Protective Layer and Current Collector Design for Interface Stabilization in Lithium-Metal Batteries
Abstract
1. Introduction
2. Li-Metal Battery
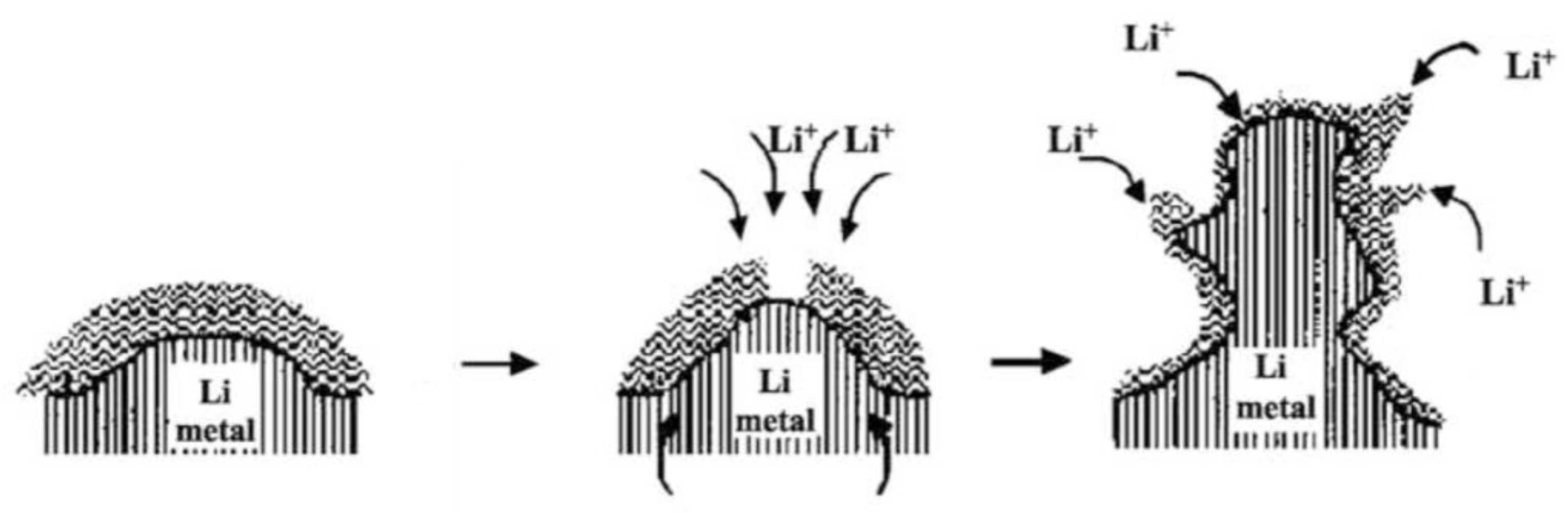
2.1. Mechanisms of Nucleation and Growth of Li Dendrites
2.2. SEI Formation and Associated Challenges in LMBs
3. Suppression of Li Dendrites: Structured Electrode Strategy
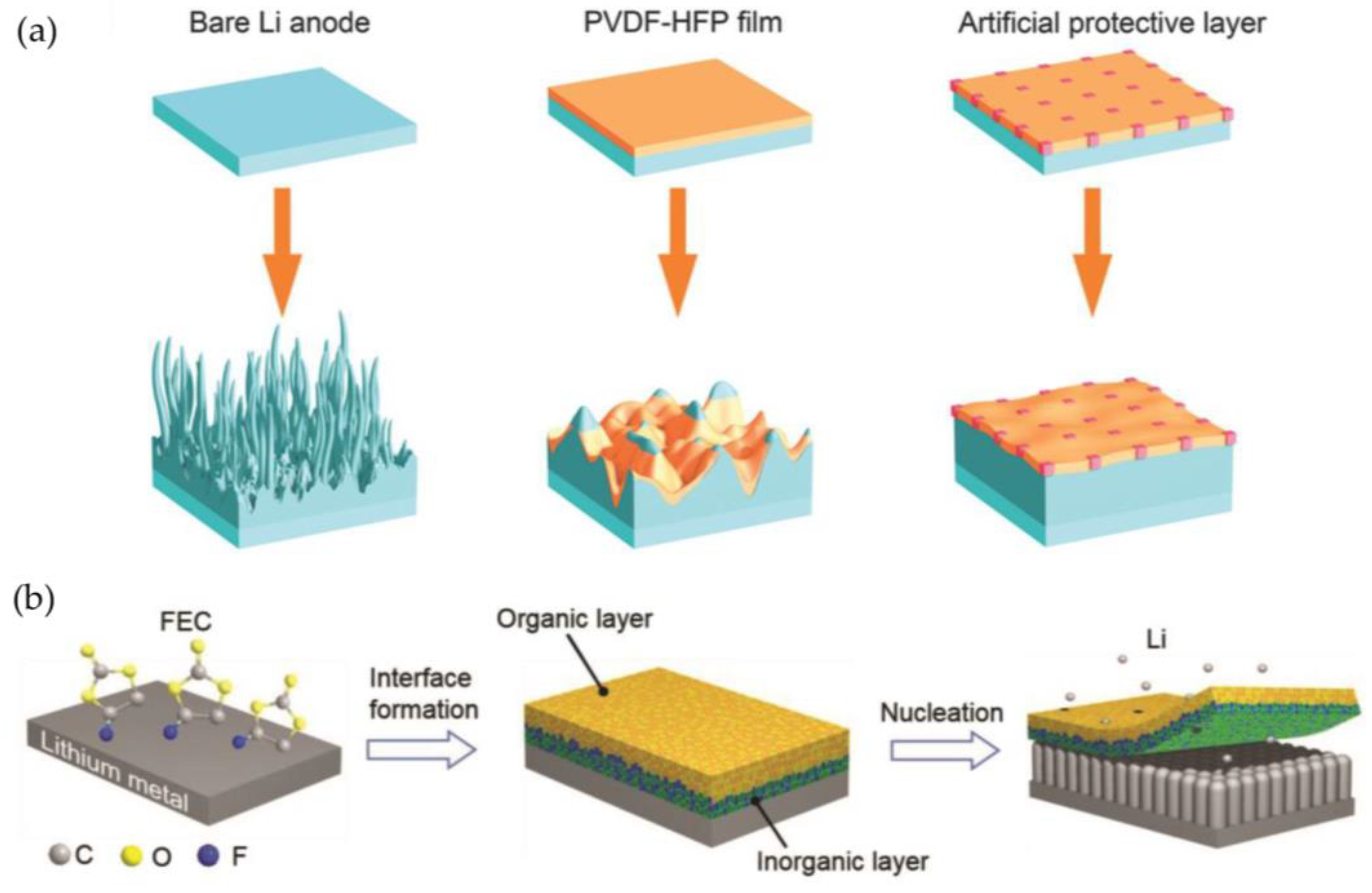
3.1. Protective Layer
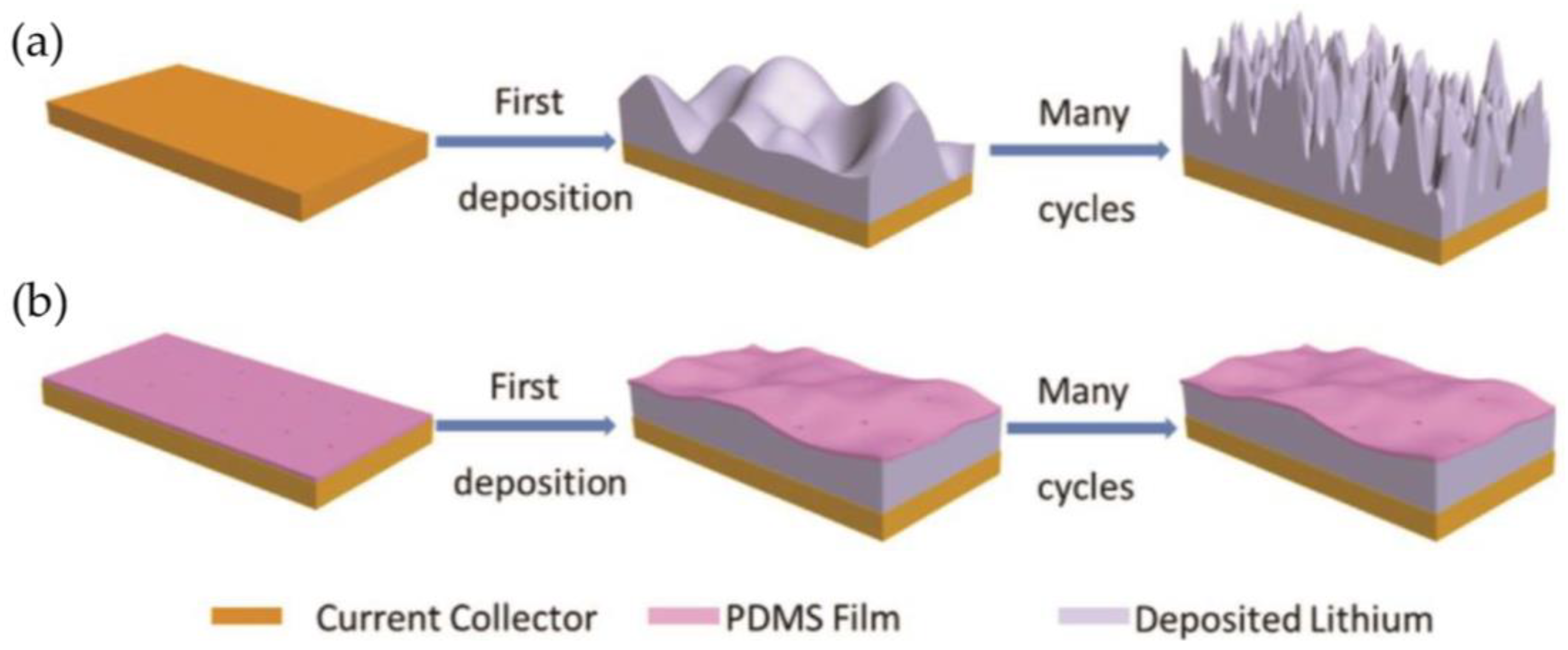
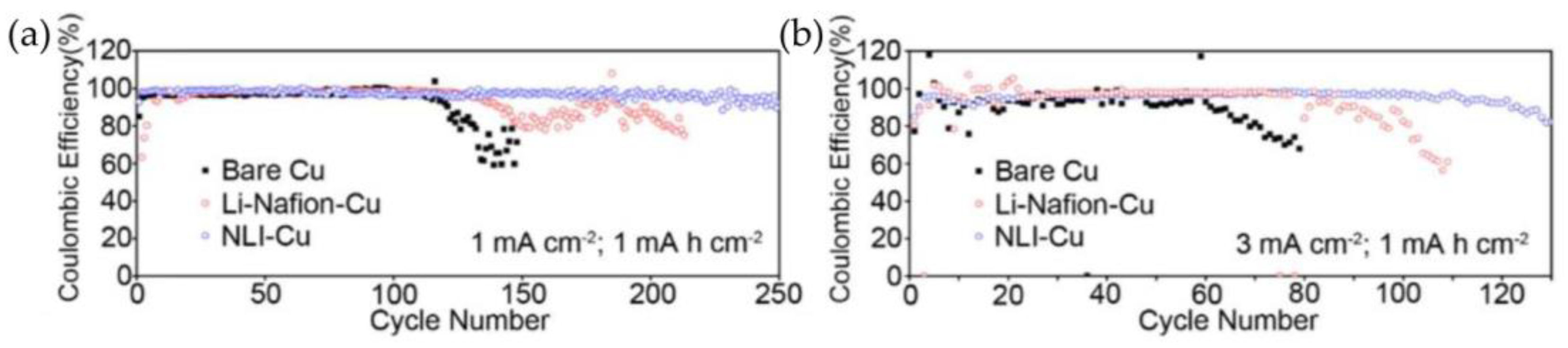
3.1.1. Polymer-Based Protective Layers
3.1.2. Inorganic-Based Protective Layers
3.1.3. Composite Protective Layers
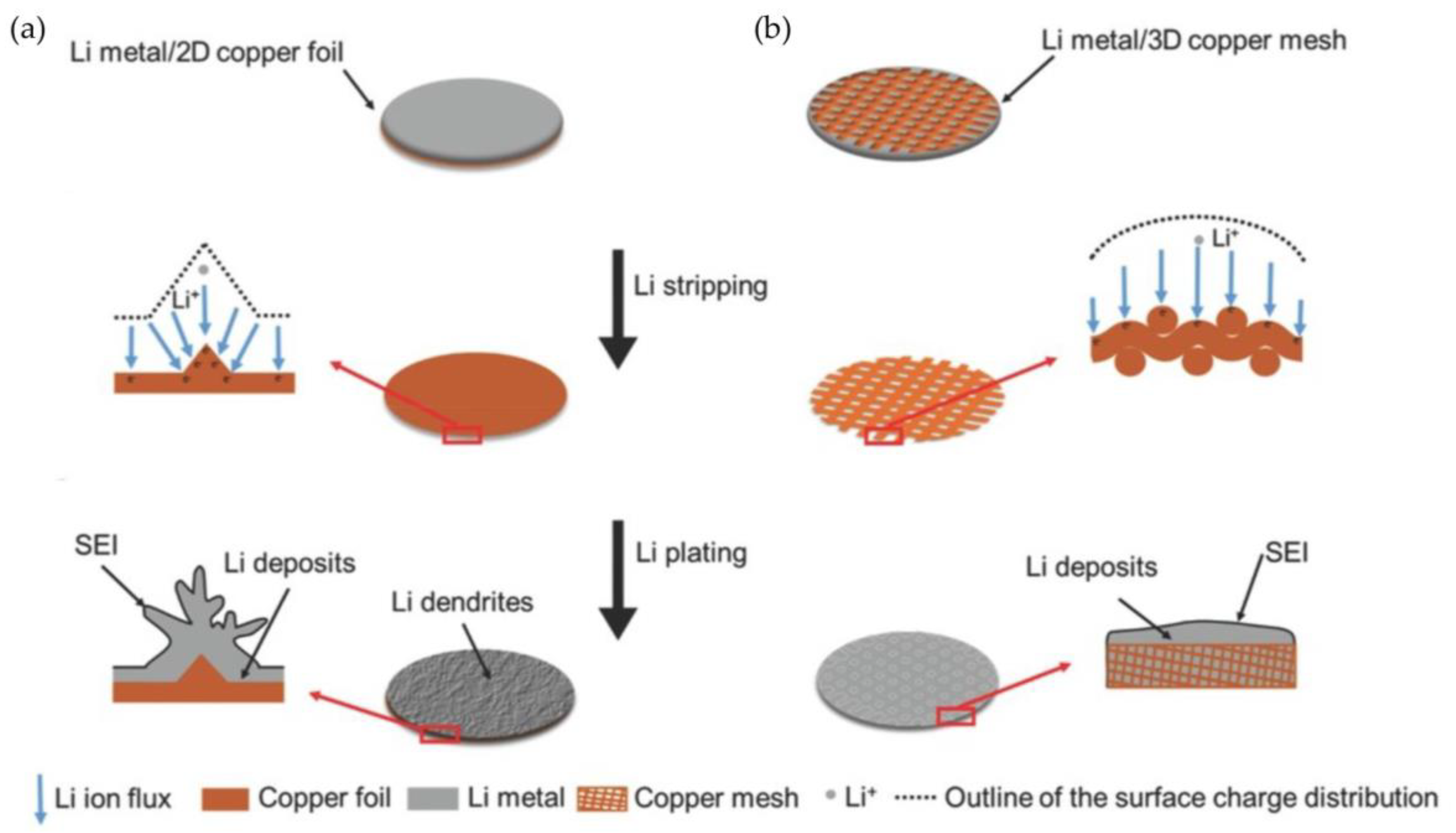
3.2. Structural Design of Current Collectors for Lithium Metal
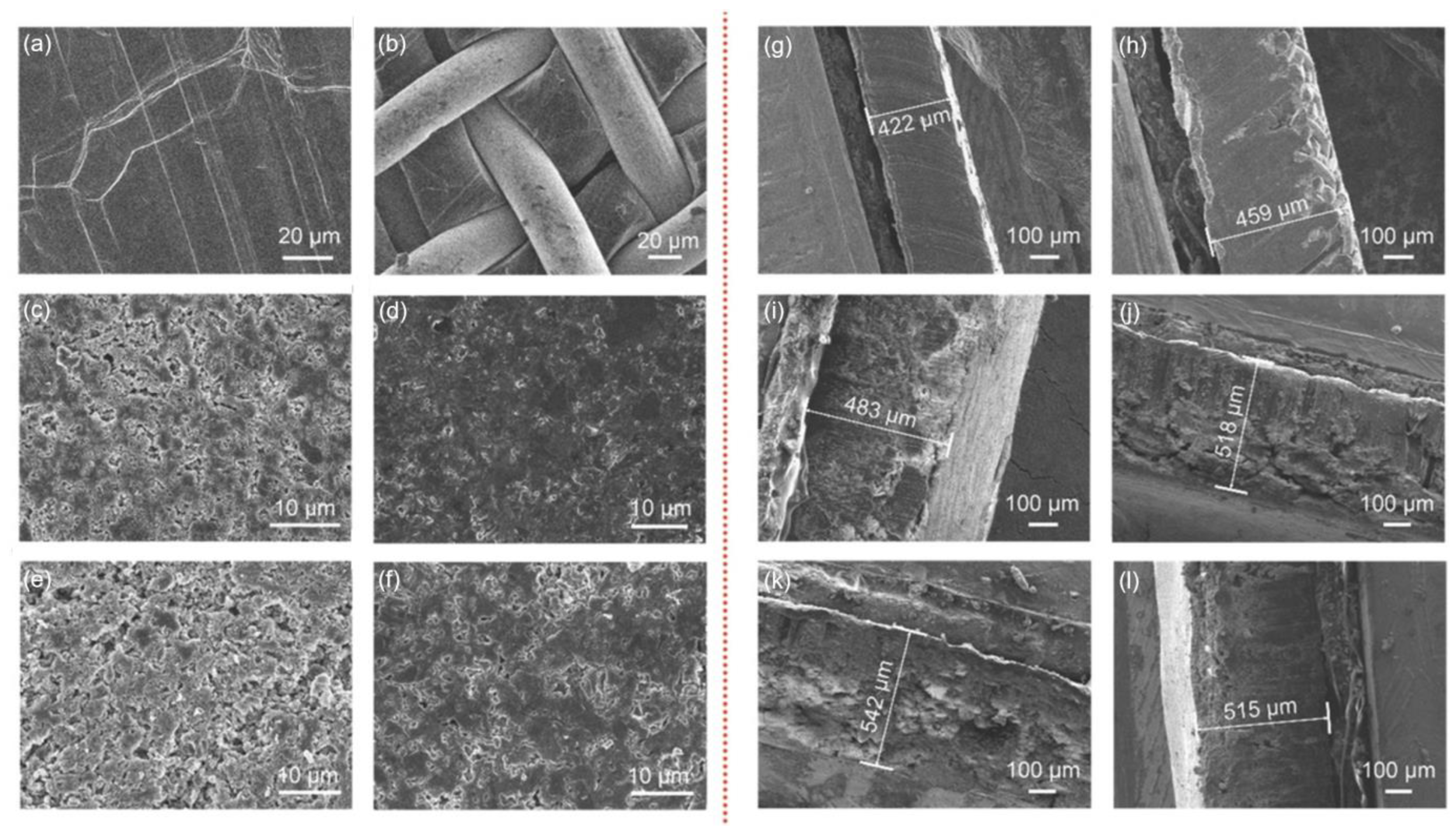
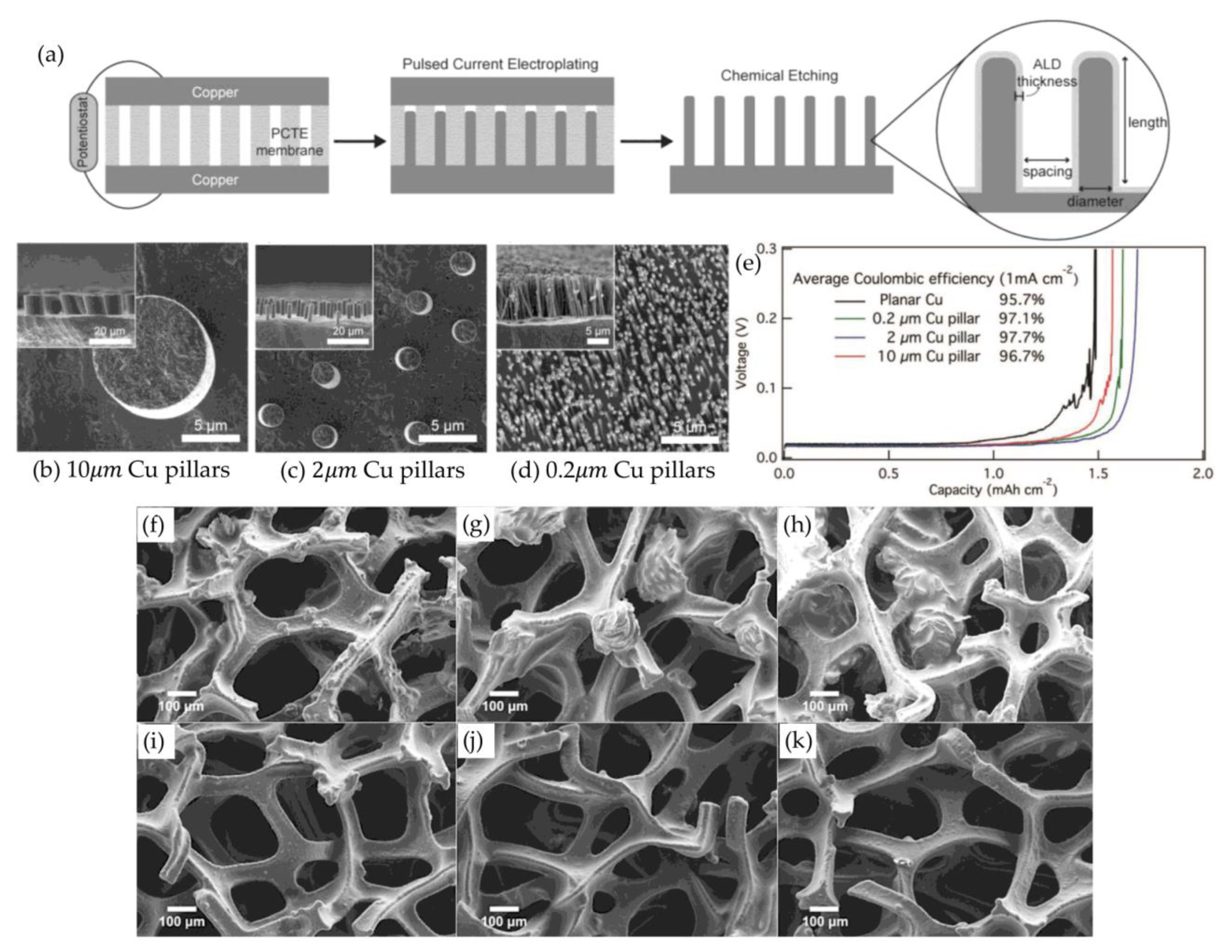
3.2.1. Porous Copper Current Collectors
3.2.2. Effects of Incorporating Lithiophilic Materials
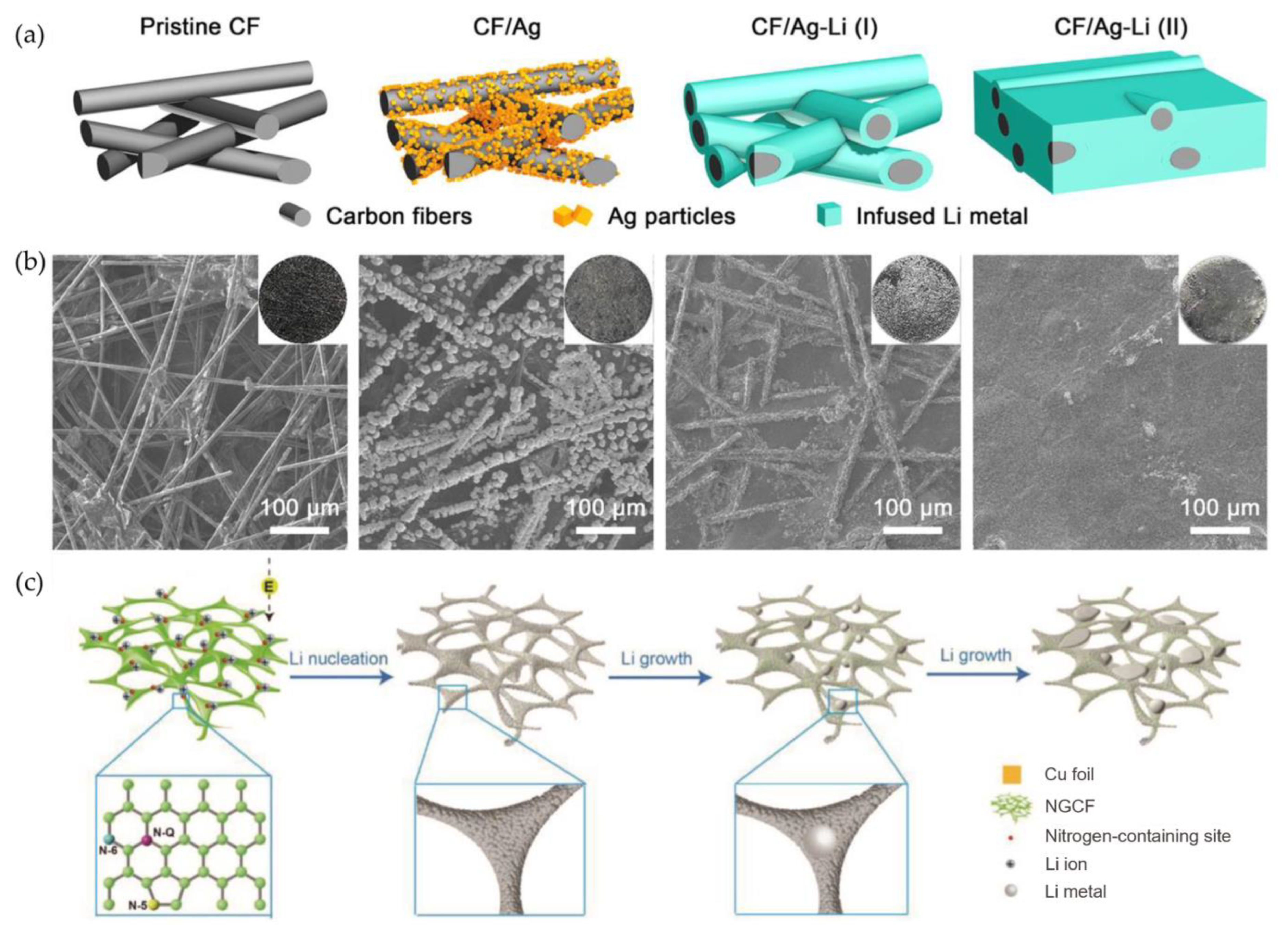
3.2.3. 3D Carbon-Based Frameworks
4. Conclusions
Funding
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I.T.; Hur, J. Highly conductive and robust telluride-carbon hybrid matrix for enhanced copper diphosphide anode in Li-ion batteries. J. Alloys Compd. 2023, 950, 169914. [Google Scholar] [CrossRef]
- Kim, Y.; Stepien, D.; Moon, H.; Schönherr, K.; Schumm, B.; Kuenzel, M.; Althues, H.; Bresser, D.; Passerini, S. Artificial Interphase Design Employing Inorganic–Organic Components for High-Energy Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 20987–20997. [Google Scholar] [CrossRef]
- Lee, D.; Cui, Z.; Goodenough, J.B.; Manthiram, A. Interphase stabilization of LiNi0.5Mn1.5O4 cathode for 5 V-class all-solid-state batteries. Small 2024, 20, 2306053. [Google Scholar] [CrossRef]
- Park, I.; Lee, H.; Chae, O.B. Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries. Batteries 2024, 10, 381. [Google Scholar] [CrossRef]
- Song, C.; Park, S.; Kim, N.; Chae, O.B.; Ryu, J.H. Enhanced Electrochemical Performance of Surface-Nitrided MnO Negative Electrode Materials in Li-Ion Batteries. J. Electrochem. Sci. Technol. 2024, 16, 105–112. [Google Scholar] [CrossRef]
- Salunkhe, T.T.; Kadam, A.N.; Kidanu, W.G.; Lee, S.-W.; Nguyen, T.L.; Kim, I.T. A diffusion encouraged core–shell heterostructured Co3Sn2@SnO2 anode towards emerging dual ion batteries with high energy density. J. Mater. Chem. A 2021, 9, 14991–15002. [Google Scholar] [CrossRef]
- So, S.; Ko, J.; Ahn, Y.N.; Kim, I.T.; Hur, J. Unraveling improved electrochemical kinetics of In2Te3-based anodes embedded in hybrid matrix for Li-ion batteries. Chem. Eng. J. 2022, 429, 132395. [Google Scholar] [CrossRef]
- Ko, J.; Kim, M.; So, S.; Kim, I.T.; Hur, J. Electron-rich hybrid matrix to enhance molybdenum oxide-based anode performance for Lithium-Ion batteries. J. Colloid Interface Sci. 2023, 647, 93–103. [Google Scholar] [CrossRef]
- Bae, J.; Salunkhe, T.T.; Hur, J.; Kim, I.T. Novel carbon-free niobium silicide/oxide nanocomposites for lithium-ion battery anodes. Appl. Mater. Today 2021, 22, 100917. [Google Scholar] [CrossRef]
- Kidanu, W.G.; Hur, J.; Kim, I.T. Gallium-indium-tin eutectic as a self-healing room-temperature liquid metal anode for high-capacity lithium-ion batteries. Materials 2021, 15, 168. [Google Scholar] [CrossRef]
- Park, S.M.; Salunkhe, T.T.; Yoo, J.H.; Kim, I.H.; Kim, I.T. Artificial Graphite-Based Silicon Composite Anodes for Lithium-Ion Batteries. Nanomaterials 2024, 14, 1953. [Google Scholar] [CrossRef] [PubMed]
- Broussely, M.; Archdale, G. Li-ion batteries and portable power source prospects for the next 5–10 years. J. Power Sources 2004, 136, 386–394. [Google Scholar] [CrossRef]
- Shen, X.; Liu, H.; Cheng, X.-B.; Yan, C.; Huang, J.-Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175. [Google Scholar] [CrossRef]
- Luo, Z.; Qiu, X.; Liu, C.; Li, S.; Wang, C.; Zou, G.; Hou, H.; Ji, X. Interfacial challenges towards stable Li metal anode. Nano Energy 2021, 79, 105507. [Google Scholar] [CrossRef]
- Acebedo, B.; Morant-Miñana, M.C.; Gonzalo, E.; Ruiz de Larramendi, I.; Villaverde, A.; Rikarte, J.; Fallarino, L. Current status and future perspective on lithium metal anode production methods. Adv. Energy Mater. 2023, 13, 2203744. [Google Scholar] [CrossRef]
- Yang, S.Y.; Park, J.-S.; Kim, J.H.; Yoon, M.; Wang, S.E.; Jung, D.S.; Kang, Y.C. Spray-assisted synthesis of ant-cave-structured Ni-rich cathode microspheres with Li-reactive coating layer for high-performance Li-ion batteries. Mater. Today Chem. 2024, 35, 101889. [Google Scholar] [CrossRef]
- Song, W.; Chae, O.B. Surface-Coating Strategies of Si-Negative Electrode Materials in Lithium-Ion Batteries. Batteries 2024, 10, 327. [Google Scholar] [CrossRef]
- Hu, S.; Pillai, A.S.; Liang, G.; Pang, W.K.; Wang, H.; Li, Q.; Guo, Z. Li-rich layered oxides and their practical challenges: Recent progress and perspectives. Electrochem. Energy Rev. 2019, 2, 277–311. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.M. Li-rich layered oxide cathodes for next-generation Li-ion batteries: Chances and challenges. J. Electrochem. Soc. 2015, 162, A2490. [Google Scholar] [CrossRef]
- Shi, J.L.; Xiao, D.D.; Ge, M.; Yu, X.; Chu, Y.; Huang, X.; Zhang, X.D.; Yin, Y.X.; Yang, X.Q.; Guo, Y.G. High-capacity cathode material with high voltage for Li-ion batteries. Adv. Mater. 2018, 30, 1705575. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Song, W.; Chae, O.B. Advances in Coating Materials for Silicon-Based Lithium-Ion Battery Anodes. Energies 2024, 17, 4970. [Google Scholar] [CrossRef]
- Chae, O.B.; Lee, S.; Park, J.H.; Song, C.H.; Go, N.; Lee, D.; Kim, J.; Jin, F.; Park, Y.D.; Mun, J. Facile self-assembled monolayer deposition on copper foil for high-performance lithium-metal batteries. Electrochim. Acta 2024, 507, 145154. [Google Scholar] [CrossRef]
- Chae, O.B.; Adiraju, V.A.; Lucht, B.L. Performance improvement of lithium metal batteries enabled By LiBF3CN as a new electrolyte additive. J. Electrochem. Soc. 2022, 169, 110506. [Google Scholar] [CrossRef]
- Weldehans, M.G.; Nguyen, T.P.; Hur, J.; Kim, I.T. Carbon-decorated hydrated V10O24 nanorods for high-performance lithium-ion battery cathodes. J. Energy Storage 2024, 101, 113888. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.; Park, J.; Lee, S.; Lee, H.; Lee, D.; Paik, U.; Song, T. Self-healing Si anodes with robust ionic and electronic conducting network by Ga-In-Sn liquid metal alloy in solid-state batteries. Energy Storage Mater. 2025, 76, 104108. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, I.; Lee, J.W.; Yoon, D.; Kim, J.H.; Lee, S.; Kim, K.; Kim, P.J.; Choi, J.; Kang, Y.C. A Novel Structured Si-Based Composite with 2D Structured Graphite for High-Performance Lithium-Ion Batteries. Small 2024, 20, 2405005. [Google Scholar] [CrossRef]
- Preman, A.N.; Aswale, S.; Salunkhe, T.T.; Lee, S.; Kim, M.C.; Devaraju, S.; Hyun, K.; Paik, H.-j.; Kim, I.T.; Ahn, S.-k. Better together: Integrating adhesion and ion conductivity in composite binders for high-performance silicon anodes. J. Mater. Chem. A 2025, 13, 8355–8367. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, T.; Chae, O.B. Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes. Micromachines 2024, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ma, J.; Zhang, X.; Li, Y.; Meng, S.; Zhang, Y.; Dong, H.; Liu, R.; Gao, R.; Feng, M. Interfacial strategies towards highly stable Li-metal anode of liquid-based Li-metal batteries. Energy Storage Mater. 2023, 64, 103084. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.; Ren, L.; Guan, X.; Wang, D.; Dong, A.; Zhang, C.; Li, G.; Luo, J. Controlling Li ion flux through materials innovation for dendrite-free lithium metal anodes. Adv. Funct. Mater. 2019, 29, 1905940. [Google Scholar] [CrossRef]
- Chae, O.B.; Lucht, B.L. Interfacial issues and modification of solid electrolyte interphase for Li metal anode in liquid and solid electrolytes. Adv. Energy Mater. 2023, 13, 2203791. [Google Scholar] [CrossRef]
- Aslam, J.; Waseem, M.A.; Lu, X.M.; Wu, S.; Sun, W.; Wang, Y. Unveiling Covalent Triazine Frameworks for Lithium Metal Anodes: Recent Developments and Prospective Advances. Small 2025, 21, 2408988. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef] [PubMed]
- Yasin, G.; Arif, M.; Mehtab, T.; Lu, X.; Yu, D.; Muhammad, N.; Nazir, M.T.; Song, H. Understanding and suppression strategies toward stable Li metal anode for safe lithium batteries. Energy Storage Mater. 2020, 25, 644–678. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Liu, P.; Wang, B.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Modified solid-electrolyte interphase toward stable Li metal anode. Nano Energy 2020, 77, 105308. [Google Scholar] [CrossRef]
- Xiao, J. How lithium dendrites form in liquid batteries. Science 2019, 366, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Chen, Y.; Ke, X.; Li, J.; Yang, Y.; Shi, Z. Lithium Host: Advanced architecture components for lithium metal anode. Energy Storage Mater. 2021, 38, 276–298. [Google Scholar] [CrossRef]
- Afzali, P.; Gibertini, E.; Magagnin, L. Improved plating/stripping in anode-free lithium metal batteries through electrodeposition of lithiophilic zinc thin films. Electrochim. Acta 2024, 488, 144190. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Xu, J.; Huang, M.; Zhang, C.; Wang, Z.; Xiao, X.; Guo, P.; Fan, Q.; Geng, S.; Lin, X.; Liang, L.; et al. Hierarchical carbon cloth with Co-Nx nanoneedle arrays: Enabling highly reversible lithium metal anode via enhanced lithiophilicity and structural confinement. Chem. Eng. J. 2025, 513, 162883. [Google Scholar] [CrossRef]
- Cha, E.; Yun, J.H.; Ponraj, R.; Kim, D.K. A mechanistic review of lithiophilic materials: Resolving lithium dendrites and advancing lithium metal-based batteries. Mater. Chem. Front. 2021, 5, 6294–6314. [Google Scholar] [CrossRef]
- Liu, D.-H.; Bai, Z.; Li, M.; Yu, A.; Luo, D.; Liu, W.; Yang, L.; Lu, J.; Amine, K.; Chen, Z. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives. Energy Storage Mater. 2020, 49, 5407–5445. [Google Scholar] [CrossRef]
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, T.; Du, C.; Liu, Q.; Tang, Y.; Zhao, J.; Wang, B.; Chen, T.; Sun, Y.; Jia, P. Lithium whisker growth and stress generation in an in situ atomic force microscope–environmental transmission electron microscope set-up. Nat. Nanotechnol. 2020, 15, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—The solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An Artificial Solid Electrolyte Interphase with High Li-Ion Conductivity, Mechanical Strength, and Flexibility for Stable Lithium Metal Anodes. Adv. Mater. 2017, 29, 1605531. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode–electrolyte interfaces in lithium-based batteries. Energy Environ. Sci. 2018, 11, 527–543. [Google Scholar] [CrossRef]
- Peled, E. Film forming reaction at the lithium/electrolyte interface. J. Power Sources 1983, 9, 253–266. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 1997, 144, L208. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.-M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef]
- Kang, D.; Hart, N.; Koh, J.; Ma, L.; Liang, W.; Xu, J.; Sardar, S.; Lemmon, J.P. Rearrange SEI with artificial organic layer for stable lithium metal anode. Energy Storage Mater. 2020, 24, 618–625. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Yu, Z.; Cui, Y.; Bao, Z. Design Principles of Artificial Solid Electrolyte Interphases for Lithium-Metal Anodes. Cell Rep. Phys. Sci. 2020, 1, 100119. [Google Scholar] [CrossRef]
- Leung, K. Two-electron reduction of ethylene carbonate: A quantum chemistry re-examination of mechanisms. Chem. Phys. Lett. 2013, 568–569, 1–8. [Google Scholar] [CrossRef]
- Chen, X.R.; Zhao, B.C.; Yan, C.; Zhang, Q. Review on Li deposition in working batteries: From nucleation to early growth. Adv. Mater. 2021, 33, 2004128. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Hao, F.; Verma, A.; Mukherjee, P.P. Mechanistic insight into dendrite–SEI interactions for lithium metal electrodes. J. Mater. Chem. A 2018, 6, 19664–19671. [Google Scholar] [CrossRef]
- Wu, B.; Chen, C.; Raijmakers, L.H.; Liu, J.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H. Li-growth and SEI engineering for anode-free Li-metal rechargeable batteries: A review of current advances. Energy Storage Mater. 2023, 57, 508–539. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, C.; Lin, Q.; Zhang, Y.; Deng, Y.; Han, J.; Wu, D.; Kang, F.; Yang, Q.H.; Lv, W. A protective layer for lithium metal anode: Why and how. Small Methods 2021, 5, 2001035. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, H.; Zhai, T. Reviving lithium-metal anodes for next-generation high-energy batteries. Adv. Mater. 2017, 29, 1700007. [Google Scholar] [CrossRef]
- Zhu, B.; Jin, Y.; Hu, X.; Zheng, Q.; Zhang, S.; Wang, Q.; Zhu, J. Poly (dimethylsiloxane) thin film as a stable interfacial layer for high-performance lithium-metal battery anodes. Adv. Mater. 2017, 29, 1603755. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.; Liu, Y.; Liu, C.; Hsu, P.-C.; Bao, Z. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, D.; Gao, Y.; Chen, T.; Huang, Q.; Wang, D. Stable Li metal anode by a polyvinyl alcohol protection layer via modifying solid-electrolyte interphase layer. Nano Energy 2019, 64, 103893. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Cui, Y.; Liu, S.; Chen, Z.; Huang, W.; Li, C.; Liu, R.; Fu, R.; Wu, D. A robust all-organic protective layer towards ultrahigh-rate and large-capacity Li metal anodes. Nat. Nanotechnol. 2022, 17, 613–621. [Google Scholar] [CrossRef]
- Zhao, B.; Xing, C.; Shi, Y.; Duan, Q.; Shen, C.; Li, W.; Jiang, Y.; Zhang, J. Construction of high elastic artificial SEI for air-stable and long-life lithium metal anode. J. Colloid Interface Sci. 2023, 642, 193–203. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Lu, W.; Sun, L.; Lin, L.; Zheng, M.; Sun, X.; Xie, H. PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries. Nano Energy 2021, 88, 106205. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, R.K. Development of ion conducting polymer gel electrolyte membranes based on polymer PVdF-HFP, BMIMTFSI ionic liquid and the Li-salt with improved electrical, thermal and structural properties. J. Mater. Chem. 2015, 3, 7305–7318. [Google Scholar]
- Zainuddin, Z.; Hambali, D.; Supa’at, I.; Osman, Z. Ionic conductivity, ionic transport and electrochemical characterizations of plastic crystal polymer electrolytes. Ionics 2017, 23, 265–273. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, X.J.; Hou, Y.Y.; Gao, X.W.; Liu, L.L.; Wu, Y.P.; Shimizu, M. A new single-ion polymer electrolyte based on polyvinyl alcohol for lithium ion batteries. Electrochim. Acta 2013, 87, 113–118. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chen, H.-H.; Wen, T.-C.; Digar, M.; Gopalan, A. Morphology and ionic conductivity of thermoplastic polyurethane electrolytes. J. Appl. Polym. Sci. 2004, 91, 1154–1167. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Agostini, M.; Enotiadis, A.; Brutti, S. A Novel Li+-Nafion-Sulfonated Graphene Oxide Membrane as Single Lithium-Ion Conducting Polymer Electrolyte for Lithium Batteries. J. Phys. Chem. C 2019, 123, 27406–27416. [Google Scholar] [CrossRef]
- Falco, M.; Castro, L.; Nair, J.R.; Bella, F.; Bardé, F.; Meligrana, G.; Gerbaldi, C. UV-Cross-Linked Composite Polymer Electrolyte for High-Rate, Ambient Temperature Lithium Batteries. ACS Appl. Energy Mater. 2019, 2, 1600. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Li, Y.C.; Huang, Q.; Mallouk, T.E.; Wang, D. Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 2017, 139, 15288–15291. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, Z.; Gray, J.L.; He, X.; Wang, D.; Chen, T.; Huang, Q.; Li, Y.C.; Wang, H.; Kim, S.H. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, G.; Gao, Y.; Wang, D.; Huang, Q.; Wang, D. Stable Li metal anode by a hybrid lithium polysulfidophosphate/polymer cross-linking film. ACS Energy Lett. 2019, 4, 1271–1278. [Google Scholar] [CrossRef]
- Ordaz, M.V.; Pavlin, N.; Gastaldi, M.; Gerbaldi, C.; Dominko, R. Protective Coating for Stable Cycling of Li-Metal Batteries Based on Cellulose and Single-Ion Conducting Polymer. ACS Appl. Mater. Interfaces 2024, 16, 68237–68246. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, X.; Liu, Z.; Zheng, Y.; Zhang, T.; Rahmati, F.; Yan, S.; Qian, L.; Dong, J.; Ma, C. Novel “sandwich” configuration with ALD-coating layers on electrode/electrolyte interfaces for durable all-solid-state lithium metal batteries with high-voltage cathodes. Energy Mater. 2025, 5, 500064. [Google Scholar] [CrossRef]
- Pathak, R.; Chen, K.; Gurung, A.; Reza, K.M.; Bahrami, B.; Pokharel, J.; Baniya, A.; He, W.; Wu, F.; Zhou, Y. Fluorinated hybrid solid-electrolyte-interphase for dendrite-free lithium deposition. Nat. Commun. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhai, P.; Wei, Y.; Yang, Z.; Chen, Q.; Zuo, J.; Gu, X.; Gong, Y. Constructing Artificial SEI Layer on Lithiophilic MXene Surface for High-Performance Lithium Metal Anodes. Adv. Sci. 2022, 9, 2103930. [Google Scholar] [CrossRef]
- You, J.; Deng, H.; Zheng, X.; Yan, H.; Deng, L.; Zhou, Y.; Li, J.; Chen, M.; Wu, Q.; Zhang, P. Stabilized and almost dendrite-free Li metal anodes by in situ construction of a composite protective layer for Li metal batteries. ACS Appl. Mater. Interfaces 2022, 14, 5298–5307. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv. Energy Mater. 2021, 11, 2003092. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, C.; Song, L.; Sun, Z. Electrical and lithium ion dynamics in three main components of solid electrolyte interphase from density functional theory study. J. Phys. Chem. C 2011, 115, 7044–7049. [Google Scholar] [CrossRef]
- Ko, J.; Yoon, Y.S. Recent progress in LiF materials for safe lithium metal anode of rechargeable batteries: Is LiF the key to commercializing Li metal batteries? Ceram. Int. 2019, 45, 30–49. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.; Chen, X.; Legut, D.; Fan, Y.; Zhang, R.; Lu, Y.; Cheng, X.; Zhang, Q. Rational design of graphitic-inorganic Bi-layer artificial SEI for stable lithium metal anode. Energy Storage Mater. 2019, 16, 426–433. [Google Scholar] [CrossRef]
- Hu, A.; Chen, W.; Du, X.; Hu, Y.; Lei, T.; Wang, H.; Xue, L.; Li, Y.; Sun, H.; Yan, Y.; et al. An artificial hybrid interphase for an ultrahigh-rate and practical lithium metal anode. Energy Environ. Sci. 2021, 14, 4115–4124. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat. Energy 2018, 3, 310–316. [Google Scholar] [CrossRef]
- Choudhury, S.; Tu, Z.; Stalin, S.; Vu, D.; Fawole, K.; Gunceler, D.; Sundararaman, R.; Archer, L.A. Electroless formation of hybrid lithium anodes for fast interfacial ion transport. Angew. Chem. Int. Ed. 2017, 56, 13070–13077. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, C.J.; Lu, P.; Dong, Y.; Wen, P.; Wu, Z.-S. Conducting and lithiophilic MXene/graphene framework for high-capacity, dendrite-free lithium–metal anodes. ACS Nano 2019, 13, 14308–14318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, S.; Li, B.; Gong, Y.; Yang, S. Horizontal growth of lithium on parallelly aligned MXene layers towards dendrite-free metallic lithium anodes. Adv. Mater. 2019, 31, 1901820. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, P.; Liu, Y.; Ouyang, Y.; Li, D.; Zhai, T.; Li, H.; Cui, Y. An autotransferable g-C3N4 Li+-modulating layer toward stable lithium anodes. Adv. Mater. 2019, 31, 1900342. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, D.J.; Kim, Y.-J.; Park, J.-K.; Kim, H.-T. A simple composite protective layer coating that enhances the cycling stability of lithium metal batteries. J. Power Sources 2015, 284, 103–108. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.; Song, J.; Ryou, M.-H.; Lee, Y.M.; Kim, H.-T.; Park, J.-K. Composite protective layer for Li metal anode in high-performance lithium–oxygen batteries. Electrochem. Commun. 2014, 40, 45–48. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial soft–rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries. ACS Energy Lett. 2017, 2, 1337–1345. [Google Scholar] [CrossRef]
- Schiele, A.; Breitung, B.; Hatsukade, T.; Berkes, B.B.; Hartmann, P.; Janek, J.r.; Brezesinski, T. The critical role of fluoroethylene carbonate in the gassing of silicon anodes for lithium-ion batteries. ACS Energy Lett. 2017, 2, 2228–2233. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Chesneau, F.; Schmidt, M.; Aurbach, D. Very stable lithium metal stripping–plating at a high rate and high areal capacity in fluoroethylene carbonate-based organic electrolyte solution. ACS Energy Lett. 2017, 2, 1321–1326. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.B.; Tian, Y.; Chen, X.; Zhang, X.Q.; Li, W.J.; Huang, J.Q.; Zhang, Q. Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 2018, 30, 1707629. [Google Scholar] [CrossRef]
- Li, S.; Fan, L.; Lu, Y. Rational design of robust-flexible protective layer for safe lithium metal battery. Energy Storage Mater. 2019, 18, 205–212. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, X.B.; Zhao, C.Z.; Peng, H.J.; Shi, J.L.; Huang, J.Q.; Wang, J.; Wei, F.; Zhang, Q. Conductive nanostructured scaffolds render low local current density to inhibit lithium dendrite growth. Adv. Mater. 2016, 28, 2155–2162. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.; Seh, Z.W.; Liu, N.; Wang, S.; Sun, J.; Lee, H.R.; Cui, Y. Graphite-encapsulated Li-metal hybrid anodes for high-capacity Li batteries. Chem 2016, 1, 287–297. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Zhou, Y.; Xu, E.; Lai, C.; Dou, P.; Tian, X.; Yuan, Z. Controllable organic/inorganic composite film enables improved long-term and high-temperature storage performance of lithium primary battery. Electrochim. Acta 2024, 477, 143776. [Google Scholar] [CrossRef]
- Lee, J.; Lim, H.-S.; Cao, X.; Ren, X.; Kwak, W.-J.; Rodríguez-Pérez, I.A.; Zhang, J.-G.; Lee, H.; Kim, H.-T. Lithium Dendrite Suppression with a Silica Nanoparticle-Dispersed Colloidal Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 37188–37196. [Google Scholar] [CrossRef]
- Yang, D.; Wu, X.; He, L.; Zhao, H.; Wang, Y.; Zhang, Z.; Qiu, J.; Chen, X.; Wei, Y. Ionic Layer Epitaxy Growth of Organic/Inorganic Composite Protective Layers for Large-Area Li and Zn Metal Anodes. Nano Lett. 2023, 23, 11152–11160. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, T.; Chae, O.B. Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries. Batteries 2024, 10, 135. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Lu, Y. 3D porous Cu current collector/Li-metal composite anode for stable lithium-metal batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, P.; Yang, W.; Wei, Y.; Xiao, J.; Deng, L.; Gong, Y. Large-scale modification of commercial copper foil with lithiophilic metal layer for Li metal battery. Small 2020, 16, 1905620. [Google Scholar] [CrossRef]
- Cho, K.-Y.; Hong, S.-H.; Kwon, J.; Song, H.; Kim, S.; Jo, S.; Eom, K. Effects of a nanometrically formed lithiophilic silver@copper current collector on the electrochemical nucleation and growth behaviors of lithium metal anodes. Appl. Surf. Sci. 2021, 554, 149578. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, C.; Zhou, Y.; Ülgüt, B.; Zhao, Y.; Qian, J. Guided lithium nucleation and growth on lithiophilic tin-decorated copper substrate. J. Energy Chem. 2022, 74, 412–419. [Google Scholar] [CrossRef]
- Tchoe, Y.; Song, M.S.; Kim, H.; Baek, H.; Park, J.Y.; Oh, H.; Lee, K.; Chung, K.; Hyun, J.K.; Yi, G.-C. Individually addressable, high-density vertical nanotube Schottky diode crossbar array. Nano Energy 2020, 76, 104955. [Google Scholar] [CrossRef]
- Chen, K.H.; Sanchez, A.J.; Kazyak, E.; Davis, A.L.; Dasgupta, N.P. Synergistic effect of 3D current collectors and ALD surface modification for high coulombic efficiency lithium metal anodes. Adv. Energy Mater. 2019, 9, 1802534. [Google Scholar] [CrossRef]
- Lu, L.-L.; Ge, J.; Yang, J.-N.; Chen, S.-M.; Yao, H.-B.; Zhou, F.; Yu, S.-H. Free-standing copper nanowire network current collector for improving lithium anode performance. Nano Lett. 2016, 16, 4431–4437. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lei, D.; Lv, W.; Zhao, Q.; Ni, B.; Liu, Y.; Li, B.; Kang, F.; He, Y.-B. Spherical Li deposited inside 3D Cu skeleton as anode with ultrastable performance. ACS Appl. Mater. Interfaces 2018, 10, 20244–20249. [Google Scholar] [CrossRef]
- Wang, S.H.; Yin, Y.X.; Zuo, T.T.; Dong, W.; Li, J.Y.; Shi, J.L.; Zhang, C.H.; Li, N.W.; Li, C.J.; Guo, Y.G. Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv. Mater. 2017, 29, 1703729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Li, G.; Jiang, T.; Tong, Y.; Yue, X.; Zhang, J.; Mao, Z.; Sun, W.; Sun, K. Inspired by the “tip effect”: A novel structural design strategy for the cathode in advanced lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 3140–3144. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, C.; Chang, N.; Song, Y.; Zhang, H.; Yin, Y.; Li, X. Act in contravention: A non-planar coupled electrode design utilizing “tip effect” for ultra-high areal capacity, long cycle life zinc-based batteries. Sci. Bull. 2021, 66, 889–896. [Google Scholar] [CrossRef]
- Li, W.; Luo, P.; Chen, M.; Lin, X.; Du, L.; Song, H.; Lu, Y.; Cui, Z. Hedging Li dendrite formation by virtue of controllable tip effect. J. Mater. Chem. A 2022, 10, 15161–15168. [Google Scholar] [CrossRef]
- Kim, G.; Seok, J.Y.; Kim, Y.U.; Kwon, S.; Kim, H.; Woo, Y.M.; Yang, W.; Park, J.H.; Park, C.; Woo, K. Ultrafast and Scalable Fabrication of Cu–CuxO Nanostructures for Stabilizing Lithium Metal Anodes via Flashlight Irradiation. ACS Appl. Nano Mater. 2024, 7, 21250–21260. [Google Scholar] [CrossRef]
- Zhan, Y.X.; Shi, P.; Ma, X.X.; Jin, C.B.; Zhang, Q.K.; Yang, S.J.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q. Failure mechanism of lithiophilic sites in composite lithium metal anode under practical conditions. Adv. Energy Mater. 2022, 12, 2103291. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Deng, W.; Liu, Z. Entrapping lithium deposition in lithiophilic reservoir constructed by vertically aligned ZnO nanosheets for dendrite-free Li metal anodes. Nano Energy 2019, 62, 55–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Pastel, G.; Kuang, Y.; Xie, H.; Li, Y.; Liu, B.; Luo, W.; Chen, C.; Hu, L. 3D wettable framework for dendrite-free alkali metal anodes. Adv. Energy Mater. 2018, 8, 1800635. [Google Scholar] [CrossRef]
- Liu, W.; Man, J.; Guo, Y.; Du, Y.; Liu, K.; Zhang, H.; Sun, X.; Zhang, N.; Fu, S.; Sun, J. Lithiophilic Sn layer via pre-electroplating to realize the uniform stripping/plating for dendrite free Li metal anodes. Chem. Eng. J. 2023, 475, 146153. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, L.; Li, T.; Zheng, X.; Chen, X.; Huang, W.; Xiong, Q.; Zhang, Y. Lithiophilic ZnCu alloy sites on copper current collector for high performance Li metal anode. Electrochim. Acta 2024, 489, 144294. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.X.; Li, J.Y.; Wang, S.H.; Guo, Y.G.; Wan, L.J. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes. Adv. Mater. 2018, 30, 1706216. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.; Shen, X.; Zhang, X.-Q.; Chen, X.-R.; Cheng, X.-B.; Yan, C.; Zhao, C.-Z.; Zhang, Q. Coralloid Carbon Fiber-Based Composite Lithium Anode for Robust Lithium Metal Batteries. Joule 2018, 2, 764–777. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef]
- Xue, P.; Liu, S.; Shi, X.; Sun, C.; Lai, C.; Zhou, Y.; Sui, D.; Chen, Y.; Liang, J. A Hierarchical Silver-Nanowire–Graphene Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Lithium-Metal Composite Anodes. Adv. Mater. 2018, 30, 1804165. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.R.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Yan, C.; Zhang, Q. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 2017, 129, 7872–7876. [Google Scholar] [CrossRef]
- Gan, H.; Wang, R.; Wu, J.; Chen, H.; Li, R.; Liu, H. Coupling a 3D lithophilic skeleton with a fluorine-enriched interface to enable stable lithium metal anode. ACS Appl. Mater. Interfaces 2021, 13, 37162–37171. [Google Scholar] [CrossRef]
- Fan, X.; Ji, X.; Han, F.; Yue, J.; Chen, J.; Chen, L.; Deng, T.; Jiang, J.; Wang, C. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 2018, 4, eaau9245. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Myung, S.; Kim, G.; Lee, D.; Son, H.; Jang, M.; Park, E.; Son, B.; Jung, Y.-G.; Paik, U.; et al. Facile ex situ formation of a LiF–polymer composite layer as an artificial SEI layer on Li metal by simple roll-press processing for carbonate electrolyte-based Li metal batteries. J. Mater. Chem. A 2020, 8, 17229–17237. [Google Scholar] [CrossRef]
- Cui, C.; Yang, C.; Eidson, N.; Chen, J.; Han, F.; Chen, L.; Luo, C.; Wang, P.F.; Fan, X.; Wang, C. A highly reversible, dendrite-free lithium metal anode enabled by a lithium-fluoride-enriched interphase. Adv. Mater. 2020, 32, 1906427. [Google Scholar] [CrossRef] [PubMed]



| Materials | Ionic Conductivity (S·cm−1 at 25 °C) | Compatibility with Li Metal | Characteristic | Ref. |
|---|---|---|---|---|
| PEO (Polyethylene oxide) | ~10−6 to ~10−1 | Moderate—unstable SEI over the long term | Widely used; limited at room temperature | [75] |
| PVDF-HFP | ~10−5 to 10−4 | Good | High dielectric constant and flexibility | [76] |
| PAN (Polyacrylonitrile) | ~10−5 | Moderate to Good | Good electrochemical stability | [77] |
| PVA (Polyvinyl alcohol) | ~10−6 to 10−5 | Good | Chemically stable, often modified | [78] |
| TPU (Thermoplastic polyurethane) | ~10−5 to 10−4 | Good | Elasticity supports volume change buffering | [79] |
| Nafion (lithiated) | ~10−4 to 10−3 | Excellent | Cation-selective; used in single-ion conductors | [80] |
| PDMS (Polydimethylsiloxane) | ~10−7 to 10−6 | Good | Inert, flexible; often used in composites | [81] |
| Strategy | Dendrite Suppression | Volume Accommodation | Cycling Stability | Fabrication Complexity | Key Limitations |
|---|---|---|---|---|---|
| Polymer-based protective layer | Moderate | Moderate | CE ~ 98% for 200–500 cycles @ 1–2 mA cm−2 | Low | Limited ionic conductivity; thermal instability |
| Inorganic-based protective layer | High | Low | CE > 98% for 300–800 cycles @ 1–3 mA cm−2 | Moderate | Brittle; prone to cracking and interfacial mismatch |
| Composite protective layer | High | Moderately High | CE > 98% for >600 cycles @ 1–5 mA cm−2 | High | Complex synthesis; integration challenges |
| Porous copper current collector | Moderate | Moderate | CE ~ 97–98% for 200–500 cycles @ 1–2 mA cm−2 | Moderate | Requires precise 3D architecture control |
| Effect of incorporating lithiophilic materials | High | Low | CE > 98% for 300–700 cycles @ 1–3 mA cm−2 | High | Costly materials; surface treatment complexity |
| Carbon-based frameworks | High | High | CE > 98% for >1000 cycles @ 1–5 mA cm−2 | Moderately high | Requires tailored interface design and conductivity balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Song, C.; Chae, O.B. Protective Layer and Current Collector Design for Interface Stabilization in Lithium-Metal Batteries. Batteries 2025, 11, 220. https://doi.org/10.3390/batteries11060220
Kim D, Song C, Chae OB. Protective Layer and Current Collector Design for Interface Stabilization in Lithium-Metal Batteries. Batteries. 2025; 11(6):220. https://doi.org/10.3390/batteries11060220
Chicago/Turabian StyleKim, Dayoung, Cheolhwan Song, and Oh B. Chae. 2025. "Protective Layer and Current Collector Design for Interface Stabilization in Lithium-Metal Batteries" Batteries 11, no. 6: 220. https://doi.org/10.3390/batteries11060220
APA StyleKim, D., Song, C., & Chae, O. B. (2025). Protective Layer and Current Collector Design for Interface Stabilization in Lithium-Metal Batteries. Batteries, 11(6), 220. https://doi.org/10.3390/batteries11060220








