Pulse-Driven Internal Resistance Dynamics Enable Dual-Function Lithium-Plating Diagnosis and Longevity Enhancement in V2G-Optimized Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Capacity Test
2.2. Low-Temperature Charging Test
2.3. Observing the Anode Using SEM
3. Results
3.1. Capacity Results
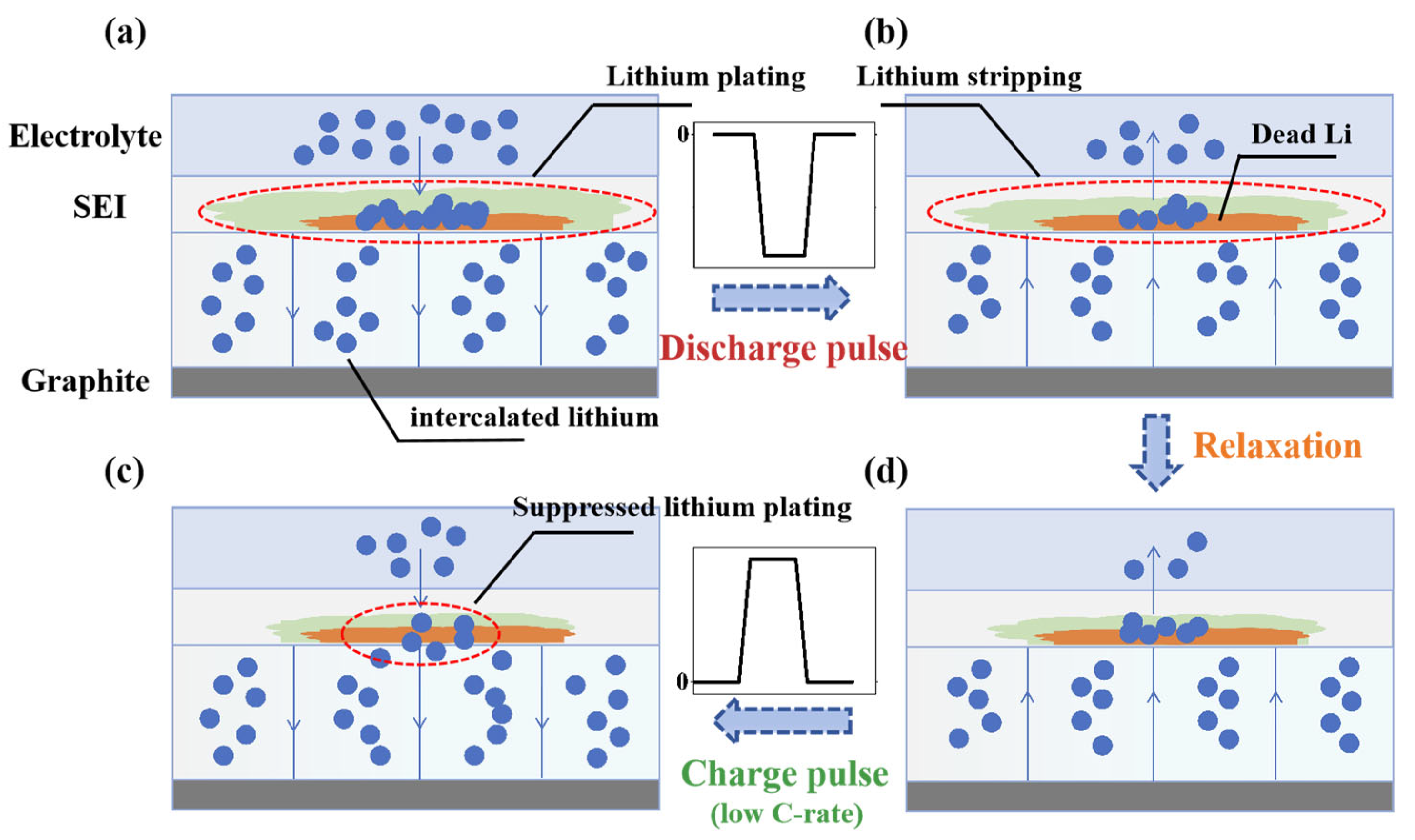
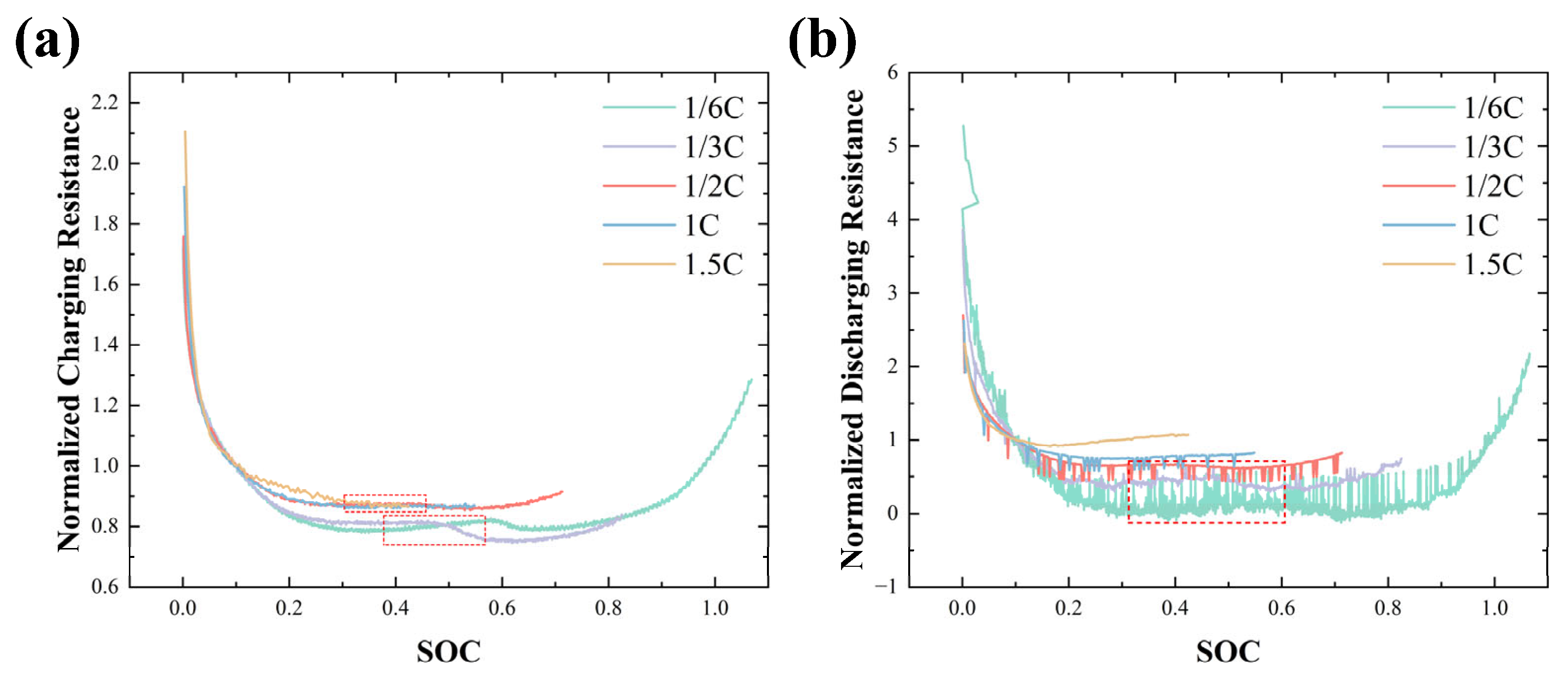
3.2. Pulse Effect Analysis
3.3. Lithium-Plating Detection Method Under Pulse Charging
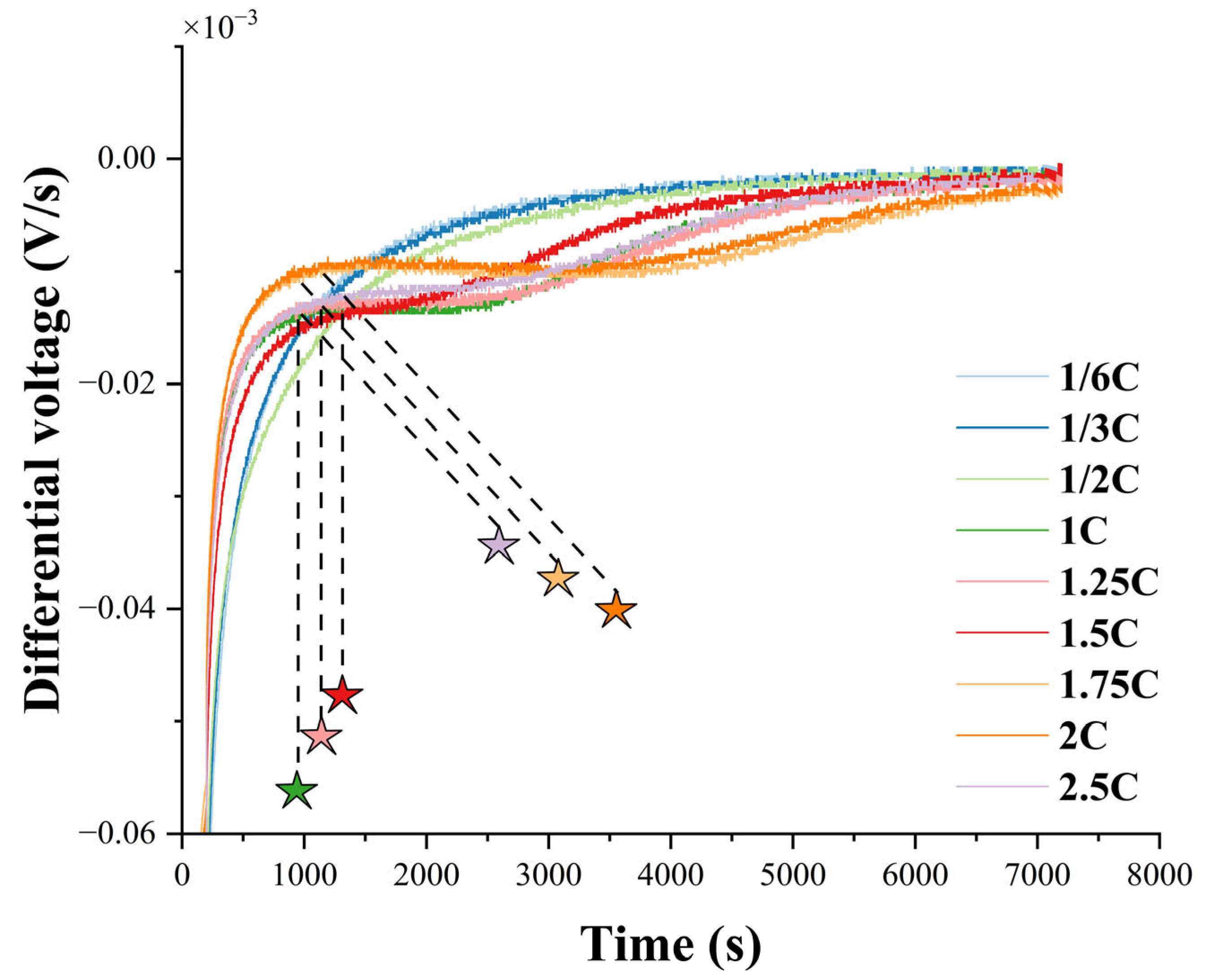
3.4. Validation of Results
3.4.1. Relaxation Voltage Method
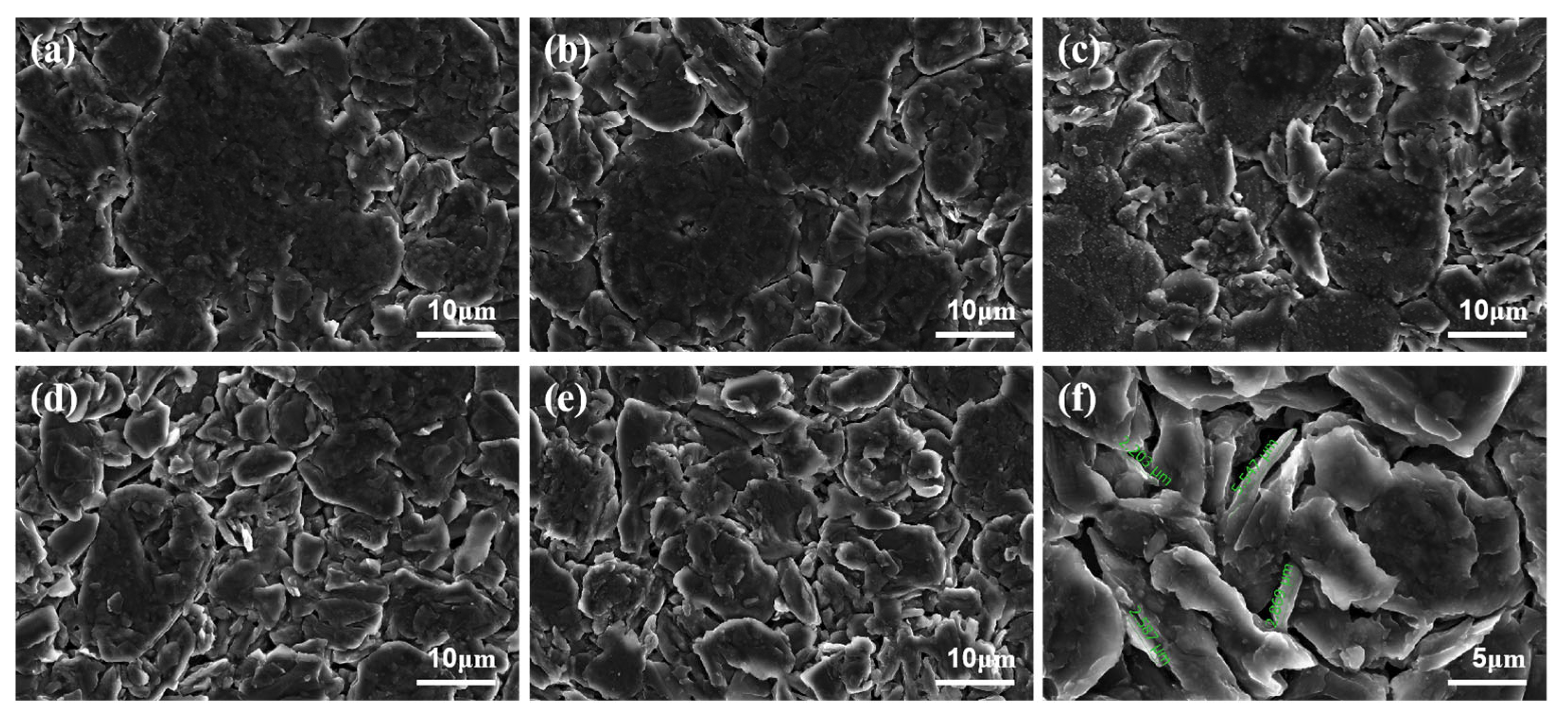
3.4.2. Ex Situ SEM Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chigbu, B.I. Advancing sustainable development through circular economy and skill development in EV lithium-ion battery recycling: A comprehensive review. Front. Sustain. 2024, 5, 1409498. [Google Scholar] [CrossRef]
- Morita, Y.; Saito, Y.; Yoshioka, T.; Shiratori, T. Estimation of recoverable resources used in lithium-ion batteries from portable electronic devices in Japan. Resour. Conserv. Recycl. 2021, 175, 105884. [Google Scholar] [CrossRef]
- Salkuti, S.R. Electrochemical batteries for smart grid applications. Int. J. Electr. Comput. Eng. 2021, 11, 1849–1856. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Yu, H. Design of high-energy-density lithium batteries: Liquid to all solid state. eTransportation 2025, 23, 100382. [Google Scholar] [CrossRef]
- Nasajpour-Esfahani, N.; Garmestani, H.; Bagheritabar, M.; Jasim, D.J.; Toghraie, D.; Dadkhah, S.; Firoozeh, H. Comprehensive review of lithium-ion battery materials and development challenges. Renew. Sustain. Energy Rev. 2024, 203, 114783. [Google Scholar] [CrossRef]
- Wu, S.; Wang, C.; Luan, W.; Zhang, Y.; Chen, Y.; Chen, H. Thermal runaway behaviors of Li-ion batteries after low temperature aging: Experimental study and predictive modeling. J. Energy Storage 2023, 66, 107451. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Hong, Y.; Xu, C.; Zheng, Y.; Jin, C.; Chen, K.; He, Y.; Feng, X.; Wei, X. Thermal safety boundary of lithium-ion battery at different state of charge. J. Energy Chem. 2024, 91, 59–72. [Google Scholar] [CrossRef]
- Tang, X.; Guo, Q.; Li, M.; Wei, C.; Pan, Z.; Wang, Y. Performance analysis on liquid-cooled battery thermal management for electric vehicles based on machine learning. J. Power Sources 2021, 494, 229727. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Fuchs, A. Influence of aging on the failing behavior of automotive lithium-ion batteries. Batteries 2021, 7, 23. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and characterization of Li(Co0.5Ni0.5)PO4 cathode for Li-ion aqueous battery applications. Electrochem. Solid-State Lett. 2011, 14, A86. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Xie, W.; He, R.; Gao, X.; Li, X.; Wang, H.; Liu, X.; Yan, X.; Yang, S. Degradation identification of LiNi0.8Co0.1Mn0.1O2/graphite lithium-ion batteries under fast charging conditions. Electrochim. Acta 2021, 392, 138979. [Google Scholar] [CrossRef]
- Hoque, M.A.; Nurmi, P.; Kumar, A.; Varjonen, S.; Song, J.; Pecht, M.G.; Tarkoma, S. Data driven analysis of lithium-ion battery internal resistance towards reliable state of health prediction. J. Power Sources 2021, 513, 230519. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Tang, A. Capacity degradation and aging mechanisms evolution of lithium-ion batteries under different operation conditions. Energies 2023, 16, 4232. [Google Scholar] [CrossRef]
- Chen, R.; Miao, S.; Peng, J.; Zhang, K.; Li, Z.; Cai, W.; Jia, Y.; Zhang, X.; Wu, F.; Zhao, J. A review of detecting Li plating on graphite anodes based on electrochemical methods. J. Mater. Chem. A 2024, 12, 33427–33447. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, H.; Yang, X.; Niu, W.; Li, S.; Chen, S. The dilemma of c-rate and cycle life for lithium-ion batteries under low temperature fast charging. Batteries 2022, 8, 234. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Han, G.; Dai, H.; Zhu, J.; Wang, X.; Tang, X.; Ye, J. Lithium plating on the anode for lithium-ion batteries during long-term low temperature cycling. J. Power Sources 2021, 484, 229312. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, X.; Huang, Z.; Meng, J.; Zhong, Y.; Ma, G.; Zhou, Y.; Shen, Y.; Ding, H.; Huang, Y. Recent progress on advanced imaging techniques for lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2000806. [Google Scholar] [CrossRef]
- Tao, M.; Xiang, Y.; Zhao, D.; Shan, P.; Yang, Y. Protocol for quantifying inactive lithium in anode-free lithium batteries by mass spectrometry titration. Commun. Mater. 2022, 3, 50. [Google Scholar] [CrossRef]
- Huang, Y.; Perlmutter, D.; Fei-Huei Su, A.; Quenum, J.; Shevchenko, P.; Parkinson, D.Y.; Zenyuk, I.V.; Ushizima, D. Detecting lithium plating dynamics in a solid-state battery with operando X-ray computed tomography using machine learning. Npj Comput. Materials 2023, 9, 93. [Google Scholar] [CrossRef]
- Min, H.; Yang, Z.; Yang, F.; Hu, W.; Lei, Y.; Luo, S. Design And Application Of A Smart Interactive Distribution Area For Photovoltaic, Energy Storage And Charging Piles. In Proceedings of the 2023 IEEE 6th International Electrical and Energy Conference (CIEEC), Hefei, China, 12–14 May 2023; pp. 2251–2256. [Google Scholar]
- Koseoglou, M.; Tsioumas, E.; Ferentinou, D.; Panagiotidis, I.; Jabbour, N.; Papagiannis, D.; Mademlis, C. Lithium plating detection using differential charging current analysis in lithium-ion batteries. J. Energy Storage 2022, 54, 105345. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, M.; Huang, T.; Yu, A. Detection of lithium plating in lithium-ion batteries by distribution of relaxation times. J. Power Sources 2021, 496, 229867. [Google Scholar] [CrossRef]
- Mojumder, M.R.H.; Ahmed Antara, F.; Hasanuzzaman, M.; Alamri, B.; Alsharef, M. Electric vehicle-to-grid (V2G) technologies: Impact on the power grid and battery. Sustainability 2022, 14, 13856. [Google Scholar] [CrossRef]
- Du, P.; Liu, T.; Chen, T.; Jiang, M.; Zhu, H.; Shang, Y.; Goh, H.H.; Zhao, H.; Huang, C.; Kong, F. Enhancing green mobility through vehicle-to-grid technology: Potential, technological barriers, and policy implications. Energy Environ. Sci. 2025. [Google Scholar] [CrossRef]
- Kumar, D.S.; Krishnan, S.B.; Sharma, A.; Tak, T.K.; Rathore, A.K. Vehicle-to-Grid (V2G) Technology: Global Scenario, Future Scope, Challenges, and Implementation. In Proceedings of the 2024 IEEE International Conference on Power Electronics, Drives and Energy Systems (PEDES), Mangalore, India, 18–21 December 2024; pp. 1–6. [Google Scholar]
- Sufyan, M.; Rahim, N.; Muhammad, M.A.; Tan, C.K.; Raihan, S.R.S.; Bakar, A. Charge coordination and battery lifecycle analysis of electric vehicles with V2G implementation. Electr. Power Syst. Res. 2020, 184, 106307. [Google Scholar] [CrossRef]
- Leippi, A.; Fleschutz, M.; Murphy, M.D. A review of ev battery utilization in demand response considering battery degradation in non-residential vehicle-to-grid scenarios. Energies 2022, 15, 3227. [Google Scholar] [CrossRef]
- Schindler, S.; Bauer, M.; Petzl, M.; Danzer, M.A. Voltage relaxation and impedance spectroscopy as in-operando methods for the detection of lithium plating on graphitic anodes in commercial lithium-ion cells. J. Power Sources 2016, 304, 170–180. [Google Scholar] [CrossRef]
- Koleti, U.R.; Dinh, T.Q.; Marco, J. A new on-line method for lithium plating detection in lithium-ion batteries. J. Power sources 2020, 451, 227798. [Google Scholar] [CrossRef]
- Pan, Y.; Ren, D.; Han, X.; Lu, L.; Ouyang, M. Lithium plating detection based on electrochemical impedance and internal resistance analyses. Batteries 2022, 8, 206. [Google Scholar] [CrossRef]
- Xu, X.; Tang, S.; Han, X.; Lu, L.; Qin, Y.; Du, J.; Wu, Y.; Li, Y.; Yu, C.; Sun, X. Flexible bidirectional pulse charging regulation achieving long-life lithium-ion batteries. J. Energy Chem. 2024, 96, 59–71. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in Lithium-ion batteries–A Post-Mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Chen, Y.; Torres-Castro, L.; Chen, K.-H.; Penley, D.; Lamb, J.; Karulkar, M.; Dasgupta, N.P. Operando detection of Li plating during fast charging of Li-ion batteries using incremental capacity analysis. J. Power Sources 2022, 539, 231601. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Su, L.; Li, Z.; Liaw, B.Y.; Zhang, J. Lithium plating detection and quantification in Li-ion cells from degradation behaviors. ECS Trans. 2017, 75, 37. [Google Scholar] [CrossRef]
- Ge, B.; Deng, J.; Wang, Z.; Liang, Q.; Hu, L.; Ren, X.; Li, R.; Lin, Y.; Li, Y.; Wang, Q. Aggregate-Dominated Dilute Electrolytes with Low-Temperature-Resistant Ion-Conducting Channels for Highly Reversible Na Plating/Stripping. Adv. Mater. 2024, 36, 2408161. [Google Scholar] [CrossRef]



| Method | Detection Accuracy | Testing Duration | V2G Application Feasibility |
|---|---|---|---|
| Arrhenius method [34] | Qualitative | Long | No |
| Incremental capacity analysis [35] | Quantitative | Short | No |
| Internal resistance-capacity method [36] | Qualitative | Long | Yes |
| Voltage relaxation method [23,24] | Quantitative | Moderate | No |
| Electrochemical impedance spectroscopy analysis [30] | Qualitative | Short | No |
| Dynamic discharge detection method [32] | Qualitative | Moderate | Yes |
| Proposed method | Qualitative | Short | Yes |
| INR21700-M50 NMC Li-Ion Batteries | |
|---|---|
| Positive electrode | NMC811 |
| Dimensions (D × H) | 21.44 mm × 70.60 mm |
| Weight | 68.2 g ± 1.0 g |
| Nominal voltage | 3.69 V |
| Nominal capacity | 5000 mAh |
| Charging cut-off voltage | 4.20 V |
| Discharging cut-off voltage | 2.50 V |
| Manufacturer | LG-Chem |
| No. | Charging C-Rate | Pulse Parameters | No. | Charging C-Rate | Pulse Parameters | ||
|---|---|---|---|---|---|---|---|
| Amplitude | Period | Amplitude | Period | ||||
| #1-1 | 1/6 C | Without Pulse | #2-1 | 1/6 C | 0.1 C | 40 s | |
| #1-2 | 1/3 C | #2-2 | 1/3 C | ||||
| #1-3 | 1/2 C | #2-3 | 1/2 C | ||||
| #1-4 | 1 C | #2-4 | 1 C | ||||
| #1-5 | 1.5 C | #2-5 | 1.5 C | ||||
| #3-1 | 1/3 C | 0.1 C | 40 s | #4-1 | 1/3 C | 0.1 C | 40 s |
| #3-2 | 0.2 C | #4-2 | 30 s | ||||
| #3-3 | 0.3 C | #4-3 | 20 s | ||||
| #3-4 | 0.4 C | #4-4 | 10 s | ||||
| Constant Current | Pulse Current | ||
|---|---|---|---|
| C-Rate | Capacity Change Ratio | C-Rate | Capacity Change Ratio |
| 1/6 C | −0.37 | 1/6 C | −0.61 |
| 1/3 C | −0.72 | 1/3 C | −1.03 |
| 1/2 C | −1.09 | 1/2 C | −0.25 |
| 1 C | −4.89 | 1 C | −0.70 |
| 1.5 C | −12.10 | 1.5 C | −3.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, Y.; Guo, D.; Han, X.; Wang, H.; Sun, L.; Ouyang, M. Pulse-Driven Internal Resistance Dynamics Enable Dual-Function Lithium-Plating Diagnosis and Longevity Enhancement in V2G-Optimized Lithium-Ion Batteries. Batteries 2025, 11, 200. https://doi.org/10.3390/batteries11050200
Li L, Wang Y, Guo D, Han X, Wang H, Sun L, Ouyang M. Pulse-Driven Internal Resistance Dynamics Enable Dual-Function Lithium-Plating Diagnosis and Longevity Enhancement in V2G-Optimized Lithium-Ion Batteries. Batteries. 2025; 11(5):200. https://doi.org/10.3390/batteries11050200
Chicago/Turabian StyleLi, Letong, Yanan Wang, Dongliang Guo, Xuebing Han, Hewu Wang, Lei Sun, and Minggao Ouyang. 2025. "Pulse-Driven Internal Resistance Dynamics Enable Dual-Function Lithium-Plating Diagnosis and Longevity Enhancement in V2G-Optimized Lithium-Ion Batteries" Batteries 11, no. 5: 200. https://doi.org/10.3390/batteries11050200
APA StyleLi, L., Wang, Y., Guo, D., Han, X., Wang, H., Sun, L., & Ouyang, M. (2025). Pulse-Driven Internal Resistance Dynamics Enable Dual-Function Lithium-Plating Diagnosis and Longevity Enhancement in V2G-Optimized Lithium-Ion Batteries. Batteries, 11(5), 200. https://doi.org/10.3390/batteries11050200









