Enhancing the Cycle Life of Silicon Oxide–Based Lithium-Ion Batteries via a Nonflammable Fluorinated Ester–Based Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrolyte
2.2. Electrochemistry
2.3. Characterization
3. Results and Discussion
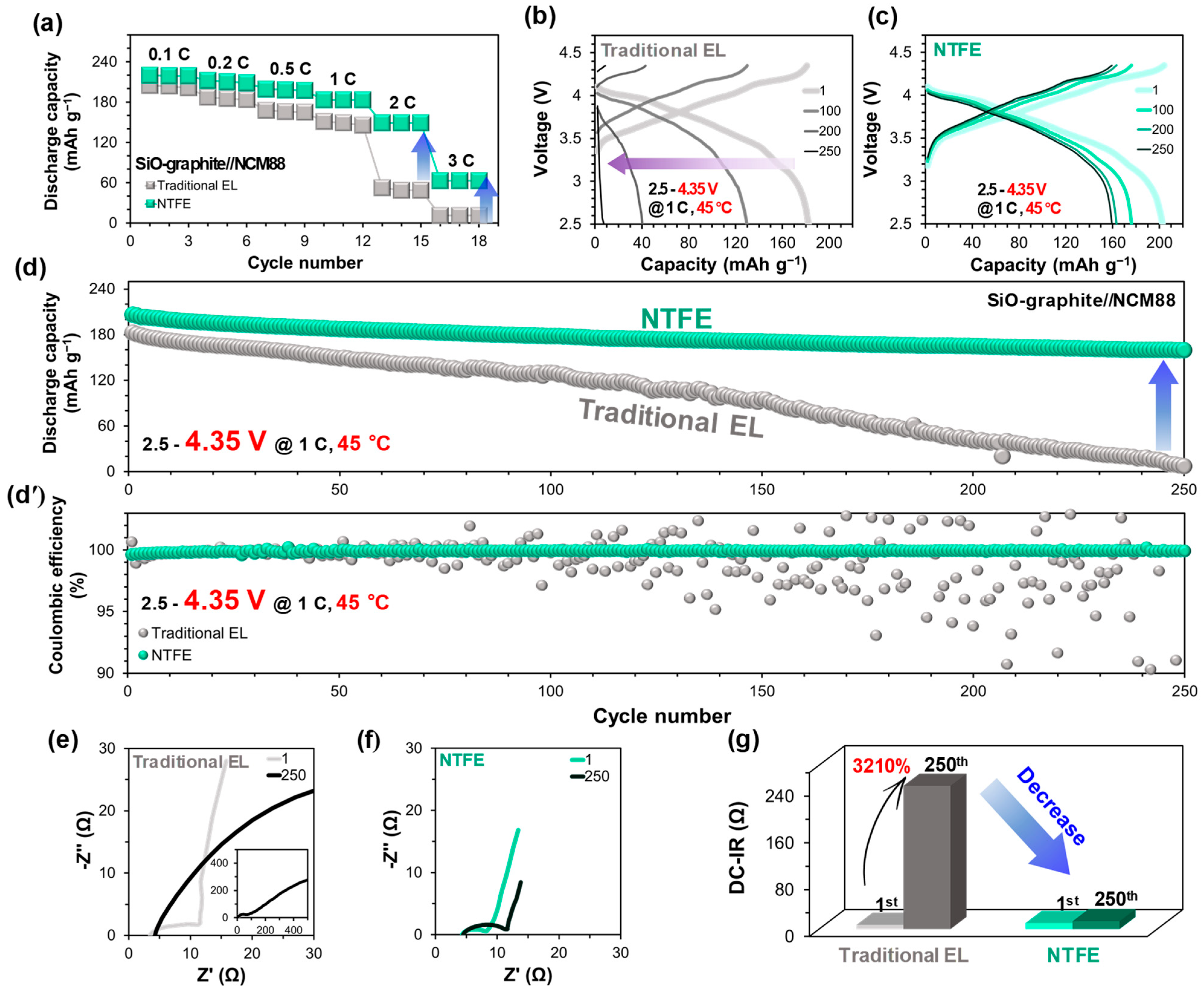
3.1. NTFE-Induced Performance Improvement and Resistance Reduction
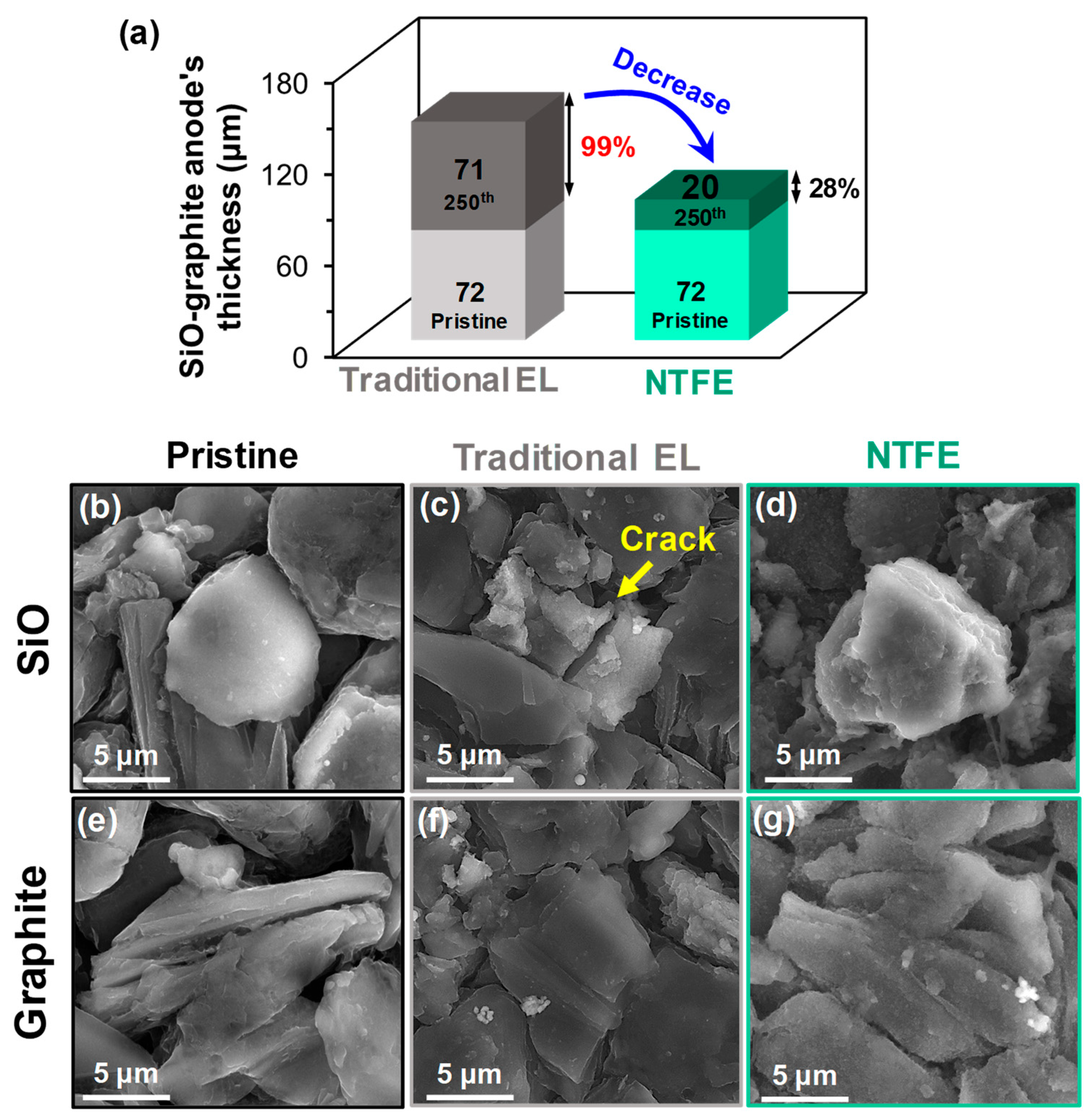
3.2. Suppressed Swelling of the SiO–graphite Anode by a Uniform and Robust SEI Layer
3.3. Mitigated Surface Changes and Metal Dissolution on the NCM88 Cathode via SEI Stabilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: The Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, J.W.; Sung, Y.-E.; Oh, S.M. Failure Modes of Silicon Powder Negative Electrode in Lithium Secondary Batteries. Electrochem. Solid-State Lett. 2004, 7, A306–A309. [Google Scholar] [CrossRef]
- Weydanz, W.J.; Wohlfahrt-Mehrens, M.; Huggins, R.A. A Room Temperature Study of the Binary Lithium−Silicon and the Ternary Lithium−Chromium−Silicon System for Use in Rechargeable Lithium Batteries. J. Power Sources 1999, 81–82, 237–242. [Google Scholar] [CrossRef]
- Beattie, S.D.; Larcher, D.; Morcrette, M.; Simon, B.; Tarascon, J.-M. Si Electrodes for Li-Ion Batteries—A New Way to Look at an Old Problem. J. Electrochem. Soc. 2008, 155, A158–A163. [Google Scholar] [CrossRef]
- Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.-G.; Liu, J. Mesoporous Silicon Sponge as an Anti-pulverization Structure for High-performance Lithium-Ion Battery anodes. Nat. Commun. 2014, 5, 4105. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Kang, D.G.; Lee, H.G.; Tran, Y.H.T.; An, K.; Choi, J.; Roh, K.C.; Kim, D.Y.; Song, S.-W. Stable Operation of Highly Loaded Pure Si-Fe Anode under Ambient Pressure via Carboxy Silane-directed Robust Solid Electrolyte Interphase. J. Energy Chem. 2024, 94, 568–576. [Google Scholar] [CrossRef]
- Nadimpalli, S.P.V.; Sethuraman, V.A.; Dalavi, S.; Lucht, B.; Chon, M.J.; Shenoy, V.B.; Guduru, P.R. Quantifying Capacity Loss due to Solid-Electrolyte-Interphase Layer Formation on Silicon Negative Electrodes in Lithium-Ion Batteries. J. Power Sources 1999, 215, 145–151. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved Performance of the Silicon Anode for Li-Ion Batteries: Understanding the Surface Modification Mechanism of Fluoroethylene Carbonate as an Effective Electrolyte Additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Yang, J.; Takeda, Y.; Imanichi, N.; Capiglia, C.; Xie, J.Y.; Yamamoto, O. SiOx-Based Anodes for Secondary Lithium Batteries. Solid State Ion. 2002, 152–153, 125–129. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Choi, H.; Song, S.-W. Roles of Oxygen and Interfacial Stabilization in Enhancing the Cycling Ability of Silicon Oxide Anodes for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2013, 160, A906–A914. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Li, Z.; Takenaka, N.; Liu, Y.; Zou, H.; Chen, W.; Du, M.; Hong, X.-J.; Shang, R.; et al. Rational Electrolyte Design to Form Inorganic–Polymeric Interphase on Silicon-Based Anodes. ACS Energy Lett. 2021, 6, 1811–1820. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, N.; Yang, D. Designing Superior Solid Electrolyte Interfaces on Silicon Anodes for High-performance Lithium-Ion Batteries. Nanoscale 2019, 11, 19086–19104. [Google Scholar]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly Elastic Binders Integrating Polyrotaxanes for Silicon Microparticle Anodes in Lithium Ion Batteries. Science 2017, 357, 279–283. [Google Scholar] [PubMed]
- Ratyński, M.; Hamankiewicz, B.; Krajewski, M.; Boczara, M.; Czerwiński, A. The Effect of Compressive Stresses on a Silicon Electrode’s Cycle Life in a Li-Ion Battery. RSC Adv. 2018, 8, 22546–22551. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.-S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.-S. Effect of Fluoroethylene Carbonate Additive on Interfacial Properties of Silicon Thin-film Electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Jo, H.; Kim, J.; Nguyen, D.T.; Kang, K.K.; Jeon, D.M.; Yang, A.-R.; Song, S.-W. Stabilizing the Solid Electrolyte Interphase Layer and Cycling Performance of Silicon–Graphite Battery Anode by Using a Binary Additive of Fluorinated Carbonates. J. Phys. Chem. C 2016, 120, 22466–22475. [Google Scholar] [CrossRef]
- Oh, M.-G.; Kwak, S.; An, K.; Tran, Y.H.T.; Kang, D.G.; Park, S.J.; Lim, G.; Kim, K.; Lee, Y.S.; Song, S.-W. Perfluoro Macrocyclic Ether as an Ambifunctional Additive for High-Performance SiO and Nickel 88%-based High-Energy Li-Ion Battery. Adv. Funct. Mater. 2023, 33, 2212890. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, L.; Jiang, T.; Liu, J.; Rashid, A.; Sun, P.; Wang, G.; Yan, C.; Zhang, L. Trifluoropropylene Carbonate-Driven Interface Regulation Enabling Greatly Enhanced Lithium Storage Durability of Silicon-Based Anodes. Adv. Funct. Mater. 2019, 29, 1906548. [Google Scholar] [CrossRef]
- Han, G.-B.; Lee, J.-N.; Choi, J.W.; Park, J.-K. Tris(pentafluorophenyl) Borane as an Electrolyte Additive for High Performance Silicon Thin Film Electrodes in Lithium Ion Batteries. Electrochim. Acta 2011, 56, 8997–9003. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, J.-G.; Lee, Y.; Jeong, M.-H.; Shin, W.C.; Ue, M.; Choi, N.-S. A bi-Functional Lithium Difluoro(oxalato)borate Additive for Lithium Cobalt Oxide/Lithium Nickel Manganese Cobalt Oxide Cathodes and Silicon/Graphite Anodes in Lithium-Ion Batteries at Elevated Temperatures. Electrochim. Acta 2014, 137, 1–8. [Google Scholar] [CrossRef]
- Nguyen, D.-T.; Kang, J.; Nam, K.-M.; Paik, Y.; Song, S.-W. Understanding Interfacial Chemistry and Stability for Performance Improvement and Fade of High-energy Li-Ion Battery of LiNi0.5Co0.2Mn0.3O2//Silicon-Graphite. J. Power Sources 2016, 303, 150–158. [Google Scholar] [CrossRef]
- De Sutter, L.; Berckmans, G.; Marinaro, M.; Wohlfahrt-Mehrens, M.; Berecibar, M.; Van Mierlo, J. Mechanical Behavior of Silicon-Graphite Pouch Cells under External Compressive Load: Implications and Opportunities for Battery Pack Design. J. Power Sources 2020, 451, 227774. [Google Scholar] [CrossRef]
- An, K.; Tran, Y.H.T.; Kwak, S.; Han, J.; Song, S.-W. Design of Fire-Resistant Liquid Electrolyte Formulation for Safe and Long-Cycled Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2106102. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, X.; Wu, B.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Bis(2,2,2-trifluoroethyl) methylphosphonate: An Novel Flame-retardant Additive for Safe Lithium-ion Battery. Electrochim. Acta 2014, 129, 300–304. [Google Scholar] [CrossRef]
- Li, X.; Yan, P.; Xiao, X.; Woo, J.H.; Wang, C.; Liu, J.; Zhang, J.G. Design of Porous Si/C–Graphite Electrodes with Long Cycle Stability and Controlled Swelling. Energy Environ. Sci. 2017, 10, 1427–1434. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Role of the LiPF6 Salt for the Long-Term Stability of Silicon Electrodes in Li-Ion Batteries—A Photoelectron Spectroscopy Study. Chem. Mater. 2013, 25, 394–404. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Allouche, J.; Lindgren, F.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Nanosilicon Electrodes for Lithium-Ion Batteries: Interfacial Mechanisms Studied by Hard and Soft X-ray Photoelectron Spectroscopy. Chem. Mater. 2012, 24, 1107–1115. [Google Scholar] [CrossRef]
- Tran, Y.H.T.; An, K.; Lim, G.; Kim, D.; Lee, Y.J.; Doh, C.; Song, S.-W. Preventing Thermal Runaway of High-nickel Li-ion battery through Nonflammable Carbonates-based Electrolyte Formulation. Mater. Sci. Eng. R Rep. 2025, 164, 100980. [Google Scholar] [CrossRef]
- Chung, G.J.; Han, J.; Song, S.-W. Fire-Preventing LiPF6 and Ethylene Carbonate-Based Organic Liquid Electrolyte System for Safer and Outperforming Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 42868–42879. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-H.; Park, K.-J.; Yoon, C.S.; Sun, Y.-K. Capacity Fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies: Tables and Charts, 2nd ed.; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Minnesota, MN, USA, 1979. [Google Scholar]
- NIST X-Ray Photoelectron Spectroscopy Database. Available online: http://srdata.nist.gov/xps/ (accessed on 15 May 2024).
- Chung, G.J.; Tran, Y.H.T.; Han, J.; Kim, K.; Lee, Y.S.; Song, S.-W. Novel Additives-Package to Mitigate the Failure Modes of High-capacity LiNi0.82Co0.11Mn0.07O2-based Lithium-Ion Battery. Chem. Eng. J. 2022, 446, 137288. [Google Scholar] [CrossRef]


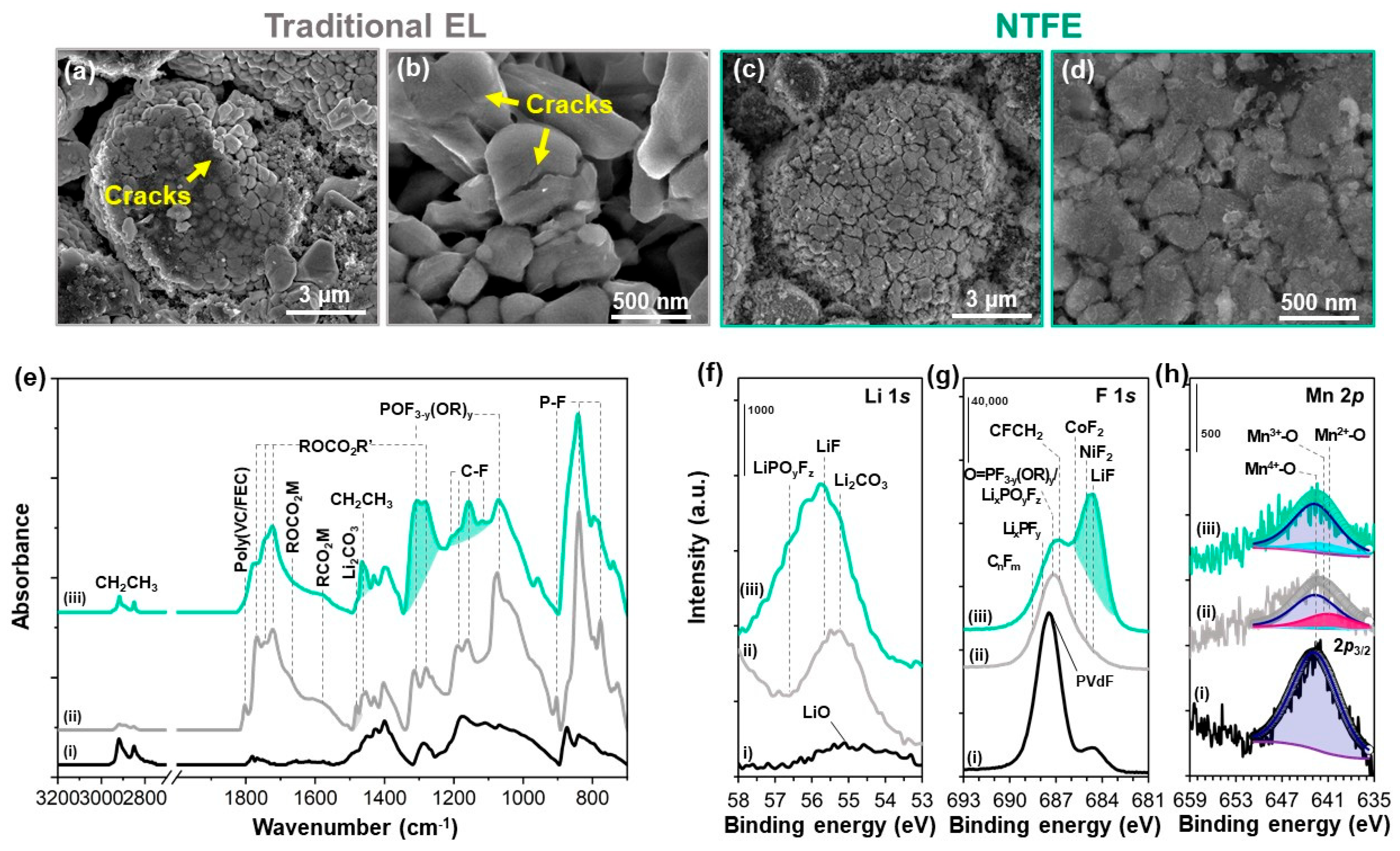
| Areal Ratio [%] | Mn2+-O | Mn3+-O | Mn4+-O |
|---|---|---|---|
| Pristine | 0 | 0 | 100 |
| Traditional EL | 29 | 4 | 67 |
| NTFE | 2 | 15 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, K.; Tran, Y.H.T.; Kang, D.G.; Song, S.-W. Enhancing the Cycle Life of Silicon Oxide–Based Lithium-Ion Batteries via a Nonflammable Fluorinated Ester–Based Electrolyte. Batteries 2025, 11, 250. https://doi.org/10.3390/batteries11070250
An K, Tran YHT, Kang DG, Song S-W. Enhancing the Cycle Life of Silicon Oxide–Based Lithium-Ion Batteries via a Nonflammable Fluorinated Ester–Based Electrolyte. Batteries. 2025; 11(7):250. https://doi.org/10.3390/batteries11070250
Chicago/Turabian StyleAn, Kihun, Yen Hai Thi Tran, Dong Guk Kang, and Seung-Wan Song. 2025. "Enhancing the Cycle Life of Silicon Oxide–Based Lithium-Ion Batteries via a Nonflammable Fluorinated Ester–Based Electrolyte" Batteries 11, no. 7: 250. https://doi.org/10.3390/batteries11070250
APA StyleAn, K., Tran, Y. H. T., Kang, D. G., & Song, S.-W. (2025). Enhancing the Cycle Life of Silicon Oxide–Based Lithium-Ion Batteries via a Nonflammable Fluorinated Ester–Based Electrolyte. Batteries, 11(7), 250. https://doi.org/10.3390/batteries11070250







