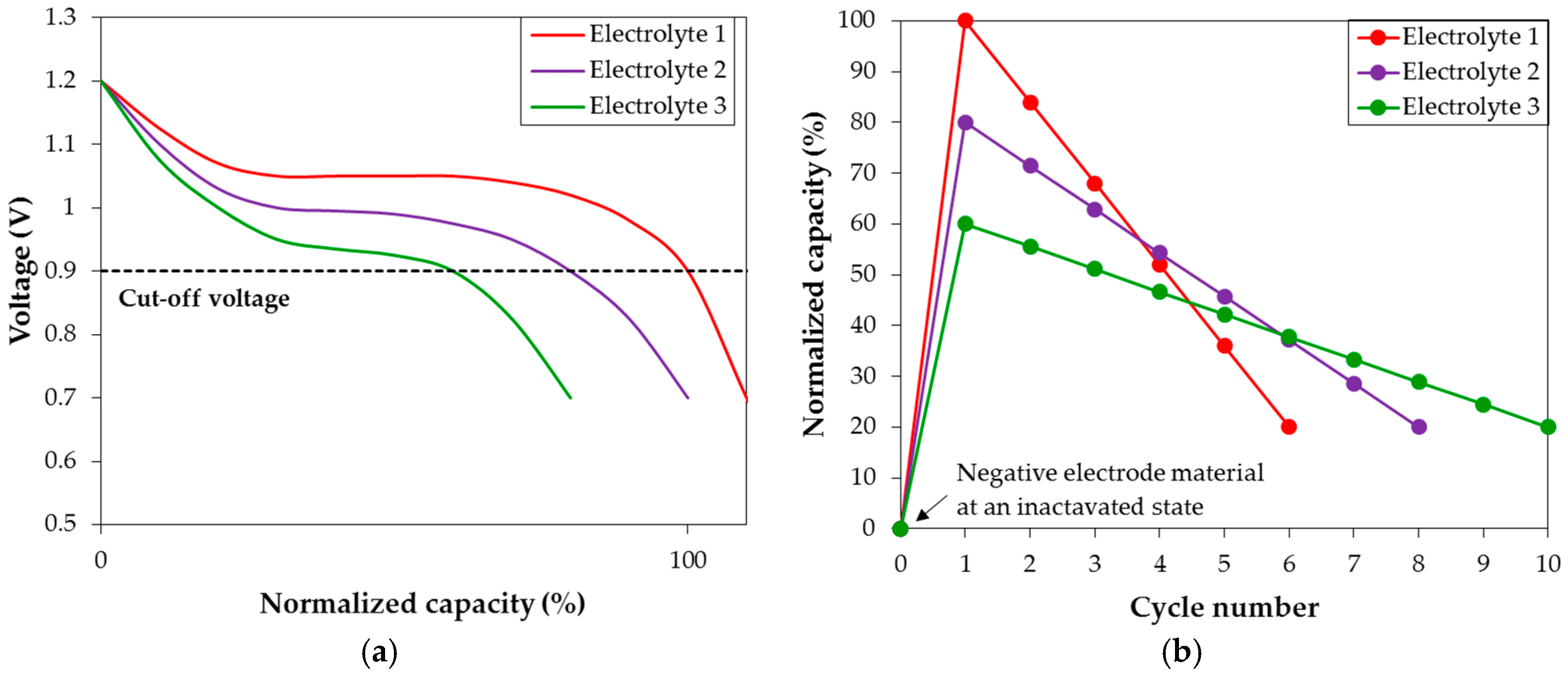
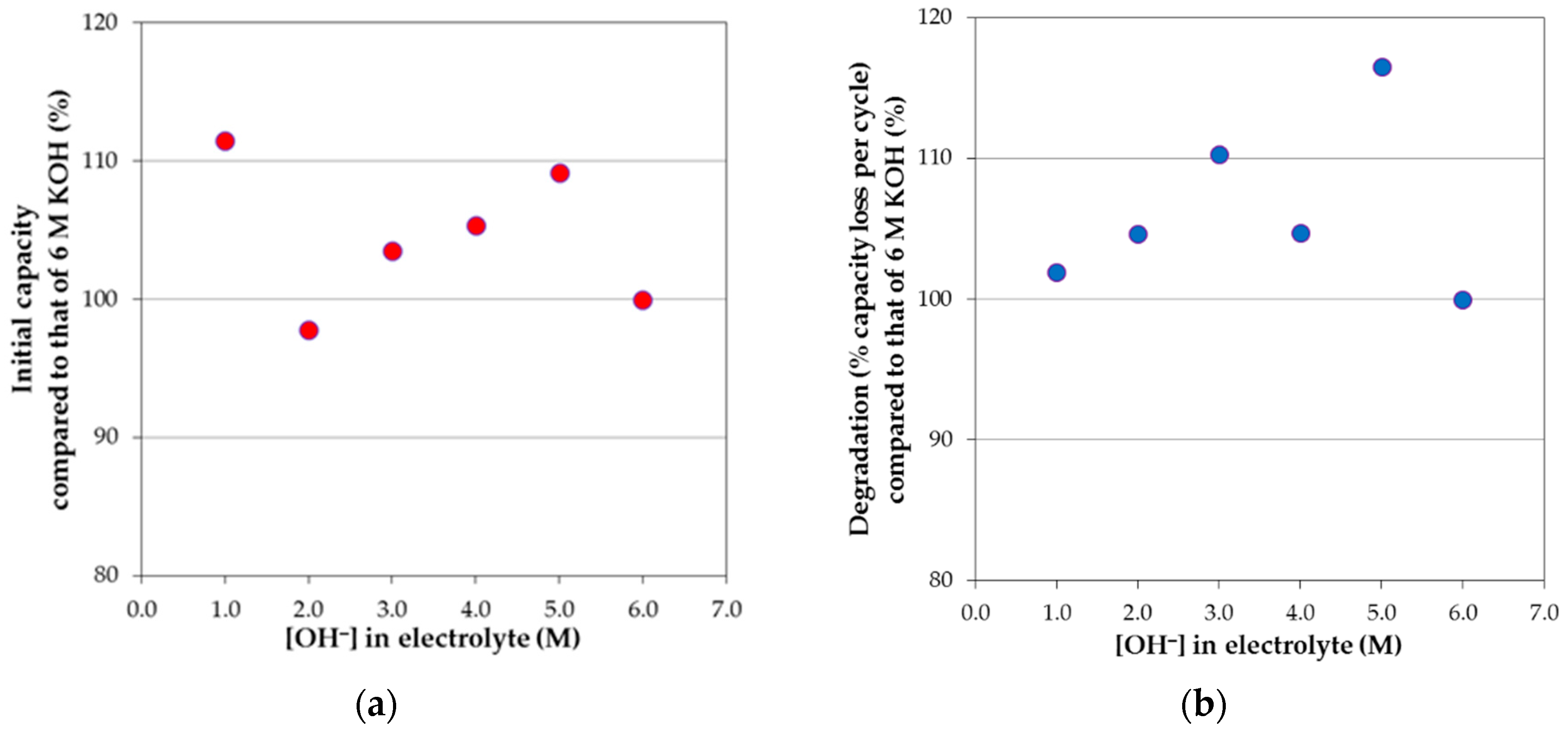
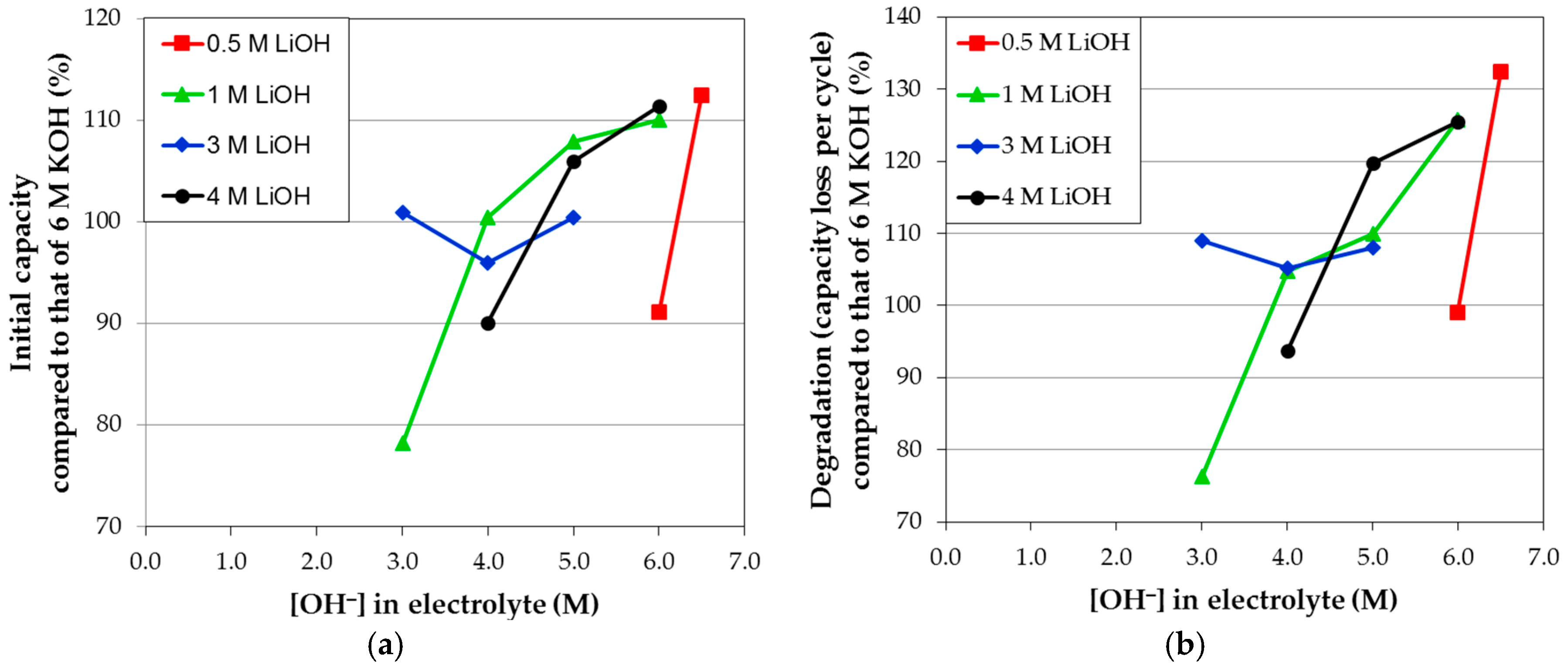
3.4. Corrosion Performances in Various Hydroxide Electrolytes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MH | Metal hydride |
| Ni/MH | Nickel/metal hydride |
| HRD | High-rate dischargeability |
| LT | Low temperature |
| RT | Room temperature |
| HT | High temperature |
| DOE | U.S. Department of Energy |
| RANGE | Robust affordable next generation EV-storage |
| RF | Radio frequency |
| MS | Melt spinning |
| MA | Mechanical alloying |
| XRD | X-ray diffractometer |
| TEM | Transmission electron microscope |
| tEAOH | Tetraethylammonium hydroxide |
| R2 | Correlation factor |
Appendix
| Content | KOH (M) | X(OH)y (M) | [OH−] (M) | Conductivity (%) | Content | KOH (M) | X(OH)y (M) | [OH−] (M) | Conductivity (%) |
|---|---|---|---|---|---|---|---|---|---|
| X = Li, y = 1 | 0.00 | 3.00 | 3.00 | 43.43 | X = Na, y = 1 | 0.00 | 1.00 | 1.00 | 32.70 |
| 0.00 | 4.00 | 4.00 | 51.15 | 0.00 | 2.00 | 2.00 | 53.15 | ||
| 1.00 | 3.00 | 4.00 | 59.61 | 0.00 | 3.00 | 3.00 | 62.71 | ||
| 1.00 | 4.00 | 5.00 | 64.96 | 0.00 | 4.00 | 4.00 | 65.23 | ||
| 2.00 | 1.00 | 3.00 | 66.64 | 0.00 | 5.00 | 5.00 | 63.33 | ||
| 2.00 | 3.00 | 5.00 | 72.41 | 0.00 | 6.00 | 6.00 | 58.47 | ||
| 2.00 | 4.00 | 6.00 | 73.06 | 1.00 | 1.00 | 2.00 | 57.48 | ||
| 3.00 | 1.00 | 4.00 | 80.02 | 1.00 | 2.00 | 3.00 | 67.98 | ||
| 4.00 | 1.00 | 5.00 | 88.65 | 1.00 | 3.00 | 4.00 | 72.19 | ||
| 5.00 | 1.00 | 6.00 | 93.29 | 1.00 | 4.00 | 5.00 | 70.02 | ||
| 5.50 | 0.50 | 6.00 | 97.23 | 1.00 | 5.00 | 6.00 | 64.41 | ||
| 6.00 | 0.50 | 6.50 | 98.03 | 2.00 | 1.00 | 3.00 | 72.13 | ||
| X = Rb, y = 1 | 0.00 | 1.00 | 1.00 | 28.79 | 2.00 | 2.00 | 4.00 | 77.44 | |
| 0.00 | 2.00 | 2.00 | 50.18 | 2.00 | 3.00 | 5.00 | 75.93 | ||
| 0.00 | 3.00 | 3.00 | 66.16 | 2.00 | 4.00 | 6.00 | 71.31 | ||
| 0.00 | 4.00 | 4.00 | 51.08 | 3.00 | 1.00 | 4.00 | 82.89 | ||
| 0.00 | 5.00 | 5.00 | 58.26 | 3.00 | 2.00 | 5.00 | 82.71 | ||
| 0.00 | 6.00 | 6.00 | 89.38 | 3.00 | 3.00 | 6.00 | 78.60 | ||
| 1.00 | 1.00 | 2.00 | 54.83 | 4.00 | 1.00 | 5.00 | 89.96 | ||
| 1.00 | 2.00 | 3.00 | 69.90 | 5.00 | 1.00 | 6.00 | 92.27 | ||
| 1.00 | 3.00 | 4.00 | 53.39 | 5.50 | 0.50 | 6.00 | 95.16 | ||
| 1.00 | 4.00 | 5.00 | 61.53 | 6.00 | 0.50 | 6.50 | 97.21 | ||
| 1.00 | 5.00 | 6.00 | 68.47 | X = Cs, y = 1 | 0.00 | 1.00 | 1.00 | 36.18 | |
| 2.00 | 1.00 | 3.00 | 74.04 | 0.00 | 3.00 | 3.00 | 78.65 | ||
| 2.00 | 2.00 | 4.00 | 83.90 | 0.00 | 4.00 | 4.00 | 78.10 | ||
| 2.00 | 3.00 | 5.00 | 88.69 | 0.00 | 5.00 | 5.00 | 53.39 | ||
| 2.00 | 4.00 | 6.00 | 70.78 | 0.00 | 6.00 | 6.00 | 100.31 | ||
| 3.00 | 1.00 | 4.00 | 84.95 | 1.00 | 1.00 | 2.00 | 59.81 | ||
| 3.00 | 2.00 | 5.00 | 91.47 | 1.00 | 2.00 | 3.00 | 50.59 | ||
| 3.00 | 3.00 | 6.00 | 94.65 | 1.00 | 4.00 | 5.00 | 61.59 | ||
| 4.00 | 1.00 | 5.00 | 93.68 | 1.00 | 5.00 | 6.00 | 71.33 | ||
| 4.00 | 2.00 | 6.00 | 96.59 | 2.00 | 1.00 | 3.00 | 79.92 | ||
| 5.00 | 1.00 | 6.00 | 98.66 | 2.00 | 3.00 | 5.00 | 60.30 | ||
| 5.50 | 0.50 | 6.00 | 99.68 | 2.00 | 4.00 | 6.00 | 71.26 | ||
| 6.00 | 0.50 | 6.50 | 100.93 | 3.00 | 1.00 | 4.00 | 88.99 | ||
| X = tEA, y = 1 | 0.00 | 0.62 | 0.62 | 20.13 | 3.00 | 3.00 | 6.00 | 69.31 | |
| 0.00 | 1.24 | 1.24 | 24.55 | 4.00 | 1.00 | 5.00 | 95.97 | ||
| 0.00 | 1.86 | 1.86 | 24.83 | 5.00 | 1.00 | 6.00 | 98.78 | ||
| 1.00 | 0.62 | 1.62 | 38.87 | 5.50 | 0.50 | 6.00 | 99.95 | ||
| 1.00 | 1.24 | 2.24 | 37.29 | 6.00 | 0.50 | 6.50 | 101.21 | ||
| 1.00 | 1.86 | 2.86 | 32.94 | X = Mg, y = 2 | 6.00 | 0.0130 | 6.0259 | 99.58 | |
| 2.00 | 0.62 | 2.62 | 52.90 | ||||||
| 2.00 | 1.24 | 3.24 | 46.27 | ||||||
| 2.00 | 1.86 | 3.86 | 38.71 | ||||||
| 2.00 | 2.48 | 4.48 | 31.31 | X = Ca, y = 2 | 6.00 | 0.0180 | 6.0360 | 98.81 | |
| 3.00 | 0.62 | 3.62 | 61.80 | ||||||
| 3.00 | 1.24 | 4.24 | 56.29 | ||||||
| 3.00 | 1.86 | 4.86 | 41.75 | X = Sr, y = 2 | 6.00 | 0.0004 | 6.0008 | 98.73 | |
| 4.00 | 0.62 | 4.62 | 69.22 | ||||||
| 4.00 | 1.24 | 5.24 | 73.48 | ||||||
| 5.00 | 0.62 | 5.62 | 75.74 | X = Ba, y = 2 | 6.00 | 0.0005 | 6.0011 | 98.74 | |
| 5.50 | 0.31 | 5.81 | 83.96 | ||||||
| 6.00 | 0.31 | 6.31 | 85.68 |
| Content | KOH (M) | X(OH)y (M) | [OH−] (M) | Degradation (%) | Content | KOH (M) | X(OH)y (M) | [OH−] (M) | Degradation (%) |
|---|---|---|---|---|---|---|---|---|---|
| X = Li, y = 1 | 1.00 | 0.00 | 1.00 | 101.92 | X = Na, y = 1 | 0.00 | 1.00 | 1.00 | 82.11 |
| 2.00 | 0.00 | 2.00 | 104.64 | 0.00 | 2.00 | 2.00 | 117.41 | ||
| 3.00 | 0.00 | 3.00 | 110.26 | 0.00 | 3.00 | 3.00 | 114.34 | ||
| 4.00 | 0.00 | 4.00 | 104.69 | 0.00 | 4.00 | 4.00 | 75.89 | ||
| 5.00 | 0.00 | 5.00 | 116.52 | 0.00 | 5.00 | 5.00 | 65.91 | ||
| 5.50 | 0.00 | 5.50 | 83.76 | 0.00 | 6.00 | 6.00 | 67.41 | ||
| 6.00 | 0.00 | 6.00 | 100.00 | 1.00 | 1.00 | 2.00 | 89.07 | ||
| 0.00 | 3.00 | 3.00 | 108.06 | 1.00 | 2.00 | 3.00 | 103.36 | ||
| 0.00 | 4.00 | 4.00 | 104.05 | 1.00 | 3.00 | 4.00 | 78.91 | ||
| 1.00 | 3.00 | 4.00 | 109.58 | 1.00 | 4.00 | 5.00 | 65.76 | ||
| 1.00 | 4.00 | 5.00 | 113.00 | 1.00 | 5.00 | 6.00 | 64.64 | ||
| 2.00 | 1.00 | 3.00 | 97.59 | 2.00 | 1.00 | 3.00 | 91.91 | ||
| 2.00 | 3.00 | 5.00 | 107.62 | 2.00 | 2.00 | 4.00 | 106.48 | ||
| 2.00 | 4.00 | 6.00 | 112.67 | 2.00 | 3.00 | 5.00 | 89.88 | ||
| 3.00 | 1.00 | 4.00 | 104.37 | 2.00 | 4.00 | 6.00 | 89.46 | ||
| 4.00 | 1.00 | 5.00 | 101.92 | 3.00 | 1.00 | 4.00 | 89.90 | ||
| 5.00 | 1.00 | 6.00 | 114.28 | 3.00 | 2.00 | 5.00 | 105.34 | ||
| 5.50 | 0.50 | 6.00 | 108.68 | 3.00 | 3.00 | 6.00 | 103.66 | ||
| 6.00 | 0.50 | 6.50 | 117.71 | 4.00 | 1.00 | 5.00 | 103.81 | ||
| X = Rb, y = 1 | 0.00 | 1.00 | 1.00 | 107.00 | 5.00 | 1.00 | 6.00 | 92.73 | |
| 0.00 | 2.00 | 2.00 | 104.29 | 5.50 | 0.50 | 6.00 | 94.45 | ||
| 0.00 | 3.00 | 3.00 | 92.36 | 6.00 | 0.50 | 6.50 | 112.35 | ||
| 0.00 | 4.00 | 4.00 | 86.38 | X = Cs, y = 1 | 0.00 | 1.00 | 1.00 | 82.36 | |
| 0.00 | 5.00 | 5.00 | 95.83 | 0.00 | 2.00 | 2.00 | 81.80 | ||
| 0.00 | 6.00 | 6.00 | 102.33 | 0.00 | 3.00 | 3.00 | 85.26 | ||
| 1.00 | 1.00 | 2.00 | 101.22 | 0.00 | 4.00 | 4.00 | 77.61 | ||
| 1.00 | 2.00 | 3.00 | 107.25 | 0.00 | 5.00 | 5.00 | 90.85 | ||
| 1.00 | 3.00 | 4.00 | 84.30 | 0.00 | 6.00 | 6.00 | 107.00 | ||
| 1.00 | 4.00 | 5.00 | 86.97 | 1.00 | 1.00 | 2.00 | 76.35 | ||
| 1.00 | 5.00 | 6.00 | 88.66 | 1.00 | 2.00 | 3.00 | 79.92 | ||
| 2.00 | 1.00 | 3.00 | 98.37 | 1.00 | 3.00 | 4.00 | 82.31 | ||
| 2.00 | 2.00 | 4.00 | 112.52 | 1.00 | 4.00 | 5.00 | 94.88 | ||
| 2.00 | 3.00 | 5.00 | 95.00 | 1.00 | 5.00 | 6.00 | 92.78 | ||
| 2.00 | 4.00 | 6.00 | 92.46 | 2.00 | 1.00 | 3.00 | 88.79 | ||
| 3.00 | 1.00 | 4.00 | 104.29 | 2.00 | 2.00 | 4.00 | 77.35 | ||
| 3.00 | 2.00 | 5.00 | 111.65 | 2.00 | 3.00 | 5.00 | 86.31 | ||
| 3.00 | 3.00 | 6.00 | 98.37 | 2.00 | 4.00 | 6.00 | 93.55 | ||
| 4.00 | 1.00 | 5.00 | 107.25 | 3.00 | 1.00 | 4.00 | 89.42 | ||
| 4.00 | 2.00 | 6.00 | 112.63 | 3.00 | 2.00 | 5.00 | 81.53 | ||
| 5.00 | 1.00 | 6.00 | 112.52 | 3.00 | 3.00 | 6.00 | 86.18 | ||
| 5.50 | 0.50 | 6.00 | 111.65 | 4.00 | 1.00 | 5.00 | 103.22 | ||
| 6.00 | 0.50 | 6.50 | 109.97 | 4.00 | 2.00 | 6.00 | 76.46 | ||
| X = tEA, y = 1 | 0.00 | 0.62 | 0.62 | 82.42 | 5.00 | 1.00 | 6.00 | 104.51 | |
| 0.00 | 1.24 | 1.24 | 76.59 | 5.50 | 0.50 | 6.00 | 97.03 | ||
| 0.00 | 1.86 | 1.86 | 76.97 | 6.00 | 0.50 | 6.50 | 104.57 | ||
| 0.00 | 2.48 | 2.48 | 85.49 | X = Mg, y = 2 | 6.00 | 0.0130 | 6.0259 | 98.38 | |
| 1.00 | 0.62 | 1.62 | 78.96 | ||||||
| 1.00 | 1.24 | 2.24 | 74.06 | ||||||
| 1.00 | 1.86 | 2.86 | 75.89 | ||||||
| 1.00 | 2.48 | 3.48 | 75.15 | ||||||
| 2.00 | 0.62 | 2.62 | 76.65 | X = Ca, y = 2 | 6.00 | 0.0180 | 6.0360 | 98.29 | |
| 2.00 | 1.24 | 3.24 | 83.52 | ||||||
| 2.00 | 1.86 | 3.86 | 85.17 | ||||||
| 2.00 | 2.48 | 4.48 | 93.69 | ||||||
| 3.00 | 0.62 | 3.62 | 81.94 | X = Sr, y = 2 | 6.00 | 0.0004 | 6.0008 | 96.13 | |
| 3.00 | 1.24 | 4.24 | 78.27 | ||||||
| 3.00 | 1.86 | 4.86 | 84.33 | ||||||
| 4.00 | 0.62 | 4.62 | 83.07 | ||||||
| 4.00 | 1.24 | 5.24 | 84.43 | X = Ba, y = 2 | 6.00 | 0.0005 | 6.0011 | 97.90 | |
| 5.00 | 0.62 | 5.62 | 93.81 | ||||||
| 5.50 | 0.31 | 5.81 | 98.44 | ||||||
| 6.00 | 0.31 | 6.31 | 97.26 |
References
- Liu, D.; Zhu, Y.; Li, L. Effect of surface oxidation on the hydriding and dehydriding of Mg2Ni alloy produced by hydriding combustion synthesis. J. Mater. Sci. 2007, 42, 9725–9729. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Redzeb, M.; Zlatanova, Z.; Spassov, T. Influence of boron on the hydriding of nanocrystalline Mg2Ni. Intermetallics 2013, 34, 63–68. [Google Scholar] [CrossRef]
- Nikkuni, F.R.; Santos, S.F.; Ticianelli, E.A. Microstructures and electrochemical properties of Mg49Ti6Ni(45−x)Mx (M = Pd and Pt) alloy electrodes. Int. J. Energy Res. 2013, 37, 706–712. [Google Scholar] [CrossRef]
- Zhang, Z.; Elkedim, O.; Balcerzak, M.; Jurczyk, M. Structural and electrochemical hydrogen storage properties of MgTiNix (x = 0.1, 0.5, 1, 2) alloys prepared by ball milling. Int. J. Hydrog. Energy 2016, 41, 11761–11766. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Cai, Y.; Hu, F.; Liu, Z.; Guo, S. Highly improved electrochemical hydrogen storage performances of the Nd-Cu-added Mg2Ni-type alloys by melt spinning. J. Alloys Compd. 2014, 584, 81–86. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wan, C.B.; Wang, R.L.; Meng, X.H.; Huang, M.F.; Ju, X. Effect of Cr substitution by Ni on the cycling stability of Mg2Ni alloy using EXAFS. Int. J. Hydrog. Energy 2014, 39, 14858–14867. [Google Scholar] [CrossRef]
- Pu, Z.; Zhu, Y.; Zhu, J.; Yuan, J.; Zhang, J.; Chen, W.; Fang, J.; Li, L. Kinetics and electrochemical characteristics of Mg2NiH4−x wt.% MmNi3.8Co0.75Mn0.4Al0.2 (x = 5, 10, 20, 40) composites for Ni-MH battery. Int. J. Hydrog. Energy 2014, 39, 3887–3894. [Google Scholar] [CrossRef]
- Hou, X.; Hu, R.; Zhang, T.; Kou, H.; Song, W.; Li, J. Microstructure and electrochemical hydrogenation/dehydrogenation performance of melt-spun La-doped Mg2Ni alloys. Mater. Charact. 2015, 106, 163–174. [Google Scholar] [CrossRef]
- Verbovytskyy, Y.; Zhang, J.; Cuevas, F.; Paul-Boncour, V.; Zavaliy, I. Synthesis and properties of the Mg2Ni0.5Cu0.5H4.4 hydride. J. Alloys Compd. 2015, 645, S408–S411. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Yang, C.; Zhang, J.; Chen, W. Enhanced electrochemical hydrogen storage properties of Mg2NiH4 by coating with nano-nickel. Int. J. Hydrog. Energy 2015, 40, 13949–13956. [Google Scholar] [CrossRef]
- Shang, J.; Ouyang, Z.; Liu, K.; Xing, C.; Liu, W.; Wang, L. Effect of Li atom infiltration by the way of electro-osmosis on electrochemical properties of amorphous Mg62Ni27La8 alloy used as negative electrode materials for the nickel-metal hydride secondary batteries. J. Non Cryst. Solids 2015, 415, 30–35. [Google Scholar] [CrossRef]
- Shao, H.; Li, X. Effect of nanostructure and partial substitution on gas absorption and electrochemical properties in Mg2Ni-based alloys. J. Alloys Compd. 2016, 667, 191–197. [Google Scholar] [CrossRef]
- Shahcheraghi, A.; Dehghani, F.; Raeissi, K.; Saatchi, A.; Enayati, M.H. Effects of TiO2 additive on electrochemical hydrogen storage properties of nanocrystalline/amorphous Mg2Ni intermetallic alloy. Iran. J. Mater. Sci. Eng. 2013, 10, 1–9. [Google Scholar]
- Ohara, R.; Lan, C.; Hwang, C. Electrochemical and structural characterization of electroless nickel coating on Mg2Ni hydrogen storage alloy. J. Alloys Compd. 2013, 580, S368–S372. [Google Scholar] [CrossRef]
- Haghighat-Shishavan, S.; Kashani-Bozorg, S.F. Nano-crystalline Mg(2−x)MnxNi compounds synthesized by mechanical alloys: Microstructure and electrochemistry. J. Ultrafine Grained Nanostruct. Mater. 2014, 47, 43–49. [Google Scholar]
- Venkateswari, A.; Nithya, C.; Kumaran, S. Electrochemical behaviour of Mg67Ni(33−x)Nbx (x = 0, 1, 2, and 4) alloy synthesized by high energy ball milling. Proc. Mater. Sci. 2014, 5, 679–687. [Google Scholar] [CrossRef]
- Rongeat, C.; Roué, L. On the cycle life improvement of amorphous MgNi-based alloy for Ni-MH batteries. J. Alloys Compd. 2005, 404–406, 679–681. [Google Scholar] [CrossRef]
- Rongeat, C.; Grosjean, M.-H.; Ruggeri, S.; Dehmas, M.; Bourlot, S.; Marcotte, S.; Roué, L. Evaluation of different approaches for improving the cycle life of MgNi-based electrodes for Ni-MH batteries. J. Power Sources 2006, 158, 747–753. [Google Scholar] [CrossRef]
- Wang, J.; Na, E.; Wu, F. Study on the improvement of cycle life of magnesium-based hydrogen storage alloy. J. Wuhan Univ. Technol. 2006, 28, 371–373. [Google Scholar]
- Kong, F.; Yan, H.; Xiong, W.; Li, B.; Li, J. Investigation on the cycling stability of Mg-based hydrogen storage electrode improved by anti-corrosion method. Chin. Rare Earths 2006, 27, 39–42. [Google Scholar]
- Yan, S.; Young, K.; Ng, K.Y.S. Effects of salt additives to the KOH electrolyte used in Ni/MH batteries. Batteries 2015, 1, 54–73. [Google Scholar] [CrossRef]
- Young, K.; Ng, K.Y.S.; Bendersky, L.A. A technical report of the robust affordable next generation energy storage system-BASF program. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Rubin, E.J.; Baboian, R. A correlation of the solution properties and the electrochemical behavior of the nickel hydroxide electrode in binary aqueous alkali hydroxides. J. Electrochem. Soc. 1971, 118, 428–433. [Google Scholar] [CrossRef]
- Barnard, R.; Randell, C.F.; Tye, F.L. Studies concerning changes nickel hydroxide electrodes. IV. Reversible potentials in LiOH, NaOH, RbOH and CdOH. J. Appl. Electrochem. 1981, 11, 517–523. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; Guibert, A. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- See, D.M.; White, R.E. Temperature and concentration dependence of the specific conductivity of concentrated solutions of potassium hydroxide. J. Chem. Eng. Data 1997, 42, 1266–1268. [Google Scholar] [CrossRef]
- Leblanc, P.; Jordy, C.; Knosp, B.; Blanchard, P. Mechanism of alloy corrosion and consequences on sealed nickel—metal hydride battery performance. J. Electrochem. Soc. 1998, 145, 860–863. [Google Scholar] [CrossRef]
- Knosp, B.; Vallet, L.; Blamchard, P. Performance of an AB2 alloy in sealed Ni-MH batteries for electric vehicles: Qualification of corrosion rate and consequences on the battery performance. J. Alloys Compd. 1999, 293–295, 770–774. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, H.G.; Jung, Y.H.; Ruhmann, H. Effect of LiOH, NaOH and KOH on Corrosion and Oxide Microstructure of Zr-Based Alloys; International Atomic Energy Agency: Wien, Austria, 1999. [Google Scholar]
- Liu, J.; Wang, D.; Liu, S.; Feng, X. Improving high temperature performance of MH/Ni battery by orthogonal design. Battery Bimon. 2003, 33, 218–220. [Google Scholar]
- Hou, X.L.; Na, J.M.; Han, D.M.; Zhao, J.F. Preparation and performance of high-rated A-type MH-Ni batteries. Chin. J. Appl. Chem. 2004, 21, 1169–1173. (In Chinese) [Google Scholar]
- Lv, J.; Liu, X.; Zhang, J.; Fan, L.; Wang, L.; Zhang, Z. Studies on high-power nickel-metal hydride battery. Chin. J. Power Sources 2005, 29, 826–830. [Google Scholar]
- Li, X.; Dong, H.; Zhang, A.; Wei, Y. Electrochemical impedance and cyclic voltammetry characterization of a metal hydride electrode in alkaline electrolytes. J. Alloys Compd. 2006, 426, 93–96. [Google Scholar] [CrossRef]
- Park, C.; Shim, J.; Jang, M.; Park, C.; Choi, J. Influences of various electrolytes on the low-temperature characteristics of Ni-MH secondary battery. Trans. Korean Hydrog. New Energy Soc. 2007, 18, 284–291. [Google Scholar]
- Chen, R.; Li, L.; Wu, F.; Qiu, X.; Chen, S. Effects of low temperature on performance of hydrogen-storage alloys and electrolyte. Min. Metall. Eng. 2007, 27, 44–46. [Google Scholar]
- Yang, D.C.; Park, C.N.; Park, C.J.; Choi, J.; Sim, J.S.; Jang, M.H. Design of additives and electrolyte for optimization of electrode characteristics of Ni-MH secondary battery at room and low temperatures. Trans. Korean Hydrog. New Energy Soc. 2007, 18, 365–373. [Google Scholar]
- Zhang, X.; Chen, Y.; Tao, M.; Wu, C. Effect of electrolyte concentration on low-temperature electrochemical properties of LaNi5 alloy electrode at 233 K. J. Rare Earths 2008, 26, 402–405. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Tao, M.; Wu, C. Effect of electrolyte on the low-temperature electrochemical properties of LaNi5 alloy electrode at 253 K. Rare Metal. Mater. Eng. 2008, 37, 2012–2015. (In Chinese) [Google Scholar]
- Pei, L.; Yi, S.; He, Y.; Chen, Q. Effect of electrolyte formula on the self-discharge properties of nickel-metal hydride batteries. J. Guangdong Univ. Technol. 2008, 25, 10–12. [Google Scholar]
- Khaldi, C.; Mathlouthi, H.; Lamloumi, J. A comparative study of 1 M and 8 M KOH electrolyte concentrations used in Ni-MH batteries. J. Alloys Compd. 2009, 469, 464–471. [Google Scholar] [CrossRef]
- Guiose, B.; Cuevas, F.; Décamps, B.; Leroy, E.; Percheron-Guégan, A. Microstructural analysis of the aging of pseudo-binary (Ti,Zr)Ni intermetallic compounds as negative electrodes of Ni-MH batteries. Electrochim. Acta 2009, 54, 2781–2789. [Google Scholar] [CrossRef]
- Qiu, Z.; Wu, A. Study on wide temperature characteristics of Ni-MH battery. J. South China Norm. Univ. 2009, S1, 79–81. (In Chinese) [Google Scholar]
- Song, M.; Chen, Y.; Tao, M.; Wu, C.; Zhu, D.; Yang, H. Some factors affecting the electrochemical performances of LaCrO3 as negative electrodes for Ni/MH batteries. Electrochim. Acta 2010, 55, 3103–3108. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, F.; Chen, J. Nickel-Metal Hydride (Ni-MH) Rechargeable Battery. In Electrochemical Technologies for Energy Storage and Conversion; Zhang, J., Zhang, L., Liu, H., Sun, A., Liu, R., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2011; p. 204. [Google Scholar]
- Ruiz, F.C.; Martínez, P.S.; Castro, E.B.; Humana, R.; Peretti, H.A.; Visintin, A. Effect of electrolyte concentration on the electrochemical properties of an AB5-type alloy for Ni/MH batteries. Int. J. Hydrog. Energy 2013, 38, 240–245. [Google Scholar] [CrossRef]
- Martínez, P.S.; Ruiz, F.C.; Visintin, A. Influence of different electrolyte concentrations on the performance of an AB2-type alloy. J. Electrochem. Soc. 2014, 161, A326–A329. [Google Scholar] [CrossRef]
- Karwowska, M.; Jaron, T.; Fijalkowski, K.J.; Leszczynski, P.J.; Rogulski, Z.; Czerwinski, A. Influence of electrolyte composition and temperature on behavior of AB5 hydrogen storage alloy used as negative electrode in Ni-MH batteries. J. Power Sources 2014, 263, 304–309. [Google Scholar] [CrossRef]
- Giza, K. Influence of electrolyte on capacity and corrosion resistance of anode material used in Ni-MH cells. Ochr. Koroz. 2016, 59, 167–169. [Google Scholar] [CrossRef]
- Young, K. Stoichiometry in Inter-Metallic Compounds for Hydrogen Storage Applications. In Stoichiometry and Materials Science—When Numbers Matter; Innocenti, A., Kamarulzaman, N., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Nayeb-Hashemi, A.A.; Clark, J.B. Mg-Ni (Magnesium-Nickel). In Binary Alloy Phase Diagram, 2nd ed.; Massalski, T.B., Okamoto, H., Subramanian, P.R., Kacprzak, L., Eds.; ASM International: Geauga County, OH, USA, 1990; Volume 3, pp. 2529–2530. [Google Scholar]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Fetcenko, M.; Koch, J.; Zelinsky, M. Nickel-Metal Hydride Batteries and Nickel-Zinc Batteries for Hybrid Electric Vehicles and Battery Electric Vehicles. In Advances in Battery Technologies for Electric Vehicles; Scrosati, B., Garche, J., Tillmet, W., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2015; pp. 107–108. [Google Scholar]

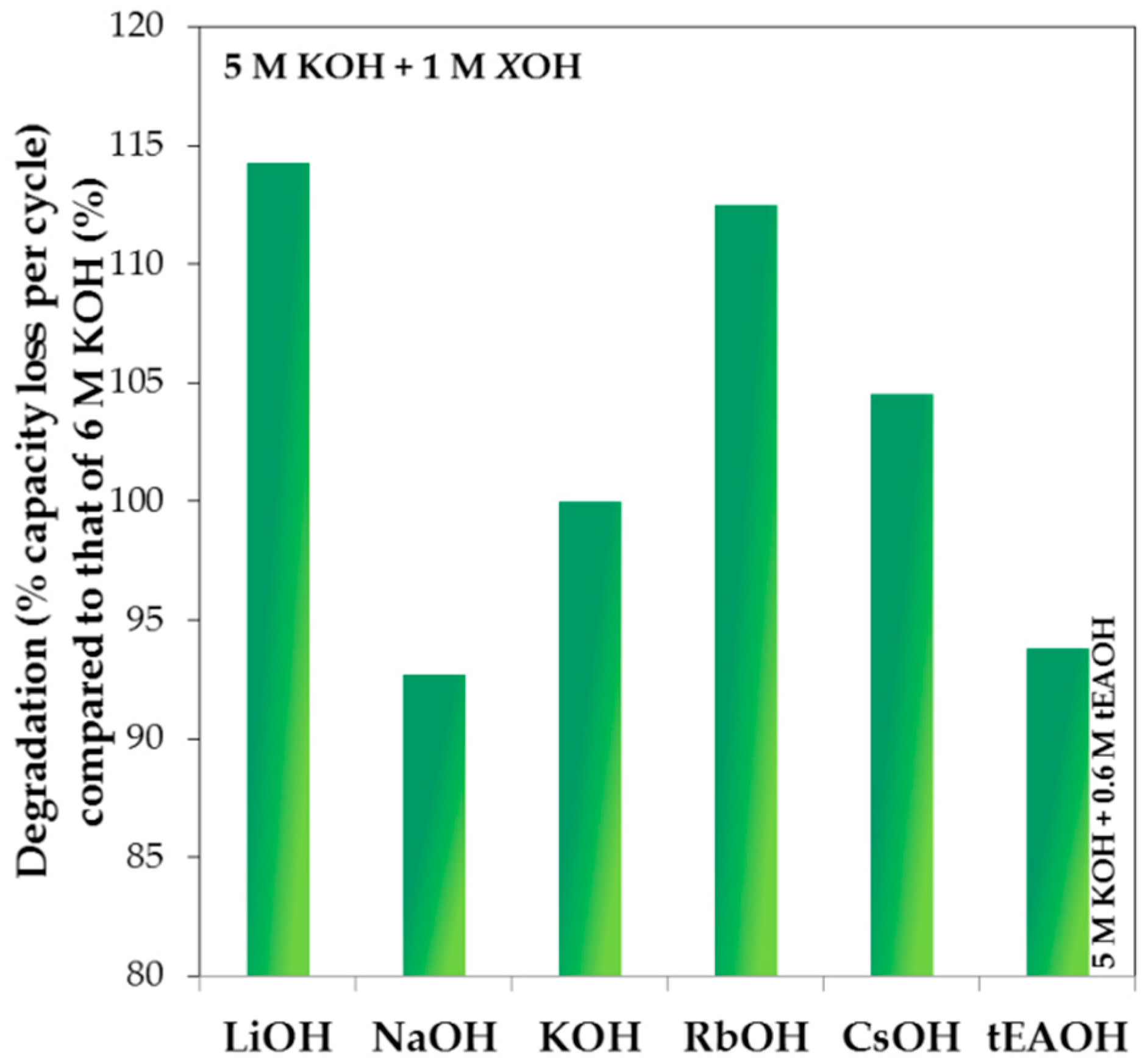
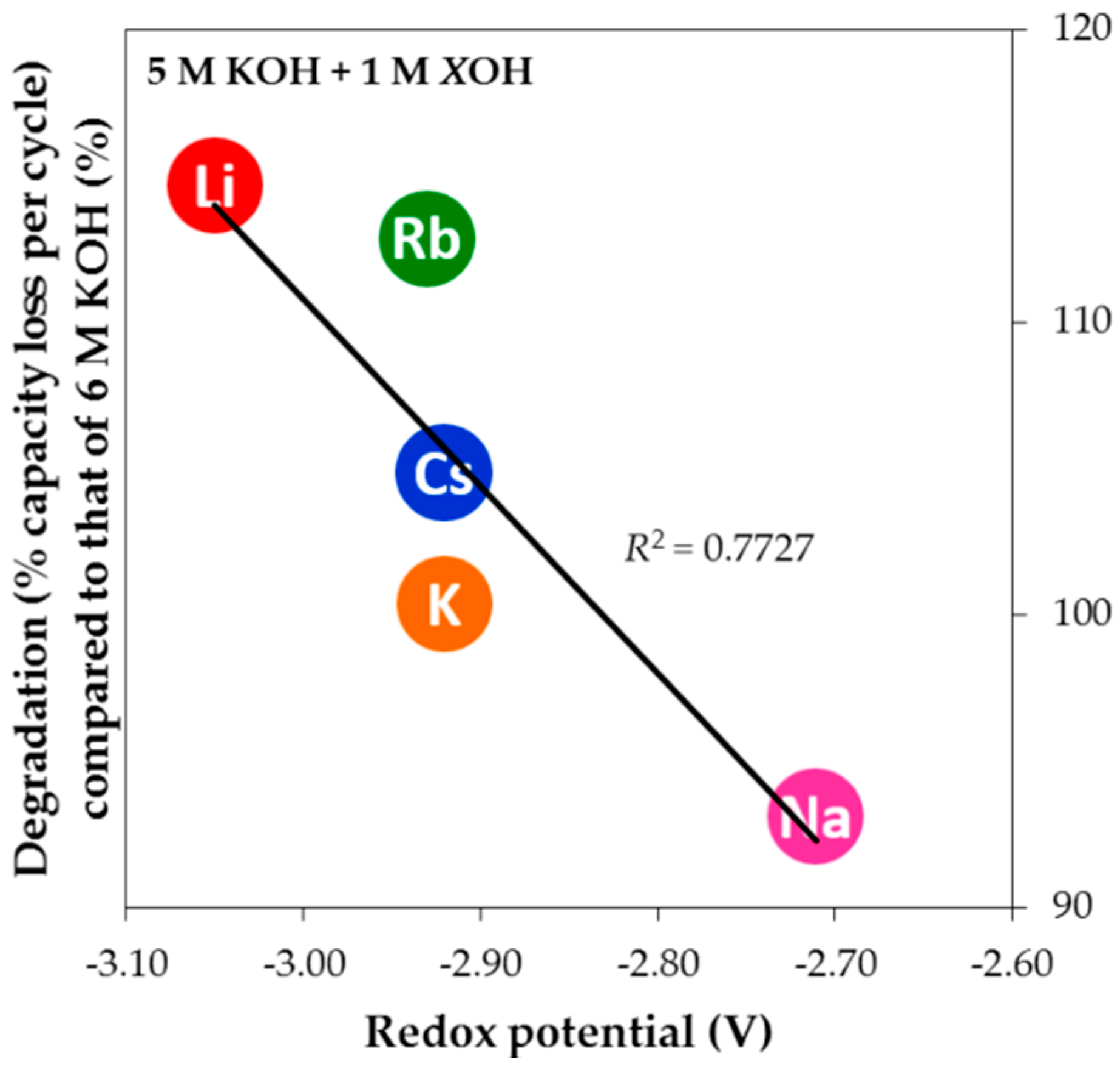
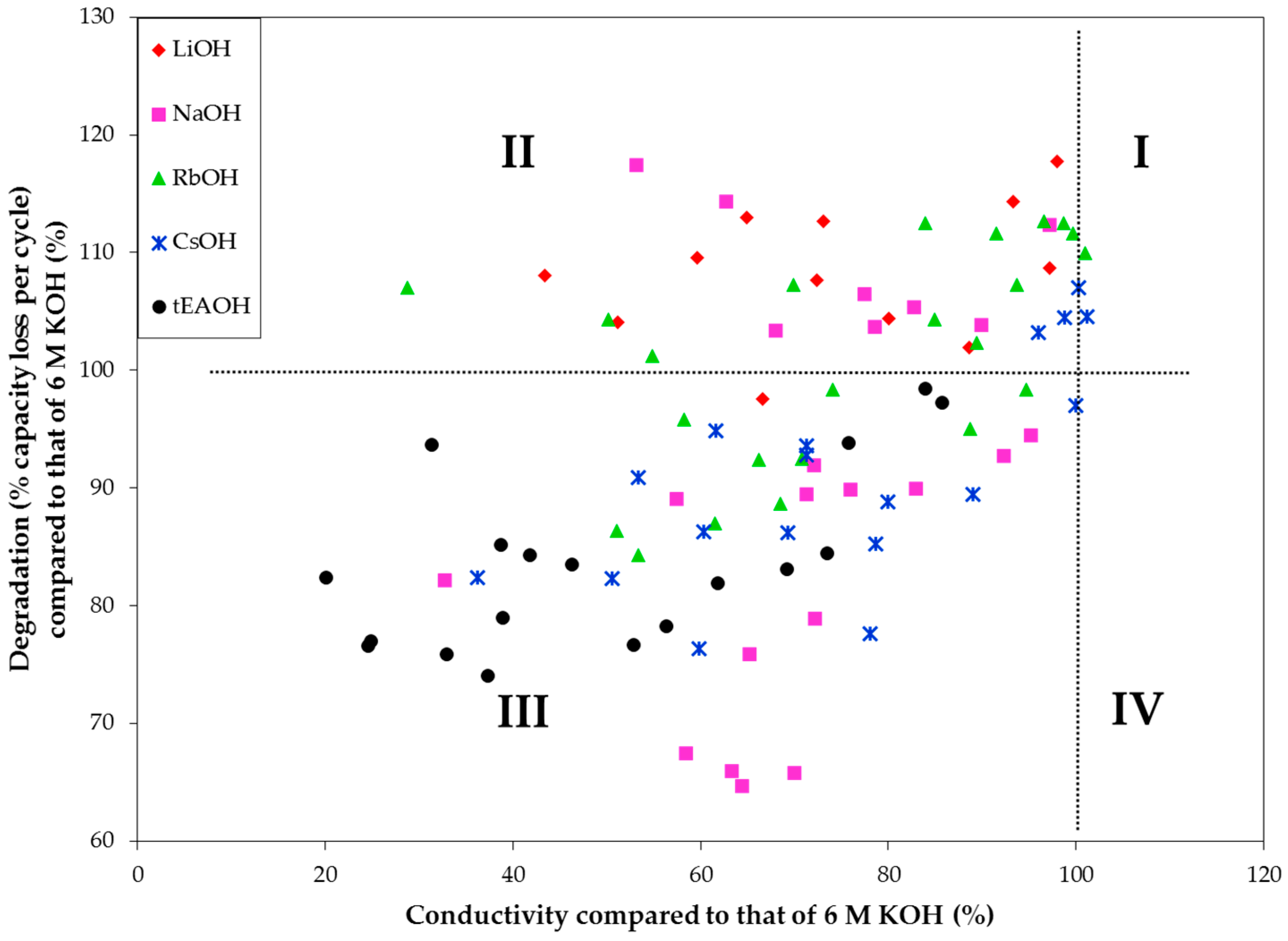
| Tested Electrode | Hydroxide | Main Findings | Reference |
|---|---|---|---|
| Ni(OH)2 | From LiOH to CsOH |
| [24] |
| Ni(OH)2 | From LiOH to CsOH |
| [25] |
| Ni(OH)2 | From LiOH to CsOH |
| [26] |
| Not specified | KOH |
| [27] |
| AB5 | 8.7 M KOH-0.5 M NaOH-0.7 M LiOH |
| [28] |
| (Ti,Zr)B2 | 8.5 M mixture of LiOH, NaOH, KOH |
| [29] |
| Zircaloy (ZrSn) | From LiOH to CsOH |
| [30] |
| Ni(OH)2 | LiOH additive |
| [31] |
| AB5 | Mixture of LiOH, NaOH, KOH |
| [32] |
| AB5 | Mixture of LiOH, NaOH, KOH |
| [33] |
| AB5 | KOH, NaOH |
| [34] |
| AB5 | From LiOH to CsOH |
| [35] |
| AB5 | Mixture of LiOH, NaOH, KOH |
| [36] |
| AB5 | Mixture of LiOH, NaOH, KOH |
| [37] |
| LaNi5 | KOH, NaOH |
| [38,39] |
| LaNi5 | KOH, NaOH |
| [40] |
| LaB5 | 1 M KOH and 8 M KOH |
| [41] |
| (Ti,Zr)Ni | 6 M KOH and 8 M KOH |
| [42] |
| AB5 | KOH, NaOH |
| [43] |
| LaCrO3 | 5.6–12.5 M KOH |
| [44] |
| AB5 | LiOH, KOH |
| [45] |
| LaB5 | 2, 4, 6, 8 M KOH |
| [46] |
| (Ti,Zr)B2 | 2, 4, 6, 8 M KOH |
| [47] |
| AB5 | From LiOH to CsOH |
| [48] |
| AB5 | LiOH, KOH |
| [49] |
| Alloy Composition | Discharge Capacity (mAh·g−1) | Microstructure |
|---|---|---|
| Mg48Ni52 | 302 | 30% microcrystalline + 70% amorphous |
| Mg52Ni48 | 327 | 20% microcrystalline + 80% amorphous |
| Mg57Ni43 | 260 | 10% polycrystalline + 90% amorphous |
| Mg61Ni39 | 75 | 20% polycrystalline + 80% amorphous |
| Mg65Ni35 | 20 | 30% polycrystalline + 70% amorphous |
| Formula Name | Composition | Thin Film Discharge Capacity (mAh·g−1) | Bulk Powder Discharge Capacity (mAh·g−1) | Power | Cycle Life |
|---|---|---|---|---|---|
| AR1 | Mg52Ni48 | 327 | - | Low | Low |
| AR2 | Mg52.1Ni45.1Co2.8 | 662 | - | Low | Low |
| AR3 | Mg52Ni39Co3Mn6 | 639 | 791 | Medium | Low |
| AR4 | Mg51.5Ni37Co6Mn4Fe1.5 | 823 | 472 | Medium | Improved |
| AR5 | Mg50Ni40Co6Mn3Zr1 | 592 | 456 | Good | Good |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nei, J.; Young, K.-H.; Rotarov, D. Studies on MgNi-Based Metal Hydride Electrode with Aqueous Electrolytes Composed of Various Hydroxides. Batteries 2016, 2, 27. https://doi.org/10.3390/batteries2030027
Nei J, Young K-H, Rotarov D. Studies on MgNi-Based Metal Hydride Electrode with Aqueous Electrolytes Composed of Various Hydroxides. Batteries. 2016; 2(3):27. https://doi.org/10.3390/batteries2030027
Chicago/Turabian StyleNei, Jean, Kwo-Hsiung Young, and Damian Rotarov. 2016. "Studies on MgNi-Based Metal Hydride Electrode with Aqueous Electrolytes Composed of Various Hydroxides" Batteries 2, no. 3: 27. https://doi.org/10.3390/batteries2030027
APA StyleNei, J., Young, K.-H., & Rotarov, D. (2016). Studies on MgNi-Based Metal Hydride Electrode with Aqueous Electrolytes Composed of Various Hydroxides. Batteries, 2(3), 27. https://doi.org/10.3390/batteries2030027