Influence of Lithium Plating on the Mechanical Properties of Automotive High-Energy Pouch Batteries
Abstract
1. Introduction
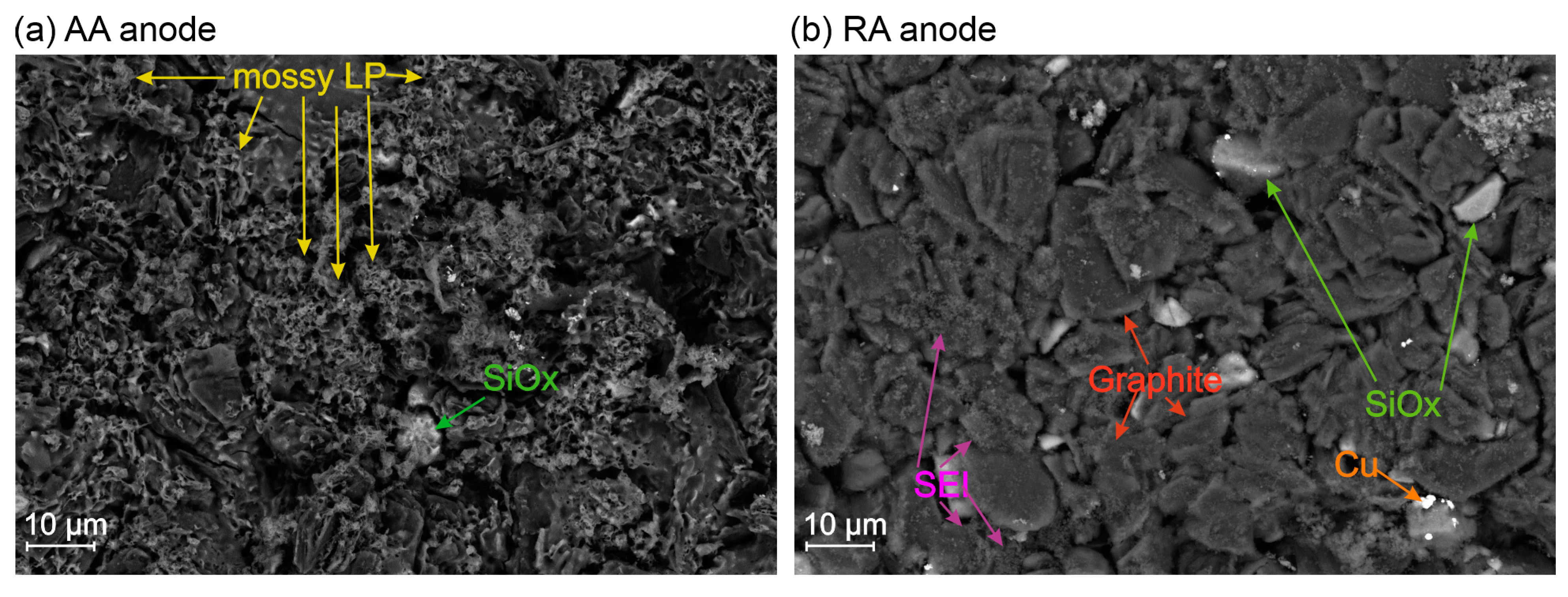
- First of all, the presence of LP must be confirmed beforehand, which requires a determination of the operational conditions under which LP forms must first be determined for the cells under study. Verification can be achieved indirectly through voltage and current signal analysis during charging, discharging, or relaxation phases, or directly via post-mortem analytical methods, as demonstrated in our previous work [40,41].
- It is in principle, Without disassembling the cell, the exact location of LP formation remains unclear, as it depends on pressure variations within the electrode stack, current density distribution, temperature, and C-rate [7,42,43]. These factors must be considered when defining the shape of the impactor and the location of mechanical loading in abuse tests.
- LP forms and dissolves throughout the operational cycle [33]. Even in fully charged state, the plated lithium content does not remain constant after charging is complete, complicating efforts to establish repeatable and robust characterization.
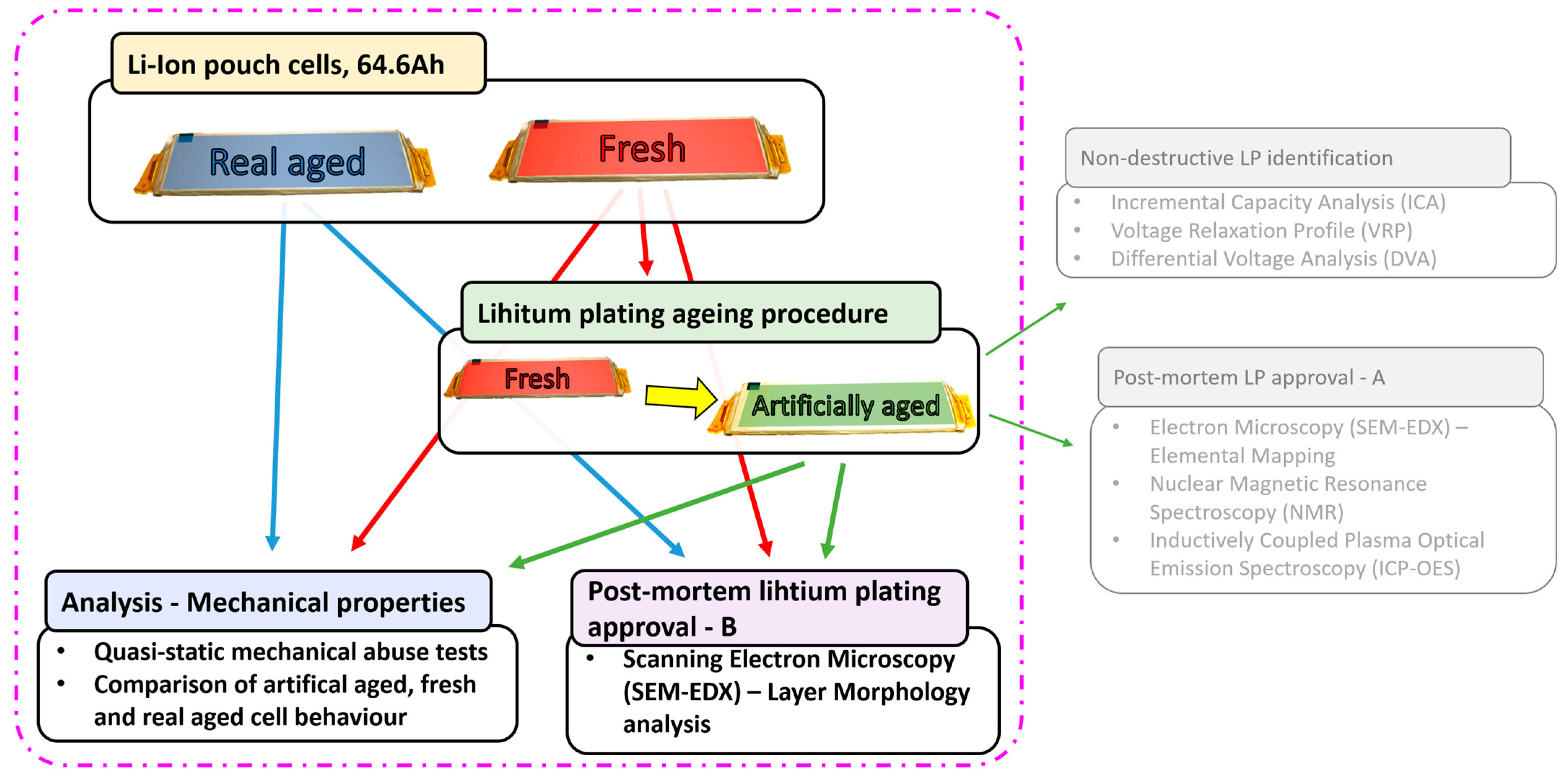
2. Materials and Methods
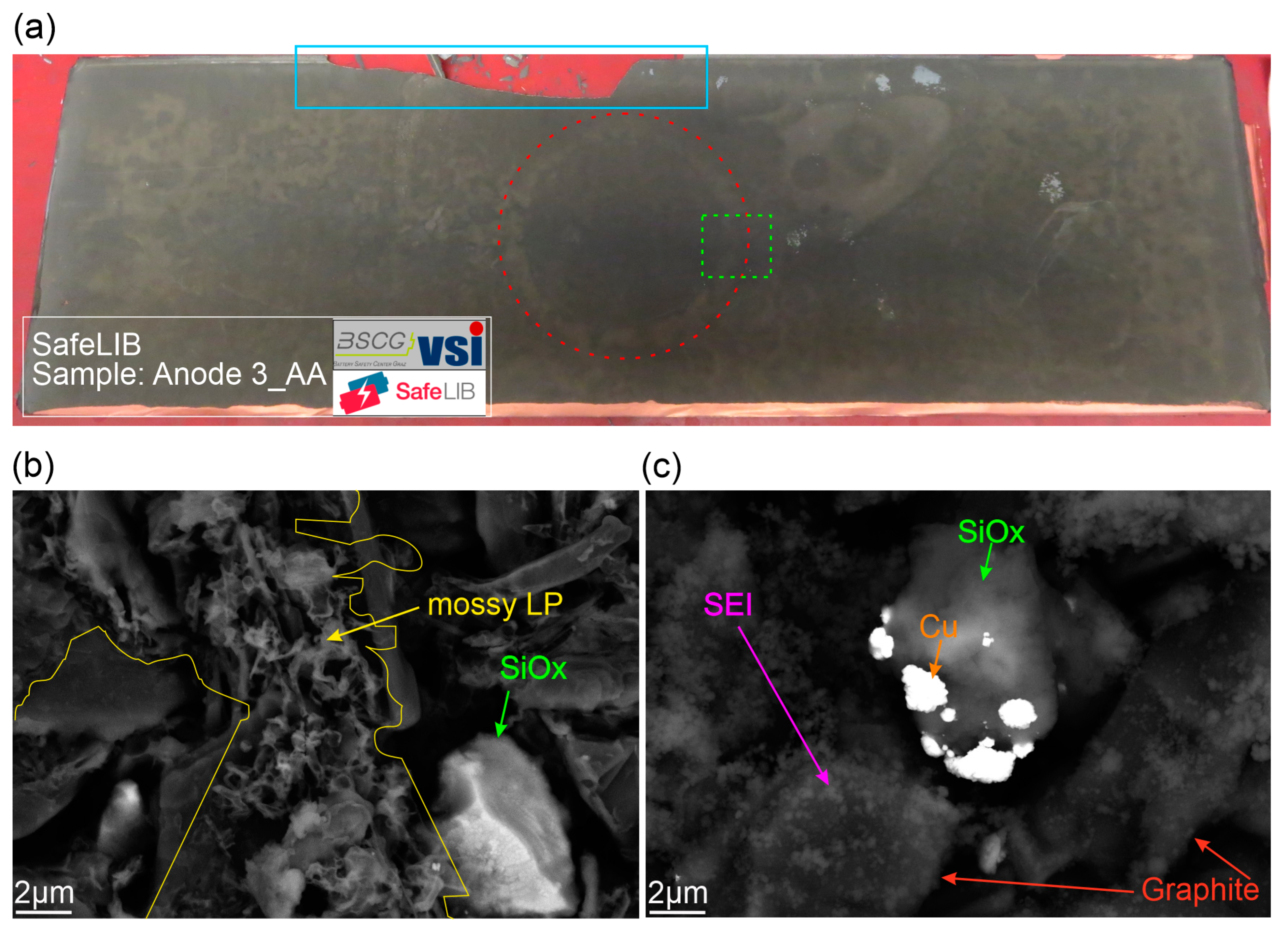
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Artificially aged |
| BCs | Boundary conditions |
| C/NMC | Carbon/nickel manganese cobalt |
| C-SiOx | Carbon–silicon oxides |
| EVs | Electric vehicles |
| F | Fresh |
| FESEM | Field emission scanning electron microscope |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| ISC | Internal short circuit |
| LAMne | Loss of active material negative electrode |
| LAMpe | Loss of active material positive electrode |
| LIBs | Lithium-ion batteries |
| LLI | Loss of lithium inventory |
| LP | Lithium plating |
| PHEVs | Plug-in hybrid electric vehicles |
| RA | Real aged |
| SEI | Solid electrolyte interface |
| SOC | State of charge |
| SOHc | State of health based on nominal capacity (ratio between actual and nominal capacity) |
| TR | Thermal runaway |
Appendix A
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. Engl. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Raël, S. Physical characterization of the charging process of a Li-ion battery and prediction of Li plating by electrochemical modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, C.; Li, H.; Du, J.; Xiong, R. Detecting undesired lithium plating on anodes for lithium-ion batteries—A review on the in-situ methods. Appl. Energy 2021, 300, 117386. [Google Scholar] [CrossRef]
- Fuchs, G.; Willenberg, L.; Ringbeck, F.; Sauer, D.U. Post-Mortem Analysis of Inhomogeneous Induced Pressure on Commercial Lithium-Ion Pouch Cells and Their Effects. Sustainability 2019, 11, 6738. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Kasper, M.; Grolleau, S.; Couceiro, C.G.; Trad, K.; Matadi, B.P.; Wohlfahrt-Mehrens, M. Interplay of Operational Parameters on Lithium Deposition in Lithium-Ion Cells: Systematic Measurements with Reconstructed 3-Electrode Pouch Full Cells. J. Electrochem. Soc. 2016, 163, A1232–A1238. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Zhang, Z.; Li, Y.; Yang, S.; He, X. Insight Understanding of External Pressure on Lithium Plating in Commercial Lithium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2406966. [Google Scholar] [CrossRef]
- Schmitt, C.; Kopljar, D.; Friedrich, K.A. Detailed investigation of degradation modes and mechanisms of a cylindrical high-energy Li-ion cell cycled at different temperatures. J. Energy Storage 2025, 120, 116486. [Google Scholar] [CrossRef]
- Smith, A.J.; Fang, Y.; Mikheenkova, A.; Ekström, H.; Svens, P.; Ahmed, I.; Lacey, M.J.; Lindbergh, G.; Furó, I.; Lindström, R.W. Localized lithium plating under mild cycling conditions in high-energy lithium-ion batteries. J. Power Sources 2023, 573, 233118. [Google Scholar] [CrossRef]
- Chen, R.; Miao, S.; Jia, Y.; Zhang, X.; Peng, J.; Zhang, K.; Wu, F.; Zhao, J.; Li, Z.; Cai, W. A review of detecting Li plating on graphite anodes based on electrochemical methods. J. Mater. Chem. A 2024, 12, 33427–33447. [Google Scholar] [CrossRef]
- Das, S.; Shrotriya, P. Electrochemical Mechanism Underlying Lithium Plating in Batteries: Non-Invasive Detection and Mitigation. Energies 2024, 17, 5930. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, e2101051. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Key, B.; Chen, H.; Best, A.S.; Hollenkamp, A.F.; Grey, C.P. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 2010, 9, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Du Pasquier, A.; Beaudoin, B.; Tarascon, J.; Trentin, M.; Langenhuizen, N.; de Beer, E.; Notten, P. In situ Scanning Electron Microscopy (SEM) observation of interfaces within plastic lithium batteries. J. Power Sources 1998, 76, 19–29. [Google Scholar] [CrossRef]
- Kovachev, G.; Ellersdorfer, C.; Gstrein, G.; Hanzu, I.; Wilkening, H.M.R.; Werling, T.; Schauwecker, F.; Sinz, W. Safety assessment of electrically cycled cells at high temperatures under mechanical crush loads. eTransportation 2020, 6, 100087. [Google Scholar] [CrossRef]
- Sprenger, M.; Dölle, N.; Schauwecker, F.; Raffler, M.; Ellersdorfer, C.; Sinz, W. Multiscale Analysis and Safety Assessment of Fresh and Electrical Aged Lithium-Ion Pouch Cells Focusing on Mechanical Behavior. Energies 2022, 15, 847. [Google Scholar] [CrossRef]
- Sprenger, M.; Kovachev, G.; Dölle, N.; Schauwecker, F.; Sinz, W.; Ellersdorfer, C. Changes in the Mechanical Behavior of Electrically Aged Lithium-Ion Pouch Cells: In-Plane and Out-of-Plane Indentation Loads with Varying Testing Velocity and State of Charge. Batteries 2023, 9, 67. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Sahraei, E. Degradation of battery separators under charge–discharge cycles. RSC Adv. 2017, 7, 56099–56107. [Google Scholar] [CrossRef]
- Wu, Z.; Cao, L.; Hartig, J.; Santhanagopalan, S. (Invited) Effect of Aging on Mechanical Properties of Lithium Ion Cell Components. ECS Trans. 2017, 77, 199–208. [Google Scholar] [CrossRef]
- Fink, K.; Santhanagopalan, S.; Hartig, J.; Cao, L. Characterization of Aged Li-Ion Battery Components for Direct Recycling Process Design. J. Electrochem. Soc. 2019, 166, A3775–A3783. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Abaza, A.; Ferrari, S.; Wong, H.K.; Lyness, C.; Moore, A.; Weaving, J.; Blanco-Martin, M.; Dashwood, R.; Bhagat, R. Experimental study of internal and external short circuits of commercial automotive pouch lithium-ion cells. J. Energy Storage 2018, 16, 211–217. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Sahraei, E.; Meier, J.; Wierzbicki, T. Characterizing and modeling mechanical properties and onset of short circuit for three types of lithium-ion pouch cells. J. Power Sources 2014, 247, 503–516. [Google Scholar] [CrossRef]
- Kermani, G.; Sahraei, E. Review: Characterization and Modeling of the Mechanical Properties of Lithium-Ion Batteries. Energies 2017, 10, 1730. [Google Scholar] [CrossRef]
- Luo, H.; Xia, Y.; Zhou, Q. Mechanical damage in a lithium-ion pouch cell under indentation loads. J. Power Sources 2017, 357, 61–70. [Google Scholar] [CrossRef]
- Chen, Y.; Santhanagopalan, S.; Babu, V.; Ding, Y. Dynamic mechanical behavior of lithium-ion pouch cells subjected to high-velocity impact. Compos. Struct. 2019, 218, 50–59. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Zhou, Q. Effect of low-temperature aging on the safety performance of lithium-ion pouch cells under mechanical abuse condition: A comprehensive experimental investigation. Energy Storage Mater. 2021, 40, 268–281. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Jaguemont, J.; van Mierlo, J. A comprehensive review of future thermal management systems for battery-electrified vehicles. J. Energy Storage 2020, 31, 101551. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Siegel, J.B.; Stefanopoulou, A.G. Robust Estimation of Battery System Temperature Distribution Under Sparse Sensing and Uncertainty. IEEE Trans. Control Syst. Technol. 2020, 28, 753–765. [Google Scholar] [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding undesirable anode lithium plating issues in lithium-ion batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Sagane, F.; Shimokawa, R.; Sano, H.; Sakaebe, H.; Iriyama, Y. In-situ scanning electron microscopy observations of Li plating and stripping reactions at the lithium phosphorus oxynitride glass electrolyte/Cu interface. J. Power Sources 2013, 225, 245–250. [Google Scholar] [CrossRef]
- Abbas, S.M.; Drießen, C.; Sprenger, M.; Ellersdorfer, C.; Hanzu, I.; Gstrein, G. Nondestructive Electrochemical Identification of Lithium Plating in High-Energy Automotive Batteries. ACS Omega 2025, 10, 13209–13217. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.M.; Jodlbauer, A.; Wilkening, M.; Wiltsche, H.; Ecker, J.V.; Ellersdorfer, C.; Gstrein, G.; Hanzu, I. Post-mortem identification of lithium plating in high energy automotive batteries. RSC—Sustain. Energy Fuels, 2025; submitted. [Google Scholar] [CrossRef]
- Mei, W.; Jiang, L.; Liang, C.; Sun, J.; Wang, Q. Understanding of Li-plating on graphite electrode: Detection, quantification and mechanism revelation. Energy Storage Mater. 2021, 41, 209–221. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. The Effects of Defects on Localized Plating in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1365–A1373. [Google Scholar] [CrossRef]
- Gstrein, G.; Abbas, S.M.; Ewert, E.; Wenzl, M.; Ellersdorfer, C. Safety-Critical Influence of Ageing on Mechanical Properties of Lithium-Ion Pouch Cells. Batteries 2025, 11, 99. [Google Scholar] [CrossRef]
- Schmid, A.; Pasquale, A.; Ellersdorfer, C.; Raffler, M.; Champaney, V.; Ziane, M.; Chinesta, F.; Feist, F. Mechanical Characterization of Li-Ion Cells and the Calibration of Numerical Models Using Proper Generalized Decomposition. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition 2023, New Orleans, LA, USA, 29 October–2 November 2023. [Google Scholar]
- Wang, Y.; Dang, D.; Wang, M.; Xiao, X.; Cheng, Y.-T. Mechanical behavior of electroplated mossy lithium at room temperature studied by flat punch indentation. Appl. Phys. Lett. 2019, 115, 043903. [Google Scholar] [CrossRef]
- Sun, K.; Li, X.; Zhang, Z.; Xiao, X.; Gong, L.; Tan, P. Pattern Investigation and Quantitative Analysis of Lithium Plating under Subzero Operation of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 36356–36365. [Google Scholar] [CrossRef]
- Chang, H.J.; Trease, N.M.; Ilott, A.J.; Zeng, D.; Du, L.-S.; Jerschow, A.; Grey, C.P. Investigating Li Microstructure Formation on Li Anodes for Lithium Batteries by in Situ 6 Li/ 7 Li NMR and SEM. J. Phys. Chem. C 2015, 119, 16443–16451. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Yang, H.-W.; Maniyazagan, M.; Naveenkumar, P.; Seung Kang, W.; Kim, S.-J. Building optimal SEI through control of morphology and chemical composition for high-performance lithium-ion batteries. Appl. Surf. Sci. 2023, 612, 155888. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, H.; Yang, Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim. Acta 2008, 53, 3539–3546. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Electronic Structure and Comparative Properties of LiNixMnyCozO2 Cathode Materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Gor, G.Y.; Cannarella, J.; Prévost, J.H.; Arnold, C.B. A Model for the Behavior of Battery Separators in Compression at Different Strain/Charge Rates. J. Electrochem. Soc. 2014, 161, F3065–F3071. [Google Scholar] [CrossRef]
- Fröhlich, K.; Abrahams, I.; Jahn, M. Determining phase transitions of layered oxides via electrochemical and crystallographic analysis. Sci. Technol. Adv. Mater. 2020, 21, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Pollard, T.P.; Borodin, O.; Xu, K.; Allen, J.L. Degradation of High Nickel Li-Ion Cathode Materials Induced by Exposure to Fully-Charged State and Its Mitigation. Adv. Energy Mater. 2023, 13, 2204360. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Han, G.; Dai, H.; Zhu, J.; Wang, X.; Tang, X.; Ye, J. Lithium plating on the anode for lithium-ion batteries during long-term low temperature cycling. J. Power Sources 2021, 484, 229312. [Google Scholar] [CrossRef]

| Cell Nomenclature | ||
|---|---|---|
| Cell Status | SOHc | History |
| Fresh (F) | 100% | Fresh cell, not used in application |
| Artificially aged (AA) | 80–82% | 20 cycles under LP conditions |
| Real aged (RA) | 93% | Used in a car for ca. 160,000 km |
| Cell Thickness (mm) | Breathing (0–100% SOC) (mm) | Swelling vs. F (0% SOC) (mm) | ||
|---|---|---|---|---|
| Cell Type | 0% SOC | 100% SOC | ||
| F | 11.25 ± 0.10 | 11.75 ± 0.06 | +0.5 (+4.4%) | - |
| AA | 12.27 ± 0.07 | 12.73 ± 0.10 | +0.46 (+3.7%) | +1.02 (+9.1%) |
| RA | 11.78 ± 0.08 | 12.05 ± 0.09 | 0.27 (+2.3%) | +0.53 (+4.7%) |
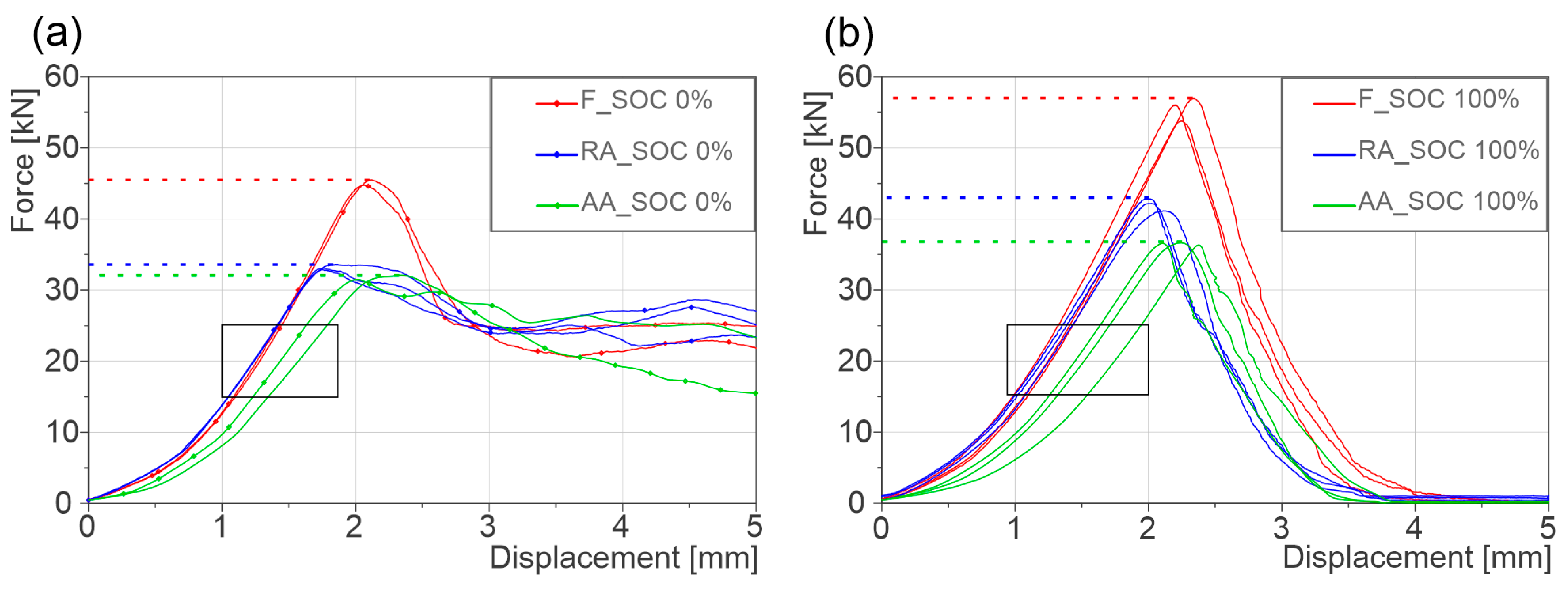
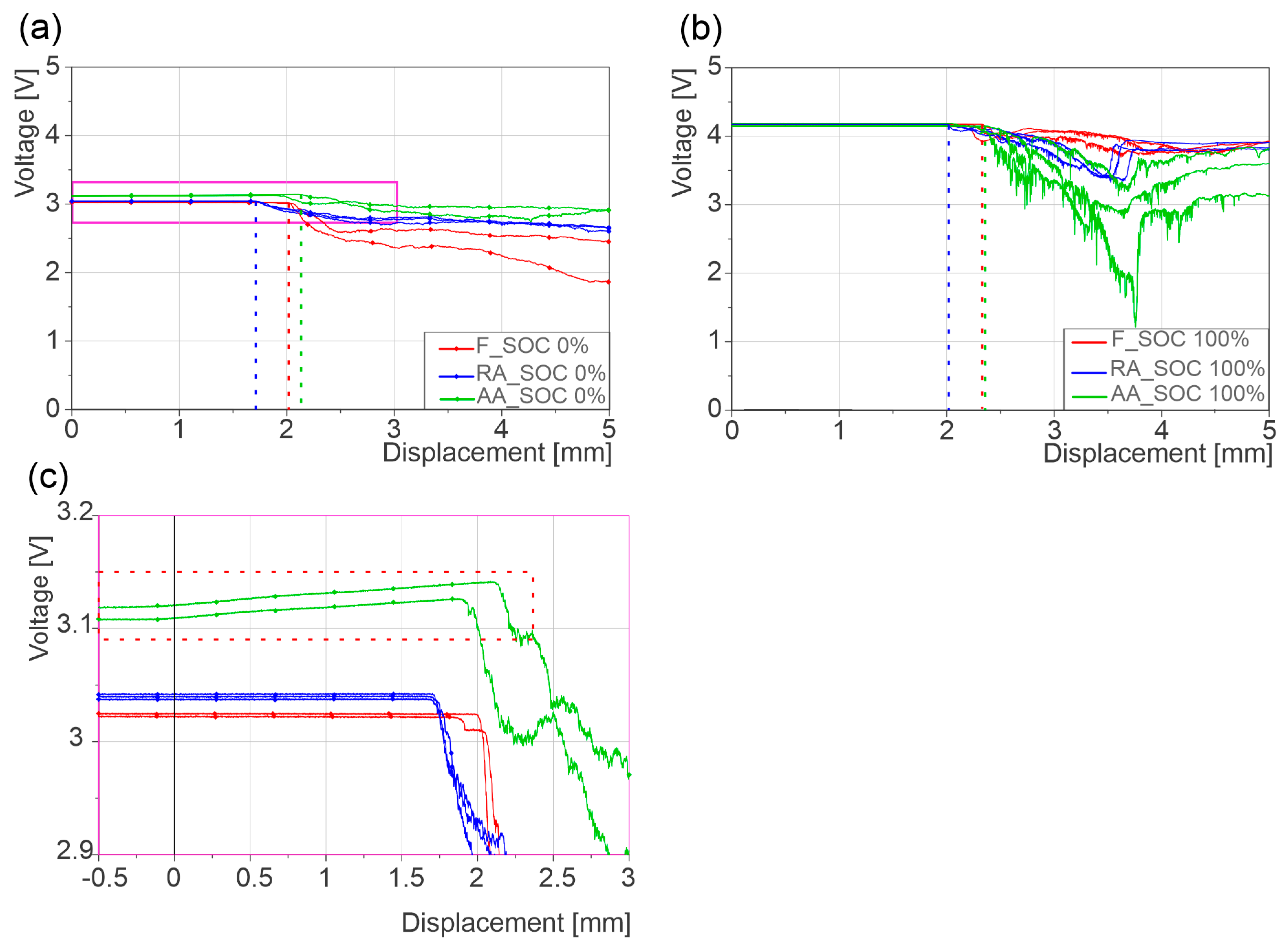
| Cell Type | Peak Failure Force (kN) | Failure Deformation (mm) | Difference to F | |||||
|---|---|---|---|---|---|---|---|---|
| 0% SOC | 100% SOC | 0% SOC | 100% SOC | Peak Failure Force (kN) | Failure Deformation (mm) | |||
| 0% SOC | 100% SOC | 0% SOC | 100% SOC | |||||
| F | 45 ± 0.5 | 57 ± 2 | 2.10 ± 0.03 | 2.24 ± 0.05 | - | - | - | - |
| AA | 32 ± 0.5 | 36 ± 0.5 | 2.24 ± 0.07 | 2.24 ± 0.1 | −13 (−28%) | −21 (−37%) | +0.14 (+6%) | 0 (0%) |
| RA | 33 ± 0.6 | 42 ± 0.8 | 1.79 ± 0.05 | 2.04 ± 0.6 | −12 (−26%) | −15 (−26%) | −0.31 (−15%) | −0.20 (−11%) |
| Cell Stiffness (kN/mm) | ||||
|---|---|---|---|---|
| Cell Type | 0% SOC | 100% SOC | ∆ Relative to Fresh | |
| 0% SOC | 100% SOC | |||
| F | 29.45 ± 0.3 | 29.04 ± 0.19 | - | - |
| AA | 24.76 ± 1.0 | 25.16 ± 0.45 | −4.7 (16%) | −3.9 (−13.5%) |
| RA | 27.27 ± 0.21 | 28.62 ± 0.20 | −2.2 (−7.4%) | −0.4 (1.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, S.M.; Gstrein, G.; Jauernig, A.D.; Schmid, A.; Michelini, E.; Hinterberger, M.; Ellersdorfer, C. Influence of Lithium Plating on the Mechanical Properties of Automotive High-Energy Pouch Batteries. Batteries 2025, 11, 330. https://doi.org/10.3390/batteries11090330
Abbas SM, Gstrein G, Jauernig AD, Schmid A, Michelini E, Hinterberger M, Ellersdorfer C. Influence of Lithium Plating on the Mechanical Properties of Automotive High-Energy Pouch Batteries. Batteries. 2025; 11(9):330. https://doi.org/10.3390/batteries11090330
Chicago/Turabian StyleAbbas, Syed Muhammad, Gregor Gstrein, Alois David Jauernig, Alexander Schmid, Emanuele Michelini, Michael Hinterberger, and Christian Ellersdorfer. 2025. "Influence of Lithium Plating on the Mechanical Properties of Automotive High-Energy Pouch Batteries" Batteries 11, no. 9: 330. https://doi.org/10.3390/batteries11090330
APA StyleAbbas, S. M., Gstrein, G., Jauernig, A. D., Schmid, A., Michelini, E., Hinterberger, M., & Ellersdorfer, C. (2025). Influence of Lithium Plating on the Mechanical Properties of Automotive High-Energy Pouch Batteries. Batteries, 11(9), 330. https://doi.org/10.3390/batteries11090330









