Characteristic Differences of Thermal Runaway Triggered by Overheating and Overcharging in Lithium-Ion Batteries and Multi-Dimensional Safety Protection Strategies
Abstract
1. Introduction
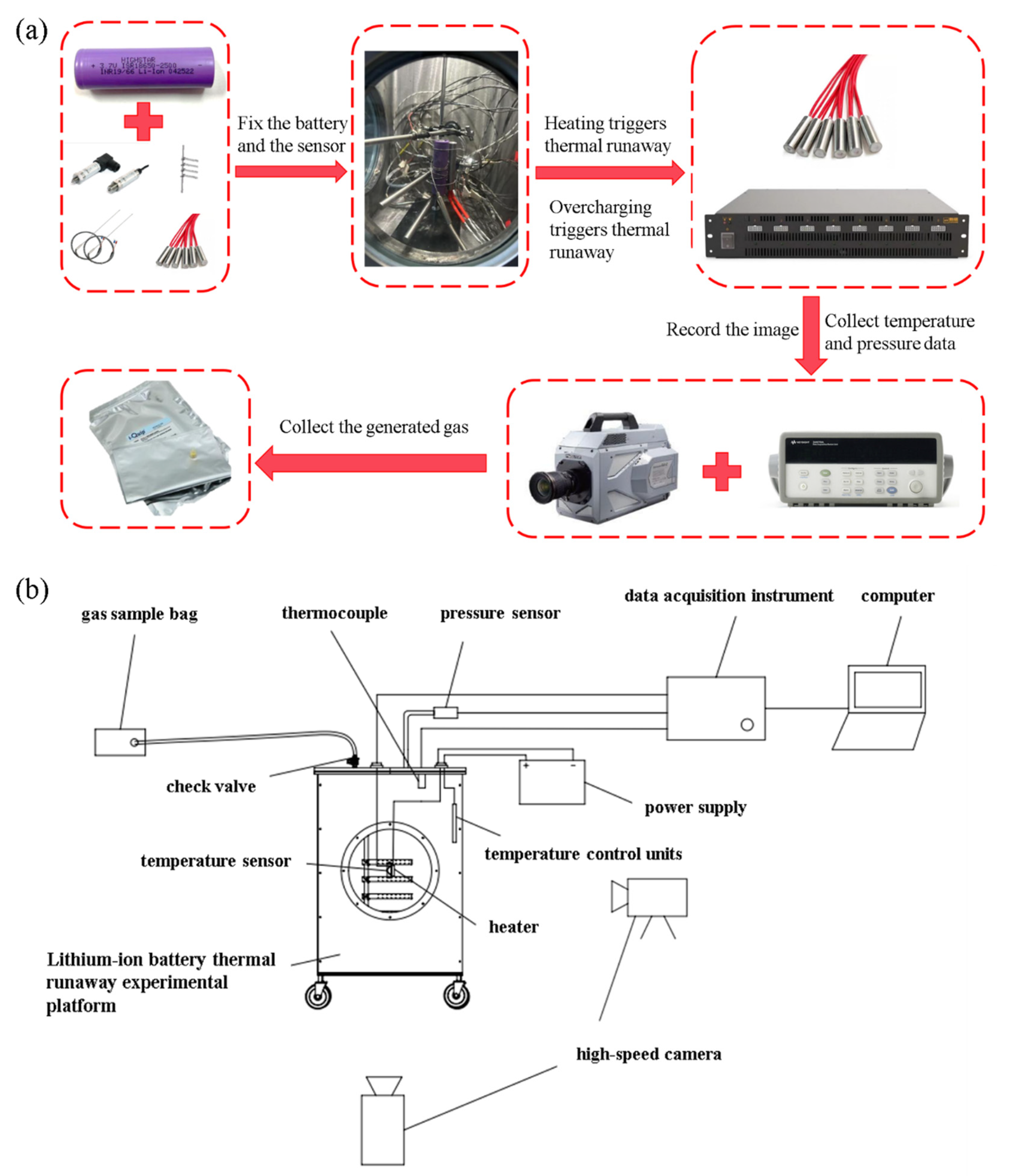
2. Materials and Methods
2.1. Experimental Materials and Sample Preparation
2.2. Experimental Procedures
2.3. Experimental Platform
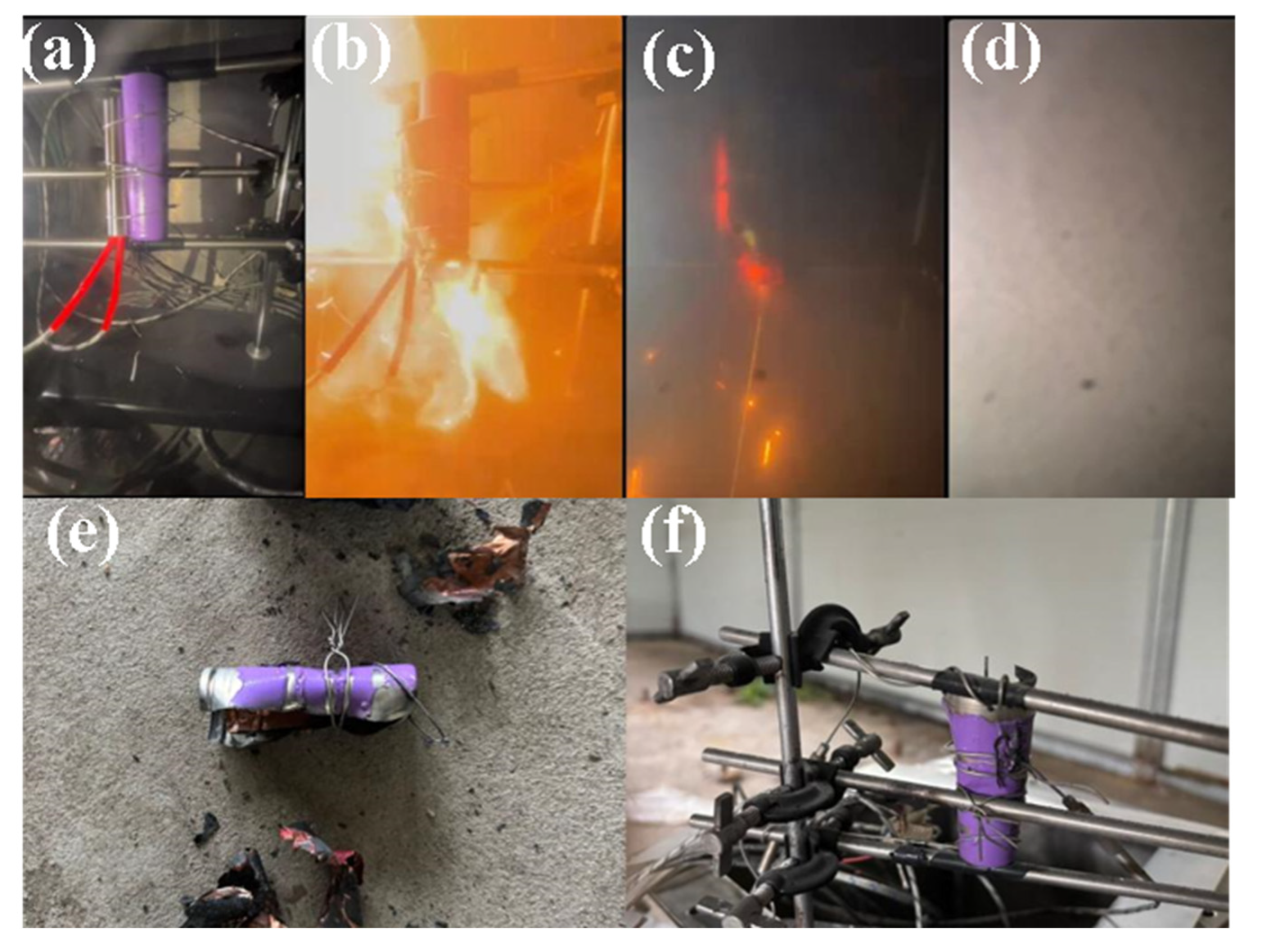
3. Results
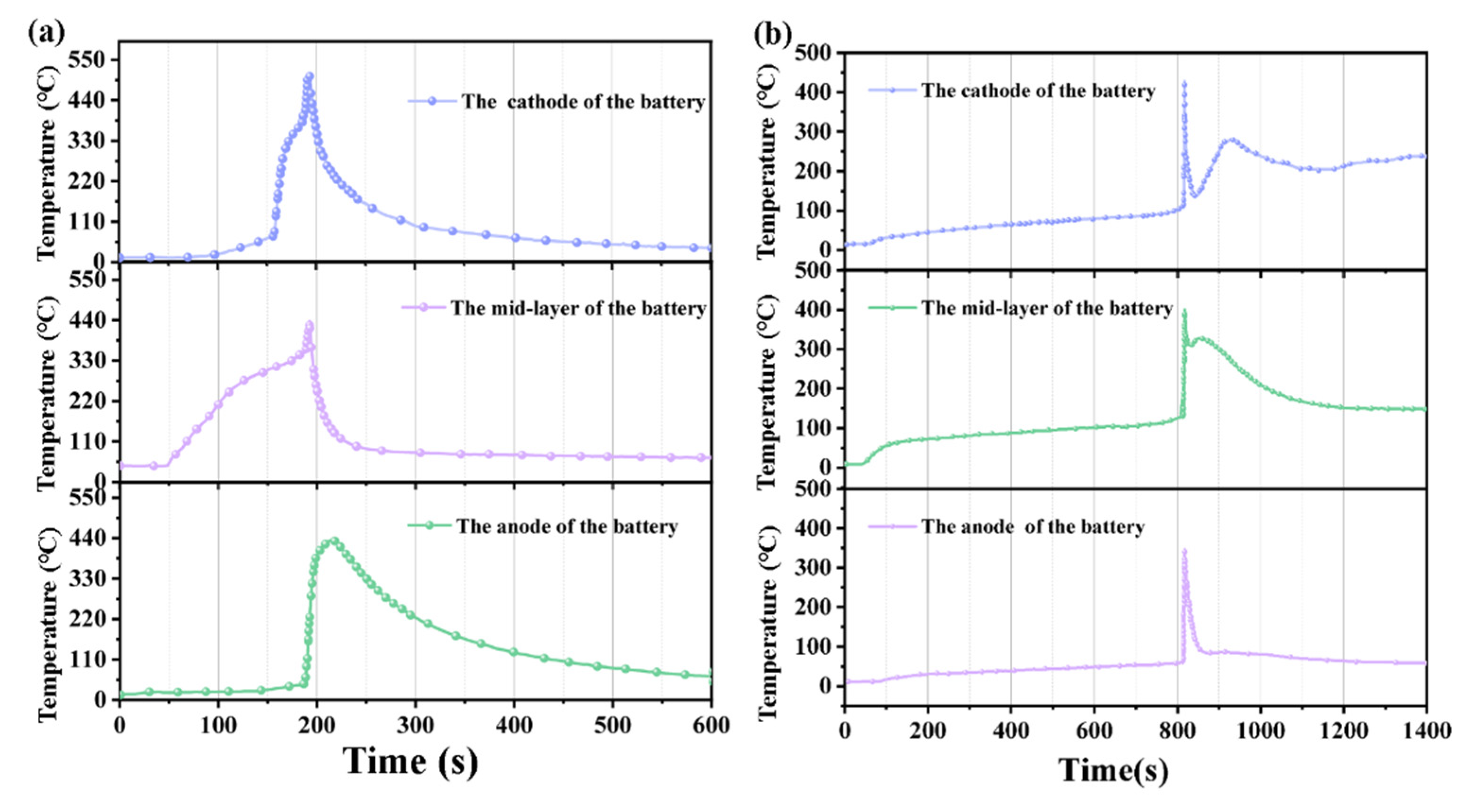
3.1. Temperature Characteristics of Lithium-Ion Batteries Under Different Thermal Runaway Conditions
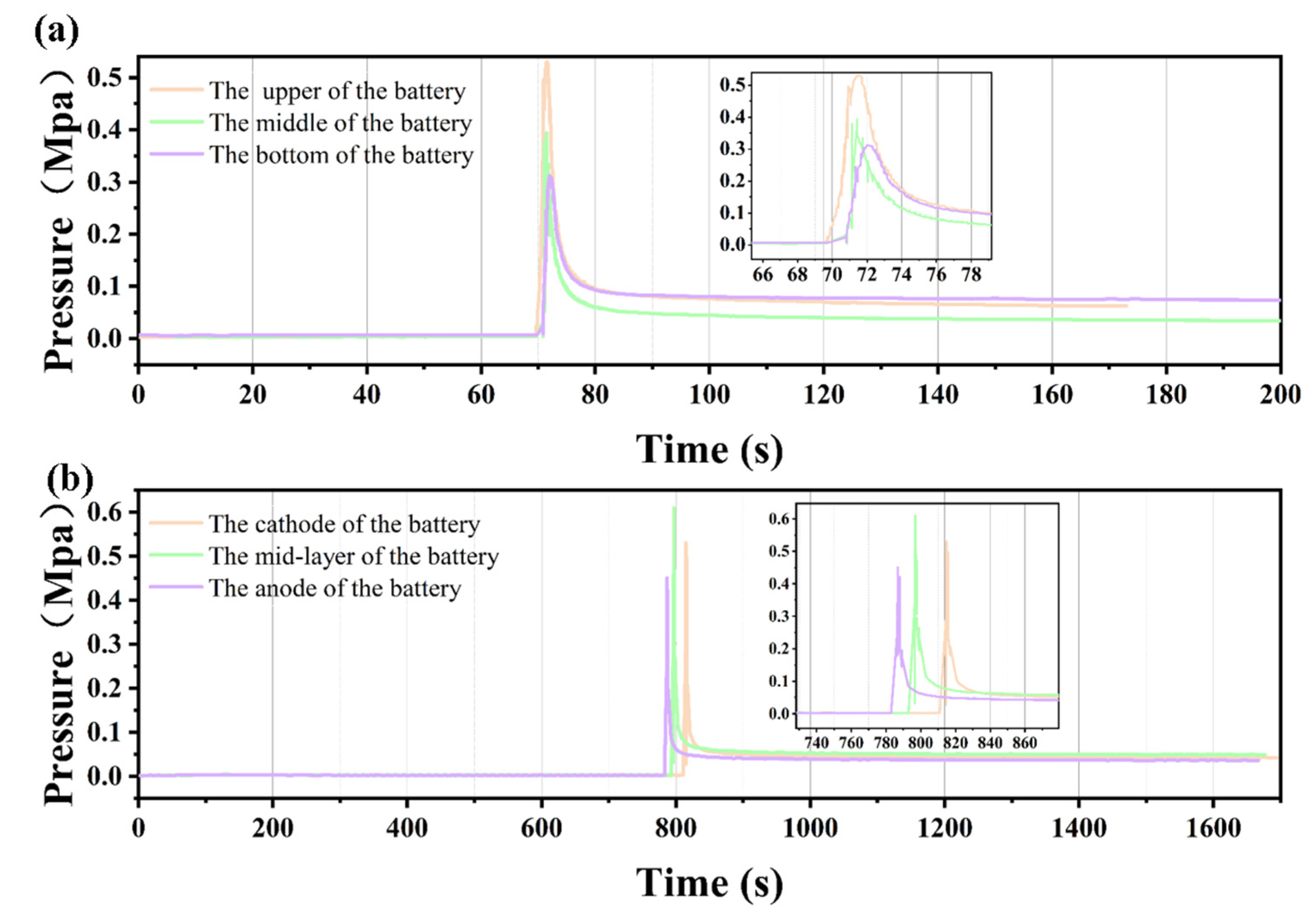
3.2. Pressure Characteristics of Lithium-Ion Batteries Under Different Thermal Runaway Conditions
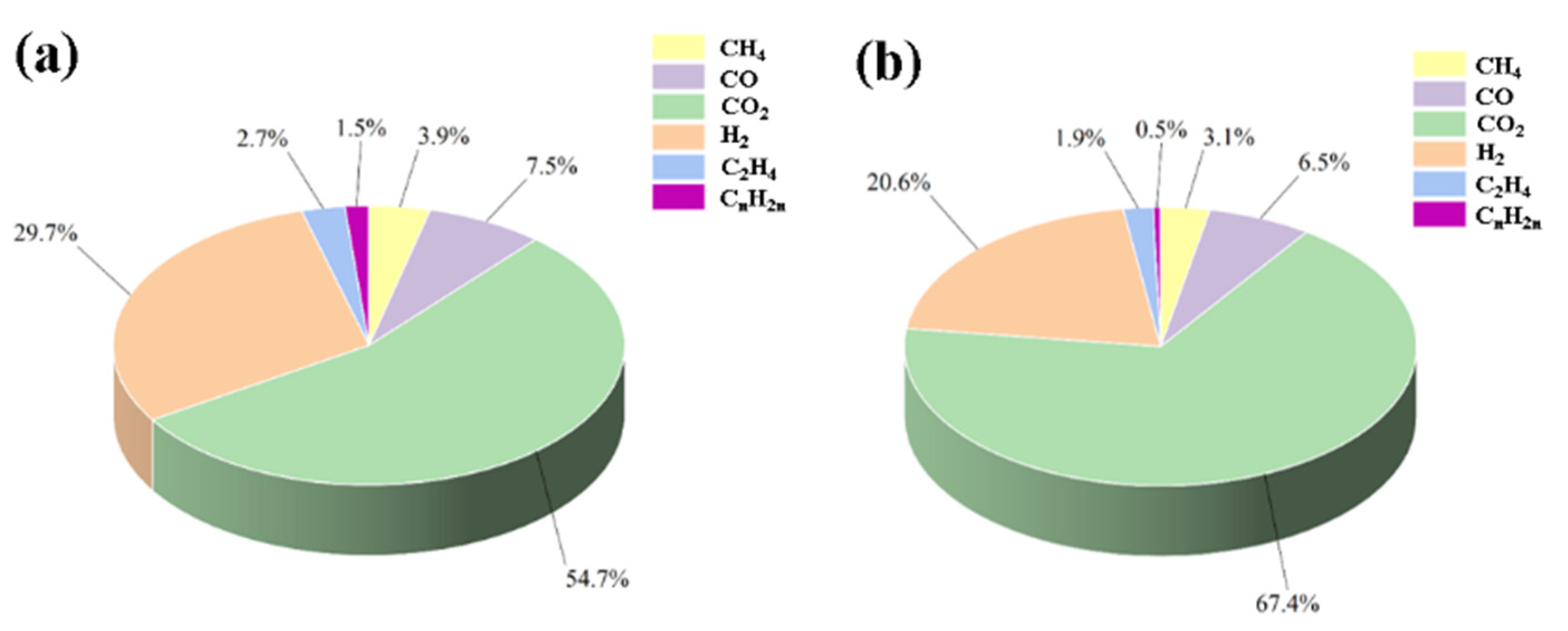
3.3. Gas Production Characteristics of Lithium-Ion Batteries Under Different Thermal Runaway Conditions
4. Discussion
4.1. Heat Release During the Thermal Runaway Process
4.2. Security Protection Strategy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800935–1809648. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.-C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating thermal runaway of lithium-ion batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Kong, D.; Lv, H.; Ping, P.; Wang, G. A review of early warning methods of thermal runaway of lithium ion batteries. J. Energy Storage 2023, 64, 107073. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Hu, X.; Gao, F.; Xiao, Y.; Wang, D.; Gao, Z.; Huang, Z.; Ren, S.; Jiang, N.; Wu, S. Advancements in the safety of Lithium-Ion Battery: The Trigger, consequence and mitigation method of thermal runaway. Chem. Eng. J. 2024, 481, 148450. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Strategies to solve lithium battery thermal runaway: From mechanism to modification. Electrochem. Energy Rev. 2021, 4, 633–679. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Causes and mechanism of thermal runaway in lithium-ion batteries, contradictions in the generally accepted mechanism. J. Energy Storage 2024, 86, 111372. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Shen, Z.; Yu, Q.; Xiong, R.; Shen, W. Towards a safer lithium-ion batteries: A critical review on cause, characteristics, warning and disposal strategy for thermal runaway. Adv. Appl. Energy 2023, 11, 100146. [Google Scholar] [CrossRef]
- He, D.; Wang, J.; Peng, Y.; Li, B.; Feng, C.; Shen, L.; Ma, S. Research advances on thermal runaway mechanism of lithium-ion batteries and safety improvement. Sustain. Mater. Technol. 2024, 41, e01017. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Yan, W.; Huang, W.; Yang, Y.; Wei, Z.; Zhen, H.; Lin, Y. Research on overcharge mitigations and thermal runaway risk of 18650 lithium-ion batteries. J. Energy Storage 2025, 120, 116372. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Meng, N.; Yu, Z.; Yang, H. Study of thermal runaway and the combustion behavior of lithium-ion batteries overcharged with high current rates. Thermochim. Acta 2022, 715, 179276. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, H.; Zhang, Q.; Yang, S.; Liu, Y.; Niu, C.; Kong, Y.; Huang, Q.; Wei, Z. Experimental study on thermal runaway and flame eruption characteristics of NCM523 lithium-ion battery induced by the coupling stimulations of overcharge-penetration. Process Saf. Environ. Prot. 2024, 191, 131–145. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.; Ren, D.; Wu, Y.; Xu, G.; Lu, L.; Hou, J.; Zhang, W. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Huang, L.; Xu, G.; Du, X.; Li, J.; Xie, B.; Liu, H.; Han, P.; Dong, S.; Cui, G.; Chen, L. Uncovering LiH triggered thermal runaway mechanism of a high-energy LiNi0.5Co0.2Mn0.3O2/graphite pouch cell. Adv. Sci. 2021, 8, 2100676. [Google Scholar] [CrossRef]
- Yue, Y.; Jia, Z.; Li, Y.; Wen, Y.; Lei, Q.; Duan, Q.; Sun, J.; Wang, Q. Thermal runaway hazards comparison between sodium-ion and lithium-ion batteries using accelerating rate calorimetry. Process Saf. Environ. Prot. 2024, 189, 61–70. [Google Scholar] [CrossRef]
- Qi, C.; Liu, Z.; Lin, C.; Liu, X.; Liu, D.; Li, Z.; Yi, A. The gas production characteristics and catastrophic hazards evaluation of thermal runaway for LiNi0.5Co0.2Mn0.3O2 lithium-ion batteries under different SOCs. J. Energy Storage 2024, 88, 111678. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, S.; Li, K.; Zhao, M.; Qiu, Z.; Han, D.; Zhang, G.; Habetler, T.G. Hazard analysis of thermally abused lithium-ion batteries at different state of charges. J. Energy Storage 2020, 27, 101065. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, K.; Wang, D.; Wang, Z.; Chen, L. Overcharge investigation of lithium-ion polymer batteries. J. Power Sources 2006, 160, 1302–1307. [Google Scholar] [CrossRef]
- Song, Y.; Liu, X.; Ren, D.; Liang, H.; Wang, L.; Hu, Q.; Cui, H.; Xu, H.; Wang, J.; Zhao, C. Simultaneously blocking chemical crosstalk and internal short circuit via gel-stretching derived nanoporous non-shrinkage separator for safe lithium-ion batteries. Adv. Mater. 2022, 34, 2106335. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Zhou, Y.; Zhang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. The application of Al2O3 in separators and solid electrolytes of lithium-ion battery: A review. Energy Storage Mater. 2024, 71, 103575. [Google Scholar] [CrossRef]
- Shang, Z.; Qi, H.; Liu, X.; Ouyang, C.; Wang, Y. Structural optimization of lithium-ion battery for improving thermal performance based on a liquid cooling system. Int. J. Heat Mass Transf. 2019, 130, 33–41. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Kalogiannis, T.; Jaguemont, J.; Jin, L.; Behi, H.; Karimi, D.; Beheshti, H.; Van Mierlo, J.; Berecibar, M. A comparative study between air cooling and liquid cooling thermal management systems for a high-energy lithium-ion battery module. Appl. Therm. Eng. 2021, 198, 117503. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Othman, A.M.; Abdussami, M.R. Review of battery management systems (BMS) development and industrial standards. Technologies 2021, 9, 28. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Ji, Z.; Su, M.; Li, H.; Wu, Y.; Su, X.; Zhang, Z. Prospective Application, Mechanism, and Deficiency of Lithium Bis (oxalate) Borate as the Electrolyte Additive for Lithium-Batteries. Adv. Energy Mater. 2023, 13, 2301422. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Peng, S.; Li, K.; Liu, P.; Zhang, W.; Wu, W.; Han, X.; Cao, Y.-c.; Wen, J.; Cheng, S. Dynamics of multidimensional signals in lithium-ion battery during thermal runaway under various oven temperatures. J. Energy Storage 2025, 108, 115071. [Google Scholar] [CrossRef]
- Song, Y.; Cui, Y.; Li, B.; Geng, L.; Yan, J.; Zhu, D.; Zhou, P.; Zhou, J.; Yan, Z.; Xue, Q. Revealing the origin of high-thermal-stability of single-crystal Ni-rich cathodes toward higher-safety batteries. Nano Energy 2023, 116, 108846. [Google Scholar] [CrossRef]
- Jia, Y.; Hou, X.; Li, K.; Wang, L.; Zhang, M.; Li, Z.; Xu, X.; Zheng, J. Unraveling the oxygen evolution in layered LiNiO2 with the role of Li/Ni disordering. Energy Storage Mater. 2024, 71, 103632. [Google Scholar] [CrossRef]
- House, R.A.; Marie, J.-J.; Pérez-Osorio, M.A.; Rees, G.J.; Boivin, E.; Bruce, P.G. The role of O2 in O-redox cathodes for Li-ion batteries. Nat. Energy 2021, 6, 781–789. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef]
- Shalnev, S.; Krzack, S.; Küster, F.; Friedrich, H.-J.; Meyer, B.; Gräbner, M. An experimental investigation into the conversion of nuclear graphite by oxygen and carbon dioxide: Kinetics and change in pore structure. Fuel 2024, 357, 129756. [Google Scholar] [CrossRef]
- Yu, R.; Zeng, W.; Zhou, L.; Van Tendeloo, G.; Mai, L.; Yao, Z.; Wu, J. Layer-by-layer delithiation during lattice collapse as the origin of planar gliding and microcracking in Ni-rich cathodes. Cell Rep. Phys. Sci. 2023, 4, 101480. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A review of gas evolution in lithium ion batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Liu, L.; Gu, J.; Xing, H.; Liu, H.; Cheng, Y.; Wang, Y.; Yue, S.; Qiu, Y.; Zhang, Z. Characteristic Differences of Thermal Runaway Triggered by Overheating and Overcharging in Lithium-Ion Batteries and Multi-Dimensional Safety Protection Strategies. Batteries 2025, 11, 242. https://doi.org/10.3390/batteries11070242
Yao Y, Liu L, Gu J, Xing H, Liu H, Cheng Y, Wang Y, Yue S, Qiu Y, Zhang Z. Characteristic Differences of Thermal Runaway Triggered by Overheating and Overcharging in Lithium-Ion Batteries and Multi-Dimensional Safety Protection Strategies. Batteries. 2025; 11(7):242. https://doi.org/10.3390/batteries11070242
Chicago/Turabian StyleYao, Yao, Lu Liu, Juan Gu, Haozhe Xing, Huachao Liu, Yihao Cheng, Youning Wang, Songlin Yue, Yanyu Qiu, and Zhi Zhang. 2025. "Characteristic Differences of Thermal Runaway Triggered by Overheating and Overcharging in Lithium-Ion Batteries and Multi-Dimensional Safety Protection Strategies" Batteries 11, no. 7: 242. https://doi.org/10.3390/batteries11070242
APA StyleYao, Y., Liu, L., Gu, J., Xing, H., Liu, H., Cheng, Y., Wang, Y., Yue, S., Qiu, Y., & Zhang, Z. (2025). Characteristic Differences of Thermal Runaway Triggered by Overheating and Overcharging in Lithium-Ion Batteries and Multi-Dimensional Safety Protection Strategies. Batteries, 11(7), 242. https://doi.org/10.3390/batteries11070242





