Interfacial Layer (“Interlayer”) Addition to Improve Active Material Utilisation in Lithium–Sulfur Batteries: Use of a Phenylsulfonated MWCNT Film
Abstract
1. Introduction
| Format | Functionalisation | Method of Addition | Cell Capacity (mA h g−1sulfur) | Improvement (mA h g−1sulfur) | C-Rate (h−1) | Refs |
|---|---|---|---|---|---|---|
| Cathode | Methylsulfonation | Strecker sulfite alkylation | ~820 (@ 100th cycle) | + ~270 (@ 100th cycle) | 0.1 | [17] |
| Cathode | PEI + | Grafted to CNT surface | ~750 (@ 100th cycle) | + ~350 (@ 100th cycle) | 0.5 | [19] * |
| Cathode | N-doped CNTs grown on Co3O4 | Spray pyrolysis on Co3O4 | ~700 (@ 400th cycle) | + ~460 (@ 400th cycle) | 1.0 | [20] * |
| Cathode | N-doped | Melamine addition during polymerisation | ~963 (@ 100th cycle) | + ~281 (@ 100th cycle) | 1.0 | [21] * |
| Cathode/ Interlayer | O or H-doping | Annealing | ~750 (@ 250th cycle) | + ~480 (@ 250th cycle) | 0.5 | [22] * |
| Separator coating | Phenylsulfonation | Diazotisation of rGO ‡ with sulfamic acid | ~930 (@ 100th cycle) | + ~286 (@ 100th cycle) | 0.5 | [23] * |
| Separator coating | rGO ‡-PEDOT:PSS ◊ | Air-controlled Electrospray | ~813 (@ 100th cycle) | + ~516 (@ 100th cycle) | 0.5 | [24] * |
| Separator coating | Mixing with PANi 0 † | Filtering a dispersion | ~400 (@ 50th cycle) | + ~50 (@ 50th cycle) | 0.2 | [25] * |
2. Materials and Methods
2.1. Chemicals and Electrolyte Composition
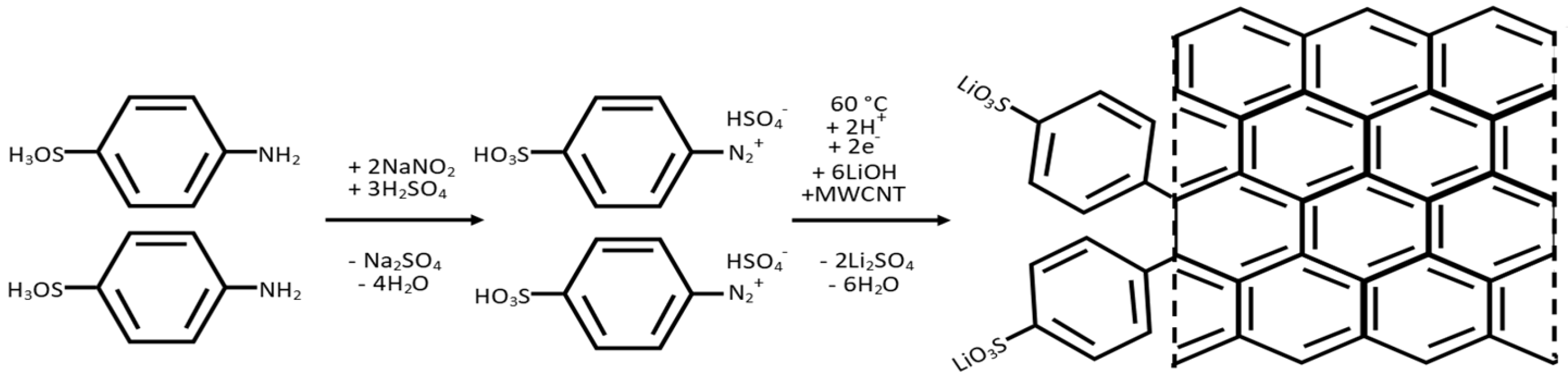
2.2. Functionalisation of Multiwalled Carbon Nanotubes
2.3. Fabrication of a Functionalised MWCNT Interlayer Film
2.4. Fabrication of an Unfunctionalised MWCNT Interlayer Film
2.5. Cell Production
2.6. Characterisation
3. Results and Discussion
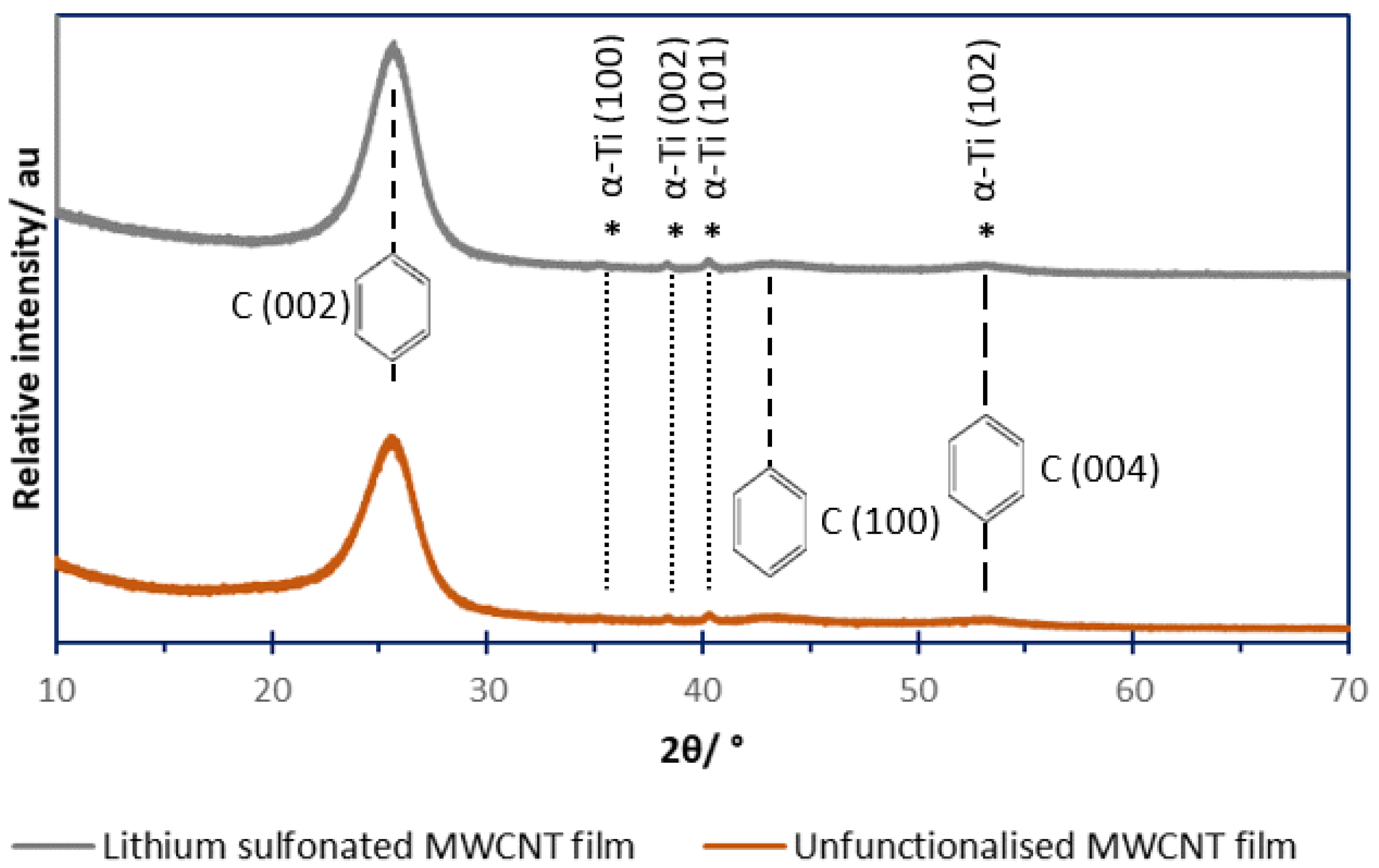
3.1. Powder X-Ray Diffractometry (XRD)
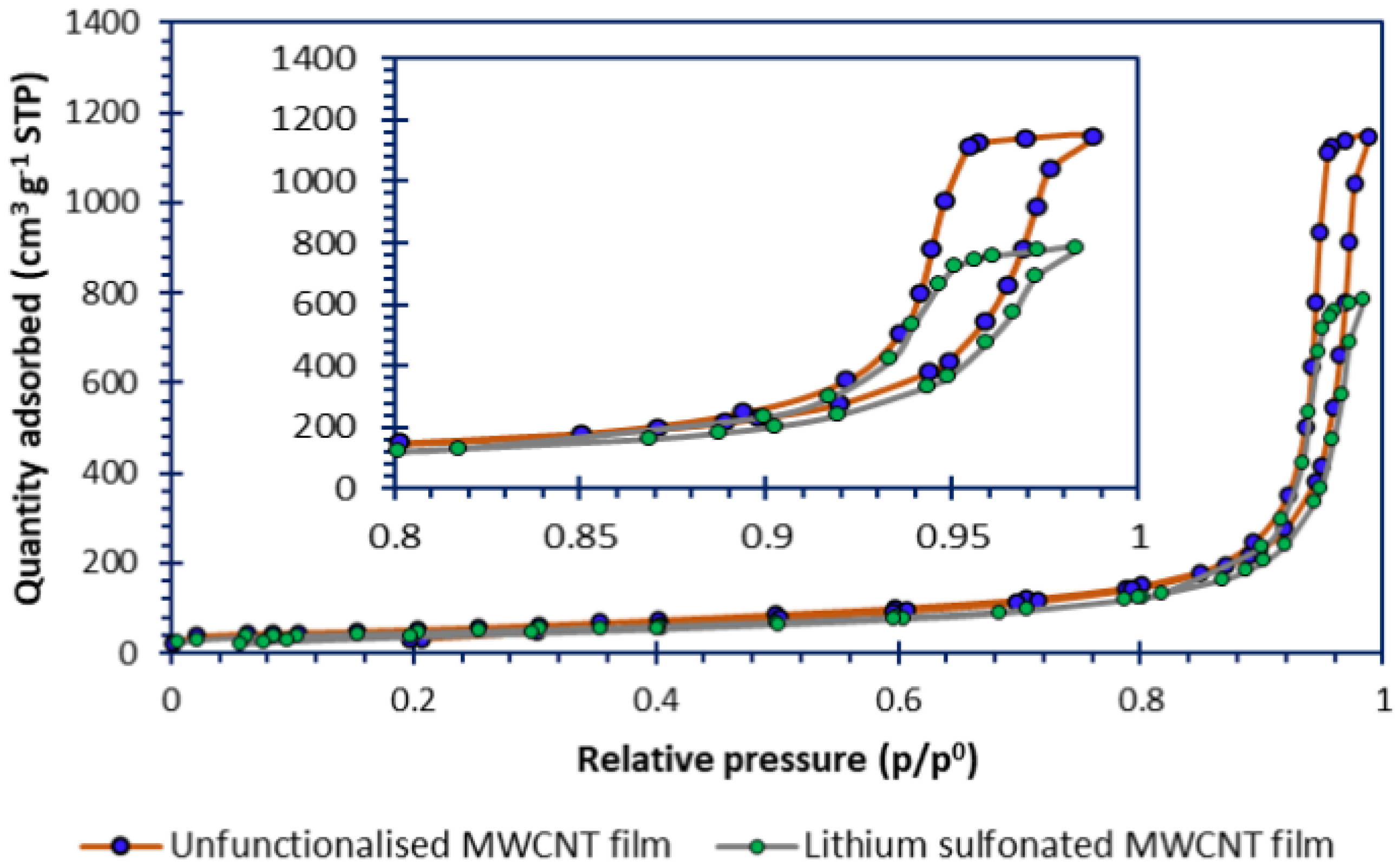
3.2. Nitrogen Sorptiometry
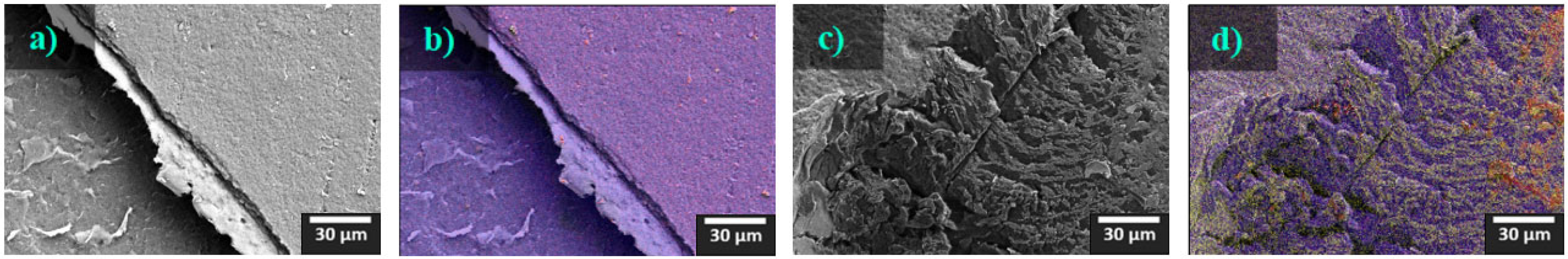
3.3. Scanning Electron Microscopy (SEM)
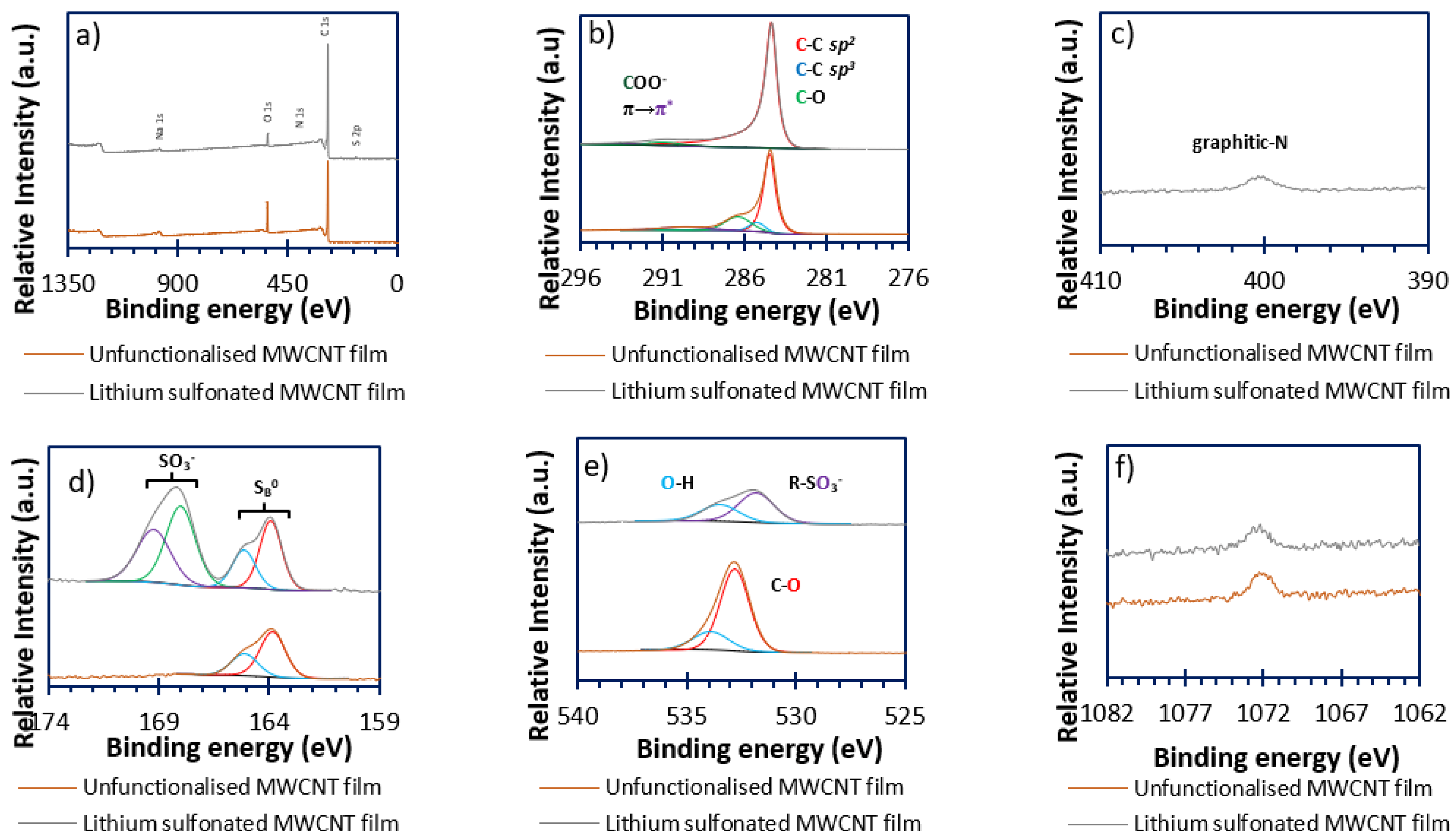
3.4. X-Ray Photoelectron Spectroscopy (XPS)
3.4.1. Carbon (Figure 5b)
3.4.2. Nitrogen (Figure 5c)
3.4.3. Sulfur (Figure 5d)
3.4.4. Oxygen (Figure 5e)
3.4.5. Sodium (Figure 5f)
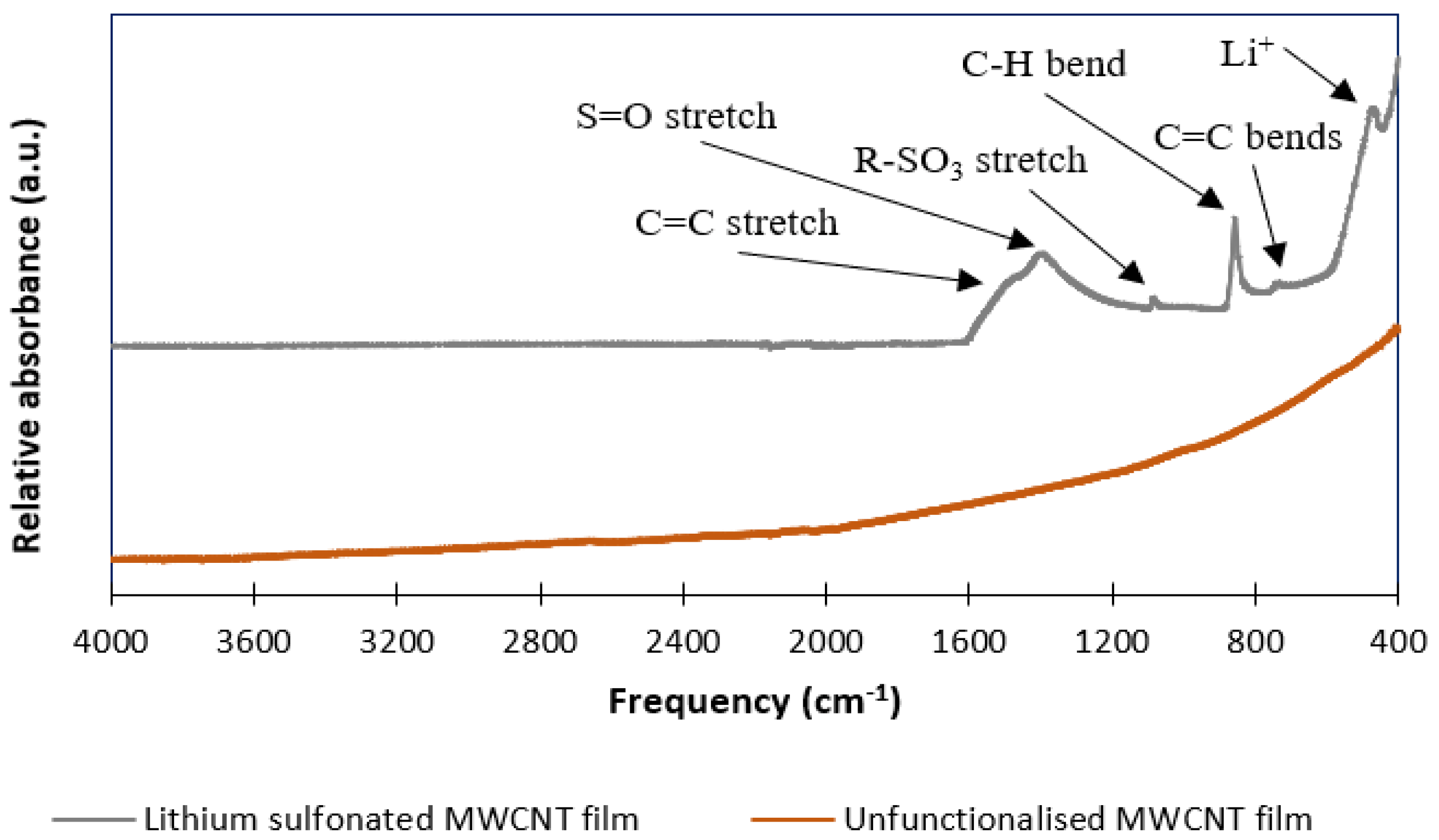
3.5. Infrared (IR) Spectroscopy
3.6. Raman Spectroscopy
3.7. Elemental Microanalysis
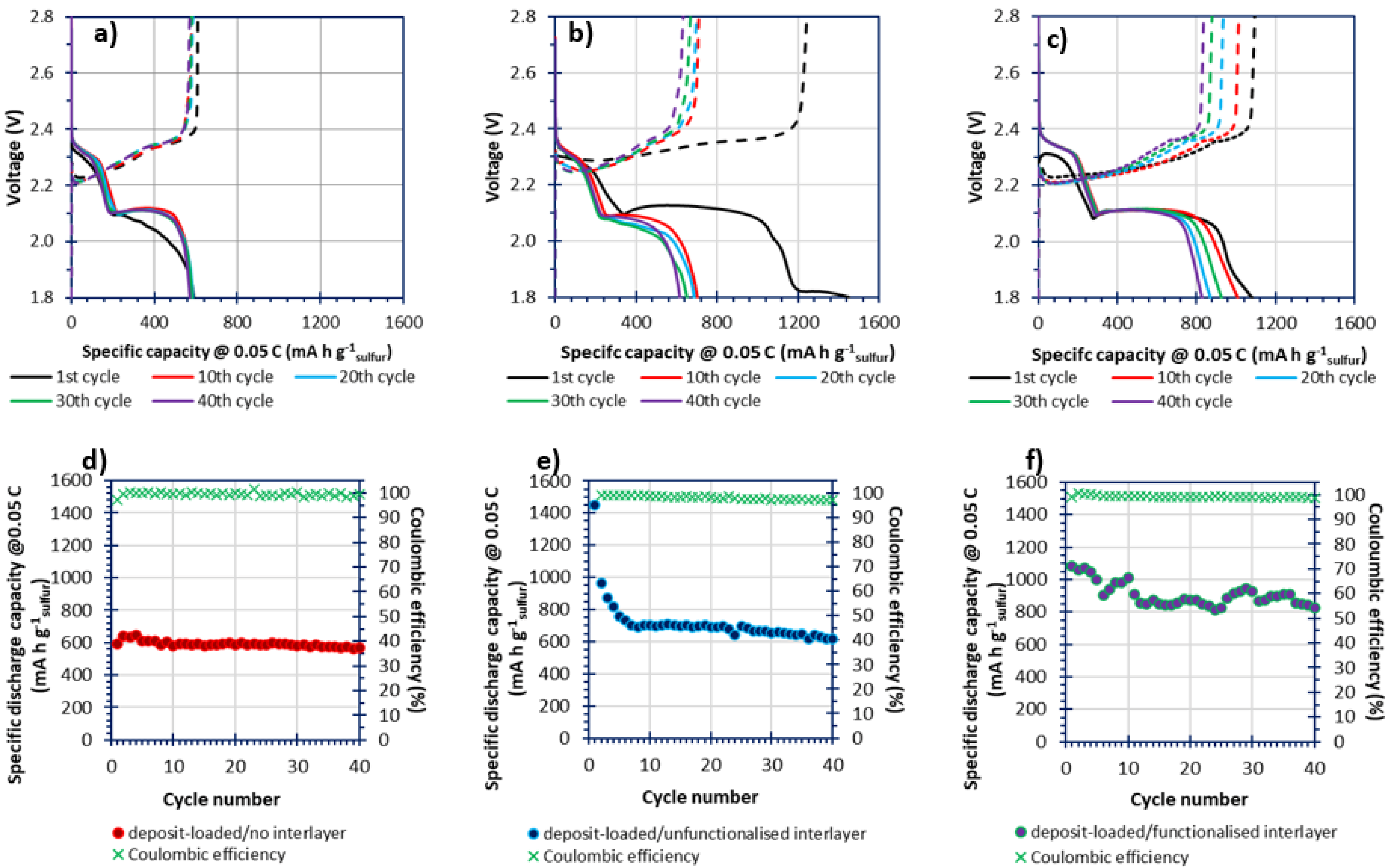
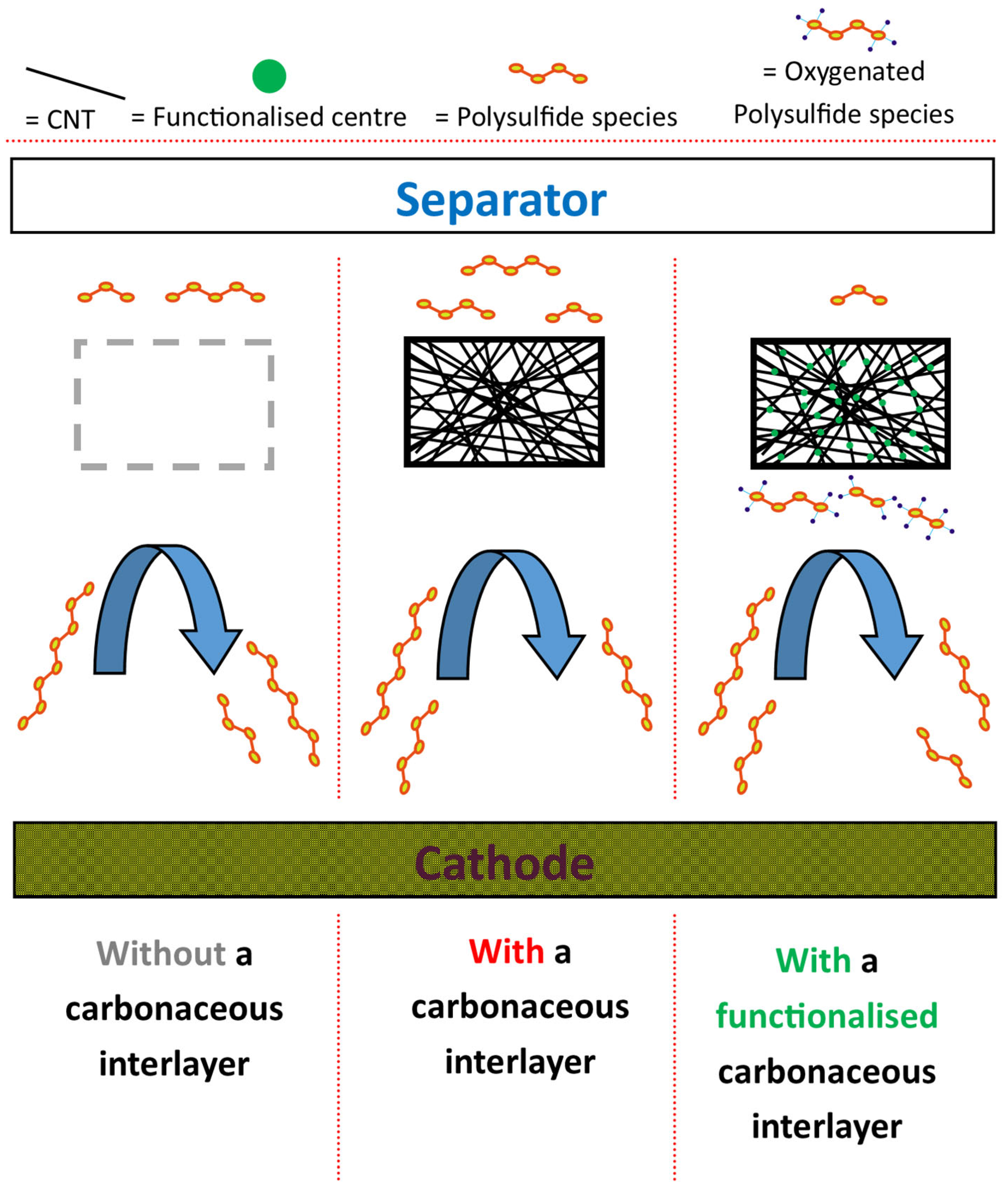
3.8. In-Cell Electrochemical Investigations via GDC Cycling
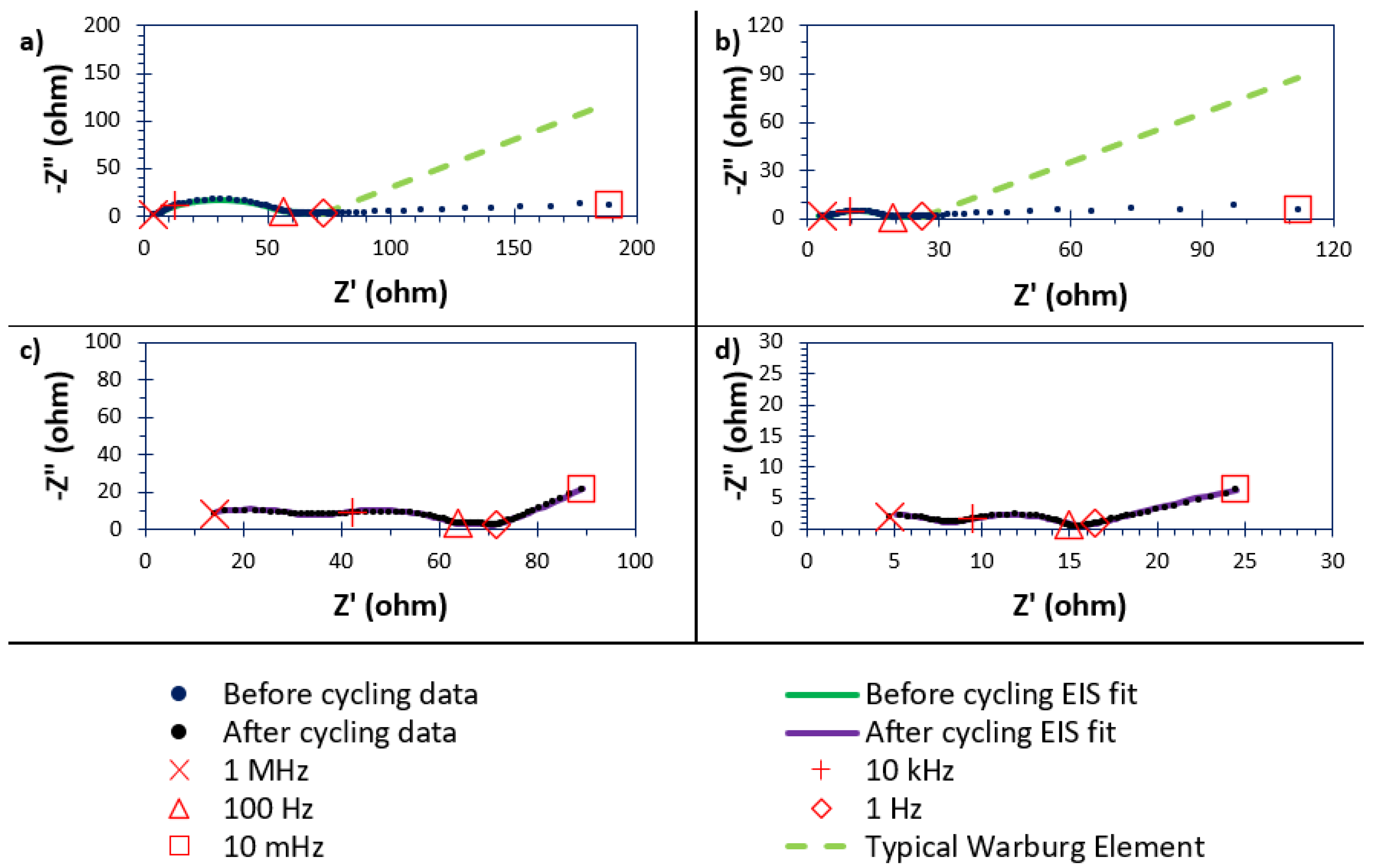
3.9. In-Cell Studies Using Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MWCNT | multiwalled carbon nanotubes |
| LSB | Lithium–sulfur battery |
| LIB | Lithium-ion battery |
| SB0 | Bridging sulfur |
| ST1− | Polysulfide termini |
| CNT | Carbon nanotubes |
| E/S | Electrolyte-to-sulfur |
| ACS | American chemical society |
| CTAB | Cetyltrimethyl ammonium bromide |
| NMP | N-methyl 2-pyrolidone |
| PVDF | Polyvinylidene difluoride |
| MW | Molecular weight |
| GPC | Gel permeation chromatography |
| DME | 1,2-Dimethoxyethane |
| DOL | 1,3-Dioxolane |
| BHT | Butylated hydroxytoluene |
| LiTFSI | Lithium bis(trifluromethanesulfonyl)imide |
| HEPA | High-efficiency particulate air |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| IR | Infrared |
| UATR | Universal attenuated total reflectance |
| FT | Fourier transform |
| SEM | Scanning electron microscopy |
| EDX | Energy-dispersive X-ray |
| CHNS | Carbon, hydrogen, nitrogen and sulfur |
| DFC | Dynamic flash combustion |
| TDC | Thermal conductivity detector |
| GDC | Galvanic discharge–charge |
| EIS | Electrochemical impedance spectroscopy |
| OCV | Open-circuit voltage |
| SEI | Solid–electrolyte interface |
| Bet | Brunauer–Emmett–Teller |
| LCO | Lithium cobalt oxide |
| LGPS | Li10GeP2S12 |
References
- Zhu, J.; Zou, J.; Cheng, H.; Gu, Y.; Lu, Z. High Energy Batteries Based on Sulfur Cathode. Green Energy Environ. 2019, 4, 345–359. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Nakamura, N.; Ahn, S.; Momma, T.; Osaka, T. Future Potential for Lithium-Sulfur Batteries. J. Power Sources 2023, 558, 232566. [Google Scholar] [CrossRef]
- Danner, T.; Latz, A. On the Influence of Nucleation and Growth of S8 and Li2S in Lithium-Sulfur Batteries. Electrochim. Acta 2019, 322, 134719. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.-W. Antimicrobial Activity of Sulfur Nanoparticles: Effect of Preparation Methods. Arab. J. Chem. 2020, 13, 6580–6588. [Google Scholar] [CrossRef]
- Xiao, R.; Yu, T.; Yang, S.; Chen, K.; Li, Z.; Liu, Z.; Hu, T.; Hu, G.; Li, J.; Cheng, H.M.; et al. Electronic Structure Adjustment of Lithium Sulfide by a Single-Atom Copper Catalyst toward High-Rate Lithium-Sulfur Batteries. Energy Storage Mater. 2022, 51, 890–899. [Google Scholar] [CrossRef]
- Feng, Z.; Kim, C.; Vijh, A.; Armand, M.; Bevan, K.H.; Zaghib, K. Unravelling the Role of Li2S2 in Lithium-Sulfur Batteries: A First Principles Study of Its Energetic and Electronic Properties. J. Power Sources 2014, 272, 518–521. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Xu, J.; Guo, Z. Advances in Polar Materials for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707520. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, S.; Zhong, L.; Wang, S.; Han, D.; Ren, S.; Xiao, M.; Meng, Y. Organic Liquid Electrolytes in Li-S Batteries: Actualities and Perspectives. Energy Storage Mater. 2021, 34, 128–147. [Google Scholar] [CrossRef]
- Shutthanandan, V.; Nandasiri, M.; Zheng, J.; Engelhard, M.H.; Xu, W.; Thevuthasan, S.; Murugesan, V. Applications of XPS in the Characterization of Battery Materials. J. Electron Spectros. Relat. Phenom. 2019, 231, 2–10. [Google Scholar] [CrossRef]
- Fan, Y.; Niu, Z.; Zhang, F.; Zhang, R.; Zhao, Y.; Lu, G. Suppressing the Shuttle Effect in Lithium-Sulfur Batteries by a UiO-66-Modified Polypropylene Separator. ACS Omega 2019, 4, 10328–10335. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Li, X.; Xiao, W.; Chen, Z.; Lu, J. Interlayer Material Selection for Lithium-Sulfur Batteries. Joule 2019, 3, 361–386. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.; Wei, C.; Wang, Z.; Xi, B.; Xiong, S.; Feng, J. Triggering the Electronic Microenvironment of Extraordinary Nitrogen-Bridged Atomic Iron Coordinated with in-Plane Nitrogen by Manipulating Phase-Reconfigured 2D Vanadium Nitride MXenes toward Invigorated Lithium–Sulfur Batteries. Energy Environ. Sci. 2024, 17, 7403–7415. [Google Scholar] [CrossRef]
- Lee, B.-J.; Zhao, C.; Yu, J.-H.; Kang, T.-H.; Park, H.-Y.; Kang, J.; Jung, Y.; Liu, X.; Li, T.; Xu, W.; et al. Development of High-Energy Non-Aqueous Lithium-Sulfur Batteries via Redox-Active Interlayer Strategy. Nat. Commun. 2022, 13, 4629. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ko, Y.i.; Kim, Y.A.; Kim, K.S.; Yang, C.M. Sulfur-Doped Carbon Nanotubes as a Conducting Agent in Supercapacitor Electrodes. J. Alloys Compd. 2021, 855, 157282. [Google Scholar] [CrossRef]
- Salice, P.; Fabris, E.; Sartorio, C.; Fenaroli, D.; Figà, V.; Casaletto, M.P.; Cataldo, S.; Pignataro, B.; Menna, E. An Insight into the Functionalisation of Carbon Nanotubes by Diazonium Chemistry: Towards a Controlled Decoration. Carbon 2014, 74, 73–82. [Google Scholar] [CrossRef]
- Fretz, S.J.; Pal, U.; Girard, G.M.A.; Howlett, P.C.; Palmqvist, A.E.C. Lithium Sulfonate Functionalization of Carbon Cathodes as a Substitute for Lithium Nitrate in the Electrolyte of Lithium–Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2002485. [Google Scholar] [CrossRef]
- Guthrie, J.P. Hydrolysis of Esters of Oxy Acids: PKa Values for Strong Acids; Brønsted Relationship for Attack of Water at Methyl; Free Energies of Hydrolysis of Esters of Oxy Acids; and a Linear Relationship between Free Energy of Hydrolysis and PKa Holding over a Ran. Can. J. Chem. 1978, 56, 2342–2354. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li-S Batteries Using Amine-Functionalized Carbon Nanotubes in the Cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, J.K.; Kang, Y.C. Yolk–Shell Structured Assembly of Bamboo-Like Nitrogen-Doped Carbon Nanotubes Embedded with Co Nanocrystals and Their Application as Cathode Material for Li–S Batteries. Adv. Funct. Mater. 2018, 28, 1705264. [Google Scholar] [CrossRef]
- Gaikwad, M.M.; Sarode, K.K.; Pathak, A.D.; Sharma, C.S. Ultrahigh Rate and High-Performance Lithium-Sulfur Batteries with Resorcinol-Formaldehyde Xerogel Derived Highly Porous Carbon Matrix as Sulfur Cathode Host. Chem. Eng. J. 2021, 425, 131521. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Kim, K.; Pol, V.G. Towards Highly Stable Lithium Sulfur Batteries: Surface Functionalization of Carbon Nanotube Scaffold. Carbon 2018, 131, 175–183. [Google Scholar] [CrossRef]
- Lu, Y.; Gu, S.; Guo, J.; Rui, K.; Chen, C.; Zhang, S.; Jin, J.; Yang, J.; Wen, Z. Sulfonic Groups Originated Dual-Functional Interlayer for High Performance Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2017, 9, 14878–14888. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, J.; Kim, S.W.; Halim, W.; Frey, M.W.; Joo, Y.L. Effective Suppression of the Polysulfide Shuttle Effect in Lithium-Sulfur Batteries by Implementing RGO-PEDOT:PSS-Coated Separators via Air-Controlled Electrospray. ACS Omega 2018, 3, 16465–16471. [Google Scholar] [CrossRef]
- Chang, C.H.; Chung, S.H.; Manthiram, A. Ultra-Lightweight PANiNF/MWCNT-Functionalized Separators with Synergistic Suppression of Polysulfide Migration for Li-S Batteries with Pure Sulfur Cathodes. J. Mater. Chem. A 2015, 3, 18829–18834. [Google Scholar] [CrossRef]
- Barter, L.D.J.; Mohammad, I.; Hinder, S.J.; Watts, J.F.; Slade, R.C.T.; Crean, C. Carbons Derived from Resole-Type Phenolic Resins for Use in Lithium–Sulfur Batteries: Templating the Resins with Sulfur Leads to Enhanced Cell Performance. Energy Adv. 2024, 3, 471–481. [Google Scholar] [CrossRef]
- Renda, C.G.; Bertholdo, R. Study of Phenolic Resin and Their Tendency for Carbon Graphitization. J. Polym. Res. 2018, 25, 241. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural Parameters from X-Ray Diffraction Peak Broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Webster, T.J. 3.32 Carbon Nanotubes: Applications for In Situ Implant Sensors. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 703–715. ISBN 978-0-08-100692-4. [Google Scholar]
- Azman, N.H.N.; Sulaiman, Y. Hierarchical Porous Materials for Supercapacitors. In Encyclopedia of Energy Storage; Cabeza, L.F., Ed.; Elsevier: Oxford, UK, 2022; pp. 622–637. ISBN 978-0-12-819730-1. [Google Scholar]
- Hippauf, F.; Nickel, W.; Hao, G.P.; Schwedtmann, K.; Giebeler, L.; Oswald, S.; Borchardt, L.; Doerfler, S.; Weigand, J.J.; Kaskel, S. The Importance of Pore Size and Surface Polarity for Polysulfide Adsorption in Lithium Sulfur Batteries. Adv. Mater. Interfaces 2016, 3, 1600508. [Google Scholar] [CrossRef]
- Chen, X.; Gross, A.J.; Giroud, F.; Holzinger, M.; Cosnier, S. Comparison of Commercial and Lab-Made MWCNT Buckypaper: Physicochemical Properties and Bioelectrocatalytic O2 Reduction. Electroanalysis 2018, 30, 1511–1520. [Google Scholar] [CrossRef]
- Khan, Z.U.; Kausar, A.; Ullah, H. A Review on Composite Papers of Graphene Oxide, Carbon Nanotube, Polymer/GO, and Polymer/CNT: Processing Strategies, Properties, and Relevance. Polym. Plast. Technol. Eng. 2016, 55, 559–581. [Google Scholar] [CrossRef]
- Ensling, D.; Stjerndahl, M.; Nytén, A.; Gustafsson, T.; Thomas, J.O. A Comparative XPS Surface Study of Li2FeSiO4/C Cycled with LiTFSI- and LiPF6-Based Electrolytes. J. Mater. Chem. 2009, 19, 82–88. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Wang, Z.; Wang, X.; Lin, S. The Removal Mechanism and Performance of Tetrabromobisphenol A with a Novel Multi-Group Activated Carbon from Recycling Long-Root: Eichhornia Crassipes Plants. RSC Adv. 2019, 9, 24760–24769. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Chen, J.; Zhao, J.; Zheng, J.; Song, C.; Li, L.; Zhu, Z. Synthesis of Highly Nitrogen-Doped Hollow Carbon Nanoparticles and Their Excellent Electrocatalytic Properties in Dye-Sensitized Solar Cells. J. Mater. Chem. 2010, 20, 10829–10834. [Google Scholar] [CrossRef]
- Lee, M.-T.; Liu, H.; Brandell, D. The Surface Chemistry of Thin Lithium Metal Electrodes in Lithium-Sulfur Cells. Batter. Supercaps 2020, 3, 1370–1376. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Ramalinga, K.; Gattu, B.; Datta, M.K.; Kumta, P.N. Sulfonic Acid Based Complex Framework Materials (CFM): Nanostructured Polysulfide Immobilization Systems for Rechargeable Lithium–Sulfur Battery. J. Electrochem. Soc. 2019, 166, A1827–A1835. [Google Scholar] [CrossRef]
- Rojas, J.V.; Toro-Gonzalez, M.; Molina-Higgins, M.C.; Castano, C.E. Facile Radiolytic Synthesis of Ruthenium Nanoparticles on Graphene Oxide and Carbon Nanotubes. Mater. Sci. Eng. B 2016, 205, 28–35. [Google Scholar] [CrossRef]
- Koushik, D.; Verhees, W.J.H.; Zhang, D.; Kuang, Y.; Veenstra, S.; Creatore, M.; Schropp, R.E.I. Atomic Layer Deposition Enabled Perovskite/PEDOT Solar Cells in a Regular n–i–p Architectural Design. Adv. Mater. Interfaces 2017, 4, 1700043. [Google Scholar] [CrossRef]
- Dubina, E.; Plank, J.; Black, L. Impact of Water Vapour and Carbon Dioxide on Surface Composition of C3A Polymorphs Studied by X-Ray Photoelectron Spectroscopy. Cem. Concr. Res. 2015, 73, 36–41. [Google Scholar] [CrossRef]
- Parker, S.F.; Revill-Hivet, E.J.; Nye, D.W.; Gutmann, M.J. Structure and Vibrational Spectroscopy of Lithium and Potassium Methanesulfonates. R. Soc. Open Sci. 2020, 7, 200776. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, S.; Wang, R.; Jiang, M.; Han, J.; Gu, T.; Liu, M.; Cheng, S.; Wang, K. Lithium Sulfonate/Carboxylate-Anchored Polyvinyl Alcohol Separators for Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18310–18315. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Caoa, H.; Chena, Y.P. Raman Spectroscopy of Graphene and Related Materials. In New Developments in Photon and Materials Research; Jang, J.I., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 403–418. ISBN 9781626183391. [Google Scholar]
- Mohan, S.; Oluwafemi, O.S.; Songca, S.P.; Rouxel, D.; Miska, P.; Lewu, F.B.; Kalarikkal, N.; Thomas, S. Completely Green Synthesis of Silver Nanoparticle Decorated MWCNT and Its Antibacterial and Catalytic Properties. Pure Appl. Chem. 2016, 88, 71–81. [Google Scholar] [CrossRef]
- Alía, J.M.; Edwards, H.G.M.; Kiernan, B.M. Raman Spectroscopy of Benzenesulfonic and 4-Toluenesulfonic Acids Dissolved in Dimethylsulfoxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Zerrin, T.; Shang, R.; Dong, B.; Aguilar, E.C.; Malvin, J.; Ozkan, M.; Ozkan, C.S. An Overlooked Parameter in Li-S Batteries: The Impact of Electrolyte-to-Sulfur Ratio on Capacity Fading. Nano Energy 2022, 104, 107913. [Google Scholar] [CrossRef]
- Wang, M.; Xia, X.; Zhong, Y.; Wu, J.; Xu, R.; Yao, Z.; Wang, D.; Tang, W.; Wang, X.; Tu, J. Porous Carbon Hosts for Lithium–Sulfur Batteries. Chem. A Eur. J. 2019, 25, 3710–3725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. A Perspective toward Practical Lithium–Sulfur Batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Adair, K.R.; Zhang, H.; Sun, X. Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application. Electrochem. Energy Rev. 2018, 1, 239–293. [Google Scholar] [CrossRef]
- Jozwiuk, A.; Berkes, B.B.; Weiß, T.; Sommer, H.; Janek, J.; Brezesinski, T. The Critical Role of Lithium Nitrate in the Gas Evolution of Lithium-Sulfur Batteries. Energy Environ. Sci. 2016, 9, 2603–2608. [Google Scholar] [CrossRef]
- Blanchard, D.; Slagter, M. In Operando Raman and Optical Study of Lithium Polysulfides Dissolution in Lithium–Sulfur Cells with Carrageenan Binder. J. Phys. Energy 2021, 3, 044003. [Google Scholar] [CrossRef]
- Bondy, A.L.; Craig, R.L.; Zhang, Z.; Gold, A.; Surratt, J.D.; Ault, A.P. Isoprene-Derived Organosulfates: Vibrational Mode Analysis by Raman Spectroscopy, Acidity-Dependent Spectral Modes, and Observation in Individual Atmospheric Particles. J. Phys. Chem. A 2018, 122, 303–315. [Google Scholar] [CrossRef]
- Xu, L.; Csekő, G.; Petz, A.; Horváth, A.K. Kinetics and Mechanism of the Oxidation of Pentathionate Ion by Chlorine Dioxide in a Slightly Acidic Medium. J. Phys. Chem. A 2014, 118, 1293–1299. [Google Scholar] [CrossRef]
- Borah, R.; Hughson, F.R.; Johnston, J.; Nann, T. On Battery Materials and Methods. Mater. Today Adv. 2020, 6, 100046. [Google Scholar] [CrossRef]
- Li, J.; Dai, L.; Wang, Z.; Wang, H.; Xie, L.; Chen, J.; Yan, C.; Yuan, H.; Wang, H.; Chen, C. Cellulose Nanofiber Separator for Suppressing Shuttle Effect and Li Dendrite Formation in Lithium-Sulfur Batteries. J. Energy Chem. 2022, 67, 736–744. [Google Scholar] [CrossRef]
- Zhe, R.; Zhu, T.; Wei, X.; Ren, Y.; Qing, C.; Li, N.; Wang, H.E. Graphene Oxide Wrapped Hollow Mesoporous Carbon Spheres as a Dynamically Bipolar Host for Lithium-Sulfur Batteries. J. Mater. Chem. A 2022, 10, 24422–24433. [Google Scholar] [CrossRef]
- Pathak, R.; Chen, K.; Gurung, A.; Reza, K.M.; Bahrami, B.; Pokharel, J.; Baniya, A.; He, W.; Wu, F.; Zhou, Y.; et al. Fluorinated Hybrid Solid-Electrolyte-Interphase for Dendrite-Free Lithium Deposition. Nat. Commun. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Andrei, P.; Zheng, J.P. Stable Cycling of Lithium-Sulfur Batteries by Optimizing the Cycle Condition. Electrochim. Acta 2019, 326, 134948. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Yang, J.; Wang, J.; Yao, L.; Yao, X.; Cui, P.; Xu, X. Interface Re-Engineering of Li10GeP2S12 Electrolyte and Lithium Anode for All-Solid-State Lithium Batteries with Ultralong Cycle Life. ACS Appl. Mater. Interfaces 2018, 10, 2556–2565. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, W.; Si, Y.; Wang, D.; Fu, Y.; Manthiram, A. Artificial Dual Solid-Electrolyte Interfaces Based on in Situ Organothiol Transformation in Lithium Sulfur Battery. Nat. Commun. 2021, 12, 3031. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef]
| Sample | Specific Surface Area # (m2 g−1) |
|---|---|
| Unfunctionalised MWCNT film | 197.4 ± 2.2 |
| Functionalised MWCNT film | 163.2 ± 0.4 |
| Sample | C (%atom) | N (%atom) | S (%atom) | O (%atom) | Na (%atom) |
|---|---|---|---|---|---|
| Unfunctionalised MWCNT film | 87.3 | <LOD | 0.4 | 12.1 | 0.1 |
| Fuctionalised MWCNT film | 92.7 | 0.6 | 1.1 | 5.5 | 0.1 |
| Sample | C (%mass) | H (%mass) | N (%mass) | S (%mass) |
|---|---|---|---|---|
| Unfunctionalised MWCNT film | 86.50 | 2.01 | <LOD | 0.66 |
| Functionalised MWCNT film | 85.98 | 2.10 | 0.62 | 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barter, L.D.J.; Hinder, S.J.; Watts, J.F.; Slade, R.C.T.; Crean, C. Interfacial Layer (“Interlayer”) Addition to Improve Active Material Utilisation in Lithium–Sulfur Batteries: Use of a Phenylsulfonated MWCNT Film. Batteries 2025, 11, 266. https://doi.org/10.3390/batteries11070266
Barter LDJ, Hinder SJ, Watts JF, Slade RCT, Crean C. Interfacial Layer (“Interlayer”) Addition to Improve Active Material Utilisation in Lithium–Sulfur Batteries: Use of a Phenylsulfonated MWCNT Film. Batteries. 2025; 11(7):266. https://doi.org/10.3390/batteries11070266
Chicago/Turabian StyleBarter, Luke D. J., Steven J. Hinder, John F. Watts, Robert C. T. Slade, and Carol Crean. 2025. "Interfacial Layer (“Interlayer”) Addition to Improve Active Material Utilisation in Lithium–Sulfur Batteries: Use of a Phenylsulfonated MWCNT Film" Batteries 11, no. 7: 266. https://doi.org/10.3390/batteries11070266
APA StyleBarter, L. D. J., Hinder, S. J., Watts, J. F., Slade, R. C. T., & Crean, C. (2025). Interfacial Layer (“Interlayer”) Addition to Improve Active Material Utilisation in Lithium–Sulfur Batteries: Use of a Phenylsulfonated MWCNT Film. Batteries, 11(7), 266. https://doi.org/10.3390/batteries11070266









