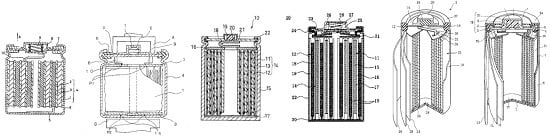
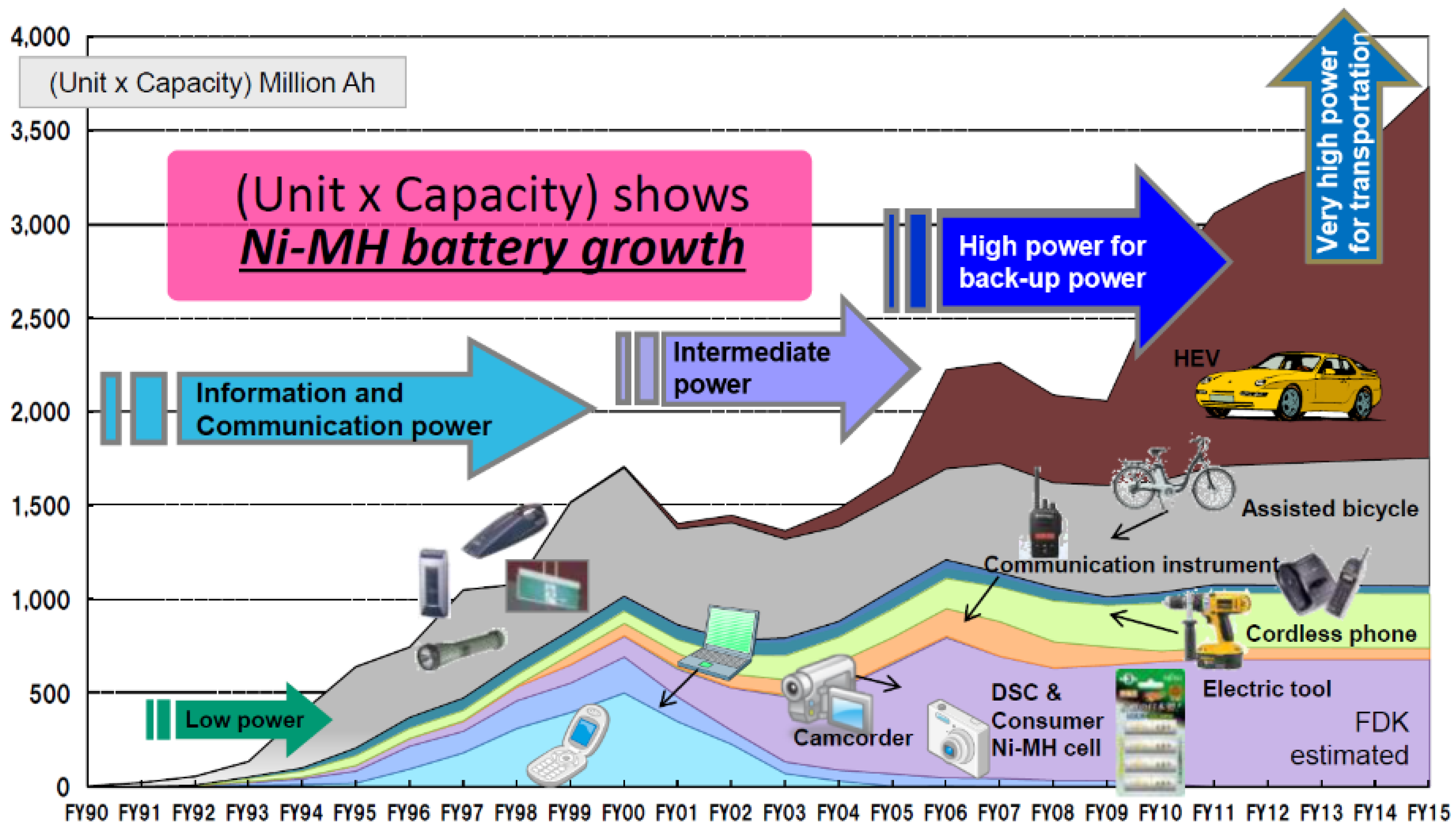
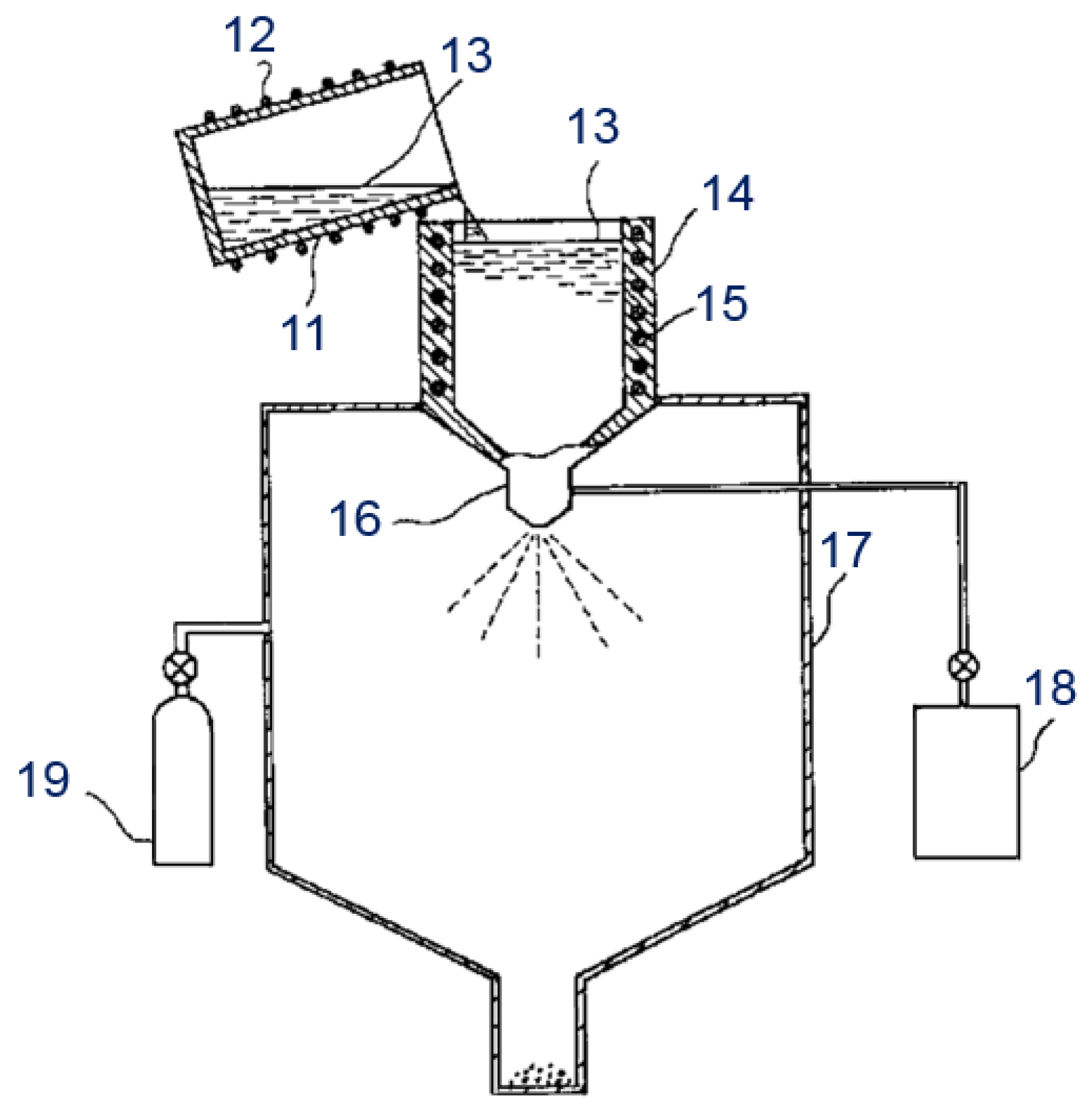
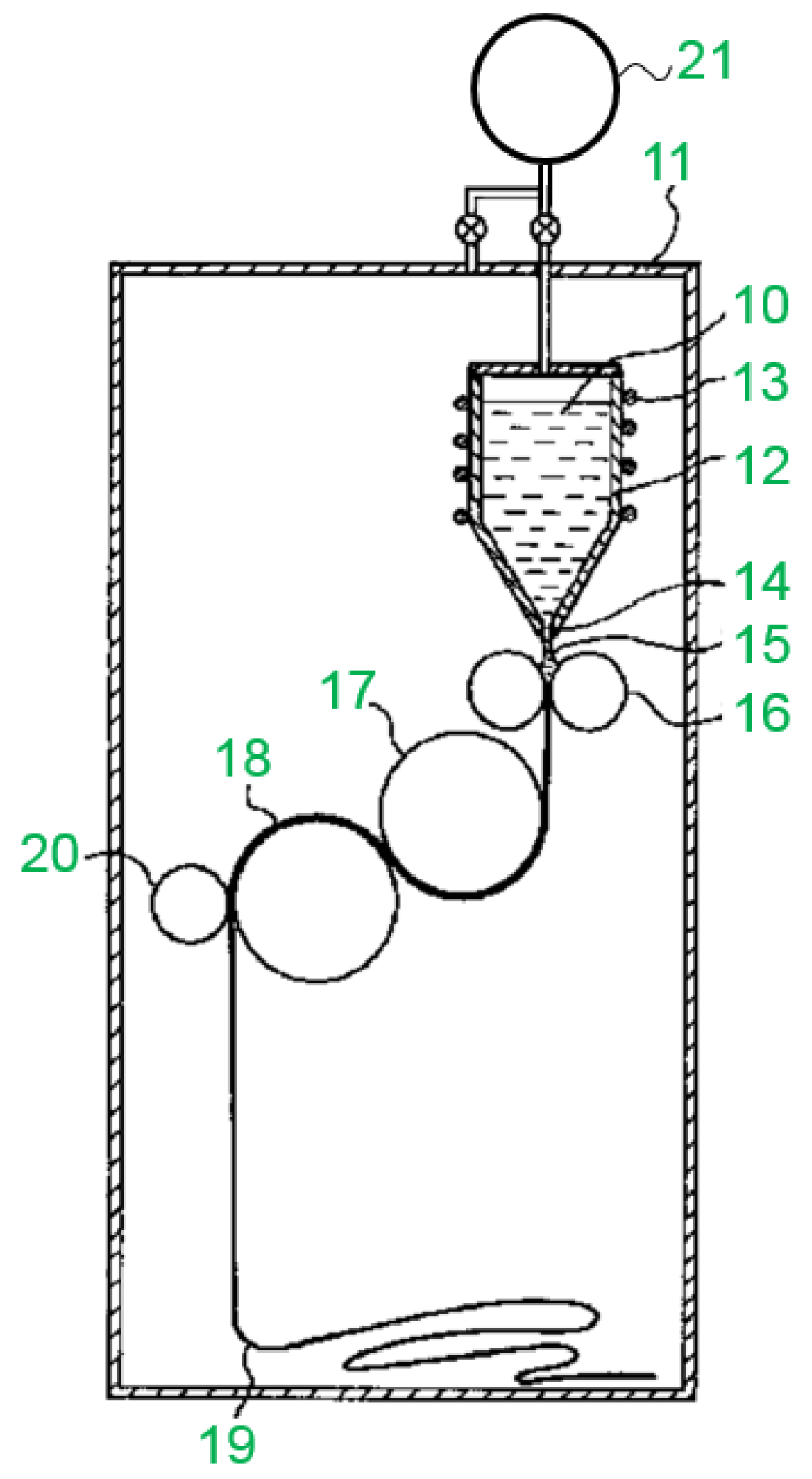
Reviews on the Japanese Patent Applications Regarding Nickel/Metal Hydride Batteries
Abstract
:1. Introduction
2. Results
2.1. Battery Manufacturers
2.1.1. Matsushita
2.1.2. Sanyo
2.1.3. Hitachi Maxell
2.1.4. Yuasa
2.1.5. Toshiba
2.1.6. FDK
2.1.7. Furukawa
2.1.8. Japan Storage
2.1.9. Shin-Kobe
2.1.10. Other Battery Manufacturers
2.2. Component Suppliers
2.2.1. Tanaka Chemical Co.
2.2.2. Mitsui Metal
2.2.3. Santoku Metal
2.2.4. Japan Metals & Chemicals Co.
2.2.5. Shin-Etsu Chemical
2.2.6. Other Suppliers
2.3. Research Institutes
2.3.1. Industrial Research Institute
2.3.2. National Institute of Advanced Industrial Science and Technology
2.3.3. Toyota Central R & D Lab
2.3.4. Other Research Institutes in Japan
2.4. Analysis
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ni/MH | Nickel/metal hydride |
| HEV | Hybrid electric vehicle |
| AIST | Agency of Industrial Science and Technology |
| PEVE | Panasonic EV Energy (now Primearth EV Energy) |
| MH | Metal hydride |
| PTFE | Polytetrafluoroethylene |
| HRD | High-rate dischargeability |
| XRD | X-ray diffractometer |
| PU | Polyurethane |
| BMS | Battery management system |
| JMC | Japan Metals & Chemicals Co. |
| PVA | Polyvinyl alcohol |
| ISI | Industrial Research Institute |
| bcc | Body-centered-cubic |
| JAXA | Japan Aerospace Exploration Agency |
| ICETT | International Center for Environmental Technology Transfer |
References
- Global xEV Batteries Market: Key Research Findings 2014; Yano Research Institute: Tokyo, Japan, 2015.
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. Patents regarding nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Webpage of Japan Platform for Patent Information. Available online: https://www4.j-platpat.inpit.go.jp/eng/tokujitsu/tkbs_en/TKBS_EN_GM101_Top.action (accessed on 24 June 2016).
- Panasonic EVOLTA NiMH. Available online: http://www.panasonic.com/global/consumer/battery/panasonic_rechargeable.html (accessed on 9 April 2016).
- Yamamoto, T.; Komori, K.; Suzuki, G.; Yamaguchi, S.; Kimura, T.; Ikoma, M.; Toyoguchi, Y. Production of Hydrogen Storage Alloy Powder. Jpn. Pat. Appl. H07-048602, 21 February 1995. [Google Scholar]
- Yamamoto, T.; Tsuji, M.; Komori, K.; Suzuki, G.; Yamaguchi, S.; Toyoguchi, Y. Production of Hydrogen Storage Alloy Powder and Nickel-Hydrogen Battery. Jpn. Pat. Appl. H07-054016, 28 February 1995. [Google Scholar]
- Furuike, K.; Ebihara, T.; Tanaka, A.; Yuasa, K.; Kaiya, H. Sealed Alkaline Battery. Jpn. Pat. Appl. H9-213319, 15 August 1997. [Google Scholar]
- Izumi, Y.; Moriwaki, Y.; Yamashita, K.; Tokuhiro, T. Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. H09-231969, 5 September 1997. [Google Scholar]
- Imamura, K.; Oomura, A.; Yuasa, K.; Kaiya, H. Hydrogen Storage Alloy Powder for Battery and Manufacture Thereof. Jpn. Pat. Appl. H09-245784, 19 September 1997. [Google Scholar]
- Hayashi, S.; Tomioka, K.; Morishita, N.; Ikeyama, S.; Ikoma, M. Nickel Positive Electrode for Alkaline Storage Battery and Nickel Hydrogen Storage Battery Using This Electrode. Jpn. Pat. Appl. H10-064537, 6 March 1998. [Google Scholar]
- Hayashi, S.; Tomioka, K.; Morishita, N.; Ikoma, M. Nickel Positive Electrode and Nickel-Hydrogen Storage Battery Using It. Jpn. Pat. Appl. H10-106556, 24 April 1998. [Google Scholar]
- Yuasa, S.; Komori, K.; Matsuda, H.; Ikoma, M. Alkaline Storage Battery. Jpn. Pat. Appl. H10-106620, 24 April 1998. [Google Scholar]
- Kasahara, H.; Yao, T.; Dansui, Y.; Konishi, H. Alkaline Storage Battery and Manufacture of Its Positive Electrode Active Material. Jpn. Pat. Appl. H11-185749, 9 July 1999. [Google Scholar]
- Kasahara, H.; Yao, T.; Suzuki, T.; Masui, M.; Konishi, H. Nickel-Hydrogen Storage Battery for Backup Power Supply. Jpn. Pat. Appl. H11-329481, 30 November 1999. [Google Scholar]
- Kikuyama, T.; Ebihara, T.; Miyahara, A.; Ou, S.; Yuasa, K. Hydrogen Storage Alloy for Battery, Its Manufacture and Alkaline Storage Battery Using It. Jpn. Pat. Appl. H11-339793, 10 December 1999. [Google Scholar]
- Ebihara, T.; Kikuyama, T.; Miyahara, A.; Ou, S.; Yuasa, K. Alkaline Storage Battery, Hydrogen Storage Alloy Electrode and Its Manufacture. Jpn. Pat. Appl. 2000-090920, 31 March 2000. [Google Scholar]
- Sakai, T. Secondary Battery with Hydrogen Storage Alloys. In Hydrogen Storage Alloys—Fundamentals and Frontier Technology; Tamura, H., Ed.; NTS Inc.: Tokyo, Japan, 1998; p. 411. [Google Scholar]
- Wikipedia, The Free Encyclopedia. Eneloop. Available online: https://en.wikipedia.org/wiki/Eneloop (accessed on 9 April 2016).
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Low Self-Discharge Nickel-Metal Hydride Battery. Available online: http://www.scribd.com/doc/9704685/Teraoka-Article-En (accessed on 24 June 2016).
- Takizawa, Y.; Ueda, T.; Kanekawa, I. Metal Hydride Storage Battery. Jpn. Pat. Appl. H05-242908, 21 September 1993. [Google Scholar]
- Shiraiwa, S.; Ueda, T.; Takizawa, Y. Metal Hydride Battery. Jpn. Pat. Appl. H07-065825, 10 March 1995. [Google Scholar]
- Yamawaki, A.; Nakahori, S.; Hamamatsu, T.; Baba, Y. Non-Sintered Nickel Electrode for Alkaline Storage Battery, Manufacture Thereof and Alkaline Storage Battery. Jpn. Pat. Appl. H08-148146, 7 June 1996. [Google Scholar]
- Nogami, M.; Matsuura, Y.; Kimoto, M.; Higashiyama, N.; Tokuda, M.; Isono, T.; Yonezu, I.; Nishio, K. Hydrogen Storage Alloy Electrode for Alkaline Storage Battery. Jpn. Pat. Appl. H09-147903, 6 June 1997. [Google Scholar]
- Ise, T. Metal Hydride Storage Battery. Jpn. Pat. Appl. H09-245782, 19 September 1997. [Google Scholar]
- Niiyama, K.; Tokuda, M.; Satoguchi, K.; Yano, M.; Nogami, M.; Yonezu, I.; Nishio, K. Alkaline Storage Battery. Jpn. Pat. Appl. H10-021904, 23 January 1998. [Google Scholar]
- Satoguchi, K.; Tokuda, M.; Niiyama, K.; Yano, M.; Nogami, M.; Yonezu, I.; Nishio, K. Manufacture of Unsintered Nickel Electrode for Alkaline Storage Battery. Jpn. Pat. Appl. H10-188970, 21 July 1998. [Google Scholar]
- Suzuki, S.; Tokuda, M.; Kimoto, M.; Yano, M.; Fujitani, S.; Nishio, K. Sealed Alkaline Storage Battery. Jpn. Pat. Appl. H10-214621, 11 August 1998. [Google Scholar]
- Matsuura, Y.; Nogami, M.; Maeda, R.; Niiyama, K.; Yonezu, I.; Nishio, K. Hydrogen Storage Alloy Electrode for Alkaline Storage Battery, and the Alkaline Storage Battery Using Thereof. Jpn. Pat. Appl. H11-031504, 2 February 1999. [Google Scholar]
- Kamiyoshi, K.; Ozaki, K.; Otsuki, K. Alkaline Storage Battery. Jpn. Pat. Appl. H09-213342, 15 August 1997. [Google Scholar]
- Niiyama, K.; Yano, M.; Maeda, R.; Nogami, M.; Yonezu, I.; Nishio, K. Non-Sintered Nickel Electrode for Alkaline Storage Battery. Jpn. Pat. Appl. H09-259878, 3 October 1997. [Google Scholar]
- Uenae, K. Hydrogen Storage Alloy Electrode and Alkaline Secondary Cell Using It. Jpn. Pat. Appl. H05-159798, 25 June 1993. [Google Scholar]
- Horiie, H.; Nagai, T. Cylindrical Alkaline Secondary Battery. Jpn. Pat. Appl. H05-226001, 3 September 1993. [Google Scholar]
- Hattori, H.; Kido, H.; Ishida, O.; Nagai, T. Alkaline Secondary Battery. Jpn. Pat. Appl. H05-290841, 5 November 1993. [Google Scholar]
- Edamoto, T.; Wada, S. Nickel Hydride Secondary Cell. Jpn. Pat. Appl. H08-050918, 20 February 1996. [Google Scholar]
- Fukunaga, H.; Nagai, T. Hydride Secondary Battery. Jpn. Pat. Appl. H08-190931, 23 July 1996. [Google Scholar]
- Fukunaga, H.; Tamakoshi, H.; Nagai, T.; Tateishi, S. Hydride Secondary Battery and Manufacture Thereof. Jpn. Pat. Appl. H10-040910, 13 February 1998. [Google Scholar]
- Takai, M.; Fukunaga, H.; Nagai, T. Assembled Battery of Hydride Secondary Battery. Jpn. Pat. Appl. H10-208768, 7 August 1998. [Google Scholar]
- Fukunaga, H.; Nagai, T. Closed Type Hydride Secondary Battery. Jpn. Pat. Appl. H11-149939, 2 June 1999. [Google Scholar]
- Fukunaga, H.; Tamakoshi, H.; Fujimoto, Y.; Nagai, T. Hydride Secondary Battery and Its Manufacture. Jpn. Pat. Appl. 2000-149934, 30 May 2000. [Google Scholar]
- Ono, H.; Tamakoshi, H.; Fukunaga, H.; Nagai, T. Alkaline Storage Battery. Jpn. Pat. Appl. 2000-353520, 19 December 2000. [Google Scholar]
- Ono, H.; Tamakoshi, H.; Fukunaga, H.; Nagai, T. Alkaline Storage Battery. Jpn. Pat. Appl. 2000-353542, 19 December 2000. [Google Scholar]
- Oshitani, M.; Yufu, H. Nickel Electrode for Alkaline Battery. Jpn. Pat. Appl. H01-272050, 31 October 1989. [Google Scholar]
- GS Yuasa. ENiTIME: Sealed Nickel-Metal Hydride Rechargeable Battery. Available online: http://pdf.directindustry.com/pdf/gs-yuasa/enitime/12414-296249.html (accessed on 9 April 2016).
- Tomita, M. Alkaline Battery. Jpn. Pat. Appl. S55-009354, 23 January 1980. [Google Scholar]
- Watada, M.; Matsumura, Y.; Miyake, N.; Oshitani, M. Electrode for Alkaline Storage Battery and Alkaline Storage Battery Using This Electrode. Jpn. Pat. Appl. H06-314567, 8 November 1994. [Google Scholar]
- Bougauchi, T.; Nakagawa, H.; Kishimoto, N.; Yamane, M. Nickel Electrode Plate and Manufacture Thereof and Alkaline Storage Battery Using It. Jpn. Pat. Appl. H07-114920, 2 May 1995. [Google Scholar]
- Tani, A. Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. H08-115739, 7 May 1996. [Google Scholar]
- Ito, T.; Harada, T.; Arahi, K.; Yufu, H. Forming Method of Sealed Alkaline Storage Battery. Jpn. Pat. Appl. H08-153543, 11 June 1996. [Google Scholar]
- Furukawa, K.; Tanaka, T.; Onishi, M.; Oshitani, M. Alkaline Storage Battery. Jpn. Pat. Appl. H09-092279, 4 April 1997. [Google Scholar]
- Tani, A.; Kurokuzuhara, M. Separator for Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. H10-031990, 3 February 1998. [Google Scholar]
- Matsumura, Y.; Tanaka, T.; Kurokuzuhara, M.; Tani, A.; Watada, M.; Oshitani, M. Sealed Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. H10-074536, 17 March 1998. [Google Scholar]
- Kanemoto, M.; Kodama, M.; Kurokuzuhara, M. Hydrogen-Storing Alloy Electrode and Nickel Hydrogen Battery Using Same. Jpn. Pat. Appl. 2001-283854, 12 October 2001. [Google Scholar]
- Kanemoto, M.; Kodama, M.; Kurokuzuhara, M.; Watada, M. Hydrogen Storage Alloy Electrode. Jpn. Pat. Appl. 2002-042800, 8 February 2002. [Google Scholar]
- Kanemoto, M.; Kurokuzuhara, M.; Kodama, M.; Sakamoto, K.; Watada, M. Hydrogen Occlusion Alloy Powder, Hydrogen Occlusion Alloy Electrode, and Nickel-Hydrogen Storage Battery Using the Same. Jpn. Pat. Appl. 2004-124132, 22 April 2004. [Google Scholar]
- Hasebe, H.; Takeno, K.; Ikeda, K.; Sato, Y. Nickel Hydrogen Secondary Cell Module. Jpn. Pat. Appl. H05-036392, 12 February 1993. [Google Scholar]
- Uchiyama, M.; Takeno, K. Alkaline Secondary Battery. Jpn. Pat. Appl. H07-211313, 11 August 1995. [Google Scholar]
- Tsuruta, S.; Kono, R.; Kanda, M. Hydrogen Storage Alloy and Alkaline Secondary Battery. Jpn. Pat. Appl. H10-251782, 22 September 1998. [Google Scholar]
- Kono, R.; Kanda, M. Hydrogen Storage Alloy, Cathode for Battery and Alkaline Secondary Battery. Jpn. Pat. Appl. H10-251791, 22 September 1998. [Google Scholar]
- Mukai, K.; Ishizuka, S.; Takeno, K. Alkaline Secondary Battery. Jpn. Pat. Appl. H10-294106, 4 November 1998. [Google Scholar]
- Hosobuchi, K.; Gama, M. Metallic Oxide Hydrogen Secondary Battery. Jpn. Pat. Appl. H07-326354, 12 December 1995. [Google Scholar]
- Hayashida, H.; Kitayama, H.; Yamamoto, M.; Sakai, I.; Kono, R.; Yoshida, H.; Inaba, T.; Inada, S.; Kanda, M. Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H11-162460, 18 June 1999. [Google Scholar]
- Kitayama, H.; Hayashida, H.; Yamamoto, M.; Sakai, I.; Kono, R.; Yoshida, H.; Inaba, T.; Inada, S.; Kanda, M. Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H11-162503, 18 June 1999. [Google Scholar]
- Kono, R.; Sakai, I.; Yoshida, H.; Inaba, T.; Yamamoto, M.; Takeno, S. Hydrogen Storage Alloy and Secondary Battery. Jpn. Pat. Appl. 2000-073132, 7 March 2000. [Google Scholar]
- Kono, R.; Sakai, I.; Yoshida, H.; Inaba, T.; Yamamoto, M. Hydrogen Storage Alloy and Secondary Battery. Jpn. Pat. Appl. 2000-265228, 26 September 2000. [Google Scholar]
- Yoshida, H.; Yamamoto, M.; Sakai, I.; Inaba, T.; Takabayashi, J.; Irie, S.; Suzuki, H.; Takeno, K. Hydrogen Storage Alloy, Alkali Secondary Battery, Hybrid Car and Electric Vehicle. Jpn. Pat. Appl. 2002-069554, 8 March 2002. [Google Scholar]
- Inaba, T.; Sakai, I.; Yoshida, H.; Takabayashi, J.; Yamamoto, M.; Suzuki, H.; Irie, S.; Takeno, K. Nickel Hydrogen Secondary Battery, Hybrid Car and Electric Vehicle. Jpn. Pat. Appl. 2002-083593, 22 March 2002. [Google Scholar]
- Kawashima, F.; Sakamoto, T.; Arai, T. Hydrogen Storage Alloy and Nickel-Hydrogen Secondary Battery Using the Same. Jpn. Pat. Appl. 2002-105563, 10 April 2002. [Google Scholar]
- Sakamoto, T.; Kawashima, F.; Arai, T. Hydrogen Storage Alloy, Its Production Method and Nickel-Hydrogen Secondary Battery Using the Same. Jpn. Pat. Appl. 2002-105564, 10 April 2002. [Google Scholar]
- Sakai, I.; Inaba, T.; Yoshida, H.; Yamamoto, M.; Irie, S.; Suzuki, H.; Takeno, K. Hydrogen Storage Alloy, Secondary Battery, Hybrid Vehicle, and Electric Vehicle. Jpn. Pat. Appl. 2002-164045, 7 June 2002. [Google Scholar]
- Saguchi, A.; Kihara, M.; Endo, T. Negative Electrode for Alkaline Secondary Battery, and Alkaline Secondary Battery Comprising the Negative Electrode. Jpn. Pat. Appl. 2012-134110, 12 July 2012. [Google Scholar]
- Ishida, J.; Yasuoka, S.; Inui, H.; Kishida, K. Hydrogen Absorbing Alloy and Alkaline Storage Battery Manufactured Using the Hydrogen Absorbing Alloy. Jpn. Pat. Appl. 2012-174639, 10 September 2012. [Google Scholar]
- Kihara, M.; Endo, T.; Sato, T.; Saguchi, A.; Wada, S.; Mugima, I.; Nakamura, T.; Asanuma, H.; Tamura, M. Negative Electrode for Nickel-Hydrogen Secondary Battery, and Nickel-Hydrogen Secondary Battery Using the Negative Electrode. Jpn. Pat. Appl. 2012-256522, 27 December 2012. [Google Scholar]
- Kihara, M.; Takei, M.; Yamane, T. Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. 2013-030345, 7 February 2013. [Google Scholar]
- Ishida, J. Hydrogen Storage Alloy and Nickel-Hydrogen Secondary Battery Using the Same. Jpn. Pat. Appl. 2013-100585, 23 May 2013. [Google Scholar]
- Ishida, J.; Yasuoka, S. Hydrogen Storage Alloy and Nickel-Hydrogen Secondary Battery Using the Same. Jpn. Pat. Appl. 2013-108105, 6 June 2013. [Google Scholar]
- Ishida, J.; Kai, T. Hydrogen Storage Alloy and Nickel Hydride Secondary Battery Using the Hydrogen Storage Alloy. Jpn. Pat. Appl. 2014-145122, 14 August 2014. [Google Scholar]
- Yamane, T.; Takei, M.; Imoto, Y.; Ito, T. Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2013-206867, 7 October 2013. [Google Scholar]
- Kai, T.; Ishida, J. Nickel Hydrogen Secondary Battery and Negative Electrode for Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2014-026844, 6 February 2014. [Google Scholar]
- Kai, T.; Ishida, J. Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2014-146557, 14 August 2014. [Google Scholar]
- Kihara, M.; Saguchi, A.; Takei, M.; Ito, T.; Imoto, Y. Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2014-089879, 15 May 2014. [Google Scholar]
- Ishida, J.; Kai, T. Negative Electrode for Nickel-Hydrogen Secondary Battery, and Nickel-Hydrogen Secondary Battery Using the Same. Jpn. Pat. Appl. 2014-207086, 30 October 2014. [Google Scholar]
- Sato, T. Nickel Hydrogen Storage Battery. Jpn. Pat. Appl. 2015-008107, 15 January 2015. [Google Scholar]
- Kai, T.; Ishida, J. Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2015-103497, 4 June 2015. [Google Scholar]
- Ishida, J.; Kai, T. Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2015-195108, 5 November 2015. [Google Scholar]
- Takasu, D. Negative Electrode for Nickel Hydrogen Secondary Battery and Nickel Hydrogen Secondary Battery Using This Negative Electrode. Jpn. Pat. Appl. 2015-201334, 12 November 2015. [Google Scholar]
- Ushara, I.; Sakai, T.; Ishikawa, H. The state of research and development for applications of metal hydrides in Japan. J. Alloys Compd. 1997, 253–254, 635–641. [Google Scholar]
- Furukawa, A. Negative Electrode for Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H06-231758, 19 August 1994. [Google Scholar]
- Furukawa, A. Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H06-231761, 19 August 1994. [Google Scholar]
- Furukawa, A. Manufacture of Sealed Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H06-251800, 9 September 1994. [Google Scholar]
- Furukawa, A. Production of Powdery Hydrogen Occluding Alloy for Negative Electrode of Nickel-Hydrogen Secondary Battery and Production of Negative Electrode for Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H06-279980, 4 October 1994. [Google Scholar]
- Furukawa, A. Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H06-283195, 7 October 1994. [Google Scholar]
- Furukawa, A. Sealed Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H06-283196, 7 October 1994. [Google Scholar]
- Murata, T. Sealed Battery. Jpn. Pat. Appl. H05-021045, 29 January 1993. [Google Scholar]
- Murata, T. Enclosed Type Nickel/Metal Hydride Storage Battery. Jpn. Pat. Appl. H05-041204, 19 February 1993. [Google Scholar]
- Murata, T. Nickel-Metal Hydride Storage Battery. Jpn. Pat. Appl. H05-121073, 18 May 1993. [Google Scholar]
- Shichimoto, K. Manufacture of Nickel Metal Hydride Cell. Jpn. Pat. Appl. H08-050919, 20 February 1996. [Google Scholar]
- Nanamoto, K. Sealed Alkaline Storage Battery. Jpn. Pat. Appl. H09-199162, 31 July 1997. [Google Scholar]
- Nomura, Y.; Ogura, T.; Kobayashi, K.; Tsuda, T. Hydrogen Storage Alloy Electrode and Manufacture Thereof. Jpn. Pat. Appl. H05-003029, 8 January 1993. [Google Scholar]
- Konuki, T.; Kobayashi, K. Hydrogen Storage Alloy Electrode and Manufacture Thereof. Jpn. Pat. Appl. H06-163043, 10 June 1994. [Google Scholar]
- Yamaguchi, T.; Watanabe, K.; Yoshida, M.; Kamigata, Y. Hydrogen Storage Electrode and Manufacture Thereof. Jpn. Pat. Appl. H08-050898, 20 February 1996. [Google Scholar]
- Kobayashi, K.; Tamagawa, T. Hydrogen Storage Alloy Electrode and Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. H08-264175, 11 October 1996. [Google Scholar]
- Minoura, S.; Kobayashi, K.; Ogura, T. Hydrogen Storage Alloy Electrode for Alkaline Storage Battery. Jpn. Pat. Appl. H10-092422, 10 April 1998. [Google Scholar]
- Minamiura, K. Method and Device for Sensing Failure in Battery Pack System. Jpn. Pat. Appl. 2003-142165, 16 May 2003. [Google Scholar]
- Minamiura, K. Controlling Method and Device for Cooling of Battery. Jpn. Pat. Appl. 2003-142166, 16 May 2003. [Google Scholar]
- Yudahira, H. Battery Power Unit and Its Current Detecting Method. Jpn. Pat. Appl. 2003-168488, 13 June 2003. [Google Scholar]
- Yudahira, H. Electric Leakage Detecting Device. Jpn. Pat. Appl. 2003-194870, 9 July 2003. [Google Scholar]
- Yudahira, H. Voltage Measurement Device and Method, as well as Battery Pack System. Jpn. Pat. Appl. 2003-197273, 11 July 2003. [Google Scholar]
- Murakami, T. Estimating Method of Polarized Voltage of Secondary Battery, Estimating Method and Device of Residual Capacity of Secondary Battery, as well as Battery Pack System. Jpn. Pat. Appl. 2003-197275, 11 July 2003. [Google Scholar]
- Ueda, T.; Morishita, N.; Okawa, K.; Nakao, Y. Remaining Capacity Arithmetic Unit and Remaining Capacity Computing Method of Secondary Battery. Jpn. Pat. Appl. 2004-361313, 24 December 2004. [Google Scholar]
- Morimoto, N. Testing Method for Relay Contact Welding in Battery Power Supply. Jpn. Pat. Appl. 2003-209907, 25 July 2003. [Google Scholar]
- Nakanishi, T.; Torii, Y. Controller for Motorized Vehicle, Motorized Vehicle Equipped with It. Jpn. Pat. Appl. 2004-048937, 12 February 2004. [Google Scholar]
- Katakura, Y.; Kajikawa, T. Manufacturing Method of Nickel Metal Hydride Storage Battery. Jpn. Pat. Appl. 2010-153261, 8 July 2010. [Google Scholar]
- Kawasaki Heavy Industries. Battery Energy Storage System-GIGACELL. Available online: https://global.kawasaki.com/en/energy/solutions/battery_energy/ (accessed on 9 April 2016).
- Tsutsumi, K. Three-Dimensional Battery. Jpn. Pat. Appl. 2002-141101, 17 May 2002. [Google Scholar]
- Tsutsumi, K.; Nishimura, K.; Mitsuta, S. Active Material for Battery and Its Manufacturing Method. Jpn. Pat. Appl. 2003-197187, 11 July 2003. [Google Scholar]
- Tsutsumi, K.; Nishimura, K.; Mitsuta, S. Electrode Using Fibrous Hydrogen Storage Alloy, Battery Using Fibrous Hydrogen Storage Alloy and Electric Double Layer Capacitor. Jpn. Pat. Appl. 2004-022332, 22 January 2004. [Google Scholar]
- Tsutsumi, K.; Nishimura, K. Battery. Jpn. Pat. Appl. 2008-041522, 21 February 2008. [Google Scholar]
- Nishimura, K.; Tsutsumi, K. Square Battery. Jpn. Pat. Appl. 2010-129360, 10 June 2010. [Google Scholar]
- Tsutsumi, K.; Nakoji, M.; Origuchi, T.; Ogawa, S.; Nakayama, N. Alkaline Storage Battery. Jpn. Pat. Appl. 2010-177071, 12 August 2010. [Google Scholar]
- Ishida, T.; Sugiyama, S. Charger for Battery in Railroad Vehicle. Jpn. Pat. Appl. 2008-172857, 24 July 2008. [Google Scholar]
- Koyano, K.; Miyamoto, Y.; Hayashi, M.; Sawai, T.; Yoshiyama, E.; Tsutsumi, K. Charged State Estimation Method and Device for Secondary Battery. Jpn. Pat. Appl. 2009-244057, 22 October 2009. [Google Scholar]
- Nishimura, K.; Tsutsumi, K. Pressure Regulating Device of Battery. Jpn. Pat. Appl. 2009-301888, 24 December 2009. [Google Scholar]
- Nishimura, K.; Takagaki, K.; Ugawa, K.; Ide, T. Battery Module. Jpn. Pat. Appl. 2013-168216, 29 August 2013. [Google Scholar]
- Nishimura, K. Battery System. Jpn. Pat. Appl. 2013-143295, 22 July 2013. [Google Scholar]
- Eguro, T.; Hashiguchi, J.; Koga, Y. Chemical Forming Method for High Energy Type Fe-Ni Battery. Jpn. Pat. Appl. H02-075168, 14 March 1990. [Google Scholar]
- Lichtenberg, F.; Kleinsorgen, K.; Hofmann, G. Nickel/Metal Hydride Secondary Battery. Jpn. Pat. Appl. H07-211317, 11 August 1995. [Google Scholar]
- Knosp, B.; Bouet, J.; Jordy, C.; Mimoun, M.; Gicquel, D. Material Capable of Hydrogenation for Negative Electrode of Nickel-Metal Hydride Storage Battery. Jpn. Pat. Appl. H08-017435, 19 January 1996. [Google Scholar]
- Kang, S. Nickel Metal Hydride Storage Battery and Manufacture Thereof. Jpn. Pat. Appl. H09-171818, 30 June 1997. [Google Scholar]
- Fetcenko, M.; Ovshinsky, S.; Chao, B.; Reichman, B. Improved Electrochemical Hydrogen Storage Alloy for Nickel Hydride Metal Battery. Jpn. Pat. Appl. 2000-144288, 26 May 2000. [Google Scholar]
- Ovshinsky, S.; Fetcenko, M.; Im, J.; Young, K.; Chao, B.; Reichman, B. Hydrogen Occluding Material Having Abnormal Site Capable of Occluding Hydrogen at High Density. Jpn. Pat. Appl. 2002-088430, 27 March 2002. [Google Scholar]
- Fetcenko, M.; Ovshinsky, S.; Chao, B.; Reichman, B. Improved Electrochemical Hydrogen Storage Alloy for Nickel Hydride Metal Battery. Jpn. Pat. Appl. 2002-088436, 27 March 2002. [Google Scholar]
- Ovshinsky, S.; Fetcenko, M.; Im, J.; Young, K.; Chao, B.; Reichman, B. Hydrogen Storage Material Having High Density of Non-Conventional Usable Hydrogen Storing Sites. Jpn. Pat. Appl. 2002-241874, 28 August 2002. [Google Scholar]
- Fetcenko, M.; Young, K.; Ovshinsky, S.; Reichman, B.; Koch, J.; Mays, W. Modified Electrochemical Hydrogen Storage Alloy Having Increased Capacity, Rate Capability and Catalytic Activity. Jpn. Pat. Appl. 2006-183148, 13 July 2006. [Google Scholar]
- Ovshinsky, S.; Fetcenko, M. Secondary Battery Fabricated of Electrochemical Hydrogen Storage Alloy and Mg-Contained Base Alloy. Jpn. Pat. Appl. 2003-217578, 31 July 2003. [Google Scholar]
- Ovshinsky, S.; Fetcenko, M.; Reichman, B.; Young, K.; Chao, B.; Im, J. Electrochemical Hydrogen Storage Alloy and Battery Fabricated from Magnesium-Containing Base Alloy. Jpn. Pat. Appl. 2003-247038, 5 September 2003. [Google Scholar]
- Fetcenko, M.; Young, K.; Ouchi, T.; Reinhout, M.; Ovshinsky, S. Mg-Ni Hydrogen Storage Composite Having High Storage Capacity and Excellent Room Temperature Kinetics. Jpn. Pat. Appl. 2007-119906, 17 May 2007. [Google Scholar]
- Fetcenko, M.; Fierro, C.; Ovshinsky, S.; Sommers, B.; Reichman, B.; Young, K.; Mays, W. Composite Positive Electrode Material and Its Manufacturing Method. Jpn. Pat. Appl. 2004-214210, 29 July 2004. [Google Scholar]
- Ovshinsky, S.; Fetcenko, M.; Fierro, C.; Gifford, P.; Corrigan, D.; Benson, P.; Martin, F. High Performance Nickel Hydroxide Positive Electrode Electrode Material for Alkaline Rechargeable Electrochemical Battery. Jpn. Pat. Appl. 2006-054190, 23 February 2006. [Google Scholar]
- Fetcenko, M.; Fierro, C.; Ovshinsky, S.; Sommers, B.; Reichman, B.; Young, K.; Mays, W. Composite Positive Electrode Material and Method for Making Same. Jpn. Pat. Appl. 2010-282973, 16 December 2010. [Google Scholar]
- Corrigan, D.; Gow, P.; Higley, L.; Muller, M.; Osgood, A.; Ovshinsky, S.; Payne, J.; Puttaiah, R. Monoblock Battery Assembly. Jpn. Pat. Appl. 2010-225591, 7 October 2010. [Google Scholar]
- Wolff, M.; Nuss, M.; Fetcenko, M.; Lijoi, A. Continuous Manufacture of Hydrogen Storage Alloy Cathode. Jpn. Pat. Appl. H01-286255, 17 November 1989. [Google Scholar]
- Imaizumi, J.; Kawasaki, Y.; Makino, T.; Iida, T. Alpha-Cobalt Hydroxide Layer-Coated Nickel Hydroxide for Alkaline Storage Battery and Manufacture Thereof. Jpn. Pat. Appl. H10-012236, 16 January 1998. [Google Scholar]
- Imaizumi, J.; Kawasaki, Y.; Makino, T.; Iida, T. Beta-Cobalt Hydroxide Layer-Coated Nickel Hydroxide for Alkaline Storage Battery and Manufacture Thereof. Jpn. Pat. Appl. H10-012237, 16 January 1998. [Google Scholar]
- Watada, M.; Oshitani, M.; Imaizumi, J.; Iida, T. Positive Electrode Active Material for Alkali Storage Battery, Its Manufacture and Positive Electrode for Alkali Storage Battery. Jpn. Pat. Appl. H10-027608, 27 January 1998. [Google Scholar]
- Usui, T.; Makino, T.; Iida, T. Nickel Hydroxide for Alkaline Storage Battery and Manufacture Thereof. Jpn. Pat. Appl. H10-097856, 14 April 1998. [Google Scholar]
- Watada, M.; Oshitani, M.; Imaizumi, J.; Iida, T. Positive Electrode Active Material for Alkaline Storage Battery and Manufacture Thereof, and Positive Electrode for Alkaline Storage Battery. Jpn. Pat. Appl. H10-188973, 21 July 1998. [Google Scholar]
- Sakai, T.; Ishihara, K.; Imaizumi, J. Nickel Positive Electrode for Alkaline Secondary Battery. Jpn. Pat. Appl. 2000-077068, 14 March 2000. [Google Scholar]
- Hosoe, A.; Imaizumi, J.; Iida, T. Nickel Positive Electrode Active Material for Alkaline Battery and Its Manufacture. Jpn. Pat. Appl. 2000-082463, 21 March 2000. [Google Scholar]
- Tanaka, T.; Makino, T.; Iida, T. Manufacture of Positive Electrode Active Material for Alkaline Storage Battery. Jpn. Pat. Appl. 2000-268820, 29 September 2000. [Google Scholar]
- Hirayama, S. Ingot for Hydrogen Storage Alloy Powder and Production of the Powder. Jpn. Pat. Appl. H04-358008, 11 December 1992. [Google Scholar]
- Kuji, T.; Kitakado, M.; Yasuda, K.; Hanawa, K.; Nitta, S.; Dobashi, M. Method for Surface-Modifying Hydrogen Storage Alloy. Jpn. Pat. Appl. H07-316610, 5 December 1995. [Google Scholar]
- Sasaki, M.; Hirayama, S.; Sumimoto, S. Hydrogen Storage Alloy and Its Production. Jpn. Pat. Appl. H09-031573, 4 February 1997. [Google Scholar]
- Yasuda, K.; Nakayama, S. Hydrogen Storage Alloy. Jpn. Pat. Appl. H09-316573, 9 December 1997. [Google Scholar]
- Sakaguchi, Y.; Nakayama, S.; Yasuda, K. Hydrogen Storage Alloy and Its Production. Jpn. Pat. Appl. H10-088261, 7 April 1998. [Google Scholar]
- Sumimoto, S.; Sakai, M.; Uchiyama, A.; Hirayama, S.; Yasuda, K.; Nakayama, S.; Ebihara, T.; Yuasa, K. Hydrogen Storage Alloy and Electrode for Nickel-Hydrogen Battery Using It. Jpn. Pat. Appl. H10-152739, 9 June 1998. [Google Scholar]
- Yasuda, K.; Hirayama, S.; Sakai, M.; Uchiyama, A.; Sakaguchi, Y.; Nakayama, S. Hydrogen Storage Alloy and Its Production. Jpn. Pat. Appl. H11-152533, 8 June 1999. [Google Scholar]
- Yasuda, K.; Sakaguchi, Y.; Uchiyama, A.; Mukai, D.; Kikukawa, S. Hydrogen Storage Alloy and Its Manufacture. Jpn. Pat. Appl. 2000-234133, 29 August 2000. [Google Scholar]
- Yasuda, K.; Sakaguchi, Y.; Kikukawa, S. Hydrogen Storage Alloy and Its Production Method. Jpn. Pat. Appl. 2001-348636, 18 December 2001. [Google Scholar]
- Toma, H. Manufacture of Hydrogen Occluding Alloy of Rare Earth Metal-Nickel System. Jpn. Pat. Appl. S59-140301, 11 August 1984. [Google Scholar]
- Yamamoto, K.; Miyake, Y.; Okada, T.; Kitatsume, N. Rare Earth-Nickel Hydrogen Storage Alloy Ingot and Its Production. Jpn. Pat. Appl. H05-320792, 3 December 1993. [Google Scholar]
- Nishigaki, N.; Kitatsume, N.; Okada, T. Method for Preserving Rare Earth-Transition Metal Alloy. Jpn. Pat. Appl. H06-264102, 20 September 1994. [Google Scholar]
- Kaneko, A. Rare Earth Metal-Nickel Based Hydrogen Storage Alloy and Its Production, and Cathode for Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. H09-025529, 28 January 1997. [Google Scholar]
- Yamamoto, K.; Okada, T. Production System of Alloy Containing Rare Earth Metal. Jpn. Pat. Appl. H09-155507, 17 June 1997. [Google Scholar]
- Oota, T.; Yamaguchi, M.; Gashiyuu, S.; Noda, K.; Oku, K.; Konno, H.; Sasai, K. Hydrogen Occluding Alloy and Its Manufacture. Jpn. Pat. Appl. S56-136957, 26 October 1981. [Google Scholar]
- Noda, K.; Oku, K.; Konno, H.; Sasai, K.; Onoe, K.; Kashiyuu, S. Material for Storage of Hydrogen. Jpn. Pat. Appl. S57-075138, 11 May 1982. [Google Scholar]
- Sasai, O.; Hayamizu, N.; Uotani, S. Hydrogen Storage Material. Jpn. Pat. Appl. S63-047345, 29 February 1988. [Google Scholar]
- Uemura, M.; Yamamoto, I.; Tsubata, A.; Hayashi, T. Production of Nickel Hydroxide. Jpn. Pat. Appl. S64-042330, 14 February 1989. [Google Scholar]
- Tomioka, H.; Matsubara, Y.; Hayamizu, N. Hydrogen Storage Alloy for Nickel/Hydrogen Battery. Jpn. Pat. Appl. H07-029570, 31 January 1995. [Google Scholar]
- Uehara, H.; Ishikawa, H.; Nishida, J.; Sakuma, T.; Saito, N. Electrode for Alkaline Secondary Battery. Jpn. Pat. Appl. H07-073885, 17 March 1995. [Google Scholar]
- Sasai, K.; Hayamizu, N.; Nakamura, M.; Kenmochi, Y.; Honda, J. Method for Recovering Valuable Material from Scrapped Nickel Hydrogen Occluding Alloy Secondary Battery. Jpn. Pat. Appl. H08-020825, 23 January 1996. [Google Scholar]
- Kamisaka, K.; Nakayama, Y.; Nishida, J.; Igarashi, K.; Sakuma, T. Electrode Material of Silver Plated Nickel Based Porous Metal and Its Production. Jpn. Pat. Appl. H08-337894, 24 December 1996. [Google Scholar]
- Takahashi, S.; Osawa, M.; Shimizu, H. Production of Magnesium-Yttrium Hydrogen Storage Alloy. Jpn. Pat. Appl. H09-125172, 13 May 1997. [Google Scholar]
- Saito, N.; Takahashi, M.; Sasai, K. Hydrogen Storage Alloy for Battery. Jpn. Pat. Appl. H09-298059, 18 November 1997. [Google Scholar]
- Obuchi, I.; Haraikawa, N. Production of Powder-Type Hydrogen-Storing Alloy. Jpn. Pat. Appl. H09-316505, 9 December 1997. [Google Scholar]
- Sanoki, H.; Sugimoto, T.; Kudo, K. Method for Stabilizing Hydrogen Storage Alloy. Jpn. Pat. Appl. H10-195503, 28 July 1998. [Google Scholar]
- Terashita, N.; Takahashi, M.; Kobayashi, K.; Sasai, K. Production of Amorphous Magnesium-Nickel Base Hydrogen Storage Alloy. Jpn. Pat. Appl. H11-269572, 5 October 1999. [Google Scholar]
- Osawa, M.; Muromachi, N. Surface Treatment of Hydrogen Storage Alloy. Jpn. Pat. Appl. H11-335867, 7 December 1999. [Google Scholar]
- Sakai, T.; Ikenaga, M.; Nishino, H.; Nishida, J. Alkaline Secondary Battery Electrode Substrate. Jpn. Pat. Appl. 2000-040516, 8 February 2000. [Google Scholar]
- Yoshikawa, T.; Osawa, M.; Muromachi, N.; Endo, T.; Ogura, H. Negative Electrode for Secondary Battery. Jpn. Pat. Appl. 2000-090919, 31 March 2000. [Google Scholar]
- Kobayashi, K.; Ogura, H.; Osawa, M.; Muromachi, N.; Harada, R.; Kimura, M.; Toyoshima, H. Hydrogen Storage Alloy Negative Electrode. Jpn. Pat. Appl. 2000-182608, 30 June 2000. [Google Scholar]
- Saito, N.; Sugimoto, T.; Haneda, T.; Osawa, M.; Yoshikawa, T. Hydrogen Storage Alloy. Jpn. Pat. Appl. 2001-279354, 10 October 2001. [Google Scholar]
- Saito, N.; Sugimoto, T.; Haneda, T.; Osawa, M.; Yoshikawa, T. Hydrogen Storage Alloy for Secondary Battery. Jpn. Pat. Appl. 2001-279355, 10 October 2001. [Google Scholar]
- Terashita, N.; Ito, N.; Osawa, M.; Takahashi, S.; Tsunokake, S.; Hamura, K. Hydrogen Storage Alloy, Its Manufacturing Method and Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2007-056309, 8 March 2007. [Google Scholar]
- Osawa, M.; Ito, N.; Terashita, N.; Takahashi, S.; Tsunokake, S. Hydrogen Storage Alloy and Nickel-Hydride Secondary Battery. Jpn. Pat. Appl. 2007-291474, 8 November 2007. [Google Scholar]
- Kobayashi, K.; Takahashi, S.; Hayamizu, N. Secondary Battery Hydrogen Storage Alloy. Jpn. Pat. Appl. 2008-210809, 11 September 2008. [Google Scholar]
- Terashita, N.; Osawa, M.; Kudo, K.; Soma, Y.; Tsunokake, S. Hydrogen Storage Alloy, and Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2012-067357, 5 April 2012. [Google Scholar]
- Sakai, T.; Saito, M.; Mukai, T.; Tsunokake, S.; Osawa, M. Hydrogen-Storage Alloy, Hydrogen-Storage Alloy Electrode, and Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. 2012-102343, 31 May 2012. [Google Scholar]
- Fukui, I.; Kuribayashi, Y. Hydrogen Storage Alloy Electrode and Manufacture Thereof. Jpn. Pat. Appl. H08-088001, 2 April 1996. [Google Scholar]
- Shintani, H.; Sugahara, Y. Hydrogen Storage Alloy-Containing Composition and Electrode Using It. Jpn. Pat. Appl. H08-329934, 13 December 1996. [Google Scholar]
- Fukui, I. Manufacture of Hydrogen Storage Alloy Electrode. Jpn. Pat. Appl. H09-171820, 30 June 1997. [Google Scholar]
- Fukui, I. Hydrogen Storage Alloy Electrode and Its Manufacture. Jpn. Pat. Appl. H11-329437, 30 November 1999. [Google Scholar]
- Kasashima, M.; Hashimoto, T.; Minowa, T. Apparatus for Producing Hydrogen-Storage Alloy and Production Thereof. Jpn. Pat. Appl. 2000-158098, 13 June 2000. [Google Scholar]
- Komata, N.; Hibi, K.; Ohashi, S.; Aizawa, A.; Yaginuma, T. Production of Nickel-Containing Hydroxide. Jpn. Pat. Appl. H11-130441, 18 May 1999. [Google Scholar]
- Hibi, K.; Wakai, E.; Oki, T.; Ban, S.; Furushima, K.; Shioda, M. Method for Producing Spherical High Density Nickel Hydroxide and Spherical High Density Nickel Hydroxide Powder for Anode Active Substance of Alkaline Secondary Battery. Jpn. Pat. Appl. 2003-002665, 8 January 2003. [Google Scholar]
- Hibi, K.; Wakai, E.; Oki, T.; Furushima, K.; Ono, M.; Shioda, M. Nickel Positive Electrode Active Material for Alkaline Secondary Battery and Manufacturing Method of Cobalt Compound-Coated Nickel Hydroxide Particle. Jpn. Pat. Appl. 2003-157840, 30 May 2003. [Google Scholar]
- Tsuji, S.; Son, H.; Uchida, Y. Spherical Nickel Hydroxide Powder and Method for Producing Same. Jpn. Pat. Appl. 2006-151795, 15 June 2006. [Google Scholar]
- Matsubara, N.; Tanaka, M.; Ishikawa, S.; Shimizu, T.; Kitamura, N. Method for Manufacturing Nickel Sintered Substrate for Alkaline Storage Battery Positive Electrode and Nickel Sintered Substrate for Alkaline Storage Battery Positive Electrode. Jpn. Pat. Appl. 2015-198061, 9 November 2015. [Google Scholar]
- Shibuya, S.; Hideno, A. Porous Hydrogen Storage Alloy. Jpn. Pat. Appl. H01-309937, 14 December 1989. [Google Scholar]
- Kojima, Y.; Yamamoto, K.; Furukawa, S. Method of Producing Hydrogen Storage Alloy Powder. Jpn. Pat. Appl. 2004-169125, 17 June 2004. [Google Scholar]
- Ishikawa, R.; Miyashita, T.; Yugamidani, M. Production of Hydrogen Occluding Alloy. Jpn. Pat. Appl. H06-088150, 29 March 1994. [Google Scholar]
- Kojima, Y.; Ikeda, H.; Furukawa, S.; Sugiyama, K.; Kobayashi, N. Hydrogen-Storage Alloy, and Electrode for Nickel-Hydrogen Battery. Jpn. Pat. Appl. 2008-184660, 14 August 2008. [Google Scholar]
- Hatakeyama, S.; Kojima, Y.; Miyashita, T.; Furukawa, S.; Sugiyama, K.; Tsuchiya, E.; Yabe, T. Manufacture of Hydrogen Storage Alloy Powder, and Ni-Hydrogen Battery. Jpn. Pat. Appl. H10-021907, 23 January 1998. [Google Scholar]
- Doi, H.; Yabuki, T. Hydrogen Storage Ni-Base Alloy and Closed Type Ni-Hydrogen Storage Battery. Jpn. Pat. Appl. H02-111836, 24 April 1990. [Google Scholar]
- Doi, H.; Yabuki, T. Hydrogen Storage Ni-Based Alloy and Closed Ni-Hydrogen Battery. Jpn. Pat. Appl. H02-194140, 31 July 1990. [Google Scholar]
- Doi, H.; Yabuki, T. Hydrogen Storage Ni-Zr Series Alloy and Closed-Type Ni-Hydrogen Storage Battery. Jpn. Pat. Appl. H02-263944, 26 October 1990. [Google Scholar]
- Nishikawa, S.; Takeshita, T. Hydrogen Storage Alloy Excellent in Corrosion Resistance and Negative Electrode for Secondary Battery Using It. Jpn. Pat. Appl. H05-156395, 22 June 1993. [Google Scholar]
- Kita, K.; Sugawara, K.; Wada, M.; Murai, T.; Isobe, T. Hydrogen Storage Alloy Enabling High Rate Discharge of Battery. Jpn. Pat. Appl. 2000-345261, 12 December 2000. [Google Scholar]
- Kita, K.; Sugawara, K.; Wada, M.; Murai, T.; Isobe, T. Hydrogen Storage Alloy. Jpn. Pat. Appl. 2000-073131, 7 March 2000. [Google Scholar]
- Okochi, T.; Omori, H.; Kanekawa, A. Production of Hydrogen Storage Alloy. Jpn. Pat. Appl. H07-188799, 25 July 1995. [Google Scholar]
- Nagase, I.; Shimizu, T.; Matsuyama, M. Production of Hydrogen Storage Alloy. Jpn. Pat. Appl. H07-305123, 21 November 1995. [Google Scholar]
- Nagase, I.; Nishinakagawa, T.; Kimura, Y. Production of Hydrogen Storage Alloy Powder. Jpn. Pat. Appl. 2000-073101, 7 March 2000. [Google Scholar]
- Matsukawa, A.; Odakawa, Y.; Fukuno, A.; Yamashita, S. Hydrogen Storage Alloy, Its Manufacture, and Secondary Battery. Jpn. Pat. Appl. 2000-265234, 26 September 2000. [Google Scholar]
- Matsukawa, A.; Odakawa, Y.; Fukuno, A.; Yamashita, S. Hydrogen Storage Alloy, Its Manufacture, and Secondary Battery. Jpn. Pat. Appl. 2000-265235, 26 September 2000. [Google Scholar]
- Sano, T.; Yamashita, S.; Cho, T.; Okada, M. Alloy Manufacturing Apparatus and Manufacturing Method of Hydrogen Storage Alloy. Jpn. Pat. Appl. 2002-331336, 19 November 2002. [Google Scholar]
- Miyaki, T.; Terao, K.; Takahashi, T.; Kabutomori, T.; Wakizaka, Y. Manufacture of Hydrogen Storage Alloy Electrode. Jpn. Pat. Appl. H09-213317, 15 August 1997. [Google Scholar]
- Miyaki, T.; Kabutomori, T.; Wakizaka, Y.; Morozumi, S.; Minegishi, T. Manufacture of Hydrogen Storage Alloy Electrode. Jpn. Pat. Appl. H08-203510, 9 August 1996. [Google Scholar]
- Miyaki, T.; Kabutomori, T. Hydrogen Storage Alloy Electrode. Jpn. Pat. Appl. H09-027321, 28 January 1997. [Google Scholar]
- Kabutomori, T.; Miyaki, T.; Terao, K. Electrode of Hydrogen Storage Alloy. Jpn. Pat. Appl. H09-213320, 15 August 1997. [Google Scholar]
- Miyaki, T.; Aoki, K.; Goto, T.; Ito, H. Hydrogen Storage Alloy, Producing Method Therefor and Hydrogen Storage Alloy Electrode Made of Same Alloy. Jpn. Pat. Appl. 2001-192756, 17 July 2001. [Google Scholar]
- Miyaki, T.; Aoki, K.; Goto, T.; Ito, H. Hydrogen Storage Alloy, Producing Method Therefor and Hydrogen Storage Alloy Electrode Made of Same Alloy. Jpn. Pat. Appl. 2001-192758, 17 July 2001. [Google Scholar]
- Miyaki, T.; Aoki, K.; Goto, T.; Ito, H. Hydrogen Storage Alloy, Producing Method Therefor and Hydrogen Storage Alloy Electrode Made of Same Alloy. Jpn. Pat. Appl. 2001-192757, 17 July 2001. [Google Scholar]
- Aoki, K.; Muro, M.; Kakihara, H. Method of Producing Hydrogen Storage Alloy and Cold Crucible Melting Apparatus. Jpn. Pat. Appl. 2010-242145, 28 October 2010. [Google Scholar]
- Hirose, Y.; Sasaki, S.; Hasegawa, H.; Hosono, U. Manufacture of Hydrogen Storage Alloy. Jpn. Pat. Appl. H09-180716, 11 July 1997. [Google Scholar]
- Nishi, T.; Oka, Y. Metal Porous Body, Its Manufacture, and Battery Electrode Plate Using the Same. Jpn. Pat. Appl. H09-153365, 10 June 1997. [Google Scholar]
- Fukui, A.; Imamura, M. Method for Recovering Valuable Metal from Scrap of Nickel Metal Hydride Secondary Battery. Jpn. Pat. Appl. 2003-041326, 13 February 2003. [Google Scholar]
- Toki, N.; Kudo, T.; Asano, S. Method of Recovering Metal from Used Nickel-Metal Hydride Battery. Jpn. Pat. Appl. 2010-174366, 12 August 2010. [Google Scholar]
- Harada, K.; Kato, M.; Saito, H.; Tsuchida, H.; Omura, T. Current Collector and Electrode Base Plate for Battery and Their Manufacturing Method. Jpn. Pat. Appl. 2006-310261, 9 November 2006. [Google Scholar]
- Sugikawa, H. Production of Metallic Sheet and Metallic Sheet Produced by This Method. Jpn. Pat. Appl. H09-287006, 4 November 1997. [Google Scholar]
- Sugikawa, H. Battery Can and Forming Material Thereof. Jpn. Pat. Appl. H05-021044, 29 January 1993. [Google Scholar]
- Sugikawa, H. Method for Manufacturing Electrode Plate for Battery, Electrode Plate Manufactured by the Method, and Battery Provided with the Electrode Plate. Jpn. Pat. Appl. 2000-173603, 23 June 2000. [Google Scholar]
- Kamiya, Y. Nickel-Metal Hydride Battery. Jpn. Pat. Appl. H08-227707, 3 September 1996. [Google Scholar]
- Oshima, Y.; Kimura, K.; Nishimura, K.; Takasaki, T.; Ikeda, T. Mixture Ink for Forming Foil-Shape Collector Positive Electrode of Nickel-Metal Hydride Secondary Battery. Jpn. Pat. Appl. 2013-138001, 11 July 2013. [Google Scholar]
- Tanaka, M.; Tokutake, N.; Kondo, Y.; Yamazaki, H.; Hirooka, M. Separator for Alkaline Battery and Alkaline Battery Using It. Jpn. Pat. Appl. H06-036753, 10 February 1994. [Google Scholar]
- Hirooka, M.; Arimura, T.; Kawatsu, Y. Separator for Alkaline Battery. Jpn. Pat. Appl. H08-273650, 18 October 1996. [Google Scholar]
- Sato, K.; Tanaka, M.; Hirooka, M. Separator for Alkaline Battery and Manufacture of the Separator. Jpn. Pat. Appl. 2000-106162, 11 April 2000. [Google Scholar]
- Abe, M.; Tokuyama, E.; Okazaki, D.; Kokaji, T. Ball Mill Device, Method for Producing Hydrogen Storage Alloy Powder Using the Device and Hydrogen Storage Alloy Powder. Jpn. Pat. Appl. 2006-111909, 27 April 2006. [Google Scholar]
- Osumi, Y.; Kato, A.; Suzuki, H.; Nakane, M.; Miyake, Y. Hydrogen absorption-desorption characteristics of mischmetal-nickel-aluminum alloys. J. Less Comm. Met. 1979, 66, 67–75. [Google Scholar] [CrossRef]
- Osumi, Y.; Suzuki, H.; Kato, A.; Oguro, K.; Nakane, M. Development of misch metal-nickel and titanium-cobalt hydrides for hydrogen storage. J. Less Comm. Met. 1980, 74, 271–277. [Google Scholar] [CrossRef]
- Osumi, Y.; Suzuki, H.; Kato, A.; Oruro, K. Hydrogen absorption-desorption characteristics of MnNi5−xAl(Mn)y−zMz and MmNi5−xAlMnyM2 alloys (Mm = misch metal). J. Less Comm. Met. 1983, 89, 287–292. [Google Scholar] [CrossRef]
- Osumi, Y. Suiso Kyuzou Goukin; Agune Co. Ltd.: Tokyo, Japan, 1993. [Google Scholar]
- Kadir, K.; Sakai, T.; Uehara, I. Synthesis and structure determination of a new series of hydrogen storage alloys; RMg2Ni9 (R = La, Cel, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers. J. Alloys Compd. 1997, 257, 115–121. [Google Scholar] [CrossRef]
- Kuriyama, N.; Sakai, T.; Miyamura, H.; Uehara, H. Manufacture of Hydrogen Storage Electrode. Jpn. Pat. Appl. H06-283164, 7 October 1994. [Google Scholar]
- Tsukahara, M.; Takahashi, K.; Mishima, T.; Isomura, A.; Sakai, T.; Miyamura, H.; Uehara, H. Hydrogen Occluding Alloy and Hydrogen Occluding Alloy Electrode. Jpn. Pat. Appl. H07-268513, 17 October 1995. [Google Scholar]
- Sakai, T.; Madono, J.; Miyamura, H.; Uehara, H. Hydrogen Storage Alloy and Electrode of It. Jpn. Pat. Appl. H07-278708, 24 October 1995. [Google Scholar]
- Sakai, T.; Iwaki, T. Manufacture of Alkaline Secondary Battery and Catalytic Electrode Body. Jpn. Pat. Appl. H07-282860, 27 October 1995. [Google Scholar]
- Kariimu, K.; Sakai, T.; Takeshita, H.; Uehara, H. New Hydrogen Storage Alloy and Hydrogen Electrode Using the Alloy. Jpn. Pat. Appl. H11-217643, 10 August 1999. [Google Scholar]
- Sakai, T.; Takeshita, H.; Uehara, H.; Yamashita, I. Alkaline Secondary Battery Negative Electrode and Alkaline Secondary Battery. Jpn. Pat. Appl. H11-307088, 5 November 1999. [Google Scholar]
- Osawa, M.; Tsunokake, S.; Katsura, S.; Iwaki, T.; Sakai, T. Hydrogen Storage Alloy and Nickel-Hydrogen Battery. Jpn. Pat. Appl. 2015-113522, 22 June 2015. [Google Scholar]
- Mishima, R.; Sekine, T.; Sakai, T.; Ishikawa, H.; Miyamura, H.; Uehara, H. Hydrogen Storage Alloy and Its Production. Jpn. Pat. Appl. H06-145851, 27 May 1994. [Google Scholar]
- Sakai, T.; Fukunaga, H.; Tanaka, T. Hydrogen Storage Alloy, and Electrode Using the Same. Jpn. Pat. Appl. 2003-059784, 6 March 2003. [Google Scholar]
- Sakai, T.; Fukunaga, H.; Matsumoto, N.; Tanaka, T. Rectangular Nickel-Hydrogen Battery. Jpn. Pat. Appl. 2005-235421, 2 September 2005. [Google Scholar]
- Kanemoto, M.; Kakeya, T.; Kurokuzuhara, M.; Watada, M.; Ozaki, T.; Sakai, T. Hydrogen Storage Alloy and Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. 2008-163421, 17 July 2008. [Google Scholar]
- Ozaki, T.; Kanemoto, M.; Kakeya, T.; Kurokuzuhara, M.; Watada, M.; Sakai, T. Nickel-Hydrogen Storage Battery. Jpn. Pat. Appl. 2009-163986, 23 July 2009. [Google Scholar]
- Kondo, T.; Yamamizu, T.; Sakai, T.; Kuriyama, N. Nickel Hydride Secondary Battery. Jpn. Pat. Appl. 2002-063889, 28 February 2002. [Google Scholar]
- Kondo, T.; Yamamizu, T.; Sakai, T. Nickel Hydrogen Secondary Battery. Jpn. Pat. Appl. 2002-157988, 31 May 2002. [Google Scholar]
- Iwaki, T.; Yao, M.; Sakai, T.; Okuno, K.; Kato, M.; Boku, T. Hydrogen Absorbing Alloy Negative Electrode for Alkaline Battery. Jpn. Pat. Appl. 2008-117579, 22 May 2008. [Google Scholar]
- Mukai, T.; Takasaki, T.; Sakai, T.; Iwaki, T.; Tsutsumi, K.; Nishimura, K. Alloy Negative Electrode for Fiber Battery. Jpn. Pat. Appl. 2010-160912, 22 July 2010. [Google Scholar]
- Takasaki, T.; Nishimura, K.; Fukunaga, H.; Tsutsumi, K.; Saito, M.; Mikai, T.; Sakai, T. Cobalt-Free Alkaline Secondary Battery. Jpn. Pat. Appl. 2012-204177, 22 October 2012. [Google Scholar]
- Hashimoto, H.; Son, M.; Abe, T. Hydrogen Storage Material, and Production Method Therefor. Jpn. Pat. Appl. 2004-204309, 22 July 2004. [Google Scholar]
- Sakai, T.; Iwaki, T. Electrode for Secondary Battery, and the Secondary Battery Using the Same. Jpn. Pat. Appl. 2003-326738, 18 September 2003. [Google Scholar]
- Towata, S.; Ito, K.; Kadoura, H. Surface Treatment of Hydrogen Occlusion Alloy Material, Activation Treatment of Hydrogen Occlusion Alloy Electrode, Activating Solution, and Hydrogen Occlusion Alloy Electrode Having Excellent Initial Activity. Jpn. Pat. Appl. H08-291391, 5 November 1996. [Google Scholar]
- Towata, S.; Ito, K.; Yamakawa, S.; Abe, K.; Oya, Y.; Morishita, S.; Kawase, Y. Surface Treatment Method of Hydrogen Storage Alloy by Steam and Alloy Obtained Thereby. Jpn. Pat. Appl. H09-180715, 11 July 1997. [Google Scholar]
- Yoshida, T.; Miyano, K.; Itou, T.; Towata, S.; Ito, K. Hydrogen Storage Alloy Unit and Manufacture Thereof. Jpn. Pat. Appl. H10-012227, 16 January 1998. [Google Scholar]
- Morishita, S.; Kondo, Y.; Towata, S.; Abe, K.; Muta, M.; Kinoshita, K. Nickel Positive Electrode Active Material for Alkali Storage Battery. Jpn. Pat. Appl. H10-074514, 17 March 1998. [Google Scholar]
- Kondo, Y.; Morishita, S.; Oya, Y.; Towata, S.; Muta, M.; Kinoshita, K. Stabilizing Method for Hydrogen Storage Material. Jpn. Pat. Appl. H10-130860, 19 May 1998. [Google Scholar]
- Muta, M.; Kinoshita, K.; Morishita, S.; Towata, S. Conductive Powder for Hydrogen Storage Alloy Negative Electrode. Jpn. Pat. Appl. H11-111299, 23 April 1999. [Google Scholar]
- Morishita, S.; Towata, S.; Imaizumi, J.; Usui, T. Nickel Hydroxide for Positive Electrode Active Material of Alkaline Secondary Battery, Alkaline Secondary Battery Using Same, Its Characteristics Evaluation Method and Manufacturing Method. Jpn. Pat. Appl. 2002-208400, 26 July 2002. [Google Scholar]
- Kobayashi, T.; Kondo, Y.; Matsuo, H.; Sasaki, I.; Ito, Y.; Nozaki, H.; Nonaka, T.; Senoo, Y.; Ukiyou, Y.; Ito, M. Alkaline Storage Battery. Jpn. Pat. Appl. 2005-347089, 15 December 2005. [Google Scholar]
- Yamawaki, K.; Kuwajima, S.; Suzuki, N.; Shirogami, T. Bipolar Metal-Hydrogen Secondary Battery. Jpn. Pat. Appl. H07-014618, 17 January 1995. [Google Scholar]
- Tsutsumi, K. Laminate Battery and Laminate Battery System. Jpn. Pat. Appl. 2013-080698, 2 May 2013. [Google Scholar]
- Ueda, K.; Tsukahara, M.; Kamiya, Y.; Kikuchi, S. Manufacturing Method for Mg-Cu Composite Material and Hydrogen Occluding Alloy. Jpn. Pat. Appl. 2005-178095, 7 July 2005. [Google Scholar]
- Kamiya, Y.; Tsukahara, M. Hydrogen Storage Alloy and Hydrogen Storage Vessel. Jpn. Pat. Appl. 2006-028632, 2 February 2006. [Google Scholar]
- Takahashi, K.; Tsukahara, M.; Mishima, T.; Isomura, A. Hydrogen Storage Alloy Electrode and Manufacture Thereof. Jpn. Pat. Appl. H08-236107, 13 September 1996. [Google Scholar]
- Tsukahara, M.; Takahashi, K.; Mishima, T.; Isomura, A. Hydrogen Storage Alloy and Hydrogen Storage Alloy Electrode. Jpn. Pat. Appl. H08-269655, 15 October 1996. [Google Scholar]
- Kamiya, Y.; Tsukahara, M.; Takahashi, K.; Isomura, A.; Sakai, T.; Takeshita, H. Hydrogen Storage Alloy, Hydrogen Storage Alloy Electrode and Production of Hydrogen Storage Alloy. Jpn. Pat. Appl. 2000-096179, 4 April 2000. [Google Scholar]
- Tsukahara, M.; Kamiya, Y.; Takahashi, K.; Isomura, A.; Sakai, T.; Kuriyama, N.; Takeshita, H. Production of Hydrogen Storage Alloy, Alloy Thereof and Electrode Using the Alloy. Jpn. Pat. Appl. H11-106847, 20 April 1999. [Google Scholar]
- Furukawa, K.; Sakamoto, K.; Mori, H.; Kishimoto, M.; Okabe, K. Sealed Nickel-Hydrogen Secondary Battery. Jpn. Pat. Appl. 2006-147327, 8 June 2006. [Google Scholar]
- Furukawa, K.; Harada, Y. Battery. Jpn. Pat. Appl. 2007-234486, 13 September 2007. [Google Scholar]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Hydrogen Storage Alloys Having Improved Cycle Life and Low Temperature Operating Characteristics. U.S. Patent 7,344,677, 18 March 2008. [Google Scholar]
- Sakamoto, K.; Bando, H.; Mori, H.; Okabe, K. Nickel Hydrogen Battery and Its Manufacturing Method. Jpn. Pat. Appl. 2007-012573, 18 January 2007. [Google Scholar]
- Morimoto, K.; Saito, H.; Inaba, Y. Method of Manufacturing Nickel Metal Hydride Storage Battery. Jpn. Pat. Appl. 2010-010097, 14 January 2010. [Google Scholar]
- Sakatani, T.; Sugui, H.; Ochi, M.; Kawase, R. Nickel Hydrogen Storage Battery and Battery System. Jpn. Pat. Appl. 2014-089896, 15 May 2014. [Google Scholar]
- Sumiyama, S.; Shibuya, N.; Okabe, A. Nickel Hydrogen Storage Battery. Jpn. Pat. Appl. 2015-173058, 1 October 2015. [Google Scholar]


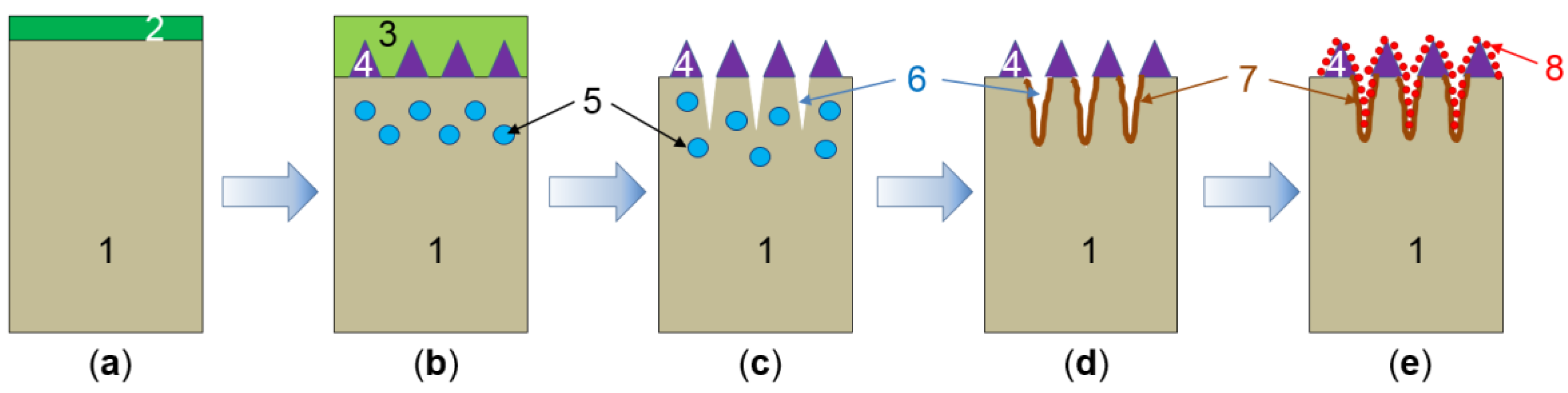

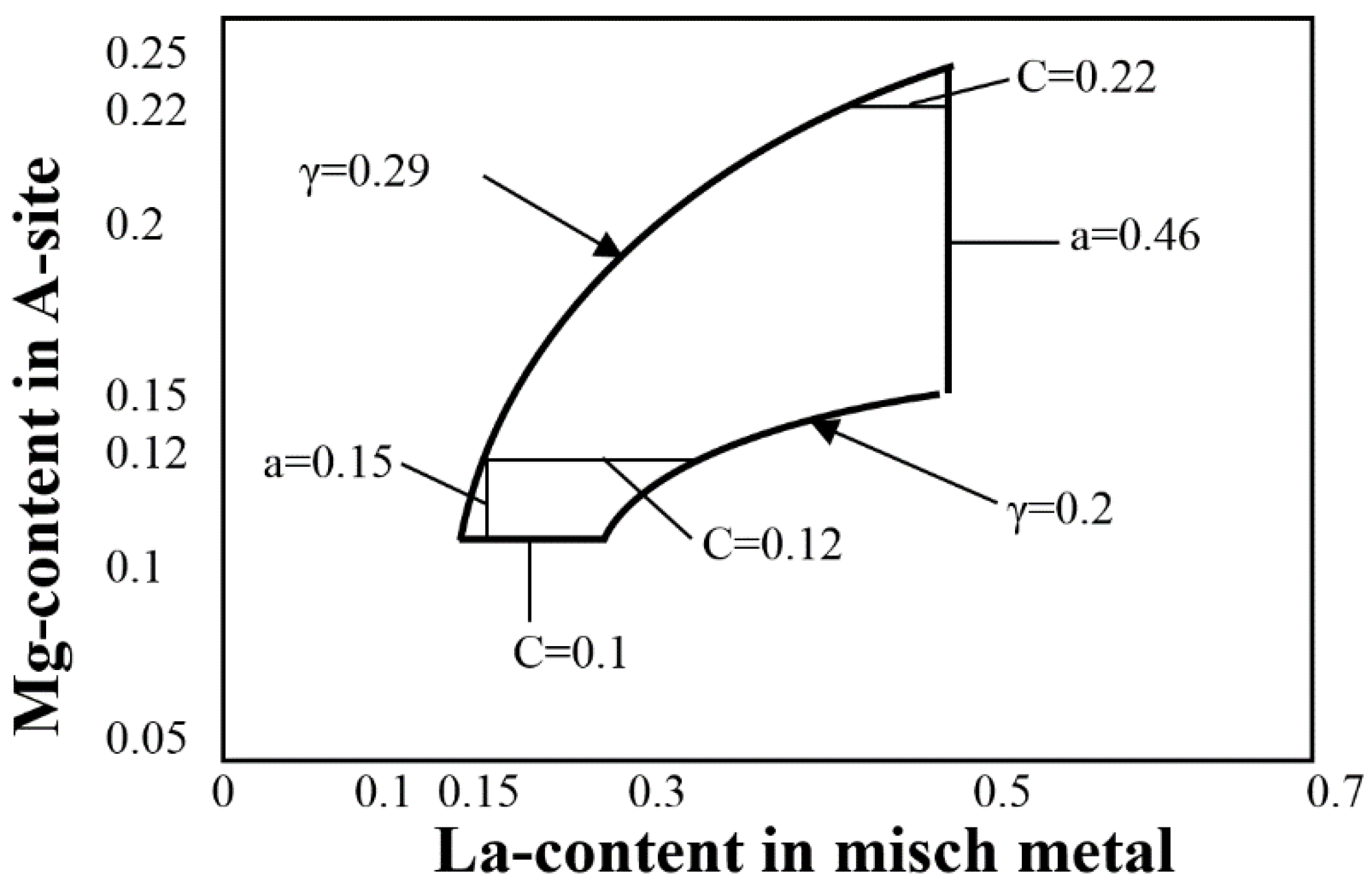

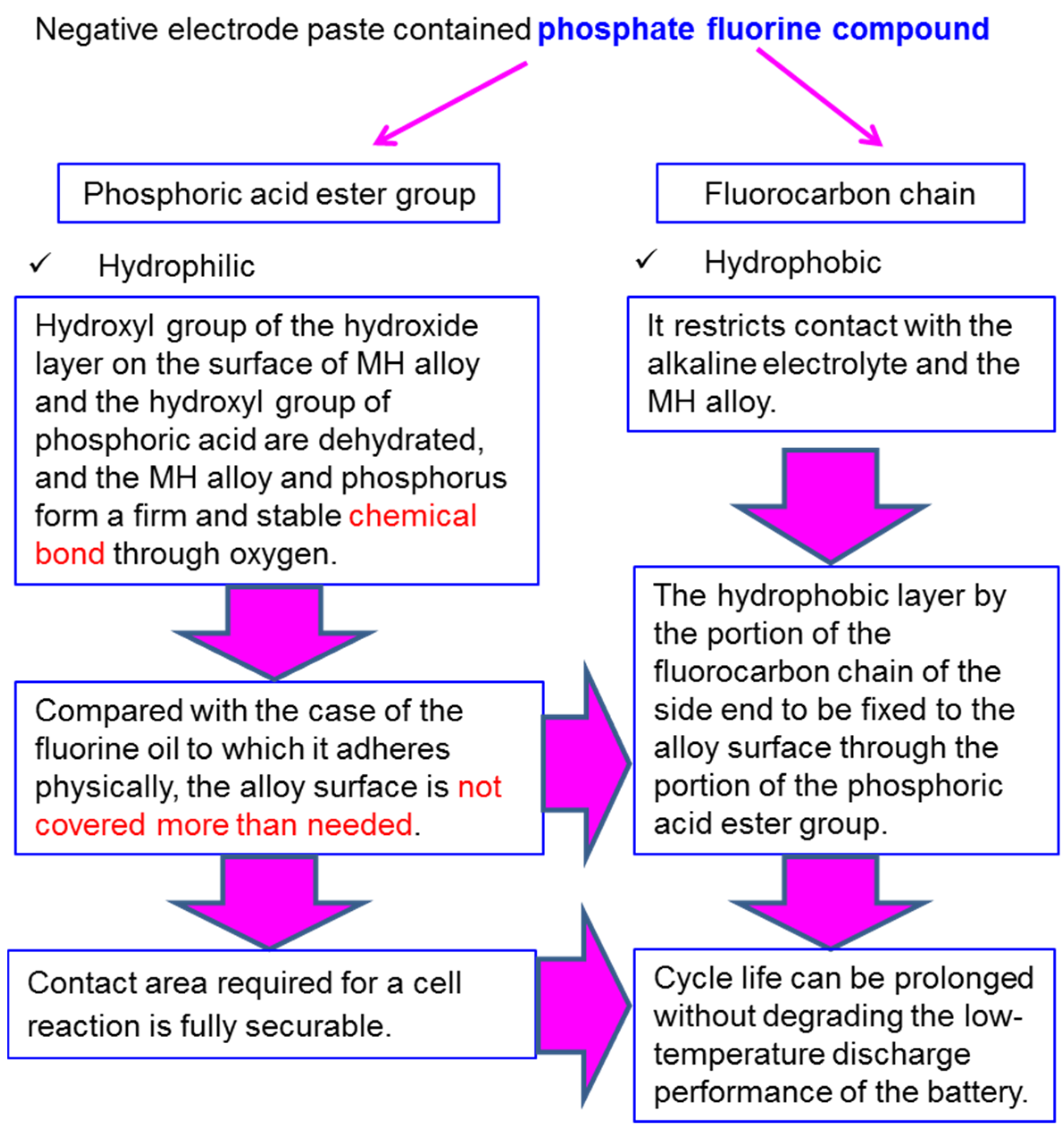
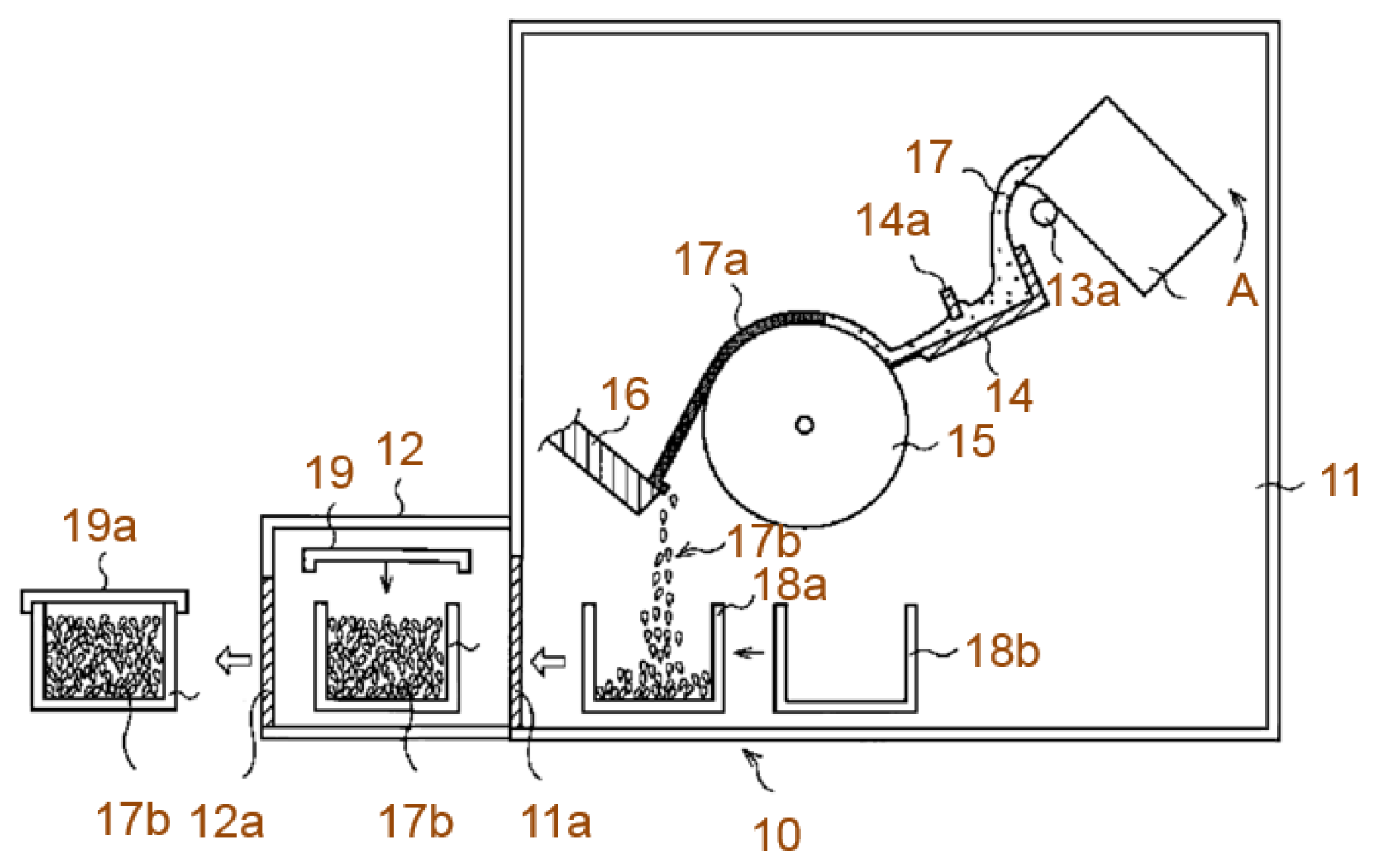

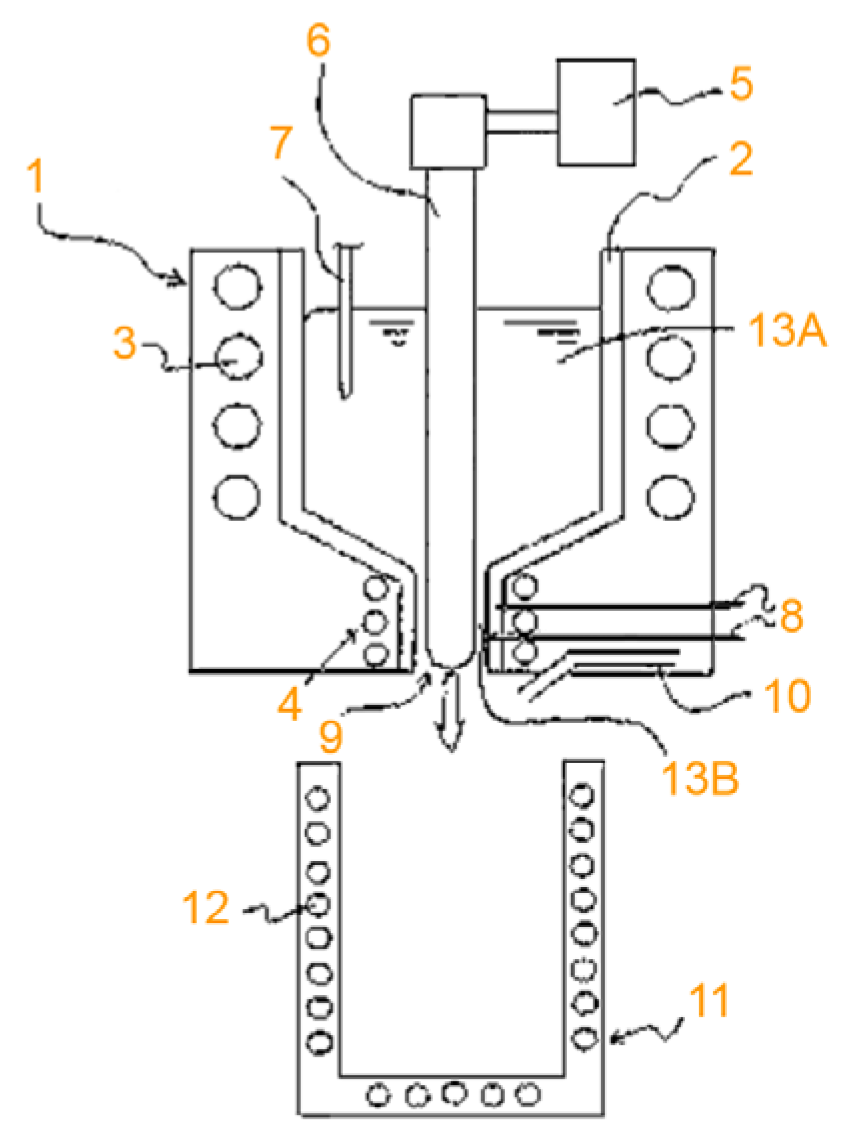
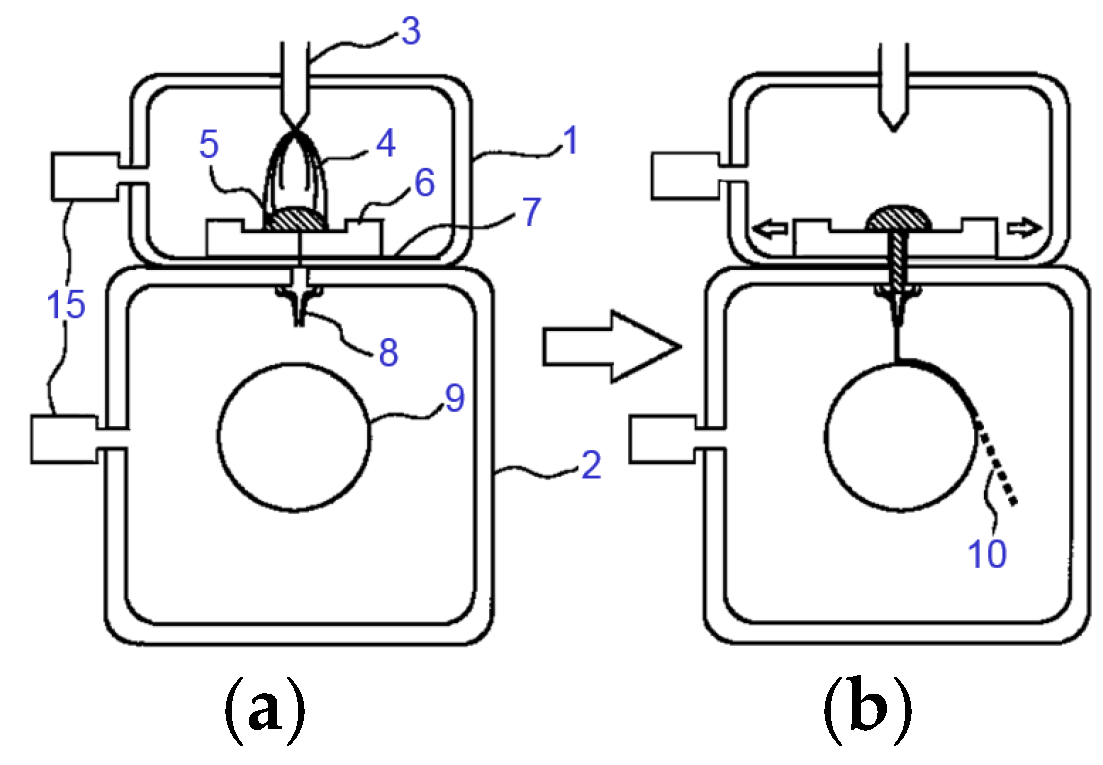
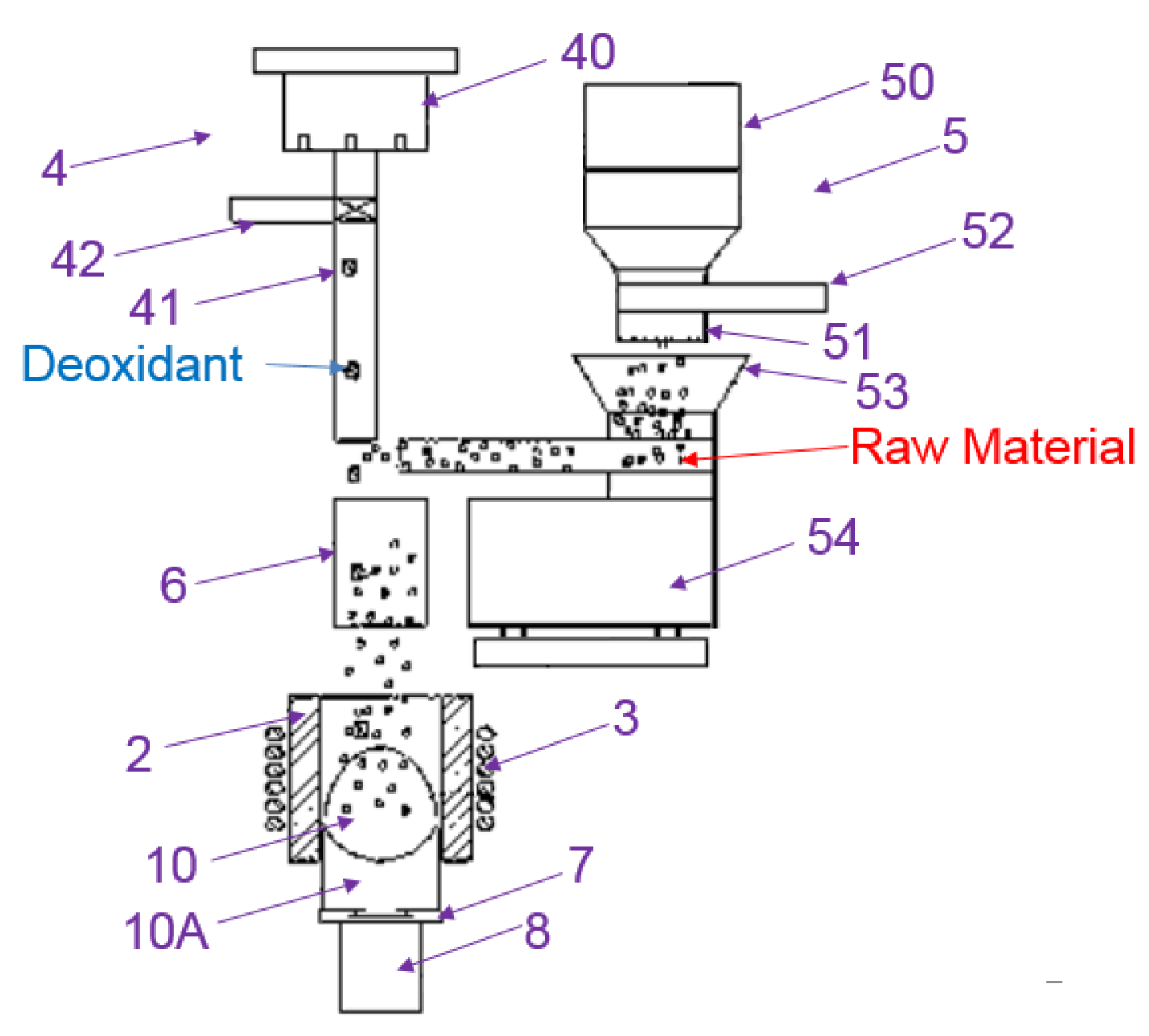
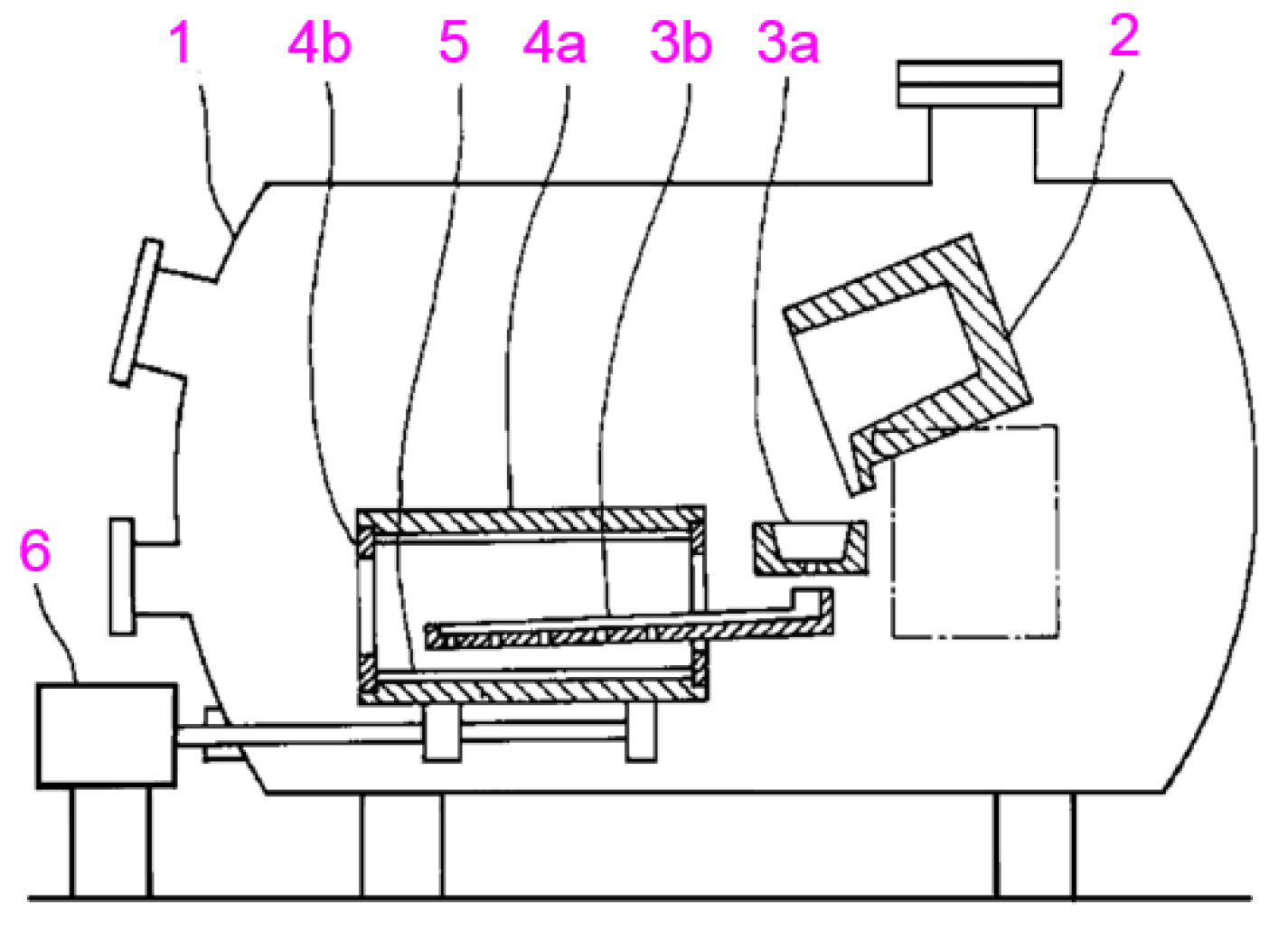
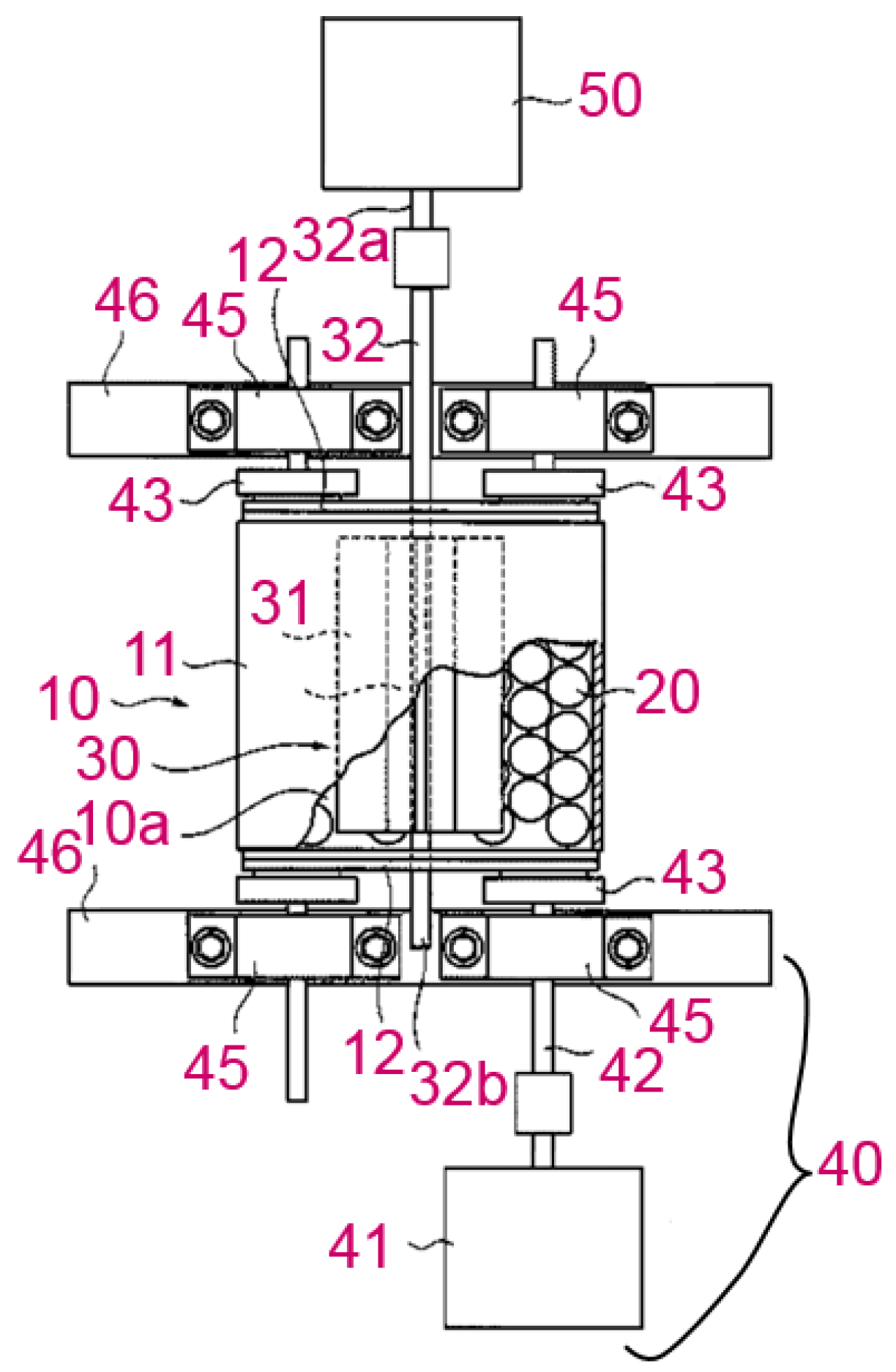
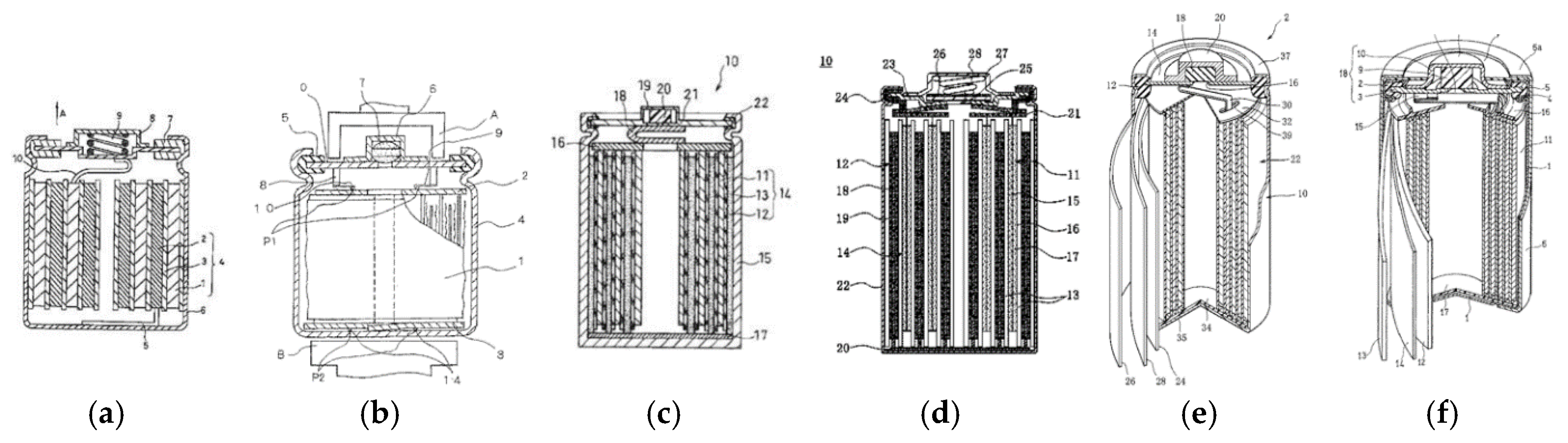
| Company Name | Established Date | Main Business | Trademark | Employee | Remark |
|---|---|---|---|---|---|
| Matsushita (Panasonic) | 1918 | Home appliances and electronics |  | Consolidated: 254,084 | - |
| SANYO Electric | 1947 | Home appliances and electronics |  | Merge into Panasonic in December 2009 | Ni/MH division was sold to FDK in January 2010 |
| Hitachi Maxell | 1960 | Energy, industrial materials and electronic appliance/consumer products |  | Consolidated: 4053 | - |
| Yuasa | 1918 | Storage batteries and other products |  | Consolidated: 14,506 as GS Yuasa | Managed integration through the establishment of a holding company (GS Yuasa Corporation) in April 2004. |
| Toshiba Battery | 1954 | Storage batteries and other products |  | Merge into Toshiba Home Appliances, and dissolved in 2009 | Ni/MH div. was sold to SANYO in 2000 |
| FDK | 1950 | Various kinds of batteries, rechargeable batteries, battery devices, electronic components, and devices |  | 4917 consolidated basis | Acquired Sanyo Energy Twicell Co., Ltd. and Sanyo Energy Tottori Co., Ltd. in 2000 |
| Furukawa Battery | 1950 | Various kinds of batteries, rechargeable batteries, battery devices, electronic components, and devices |  | Consolidated: 1999 | - |
| Japan Storage Battery | 1917 | Storage batteries and other products |  | Consolidated: 14,506 as GS Yuasa | Managed integration through the establishment of a holding company (GS Yuasa Corporation) in April 2004. |
| Shin-Kobe Electric Machinery | 1969 (consolidation) | Various kinds of batteries, rechargeable batteries, battery devices, electronic components and devices, and polymer science materials |  | Merge into Hitachi Chemical, and dissolved in Jan. 2016 | Subsidiary of Hitachi Chemical in March 2012 |
| Tanaka Chemical | 1957 | Positive electrode materials for rechargeable batteries, catalyst materials, inorganic chemical products |  | 180 | - |
| Mitsui Kinzoku | 1950 | Manufacturing and sales of functional materials, electronic materials, and automotive parts/components, etc. |  | Consolidated: 10,804 | - |
| Santoku | 1949 | Rare earths |  | 229 | - |
| JMC | 1917 | Ferroalloy, MH and non-ferrous metals, ferrite and ceramic products, geothermal energy consulting service, and electric power generation |  | Consolidated: 921 | - |
| Shin-Etsu Chemical | 1926 | PVC/Chlor-Alkali, silicones, specialty chemicals, electronics & functional material, and diversified |  | Consolidated: 18,276 | - |
| AIST | 1952 | AIST takes initiative as a leading research institute for innovation while placing its major emphasis on research that offers practical benefits to the world |  | 2929 | Reformed in 2001 (National Institute of Advanced Industrial Science and Technology) |
| Type | MHS | MHM | |
|---|---|---|---|
| Main components | Positive plate | Oxy nickel hydroxide (pocket type) | |
| Negative plane | Metal hydride (pocket type) | ||
| Separator | Synthetic resin | ||
| Cell container | Synthetic resin (translucent) | ||
| Electrolyte | Potassium hydroxide | ||
| Specific gravity (20 °C) | 1.21 | ||
| Capacity (Ah) | Range | 20–1000 | |
| Discharge rate (h) | 5 | ||
| Voltage (V/cell) | Nominal | 1.2 | |
| Floating charge | 1.39 | 1.38 | |
| Recommended back-up time | More than 60 min | More than 30 min | |
| Expected life | More than 15 years | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouchi, T.; Young, K.-H.; Moghe, D. Reviews on the Japanese Patent Applications Regarding Nickel/Metal Hydride Batteries. Batteries 2016, 2, 21. https://doi.org/10.3390/batteries2030021
Ouchi T, Young K-H, Moghe D. Reviews on the Japanese Patent Applications Regarding Nickel/Metal Hydride Batteries. Batteries. 2016; 2(3):21. https://doi.org/10.3390/batteries2030021
Chicago/Turabian StyleOuchi, Taihei, Kwo-Hsiung Young, and Dhanashree Moghe. 2016. "Reviews on the Japanese Patent Applications Regarding Nickel/Metal Hydride Batteries" Batteries 2, no. 3: 21. https://doi.org/10.3390/batteries2030021
APA StyleOuchi, T., Young, K.-H., & Moghe, D. (2016). Reviews on the Japanese Patent Applications Regarding Nickel/Metal Hydride Batteries. Batteries, 2(3), 21. https://doi.org/10.3390/batteries2030021