Abstract
In recent years, the energy revolution has driven the rapid development of lithium-ion batteries (LIBs). A fire suppression system capable of rapidly and effectively extinguishing LIB fires constitutes the last line of defense for ensuring the safe operation of the LIB industry. In this study, an experimental platform simulating the storage environment of LIBs in energy-storage stations was constructed, and liquid nitrogen (LN) was employed to conduct fire suppression tests on LIBs. The effective utilization of 17.4 kg of LN during the suppression process inside the battery module was quantified. In addition, fire compartments were established within the battery module, and a strategy for enhancing the LN suppression effectiveness was proposed. The results indicate that, without intervention, the thermal runaway propagation (TRP) rate within the LIB module gradually accelerates. After LN injection, the effective utilization of LN for extinguishing individual LIBs decreases progressively along the sequence of TRP. Creating fire compartments inside the PACK using 6 mm aerogel blankets effectively reduces the transfer of energy from the region undergoing thermal runaway (TR) to other regions, while simultaneously enhancing the extinguishing performance of LN. Under the same LN dosage, the introduction of fire compartments increases the effective utilization from 0.037 to 0.051. However, as the compartment volume decreases, the degree of improvement in LN utilization is reduced. This work is expected to provide guidance for the engineering application of LN-based fire suppression systems to inhibit LIB TR and its propagation.
1. Introduction
Driven by the energy crisis and climate change, carbon-neutrality targets have been proposed in more than half of the countries worldwide [1,2], and sustainable, low-carbon energy resources are being actively pursued [3]. As a representative of emerging energy carriers, LIBs are characterized by high energy density, long service life, excellent cycling performance, and negligible memory effect [4,5,6], and have therefore been widely deployed in electric vehicles and stationary energy-storage systems. However, owing to their specific chemical compositions and electrochemical energy-storage characteristics, LIBs may undergo rupture, ignition, or even explosion when subjected to abusive conditions such as overheating, overcharging, penetration, or compression [7]. With the rapid development of energy-storage stations toward larger scale and higher energy density, the consequences of fire incidents have become increasingly severe. For example, a fire occurred at the Gateway energy-storage station in California, USA, on 15 May 2024, and persisted for nearly two weeks with multiple re-ignitions. Therefore, it is of critical importance to develop fire-suppression strategies capable of rapidly and effectively mitigating TR.
For LIB fire suppression technologies, current research can be broadly classified into solid extinguishing agents, gaseous extinguishing agents, water-based agents, and novel agents. Solid agents such as dry powder and aerosol have been shown to lack sufficient cooling capability. Meng et al. [8] attempted to extinguish LiFePO4 battery fires using a dry-powder agent and found that, under certain conditions, the fire could be extinguished; however, the suppression effect was limited. Only the regions in direct contact with the battery experienced noticeable cooling, and the internal thermal runaway reactions could not be completely suppressed. In addition, shorter discharge distance and longer discharge duration were found to enhance the suppression performance, whereas the spray angle had little influence.
Gaseous extinguishing agents mainly include CO2, heptafluoropropane (HFC-227ea) and perfluorohexanone (Novec1230). Xu et al. [9] conducted experiments on the combustion and explosion characteristics and fire suppression of 94 Ah Li(NiCoMn)O2 batteries to evaluate the performance of CO2, HFC-227ea and water mist. The results indicated that all three agents were capable of suppressing LIB fires; however, water mist outperformed the other two agents in both suppression effectiveness and cooling capability. Liu et al. [10] reported that Novec1230 initially exhibited a negative inhibition effect in LIB fires. It first promoted the progression of thermal runaway; as the injection quantity increased, this negative effect was converted into a positive inhibition effect and was gradually enhanced. Zhang et al. [11] proposed a synergistic suppression strategy in which gaseous agents (Novec1230, CO2 and HFC-227ea) were combined with water mist to suppress LIB fires. Experimental results showed that the composite agents provided stronger suppression than any single agent, and among the composite agents, the combination of Novec1230 and water mist was the most effective. Although gaseous agents can effectively inhibit flaming combustion, they are not suitable for LIB fires if adequate cooling cannot be provided.
Water-based extinguishing agents are typically formulated by adding additives to water to enhance its fire-extinguishing performance, such as water gel, F-500 and foam concentrates. Liu et al. [12] compared the suppression effects of water and water gel on LIB TR, and it was shown that, owing to its special three-dimensional network structure, water gel exhibited superior performance in extinguishing LIB fires. F-500 encapsulator technology, developed by Hazard Control Technologies (HCT) in the United States, has been shown to significantly enhance the extinguishing effectiveness of plain water and to improve its penetration and cooling capabilities when F-500 is mixed with water at an appropriate ratio. In comparative experiments [13,14], F-500 aqueous solutions demonstrated better extinguishing and cooling performance than pure water, while requiring a smaller amount of water. Andersson et al. [15] evaluated the performance of foam extinguishing agents on LiFePO4 battery fires and found that foam agents could extinguish open flames; low-expansion foam, with its higher water content compared with high-expansion foam, provided superior cooling. Overall, compared with solid and gaseous agents, liquid extinguishing agents offer greater advantages in suppressing lithium-ion battery fires.
Fire suppression agents represented by LN have emerged in recent years as a novel technology for extinguishing LIB fires. Owing to its strong cooling and inerting capabilities, LN has been widely applied in confined spaces such as coal mines and cable tunnels [16,17,18]. Wang et al. [19] selected ten commonly used extinguishing agents—water, water mist, dry powder, HFC-227ea, CO2, a water-based agent, 3% aqueous film-forming foam, HFC-227ea, water gel, and LN—and conducted comparative tests under identical experimental conditions. By establishing an evaluation and scoring system, LN was verified to provide the best overall suppression performance. To obtain a comprehensive understanding of the suppression mechanism of LN on LIB fires, Huang et al. [20] carried out extinguishing experiments on a commercial 2200 mAh 18650-type LIB module. The following conclusions were drawn: LN can successfully slow down or even block heat transfer between cells, thereby delaying the onset of TRP; cells located further downstream in the propagation chain require less LN for cooling; during the experiments, the maximum cooling rate of LN reached 34.25 °C/s; and the suppression mechanism of LN is mainly reflected in two aspects, namely protecting unreacted cells and cooling already reacted high-temperature cells, thus interrupting the rapid TR propagation between cells. Wang et al. [21] further investigated the effects of LN injection mass, injection position, state of charge (SOC), and heating power on the suppression of LIB TR in a confined space. The following conclusions were obtained: (1) the average cooling rate of cells in a confined space is lower than that in an open space, indicating more difficult heat dissipation; (2) LN can cool not only the TR cell but also its neighboring cells; (3) the cooling performance of LN is enhanced as the injection mass increases; (4) top injection is more effective than side injection; (5) the average cooling rate of LN is negatively correlated with SOC and positively correlated with heating power; and (6) reducing the spacing between cells enhances inter-cell heat transfer.
Previous studies on fire suppression agents have mainly focused on conventional agents, all of which exhibit certain limitations when dealing with LIB fires. Although extensive research has been conducted on liquid nitrogen fire extinguishing agents. These studies have confirmed the feasibility of liquid nitrogen in mitigating battery thermal runaway. However, liquid nitrogen is highly prone to vaporization at room temperature, and few studies have focused on how to maximize the cooling efficiency of liquid nitrogen. LN is stored at a very low temperature (−196 °C) and vaporizes rapidly after being released into the ambient air, improving its effective utilization on the basis of existing studies has become a new research focus. In this work, by establishing fire compartments, the effective action area of LN is reduced, its cooling capacity per unit volume is increased, and thus the cooling effectiveness of LN is improved to varying degrees. This study proposes a novel synergistic strategy that employs physical compartmentalization to overcome the rapid vaporization of LN.
2. Experimental Section
2.1. Battery Parameters
LIBs with 100 Ah capacity (EVE Energy Co., Ltd., Huizhou, China) were employed in this study, configured with a LiFePO4 cathode and a carbon-based anode. The electrolyte used in the cell is a mixture of lithium salt solution (LiPF6) and organic solvents. The cell had a nominal voltage of 3.2 V. It measured 135 mm in length, 29 mm in width, and 215 mm in height, with a mass of 1.735 ± 0.001 kg. Charge–discharge protocols were administered through a Neware battery test system, where cells were first discharged at 1C-rate until a 2.0 V cutoff voltage was reached, followed by constant-current charging at 1C-rate until 100% SOC. Each experimental group comprised four cells as a module.
2.2. Experimental Apparatus
Figure 1 illustrates a schematic of the LN fire suppression experimental platform. A custom-designed battery rack was used as the dedicated test platform and was placed inside a container equipped with ventilation and smoke-exhaust functions. A single battery box was selected as the test object, as shown in Figure 1b. The battery box is 0.62 m in length, 0.41 m in width, and 0.26 m in height, and was positioned at the center of the rack to facilitate observation. The LN fire suppression system consists of an LN storage unit and a driving unit, mainly comprising a 300 L Dewar tank and four nitrogen cylinders. A liquid level gauge was installed on the Dewar tank to monitor the remaining LN volume in the storage tank; the LN nozzle was a commercial 8 mm stainless steel nozzle, which was horizontally injected into the battery box through the top opening. Before the experiment, nitrogen cylinders were opened, the operating pressure was adjusted to 2 MPa, and the liquid outlet valve of the Dewar tank was opened to initiate injection. Based on the changes in the liquid level gauge and recorded injection time, the mass flow rate of LN in this experiment was determined to be 0.063 kg/s; the density of LN was 810 kg/m3, its dynamic viscosity was 0.038 mPa·s, assuming continuous flow of LN in the pipeline, the calculated Reynolds number was 1.4 × 105, and the exit velocity of LN from the nozzle was 0.44 m/s. A 450 W heating plate was used to heat the battery and trigger TR. During the tests, the heating plate was attached to the front face of the battery, and the battery was fixed on both sides using clamps. The temperature and voltage acquisition system mainly consists of data acquisition devices, thermocouples, a thermocouple spot welder, and electrical wiring. The temperature and voltage signals were recorded using an LR8450 data logger (HIOKI, Tokyo, Japan) and an MIK-R6000C (Meacon, Hangzhou, China) paperless recorder, respectively. Temperature data were recorded every 1 s by the paperless recorder. Thermocouple wires were welded using a thermocouple spot welder and were employed for temperature measurement. K-type thermocouples were used in the experiments, and electrical wires were used to transmit the voltage signals in real time. According to the information provided by the manufacturer, the optimal measurement range of the K-type thermocouple used in the experiments is −50 °C to 800 °C, and data beyond this range can be measured by it for a short period of time. Thermocouple probes were tightly fixed to the battery surfaces using high-temperature-resistant Teflon tape during the experiments, and then covered with a layer of high-temperature-resistant fiberglass aluminum foil tape to ensure that the thermocouple probes would not fall off during the experiments and avoid direct contact between the thermocouples and LN. A high-definition video camera was used to record the entire experimental process.
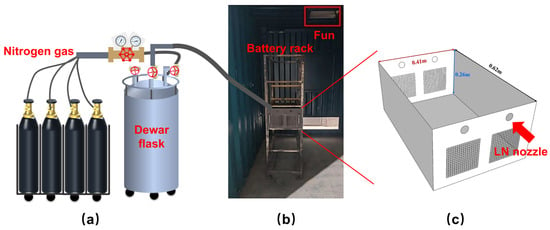
Figure 1.
Schematic diagram of LN fire extinguishing experimental platform: (a) LN fire extinguishing system, (b) battery rack, and (c) geometry of battery box.
2.3. Experimental Setup
According to previous studies, 10–20 kg of LN can effectively suppress TRP within a module [22]. In this study, an LN dosage of 17.4 kg was selected based on the level indicator of the Dewar tank. The experimental setup is shown in Table 1. Test 1 was designed as a baseline test without LN, in order to evaluate the TR behavior and TRP characteristics. Test 2 was used to investigate the suppression effect of 17.4 kg LN on TRP in the LIB module. Tests 3–5 were designed to examine the enhancement of LN suppression performance by different compartment configurations, in which a commercial 6 mm aerogel blanket was adopted as the barrier material. Four layouts of fire compartments were proposed, as illustrated in Figure 2. It should be noted that once TR occurred in a cell, the heating plate was switched off. In this experiment, when the following conditions are simultaneously met: the decrease in battery voltage exceeds 25% of the initial voltage, the temperature rise rate of the battery side temperature Ts exceeds 1 °C/s, and lasts for more than 3 s, the battery is considered to have undergone thermal runaway. In the suppression tests, LN injection was initiated immediately after the onset of TR. In the compartment experiments, two thermocouples were installed in each compartment, and the average of their readings was taken as the gas temperature within that compartment. A battery module consisting of four cells was used as the test object, and the mass of each cell was measured prior to the experiments. The module was placed at the center of the PACK. The thermocouple arrangement is shown in Figure 3. For each cell, thermocouples were mounted on the front, side, rear, and safety-valve positions, and denoted sequentially from cell 1# to cell 4# as , , , , , , , , , , , and , , , . Two additional thermocouples were installed above and below the PACK to record the ambient temperature, denoted as and , respectively. The voltages of the four cells were recorded as , , and . Each experimental group was repeated at least three times, and key parameters such as the heat absorption of liquid nitrogen and effective utilization rate during the fire extinguishing process were evaluated. Relative Standard Deviation (RSD) was used to quantify uncertainty; a smaller RSD indicates higher reliability and accuracy of the data. During the tests, the calculated RSD values of most key parameters were less than 15%, while only the RSD of the maximum surface temperature of the battery was approximately 20% to 30%, indicating that the experimental results were consistent and reproducible under the given conditions. To ensure the coherence of experimental data, the test closest to the average value was selected as the optimal test for analysis under the corresponding experimental condition.

Table 1.
Experimental testing setup.
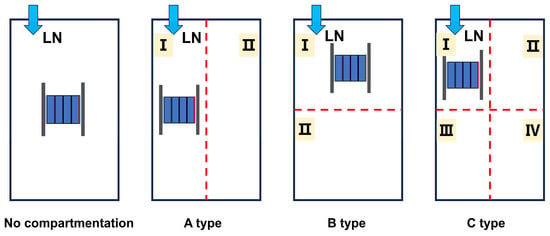
Figure 2.
Layout of fire compartments.
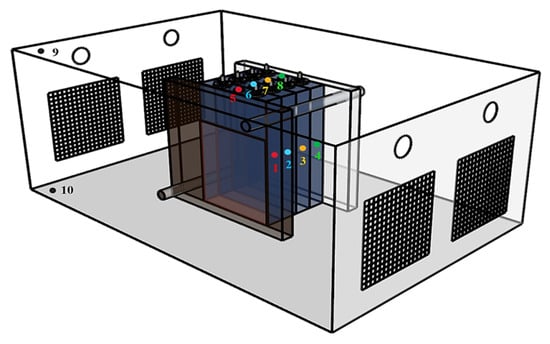
Figure 3.
Thermocouple arrangement method.
3. Results and Discussion
3.1. Thermal Runaway Without LN
3.1.1. TRP Characteristic
As heating proceeded, the temperature of cell 1# gradually increased and heat continuously accumulated inside the cell. Eventually, at 1074 s, the safety valve was triggered, and high-temperature gases and electrolyte were violently ejected, followed by intense flaming combustion. At this moment, the instantaneous temperature of the cell surface and the inner surface of the pack box approaching 400 °C. The temperature then began to decrease; however, due to the continued combustion of adhesive tape, wires, and other materials attached to the neighboring cells, the stabilized burning temperature did not fall but remained at approximately 350 °C, forming a high-temperature zone in the upper part of the box. Under this thermal environment, the temperature of cell 2# gradually increased, and its safety valve opened at 1284 s. The flammable vapors and electrolyte released were rapidly ignited by the existing flames, causing the instantaneous temperature of the cell and the inner surface of the box to again exceed 400 °C. The temperature then decreased gradually, but under the influence of the high-temperature zone, the maximum temperature of the cell remained at about 410 °C after the flame became quasi-steady. The safety valve of cell 3# opened at 1708 s, and the released gases caused the flame to expand once more; under the already elevated thermal conditions, the instantaneous temperature of the cell and the box inner surface exceeded 450 °C, and after the flame stabilized, the cell temperature remained around 420 °C. For cell 4#, the safety valve opened at 1980 s, and the instantaneous temperature of the cell and the box inner surface rose above 600 °C. The flame then gradually stabilized, and the cell temperature was maintained at approximately 420 °C. In addition, the recorded images clearly show that the upper flames directly impinged on the cells, which further increased the cell temperatures and accelerated the reaction processes.
3.1.2. Temperature Characteristic Analysis
Figure 4 presents the variations in cell voltage and surface temperature during the test, and Figure 5 shows the evolution of the gas temperature inside the experimental module. Before the start of the experiment, all cells were at 27 °C. As heating proceeded, the safety valve of cell 1# opened at 1074 s, at which time the temperature was 171 °C; TR occurred at 1122 s, with a corresponding temperature of 193 °C; the peak temperature was reached at 1228 s, reaching 368 °C. For cell 2#, the safety valve opened at 1284 s at a temperature of 176 °C; full TR occurred at 1550 s, when the temperature reached 202 °C; the peak temperature appeared at 1700 s and reached 421 °C. For cell 3#, the time and temperature corresponding to safety-valve activation were 1708 s and 184 °C, respectively; TR occurred at 1794 s with a temperature of 208 °C; the peak temperature appeared at 2186 s and reached 469 °C. For cell 4#, the times for safety-valve activation, onset of TR, and peak temperature were 1980 s, 2016 s, and 2218 s, respectively, with corresponding temperatures of 189 °C, 210 °C, and 472 °C. In Figure 5, four distinct temperature-rise stages can be observed, corresponding exactly to the TR processes of the four cells. When the first cell underwent TR, the maximum temperatures in the upper and lower spaces were 371 °C and 213 °C, respectively; when the second cell underwent TR, the maximum upper and lower space temperatures were 436 °C and 222 °C, respectively; when the third cell underwent TR, these temperatures increased to 487 °C and 227 °C; and when the fourth cell underwent TR, the maximum upper and lower space temperatures further increased to 701 °C and 249 °C, respectively. With the gradual increase in temperature inside the box and the continuous flame impingement on the cells, the safety-valve activation temperature, the initial TR temperature, the peak TR temperature of each cell, as well as the gas temperature in each stage, all exhibited continuous increases. A similar trend was observed for the temperatures on the rear surfaces of the cells: the maximum rear-surface temperatures of cells 1#, 2#, and 3# all remained above 400 °C. For cell 4#, the rear-surface temperature was relatively lower because it was in direct contact with a steel clamp with high thermal conductivity, but still exceeded 360 °C. Therefore, it can be concluded that the persistent high-temperature field and sustained flame impingement not only elevated the overall temperature of the cells, greatly increasing the risk of TR, but also raised the bulk gas temperature inside the box, thereby posing a serious threat to the safety of the remaining cells.
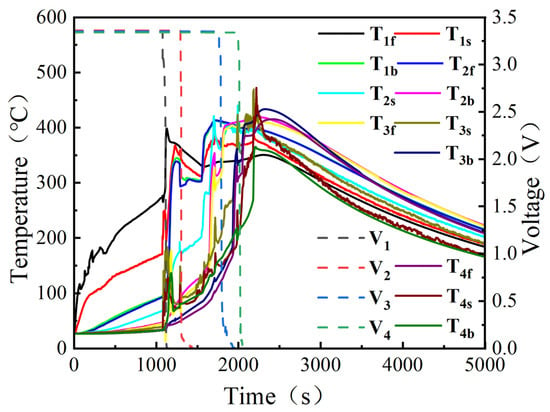
Figure 4.
Changes in cell voltage and surface temperature in Test 1.
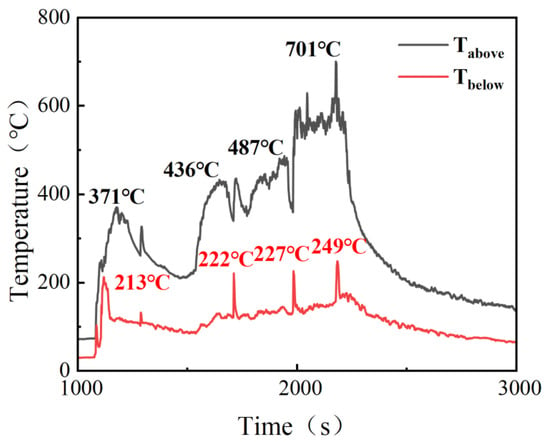
Figure 5.
Changes in temperature inside the module in Test 1.
For TRP within the battery module, the process from the onset of TR in one cell to the initiation of TR in an adjacent cell, the propagation time and propagation velocity are analyzed. Assuming that TR propagates from cell i# to cell (i + 1)#, the onset times of TR in cells i# and (i + 1)# are denoted as and , respectively; the propagation time is denoted as , the propagation velocity as , and the propagation distance as . In this study, the cell width is taken as 29 mm. The relationships among these parameters are given by the corresponding equations, and the calculated results are summarized in Table 2 [23]. It is important to note that the propagation velocity () calculated here is based on the specific geometric constraint where equals the cell thickness due to the tightly clamped arrangement. This definition assumes a direct, zero-gap conductive path. Consequently, this calculated ‘effective velocity’ serves as a characteristic parameter for this specific dense-packing configuration and should be interpreted with caution when compared to modules with air gaps or different structural constraints, where the propagation path length may differ from the geometric cell width.

Table 2.
TRP parameter table.
As shown in Table 2, from the onset of TR in cell 1# to that in cell 4#, the TRP time becomes progressively shorter, whereas the propagation velocity increases. This behavior can be attributed to the following factors. First, the continuous action of the high-temperature field and persistent flame impingement causes a sharp rise in cell temperature while simultaneously increasing the gas temperature within the enclosure, so that the time required to trigger TR in each subsequent cell is gradually reduced. Second, during heating, cell 1# undergoes deformation and bulging, which reduces the contact area between cells 1# and 2# and weakens the heat transfer between them, thereby increasing the TRP time from cell 1# to cell 2#. However, the width of the battery module is constrained by the clamps on both sides; as cell 1# continues to swell, cells 2#, 3#, and 4# are progressively compressed, the inter-cell spacing decreases, and heat transfer between cells is continuously enhanced. Consequently, the TRP time between subsequent cells becomes shorter, and the propagation velocity increases accordingly.
3.1.3. Thermal Characteristics
Heat transfer occurs in three forms: conduction, convection, and radiation. In this study, the focus is placed on heat conduction between adjacent cells. The instantaneous heat flux between the centers of the side surfaces of two neighboring cells at time t is denoted as . Because the battery module is constrained by clamps on both sides, the contact surfaces can be regarded as tightly bonded, and the heat-transfer path is taken as the thickness of a single cell. The heat transferred between cells during TRP, the heat transferred from cell i# to cell (i + 1)# over the period from to , is denoted as and can be expressed as follows:
where is the temperature difference between the centers of the side surfaces of the two cells, °C; is the heat transfer distance between the cells, m, taken as 0.029 m in this study; is the contact area between the cells, m2, taken as 0.029 m2; and is the thermal conductivity of the cell in the heat-transfer direction, taken as 1.1 W/(m·K) [24].
, and were obtained as 71.86 kJ, 41.58 kJ, and 27.07 kJ, respectively. As heat transfer proceeds, the amount of heat conducted between cells shows a decreasing trend. On the one hand, the reduction in TRP time directly diminishes the total heat transferred. On the other hand, under the continuous influence of the high-temperature field and flame impingement, the heating rate of cell (i + 1)# increases, causing its temperature to rise more rapidly and thereby reducing the temperature difference with cell i#, which in turn lowers the conductive heat transfer between the two cells. In addition, the energy required to trigger TR in cell (i + 1)# originally originates predominantly from inter-cell heat conduction. After cell (i + 1)# absorbs a large amount of heat from the high-temperature field and flames, energy accumulates more rapidly, causing the cell to enter TR earlier. This further shortens the inter-cell heat transfer duration. Together with heat losses during the conduction process, these effects lead to a progressive decrease in the net heat transferred between cells.
3.1.4. Analysis of Experimental Residues
As shown in Table 3, the mass losses of cells 1#, 2#, 3#, and 4# between before and after the experiment were 20.7%, 20.3%, 20.1%, and 19.8%, respectively, all of which are greater than the 19.3% mass loss observed for a single cell undergoing TR, indicating that the reaction of the battery module was more intense and the cell damage was more severe [25]. As shown in Figure 6, the upper surfaces of the cells are covered with residues from material combustion and exhibit a dark brown color, which indirectly reflects the severity of the flame burning during the test.

Table 3.
Changes in battery quality.

Figure 6.
Battery image after testing.
3.2. Suppression of TR in LIBs with LN
3.2.1. Suppression Effectiveness of LN
As displayed in Figure 7, for test 2, the initial temperature of all cells was 8 °C. At the early stage of TR in cell 1#, 17.4 kg of LN was injected over a duration of 295 s. During this period, the temperature of cell 1# was reduced from a maximum of 287 °C to 123 °C. After the end of LN injection, the cell temperature increased and finally stabilized at 141 °C, with the subsequent temperature rise rate remaining below 1 °C/s and no re-ignition being observed. The temperature of cell 2# decreased from 59 °C to a minimum of −38 °C and then rose to 14 °C. For cell 3#, the maximum temperature was 34 °C, the minimum temperature during LN injection was −76 °C, and the temperature later increased to 8 °C. The temperature of cell 4# decreased from 32 °C to −118 °C and eventually returned to 8 °C. The maximum and minimum gas temperatures in the upper space were 120 °C and −195 °C, respectively, while those in the lower space were 93 °C and −195 °C, respectively.
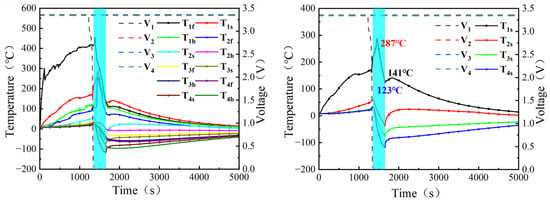
Figure 7.
Changes in cell voltage and surface temperature in Test 2.
To better describe the temperature evolution during LN-based fire suppression, the LN injection mass is defined as , the injection duration as , the initial cell temperature as , the maximum cell temperature as , the minimum cell temperature as , the rebound temperature as , the maximum temperature rise as , the maximum temperature drop as , the maximum rebound amplitude as , and the effective cooling amplitude as . In addition, the maximum space temperature, minimum space temperature, and maximum space cooling amplitude are defined as , , and , respectively. These parameters can be expressed as follows, and the outcomes are summarized in Table 4:

Table 4.
Changes in battery temperature.
3.2.2. Analysis of Thermal Characteristics
For the battery module, the heat removed by LN is equal to the sum of the heat reductions in all individual cells; that is, the heat absorbed by LN corresponds to the total decrease in cell heat. The total heat of the battery is defined as . The heat that LN is theoretically expected to absorb is denoted as . The saturation temperature of LN is . The actual heat reduction of each cell is defined as , the total heat absorbed by LN as . The cooling rate of LN is set as the ratio of the actual heat absorbed by LN to the total heat of the cell, denoted as . the overall effective utilization of LN as , and the effective utilization of LN for each cell as . These parameters satisfy the following relationships [12,26]:
where is the specific heat capacity of the cell, 1.1 kJ/kg·K−1, is the mass of the cell; is defined as the latent heat of vaporization of LN, with a value of 199 kJ/kg; is defined as the constant-pressure specific heat capacity of liquid nitrogen, with a value of 1.03841 kJ/(kg·K); is defined as the saturation temperature of liquid nitrogen, with a value of −195.8 °C. For test 2, the residual masses of cells 1#, 2#, 3#, and 4# were 1.6713 kg, 1.7356 kg, 1.7354 kg, and 1.7357 kg, respectively. These values were substituted into the above equations, and the calculated results are summarized in Table 5.

Table 5.
Battery heat parameter table of battery module.
Considering the extreme cryogenic environment, a sensitivity analysis was conducted to evaluate the potential impact of systematic measurement errors on the effective utilization rate . Although protective measures were implemented, intense LN vaporization could theoretically cause the thermocouple readings to be slightly lower than the actual cell casing temperature. Assuming a worst-case scenario where the measured temperature drop is overestimated by 10% due to local impingement cooling, the calculated absolute heat absorption would notably decrease. However, since this systematic deviation applies consistently across both the baseline (Test 2) and compartmentalized tests (Tests 3–5), the relative trend remains unaffected. Even after correcting for this potential error, the improvement in provided by the fire compartments persists, confirming that the observed efficiency enhancement is a physical result of the volume confinement rather than a measurement artifact.
3.3. Enhancement of LN Fire Suppression Performance by Fire Compartmentalization
3.3.1. Analysis of Temperature Characteristics
Figure 8 presents the temperature and voltage profiles for tests 3, 4, and 5. Compared with test 2, the incorporation of fire compartmentalization in test 3 increased the maximum temperature drop of cell 1# from 164 °C to 245 °C, while the effective cooling amplitude increased from 146 °C to 236 °C. In addition, cells 2#–4# also exhibited substantial increases in maximum temperature drop. Because the rebound temperatures of the cells were essentially equal to their initial temperatures and the peak temperatures were very similar, the differences in effective cooling amplitude among the cells were relatively small. From the above analysis, it can be concluded that the fire compartments markedly enhance the fire-suppression performance of LN, allowing its cooling capability to be more fully exploited. This enhancement is mainly attributed to the fact that the fire compartments reduce the effective action volume of LN, enabling a larger fraction of LN to directly interact with the battery module and thereby strengthening the overall suppression effect.
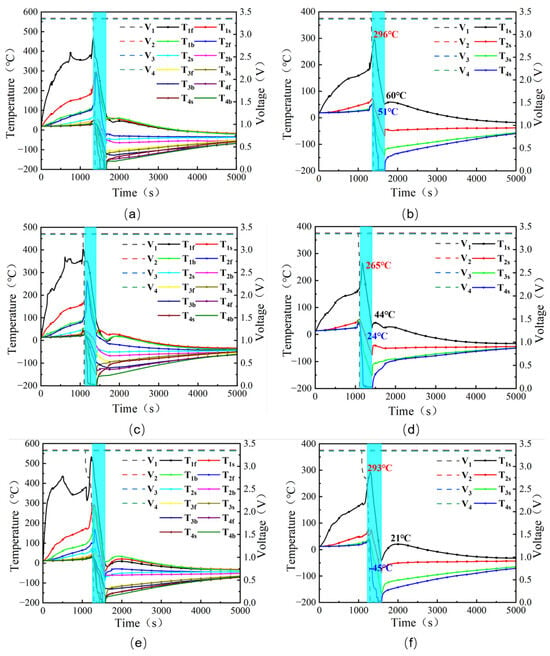
Figure 8.
Changes in cell voltage and surface temperature during the testing process: (a,b) Test 3, (c,d) Test 4, (e,f) Test 5.
3.3.2. Analysis of Heat Characteristics
The initial temperatures of the cells in Tests 2, 3, 4, and 5 were 8 °C, 20 °C, 14 °C, and 13 °C, respectively. The mass of the batteries under each condition is shown in Table 6. The calculated results are summarized in Table 7. Figure 9 shows the average temperature of different partitioned spaces during the test. Among them, the partitioning situation is divided according to Figure 2.

Table 6.
Battery quality parameter table of fireproof unit experiment.

Table 7.
Battery heat parameter table of fireproof unit experiment.
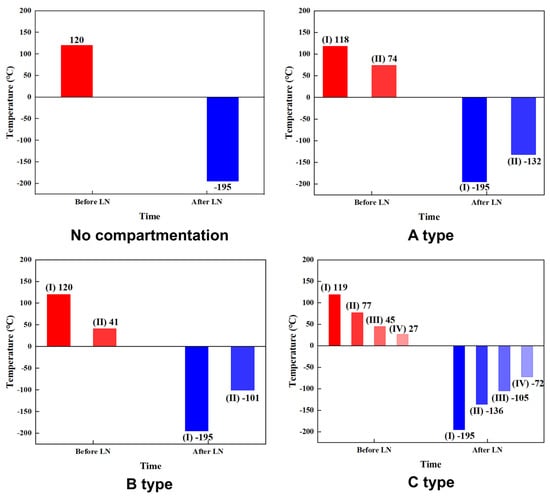
Figure 9.
Temperature in different compartmentation spaces during testing.
Referring to Table 7, comparing test 1 and test 2, under the same LN dosage, the addition of fire compartments increased the total heat absorbed by LN from 449.78 kJ to 628.70 kJ, while the effective utilization rate increased from 0.037 to 0.051. The overall cooling rate also improved, reflecting that fire compartments significantly enhance the fire-suppression performance of LN, thereby further strengthening the overall suppression effectiveness. Comparing Test 2 and Test 3, the total heat absorbed by LN and the effective utilization rate () show limited variation. Although slight differences in initial temperatures existed, critical injection parameters were strictly controlled—specifically, the 8 mm nozzle diameter 1 and the constant mass flow rate of 0.063 kg/s—to ensure experimental consistency. These results suggest that, under the specific experimental configuration and fixed injection parameters of this study, the reduction in effective cooling volume appears to be the primary driver for enhancing LN utilization. While the geometric shape of the compartments likely influences local flow fields, its impact on the macroscopic cooling efficiency was less pronounced than the effect of volume confinement in these tests. Furthermore, comparing all four conditions, it can be observed that as more fire compartments are created, the volume of each compartment becomes smaller. While this further enhances the fire-suppression effect of LN, the increase in effective utilization rate becomes gradually smaller. The effective utilization rate in Test 2 compared to Test 1 increased by 1.4%, while in Test 4 compared to Test 2, the effective utilization rate only increased by 0.7%. On one hand, this is because when the entire box is divided into two fire compartments, the space available for LN action is halved. When the number of fire compartments increases from two to four, although the LN action space is again halved, the actual reduction is only one-quarter of the total box space. As more fire compartments are added, the net reduction in space becomes smaller, and thus the ability to enhance the LN suppression effect naturally weakens. On the other hand, the reduction in space also increases heat accumulation. The smaller the space, the more heat is accumulated per unit volume for the same total amount of heat, making it more difficult for LN to suppress the fire and reduce the temperature. This thermodynamic constraint suggests that volume is the governing variable. Consequently, although the partition shape inevitably influences the internal flow field and local heat transfer coefficients, the experimental data suggests that these effects are secondary compared to the volume confinement effect. The significant improvement in is primarily driven by the reduction in the effective cooling volume, which concentrates the LN enthalpy change around the thermal runaway cells. Therefore, the variations observed between Types A, B, and C indicate that while geometry plays a role, the volume reduction factor is dominant in enhancing the overall suppression efficiency in this study.
4. Conclusions
This study focuses on 100 Ah LiFePO4 batteries to investigate the impact of fire compartmentalization on the LN fire suppression effectiveness in LIBs. The main conclusions are as follows:
(1) The TRP can be divided into four stages: the external heating stage, safety valve opening stage, self-heating stage, and cooling phase. Among these, the self-heating stage generates the most heat, with the fastest heat accumulation rate, and the highest danger level. Without fire suppression intervention, the TRP rate within the LIB module gradually accelerates.
(2) LN absorbs a large amount of heat through vaporization, rapidly lowering the temperature and interrupting the internal side reactions of the cell, as the temperature falls below the required reaction threshold, thus halting the TR reaction. The effective utilization rate of 17.4 kg of LN is 0.037, and the effective utilization rate for each cell in the module gradually decreases with the sequence of TRP.
(3) Fire compartments effectively enhance LN fire suppression. Under the controlled LN flow rate of 0.063 kg/s, the addition of fire compartments increased the effective utilization rate from 0.037 to 0.051. The data indicates that for the tested module configurations, the volume reduction effect outweighs the influence of compartment geometry, although the marginal gain in utilization diminishes as the compartment volume decreases further. Future quantitative flow simulations are recommended to strictly decouple these effects.
This study examined the impact of three different fire compartment configurations on the suppression of TRP in large-capacity LIB modules. A method to enhance LN fire suppression effectiveness based on fire compartmentalization was proposed. Future research could explore more types of compartment design methods, as this enhanced design could improve LN fire suppression performance in engineering applications. Moreover, future research will extend the application of this LN suppression strategy to higher energy-density systems, such as high-nickel layered cathodes and lithium metal anodes, to evaluate the method’s efficacy under more aggressive thermal runaway conditions. In addition, future investigations will explore the suppression performance of LN under dynamic operating conditions, including high C-rate charging and active discharge cycles, to assess the system’s effectiveness under varying internal thermal stress levels.
Author Contributions
D.X.: Writing—original draft, Methodology, Data curation, Formal analysis, Conceptualization, Funding acquisition. X.D.: Writing—review and editing, Resources, Project administration. L.S.: Software, Data curation. X.Z.: Validation, Supervision, Project administration. X.X.: Methodology, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the financial support from the State Grid Jiangsu Electric Power Co., Ltd. Technology Project (J2024183).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Authors Dunbin Xu, Xing Deng, Lingdong Su and Xiao Zhang are employed by the State Grid Xuzhou Power Supply Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Yang, X.; Wen, H.; Lin, Y.; Zhang, H.; Liu, Y.; Fu, J.; Liu, Q.; Jiang, G. Emerging research needs for characterizing the risks of global lithium pollution under carbon neutrality strategies. Environ. Sci. Technol. 2023, 57, 5103–5106. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mu, X.; Yang, S.; Wang, D.; Wang, Y.; Zhou, H.; He, P. Artificial Carbon Neutrality Through Aprotic CO2 Splitting. Angew. Chem. Int. Ed. Engl. 2025, 64, e202422888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.; Zhou, X.; Ju, X.; Yang, L. Investigating thermal runaway characteristics and trigger mechanism of the parallel lithium-ion battery. Appl. Energy 2023, 349, 121690. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mecha-nisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S. A review of power battery thermal energy management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M. A review of energy storage types, applications and rec-ent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Giovanniello, M.A.; Wu, X.-Y. Hybrid lithium-ion battery and hydrogen energy storage systems for a wind-supplied microgrid. Appl. Energy 2023, 345, 121311. [Google Scholar] [CrossRef]
- Meng, X.; Yang, K.; Zhang, M.; Gao, F.; Liu, Y.; Duan, Q.; Wang, Q. Experimental study on combustion behavior and fire extinguishing of lithium iron phosphate battery. J. Energy Storage 2020, 30, 101532. [Google Scholar] [CrossRef]
- Xu, J.; Guo, P.; Duan, Q.; Yu, X.; Zhang, L.; Liu, Y.; Wang, Q. Experimental study of the effectiveness of three kinds of extinguishing agents on suppressing lithium-ion battery fires. Appl. Therm. Eng. 2020, 171, 115076. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Chen, H.; Lu, W.; Wang, Q. Experimental study on the efficiency of dodecafluoro-2-methylpentan-3-one on suppressing lithium-ion battery fires. RSC Adv. 2018, 8, 42223–42232. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Duan, Q.; Chen, M.; Xu, J.; Zhao, C.; Sun, J.; Wang, Q. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, G.; Yuan, D.; Jiang, L.; Fan, Y.; Kong, D. Experimental study on the efficiency of hydrogel on suppressing thermal runaway propagation of lithium-ion battery. Fire Technol. 2024, 61, 3979–4000. [Google Scholar] [CrossRef]
- Egelhaaf, M.; Kress, D.; Wolpert, D.; Lange, T.; Justen, R.; Wilstermann, H. Fire fighting of li-ion traction batteries. SAE Int. J. Altern. Powertrains 2013, 2, 37–48. [Google Scholar] [CrossRef]
- Luo, W.-T.; Zhu, S.-B.; Gong, J.-H.; Zhou, Z. Research and development of fire extinguishing technology for power Lithium batteries. Procedia Eng. 2018, 211, 531–537. [Google Scholar] [CrossRef]
- Andersson, P.; Arvidson, M.; Evegren, F.; Jandali, M.; Larsson, F.; Rosengren, M. Lion Fire: Extinguishment and Mitigation of Fires in Li-ion Batteries at Sea; RISE Research Institutes of Sweden: Kista, Sweden, 2018. [Google Scholar]
- Zhou, F.; Shi, B.; Cheng, J.; Ma, L. A new approach to control a serious mine fire with using liquid nitrogen as extinguishing media. Fire Technol. 2015, 51, 325–334. [Google Scholar]
- Guo, D.; Zhang, G.; Zhu, G.; Jia, B.; Zhang, P. Applicability of liquid nitrogen fire extinguishing in urban underground utility tunnel. Case Stud. Therm. Eng. 2020, 21, 100657. [Google Scholar] [CrossRef]
- Meng, J.; Wang, T.; Li, G.; Kang, J. Simulation test on cooling and fire suppression with liquid nitrogen in computer room of data center. Fire 2023, 6, 116. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, G.; Zhang, Z.; Zhang, Z.; Zhao, Z. Study on the fire suppression efficiency of common extinguishing agents for lithium iron phosphate battery fires. Fire Technol. 2025, 61, 4059–4079. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Song, L.; Duan, Q.; Sun, J.; Mei, W.; Wang, Q. Preventing effect of liquid nitrogen on the thermal runaway propagation in 18650 lithium ion battery modules. Process Saf. Environ. Prot. 2022, 168, 42–53. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Wang, J.; Yang, Y.; Zhu, Y.; Bai, W. Inhibition effect of liquid nitrogen on thermal runaway propagation of lithium ion batteries in confined space. J. Loss Prev. Process Ind. 2022, 79, 104853. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, B.; Ruan, H.; Shi, B.; Huang, D.; Liu, H.; Li, Z. Revealing suppression effects of injection location and dose of liquid nitrogen on thermal runaway in lithium iron phosphate battery packs. Int. J. Heat Mass Transf. 2024, 219, 124866. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Wang, J. Alleviation of thermal runaway propagation in thermal management modules using aerogel felt coupled with flame-retarded phase change material. Energy Convers. Manag. 2019, 200, 112071. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Li, Y.; Wang, G.; Wang, J. Thermal runaway and fire behaviors of large-scale lithium ion batteries with different heating methods. J. Hazard. Mater. 2019, 379, 120730. [Google Scholar] [CrossRef]
- Chen, M.; Dongxu, O.; Liu, J.; Wang, J. Investigation on thermal and fire propagation behaviors of multiple lithium-ion batteries within the package. Appl. Therm. Eng. 2019, 157, 113750. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, P.; Duan, Q.; Zhao, C.; Wang, Q. Experimental investigation on the cooling and suppression effects of liquid nitrogen on the thermal runaway of lithium ion battery. J. Power Sources 2021, 495, 229795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.